Abstract
Hypertension (HTN) is a worldwide problem with major impacts on health including morbidity and mortality, as well as consumption of health care resources. Nearly 50% of American adults have high blood pressure, and this rate is rising. Even with multiple antihypertensive drugs and aggressive lifestyle modifications, blood pressure is inadequately controlled in about 1 of 5 hypertensive individuals. This review highlights a hypothesis for HTN that suggests alternative mechanisms for blood pressure elevation and maintenance. A better understanding of these mechanisms could open avenues for more successful treatments.
The hypothesis accounts for recent understandings of the involvement of gut physiology, gut microbiota, and neuroinflammation in HTN. It includes bidirectional communication between gut microbiota and gut epithelium in the gut-brain axis that is involved in regulation of autonomic nervous system activity and blood pressure control. Dysfunction of this gut-brain axis, including dysbiosis of gut microbiota, gut epithelial dysfunction and deranged input to the brain, contributes to HTN via inflammatory mediators, metabolites, bacteria in the circulation, afferent information alterations, etc. resulting in neuroinflammation and unbalanced autonomic nervous system activity that elevates blood pressure. This in turn negatively affects gut function and its microbiota exacerbating the problem.
We focus this review on the gut-brain axis hypothesis for HTN, and possible contribution to racial disparities in HTN. A novel idea, that immunoglobulin A-coated bacteria originating in the gut with access to the brain could be involved in HTN, is raised. Finally, minocycline, with its anti-inflammatory and anti-microbial properties, is evaluated as a potential anti-hypertensive drug acting on this axis.
Keywords: Hypertension, neuroinflammation, gut microbiota, gut epithelium
Introduction
Hypertension (HTN) is the leading modifiable risk factor for cardiovascular disease and related morbidity and mortality, worldwide. Over the past 30 years the number of adults with HTN has almost doubled and accounts for ~8 million deaths each year worldwide(1). Racial and ethnic disparities, sedentary lifestyles and diets with high fat and low fiber content, have all contributed to this ever-rising prevalence of HTN. More troubling, rates of blood pressure (BP) control are stagnant, if not declining, in the United States(2). Despite recent advances in use of a combination of antihypertensives, titration to the most effective doses for individual patients, and assertive guidance for life-style changes, successful control and treatment of HTN remains an art rather than an evidence-based practice. This situation is further complicated by evidence that about one in five of all HTN patients do not achieve BP goals even when treated with ≥3 antihypertensive medications, thus have treatment resistant HTN.
Therefore, there is a compelling need for the development of innovative mechanism-based concepts that would provide novel targets for HTN treatment. Involvement of the gut and its microbiota in BP homeostasis and their dysfunction in HTN have been important discoveries in the field and could offer one such innovation. Rapid progress in the last 6–7 years implicating an impaired gut-brain axis in the pathophysiology of HTN and validation of this basic concept by multiple investigators emphasizes the implications for new therapeutic targets. Therefore, our objective in this review is to present succinct summaries of involvement of the gut and its microbiota in HTN, particularly human HTN. We will also address racial/ethnic changes in microbiota and the role of gut epithelium-microbiota communication in HTN. We also discuss several new concepts in the hypertension field, that while still in their infancy, may guide future research and treatment efforts. Our overall approach is to synthesize available literature into critical issues that we believe important in translating the mechanism-based knowledge in this field to development of new strategies for HTN management.
Involvement of the Gastrointestinal Tract
The involvement of the gastrointestinal tract (GI) tract in HTN should have been realized perhaps sooner considering that long-known risk factors for HTN such as low fiber diet, high salt diet, alcohol consumption, and constipation (in older women) directly impact the GI tract and its microbiota. Nevertheless, this is now an area of active research and has the potential to offer key insights for HTN mitigation.
The GI tract is able to digest and absorb organic nutrients, electrolytes and water necessary for health of the host whilst maintaining a tight barrier between the “outside world” of its lumen and the host. It accomplishes this by several means. A mucus layer, involving goblet cells, separates the contents of the gut lumen from the gut epithelium. Few members of the microbiota penetrate or live in this layer under healthy conditions. The epithelial cells form a barrier with tight junctions and other mechanisms and secrete antimicrobial factors to further protect the host. The gut has an immune system that actively surveilles the luminal contents and epithelium and initiates immune responses against pathogens, while maintaining tolerance to the core commensal microbiome of the host that is central to host health by a variety of mechanisms such as vitamin production, bile salt cycling, short chain fatty acid synthesis etc. Meanwhile, it has a complex array of transporters that move nutrients etc. into the host.
The earliest studies of the gut in HTN were performed in hypertensive rats and described dysbiosis in the gut microbiome that was confirmed in hypertensive people(3,4). In rats this was associated with gut pathology including changes in blood flow indicative of inflammation(5), increased gut stiffness(6), increased fibrosis in the muscular layers of the gut, decreased goblet cells, increased norepinephrine containing fibers in the mucosal layer, decreased villus length in the small intestine, changes in the proteins involved in sealing the gut barrier and altered sympathetic flow to the gut. The leaky gut barrier in angiotensin II (Ang II)-induced hypertensive mice could be improved with butyrate supplementation(7). Studies in hypertensive rats suggest that the gut epithelium has altered immune function. Intestinal epithelial cells act as antigen presenting cells, transcriptomic analyses suggest decreased presentation in hypertension, likewise T cell receptor-mediated responses were decreased (8,9). Furthermore, these transcriptional changes are maintained in colon organoid cultures made from hypertensive rats(9). These data suggest gut pathology is present in HTN and could contribute to initiation and or maintenance of HTN by the interruption in barrier function (mucus and epithelial cells involvement), altered immune responses, altered sympathetic input, blood flow, and stiffness.
Altered Gut Microbiota in Animal Models of HTN
There is general agreement for an altered gut microbiota in many animal models of HTN including the spontaneously hypertensive rat(3), stroke-prone spontaneously hypertensive rats(10), AngII-induced hypertensive rats and mice(3,5,7), Dahl salt-sensitive rats(11), mice made hypertensive by high salt diet(12), DOCA-salt induced hypertensive rats(13), obstructive sleep apnea-induced hypertensive rats and mice(14,15), and mice made hypertensive by transfer of fecal matter from hypertensive humans(4). In most cases where it has been tried fecal matter transfer from these animals causes HTN in the normotensive recipient, except for the Dahl-salt sensitive and resistant rat strains that responded paradoxically(11).
Mice hypertensive due to a high salt diet(16) have specific depletion of Lactobacillus murinus, and supplementation with this bacterium prevented HTN through mechanisms related to Th17-mediated immunity. As the name implies, these bacteria are found only in the gut of mice. Rodents have a stratified, squamous epithelia in their gut that allows specific adhesion of Lactobacilli in biofilms. Similar colonization of the gut by Lactobacilli is not observed in humans, though it is in oral cavity, etc., where this type of epithelium is found (17). Depletion of Lactobacilli in the gut microbiota in some people on high salt diets has been observed(16). But there is debate about whether Lactobacilli are normal residents of the human gut or occur there from diet or the use of proton pump inhibitors, that allow these acid-intolerant bacteria to descend lower into the GI tract than usual.
It would be useful if studies revealed a specific bacterium that was either overabundant or depleted in hypertension so that tailored therapy could be performed to correct the imbalance. However, in animal models and humans with hypertension, specific bacteria were not usually associated with hypertension, rather the function of the entire gut microbiota was altered. For example, dysbiotic microbiota of HTN are depleted of short chain fatty acid-producing bacteria, but since many bacteria have this function and different ones populate individuals’ microbiomes, specifically depleted or overabundant bacteria species or strains were not observed in HTN (1,2,3,5,8–14).
Our studies, and those of others (4,18) showed that functions of the gut microbiota are more different in hypertensive and normotensive animals and people than the bacteria present(7). This suggests that the gut environment in HTN either promotes or permits shifts in what the microbiota does and how the bacterial communities interact. Most available information about gut microbiota function relates to metabolism; HTN has been associated with increases in microbial membrane transport, lipopolysaccharide biosynthesis and steroid degradation, but decreases in vitamin synthesis, amino acid and co-factor metabolism(18). This information is cost prohibitive sometimes, requiring whole genome (shotgun) sequencing rather than the cheaper 16S rRNA sequencing. However, as the cost of sequencing continues to decline, combined with recognition of their value, these data and their inferences should become more widely available.
Metabolic function of the gut microbiota can also be assessed by measurement of circulating metabolites. This is an emerging field that is restricted by the lack of databases attributing metabolites origins to the gut microbiota and the many unidentified metabolites that are measured by the techniques. Metabolites of the gut microbiota, the result of its digestion of multiple energy sources, nonetheless are important in HTN(4,19–22). Gut microbiota-dependent metabolites were altered in HTN induced by Ang II in mice. Some metabolites were altered in a fashion suggesting they were host-protective and produced by the gut microbiota in response to HTN, while others were potentially harmful (23).
Brain, Neuroinflammation and Autonomic Nervous System in Hypertension
The autonomic nervous system (ANS) controls BP via sympathetic and parasympathetic outflow. Signals are integrated in the brain in the central autonomic network (24) to affect ANS activity and therefore BP. Neuroinflammation is particularly apparent in the hypothalamic paraventricular nucleus (PVN) of hypertensive rodents(23,24), part of the central autonomic network that houses pre-autonomic neurons. But it is also seen in brainstem areas of the central autonomic network, for example in the nucleus of the solitary tract and rostroventrolateral medulla in animal models of HTN. The pre-autonomic neurons in the PVN receive input from multiple higher brain areas, including areas outside of the blood brain barrier. Thus, they are responsive to circulating factors, as well as afferent inputs via, for example, the nucleus of the solitary tract from organs including the GI tract. Information is integrated in the PVN and passed to brainstem nuclei housing autonomic neurons. Functional MRI (fMRI) is now being used to confirm components of the central autonomic network in humans that previously were only understood through lesion and autopsy information. Brain areas previously not thought to be involved in BP regulation are also being discovered with fMRI, particularly those related to, for example, stress-related BP reactivity(27,28). It will be interesting to understand if neuroinflammation is also present in these brain areas in human HTN. This is an area of research that could be highly relevant but is poorly explored to date.
Increasing evidence supports the concept that neuroinflammation plays an important role in HTN by a multifaceted mechanism(29–36). Enhanced sympathetic nervous system activity is one such facet. Evidence supporting this include: neuroinflammation is intimately linked with elevated sympathetic drive in animal models of HTN(32,37,38). Additionally, increased sympathetic drive is implicated in human HTN(39–43). Central administration of proinflammatory cytokines (e.g., IL1-β, TNF-α) increases SNA and BP in animal models(25,44–46). Furthermore, increased expression of proinflammatory cytokines in cardio-regulatory brain regions has been noted in hypertensive animals(25,47,48). In contrast, administration of the anti-inflammatory cytokine, IL-10, or minocycline exerts an antihypertensive response(25). Also, central IL-10 overexpression reduces TNF-α, IL-1β in the PVN and attenuates hypothalamic inflammation and sympathoexcitation in a rat heart failure model(25,49).
Disruption of the blood-brain barrier (BBB) is another aspect that contributes to neuroinflammation. This allows access of peripheral inflammatory molecules and signals to autonomic brain regions. The integrity of the BBB is tightly regulated by interplay among the participants of the neurovascular unit involving neurons, astrocytes, pericytes, endothelial and microglial cells. Hypertensive stimuli such as Ang II, are known to interrupt neurovascular unit integrity to increase BBB permeability in HTN(50–52). For example, gut dysbiosis and a dysregulated gut epithelial-microbiota-mediated generation of bacterial and other gut metabolites in HTN would have free access to the brain to initiate neuroinflammation. This view is supported by evidence that bacterial products (i.e., tryptophan metabolites, LPS, cytokines, butyrate etc.) that are altered in HTN, are also known to influence neuroinflammation, thus, affecting the glial-neuronal functional unit.
Microglia activation is an integral part of neuroinflammation in HTN. Hypertensive stimuli directly, or indirectly via microbiota-influenced metabolites and a leaky BBB, activate microglia to impair neuronal-microglial communication. In fact, prolonged microglial activation results in their destruction of astrocytic end feet, an important component of the BBB, thus exacerbating this process(53). Any brain insult activates microglia to the M1 state, M1-activated microglia release proinflammatory cytokines and many cytotoxic substances such as quinolinic acid and glutamate. Thus, the M1 microglial phenotype is responsible for creating an inflammatory milieu (36). This phenotype consequently shifts to M2 anti-inflammatory phase, which is responsible for directing tissue repair, ECM formation, debris clearance to return to normal homeostatic balance following insults. We propose that the transition of M1 to M2 activation state of microglia is disrupted creating a persistent inflammatory environment in HTN and would be an important line of further investigation. A similar transition has been reported for other neural diseases including Alzheimer’s disease, multiple sclerosis and encephalomyelitis(54–56). The concept of involvement of activated microglia in HTN has been gaining strength in recent years and our studies were among the first to provide experimental support(25). They showed that induction of HTN by chronic Ang II infusion resulted in an increase in activated microglia predominantly in the PVN, a finding now supported by others(57). Inhibition of microglia activation by central minocycline infusion attenuated HTN(25). This antihypertensive effect of minocycline is unlikely to be due to its antibiotic activity since central administration of CMT-3, an anti-inflammatory tetracycline derivative devoid of antimicrobial activity, also ameliorates HTN(33). Finally, depletion of microglia attenuates HTN(58). Despite this strong evidence in animal models, the role of microglia in human HTN is only now evolving. Our recent pilot trial suggested that treatment with minocycline results in a significant decrease in BP in treatment-resistant HTN patients. This appears to also decrease activated microglia in brain sympathetic region in PET scans(59). This needs to be confirmed by a larger double-blind randomized study.
