Case progress
On further history, it was discovered that the family had been using alkaline water as an alternate source of oral fluids given its reported health benefits.
Alkaline water is advertised as negatively charged water with a pH of 8–10. These properties are marketed to provide significant health benefits including increased energy levels, reduced effects of ageing and stress and reduced incidence of illness and disease. Alkaline water contains calcium and magnesium carbonate, both exogenous sources of absorbable alkaline.
The consumption of alkaline water in this case resulted in a milk-alkali syndrome with hypercalcaemia, metabolic alkalosis and an acute on chronic kidney injury. It was not detected for several months, and as a result, had a significant negative impact on this patient and his family. He required frequent venepuncture, increased clinical reviews (further complicated by the current COVID-19 pandemic) and regular titration of medications. Fortunately, on cessation of the alkaline water, his creatinine returned to baseline and calcium and acid–base normalised.
Answers
-
What clinical syndrome does this represent?
The triad of hypercalcaemia, metabolic alkalosis and acute kidney injury is consistent with milk-alkali, or more appropriately termed calcium-alkali syndrome.
-
What is the aetiology of this syndrome?
This syndrome occurs in the setting of excessive exogenous calcium and absorbable alkaline. In this case it was alkaline water in conjunction with calcium carbonate as a phosphate binder.
-
How would you manage this patient?
The first management strategy is cessation of the offending agent, in this case alkaline water, and supportive therapy with adequate hydration (often intravenous). Kidney replacement therapy (KRT) may be required to treat refractory hypercalcaemia, and intravenous bisphosphonates have been utilised; however, they convey a risk of significant hypocalcaemia.
Discussion
This patient presented with the clinical triad of hypercalcaemia, acute kidney injury and metabolic alkalosis in the context of ingestion of calcium carbonate as a phosphate binder and absorbable alkali in the form of alkaline water. This is consistent with milk-alkali or more recently described as calcium-alkali syndrome [1, 2].
Milk-alkali syndrome was first defined in the early twentieth century as a complication of peptic ulcer therapy [3–6]. Sippy proposed a treatment for peptic ulceration that aimed to neutralise and therefore decrease the effect of gastric acid [7]. He prescribed a solution of milk and cream (high in calcined magnesium and sodium bicarbonate) up to hourly with significant improvement in symptoms [7]. In the years that followed up to 18% of individuals treated with this regimen developed metabolic alkalosis with hypercalcaemia and acute kidney injury [2]. These patients presented with a myriad of symptoms including headache, irritability, confusion, psychosis, vertigo, nausea, vomiting and anorexia in addition to the biochemical changes noted above [3–6]. Symptoms often resolved on cessation of the treatment; however, kidney impairment persisted, and in 1 case series, 5 out of 6 patients developed new onset or worsening CKD [5].
The incidence of milk-alkali syndrome declined with the introduction of H2-receptor blockers to treat peptic ulcer disease, and by the 1970s, it accounted for less than 1% of hypercalcaemia in hospitalised patients [2, 8, 9]. A modern version of milk-alkali syndrome, calcium-alkali syndrome, developed in the late twentieth century with the use of calcium carbonate to prevent osteoporosis associated with corticosteroid therapy or menopause and as a phosphate binder in those with CKD [2, 8, 10, 11]. Calcium-alkali syndrome is now considered the third most common cause of hypercalcaemia in patients admitted to hospital [2, 8, 10, 11].
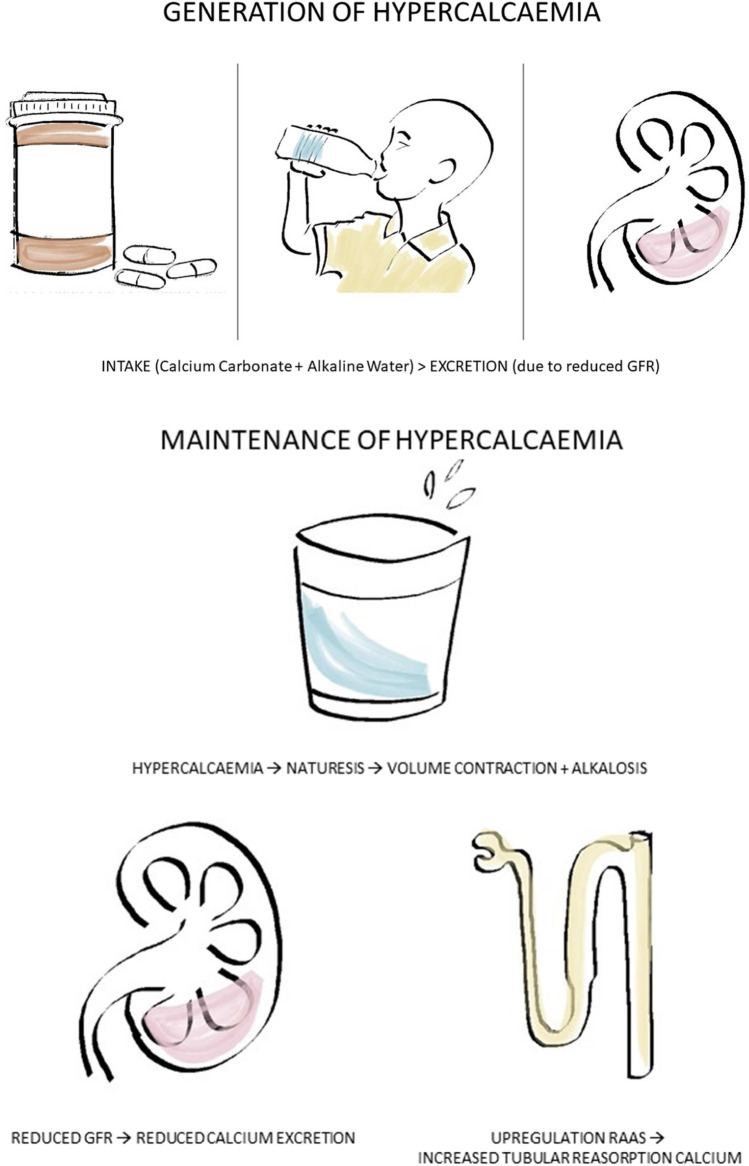
