Abstract
Purpose
While the association between diet quality and mortality has been previously demonstrated, the association between frailty and diet quality has not been evaluated well. This study aimed to investigate the association between diet quality and prevalence of both physical and comprehensive frailty, using two validated tools, in a community-based cohort of older adults.
Methods
We conducted cross-sectional analyses using baseline data of 7022 participants aged ≥ 65 years in the Kyoto-Kameoka study. Diet quality was assessed by calculating the adherence scores to the Japanese Food Guide Spinning Top using a validated questionnaire; the participants were stratified into quartile groups based on these scores. Physical and comprehensive frailty was assessed using the Fried phenotype model-based Frailty Screening Index and the Kihon Checklist, respectively. Multivariable logistic regression and the restricted cubic spline model were used to calculate odds ratios (ORs) and their 95% confidence intervals (CIs) for associations between adherence scores and frailty prevalence.
Results
Higher adherence scores signified a higher intake of vitamin C, vegetables, dairy products, and fruits. Physical and comprehensive frailty prevalence was 14.2 and 35.8%, respectively. In a multivariable adjusted model, compared with the bottom adherence score quartile, the top quartile was associated with lower ORs of physical (OR 0.64; 95% CI 0.52–0.80) and comprehensive frailty (OR 0.60; 95% CI 0.51–0.71). These relationships were similar to results in the spline model.
Conclusions
This study shows an inverse dose–response relationship between diet quality and prevalence of both physical and comprehensive frailty in older adults.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-022-02819-w.
Keywords: Diet quality, Physical frailty, Comprehensive frailty, Japanese Food Guide Spinning Top, Cross-sectional study
Introduction
Frailty is a condition in which multiple physiological systems decline in function due to loss of homeostasis against the stress response [1, 2] and/or increased risk of adverse health outcomes [3] and is characterized by a multidimensional factor such as physical, psychosocial, and cognitive ability playing a part in its development [1–3]. It is an important global public health problem among older adults [3], and it has been reported that frailty is associated with adverse outcomes such as mortality [4, 5], fractures [5, 6], and falls [5]. Therefore, frailty must be prevented to ensure a healthy life expectancy without disease [7] and reduce the burden of healthcare-related costs [8]. Lifestyle care should shift towards more appropriate strategies that prevent frailty [1].
Diet quality is important for promoting health and lowering the risk of major diet-related diseases such as cancer [9–11]. Diet quality is measured using various adherence scores based on previous studies or national dietary guidelines [10, 11]. The use of the Mediterranean diet intake [12–14], the Healthy Eating Index (HEI) [14, 15], or Dietary Approaches to Stop Hypertension (DASH) [14] may decrease the risk or odds ratios (ORs) of frailty. In Japan, the Japanese Food Guide Spinning Top was developed by the Ministry of Health, Labour, and Welfare and the Ministry of Agriculture, Forestry and Fisheries to promote healthy dietary habits [16]. While adherence to this guideline is inversely associated with mortality in middle-aged and older adults [17], it is unclear whether this diet quality is associated with frailty prevalence in older people.
Previous studies have reported an association between diet quality and defined frailty, using specific assessment tools [12–15]. Although the term “frailty” appears straightforward, its evaluation has varied among studies because many such assessment tools exist [18]. This can result in too much heterogeneity in predictive ability and classification. Therefore, measuring the association between diet quality and frailty prevalence needs to be done using a well-validated method. Notably, a previous study demonstrated that disease outcomes in patients admitted to the hospital with COVID-19 [19] were better predicted by frailty than by either age or comorbidity. This underlines the importance of assessing the impact of diet quality on frailty prevalence. Thus, we aimed to investigate the association between diet quality and prevalence of physical and comprehensive frailty using two validated assessment tools.
Subjects and methods
Study population and baseline characteristic assessment
The Kyoto-Kameoka study is an ongoing population-based cohort study of community-dwelling residents aged ≥ 65 years in Kameoka City, Japan. Residents were invited to participate in a baseline survey that included the Fried phenotype (FP) model-based Frailty Screening Index (FSI) and the Kihon Checklist (KCL) for the assessment of frailty status. The baseline survey took place on July 29, 2011, and details have been published elsewhere [20–27]. Our group conducted an additional survey, including the administration of the Food Frequency Questionnaire (FFQ), on February 14, 2012. Results of both surveys were collected via postal mail, and informed consent was obtained from participants along with their responses to these questionnaires. Information on medical history, socioeconomic status, health-related lifestyle behaviours, including smoking and drinking status, dietary habits, and physical activity, were obtained on each survey. The study was approved by the Ethics Committees of Kyoto Prefectural University of Medicine (RBMR-E-363), Kyoto University of Advanced Science (No. 20–1), and the National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN-76–2). This study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)-Nutritional Epidemiology criteria [28].
Out of the total number of participants at baseline (n = 13,294), 8319 responded to our additional survey. Those with an incomplete KCL or FSI (n = 1089), who had self-reported “needed long-term care” (n = 122), and who had extremely high or low energy intakes (standard deviation [SD] of 3 from the mean energy intake) [23] in each sex group (n = 86), were excluded. Ultimately, 7022 participants were included in the present analysis.