Finally, it will be important to investigate whether neuroinflammatory pathways are causative or consequential in HTN. The decrease in BP and attenuation of HTN by anti-inflammatory drugs such as minocycline and CMT-3, the microglia depletion experiment and the increase in BP by ICV administration of inflammatory cytokines all support a causative relationship. Additional study is warranted to solidify the concept.
Hypothesis: Gut-Brain Axis Dysfunction Causes HTN
The previous sections of this review highlighted strong evidence for roles of the gut, its microbiota and neuroinflammation in HTN. These studies led us to propose a gut-brain bidirectional communication hypothesis for BP control and impaired interactions within this axis in HTN and is presented in Figures 1 and 2. Hypertensive stimuli such as stress, diet, salt, maternal factors, environmental toxins etc. influence autonomic brain regions to affect neural, endocrine and immune pathways. During HTN the neural pathway exhibits increased sympathetic and attenuated parasympathetic activities. The pro-hypertensive endocrine pathway primarily results from activation of the hypothalamic-pituitary-adrenal axis while the immune pathway is disordered interplay of the complex interactions of bone marrow, gut, spleen and other immune regulatory organs. These pathways can act rapidly to activate blood vessel tone and contraction of vascular smooth muscle increasing peripheral resistance and BP. Increased neural activity could also trigger neuroinflammatory events that activate microglia, increase proinflammatory molecules and create an inflammatory milieu in autonomic brain regions, primarily in the hypothalamic paraventricular region.
Figure 1. Bidirectional gut-brain axis in hypertension.
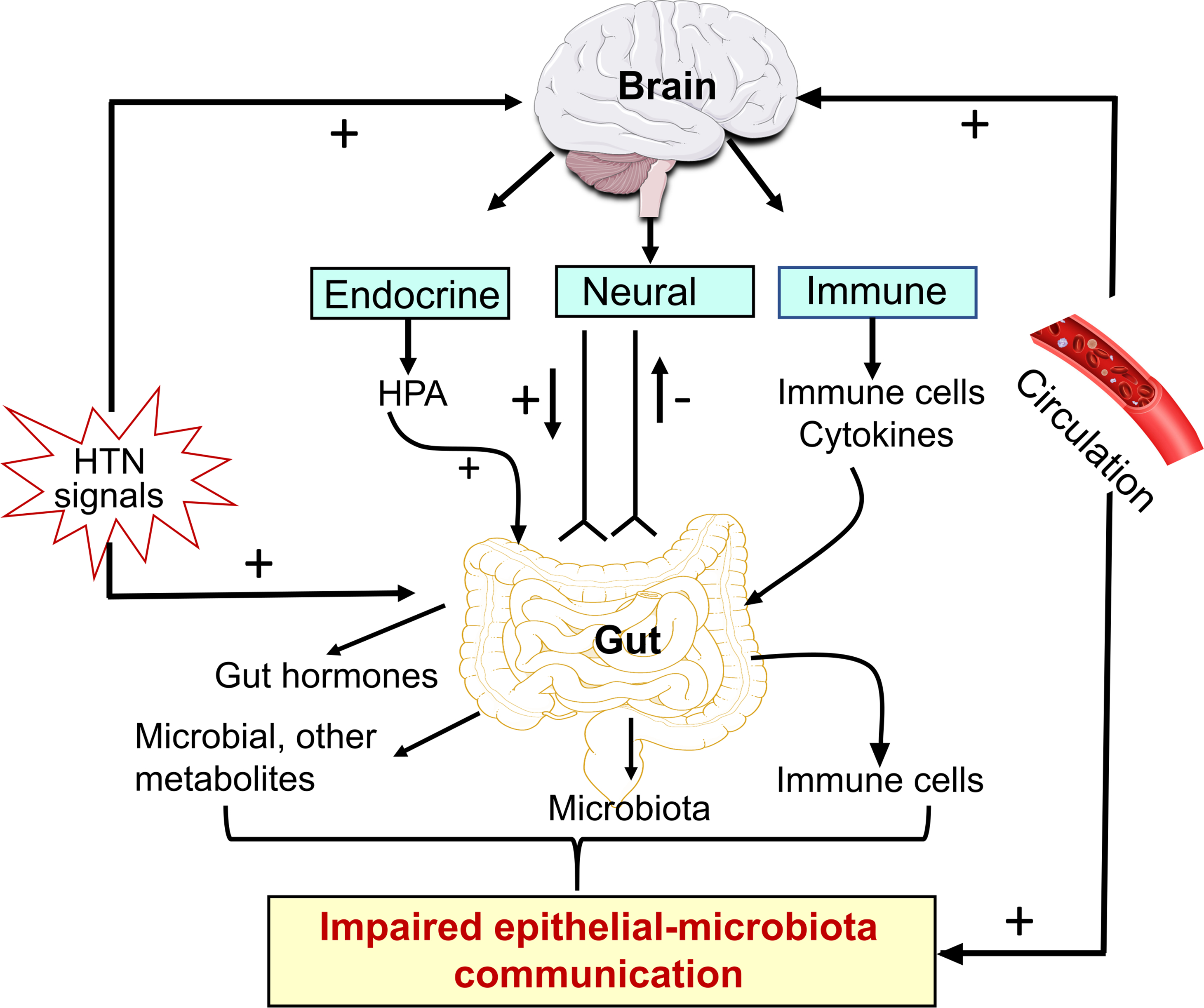
HTN stimuli influence both autonomic brain regions and the gut in the regulation of BP. They stimulate brain endocrine, neural and immune system associated pathways which converge on to the gut to regulate secretion of gut hormones, microbial communities, metabolites and gut immune status. These culminate in impaired epithelial-microbiota communication in HTN. Furthermore, HTN-associated dysbiosis-linked altered gut metabolites are released into the blood through leaky gut, access the brain through leaky BBB, and influence neuronal-microglial communication increasing neuroinflammation. HTN stimuli can also influence the gut directly to initiate alteration in epithelial-microbiota interactions. Illustration Credit: Ben Smith.
Figure 2. Salient steps associated with a dysfunctional gut-brain axis in hypertension.
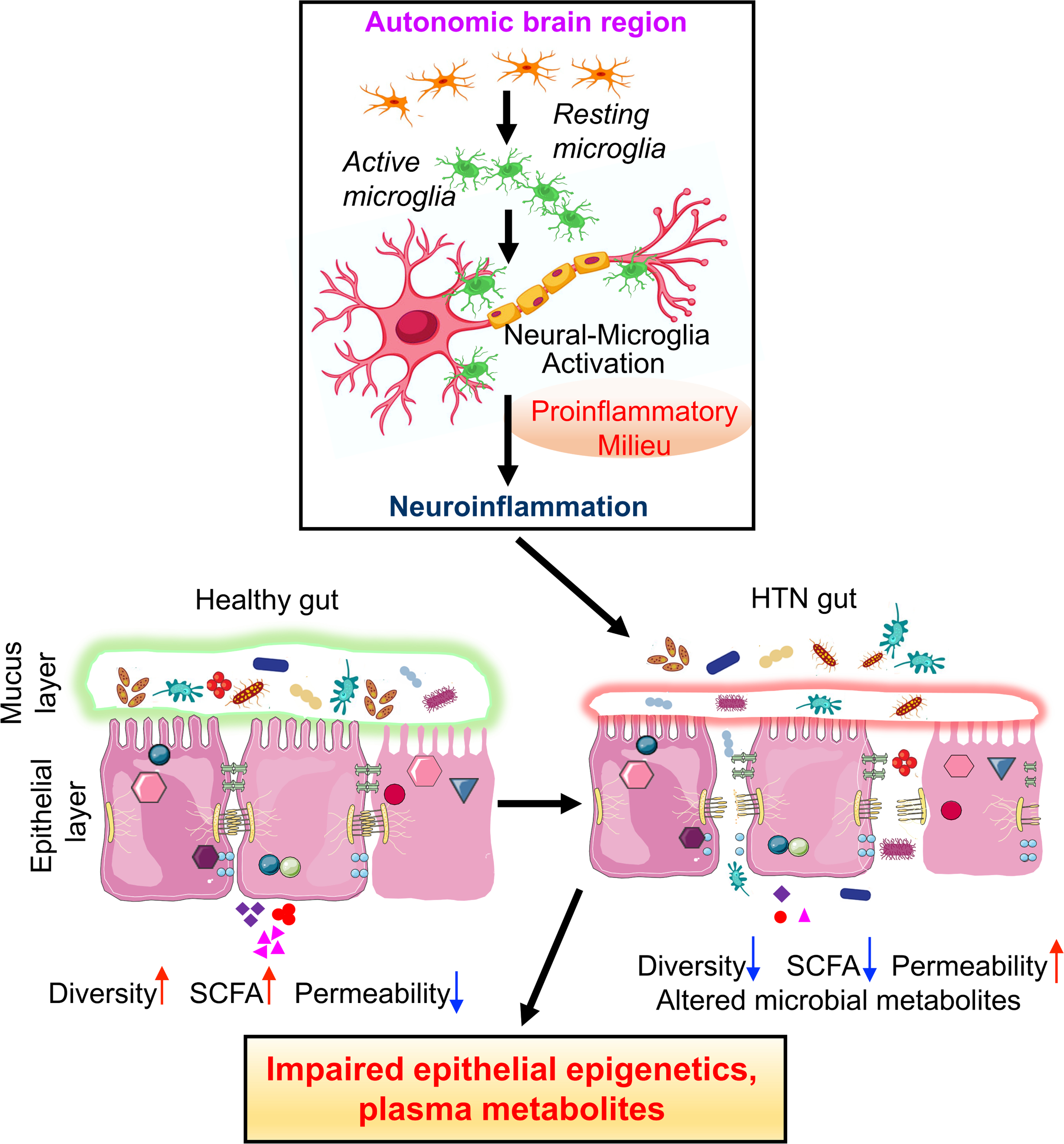
In the autonomic brain regions, primarily in the hypothalamic paraventricular nucleus, HTN stimuli initiate a cascade of signaling events that include activation of resident microglia, altered microglia-neurons interactions that results in an increase in proinflammatory milieu. Establishment of neuroinflammation would be associated with impaired hormonal, neural and immune influences impacting gut pathology and functions. This would include dysbiosis, reduction in mucin and mucus layer, increased gut wall permeability, pathology and decrease in SCFA. This would result in a proinflammatory plasma profile that would directly or indirectly influence autonomic brain areas to perpetuate hypertensive responses. Illustration Credit: Ben Smith.
Hypertensive stimuli, directly and/or via their effects on the brain, have profound effects on the gut causing pathophysiology associated with development and establishment of HTN. Key changes in the gut include microbial dysbiosis, impaired epithelial gene expression, wall pathology and inflammation. These changes are the basis of a dysfunctional gut-microbiota communication that consequently leads to altered gut motility and increased permeability. These events alter metabolites of host, bacteria and other organisms in the microbiota, immune-modulating cells and molecules in the circulation where they can negatively affect neural activity, peripheral inflammation and other cardiovascular-relevant organs to contribute to the establishment of HTN.
The bidirectional aspect of the hypothesis proposes a coordinated communication between the gut and the brain following HTN stimuli in perpetuation of HTN. For example, metabolic products circulating as a result of dysbiosis and impaired gut-microbiota interaction, could influence neuronal activity, neuroinflammation, heart, kidney and blood vessels as well as autonomic nervous system-driven results of HTN stimuli.
Gut Microbial Dysbiosis and Gut-Brain Axis Dysfunction in Human HTN
Normotensive vs Hypertensive
Since the first suggestion of a role for gut microbial dysbiosis in HTN(3), numerous studies have supported and expanded upon its role and were reviewed recently(60). Evidence was initially correlative, such as studies of exercise that both decreases BP and alters the gut microbiome(61) but a secure footing developed when it became clear that transfer of dysbiotic gut microbiota also transferred the hypertensive phenotype(4). This was found to be true when transfer was from human hypertensive subjects to animals, and animal to animal. Transfer of a healthy microbiome to hypertensive animals decreases BP and attenuates many detrimental outcomes of HTN, (e.g., neuroinflammation, increased sympathetic activity, vascular dysfunction, etc.(62)(63).
