Abstract
Mainly because of the large number of people affected and associated significant health policy implications, the Robert Koch Institute (RKI) is developing a public health surveillance system using diabetes as an example. In a first step to ensure long-term and comparable data collection and establish efficient surveillance structures, the RKI has defined a set of relevant indicators for diabetes surveillance. An extensive review of the available literature followed by a structured process of consensus provided the basis for a harmonised set of 30 core and 10 supplementary indicators. They correspond to the following four fields of activity: (1) reducing diabetes risk, (2) improving diabetes early detection and treatment, (3) reducing diabetes complications, (4) reducing the disease burden and overall costs of the disease. In future, in addition to the primary data provided by RKI health monitoring diabetes surveillance needs to also consider the results from secondary data sources. Currently, barriers to accessing this data remain, which will have to be overcome, and gaps in the data closed. The RKI intentends to continuously update this set of indicators and at some point apply it also to further chronic diseases with high public health relevance.
Keywords: PUBLIC HEALTH, SURVEILLANCE, DIABETES MELLITUS, INDICATORS, NCD
1. Background
Public health surveillance is the systematic collection and analysis of relevant health data for the provision of up-to-date information and therefore provides an important basis for decisions by diverse (health) political stakeholders to protect and improve the health of the population [1-3]. Surveillance consequently is one of the key fields of public health [4] and is no longer limited merely to infectious diseases. In the 21st century, preventing chronic, noncommunicable diseases (NCD) and providing care to patients has become one of the great health challenges globally [5-7]. Cardiovascular diseases, cancer, chronic lung diseases and diabetes mellitus [8] are now ailments faced not only by wealthy countries, but increasingly and significantly also contribute to premature mortality in low and middle income countries, which has caused the World Health Organization (WHO) to define clear goals in its Global Action Plan 2013-2020 on NCD prevention and control.
Diabetes mellitus in particular has become a serious issue for healthcare systems globally [9-11]. Due to high incidence rates and the great potential offered by prevention measures, type 2 diabetes is currently a focus. The known type 2 diabetes risk factors specifically include obesity, lack of physical activity, unhealthy diet, smoking, stress and social deprivation [12]. However, the role played by environmental and psycho-social factors in type 2 diabetes is still not fully understood [13, 14]. Equally, the complex interactions between inherited and acquired risk factors over the course of a person’s life necessitate further research. During gestation and infancy epigenetic mechanisms possibly leave their mark on metabolic processes later in life [15]. Gestational diabetes potentially plays a particularly important role here. Although in most cases women recover from this form of diabetes after pregnancy, the disease is related to a greater risk of complications during pregnancy as well as an increased risk for both the mother and the baby to develop type 2 diabetes at a later stage in life [16-18].
Importantly, the life-style related risk factors for type 2 diabetes also play a key role in other important NCDs [5, 19]. Often, the large socio-economic implications of type 2 diabetes mean that the great public health relevance of type 1 diabetes, which is a far rarer form and an autoimmune disease, is also often overlooked. Mostly, this form develops at childhood or adolescent age and requires patients to take insulin for the rest of their life, with a correspondingly severe impact on their quality of life. Moreover, over the past decade a so far unexplained global increase in the number of new cases of type 1 diabetes is recognised [20, 21].
According to data from the Robert Koch Institute (RKI), an estimated 6.7 million adults in Germany are affected by diagnosed or undiagnosed diabetes [22, 23]. For adults in Germany, the data from national health monitoring suggests that around one in five cases of diabetes goes unrecognised, with a significant decrease in this figure being registered since the end of the 1990s [23]. Notwithstanding the advances in early detection and treatment, diabetes mellitus continues to entail severe complications in many patients mainly due to the damages to small and large blood vessels, as well as the peripheral and autonomic nervous system. Cardiac insufficiency, infarction and stroke, diabetic foot and amputations of the lower extremities, diabetic eye diseases and blindness, renal insufficiency and dialysis, as well as diabetic neuropathy are among the most frequent complications of diabetes mellitus. Furthermore, in women, diabetes increases the risk for complications during pregnancy [24], as well as for depression and possibly dementia [25] and is associated with an increased risk for certain types of cancer [26]. Overall mortality for adults with diabetes remains significantly higher than for people of the same age without diabetes, although the diabetes-related excess mortality varies depending on age and gender [9, 27, 28].
This explains the central role played by diabetes mellitus as an NCD [19] and why the RKI, on behalf of the Federal Ministry of Health, chose diabetes as a model disease for developing an NCD public health surveillance. Public health surveillance [19] requires a scientific framework concept with defined goals and fields of activity, evidence-based and health politically relevant indicators [29-32], as well as an integration into the established health information systems [33]. This has not yet been achieved in Germany. While the indicator set of the federal states' health reporting [34] by default includes indicators to account for diabetes-related hospital admissions, early retirements and deaths, not all federal states so far have implemented this set of indicators. Some states have additional indicators that cover, for example, outpatient care. Moreover, some federal states have already published reports on diabetes [35]. No federal state has so far established a global concept for diabetes surveillance [36]. Besides reviewing, analysing and gathering available sources of data, establishing diabetes surveillance in Germany will depend primarily on developing a corresponding scientific framework concept and selecting suitable indicators [37].
Following Germany’s 2003 national health target Diabetes mellitus type 2 [38, 39], four fields of activity were identified: (1) reducing diabetes risk, (2) improving diabetes early detection and treatment, (3) reducing diabetes complications, (4) reducing the disease burden and overall costs of the disease [37]. The focus of this article is the methodological approach, which the RKI applied to select and define its set of indicators for diabetes surveillance in Germany.
2. Methods
The selection and definition of indicators relevant to health policy for diabetes was a multi-stage process, which was based on two independent reviews of the literature, as well as a structured consensus process that involved a national and international level interdisciplinary panel of experts (Annex Table 1).