Dietary assessment
Dietary nutrient and food intakes were estimated using the FFQ [24, 25, 29–31]. We have previously validated the FFQ using dietary records [24, 29, 31] and doubly labelled water (DLW) methods [25, 32] in a subpopulation of participants of the Kyoto-Kameoka study. On the FFQ, participants were asked to report their intake frequency of 47 food and beverage items over the past year. Food and nutrient intakes were then calculated using the frequency of appearance of each food and beverage category and the portion size for each sex group [30] or using a program based on the Standard Tables of Food Composition in Japan [33]. Energy intake was calibrated using our previously developed equation that uses total energy expenditure (as measured by the DLW method) [25]. The equation used for this calculation attenuates the impact of age, sex, and body mass index (BMI)-related self-reporting bias on estimated energy intake in older adults, assessed via the FFQ [32].
Calculation of diet quality score
To assess diet quality, we calculated participants’ adherence scores from the Japanese Food Guide Spinning Top (the food-based Japanese dietary guidelines) [16]. The details of this measure have been published elsewhere [16, 17, 27, 34]. Recommended serving amounts for each category and recommended total energy intake are specified according to sex, age, and level of physical activity (i.e., participation in manual labour or walking for at least 1 h a day were labelled as moderately physically active; other activity levels were labelled as sedentary) [16, 17, 27, 34]. We calculated the number of servings according to the food guide criteria. One serving of each was defined as follows: a grain dish (40 g of carbohydrates), vegetable dish (weight, 70 g), fish or meat dish (6 g of protein), milk (100 mg calcium), and fruit (weight of 100 g) [16, 17, 27, 34]. We determined food guide adherence scores using a method described by Kurotani et al. [17, 27]. To calculate the scores for each component, food items were categorised as follows: grain dishes (rice, bread, and noodles); vegetable dishes (potatoes, pumpkin/squash, carrots, broccoli, green-leaved vegetables, other green/yellow vegetables, cabbage, daikon (Japanese radish), kiriboshi-daikon (dry strips of Japanese radish), burdock, bamboo shoots, other vegetables, mushrooms, seaweed, peanuts, and almonds); fish and meat dishes (tofu (soybean curd), natto (fermented soybeans), soybeans, eggs, chicken, beef, pork, liver, ham, sausage, bacon, salami sausage, fish, bone-edible small fish, canned tuna, cuttlefish, squid, octopus, shrimp, crab, shellfish, fish eggs, fish paste products, ganmodoki (fried tofu paste), and nama-age (fried tofu); [soybeans were included in the fish and meat dish category, based on their high-protein nutrient profile]); milk products (milk and yogurt); fruits (citrus and other fruits); and snacks and alcoholic beverages (western-style confectioneries, Japanese-style confectioneries, and alcohol). In addition to the Japanese Food Guide Spinning Top criteria, we calculated adherence scores based on the intake ratio of white to red meat as a component of the score [17, 27]. White meat included chicken, fish, bone-edible small fish, canned tuna, cuttlefish, squid, octopus, shrimp, crab, shellfish, fish eggs, and fish paste products. Red meat included beef, pork, liver, ham, sausage, bacon, and salami sausage. Each component was given a score between 0 and 10, and to obtain a total score that ranged from 0 (lowest adherence) to 80 (highest adherence), all the component scores were summed.
Definition of frailty
Frailty was assessed using the FP model-based five-item FSI [7, 21–23] and the 25-item KCL [21–23, 35, 36], both of which have been validated according to the risk of Long-Term Care Insurance Certification [7, 35]. Physical frailty was evaluated using the FSI, focusing on the physical aspects, and was defined as a score of 3 or more points out of a maximum of 5 (weight loss, slow gait speed, cognition, exhaustion, and low physical activity) [7, 21–23]. Comprehensive frailty was evaluated using the KCL. It includes cognitive and social aspects, as well as physical factors, and was defined as a score of 7 or higher out of a possible 25 points [21–23, 35, 36]. Additionally, we investigated possible associations between the FSI and KCL subdomains and the adherence score. The KCL consists of seven subdomains: instrumental activity of daily living (IADL) disability, physical, nutrition, oral, social, cognitive, and depression. Detailed methods of the subdomain investigation are provided elsewhere [26].
Statistical analysis
Adherence scores to the Japanese Food Guide Spinning Top were divided into quartiles (Qs). Continuous and categorical variables are shown as means with SDs and numbers with percentages, respectively. Where information pertaining to the BMI (n = 35), alcohol status (n = 102), smoking status (n = 385), physical activity (n = 359), family structure (n = 468), socioeconomic status (n = 235), education attainment (n = 645), denture use (n = 82), or medications (n = 387) was missing, we performed multiple imputation on five data sets, using chained equations from R statistical software [37] to prevent a systematic error appearing due to selection bias. Missing data were assumed to be missing at random. Nutrient intake was adjusted for energy intake per 1000 kcal via the nutrient density method, using uncalibrated energy intake [38]. These values are shown as the median of each quartile group. The association between adherence score and nutrient intake was confirmed using Spearman’s correlation analysis.