One study shows that fecal matter transfer (FMT) from healthy donors lowers BP in hypertensive subjects by either nasogastric tube or lower GI tract administration (64). This is now being tested in a pilot study as a multicenter, randomized, double-blinded, placebo-controlled clinical trial (clinicaltrials.gov identifier NCT04406129). These findings suggest that gut microbial dysbiosis is a key feature of HTN and open possibilities for more widespread exploitation to treat HTN.
Dysbiosis: What is the difference between the microbiome of normotensive individuals and those with HTN? One common difference is the depletion of short chain fatty acid-producing bacteria, such as Eubacterium rectale(7) Roseburia and Ruminococcaceae(4,18,65,66) in gut microbiota of HTN subjects as compared to that of normotensive subjects and reduction of bacterial genes for butyrate production in HTN (67). Interestingly, lower fecal content of SCFA is associated with lower BP(66).
SCFA are important in maintaining the health of gut epithelial cells, in turn maintaining the gut barrier against the “outside world” of the gut contents. Gut epithelial cells use SCFAs as an energy source, consuming most of the SCFAs, especially butyrate, produced by the gut microbiota. Those SCFA that reach the circulation have receptors located in organs important for the maintenance of BP homeostasis, including the kidney, sympathetic nervous system and blood vessels(19–21)(68) and activating these receptors affects BP. Supplementation with SCFAs, either directly or via a high fiber diet (the raw material for gut microbial production of SCFAs) decreases BP (69–72). SCFAs such as acetate act as histone acetylases, and thereby modulate gene expression important for BP control(73,74) via inhibition of histone deacetylase activity and expression. SCFA are also neuroprotective (75,76), reducing LPS-induced neuroinflammation(77). They decrease gut inflammation by reducing inflammatory T cells that home to the gut(78). Finally, butyrate signals the brain via the vagal nerve to affect BP(79).
Another difference between microbiota of normotensive and hypertensive subjects is the increased potential for LPS production(4,18). Veillonellae, are examples of LPS-producing bacteria that are enriched in human hypertensives’ gut microbiota(80). This bacterium also impacts biofilm formation. Biofilms encase communities of bacteria in bacteria-produced extracellular matrix, increasing their adherence and allowing the community to create an environment more suited to their growth, and where they are protected from outside influences such as antibiotics. LPS-producing bacteria in biofilms are more inflammatory than Gram negative bacteria without the protection of a biofilm(81,82). This suggests that the hypertensive microbiota is more inflammatory both systemically and since LPS reaches the brain, centrally, contributing to the potential for autonomic nervous system activation and increased BP.
Depletion of Lactobacilli from the gut microbiota of both humans and animals with high salt diet-induced HTN has been reported(16,83,84). Although this was not confirmed in other studies (66). Lactobacilli are not detected in the gut microbiota of all healthy individuals, thus depletion of Lactobacilli cannot be the common pathway of high salt diet dependent-HTN (85,86) But, Lactobacilli were shown to be associated with the lowest quartile of ambulatory aortic stiffness index, generally considered to be indicative of lower BP(87). Taken together, although there is consensus for the bacterial communities involved in biofilm formation, SCFA production and LPS in HTN, characterization of specific bacterial genera and species remains to be established.
Few studies have examined functions associated with the gut microbiome as permitted by whole genome sequencing. Our studies showed that examination of function distinguished hypertensive from normotensive gut microbiomes better than microbial composition (7). Others using similar methods showed enrichment of membrane transport, LPS biosynthesis and steroid degradation in the gut microbiota of hypertensive subjects (4,18). Along with these increases there were significant decreases in essential functions associated with health, including branch-chain amino acid biosynthesis and transport, ketone body biosynthesis, two component regulatory system, degradation of methionine and purine (4,60) and Vitamin D3 production(88). These data suggest that functions of the gut microbiota community may be more important in determining hypertensive status than bacterial composition.
Hypertension in Black African Americans (AA) and Gut Microbiota
The prevalence of HTN in non-Hispanic AA remains significantly higher (56%) than non-Hispanic white (WA, 47%), non-Hispanic Asian (45%) and Hispanic (44%) Americans (89). AA develop high BP at an earlier age and the resulting HTN tends to be more severe and resistant to treatment. Treatment-resistant HTN has been defined as BP above the goal of ≥130/90 mmHg, despite lifestyle modifications and adequate doses of ≥3 antihypertensive drugs, including a diuretic(90). In addition, AA patients are more likely to manifest non-dipping BP, increased prevalence of left ventricular hypertrophy, and increased stroke risk. As a result, ~50% AA hypertensives have uncontrolled HTN (91). Thus, AA accumulate significantly greater burdens from CVD, end-stage renal disease and stroke leading to higher morbidity and mortality(91).
Genetics, obesity, higher salt sensitivity, low plasma renin, abnormal vascular function, and environmental factors (diet, sedentation, stresses related to racial inequality and poverty, exposure to particulate matter air pollution) are all important contributors to this disparity. For example, a study of 30,000 participants followed for nine years showed that consumption of a typical southern diet that includes fried food, organ and processed meats was the greatest mediator of differences in HTN incidence between AA and WA subjects(92). The Dietary Approach to Stop Hypertension (DASH) diet, rich in fruits, vegetables, whole grains, and low fat, lowers BP more effectively in AA compared with WA (93). Additionally, the Jackson Heart Study, a large ongoing study, suggests genetic and environmental risk factors disproportionally burden AA with CVD and HTN. Its principal observations, with those of the Barbershop cluster-randomized trial, suggest that public health efforts to increase awareness and treatment help control HTN in AA (94), (95). None the less, because of the multifactorial influences on BP and difficulties adapting currently available therapies to this group of patients, challenges to successful control and management of HTN in AA remain. Interestingly, recent advances in metabolomics and metagenomics coupled with evidence of gut dysbiosis in HTN begs the question: Is gut microbiota and its communication with the gut epithelium the Holy Grail in delineating ethnic disparities in AA? Below we summarize current knowledge on microbiota and HTN in AA patients and provide our perspective of its clinical implications.
The initial indication of the involvement of the gut and its microbiota was derived from studies of Zheng et. al. who determined metabolomic profiles in a subsample of randomly selected AA participants in Atherosclerosis Risk in Communities (ARIC) study. The key observation was that each increment of 1 standard deviation from baseline in 4-hydroxyhippurate, a product of the gut microbiota and host metabolism, was associated with 18% higher risk of HTN. Our small cohort study that combined metabolomics and microbiota analyses showed that HBP in AA patients was associated with higher oxidative stress, insulin resistance and gut ischemia(96). Furthermore, multivariate analyses reveal significant differences in gut bacterial populations of AA vs. WA hypertensives (Figure 3). Chen et.al. tested the concept that reduction in BP due to dietary sodium reduction was associated with beneficial effects on SCFAs that are primarily products of gut microbiota(97,98). The basis for this prediction was evidence that salt exerts significant effects on gut microbiota and there is a greater salt sensitivity and impaired salt handling by AA HTN patients(16,91). The randomized, double blind, placebo-controlled cross-over trial with 145 participants (42% AA) showed that sodium reduction increased 8 SCFAs including butyrate and isobutyrate.
Figure 3. Principle component analysis of gut microbiome shows distinct clustering of gene differences among African American (AA) and White American (WA) reference and hypertensive subjects.
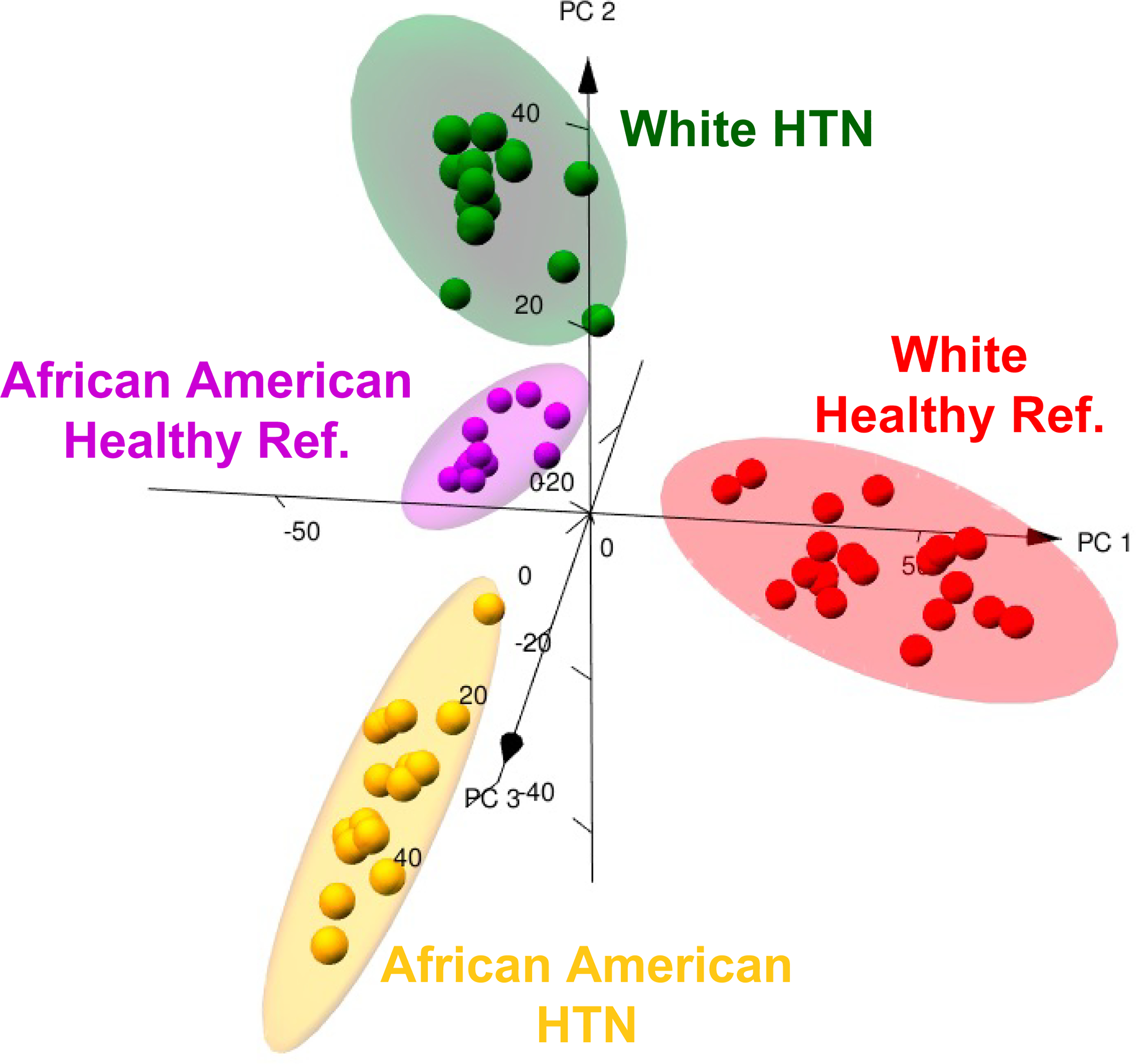
Three-dimentional PCA is shown with 95% ellipsoids based on centered log ratio transformed de-noised prevalence data from shotgun sequencing (1,817 total genes pooled from all 63 patients) obtained by machine learning. Each point represents an individual subject. Mean BPs were: WA-REF= 121/73 mmHg, WA-HTN=149/79 mmHg, AA-REF= 125/77 mmHg, and AA-HTN=151/85 mmHg.
Epithelia-Microbiota Communication in HTN
Intestinal epithelium is paramount in regulation of overall body homeostasis involving the gut-brain axis. Normal epithelial cell function confers protection of vital organs from harmful effects by maintaining gut barrier properties and sequestering adverse molecules, microbial metabolites, immune cells, and inflammatory molecules. Thus, any structural or functional compromise of epithelial cells leads to loss of the epithelial seal of the gut wall and increased leakiness. Indeed, increased gut leakiness has been associated with pathogenesis of chronic diseases such as Parkinson’s and Alzheimer’s diseases, obesity, IBD, other gut inflammatory conditions and even aging (99–102). Parts of the gut microbial community are in intimate contact with the gut thus, ideally positioned to exert epigenetic influences on epithelial cells (103). This gut-microbiota communication is proposed to be bidirectional. For example, multiple signaling and metabolic pathways from microbiota have been shown to influence epithelial genes and functions. Microbial metabolites such as SCFA and catabolic products of tryptophan are two established candidates in microbiota’s repertoire that effect epithelial cells. SCFA influence tight junction proteins expression, inhibit histone deacetylase, and immune signaling-relevant genes affecting epithelial cell turnover, growth and cellular functions(104) while tryptophan metabolites effect anti-inflammatory and protective barrier functions. Regulation of mucin secretion and production of antimicrobial peptides (AMP) are additional means of microbial communication that involve pattern recognition receptor/MYD88(105). Another signal transmission component is the inflammasome. These are multimeric, enzymatically active protein complexes assembled in the cytosol following perception of bacterial-derived molecular patterns and activate inflammatory cytokines within innate immune cells. Furthermore, a diverse, healthy microbiota provides signals for activation of the NLRP6 inflammasome class, a process likely to be impaired with dysbiosis, thus initiating a cascade of signals decreasing production of AMPs and promoting inflammation(106). Finally, microbiota is important source of polyamines (spermine, putrescine, histamine etc.) whose anti-inflammatory activity is counteracted by other microbial products such as taurine(107). Taken together, these observations form the basis for the concept of microbiota-driven influences on gut epithelial functions.