2.1 Review of the literature
Diabetes mellitus and NCD indicators
Regarding diabetes mellitus and NCDs, the RKI conducted a selective review of publications and information systems at the population level between January and June 2016. The search covered the current international health indicator systems of the Organisation for Economic Cooperation and Development (OECD), the European Union (European Community Health Indicators and Monitoring, ECHI) and the World Health Organization (WHO) [30, 32, 40]. A further focus were indicator-based scientific publications, reports and online information systems on diabetes mellitus and NCDs in OECD member states. The search considered publications regardless of date of publication, but was limited to publications and information in either German or English. For Germany, the health reporting, prevention, rehabilitation and social medicine working group of the Permanent Working Group of the Highest State Health Authorities (AOLG) in addition identified indicator-based, federal state level reports and publications on diabetes mellitus. Furthermore, a working group of experts from Baden-Württemberg proposed an initial indicator set [41]. Moreover, current national and selected international diabetes mellitus treatment guidelines [42, 43], cardiovascular disease prevention guidelines [44], as well as the quality targets of the type 1 and type 2 diabetes disease management programmes (DMP) were reviewed [45].
The scope of indicators considered was limited per definition to: (1) indicators on type 1 and type 2 diabetes, as well as gestational diabetes, (2) indicators related to one of the four defined fields of diabetes surveillance in Germany, (3) indicators defined in either German or English.
Indicators were excluded if they fell into one of the following categories: (1) indicators for rare forms of diabetes mellitus, (2) indicators for only specific target groups (such as patients with particular accompanying diseases), (3) and indicators that are not fully compatible with Germany’s healthcare system.
Quality indicators for type 2 diabetes care
A joint scientific cooperation project on diabetes surveillance [46], which was guided by the Institute for Applied Quality Improvement and Research in Health Care GmbH (aQua) in Göttingen, conducted an additional systematic review of the literature with a focus on type 2 diabetes and quality of care.
This review was conducted in the literature databases of Embase, Medline and the Cochrane Library, as well as in the indicator databases of the US Agency for Healthcare Research and Quality (AHRQ) and the British Health and Social Care Information Centre (HSCIC). Further international institutions and agencies that develop, compile or publish indicators were also reviewed. To take account of the German context, a partially automated text search was conducted of Germany’s national guidelines (NVL) on diabetes treatment [43].
The following inclusion criteria were defined: (1) indicators for adults with type 2 diabetes, (2) indicators defined in either German or English.
The criteria to exclude indicators were defined as: (1) indicators that are incompatible with the German health system, (2) indicators without clearly defined numerators and denominators, (3) indicators that relate exclusively to the care aspects of diabetes in certain population groups (e.g. African Americans in the USA).
2.2 Selecting and assessing indicators
At the RKI, the diabetes surveillance working group reviewed all of the indicators identified based on these parameters and supplemented them with indicators for: diabetes risk tests, rehabilitation, patient satisfaction, avoidable hospital admissions, screenings (gestational diabetes) and disease burden.
To ensure international compatibility, a national and international panel of experts conducted a structured assessment of the indicators provided by the RKI (Annex Table 1a); a process, which included a written assessment of indicators regarding their relevance to national diabetes surveillance. All members of the external panel of experts (n=35) received an email with an evaluation template attached to assess the relevance of indicators (essential, important, additional and negligible) that included blank boxes for further comments. The diabetes surveillance working group received back 17 assessment forms, which represents a 49% response rate. The results of this initial assessment were prepared in the run-up to an international scientific workshop at the RKI in July 2016 and then discussed and finalised during the workshop [37, 47].
The following step consisted of evaluating the health policy relevance of the selected indicators for diabetes surveillance in Germany. This was based on a two-tier Delphi method, which drew on the approach financed and started by the EU in the Joint Action on Chronic Diseases [48] programme, as well as the RAND/UCLA Appropriateness Method (RAM) [49].
In a first step, based on a nine-step scale, the 20 members of the diabetes surveillance scientific board (Annex Table 1b) were asked to rank all of the indicators according to their relevance for health policy. The members of the board received a corresponding assessment form as an email attachment and asked to return it to the RKI. The form permitted them to comment on the clarity, health political influenceability and international comparability of indicators. The RKI received 13 assessment forms back, which represents a 65% response rate. Subsequently these forms were evaluated and the results for each indicator comprehensively prepared:
► Indicators were classified as highly relevant if they received at least 75% from the top three grades (7-9).
► Indicators were classified as relevant if they received between 50% and under 75% of ratings from the top three grades (7-9).
► Indicators were classified as being of medium relevance if at least 50% of ratings were from the medium rates (4-6).
► Indicators were classified as being of low relevance if at least 50% of ratings were from the lowest rates (1-3) (Figure 1).
Figure 1.
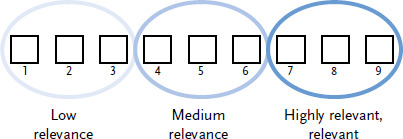
Evaluation template on indicator selection and relevance assessment
Own diagram
In the second step of the Delphi method, the members of the scientific board were invited to a meeting in Berlin. 16 members participated in the meeting in March 2017, which corresponds to an attendance rate of 80%. During the meeting the participating board members were presented with the results of the first assessment round and provided with the opportunity to discuss or clarify open questions or unclear definitions for each indicator. Following the discussion, all board members were asked to rate once again the indicators regarding their relevance for diabetes surveillance in writing and anonymously. The format of the assessment forms and evaluation criteria was identical to that of the first step of the procedure.
In a separate move, an additional panel of experts provided an evaluation of the indicators of the cooperation project on type 2 diabetes quality of care (Annex Table 1c). Here too, the evaluation of indicators was based on a two-tier Delphi method with a focus on type 2 diabetes quality of care. This was based on a modified RAND-UCLA appropriateness method (RAM) provided by the aQua institute [49, 50]. Relevance in this case too was assessed using the nine-step scale described in Figure 1. Both stages of the assessment procedure were conducted in writing and anonymously and included both filling out an online evaluation and an evaluation provided during a face-to-face meeting following a discussion of the results of the online evaluation.
For a final selection of indicators, the RKI evaluated the results from these review strategies. Initially, indicators from both indicator sets, which were rated as either highly relevant or relevant became part of the final selection and indicators with a medium or low relevance rating were excluded. In addition, from the indicators provided by the cooperation project those considering only specific aspects of treatment (such as amputations without vascular procedures, major amputations without a second opinion procedure) were also excluded. The remaining indicators were grouped into core and supplementary indicators for diabetes surveillance in Germany at the population level based on the following criteria:
Core indicators:
► Indicators classified as highly relevant, regardless as to whether they were classified as indicators for type 2 diabetes quality of care or not.