The number of frailty cases is shown as a case (%). Multivariate logistic regression analysis was performed, considering several baseline covariate variables. Multivariate adjusted analyses were verified by two models: Model 1 was adjusted for age (continuous), sex (female or male), and population density (≥ 1000 or < 1000 people/km2). In model 2, in addition to the factors adjusted for in model 1, we adjusted for body mass index (continuous), physical activity (yes or no), denture use (yes or no), smoking status (never smoker, past smoker, or current smoker), alcohol intake status (every day, sometimes, seldom, or never), educational attainment (< 9, 10–12, or ≥ 13 years), medication use (continuous), living alone (yes or no), socioeconomic status (high or low), green tea consumption (frequency), coffee consumption (frequency), and history of the disease (hypertension, diabetes, dyslipidaemia, heart disease, and stroke; yes or no). These variables were selected according to covariates used in a previous study [21–23, 26]. ORs for frailty are presented as OR (95% confidence interval [CI]), using the first quartile group (lowest diet quality) as the referent group. Diet quality is better among women [17] and affluent people [39]. Thus there is a possibility of evaluating primarily sex or socioeconomic status-specific influences when ranking individuals according to adherence scores. Therefore, subgroup analyses were performed separately by sex and socioeconomic status (high or low). Additionally, we evaluated possible associations between the adherence score and the subdomains that were evaluated using the FSI and KCL.
To evaluate the curve association of diet quality and frailty prevalence, we used a restricted cubic spline model considering three data points (5th, 50th, and 95th percentiles), based on the distribution of the recommended adherence score. Because the data were sparse, we truncated the analysis at 29 points (1% of the distribution) [22]. We calculated the ORs for frailty prevalence associated with adherence score, using the 43 points of first quartile value as the reference in the restricted cubic spline model [21]. Moreover, we considered the association between frailty prevalence and the adherence score of each component of the Japanese Food Guide Spinning Top. A two-sided p value < 0.05 was considered significant. Linear trends were computed by treating adherence score exposure as a continuous variable. Statistical analyses were performed using STATA MP, version 15.0 (Stata Corp LP, College Station, TX), and/or R software 3.4.3 (R Core Team, Vienna, Austria).
Results
The mean score on the adherence to the Japanese Food Guide Spinning Top was 54.3 (SD 8.4). Compared to participants with lower adherence scores, participants with higher scores were likelier to be women, live alone, have higher educational attainment and socioeconomic status, and have a history of hyperlipidaemia. They were less likely to be current smokers and alcohol drinkers (Table 1).
Table 1.
Baseline participant characteristics according to quartile of adherence to the Japanese Food Guide Spinning Top
| Total (n = 7022) | Quartile of the Japanese Food Guide Spinning Top score | p value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 1756) | Q2 (n = 1756) | Q3 (n = 1755) | Q4 (n = 1755) | ||||||||
| Age [years]a | 73.3 | (6.1) | 73.5 | (6.2) | 73.2 | (6.2) | 73.0 | (6.0) | 73.4 | (6.0) | 0.039 |
| Women [n (%)]b | 3709 | (52.8) | 551 | (31.4) | 874 | (49.8) | 1044 | (59.5) | 1240 | (70.7) | < 0.001 |
| PD ≥ 1000 people/km2 [n (%)]b | 3232 | (46.0) | 738 | (42.0) | 781 | (44.5) | 814 | (46.4) | 899 | (51.2) | < 0.001 |
| BMI [kg/m2]a | 22.6 | (3.0) | 22.7 | (3.1) | 22.7 | (3.1) | 22.7 | (3.0) | 22.3 | (2.9) | < 0.001 |
| Alcohol drinker [n (%)]b | 4595 | (65.4) | 1309 | (74.5) | 1174 | (66.9) | 1077 | (61.4) | 1035 | (59.0) | < 0.001 |
| Current smoker [n (%)]b | 754 | (10.7) | 335 | (19.1) | 212 | (12.1) | 115 | (6.6) | 92 | (5.3) | < 0.001 |
| MVPA [n (%)]b | 3114 | (44.4) | 746 | (42.5) | 802 | (45.7) | 788 | (44.9) | 778 | (44.3) | 0.268 |
| Living alone [n (%)]b | 789 | (11.2) | 171 | (9.7) | 167 | (9.5) | 214 | (12.2) | 237 | (13.5) | < 0.001 |
| HSES [n (%)]b | 2394 | (34.1) | 507 | (28.9) | 546 | (31.1) | 623 | (35.5) | 718 | (40.9) | < 0.001 |
| Education ≥ 13 y [n (%)]b | 1576 | (22.4) | 359 | (20.4) | 386 | (22.0) | 414 | (23.6) | 417 | (23.8) | < 0.001 |
| Denture use [n (%)]b | 4212 | (60.0) | 1099 | (62.6) | 1074 | (61.2) | 1015 | (57.8) | 1024 | (58.4) | 0.010 |
| No medication [n (%)]b | 1575 | (22.4) | 405 | (23.1) | 431 | (24.5) | 367 | (20.9) | 372 | (21.2) | < 0.001 |
| Hypertension [n (%)]b | 2731 | (38.9) | 656 | (37.4) | 677 | (38.6) | 709 | (40.4) | 689 | (39.3) | 0.308 |
| Stroke [n (%)]b | 251 | (3.6) | 71 | (4.0) | 78 | (4.4) | 54 | (3.1) | 48 | (2.7) | 0.020 |
| Heart disease [n (%)]b | 869 | (12.4) | 247 | (14.1) | 228 | (13.0) | 175 | (10.0) | 219 | (12.5) | 0.002 |
| Diabetes [n (%)]b | 736 | (10.5) | 193 | (11.0) | 190 | (10.8) | 180 | (10.3) | 173 | (9.9) | 0.677 |
| Hyperlipidemia [n (%)]b | 711 | (10.1) | 112 | (6.4) | 150 | (8.5) | 193 | (11.0) | 256 | (14.6) | < 0.001 |
Bold p values are statistically significant (p < 0.05). Data of participants with missing values underwent multiple imputation: body mass index (n = 35); alcohol status (n = 102); smoking status (n = 385); physical activity (n = 359); family structure (n = 468); socioeconomic status (n = 235); education attainment (n = 645); denture use (n = 82); medications (n = 387). BMI, body mass index; HSES, high socioeconomic status; MVPA, moderate to vigorous physical activity; PD, population density. Q1 through Q4 includes Japanese Food Guide Spinning Top scores of < 49.5, 49.5–54.8, 54.9–60.1, and ≥ 60.2
aContinuous values are shown as mean (standard deviation) and are analyzed using variance analysis
bCategorical values are shown as number (percentage) and are analyzed using the Chi-square test. MVPA means reported MVPA exercise habits from a questionnaire
The associations between adherence scores and nutrient and food intake are shown in Table 2. Adherence scores were moderately correlated to the intake of the following: vitamin C (r = 0.46), vegetables (r = 0.42), fruits (r = 0.56), and dairy products (r = 0.46). Participants with higher adherence scores tended to have a higher intake of fat, folate, and calcium (≥ r = 0.30). Therefore, participants with higher adherence scores have characteristics of high vitamin C, vegetables, fruits, and dairy products intake. However, energy, protein, and carbohydrate intake were not observed to be significantly different.