There is limited but important evidence for epithelium-initiated gut to microbiota communication. Epithelial cells are among the first to receive environmental, physical, and neural cues with drastic effects on gut microbial homeostasis. One such effector is 5-hydroxytryptamine (5-HT), ~ 95% of which is housed in the gut. Predominantly produced by enterochromaffin cells in the gut epithelium, 5-HT is an important regulator of enteric nervous system, gut motility and inflammation primarily by dampening gut vagal afferents(108). The fact that altered levels of this biogenic amine are found in many gut inflammatory diseases involving dysbiosis has led to the suggestion that 5-HT could have a direct influence on microbial communities(109). This is supported by evidence that tryptophan hydroxylase-deficient mice with significantly reduced gut 5-HT demonstrate altered gut microbiota and reduced severity of colitis(110). In addition, 5-HT is shown to inhibit certain and stimulate other commensal bacterial communities(111).
Finally, the role of microRNAs in epithelial-microbiota cross talk is a rapidly emerging area of investigation. The following findings support this view: i) miRNAs enter bacteria and co-localize with bacterial nucleic acid suggesting their influence on microbiota(112); ii) miRNA profiles in epithelial cells were demonstrated to be related to microbial status(113); iii) human miRNAs such as miR-515–5p and miR-1226–5p were shown to induce changes in 16S RNA transcripts when introduced into F. nucleatum and E. coli (112) and; iv) fecal miRNA transplantation induced significant microbial changes(112). miR-21 aggravates DSS-induced colitis by effects on gut microbiota (114) and differentially expressed miRNAs (miR-199a, miR-1226, miR-548a and miR-515–5p) affect growth of F. nucleatum and E. coli that are linked to increased IBD (114).
Is there evidence of an impaired bidirectional epithelial-microbiota communication in HTN? Emerging data supports this contention: i) Gut leakiness had been observed in both animal models and in human HTN establishing an important clue for dysfunctional sequalae. This is associated with altered gene expression for tight junction proteins (5,7,29,66). ii) Consequences of leaky gut are increased translocation of certain metabolites, toxins, and IgA-coated bacteria into the systemic circulation with profound adverse outcomes on vascular, inflammatory and neural mechanisms. Decreased bacterial communities associated with butyrate and subsequent effects on epithelium, plasma butyrate and BP are well-established (3,7,78). Increased LPS and a positive correlation between plasma levels and HTN has been found(7,66). Altered tryptophan metabolites including 5-HT have been implicated in high BP and chronic kidney disease although detailed mechanisms remain elusive (96,111,115). iii) Transcriptomics of colon and colon organoids are beginning to shed light on HTN-linked epigenetic changes in the epithelium and identification of candidate genes with potential to interact/influence microbiota. Our data show that colonic epithelium from the SHR exhibits a distinct gene expression profile compared to its normotensive controls(8,9). For example, genes associated with T cell receptor signaling and those involved in immunity, barrier function and dysbiosis were decreased implicating, for the first time, their potential role in altered bacterial communities in HTN. We next used colon organoid cultures to set the stage for identification of epithelial genes interacting with microbiota (9). We found significant decreases in genes relevant to antigen presentation, immune response, virus defense response pathways in the SHR (9). This observation led us to suggest that decreased expression of genes of these pathways likely compromises mounting immune and other defense responses, altered epithelial cell proliferation, differentiation and metabolism in the SHR (9). We performed correlation analyses to investigate associations between gene expression and abundance of gut bacterial communities relevant to HTN in the SHR. We observed that enrichment of Allobaculum, Bifdobacterium, Blautia and Gordonibacter in WKY was significantly correlated with increased expression of most gene sets such as antigen presentation (RT.Ba, RT.Bb), immune response (Cd69, Ccl20) and cell junction organization (Cldn10, Plec) in gut epithelium compared to SHR (Figure 4; correlation coefficients (r) cutoff value is 0.81, p<0.05). Interestingly, Bifdobacterium, Allobaculum and Blautia are butyrate-producing bacteria whose populations are depleted in HTN and this is consistent with evidence of decreased HTN-associated plasma butyrate, butyrate-producing bacterial communities and alleviation of HTN pathophysiology by butyrate treatment (3,7,116,117). In contrast, the enrichment of Turicibacter could be linked to downregulation of antigen presentation genes in the SHR. Collectively, such correlation analysis points out the potential of bioinformatics in combination with metagenomics of organoids in identification of key targets in epithelial-microbiota crosstalk (Figure 4). In conclusion, although limited data support the view of altered epithelial-microbiota communication in HTN, much more investigation is needed to delineate details.
Figure 4. Alteration of gut microbiota composition correlates with gene expression for functional gene sets in gut epithelium of WKY rats and SHR.
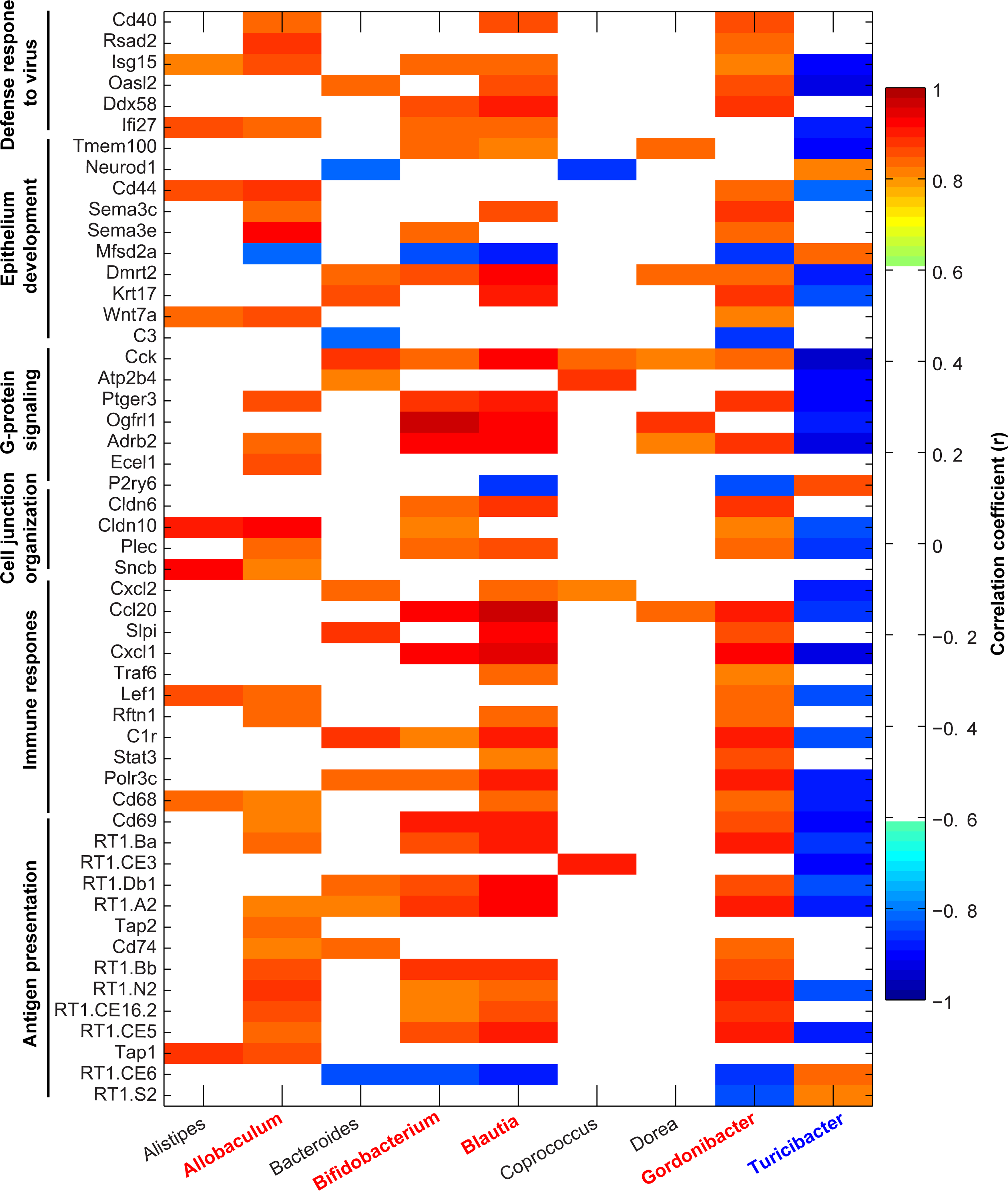
Correlation analyses were performed on the normalized abundance of bacterial genera data (3) and gene expression values from RNA-seq data(8). Pearson’s correlation coefficients (r) were generated for all samples regardless of group using MATLAB software with a cutoff value of 0.811 (n = 5, p < 0.05) and displayed using pixel maps. Red-highlighted genera are positively correlated, and blue-highlighted genera are negatively correlated.
Gut Microbiota-Immunoglobulin A Trafficking: Evolving Concept for Neuroinflammation in HTN
Immunoglobulin A (IgA) is emerging as an important molecule in dynamic gut-microbiota communication and regulation of neuroinflammatory signaling in diseases involving dysfunction in the gut-brain axis. IgA isotype antibody-producing cells are B lymphocytes that originate from hematopoietic stem cells in bone marrow. Immature B cells leave bone marrow and migrate to lymphoid organs such as spleen, lymph nodes and gut-associated lymphoid tissues where they are activated and differentiate into plasma cells in response to antigens. These plasma cells reside in the lamina propria of the gut where they produce large quantities of antibodies in response to the gut environment including bacterial communities(118,119). Secretory IgA is generated basolaterally, transported across epithelial cells, and released into the lumen where it is in an ideal position to interact with bacteria, (Figure 5). IgA and IgA+ plasma cells circulate in the blood and can target relevant organs such as lungs, kidneys, blood vessels, and brain. Thus, plasma cells and IgA are important in regulating gut mucosal immunity. IgA’s binding to specific gut bacterial taxa, termed “IgA coating” serves multiple functions. This includes providing protection against pathogens and toxins, facilitating host colonization with beneficial bacteria and altering bacterial and viral adhesion to epithelial cells influencing epithelial-microbiota communication(120,121). Recent studies combining bacterial flow cytometry with high throughput 16S sequencing have led to the characterization of bacterial taxa coated by IgA (122–126). They are diverse with four major phyla (Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria) suggesting that this diversity in IgA-coating may be important in defining immunopathology of gut-linked diseases.
Figure 5. Influence of immunoglobulin A (IgA) on gut microbiota in hypertension.