► Indicators classified as relevant, which in addition were classified as relevant for type 2 diabetes quality of care.
► Indicators classified as relevant for type 2 diabetes quality of care with a clear link to diabetes surveillance at the population level, which had so far not been included in the set of indicators used by the diabetes surveillance working group.
Supplementary indicators:
► Indicators classified as relevant, which were however not identified as indicators for type 2 diabetes quality of care.
The consensus-oriented closing discussion round prepared the indicators in line with the ECHI model [51] regarding the following aspects: operationalisation (definition, reference population), available stratification variables (such as age, gender and socioeconomic status), data availability, type and periodicity of appropriate data sources, scientific background, references, comments on specificities regarding definition or use. Moreover, indicators were pre-assigned to one of the four defined fields of activity in line with the 2003 national health target diabetes mellitus type 2 [38, 39].
3. Results
Initially, the described search strategy regarding the established diabetes and NCD reporting systems served to identify 32 indicators and/or groups of indicators. In addition, 13 indicators covering further hitherto insufficiently described fields were also adopted. This established an initial set of 45 indicators.
During the structured consensus process, in an initial evaluation round, the international panel of experts assessed 18 indicators as being ‘essential’, 14 indicators as being ‘important’, and 8 further indicators as being ‘additional’ indicators. Five indicators were rated heterogeneously, no consensus was achieved and they could therefore not be assigned to one of the categories. No indicator was classified as ‘negligible’. Furthermore, the members of the diabetes surveillance scientific board proposed four additional indicators (gestational diabetes prevalence, early retirement, hypoglycaemia incidence and information needs). They also proposed to split the indicator ‘environmental factors’ into three separate indicators (traffic exposure, walkability and social deprivation). This produced an indicator set of 51 indicators. The following national level two-tier Delphi method assessed 18 indicators as being highly relevant, 18 as being relevant and 15 as being of medium relevance. No indicator was assessed as being of low relevance.
3.1 Final selection of indicators
The final selection considered only indicators in the highly relevant (n=18) and relevant (n=18) groups. This step therefore excluded the 15 medium relevance indicators.
During the final step of the procedure, the remaining 36 indicators were compared to the results of the cooperation project on indicators for type 2 diabetes quality of care. Four additional indicators on relevant aspects of diabetes treatment were then added to the diabetes surveillance indicator set (age at diagnosis, renal replacement therapy, diabetic neuropathy, and diabetic foot syndrome). Moreover, core and supplementary indicators were defined based on the described criteria. The flowchart (Figure 2) provides a detailed step-by-step description of the indicators that were identified over the course of the selection process.
Figure 2.
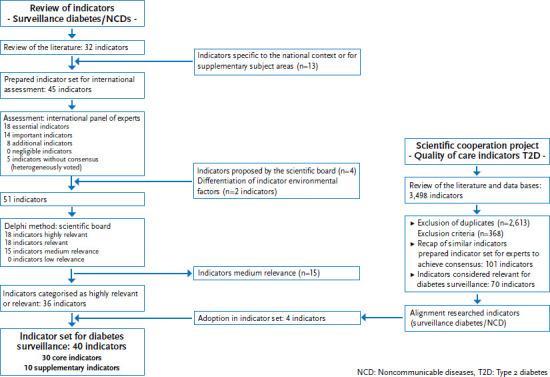
Flowchart diabetes surveillance indicator selection
Own diagram
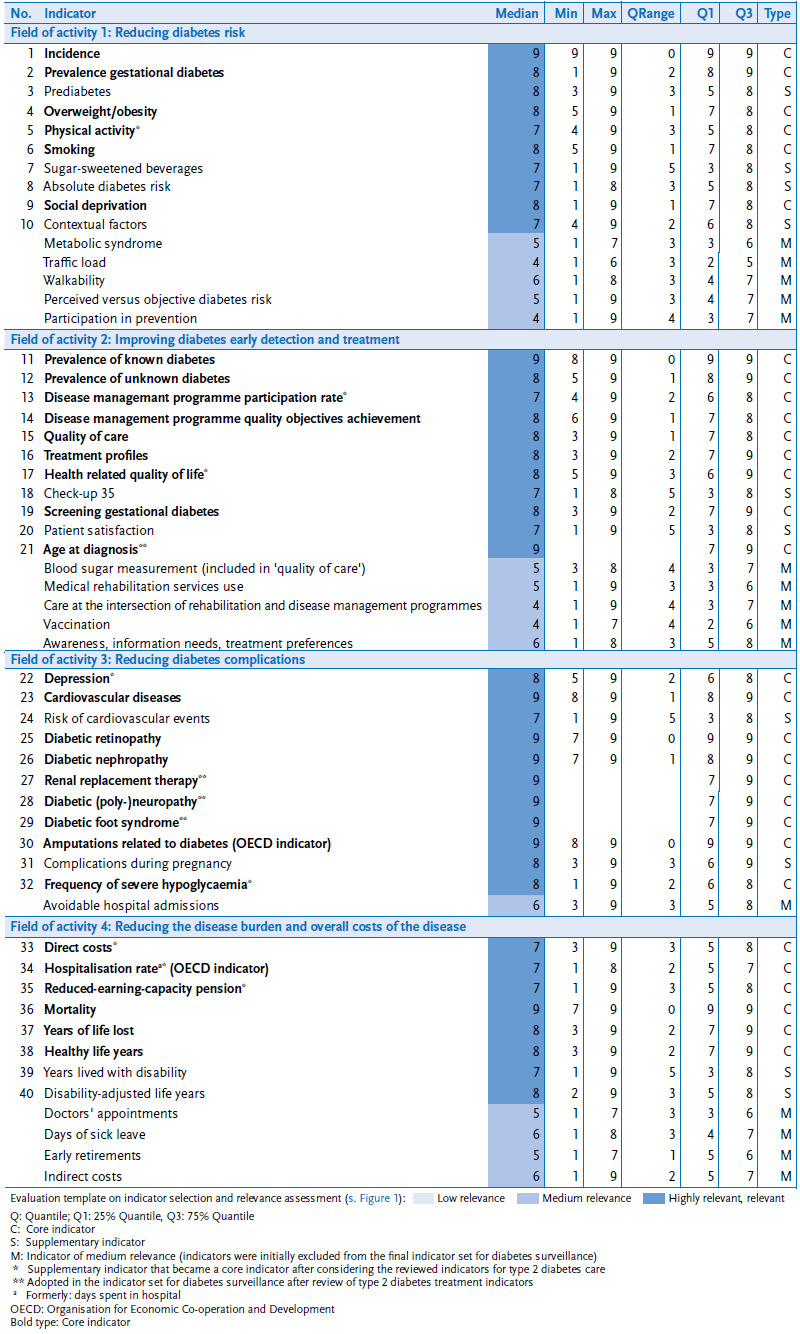
As a result, the resulting set of 30 core and 10 supplementary indicators/indicator groups for the four fields of activity were presented to the scientific board and approved in unanimous consensus (Table 1). The complete prepared indicator set including information on indicator operationalisation is available via the internet pages of the RKI (www.rki.de/diabsurv). Furthermore, the detailed assessments underlying the selection of indicators is included for all indicators as well as their division into core and supplementary indicators (Annex Table 2).