Table 2.
Association between energy and nutrient intake and adherence to the Japanese Food Guide Spinning Top
| Quartile of the Japanese Food Guide spinning top score | ra | ||||
|---|---|---|---|---|---|
| Q1 (n = 1756) | Q2 (n = 1756) | Q3 (n = 1755) | Q4 (n = 1755) | ||
| Nutrients | |||||
| Calibrated EI [kcal/day]b | 2294 | 2151 | 2047 | 1995 | − 0.23 |
| Uncalibrated EI [kcal/day] | 1832 | 1722 | 1702 | 1697 | − 0.05 |
| Protein [% energy/day] | 10.7 | 12.0 | 12.4 | 12.7 | 0.28 |
| Fat [% energy/day] | 19.1 | 23.3 | 25.9 | 28.7 | 0.35 |
| Carbohydrate [% energy/day] | 55.9 | 56.0 | 55.7 | 55.6 | − 0.01 |
| SFA [g/1000 kcal/day] | 5.4 | 6.4 | 6.9 | 7.4 | 0.26 |
| MUFA [g/1000 kcal/day] | 8.2 | 9.6 | 10.7 | 12.0 | 0.29 |
| PUFA [g/1000 kcal/day] | 7.0 | 8.3 | 8.9 | 9.8 | 0.27 |
| n-6 PUFA [g/1000 kcal/day] | 6.0 | 7.3 | 7.8 | 8.7 | 0.28 |
| n-3 PUFA [g/1000 kcal/day] | 1.0 | 1.2 | 1.3 | 1.5 | 0.29 |
| Cholesterol [mg/1000 kcal/day] | 108 | 119 | 122 | 125 | 0.12 |
| Vitamin A [µg RE/1000 kcal/day]c | 393 | 477 | 520 | 492 | 0.11 |
| Vitamin D [µg/1000 kcal/day] | 2.3 | 2.5 | 2.5 | 2.4 | 0.03 |
| α-Tocopherol [mg/1000 kcal/day] | 4.5 | 5.4 | 5.8 | 6.4 | 0.28 |
| Folate [µg/1000 kcal/day] | 143 | 174 | 191 | 216 | 0.34 |
| Vitamin C [mg/1000 kcal/day] | 41 | 52 | 61 | 75 | 0.46 |
| Sodium [mg/1000 kcal/day] | 897 | 972 | 978 | 981 | 0.08 |
| Potassium [mg/1000 kcal/day] | 978 | 1101 | 1161 | 1226 | 0.29 |
| Iron [mg/1000 kcal/day] | 3.2 | 3.7 | 3.8 | 4.0 | 0.19 |
| Calcium [mg/1000 kcal/day] | 224 | 271 | 309 | 331 | 0.31 |
| Total dietary fiber [g/1000 kcal/day] | 5.0 | 5.8 | 6.1 | 6.6 | 0.28 |
| Alcohol [g/1000 kcal/day] | 0.8 | 0.0 | 0.0 | 0.0 | − 0.22 |
| Food | |||||
| Grains [g/1000 kcal/day] | 272 | 262 | 253 | 247 | − 0.15 |
| Vegetables [g/1000 kcal/day] | 59 | 85 | 99 | 126 | 0.42 |
| Fruits [g/1000 kcal/day] | 13 | 25 | 44 | 70 | 0.56 |
| Dairies [g/1000 kcal/day] | 6 | 42 | 81 | 95 | 0.46 |
| Red meats [g/1000 kcal/day] | 11 | 12 | 12 | 9 | − 0.03 |
| White meats [g/1000 kcal/day] | 34 | 44 | 46 | 47 | 0.19 |
| Confectionery [g/1000 kcal/day] | 10 | 9 | 9 | 8 | − 0.13 |
All values are shown medians or correlation coefficients. Nutrient intake was adjusted for energy intake via the nutrient density method, using uncalibrated energy intake. Values are shown as medians in each quartile group. Q1 through Q4 included Japanese Food Guide Spinning Top scores of < 49.5, 49.5–54.8, 54.9–60.1, and ≥ 60.2. EI, energy intake; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid
aSpearman’s correlation analysis was used to evaluate the relationship between nutrient intake and adherence score
bCalibrated EI was calculated using an equation developed by the authors
cSum of retinol, β-carotene/12, α-carotene/24, and cryptoxanthin/24
Table 3 shows the associations between adherence score and physical and comprehensive frailty prevalence. Physical and comprehensive frailty prevalence was 14.2 and 35.8%, respectively. We found that adherence score was negatively associated with the OR of prevalence of physical frailty, as defined by the FP model, after adjusting for baseline potential confounding factors (Q1: reference; Q2: OR 0.87; 95% CI 0.72–1.06; Q3: OR 0.84; 95% CI 0.69–1.03; Q4: OR 0.64; 95% CI 0.52–0.80); P for trend < 0.001). Similar findings were observed with comprehensive frailty. In subgroup analyses, comparable results were observed in both the sex and socioeconomic status-stratified models (Supplemental Tables 1 and 2). Furthermore, we demonstrated that the adherence score was negatively associated with ORs for the prevalence of slow gait speed, cognition, exhaustion, and low physical activity, after evaluation using the FP model subdomains (Supplementary Table S3). These indicated a negative relationship with ORs for the prevalence of IADL disability, social frailty, cognitive frailty, and depression after evaluation using the KCL subdomains (Supplementary Table S4).