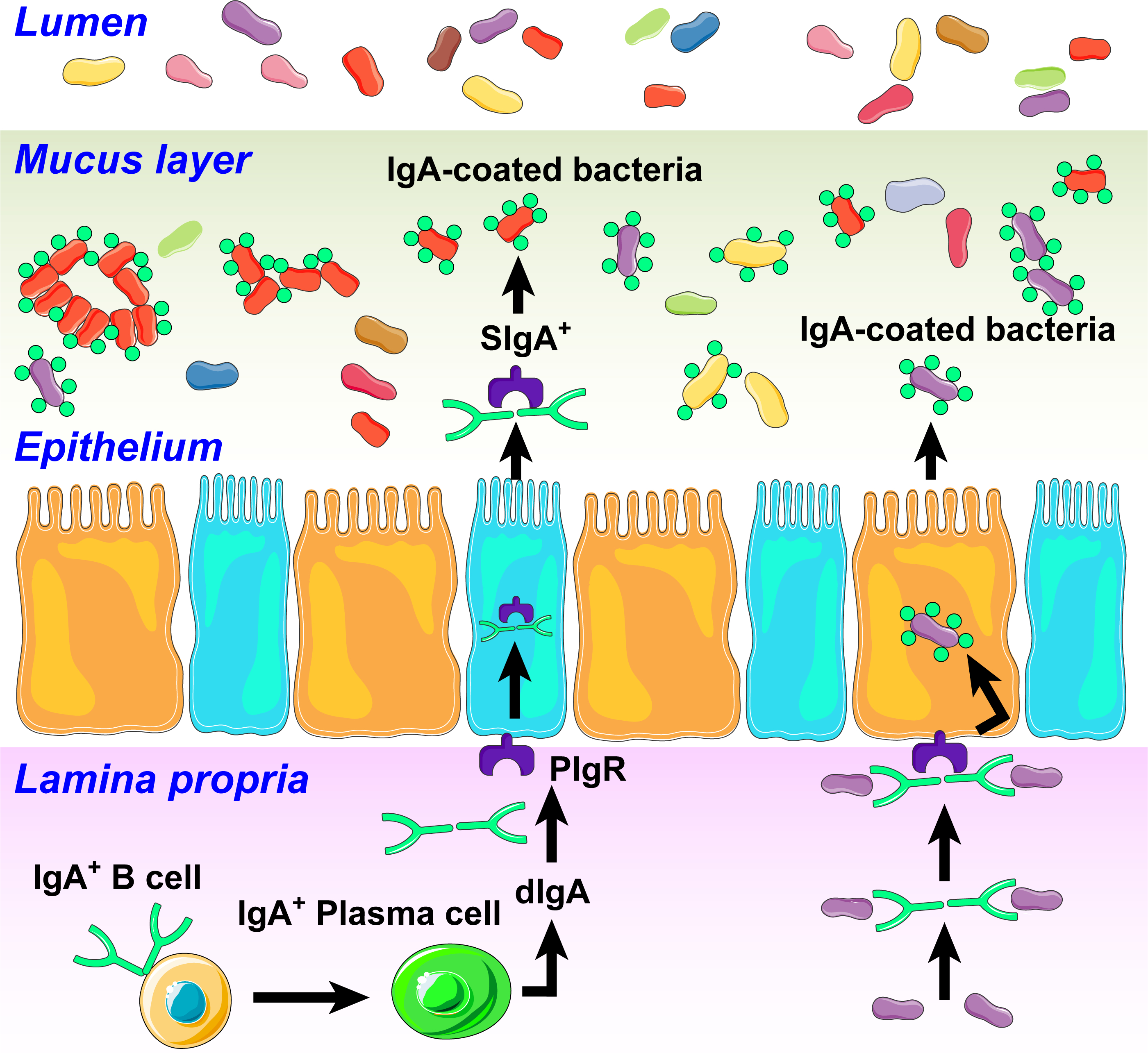
In response to antigenic signals, in part generated by an altered microbiota, IgA B cells are activated into plasma cells to produce dimeric IgA (dIgA). dIgAs bind to polymeric Ig receptors (PIgR) expressed on the basolateral side of the epithelial cells. This complex is then transported to the apical surface, where PIgR is cleaved to generate secretory IgA (SIgA). SIgA binds and coats bacteria in an antigen-specific manner altering their functions. This could include growth inhibition, clustering and elimination of beneficial bacteria and affecting the proliferation of harmful bacteria and production of toxins. We also propose that HTN-specific IgA bound bacteria taxa could translocate to the brain through leaky gut and BBB to induce neuroinflammation.
Studies of Rojas et.al. were among the first to demonstrate the role of IgA+ PC and dysbiosis in experimental autoimmune encephalomyelitis (EAE) and in multiple sclerosis (MS) (127). They demonstrated that some plasma cells in the brain of mice with EAE originate from the gut and produced high levels of IgA. A significant decrease in IgA+ plasma cells in the gut of mice and reduced IgA-coated fecal bacteria was observed and further depletion exacerbated disease. This suggested their involvement in microbial dysbiosis and brain inflammatory processes(127). Interestingly, the study also showed that MS patients exhibited a reduction in fecal bacterial-bound IgA during MS relapses due to mobilization of IgA+ cells out of the gut. Together, the evidence supports the contention that IgA+ plasma cells are involved in dampening EAE-associated neuroinflammation. Recent evidence from Probstel et. al. (128) supports this view. The proportion of IgA bound bacterial taxa (operational taxonomic units, OTUs) was significantly greater in MS and translocation of gut-associated IgA+ B cells and plasma cells to the CNS attenuated neuroinflammation.
Evidence is also emerging implicating IgA and plasma cells in type-1 diabetes (T1D). Huang et.al. demonstrated that newly diagnosed individuals with T1D have altered microbiota and associated increase in IgA bound to gut bacteria(129). Fecal transplant from T1D donors into germ-free non-obese diabetic mice enhanced gut permeability, gut antimicrobial peptide expression, decreased IgA+ B cells in pancreas and increased IgA-bound bacteria in cecum, colon and stool. Together, this was the first body of evidence linking IgA immune responses and gut microbiota regulated by SCFA (acetate) in the pathogenesis of T1D. Finally, the role of IgA in regulation of gut homeostasis in irritable bowel syndrome (IBS) has been extensively studied(120,130–132). Increases in fecal IgA and altered microbiota composition was demonstrated in 44 patients with diarrhea-predominant IBS(133). This was associated with increases in IgA-coated bacteria particularly Escherichia-Shigella, Granulicatella and Hemophilus in IBS compared with healthy controls. These findings suggest that dysbiosis in IBS results in higher IgA production, increased IgA-coated bacteria leading to a local inflammatory responses and manifestations of IBS. A common theme is emerging from the above studies regarding involvement if IgA and plasma cells in the gut-linked neural and other chronic diseases. Altered microbiota seems to be an important triggering step in activation of gut PB cells into plasma cells that produce large quantities of IgA. This selectively coats responsible bacterial taxa altering their composition and microbiota-epithelial interaction. Alternatively, the possibility of dysfunctional plasma cells overproducing IgA resulting in greater IgA coating of bacteria cannot be ruled out. Increased IgA and plasma cells could also enter the circulation because of leaky gut, translocate into the brain and other relevant organs to influence immune status.
Are IgA and IgA-producing plasma cells relevant in HTN since both dysbiosis and neuroinflammation are key factors of the disease? Despite an early report of increased circulating IgA in the SHR little progress has been made in delineating their involvement(134). Increases in IgA in prehypertensive SHR clearly indicate that its contribution is not secondary to HTN. Contribution of plasma cells to HTN was demonstrated by a depletion experiment. Taylor et.al. showed that depletion of plasma cells by bortezomib attenuated HTN, renal injury and B and T cell infiltration into the kidneys(135). A cross-sectional study with 12,373 participants showed that increased circulating IgA was associated with higher prevalence of HTN and correlated with BP(136). Another study with 61 HTN and 55 control subjects demonstrated significantly increased IgA among other immunoglobulins(137). Despite these tantalizing data a major gap in knowledge exists in delineating the involvement and importance of IgA and plasma cells in the pathophysiology of HTN, including whether the primary event is gut microbial dysbiosis or malfunctioning plasma cells. We have conducted a “proof of principle” study with a small cohort of HTN (N=13) and normal (N=9) subjects. IgA-coated bacteria from stool samples revealed a trend towards an increase in HTN subjects, (Figure 6) providing first indication of similar involvement of IgA and associated bacteria in HTN.
Figure 6: IgA-coated bacteria are enriched in HTN subjects.
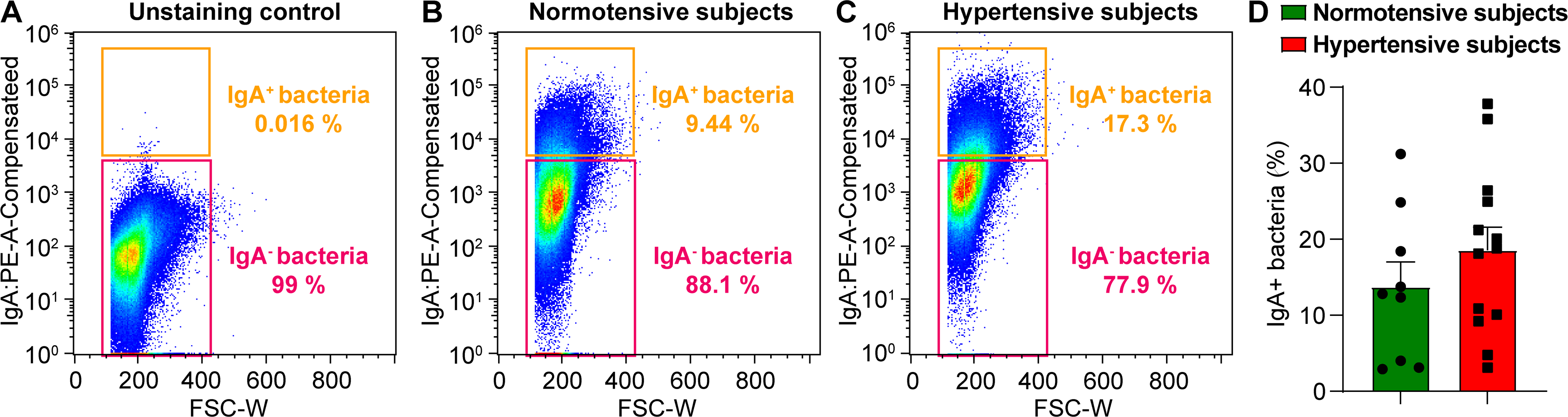
Fecal bacteria isolated as described elsewhere (131) from stool samples from RBP (N= 9, SBP 115 mmHg) and HTN (N=13, SBP 145 mmHg) subjects were stained with PE-conjugated Anti-Human IgA to measure the percentage of IgA-coated bacteria in stool by flow cytometry. A, Unstained control. B and C, Representative IgA-staining of fecal bacteria from a normotensive (B) and a hypertensive subject (C). D, Cumulative data, percentage of IgA-coated and non-coated bacteria in stool of 9 normotensive and 13 hypertensive subjects determined by flow cytometry (mean ± standard error of the mean).
Minocycline: an antihypertensive drug?
Minocycline is an antibiotic of the tetracycline family that also possess anti-inflammatory properties. Its ability to penetrate the blood-brain barrier, rapid absorbability from the gut, very good safety record over several decades of clinical use and its dual antibiotic and anti-inflammatory properties, have resulted in its evaluation to mitigate many neural diseases involving neuroinflammatory pathways(138). Interestingly, despite success in animal models of stroke, multiple sclerosis, Parkinson’s disease, ALS and Huntington’s disease, clinical trials of minocycline for neuroinflammatory disorders have met with limited success.
We decided to test the efficacy of minocycline in treatment-resistant HTN based on the following: a) Extensive animal experiments have established that minocycline attenuates high BP and HTN pathophysiology(25,32,33). These effects are associated with decreases in active microglia in the PVN and other autonomic brain regions and decrease in sympathetic nerve activity. b) Minocycline rebalances the gut microbiota composition of hypertensive rats towards that found in normotensive animals. The effect on microbiota in HTN is consistent with minocycline’s influences on gut microbiota and neuroinflammation in other diseases including depression, anxiety and Alzheimer’s disease(139–143). c) Promising results of this drug are reported in experimental colitis and other gut inflammatory diseases (144) where it works by inducing mucosal healing and reducing gut inflammation(145). d) Minocycline prevents development of diabetic retinopathy in rodent models (146). Additionally, an observational study of morbidly obese type 2 diabetic patients demonstrated that it decreased hemoglobin A1c, reduced BP and improved visual acuity and symptoms associated with neuropathy in a small cohort of morbidly obese type 2 diabetic patients (147). Taken together, these observations demonstrate that minocycline could be beneficial against neuroinflammation, gut microbiota and its pathophysiology, gut immunity, and proinflammatory mechanisms, processes all also associated with HTN.
An open label study conducted in high-risk treatment-resistant HTN patients supports this contention. (59). We observed that 16 of 26 patients (62%) responded to minocycline with a mean reduction in daytime ambulatory systolic BP (dAMBP) (135/74 to 124/68 mmHg)(59). Decreased dAMBP was associated with reductions in circulating CD4+ cells containing CD161+IL17+ and CCR6+ITGb7+CD161+IL17+ markers. This suggested that gut inflammatory cell populations were decreased by minocycline treatment (59). These data are tantalizing but clearly more work is needed to clarify the usefulness of minocycline in treatment-resistant HTN.
Is minocycline uniquely poised compared to other drugs with only anti-inflammatory or antibiotic properties for its antihypertensive effects in treatment-resistant HTN patients? There is little evidence to support this contention. One would predict that these classes of drugs would influence BP since inflammation and gut microbial dysbiosis are hallmark events in HTN. However, results have been discouraging, in part because nonsteroidal anti-inflammatory drugs have been implicated in adverse cardiovascular effects (148), leading to caution in testing other anti-inflammatory drugs with BP-reducing properties in animal models in human HTN (149). Therefore, the usefulness of anti-inflammatory/immunosuppressive drugs for HTN remains questionable at the present time.
Evidence for the influence of antibiotics on BP is primarily derived from animal studies, mostly indirect and sometimes disparate. An early study showed that antibiotic treatment significantly attenuated the corticosterone-induced increase in BP(150). Doxycycline treatment reduced BP in SHR in one (151) but had no effect in another(152) study although the antibiotic attenuated vascular remodeling. Vancomycin decreased while neomycin did not change BP in the SHR(153). Recently, Galla et.al. demonstrated that amoxicillin treatment lowered BP in the SHR and altered gut microbiota (154). Interestingly, this BP-lowering effect persisted after antibiotic treatment was discontinued. They concluded that amoxicillin reshapes gut microbiota with long-term antihypertensive effects extending even to their offspring. The concept of transmission of gut microbiota imprinting from dams to offspring is supported by our study (30). A few studies have implicated a BP-lowering effect of certain antibiotics, although indirectly, in human HTN. For example, cotreatment with ampicillin caused an enhanced antihypertensive response of amlodipine, possibly by increasing its bioavailability(155). In a retrospective study of 7,100 older patients receiving a calcium channel blocker, erythromycin and clarithromycin were strongly associated with increased risk of hypotension(156). Finally, our case study showed a pronounced BP lowering effect with a combination of antibiotics in a 69-year-old woman with a long history of HTN (157).