Table 1.
Subject areas for indicators for diabetes surveillance in four fields of activity
Own diagram
| (1) Reducing diabetes risk | (2) Improving diabetes early detection and treatment |
|---|---|
|
Core indicators Incidence Prevalence gestational diabetes Overweight/obesity Physical activity Smoking Social deprivation |
Core indicators Prevalence of known diabetes Prevalence of unknown diabetes Disease management programme participation rate Disease management programme quality objectives achievement Quality of care Treatment profiles Health related quality of life Screening gestational diabetes Age at diagnosis |
|
Supplementary indicators Prediabetes Sugary beverages consumption Absolute diabetes risk (based on the German diabetes risk test) Contextual factors |
Supplementary indicators Check-up 35 Patient satisfaction |
| (3) Reducing diabetes complications | (4) Reducing the disease burden and overall costs of the disease |
|
Core indicators Depression Cardiovascular diseases Diabetic retinopathy Diabetic nephropathy Renal replacement therapy Diabetic (poly-)neuropathy Diabetic foot syndrome Amputations related to diabetes Frequency of severe hypoglycaemia |
Core indicators Direct costs Hospitalisation rate Reduced-earning-capacity pension Mortality Years of life lost Healthy life years |
|
Supplementary indicators Risk of cardiovascular events Complications during pregnancy |
Supplementary indicators Years lived with disability Disability-adjusted life years (DALYs) |
Where possible, and depending on the available data, the diabetes types are differentiated, and, depending on the available data, the results also stratified by age, gender, social status, education and region.
4. Discussion and outlook
The selected set of indicators for diabetes surveillance in Germany aims to collect detailed, comparable and successive data on diabetes mellitus in Germany. This should establish a firm basis for diabetes surveillance, and aims to ensure an up to date and comprehensive analysis of disease dynamics, potential for prevention and care needs. The epidemiologic development of incidence and prevalence will be covered, as will the development of the known risk factors, associated diseases and quality of care that can largely be influenced. What is crucial here is a differentiation by gender, age, socioeconomic status and region. This last factor establishes a bridge to federal state level health reporting.
In Germany, potentially useful and ample data is already available. As each source of data has its specific advantages and disadvantages, the described consensually agreed indicator set references different sources of data for calculating indicators depending on the availability of such data. Primary RKI health survey data for example permits an analysis stratified by socioeconomic status and further individual level aspects, and, by referring to laboratory analysis, allows estimating the prevalence of unknown diabetes in the population. Furthermore, information on a set of risk factors is collected, which facilitate predicting the development of a type 2 diabetes by means of established risk models [52]. Furthermore, population representative estimates on quality of life and a comparison of the functional limitations faced by adults with and without diabetes mellitus are possible, as are estimates on diabetes comorbidity [53] and for selected indicators on the quality of care for adults with diabetes mellitus [54]. Secondary sources of data often offer in particular the advantage of providing information on a large number of cases, the regular availability of this data and a lack of distortion effects due to non-participation. Data from statutory health insurances (GKV) for example or the associations of statutory health insurance physicians (KVen) provide up to date, periodically recurrent estimates on the development of the incidence of medically diagnosed diabetes including the possibility of differentiating between types of diabetes and region [55, 56]. An assessment of the quality of care is possible, based as much on the results of regular DMP analyses [45, 57] as on GKV routine data provided on out and inpatient treatment [58, 59].
Diabetes surveillance will therefore also need to establish the specific strengths and weaknesses of each source of data. This should also lead to recommendations regarding the use of this data and its application to other diseases for a broader surveillance of NCDs in the future. Implementation of the data transparency act (DaTraV) in particular, which was begun by the German Institute of Medical Documentation and Information (DIMDI) in 2014, meanwhile provides data for morbidity-oriented risk structure compensation (Morbi-RSA) from all statutory health insurers for research. Processing of this data constitutes a first and important step towards using routine data for surveillance. In spite of providing data for everybody insured in the GKV, the data set currently remains limited to outpatient and inpatient diagnoses and the outpatient provision of medicines. Further data on outpatient services, as well as on operations, procedures or general measures in inpatient care is not included. Certain process-oriented indicators, however, require such information. A satisfactory solution to accessing sources of data that include such information (GKV and KV routine data, as well as DMP documentation data) is still lacking. Generally, these bodies of data are only accessible in the context of research cooperation projects. Surveillance, however, would demand a regular access to such sources of data. Clearing these obstacles in a dialogue with the holders and users of such data is desirable. In addition, the indicators of quality deducted from DMP are subject to a certain degree of change over time regarding their definition and target population (definition of the numerator and denominator sample).
To define indicator sets, this process of seeking consensus seems appropriate. Establishing an interdisciplinary advisory committee has meant that indicators for relevant fields of activity in the areas of public health and prevention as well as medical treatment could be defined. Combining online assessments in writing and consensus-oriented discussion rounds during the bi-annual face-to-face board meetings is evidently a practicable and efficient approach. The established indicator set now for the first time provides a concept for a German surveillance system for one chronic, noncommunicable disease – diabetes mellitus. Initial analyses based on these newly established indicators will now begin and health reporting formats will be developed to ensure that the results are adequately prepared for the different target groups (universities, politics, patients, the general public etc.).