Table 3.
Multivariable adjusted odds ratios and 95% confidence intervals of the prevalence of physical and comprehensive frailty according to Japanese Food Guide Spinning Top adherence score
| Quartile of the Japanese Food Guide Spinning Top score | 10 points increment | p for trenda | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 1756) | Q2 (n = 1756) | Q3 (n = 1755) | Q4 (n = 1755) | ||||||||
| Mean (SD) score | 43.2 | (5.9) | 52.3 | (1.6) | 57.5 | (1.5) | 64.1 | (3.0) | |||
| Physical frailty | |||||||||||
| Case [n (%)] | 302 | (17.2) | 259 | (14.7) | 241 | (13.7) | 196 | (11.2) | |||
| Model 1b | 1.00 | (Ref) | 0.81 | (0.68–0.98) | 0.75 | (0.62–0.90) | 0.56 | (0.45–0.68) | 0.79 | (0.71–0.87) | < 0.001 |
| Model 2c | 1.00 | (Ref) | 0.87 | (0.72–1.06) | 0.84 | (0.69–1.03) | 0.64 | (0.52–0.80) | 0.85 | (0.76–0.93) | < 0.001 |
| Comprehensive frailty | |||||||||||
| Case [n (%)] | 744 | (42.4) | 658 | (37.5) | 593 | (33.8) | 519 | (29.6) | |||
| Model 1b | 1.00 | (Ref) | 0.79 | (0.68–0.91) | 0.66 | (0.57–0.76) | 0.49 | (0.42–0.57) | 0.70 | (0.64–0.76) | < 0.001 |
| Model 2c | 1.00 | (Ref) | 0.86 | (0.73–1.00) | 0.77 | (0.66–0.90) | 0.60 | (0.51–0.71) | 0.79 | (0.72–0.86) | < 0.001 |
All values are shown means (SDs), numbers (%), or relative ORs (95% CI). All estimates were derived from a multivariable logistic regression model. Physical and comprehensive frailty was assessed using the validated Fried phenotype model-based Frailty Screening Index and the Kihon Checklist. Bold p values are statistically significant (p < 0.05). Q1 through Q4 included Japanese Food Guide Spinning Top scores of < 49.5, 49.5–54.8, 54.9–60.1, and ≥ 60.2
CI, confidence interval; OR, odds ratio; Ref, reference; SD, standard deviation
aLinear trend p values were calculated with the likelihood ratio test using continuous variables of adherence scores
bModel 1 was adjusted for age (continuous), sex (female or male), and population density (≥ 1000 or < 1000 people/km2)
cModel 2 was Model 1 with mutual adjustments for body mass index (continuous), physical activity (yes or no), denture use (yes or no), smoking status (never smoker, past smoker, and current smoker), alcohol intake status (every day, sometimes, seldom, or never), educational attainment (< 9, 10–12, or ≥ 13 years), medication use (continuous), living alone (yes or no), socioeconomic status (high or low), green tea consumption (frequency), coffee consumption (frequency), and history of disease (hypertension, diabetes, dyslipidaemia, heart disease, and stroke; yes or no)
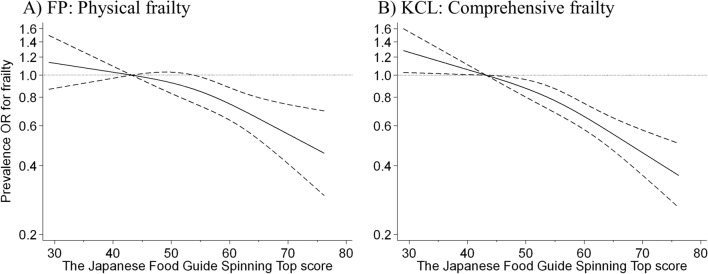
To evaluate the curve of the association between frailty prevalence and adherence score, we used a restricted cubic spline model (Fig. 1). When the first quartile (43 points) for adherence score was used as the reference, the shape of the curve showed a modest and negative association between adherence score and prevalence of each type of frailty, defined by the FP model or the KCL, up to approximately 55 points. This is the average adherence score of the current study population. Beyond this, an adherence score of up to approximately 76 points showed a strong dose-dependent negative association with frailty prevalence.