In conclusion, it is apparent that drugs that target both inflammation and gut microbiota may have greater probability to decrease BP in HTN, particularly in patients with treatment-resistant HTN. Would minocycline, with its long safety record, be the drug of choice? This remains an open question and two ongoing clinical trials (NCT02133872, NCT04478500). are likely to resolve this issue.
Conclusions and Future Directions
Significant progress has been made and new concepts have been presented since the first demonstration of dysbiosis in HTN less that ten years ago. The evidence that microbiota is involved in the initiation of high BP has been presented. Also, the supposition for bidirectional communication in BP control and a dysfunctional gut-brain axis in HTN has gained basic support. Evidence is also emerging in support of an active crosstalk between gut epithelium and bacterial communities which may be impaired in HTN. We have presented provocative ideas for epithelial-bacterial communication involving antigen presenting genes and IgA+ cells in systemic and neuroinflammation. Human studies are increasingly implicating microbiota in ethnic HTN disparity. Finally, translational implications of a dysfunctional gut-brain axis have been realized by a small trial showing that minocycline treatment decreased BP in treatment-resistant HTN patients.
Moving forward, we propose that microbiome and HTN research should focus on the following critical issues to continue advancing basic science mechanisms and to develop innovative strategies for the control of HTN:
Epithelial epigenetics certainly plays an important role in physiological homeostasis. How are these epigenetic influences altered in HTN?
Involvement of IgA and plasma cells in the transmission of inflammatory signals to cardiovascular-relevant organs (i.e., heart, kidneys, brain etc.) would be an important avenue to investigate.
Experiments with animal models are unquestionably important and provide the backbone of the mechanistic information. However, considering insurmountable limitations with animal models, efforts should be continued to validate concepts in human HTN. For example, can one utilize tissues from colonic biopsies and make 3D organoid cultures or colon-on-a-chip to investigate epithelial genes and their interacting bacterial partners in HTN subjects? IgA bacteria can be analyzed from the stool of subjects to home-in on taxa involved in systemic and neuroinflammation.
Sex-linked differences in BP regulation are well-established but little is known about the involvement of gut microbiota in this. It would be a relevant future direction in view of the evidence for i) differential regulation of microbiota by sex hormones in men and women and different metabolism of sex hormones by their gut microbiota. ii) the heavy influence of a mother’s gut microbiota on her offspring’s that may play a role in transgenerational transmission of hypertension susceptibility.
An appropriately powered, trial is needed to confirm beneficial effects of minocycline in treatment-resistant HTN.
Funding Sources:
National Institutes of Health National Heart, Lung, and Blood Institute grants HL033610, HL110170, and HL132448
Non-Standard Abbreviations and Acronyms
- AA
Black, non-Hispanic African American
- Ang II
Angiotensin II
- ANS
Autonomic nervous system
- BBB
Blood brain barrier
- BP
Blood pressure
- CMT-3
Chemically modified tetracycline 3
- dAMBP
daytime ambulatory systolic blood pressure
- DOCA
Deoxycorticosterone acetate
- EAE
Experimental Autoimmune Encephalitis
- fMRI
Functional magnetic resonance imaging
- FMT
Fecal matter transfer
- GI
Gastrointestinal
- HTN
Hypertension
- IBS
Inflammatory bowel syndrome
- ICV
Intracerebroventricular
- IgA
Immunoglobulin A
- IL1β
Interleukin 1 beta
- IL-10
Interleukin 10
- LPS
Lipopolysaccharides
- MS
Multiple Sclerosis
- NTS
Nucleus tractus solitarius or Nucleus of the solitary tract
- OVLT
Organum vasculosum of the lamina terminalis
- PET
Positron emission tomography
- PVN
Paraventricular nucleus of the hypothalamus
- SCFA
Short chain fatty acid
- SHR
Spontaneously hypertensive rat
- SNA
Sympathetic nerve activity
- TNFα
Tumor necrosis factor alpha
- WA
non-Hispanic White American
- WKY
Wistar Kyoto
Footnotes
Disclosures: Conflicts of Interest: None
References
- 1.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet (London, England). 2021;398:957–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut Dysbiosis Is Linked to Hypertension. Hypertension. 2015;65:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-Linked Pathophysiological Alterations in the Gut. Circulation Research. 2017;120:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart DC, Rubiano A, Santisteban MM, Shenoy V, Qi Y, Pepine CJ, Raizada MK, Simmons CS. Hypertension-linked mechanical changes of rat gut. Acta Biomaterialia. 2016;45:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clinical Science (London, England: 1979). 2018;132:701–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang T, Li H, Oliveira AC, Goel R, Richards EM, Pepine CJ, Raizada MK. Transcriptomic signature of gut microbiome-contacting cells in colon of spontaneously hypertensive rats. Physiological Genomics. 2020;52:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Richards EM, Handberg EM, Pepine CJ, Raizada MK. Distinct Gene Expression Profiles in Colonic Organoids from Normotensive and the Spontaneously Hypertensive Rats. Cells. 2021;10:1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiological Genomics. 2017;49:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiological Genomics. 2015;47:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J, Luo H, Wang J, Tang W, Lu J, Wu S, Xiong Z, Yang G, Chen Z, Lan T, Zhou H, Nie J, Jiang Y, Chen P. Enteric dysbiosis-linked gut barrier disruption triggers early renal injury induced by chronic high salt feeding in mice. Experimental & Molecular Medicine. 2017;49:e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robles-Vera I, Visitación N, Toral M, Sánchez M, Romero M, Gómez-Guzmán M, Yang T, Izquierdo-García JL, Guerra-Hernández E, Ruiz-Cabello J, Raizada MK, Pérez-Vizcaíno F, Jiménez R, Duarte J. Probiotic Bifidobacterium breve prevents DOCA-salt hypertension. The FASEB Journal. 2020;34:13626–13640. [DOI] [PubMed] [Google Scholar]
- 14.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RM. Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Hypertension. 2016;67:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno-Indias I, Torres M, Montserrat JM, Sanchez-Alcoholado L, Cardona F, Tinahones FJ, Gozal D, Poroyko VA, Navajas D, Queipo-Ortuño MI, Farré R. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. The European Respiratory Journal. 2015;45:1055–1065. [DOI] [PubMed] [Google Scholar]
- 16.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter J Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Applied and Environmental Microbiology. 2008;74:4985–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, Han X, Huang Y, Zhao L, Li P, Fang Z, Zhou J, Guan X, Ding Y, Wang S, et al. Alterations of the Gut Microbiome in Hypertension. Frontiers in Cellular and Infection Microbiology. 2017;7:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan L-X, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proceedings of the National Academy of Sciences. 2013;110:4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiological Genomics. 2016;48:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pluznick JL. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Current Hypertension Reports. 2017;19:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakai M, Ribeiro RV, Stevens BR, Gill P, Muralitharan RR, Yiallourou S, Muir J, Carrington M, Head GA, Kaye DM, Marques FZ. Essential Hypertension Is Associated With Changes in Gut Microbial Metabolic Pathways: A Multisite Analysis of Ambulatory Blood Pressure. Hypertension. 2021;78:804–815. [DOI] [PubMed] [Google Scholar]
- 23.Cheema MU, Pluznick JL. Gut Microbiota Plays a Central Role to Modulate the Plasma and Fecal Metabolomes in Response to Angiotensin II. Hypertension. 2019;74:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sklerov M, Dayan E, Browner N. Functional neuroimaging of the central autonomic network: recent developments and clinical implications. Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society. 2019;29:555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain Microglial Cytokines in Neurogenic Hypertension. Hypertension. 2010;56:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zubcevic J, Jun JY, Kim S, Perez PD, Afzal A, Shan Z, Li W, Santisteban MM, Yuan W, Febo M, Mocco J, Feng Y, Scott E, Baekey DM, Raizada MK. Altered Inflammatory Response Is Associated With an Impaired Autonomic Input to the Bone Marrow in the Spontaneously Hypertensive Rat. Hypertension. 2014;63:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SWG, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension. 2007;49:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naumczyk P, Sabisz A, Witkowska M, Graff B, Jodzio K, Gąsecki D, Szurowska E, Narkiewicz K. Compensatory functional reorganization may precede hypertension-related brain damage and cognitive decline: a functional magnetic resonance imaging study. Journal of Hypertension. 2017;35:1252–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Raizada MK, Richards EM. Gut-brain-bone marrow axis in hypertension. Current Opinion in Nephrology and Hypertension. 2021;30:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H-B, Yang T, Richards EM, Pepine CJ, Raizada MK. Maternal Treatment With Captopril Persistently Alters Gut-Brain Communication and Attenuates Hypertension of Male Offspring. Hypertension. 2020;75:1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain–Gut–Bone Marrow Axis: Implications for Hypertension and Related Therapeutics. Circulation Research. 2016;118:1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J. Involvement of bone marrow cells and neuroinflammation in hypertension. Circulation Research. 2015;117:178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma RK, Yang T, Oliveira AC, Lobaton GO, Aquino V, Kim S, Richards EM, Pepine CJ, Sumners C, Raizada MK. Microglial Cells Impact Gut Microbiota and Gut Pathology in Angiotensin II-Induced Hypertension. Circulation Research. 2019;124:727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donertas Ayaz B, Zubcevic J. Gut microbiota and neuroinflammation in pathogenesis of hypertension: A potential role for hydrogen sulfide. Pharmacological Research. 2020;153:104677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haase S, Wilck N, Haghikia A, Gold R, Mueller DN, Linker RA. The role of the gut microbiota and microbial metabolites in neuroinflammation. European Journal of Immunology. 2020;50:1863–1870. [DOI] [PubMed] [Google Scholar]
- 36.Mowry FE, Biancardi VC. Neuroinflammation in hypertension: the renin-angiotensin system versus pro-resolution pathways. Pharmacological Research. 2019;144:279–291. [DOI] [PubMed] [Google Scholar]
- 37.Cao Y, Yu Y, Xue B, Wang Y, Chen X, Beltz TG, Johnson AK, Wei S-G. IL (Interleukin)-17A Acts in the Brain to Drive Neuroinflammation, Sympathetic Activation, and Hypertension. Hypertension. 2021;78:1450–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmari N, Santisteban MM, Miller DR, Geis NM, Larkin R, Redler T, Denson H, Khoshbouei H, Baekey DM, Raizada MK, Zubcevic J. Elevated bone marrow sympathetic drive precedes systemic inflammation in angiotensin II hypertension. American Journal of Physiology-Heart and Circulatory Physiology. 2019;317:H279–H289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esler M The sympathetic nervous system through the ages: from Thomas Willis to resistant hypertension. Experimental Physiology. 2011;96:611–622. [DOI] [PubMed] [Google Scholar]
- 40.Mancia G, Grassi G. The Autonomic Nervous System and Hypertension. Circulation Research. 2014;114:1804–1814. [DOI] [PubMed] [Google Scholar]
- 41.Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. Journal of the American Society of Hypertension: JASH. 2016;10:457–466. [DOI] [PubMed] [Google Scholar]
- 42.Guyenet PG, Stornetta RL, Souza GMPR, Abbott SBG, Brooks VL. Neuronal Networks in Hypertension: Recent Advances. Hypertension. 2020;76:300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guyenet PG. The sympathetic control of blood pressure. Nature Reviews Neuroscience. 2006;7:335–346. [DOI] [PubMed] [Google Scholar]
- 44.Wei S-G, Yu Y, Zhang Z-H, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65:1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi H, Nishimura M, Sakamoto M, Ikegaki I, Nakanishi T, Yoshimura M. Effects of interleukin-1 beta on blood pressure, sympathetic nerve activity, and pituitary endocrine functions in anesthetized rats. American Journal of Hypertension. 1992;5:224–229. [DOI] [PubMed] [Google Scholar]
- 46.Sriramula S, Haque M, Majid DSA, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clinical and Experimental Pharmacology & Physiology. 2010;37:e52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi J, Zhao X-F, Yu X-J, Yi Q-Y, Shi X-L, Tan H, Fan X-Y, Gao H-L, Yue L-Y, Feng Z-P, Kang Y-M. Targeting Interleukin-1 beta to Suppress Sympathoexcitation in Hypothalamic Paraventricular Nucleus in Dahl Salt-Sensitive Hypertensive Rats. Cardiovascular Toxicology. 2016;16:298–306. [DOI] [PubMed] [Google Scholar]