In the future, diabetes surveillance should serve as a model for a broader surveillance of NCDs, whereby the transferability of the model to other diseases still needs to be tested. Importantly, the indicator set should not be too rigid and developed further. This applies for example to the indicators for diabetes-related health competency in the overall population and among adults, who already have diabetes. Currently, a population representative telephone survey on disease knowledge and information needs is being conducted by the RKI in close cooperation with Germany’s Federal Centre for Health Education (BZgA). Furthermore, for a surveillance of NCDs, describing social and health policy context factors and the implementation of measures through indicators will be key. Moreover, the indicator system should be continuously further developed regarding compatibility with the nascent health information systems at the international level (WHO, EU). This applies in particular to activity field 1 (reducing diabetes risks), which will require the indicator group ‘context factors’ to be developed. Indicators in activity fields 2 and 3 (reducing diabetes complications, diabetes early detection and treatment) are defined relatively completely as regards the current quality target criteria (DMP, St Vincent criteria). Minor modifications, however, owing to the continuous updating of current definitions must be expected.
Regarding a future broader surveillance of NCDs, the goal remains to also define lead indicators analogous to the Swiss model over and above the differentiation between core and supplementary indicators which have already been established [60]. Lead indicators are defined as having an overarching relevance in terms of indicators to NCD surveillance and are collected at regular intervals and reliably in the context of a national NCD targets yet to be defined. From the current set of indicators, the epidemiological figures for prevalence, incidence and mortality, the regularly published treatment indicators of the OECD on diabetes-related amputations [30], as well as the indicators to quantify the disease burden such as disease costs or the years of life lost due to disease could serve as such lead indicators.
Key statements
Due to the high social relevance of diabetes and its sequelae, the Robert Koch Institute has started to establish a national diabetes surveillance system.
Surveillance systems require indicators that are evidence-based and consented among relevant stakeholders.
Using a multi-stage consensus process, 30 core and 10 supplementary indicators for surveillance were consented and assigned to four fields of activity.
Data from the nationwide health monitoring of the Robert Koch Institute and routine data will be used for continuous data analysis and timely reports of results.
The consented set of indicators is to be continously adapted and the methodological approach could be applied to other noncommunicable diseases of high public health impact.
Annex Table 1. (in German) Participants Expert Committees a) International Panel of Experts
| Prof. Dr. Winfried Banzer | Johann Wolfgang Goethe-Universität Frankfurt am Main, Institut für Sportwissenschaften |
| Prof. Dr. Michael Böhme | Landesgesundheitsamt Baden-Württemberg, Stuttgart |
| Dr. Brigitte Borrmann | Landeszentrum Gesundheit Nordrhein-Westfalen (LZG.NRW) |
| Dr. Ralph Brinks | Deutsches Diabetes-Zentrum (DDZ), Leibniz-Zentrum für Diabetes-Forschung an der Heinrich-Heine-Universität Düsseldorf, Institut für Biometrie und Epidemiologie |
| Prof. Dr. Reinhard Busse | Technische Universität Berlin, Fachgebiet Management im Gesundheitswesen |
| Prof. Dr. Fabrizio Carinci | University of Surrey, Guildford, School of Health Sciences |
| Diana Droßel | diabetesDE – Deutsche Diabetes-Hilfe |
| Prof. Dr. Saskia E. Drösler | Hochschule Niederrhein, University of Applied Sciences, Medizin, Medizin-Controlling und Informationssysteme |
| Prof. Dr. Michael Freitag | Deutsche Gesellschaft für Allgemeinmedizin (DEGAM) Carl von Ossietzky Universität Oldenburg, Department für Versorgungsforschung |
| Prof. Dr. Edward W. Gregg | Centers for Disease Control and Prevention (CDC), Division of Diabetes Translation |
| Dr. Bernd Hagen | Zentralinstitut für die kassenärztliche Versorgung in Deutschland (Zi) |
| Prof. Dr. Hans-Ulrich Häring | Universitätsklinikum Tübingen, Medizinische Klinik IV |
| Dr. Dirk Hochlenert | Deutsche Diabetes Gesellschaft |
| Prof. Dr. Reinhard Holl | Universität Ulm, Institut für Epidemiologie und medizinische Biometrie |
| Prof. Dr. Rolf Holle | Helmholtz Zentrum München, Deutsches Forschungszentrum für Gesundheit und Umwelt (GmbH), Institut für Gesundheitsökonomie und Management im Gesundheitswesen |
| Prof. Dr. Andrea Icks | Heinrich-Heine-Universität Düsseldorf, Medizinische Fakultät, Institut für Versorgungsforschung und Gesundheitsökonomie |
| Dr. Michael Jecht | Medizinisches Versorgungszentrum Havelhöhe |
| Prof. Dr. Jeffrey Johnson | University of Alberta, School of Public Health |