Fig. 1.
Relationship between the Japanese Food Guide Spinning Top adherence score and frailty, based on A fried phenotype (FP) model and B the Kihon Checklist (KCL), using a restricted cubic spline logistic regression model. Frailty, according to the FP model-based self-administered frailty screening index (FSI), was defined as a score of ≥ 3 out of 5 points. Frailty, according to the KCL, was defined as a score ≥ 7 out of 25 points. Solid lines represent odds ratios (ORs), and dashed lines represent 95% confidence intervals (CIs). We calculated ORs for frailty prevalence using a first quartile value of 43 points as the reference. This analysis included 6954 participants. We estimated that p < 0.05 when 95% CI of the OR did not exceed 1.00, and p ≥ 0.05 when 95% CI of the OR exceeded 1.00. Analyses were adjusted for age, sex, population density, body mass index, physical activity, denture use, smoking status, alcohol intake status, educational attainment, medication use, living alone, socioeconomic status, green tea consumption, coffee consumption, and history of disease (hypertension, diabetes, dyslipidaemia, heart disease, and stroke)
The association of the adherence score of each component with physical frailty prevalence is shown in Table 4. After adjusting for confounders, the top adherence score quartile revealed a significant association with a lower OR of physical frailty (reference, lowest quartile of adherence scores): vegetable dishes (OR 0.86 [95% CI 0.70–1.06], adjusted p for trend = 0.041) and milk (OR 0.79 [95% CI 0.65–0.97], adjusted p for trend = 0.003). Similar findings were observed with comprehensive frailty as defined by the KCL (Supplemental Tables 5). Furthermore, a high score on adherence to fruits and snacks and alcoholic beverages was associated with a low prevalence of comprehensive frailty, while a high score on adherence to energy intake showed a positive relationship with the prevalence of comprehensive frailty.
Table 4.
Multivariable adjusted odds ratios and 95% confidence intervals of the prevalence of physical frailty according to adherence score of each component on the Japanese Food Guide Spinning Top
| Quartile of the Japanese Food Guide Spinning Top score | 1 points increment | p for trenda | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 1756) | Q2 (n = 1756) | Q3 (n = 1755) | Q4 (n = 1755) | ||||||||
| Grain dishes | |||||||||||
| Case [n (%)] | 250 | (14.2) | 257 | (14.6) | 243 | (13.8) | 248 | (14.1) | |||
| Model 1b | 1.00 | (Ref) | 1.01 | (0.84 to 1.23) | 0.92 | (0.76–1.11) | 0.88 | (0.73–1.07) | 0.97 | (0.95–1.00) | 0.079 |
| Model 2c | 1.00 | (Ref) | 1.02 | (0.84–1.24) | 0.92 | (0.76–1.12) | 0.86 | (0.70–1.04) | 0.98 | (0.95–1.00) | 0.098 |
| Vegetable dishes | |||||||||||
| Case [n (%)] | 273 | (15.5) | 265 | (15.1) | 231 | (13.2) | 229 | (13.0) | |||
| Model 1b | 1.00 | (Ref) | 0.97 | (0.81–1.17) | 0.79 | (0.65–0.96) | 0.73 | (0.60–0.89) | 0.95 | (0.92–0.97) | < 0.001 |
| Model 2c | 1.00 | (Ref) | 1.03 | (0.85–1.25) | 0.87 | (0.71–1.07) | 0.86 | (0.70–1.06) | 0.97 | (0.95–0.99) | 0.041 |
| Fish and meat dishes | |||||||||||
| Case [n (%)] | 269 | (15.3) | 266 | (15.1) | 225 | (12.8) | 238 | (13.6) | |||
| Model 1b | 1.00 | (Ref) | 1.07 | (0.89–1.29) | 0.92 | (0.76–1.12) | 0.99 | (0.82–1.20) | 1.00 | (0.98–1.02) | 0.801 |
| Model 2c | 1.00 | (Ref) | 1.02 | (0.85–1.24) | 0.87 | (0.71–1.06) | 0.95 | (0.78–1.15) | 0.99 | (0.97–1.01) | 0.294 |
| Milk | |||||||||||
| Case [n (%)] | 307 | (17.5) | 264 | (15.0) | 204 | (11.6) | 223 | (12.7) | |||
| Model 1b | 1.00 | (Ref) | 0.90 | (0.75–1.09) | 0.66 | (0.54–0.80) | 0.71 | (0.59–0.86) | 0.96 | (0.94–0.98) | < 0.001 |
| Model 2c | 1.00 | (Ref) | 0.92 | (0.77–1.11) | 0.71 | (0.58–0.86) | 0.79 | (0.65–0.97) | 0.97 | (0.95–0.99) | 0.003 |
| Fruits | |||||||||||
| Case [n (%)] | 300 | (17.1) | 246 | (14.0) | 221 | (12.6) | 231 | (13.2) | |||
| Model 1b | 1.00 | (Ref) | 0.79 | (0.65–0.95) | 0.67 | (0.55–0.81) | 0.68 | (0.56–0.83) | 0.96 | (0.93–0.98) | < 0.001 |
| Model 2c | 1.00 | (Ref) | 0.87 | (0.72–1.06) | 0.78 | (0.64–0.95) | 0.83 | (0.68–1.02) | 0.98 | (0.96–1.00) | 0.109 |
| Total energy | |||||||||||