- 49.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. TNF-alpha blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. American Journal of Physiology. Heart and Circulatory Physiology. 2007;293:H599–609. [DOI] [PubMed] [Google Scholar]
- 50.Pelisch N, Hosomi N, Mori H, Masaki T, Nishiyama A. RAS inhibition attenuates cognitive impairment by reducing blood- brain barrier permeability in hypertensive subjects. Current Hypertension Reviews. 2013;9:93–98. [DOI] [PubMed] [Google Scholar]
- 51.Biancardi VC, Stern JE. Compromised blood-brain barrier permeability: novel mechanism by which circulating angiotensin II signals to sympathoexcitatory centres during hypertension. The Journal of Physiology. 2016;594:1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Setiadi A, Korim WS, Elsaafien K, Yao ST. The role of the blood-brain barrier in hypertension. Experimental Physiology. 2018;103:337–342. [DOI] [PubMed] [Google Scholar]
- 53.Haruwaka K, Ikegami A, Tachibana Y, Ohno N, Konishi H, Hashimoto A, Matsumoto M, Kato D, Ono R, Kiyama H, Moorhouse AJ, Nabekura J, Wake H. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nature Communications. 2019;10:5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2016;12:719–732. [DOI] [PubMed] [Google Scholar]
- 55.González H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. Journal of Neuroimmunology. 2014;274:1–13. [DOI] [PubMed] [Google Scholar]
- 56.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Wei B, Liu X, Shen XZ, Shi P. Microglia, autonomic nervous system, immunity and hypertension: Is there a link? Pharmacological Research. 2020;155:104451. [DOI] [PubMed] [Google Scholar]
- 58.Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P, Shi P. Microglia participate in neurogenic regulation of hypertension. Hypertension. 2015;66:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pepine CJ, Thiel A, Kim S, Handberg EM, Richards EM, Dasa O, Mohammed M, Smith SM, Cooper-DeHoff RM, Raizada MK. Potential of Minocycline for Treatment of Resistant Hypertension. The American Journal of Cardiology. 2021;156:147–149. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Yang X, Zhou X, Cai J. The Role and Mechanism of Intestinal Flora in Blood Pressure Regulation and Hypertension Development. Antioxidants & Redox Signaling. 2021;34:811–830. [DOI] [PubMed] [Google Scholar]
- 61.Xia W-J, Xu M-L, Yu X-J, Du M-M, Li X-H, Yang T, Li L, Li Y, Kang KB, Su Q, Xu J-X, Shi X-L, Wang X-M, Li H-B, Kang Y-M. Antihypertensive effects of exercise involve reshaping of gut microbiota and improvement of gut-brain axis in spontaneously hypertensive rat. Gut Microbes. 2021;13:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toral M, Robles-Vera I, de la Visitación N, Romero M, Yang T, Sánchez M, Gómez-Guzmán M, Jiménez R, Raizada MK, Duarte J. Critical Role of the Interaction Gut Microbiota – Sympathetic Nervous System in the Regulation of Blood Pressure. Frontiers in Physiology. 2019;10:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toral M, Robles-Vera I, Visitación N, Romero M, Sánchez M, Gómez-Guzmán M, Rodriguez-Nogales A, Yang T, Jiménez R, Algieri F, Gálvez J, Raizada MK, Duarte J. Role of the immune system in vascular function and blood pressure control induced by faecal microbiota transplantation in rats. Acta Physiologica. 2019:e13285. [DOI] [PubMed] [Google Scholar]
- 64.Zhong H-J, Zeng H-L, Cai Y-L, Zhuang Y-P, Liou Y-L, Wu Q, He X-X. Washed Microbiota Transplantation Lowers Blood Pressure in Patients With Hypertension. Frontiers in Cellular and Infection Microbiology. 2021;11:679624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR, Shikany JM, Lloyd-Jones DM, Launer LJ, Fodor AA, Meyer KA. Gut Microbiota Composition and Blood Pressure: The CARDIA Study. Hypertension. 2019;73:998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verhaar BJH, Prodan A, Nieuwdorp M, Muller M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients. 2020;12:2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M, SPRING Trial Group. Increased Systolic and Diastolic Blood Pressure Is Associated With Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension. 2016;68:974–981. [DOI] [PubMed] [Google Scholar]
- 68.Won Y-J, Lu VB, Puhl HL, Ikeda SR. -Hydroxybutyrate Modulates N-Type Calcium Channels in Rat Sympathetic Neurons by Acting as an Agonist for the G-Protein-Coupled Receptor FFA3. Journal of Neuroscience. 2013;33:19314–19325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marques FZ. Missing Heritability of Hypertension and Our Microbiome: Have We Been Searching in the Wrong Place? Circulation. 2018;138:1381–1383. [DOI] [PubMed] [Google Scholar]
- 70.Marques FZ, Nelson E, Chu P-Y, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017;135:964–977. [DOI] [PubMed] [Google Scholar]
- 71.Marques FZ, Mackay CR, Kaye DM. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nature Reviews Cardiology. 2018;15:20–32. [DOI] [PubMed] [Google Scholar]
- 72.Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, Haase N, Kräker K, Hering L, Maase M, Kusche-Vihrog K, et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation. 2019;139:1407–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soliman ML, Rosenberger TA. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Molecular and Cellular Biochemistry. 2011;352:173–180. [DOI] [PubMed] [Google Scholar]
- 74.Soliman ML, Smith MD, Houdek HM, Rosenberger TA. Acetate supplementation modulates brain histone acetylation and decreases interleukin-1β expression in a rat model of neuroinflammation. Journal of Neuroinflammation. 2012;9:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reisenauer CJ, Bhatt DP, Mitteness DJ, Slanczka ER, Gienger HM, Watt JA, Rosenberger TA. Acetate supplementation attenuates lipopolysaccharide-induced neuroinflammation: Neuroprotective effect of acetate. Journal of Neurochemistry. 2011;117:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang T, Rodriguez V, Malphurs WL, Schmidt JT, Ahmari N, Sumners C, Martyniuk CJ, Zubcevic J. Butyrate regulates inflammatory cytokine expression without affecting oxidative respiration in primary astrocytes from spontaneously hypertensive rats. Physiological Reports. 2018;6:e13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matt SM, Allen JM, Lawson MA, Mailing LJ, Woods JA, Johnson RW. Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated With Aging in Mice. Frontiers in Immunology. 2018;9:1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chakraborty S, Galla S, Cheng X, Yeo J-Y, Mell B, Singh V, Yeoh B, Saha P, Mathew AV, Vijay-Kumar M, Joe B. Salt-Responsive Metabolite, β-Hydroxybutyrate, Attenuates Hypertension. Cell Reports. 2018;25:677–689.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Onyszkiewicz M, Gawrys-Kopczynska M, Konopelski P, Aleksandrowicz M, Sawicka A, Koźniewska E, Samborowska E, Ufnal M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Archiv: European Journal of Physiology. 2019;471:1441–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mushtaq N, Hussain S, Zhang S, Yuan L, Li H, Ullah S, Wang Y, Xu J. Molecular characterization of alterations in the intestinal microbiota of patients with grade 3 hypertension. International Journal of Molecular Medicine. 2019. 44:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hetemäki I, Jian C, Laakso S, Mäkitie O, Pajari A-M, de Vos WM, Arstila TP, Salonen A. Fecal Bacteria Implicated in Biofilm Production Are Enriched and Associate to Gastrointestinal Symptoms in Patients With APECED - A Pilot Study. Frontiers in Immunology. 2021;12:668219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Srivastava A, Gupta J, Kumar S, Kumar A. Gut biofilm forming bacteria in inflammatory bowel disease. Microbial Pathogenesis. 2017;112:5–14. [DOI] [PubMed] [Google Scholar]
- 83.Haase S, Wilck N, Kleinewietfeld M, Müller DN, Linker RA. Sodium chloride triggers Th17 mediated autoimmunity. Journal of Neuroimmunology. 2019;329:9–13. [DOI] [PubMed] [Google Scholar]
- 84.Palmu J, Salosensaari A, Havulinna AS, Cheng S, Inouye M, Jain M, Salido RA, Sanders K, Brennan C, Humphrey GC, Sanders JG, Vartiainen E, Laatikainen T, Jousilahti P, Salomaa V, et al. Association Between the Gut Microbiota and Blood Pressure in a Population Cohort of 6953 Individuals. Journal of the American Heart Association. 2020;9:e016641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tannock GW, Munro K, Harmsen HJ, Welling GW, Smart J, Gopal PK. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Applied and Environmental Microbiology. 2000;66:2578–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kimura K, McCartney AL, McConnell MA, Tannock GW. Analysis of fecal populations of bifidobacteria and lactobacilli and investigation of the immunological responses of their human hosts to the predominant strains. Applied and Environmental Microbiology. 1997;63:3394–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dinakis E, Nakai M, Gill PA, Yiallourou S, Sata Y, Muir J, Carrington M, Head GA, Kaye DM, Marques FZ. The Gut Microbiota and Their Metabolites in Human Arterial Stiffness. Heart, Lung & Circulation. 2021;30:1716–1725. [DOI] [PubMed] [Google Scholar]
- 88.Zuo K, Li J, Xu Q, Hu C, Gao Y, Chen M, Hu R, Liu Y, Chi H, Yin Q, Cao Y, Wang P, Qin Y, Liu X, Zhong J, et al. Dysbiotic gut microbes may contribute to hypertension by limiting vitamin D production. Clinical Cardiology. 2019;42:710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferdinand KC, Harrison D, Johnson A. The NEW-HOPE study and emerging therapies for difficult-to-control and resistant hypertension. Progress in Cardiovascular Diseases. 2020;63:64–73. [DOI] [PubMed] [Google Scholar]
- 90.Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension 2018;72:e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maraboto C, Ferdinand KC. Update on hypertension in African-Americans. Progress in Cardiovascular Diseases. 2020;63:33–39. [DOI] [PubMed] [Google Scholar]
- 92.Howard G, Cushman M, Moy CS, Oparil S, Muntner P, Lackland DT, Manly JJ, Flaherty ML, Judd SE, Wadley VG, Long DL, Howard VJ. Association of Clinical and Social Factors With Excess Hypertension Risk in Black Compared With White US Adults. JAMA. 2018;320:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ, DASH Collaborative Research Group. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. The American Journal of Cardiology. 2004;94:222–227. [DOI] [PubMed] [Google Scholar]
- 94.Wyatt SB, Akylbekova EL, Wofford MR, Coady SA, Walker ER, Andrew ME, Keahey WJ, Taylor HA, Jones DW. Prevalence, awareness, treatment, and control of hypertension in the Jackson Heart Study. Hypertension. 2008;51:650–656. [DOI] [PubMed] [Google Scholar]
- 95.Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, Rashid M, Hsu B, Foxx-Drew D, Moy N, Reid AE, Elashoff RM. A Cluster-Randomized Trial of Blood-Pressure Reduction in Black Barbershops. The New England Journal of Medicine. 2018;378:1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walejko JM, Kim S, Goel R, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Gut microbiota and serum metabolite differences in African Americans and White Americans with high blood pressure. International Journal of Cardiology. 2018;271:336–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen L, He FJ, Dong Y, Huang Y, Wang C, Harshfield GA, Zhu H. Modest Sodium Reduction Increases Circulating Short-Chain Fatty Acids in Untreated Hypertensives: A Randomized, Double-Blind, Placebo-Controlled Trial. Hypertension. 2020;76:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen L, He FJ, Dong Y, Huang Y, Harshfield GA, Zhu H. Sodium Reduction, Metabolomic Profiling, and Cardiovascular Disease Risk in Untreated Black Hypertensives. Hypertension. 2019;74:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Camilleri M Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qi Y, Goel R, Kim S, Richards EM, Carter CS, Pepine CJ, Raizada MK, Buford TW. Intestinal Permeability Biomarker Zonulin is Elevated in Healthy Aging. Journal of the American Medical Directors Association. 2017;18:810.e1–810.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pasini E, Corsetti G, Assanelli D, Testa C, Romano C, Dioguardi FS, Aquilani R. Effects of chronic exercise on gut microbiota and intestinal barrier in human with type 2 diabetes. Minerva Medica. 2019;110:3–11. [DOI] [PubMed] [Google Scholar]
- 102.Peters A, Wekerle H. Autoimmune diabetes mellitus and the leaky gut. Proceedings of the National Academy of Sciences of the United States of America. 2019;116:14788–14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Soderholm AT, Pedicord VA. Intestinal epithelial cells: at the interface of the microbiota and mucosal immunity. Immunology. 2019;158:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer R-J. Review article: the role of butyrate on colonic function. Alimentary Pharmacology & Therapeutics. 2008;27:104–119. [DOI] [PubMed] [Google Scholar]