| Dr. Matthias Kaltheuner | Gemeinschaftspraxis Kaltheuner – v. Boxberg, Leverkusen |
| Dr. Klaus Koch | Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG), Ressort Gesundheitsinformation |
| Prof. Dr. Bernhard Kulzer | Diabetes Zentrum Mergentheim |
| Dr. Joseph Kuhn | Bayerisches Landesamt für Gesundheit und Lebensmittelsicherheit, Dienststelle Oberschleißheim |
| Prof. Dr. Oliver Kuß | Deutsches Diabetes-Zentrum (DDZ), Leibniz-Zentrum für Diabetes-Forschung an der Heinrich-Heine-Universität Düsseldorf, Institut für Biometrie und Epidemiologie |
| Prof. Dr. Gunter Laux | Universitätsklinikum Heidelberg, Abteilung Allgemeinmedizin und Versorgungsforschung |
| Prof. Dr. Jan Mainz | Aarhus University, Studienævnene på HE – Studies in Health Science |
| Dr. Wolfgang Rathmann | Deutsches Diabetes-Zentrum (DDZ), Leibniz-Zentrum für Diabetes-Forschung an der Heinrich-Heine-Universität Düsseldorf, Institut für Biometrie und Epidemiologie |
| Helmut Schröder | Wissenschaftliches Institut der AOK (WIdO) |
| Dr. Ingrid Schubert | PMV forschungsgruppe, Klinik und Poliklinik für Psychiatrie und Psychotherapie des Kindes- und Jugendalters, Universität zu Köln |
| Prof. Dr. Jochen Seufert | Deutsche Diabetes Gesellschaft Universitätsklinikum Freiburg, Abteilung Endokrinologie und Diabetologie |
| Dr. Dominik Graf von Stillfried | Zentralinstitut für die kassenärztliche Versorgung in Deutschland (Zi) |
| Dr. Heidrun Thaiss | Bundeszentrale für gesundheitliche Aufklärung (BZgA) |
| Dr. Til Uebel | Deutsche Gesellschaft für Allgemeinmedizin (DEGAM) Praxisgemeinschaft Uebel, Ittlingen |
| Dr. Dietmar Weber | Deutsche Diabetes Gesellschaft |
| Prof. Dr. Sarah Wild | Centre for Population Health Sciences, University of Edinburgh |
Annex Table 1. (in German) Participants Expert Committees b) Scientific Advisory Board
| Prof. Dr. Winfried Banzer | Johann Wolfgang Goethe-Universität Frankfurt am Main, Institut für Sportwissenschaften |
| Prof. Dr. Michael Böhme | Landesgesundheitsamt Baden-Württemberg, Stuttgart |
| Dr. Brigitte Borrmann | Landeszentrum Gesundheit Nordrhein-Westfalen (LZG.NRW) |
| Prof. Dr. Reinhard Busse | Technische Universität Berlin, Fachgebiet Management im Gesundheitswesen |
| Diana Droßel | diabetesDE – Deutsche Diabetes-Hilfe |
| Prof. Dr. Michael Freitag | Deutsche Gesellschaft für Allgemeinmedizin (DEGAM) Carl von Ossietzky Universität Oldenburg, Department für Versorgungsforschung |
| Dr. Bernd Hagen | Zentralinstitut für die kassenärztliche Versorgung in Deutschland (Zi) |
| Prof. Dr. Hans-Ulrich Häring | Universitätsklinikum Tübingen, Medizinische Klinik IV |
| Prof. Dr. Reinhard Holl | Universität Ulm, Institut für Epidemiologie und medizinische Biometrie |
| Prof. Dr. Andrea Icks | Heinrich-Heine-Universität Düsseldorf, Medizinische Fakultät, Institut für Versorgungsforschung und Gesundheitsökonomie |
| Dr. Matthias Kaltheuner | Gemeinschaftspraxis Kaltheuner – v. Boxberg, Leverkusen |
| Dr. Klaus Koch | Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG), Ressort Gesundheitsinformation |
| Dr. Joseph Kuhn | Bayerisches Landesamt für Gesundheit und Lebensmittelsicherheit, Dienststelle Oberschleißheim |
| Prof. Dr. Oliver Kuß | Deutsches Diabetes-Zentrum (DDZ), Leibniz-Zentrum für Diabetes-Forschung an der Heinrich-Heine-Universität Düsseldorf, Institut für Biometrie und Epidemiologie |
| Dr. Wolfgang Rathmann | Deutsches Diabetes-Zentrum (DDZ), Leibniz-Zentrum für Diabetes-Forschung an der Heinrich-Heine-Universität Düsseldorf, Institut für Biometrie und Epidemiologie |
| Helmut Schröder | Wissenschaftliches Institut der AOK (WIdO) |
| Dr. Ingrid Schubert | PMV forschungsgruppe, Klinik und Poliklinik für Psychiatrie und Psychotherapie des Kindes- und Jugendalters, Universität zu Köln |
| Prof. Dr. Jochen Seufert | Deutsche Diabetes Gesellschaft Universitätsklinikum Freiburg, Abteilung Endokrinologie und Diabetologie |
| Dr. Heidrun Thaiss | Bundeszentrale für gesundheitliche Aufklärung (BZgA) |
| Dr. Til Uebel | Deutsche Gesellschaft für Allgemeinmedizin (DEGAM) Praxisgemeinschaft Uebel, Ittlingen |
Annex Table 1. (in German) Participants Expert Committees c) Expert Panel on Type 2 Diabetes Care
| Prof. Dr. Michael Freitag | Deutsche Gesellschaft für Allgemeinmedizin (DEGAM) Carl von Ossietzky Universität Oldenburg, Department für Versorgungsforschung |
| Prof. Dr. Michael Böhme | Landesgesundheitsamt Baden-Württemberg, Stuttgart |
| Dr. Dirk Hochlenert | Deutsche Diabetes Gesellschaft |
| Dr. Dietmar Weber | Deutsche Diabetes Gesellschaft |
| Dr. Petra Kaufmann-Kolle | AQUA-Institut, Göttingen |
Annex Table 2. Template for consensus on the diabetes surveillance indicator set: Own diagram
|
Funding Statement
The project ‘Establishing national diabetes surveillance at the Robert Koch Institute’ receives funding from Germany’s Federal Ministy of Health, funding note: GE 2015 03 23.
Footnotes
Conflicts of interest
Joachim Szecsenyi is CEO and shareholder of the aQua Institute. For the other co-authors and for herself, the Corresponding author declares that there is no conflict of interest.