| Case [n (%)] | 230 | (13.1) | 243 | (13.8) | 278 | (15.8) | 247 | (14.1) | |||
| Model 1b | 1.00 | (Ref) | 1.31 | (1.05–1.62) | 1.44 | (1.14–1.82) | 1.17 | (0.91–1.51) | 1.16 | (1.05–1.29) | 0.004 |
| Model 2c | 1.00 | (Ref) | 1.19 | (0.95–1.48) | 1.19 | (0.93–1.53) | 1.07 | (0.82–1.39) | 1.09 | (0.98–1.22) | 0.108 |
| Snacks and alcohol | |||||||||||
| Case [n (%)] | 270 | (15.4) | 263 | (15.0) | 234 | (13.3) | 231 | (13.2) | |||
| Model 1b | 1.00 | (Ref) | 0.97 | (0.80–1.17) | 0.81 | (0.66–0.98) | 0.77 | (0.63–0.95) | 1.00 | (0.96–1.04) | 0.945 |
| Model 2c | 1.00 | (Ref) | 0.94 | (0.77–1.14) | 0.84 | (0.68–1.02) | 0.86 | (0.70–1.06) | 0.99 | (0.96–1.03) | 0.655 |
| White–red meat | |||||||||||
| Case [n (%)] | 262 | (14.9) | 242 | (13.8) | 276 | (15.7) | 218 | (12.4) | |||
| Model 1b | 1.00 | (Ref) | 0.94 | (0.78–1.14) | 1.05 | (0.87–1.26) | 0.76 | (0.63–0.93) | 0.99 | (0.96–1.01) | 0.271 |
| Model 2c | 1.00 | (Ref) | 0.96 | (0.79–1.17) | 1.02 | (0.84–1.23) | 0.81 | (0.66–0.99) | 0.99 | (0.96–1.01) | 0.389 |
All values are shown numbers (%), or relative ORs (95% CI). All estimates were derived from a multivariable logistic regression model. Bold p values are statistically significant (p < 0.05). The range of Q1 through Q4 for each food score is shown following; < 7.1, 7.1–8.9, 9.0–9.8, and ≥ 9.9 score for grain dishes, < 2.7, 2.7–4.3, 4.4–6.4, and ≥ 6.5 score for vegetable dishes, < 4.8, 4.8–7.7, 7.8–9.7, and ≥ 9.8 score for fish and meat dishes, < 0.6, 0.6–5.2, 5.3–7.7, and ≥ 7.8 score for milk, < 1.3, 1.3–3.1, 3.2–5.5, and ≥ 5.6 score for fruits, < 8.8, 8.8–9.3, 9.4–9.8, and ≥ 9.9 score for total energy intake, < 8.7, 8.7–9.5, 9.6–9.8, and ≥ 9.9 score for snacks and alcohol, and < 5.8, 5.8–8.9, 9.0–9.8, and ≥ 9.9 score for white to red meat
CI, confidence interval; OR, odds ratio; Ref, reference
aLinear trend p values were calculated with the likelihood ratio test using the continuous variables of adherence scores
bModel 1 was adjusted for age (continuous), sex (female or male), and population density (≥ 1000 or < 1000 people/km2)
cModel 2 was Model 1 with mutual adjustment for body mass index (continuous), physical activity (yes or no), denture use (yes or no), smoking status (never smoker, past smoker, and current smoker), alcohol intake status (every day, sometimes, seldom, or never), educational attainment (< 9, 10–12, or ≥ 13 years), medication use (continuous), living alone (yes or no), socioeconomic status (high or low), green tea consumption (frequency), coffee consumption (frequency), and history of disease (hypertension, diabetes, dyslipidaemia, heart disease, and stroke; yes or no)
Discussion
Our study showed an inverse dose–response relationship between scores on adherence to the Japanese Food Guide Spinning Top and the prevalences of physical and comprehensive frailty. Higher scores of adherence to vegetable dishes and milk intake were inversely associated with both frailty types. To the best of our knowledge, this is the first study to examine the association between diet quality and prevalence of both physical and comprehensive frailty in older adults, defined by two different validation methods. Our results may corroborate the essential role of adherence to dietary guidelines in individual health.
To evaluate physical and comprehensive frailty, we used two different methods: an FP model and the KCL. The prevalence of comprehensive frailty defined by the KCL (35.8%) was significantly higher than that defined by the FP model (14.2%). This is consistent with previous findings [20, 36]. The people identified as physical and comprehensive frailty may not necessarily match [40]. Previous studies comparing comprehensive frailty, which considers multiple factors, and physical frailty, which focuses on physical factors only, reported that comprehensive frailty had higher accuracy in predicting the risk of mortality [41, 42]. The reason for this is that comprehensive frailty indices that assess frailty using a multi-faceted model have a positive linear correlation with age and thus reflect biological ageing [43]. Therefore, it is also needed to evaluate the association between diet quality, physical frailty, and comprehensive frailty.