- 105.Zong X, Fu J, Xu B, Wang Y, Jin M. Interplay between gut microbiota and antimicrobial peptides. Animal Nutrition. 2020;6:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. Regulation of inflammation by microbiota interactions with the host. Nature Immunology. 2017;18:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig Y, Pevsner-Fischer M, Shapiro H, Christ A, Harmelin A, Halpern Z, et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell. 2015;163:1428–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Terry N, Margolis KG. Serotonergic Mechanisms Regulating the GI Tract: Experimental Evidence and Therapeutic Relevance. Handbook of Experimental Pharmacology. 2017;239:319–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manocha M, Khan WI. Serotonin and GI Disorders: An Update on Clinical and Experimental Studies. Clinical and Translational Gastroenterology. 2012;3:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghia J-E, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Côté F, Mallet J, Khan WI. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. [DOI] [PubMed] [Google Scholar]
- 111.Kwon YH, Wang H, Denou E, Ghia J-E, Rossi L, Fontes ME, Bernier SP, Shajib MS, Banskota S, Collins SM, Surette MG, Khan WI. Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. Cellular and Molecular Gastroenterology and Hepatology. 2019;7:709–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L, Comstock LE, Gandhi R, Weiner HL. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host & Microbe. 2016;19:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peck BCE, Mah AT, Pitman WA, Ding S, Lund PK, Sethupathy P. Functional Transcriptomics in Diverse Intestinal Epithelial Cell Types Reveals Robust MicroRNA Sensitivity in Intestinal Stem Cells to Microbial Status. The Journal of Biological Chemistry. 2017;292:2586–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johnston DGW, Williams MA, Thaiss CA, Cabrera-Rubio R, Raverdeau M, McEntee C, Cotter PD, Elinav E, O’Neill LAJ, Corr SC. Loss of MicroRNA-21 Influences the Gut Microbiota, Causing Reduced Susceptibility in a Murine Model of Colitis. Journal of Crohn’s & Colitis. 2018;12:835–848. [DOI] [PubMed] [Google Scholar]
- 115.Hsu C-N, Tain Y-L. Developmental Programming and Reprogramming of Hypertension and Kidney Disease: Impact of Tryptophan Metabolism. International Journal of Molecular Sciences. 2020;21:E8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu P-C, Hsu C-N, Lin I-C, Lo M-H, Yang M-Y, Tain Y-L. The Association Between Changes in Plasma Short-Chain Fatty Acid Concentrations and Hypertension in Children With Chronic Kidney Disease. Frontiers in Pediatrics. 2020;8:613641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaye DM, Shihata WA, Jama HA, Tsyganov K, Ziemann M, Kiriazis H, Horlock D, Vijay A, Giam B, Vinh A, Johnson C, Fiedler A, Donner D, Snelson M, Coughlan MT, et al. Deficiency of Prebiotic Fiber and Insufficient Signaling Through Gut Metabolite-Sensing Receptors Leads to Cardiovascular Disease. Circulation. 2020;141:1393–1403. [DOI] [PubMed] [Google Scholar]
- 118.Brandtzaeg P, Johansen F-E. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunological Reviews. 2005;206:32–63. [DOI] [PubMed] [Google Scholar]
- 119.Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annual Review of Immunology. 2010;28:243–273. [DOI] [PubMed] [Google Scholar]
- 120.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, Ruggiero E, Cho JH, Goodman AL, Flavell RA. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bollinger RR, Everett ML, Wahl SD, Lee Y-H, Orndorff PE, Parker W. Secretory IgA and mucin-mediated biofilm formation by environmental strains of Escherichia coli: role of type 1 pili. Molecular Immunology. 2006;43:378–387. [DOI] [PubMed] [Google Scholar]
- 122.Bunker JJ, Erickson SA, Flynn TM, Henry C, Koval JC, Meisel M, Jabri B, Antonopoulos DA, Wilson PC, Bendelac A. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science. 2017;358:eaan6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kubinak JL, Stephens WZ, Soto R, Petersen C, Chiaro T, Gogokhia L, Bell R, Ajami NJ, Petrosino JF, Morrison L, Potts WK, Jensen PE, O’Connell RM, Round JL. MHC variation sculpts individualized microbial communities that control susceptibility to enteric infection. Nature Communications. 2015;6:8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kau AL, Planer JD, Liu J, Rao S, Yatsunenko T, Trehan I, Manary MJ, Liu T-C, Stappenbeck TS, Maleta KM, Ashorn P, Dewey KG, Houpt ER, Hsieh C-S, Gordon JI. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Science Translational Medicine. 2015;7:276ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Planer JD, Peng Y, Kau AL, Blanton LV, Ndao IM, Tarr PI, Warner BB, Gordon JI. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature. 2016;534:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.D’Auria G, Peris-Bondia F, Džunková M, Mira A, Collado MC, Latorre A, Moya A. Active and secreted IgA-coated bacterial fractions from the human gut reveal an under-represented microbiota core. Scientific Reports. 2013;3:3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rojas OL, Pröbstel A-K, Porfilio EA, Wang AA, Charabati M, Sun T, Lee DSW, Galicia G, Ramaglia V, Ward LA, Leung LYT, Najafi G, Khaleghi K, Garcillán B, Li A, et al. Recirculating Intestinal IgA-Producing Cells Regulate Neuroinflammation via IL-10. Cell. 2019;176:610–624.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pröbstel A-K, Zhou X, Baumann R, Wischnewski S, Kutza M, Rojas OL, Sellrie K, Bischof A, Kim K, Ramesh A, Dandekar R, Greenfield AL, Schubert RD, Bisanz JE, Vistnes S, et al. Gut microbiota-specific IgA+ B cells traffic to the CNS in active multiple sclerosis. Science Immunology. 2020;5:eabc7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang J, Pearson JA, Peng J, Hu Y, Sha S, Xing Y, Huang G, Li X, Hu F, Xie Z, Xiao Y, Luo S, Chao C, Wong FS, Zhou Z, et al. Gut microbial metabolites alter IgA immunity in type 1 diabetes. JCI insight. 2020;5:135718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin R, Chen H, Shu W, Sun M, Fang L, Shi Y, Pang Z, Wu W, Liu Z. Clinical significance of soluble immunoglobulins A and G and their coated bacteria in feces of patients with inflammatory bowel disease. Journal of Translational Medicine. 2018;16:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang T, Ding C, Zhao M, Dai X, Yang J, Li Y, Gu L, Wei Y, Gong J, Zhu W, Li N, Li J. Sodium Butyrate Reduces Colitogenic Immunoglobulin A-Coated Bacteria and Modifies the Composition of Microbiota in IL-10 Deficient Mice. Nutrients. 2016;8:E728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Viladomiu M, Kivolowitz C, Abdulhamid A, Dogan B, Victorio D, Castellanos JG, Woo V, Teng F, Tran NL, Sczesnak A, Chai C, Kim M, Diehl GE, Ajami NJ, Petrosino JF, et al. IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote TH17-dependent inflammation. Science Translational Medicine. 2017;9:eaaf9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Y, Yuan X, Li L, Lin L, Zuo X, Cong Y, Li Y. Increased Ileal Immunoglobulin A Production and Immunoglobulin A-Coated Bacteria in Diarrhea-Predominant Irritable Bowel Syndrome. Clinical and Translational Gastroenterology. 2020;11:e00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen CM, Schachter D. Elevation of plasma immunoglobulin A in the spontaneously hypertensive rat. Hypertension. 1993;21:731–738. [DOI] [PubMed] [Google Scholar]
- 135.Taylor EB, Barati MT, Powell DW, Turbeville HR, Ryan MJ. Plasma Cell Depletion Attenuates Hypertension in an Experimental Model of Autoimmune Disease. Hypertension. 2018;71:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X, Li Y, Li H, Gu Y, Song Y, Zhang Q, Liu L, Meng G, Yao Z, Wu H, Xia Y, Bao X, Shi H, Su Q, Fang L, et al. Relationship of serum immunoglobulin levels to blood pressure and hypertension in an adult population. Journal of Human Hypertension. 2018;32:212–218. [DOI] [PubMed] [Google Scholar]
- 137.Sereti E, Stamatelopoulos KS, Zakopoulos NA, Evangelopoulou A, Mavragani CP, Evangelopoulos ME. Hypertension: An immune related disorder? Clinical Immunology (Orlando, Fla.). 2020;212:108247. [DOI] [PubMed] [Google Scholar]
- 138.Bandini F Minocycline in neurological diseases. The Lancet. Neurology. 2005;4:138–139. [DOI] [PubMed] [Google Scholar]
- 139.Yang Q, Luo L, Sun T, Yang L, Cheng L-F, Wang Y, Liu Q-Q, Liu A, Liu H-Y, Zhao M-G, Wu S-X, Feng B. Chronic minocycline treatment exerts antidepressant effect, inhibits neuroinflammation, and modulates gut microbiota in mice. Psychopharmacology. 2020;237:3201–3213. [DOI] [PubMed] [Google Scholar]
- 140.Schmidtner AK, Slattery DA, Gläsner J, Hiergeist A, Gryksa K, Malik VA, Hellmann-Regen J, Heuser I, Baghai TC, Gessner A, Rupprecht R, Di Benedetto B, Neumann ID. Minocycline alters behavior, microglia and the gut microbiome in a trait-anxiety-dependent manner. Translational Psychiatry. 2019;9:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wong M-L, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, Kentish S, Xie P, Morrison M, Wesselingh SL, Rogers GB, Licinio J. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Molecular Psychiatry. 2016;21:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shen H, Guan Q, Zhang X, Yuan C, Tan Z, Zhai L, Hao Y, Gu Y, Han C. New mechanism of neuroinflammation in Alzheimer’s disease: The activation of NLRP3 inflammasome mediated by gut microbiota. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2020;100:109884. [DOI] [PubMed] [Google Scholar]
- 143.Schmidt EKA, Raposo PJF, Torres-Espin A, Fenrich KK, Fouad K. Beyond the lesion site: minocycline augments inflammation and anxiety-like behavior following SCI in rats through action on the gut microbiota. Journal of Neuroinflammation. 2021;18:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang T-Y, Chu H-C, Lin Y-L, Lin C-K, Hsieh T-Y, Chang W-K, Chao Y-C, Liao C-L. Minocycline attenuates experimental colitis in mice by blocking expression of inducible nitric oxide synthase and matrix metalloproteinases. Toxicology and Applied Pharmacology. 2009;237:69–82. [DOI] [PubMed] [Google Scholar]
- 145.Garrido-Mesa J, Rodríguez-Nogales A, Algieri F, Vezza T, Hidalgo-Garcia L, Garrido-Barros M, Utrilla MP, Garcia F, Chueca N, Rodriguez-Cabezas ME, Garrido-Mesa N, Gálvez J. Immunomodulatory tetracyclines shape the intestinal inflammatory response inducing mucosal healing and resolution. British Journal of Pharmacology. 2018;175:4353–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu P, Thinschmidt JS, Yan Y, Hazra S, Bhatwadekar A, Caballero S, Salazar T, Miyan JA, Li W, Derbenev A, Zsombok A, Tikhonenko M, Dominguez JM, McGorray SP, Saban DR, et al. CNS inflammation and bone marrow neuropathy in type 1 diabetes. The American Journal of Pathology. 2013;183:1608–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yellowlees Douglas J, Bhatwadekar AD, Li Calzi S, Shaw LC, Carnegie D, Caballero S, Li Q, Stitt AW, Raizada MK, Grant MB. Bone marrow-CNS connections: implications in the pathogenesis of diabetic retinopathy. Progress in Retinal and Eye Research. 2012;31:481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA, American Heart Association. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642. [DOI] [PubMed] [Google Scholar]
- 149.Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. BioMed Research International. 2014;2014:406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Honour JW, Borriello SP, Ganten U, Honour P. Antibiotics attenuate experimental hypertension in rats. The Journal of Endocrinology. 1985;105:347–350. [DOI] [PubMed] [Google Scholar]
- 151.Antonio RC, Ceron CS, Rizzi E, Coelho EB, Tanus-Santos JE, Gerlach RF. Antioxidant effect of doxycycline decreases MMP activity and blood pressure in SHR. Molecular and Cellular Biochemistry. 2014;386:99–105. [DOI] [PubMed] [Google Scholar]
- 152.Pires PW, Rogers CT, McClain JL, Garver HS, Fink GD, Dorrance AM. Doxycycline, a matrix metalloprotease inhibitor, reduces vascular remodeling and damage after cerebral ischemia in stroke-prone spontaneously hypertensive rats. American Journal of Physiology. Heart and Circulatory Physiology. 2011;301:H87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Galla S, Chakraborty S, Cheng X, Yeo J, Mell B, Zhang H, Mathew AV, Vijay-Kumar M, Joe B. Disparate effects of antibiotics on hypertension. Physiological Genomics. 2018;50:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Galla S, Chakraborty S, Cheng X, Yeo J-Y, Mell B, Chiu N, Wenceslau CF, Vijay-Kumar M, Joe B. Exposure to Amoxicillin in Early Life Is Associated With Changes in Gut Microbiota and Reduction in Blood Pressure: Findings From a Study on Rat Dams and Offspring. Journal of the American Heart Association. 2020;9:e014373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yoo HH, Kim IS, Yoo D-H, Kim D-H. Effects of orally administered antibiotics on the bioavailability of amlodipine: gut microbiota-mediated drug interaction. Journal of Hypertension. 2016;34:156–162. [DOI] [PubMed] [Google Scholar]
- 156.Wright AJ, Gomes T, Mamdani MM, Horn JR, Juurlink DN. The risk of hypotension following co-prescription of macrolide antibiotics and calcium-channel blockers. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2011;183:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Qi Y, Aranda JM, Rodriguez V, Raizada MK, Pepine CJ. Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension — A case report. International Journal of Cardiology. 2015;201:157–158. [DOI] [PMC free article] [PubMed] [Google Scholar]