Disclaimer
Note: External contributions do not necessarily reflect the opinions of the Robert Koch Institute
References
- 1.Institute of Medicine (1988) The future of public health. National Academy Press. Washington, DC [Google Scholar]
- 2.Centers for Disease Control and Prevention (2012) CDC’s Vision for Public Health Surveillance in the 21st Century. MMWR (61(Suppl; July 27)):1-40 [PubMed] [Google Scholar]
- 3.World Health Organization (2015) Integrated surveillance of non-communicable diseases (iNCD). Copenhagen, Denmark [Google Scholar]
- 4.World Health Organization (2018) The 10 Essential Public Health Operations. http://www.euro.who.int/en/health-topics/Health-systems/public-health-services/policy/the-10-essential-public-health-operations (As at 16.02.2018)
- 5.World Health Organization (2013) Global action plan for the prevention and control of noncommunicable diseases 2013-2020. WHO, Geneva [Google Scholar]
- 6.Choi BC. (2012) The past, present, and future of public health surveillance. Scientifica (Cairo) 2012:875253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebrahim S. (2011) Surveillance and monitoring: a vital investment for the changing burdens of disease. Int J Epidemiol 40(5):1139-1143 [DOI] [PubMed] [Google Scholar]
- 8.Bommer C, Heesemann E, Sagalova V, et al. (2017) The global economic burden of diabetes in adults aged 20–79 years: a cost-of-illness study. Lancet Diabetes & Endocrinol 5(6):423-430 [DOI] [PubMed] [Google Scholar]
- 9.Heidemann C, Scheidt-Nave C. (2017) Prevalence, incidence and mortality of diabetes mellitus in adults in Germany - A review in the framework of the Diabetes Surveillance in Germany. Journal of Health Monitoring 2(3):98-121 https://edoc.rki.de/handle/176904/2819 (As at 13.09.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs E, Hoyer A, Brinks R, et al. (2017) Healthcare costs of Type 2 diabetes in Germany. Diabet Med 34(6):855-861 [DOI] [PubMed] [Google Scholar]
- 11.Statistisches Bundesamt (Destatis) (2017) Krankheitskosten. Fachserie 12 Reihe 721, 2015. [Google Scholar]
- 12.Ley SH, Ardisson Korat AV, Sun Q, et al. (2016) Contribution of the Nurses’ Health Studies to uncovering risk factors for type 2 diabetes: diet, lifestyle, biomarkers, and genetics. Am J Public Health 106(9):1624-1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balti EV, Echouffo-Tcheugui JB, Yako YY, et al. (2014) Air pollution and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 106(2):161-172 [DOI] [PubMed] [Google Scholar]
- 14.Hackett RA, Steptoe A. (2017) Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol 13(9):547-560 [DOI] [PubMed] [Google Scholar]
- 15.Barrès R, Zierath JR. (2016) The role of diet and exercise in the transgenerational epigenetic landscape of T2DM. Nat Rev Endocrinol 12(8):441-451 [DOI] [PubMed] [Google Scholar]
- 16.Bellamy L, Casas JP, Hingorani AD, et al. (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373(9677):1773-1779 [DOI] [PubMed] [Google Scholar]
- 17.Rayanagoudar G, Hashi AA, Zamora J, et al. (2016) Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia 59(7):1403-1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damm P, Houshmand-Oeregaard A, Kelstrup L, et al. (2016) Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia 59(7):1396-1399 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (2005) Preventing Chronic Diseases. A Vital Investment: WHO Global Report. WHO Press, Geneva [Google Scholar]
- 20.Bendas A, Rothe U, Kiess W, et al. (2015) Trends in incidence rates during 1999-2008 and prevalence in 2008 of childhood type 1 diabetes mellitus in Germany–model-based national estimates. PLoS One 10(7):e0132716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. (2017) Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. New Engl J Med 376(15):1419-1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidemann C, Du Y, Schubert I, et al. (2013) Prevalence and temporal trend of known diabetes mellitus: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 56(5-6):668-677 https://edoc.rki.de/handle/176904/1520 (As at 28.05.2018) [DOI] [PubMed] [Google Scholar]
- 23.Heidemann C, Du Y, Paprott R, et al. (2016) Temporal changes in the prevalence of diagnosed diabetes, undiagnosed diabetes and prediabetes: findings from the German Health Interview and Examination Surveys in 1997-1999 and 2008-2011. Diabet Med 33(10):1406-1414 [DOI] [PubMed] [Google Scholar]
- 24.Beyerlein A, Von Kries R, Hummel M, et al. (2010) Improvement in pregnancy related outcomes in the offspring of diabetic mothers in Bavaria, Germany, during 1987–2007. Diabet Med 27(12):1379-1384 [DOI] [PubMed] [Google Scholar]
- 25.Kulzer B, Albus C, Herpertz S, et al. (2013) Psychosoziales und Diabetes (Teil 1) S2-Leitlinie Psychosoziales und Diabetes - Langfassung. Diabetologie und Stoffwechsel 8(03):198-242 [Google Scholar]
- 26.Tsilidis KK, Kasimis JC, Lopez DS, et al. (2015) Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350:g7607. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs E, Hoyer A, Brinks R, et al. (2017) Burden of Mortality Attributable to Diagnosed Diabetes: A Nationwide Analysis Based on Claims Data From 65 Million People in Germany. Diabetes Care 40(12):1703-1709 [DOI] [PubMed] [Google Scholar]
- 28.Röckl S, Brinks R, Baumert J, et al. (2017) All-cause mortality in adults with and without type 2 diabetes: findings from the national health monitoring in Germany. BMJ Open Diabetes Res Care 5(1):e000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattke S, Epstein AM, Leatherman S. (2006) The OECD health care quality indicators project: history and background. Int J Qual Health Care 18(Suppl 1):1-4 [DOI] [PubMed] [Google Scholar]
- 30.OECD (2017) Health at a Glance 2017: OECD Indicators. OECD Publishing, Paris [Google Scholar]
- 31.Arah OA, Westert GP, Hurst J, et al. (2006) A conceptual framework for the OECD health care quality indicators project. Int J Qual Health Care 18(suppl 1):5-13 [DOI] [PubMed] [Google Scholar]