The pathogenesis of calcium-alkali syndrome is complex and not yet fully understood. The syndrome likely consists of two stages: the generation, and the maintenance of hypercalcaemia [9]. Hypercalcaemia is generated when intake exceeds excretion. In this case, the ingestion of calcium carbonate and alkaline water in the setting of a reduced glomerular filtration rate (GFR) secondary to established CKD. The “maintenance” phase is then mediated via multiple factors. Hypercalcaemia leads to natriuresis, diuresis and subsequent volume contraction. The net effect is a further reduction in GFR and thus a reduced kidney excretion of calcium [9]. Simultaneously, volume depletion and alkalosis lead to an increased tubular reabsorption of calcium from upregulation of the renin-aldosterone system and distal tubular acidification [1, 9] (Fig. 1). Phosphate also plays a role in the maintenance of hypercalcaemia. In calcium-alkali syndrome, calcium carbonate acts as a phosphate binder, and therefore, these individuals are often hypo- or normophosphataemic. Hypophosphataemia stimulates 1,25(OH) vitamin D3 production with the downstream effect of increased intestinal absorption of calcium and calcium release from bone [9].
Fig. 1.
Patholophysiology of calcium-alkali syndrome
These biochemical changes were all evident in this patient. His initial bloods demonstrated a mild acute on chronic kidney injury that worsened over the subsequent months. This was likely to be secondary to progressive volume contraction leading to a further reduction in his GFR and reduced kidney excretion of calcium. He was also hypophosphataemic which, as explained above, would have contributed to the hypercalcaemia perpetuating the kidney injury.
The management of calcium-alkali syndrome is supportive [1, 2, 8]. It involves withdrawal of the offending agent. In this case, it was the identification and cessation of the alkaline water that led to improvement in biochemical derangement. Following this, it is important to re-establish intravascular volume with intravenous saline and monitor for potential hypocalcaemia. In severe cases, KRT may be required and intravenous bisphosphonates have been utilised for resistant hypercalcaemia [2, 9]. Complications of severe, persistent, calcium-alkali syndrome relate primarily to hypercalcaemia and include cardiac arrythmias, nephrocalcinosis and ocular calcifications (band keratopathy) [9]. These were not observed in our case but should be considered in patients who present with this syndrome and have persistently deranged kidney function or where it is suspected to be chronic.
Following a more detailed history, it was determined that this family was utilising alkaline water as a form of complementary medicine. It is unclear if alkaline water was sought by the family due to the reported health benefits or as an attempt to treat the acidosis associated with CKD. Either way, the use of complementary and alternative medicine has been increasing significantly with up to half the Australian population purchasing these therapies [12, 13]. The commonly reported reasons for seeking complementary and alternative medicine include fear of or dissatisfaction with conventional medicine, a more personalised approach to health care and desire for improved health outcomes [12, 13].
Alkaline water is described as water with a pH of greater than 8 with a variable ingredient list including sodium, bicarbonate, calcium, magnesium, potassium and minerals such as selenium and silica (see Table 1). Alkaline water has been available for decades; however, focused marketing has increased its recent household use. The alkaline water industry is now worth billions of dollars with over 600 million litres sold annually around the world [14]. Reported benefits include improved water absorption, regulation of body pH, improved energy levels, improved gastroesophageal reflux symptoms, reduction in chronic illness and minimizing risk of osteoporosis, a claim that has been denied by the US Food and Drug Administration [15].
Table 1.
Typical analysis of drinking water in Australia, Hydralyte™, a common oral rehydration solution and alkaline water
| Drinking water | Hydralyte™ | Alkaline water | |
|---|---|---|---|
| pH | 7.5–7.9 | 7–8 | 8–10 |
| Sodium | 14 mg/L | 1060 mg/L | 98 mg/L |
| Alkalinity | 35–50 mg/L CaCO3 | 480 mg/100 mL citric acid monohydrate | 322 mg/L HCO3 |
| Calcium | 10–20 mg/L | 0 mg/L | 72 mg/L |
| Magnesium | 3–6 mg/L | 0 mg/L | 23 mg/L |
| Potassium | 2–3 mg/L | 860 mg/L | 3 mg/L |
In this case, when ingested in combination with calcium carbonate as a phosphate binder and in a setting of reduced GFR, alkaline water led to the development of calcium-alkali syndrome. The result was a period of instability for this patient with increased phlebotomy, hospital visits and intervention. Once identified as the cause for concern, the alkaline water was ceased and biochemical changes resolved. The family was not discouraged to pursue further complementary or alternative medicine but were counselled on the importance of disclosing these therapies to the treating team in future.
This case highlights that milk-alkali or now more adequately described as calcium-alkali syndrome is still an important diagnostic consideration for hypercalcaemia. It also highlights the importance of an adequate history, particularly the consideration of complementary and alternative medicine and how these may interact with more conventional medicine.
Declarations
Ethics approval
The participant’s legal guardian has consented to the submission of the case report to the journal.
Competing interests
The authors declare no competing interests.
Footnotes
This refers to the article that can be found at http://dx.doi.org/10.1007/s00467-022-05454-z.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Picolos MK, Orlander PR. Calcium carbonate toxicity: the updated milk-alkali syndrome; report of 3 cases and review of the literature. Endocr Pract. 2005;11:272–280. doi: 10.4158/EP.11.4.272. [DOI] [PubMed] [Google Scholar]
- 2.Medarov BI. Milk-alkali syndrome. Mayo Clin Proc. 2009;84:261–267. doi: 10.4065/84.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardt LLRA. Toxic manifestations following the alkaline treatment of peptic ulcer. Arch Intern Med. 1923;2:171–180. doi: 10.1001/archinte.1923.00110140023003. [DOI] [Google Scholar]
- 4.Cope CL (1936) Base changes in the alkalosis produced by the treatment of gastric ulcer with alkalies. Clin Sci 287–300
- 5.Burnett CH, Commons RR, Albright F, Howard JE. Hypercalcemia without hypercalcuria or hypophosphatemia, calcinosis and renal insufficiency; a syndrome following prolonged intake of milk and alkali. N Engl J Med. 1949;240:787–794. doi: 10.1056/NEJM194905192402001. [DOI] [PubMed] [Google Scholar]
- 6.Wenger J, Kirsner JB, Palmer WL. The milk-alkali syndrome; hypercalcemia, alkalosis and azotemia following calcium carbonate and milk therapy of peptic ulcer. Gastroenterology. 1957;33:745–769. doi: 10.1016/S0016-5085(19)35627-6. [DOI] [PubMed] [Google Scholar]
- 7.Sippy BW. Landmark article May 15, 1915: Gastric and duodenal ulcer. Medical cure by an efficient removal of gastric juice corrosion. By Bertram W Sippy JAMA. 1983;250:2192–2197. doi: 10.1001/jama.250.16.2192. [DOI] [PubMed] [Google Scholar]
- 8.Waked A, Geara A, El-Imad B. Hypercalcemia, metabolic alkalosis and renal failure secondary to calcium bicarbonate intake for osteoporosis prevention–‘modern’ milk alkali syndrome: a case report. Cases J. 2009;2:6188. doi: 10.4076/1757-1626-2-6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felsenfeld AJ, Levine BS. Milk alkali syndrome and the dynamics of calcium homeostasis. Clin J Am Soc Nephrol. 2006;1:641–654. doi: 10.2215/CJN.01451005. [DOI] [PubMed] [Google Scholar]
- 10.Beall DP, Henslee HB, Webb HR, Scofield RH. Milk-alkali syndrome: a historical review and description of the modern version of the syndrome. Am J Med Sci. 2006;331:233–242. doi: 10.1097/00000441-200605000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Kapsner P, Langsdorf L, Marcus R, Kraemer FB, Hoffman AR. Milk-alkali syndrome in patients treated with calcium carbonate after cardiac transplantation. Arch Intern Med. 1986;146:1965–1968. doi: 10.1001/archinte.1986.00360220119021. [DOI] [PubMed] [Google Scholar]
- 12.Kemper KJ, Vohra S, Walls R. American Academy of Pediatrics. The use of complementary and alternative medicine in pediatrics. Pediatrics. 2008;122:1374–1386. doi: 10.1542/peds.2008-2173. [DOI] [PubMed] [Google Scholar]
- 13.Bensoussan A, Lewith GT. Complementary medicine research in Australia: a strategy for the future. Med J Aust. 2004;181:331–333. doi: 10.5694/j.1326-5377.2004.tb06303.x. [DOI] [PubMed] [Google Scholar]
- 14.Michail N. Alkaline water market potential. Fi Global Insights. 2020;31:1–5. [Google Scholar]
- 15.U.S. Food and Drug Administration (2007) Health Claims: Letter of Denial - Alkaline and Earth Alkaline Citrates Minimizing the Risk of Osteoporosis. https://www.fda.gov/food/food-labeling-nutrition/health-claims-letter-denial-alkaline-and-earth-alkaline-citrates-minimizing-risk-osteoporosis. Accessed 8 Dec 2021