- 32.Verschuuren M, Gissler M, Kilpeläinen K, et al. (2013) Public health indicators for the EU: the joint action for ECHIM (European Community Health Indicators & Monitoring). Arch Public Health 71(1):12-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroll M, Phalkey RK, Kraas F. (2015) Challenges to the surveillance of non-communicable diseases--a review of selected approaches. BMC Public Health 15:1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arbeitsgemeinschaft der Obersten Landesgesundheitsbehörden (AOLG) (2003) Indikatorensatz für die Gesundheitsberichterstattung der Länder. 3. neu bearbeitete Fassung. Ministerium für Gesundheit, Soziales, Frauen und Familie des Landes Nordrhein-Westfalen, Bielefeld [Google Scholar]
- 35.Ministerium für Arbeit und Sozialordnung, Familie Frauen und Senioren Baden-Württemberg, (2015) Diabetes mellitus Typ 2 und Schwangerschaftsdiabetes - Maßnahmenplan zur Umsetzung des Gesundheitsziels “Diabetes mellitus Typ 2 Risiko senken und Folgen reduzieren” auf Landesebene Baden-Württemberg. Sozialministerium Baden-Württemberg, Stuttgart [Google Scholar]
- 36.Gabrys L, Heidemann C, Teti A, et al. (2017) Regionalisierung der Gesundheitsberichterstattung am Beispiel Diabetes-Surveillance. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 60(10):1147-1152 [DOI] [PubMed] [Google Scholar]
- 37.Gabrys L, Schmidt C, Heidemann C, et al. (2017) Diabetes-Surveillance in Germany - Background, concept and prospects. Journal of Health Monitoring 2(1):83-95 https://edoc.rki.de/handle/176904/2602 (As at 28.052018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenner G, Altenhofen L, Knoepnadel J, et al. (2003) Nationale Gesundheitsziele: Diabetes mellitus Typ 2 als Zielbereich. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 46(2):134-143 [Google Scholar]
- 39.Nationale Gesundheitsziele (2003) Diabetes mellitus Typ 2: Erkrankungsrisiko senken, Erkrankte früh erkennen und behandeln. www.gesundheitsziele.de (As at 15.06.2018)
- 40.World Health Organization (2015) Global reference list of 100 core health indicators. WHO, Geneva [Google Scholar]
- 41.Landesgesundheitsamt Baden-Württemberg (2013) Bericht der Expertenarbeitsgruppe Indikatoren zum Diabetes mellitus Typ 2. LGA Baden-Würrtemberg, Stuttgart [Google Scholar]
- 42.American Diabetes Association (2012) Executive summary: Standards of medical care in diabetes--2012. Diabetes Care 35 (Suppl 1):S4-S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) (2013) Nationale VersorgungsLeitlinie Therapie des Typ-2-Diabetes – Langfassung, 1. Auflage. Version 4, zuletzt geändert: November 2014. https://www.leitlinien.de/nvl/diabetes/therapie (As at 15.05.2018)
- 44.Piepoli MF, Hoes AW, Agewall S, et al. (2016) 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 37(29):2315-2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagen B, Groos S, Kretschmann J, et al. (2015) Qualitätssicherungsbericht 2014. Disease-Management-Programme in Nordrhein. Nordrheinische Gemeinsame Einrichtung Disease-Management-Programme GbR, Düsseldorf [Google Scholar]
- 46.Schmidt C, Bätzing-Feigenbaum J, Bestmann A, et al. (2017) Integration von Sekundärdaten in die Nationale Diabetes-Surveillance: Hintergrund, Ziele und Ergebnisse des Sekundärdaten-Workshops am Robert Koch-Institut. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 60(6):656-661 [DOI] [PubMed] [Google Scholar]
- 47.Teti A, Gabrys L, Ziese T, et al. (2017) Proceedings of the International Workshop ‘Development of a National Diabetes Surveillance System in Germany - Core Indicators and Conceptual Framework’. BMC Proceedings 11(3):3 [Google Scholar]
- 48.Garcia-Armesto S, Vicente-Edo M, Estupinan-Romero F, et al. (2016) European consensus for the assessment of good practices on Diabetes. CHRODIS. http://chrodis.eu/outcomes-results (As at 17.05.2018)
- 49.Fitch K, Bernstein SJ, Aguilar MD, et al. (2001) The RAND/UCLA appropriateness method user’s manual. RAND Corp. Santa Monica CA (USA) [Google Scholar]
- 50.AQUA (2015) Allgemeine Methoden im Rahmen der sektorenübergreifenden Qualitätssicherung im Gesundheitswesen nach § 137a SGB V Version 4.0 (Stand: 17. Februar 2015). AQUA - Institut für Qualitätsförderung und Forschung im Gesundheitswesen GmbH, Göttingen [Google Scholar]
- 51.Verschuuren M, Achterberg P, Gijsen R, et al. (2012) ECHI Indicator Develpment and Documentation - Joint Action for ECHIM Final Report Part II. Bilthoven, Netherlands [Google Scholar]
- 52.Paprott R, Mühlenbruch K, Mensink GB, et al. (2016) Validation of the German Diabetes Risk Score among the general adult population: findings from the German Health Interview and Examination Surveys. BMJ Open Diabetes Res Care 4(1):e000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du Y, Heidemann C, Gößwald A, et al. (2013) Prevalence and comorbidity of diabetes mellitus among non-institutionalized older adults in Germany-results of the national telephone health interview survey ‘German Health Update (GEDA)’ 2009. BMC Public Health 13(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du Y, Heidemann C, Schaffrath Rosario A, et al. (2015) Changes in diabetes care indicators: findings from German National Health Interview and Examination Surveys 1997-1999 and 2008-2011. BMJ Open Diabetes Research & Care 3(1):e000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamayo T, Brinks R, Hoyer A, et al. (2016) The Prevalence and Incidence of Diabetes in Germany. Dtsch Arztebl Int 113(11):177-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goffrier B, Schulz M, Bätzing-Feigenbaum J. (2017) Administrative Prävalenzen und Inzidenzen des Diabetes mellitus von 2009 bis 2015. Versorgungsatlas. Zentralinstitut der kassenärztlichen Vereinigung (Zi), Berlin [Google Scholar]
- 57.Gross S, Kretschmann J, Macare C, et al. (2016) Qualitätsbericht 2015. Disease-Management-Programme in Nordrhein. Nordrheinische Gemeinsame Einrichtung Disease-Management-Programme GbR, Düsseldorf [Google Scholar]
- 58.Kröger K, Berg C, Santosa F, et al. (2017) Lower limb amputation in Germany - an analysis of data from the German Federal Statistical Office between 2005 and 2014. Dtsch Arztebl Int (114):130-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pollmanns J, Romano PS, Weyermann M, et al. (2017) Impact of Disease Prevalence Adjustment on Hospitalization Rates for Chronic Ambulatory Care–Sensitive Conditions in Germany. Health Serv Res 53(2):1180-1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bundesamt für Gesundheit in der Schweiz (2017) Indikatoren-Set für das Monitoring-System NCD. Ergänzendes Dokument zur Nationalen Strategie Prävention nichtübertragbarer Krankheiten - 2017-2024. Bundesamt für Gesundheit, Bern [Google Scholar]