In our study, higher scores on adherence were inversely associated with both physical and comprehensive frailty prevalence after adjustment for potential confounding variables. Previous studies on associations between diet quality and frailty focused mainly on the physical components [12, 13, 15]. Our findings were consistent across both types of frailty, despite several differences in indices. Despite differences in dietary measures, our findings, taken together with those from previous studies, suggest that a healthy dietary pattern is associated with a lower OR of frailty. Moreover, we also observed an inverse association between adherence scores and ORs of subdomains in the FP model and KCL. Given that the risk of the incidence of dementia and depression tends to be higher in older adults [44], it is necessary to consider the association between diet quality and physical frailty and the risk factors for comprehensive frailty. Therefore, our results may help shed more light on key dietary components in further investigations into untangling the relationship between diet and frailty.
Although it is unclear the detail of mechanism that diet quality is inversely associated with frailty prevalence, two reasons can be considered from the previous studies. The first is that some nutrient intake is negatively associated with the risk of frailty [45, 46]. Our results indicate a positive correlation between scores on adherence and vitamin C. Actually, a previous prospective cohort study has reported that low vitamin C intake is associated with the risk of frailty in Spanish older adults [46]. The second is that people with high diet quality maintain better nutritional status. Many researchers have considered that healthy dietary pattern is inversely associated with the risk of the double burden of malnutrition, such as underweight and obesity [47]. We have previously reported that the lowest prevalence of both physical and comprehensive frailty was found at approximately 40 kcal/kg IBW/day (ideal body weight = 22 × height2) with DLW-calibrated energy intake [23] and BMI 21.4–25.7 kg/m2 [22]. Such facts should support our data, showing that frailty prevalence is lower among people with high diet quality. These previous studies may support our results. Although there are multiple mechanisms, such as antioxidant effects and metabolic and cardiovascular benefits, through which a healthy diet may contribute to a lower risk of frailty [14], causal relationships and detailed mechanisms must be clarified with further basic and intervention studies.
A strength of this study is that it provides additional evidence of the association between diet quality and physical and comprehensive frailty, using two validated assessment tools. These results indicate a potential robust association between diet quality and frailty. However, some methodologic limitations should be mentioned. First, our study utilised a cross-sectional study design. Therefore, no temporal or direct causal relationships of the observed associations between diet quality and frailty prevalence can be inferred. Second, although we have used previously validated FFQ [24, 25, 29–31], given that the adherence scores to the Japanese Food Guide Spinning Top are based on specific absolute cut-offs for dietary components, it may be insufficient to evaluate the overall diet quality because we evaluated it using the FFQ, consisting of 47 food and beverage items. However, we have previously reported that the adherence scores to the Japanese Food Guide Spinning Top estimated from our used FFQ are inversely associated with the prevalence of poor oral health-related quality of life [27]. Third, owing to the observational nature of the study, we could not fully exclude residual confounding factors from self-report or unmeasured variables. For example, women and individuals with higher socioeconomic status may have greater opportunities to consume fruits, vegetables, and dairy products [48]. In the sex and socioeconomic status-stratified models, we observed an inverse association between diet quality and the prevalence of comprehensive frailty across all groups (Supplemental Tables 1 and 2). Therefore, the inverse association between diet quality and frailty could not be fully explained by only socioeconomic status or sex alone. Finally, our study included individuals with a history of disease, including hypertension, diabetes, and dyslipidaemia. Extrapolation to participants with attributes other than those included in the study should be performed with caution because such individuals may have received dietary advice for their disease and modified their diets accordingly. However, our results were similar after excluding participants with such diseases. Considering these limitations, rigorous longitudinal studies that further evaluate whether a healthier diet is a modifiable risk factor for frailty are needed.
In recent years, the degree of frailty increased across most adult age groups in the United States between 1999 and 2018 [49]. Our findings indicated that overall adherence scores are strongly negatively associated with ORs of frailty than any individual dietary component in the food-based Japanese dietary guidelines, and these results were similar to the previous study that reported an association between adherence to the Mediterranean diet and mortality [50]. Therefore, our results may provide useful insights for improving and preventing frailty by adherence to the food-based Japanese dietary guidelines in older adults.
In conclusion, the results of this study indicate that higher diet quality with strong adherence to the Japanese Food Guide Spinning Top is associated with a lower prevalence of both physical and comprehensive frailty. The results also show a dose–response relationship between better diet quality and a lower frailty prevalence among community-dwelling older adults. Given the rapid increase in frailty prevalence worldwide, these findings may be encouraging to older adults, who are a high-risk population for frailty.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all members of the Kyoto-Kameoka study group for their valuable contributions. We thank Shinkan Tokudome, former Director of the National Institute of Nutrition and Health, Japan. We would like to thank Editage (www.editage.jp) for English-language editing. We thank Mayu Sugita, Yuki Okabe, Yoshizu Nozawa, Miho Ono, Tomonori Koizumi, and Hisamine Kobayashi, Ajinomoto Co., Inc., for providing funding to conduct this study.
Author contributions
MK and YY: designed the research (project conception, development of overall research plan, and study oversight); TY, YW, HD, AI, KI-T, MK, and YY: conducted the research (data collection); DW and KK: analysed data or performed statistical analysis; DW, KK, HN, and CG: data interpretation; DW, KK, and YY: literature review; DW and KK: wrote the paper; DW, KK, TY, MM, and YY: had primary responsibility for final content. All authors have read and approved the final manuscript.
Funding
This work was supported by a JSPS KAKENHI research grant provided to Misaka Kimura (24240091), Yosuke Yamada (15H05363), and Daiki Watanabe (21K17699). The current study was funded by Ajinomoto Co., Inc., Tokyo, Japan. However, this study is not related to any particular products of a company, and the results do not recommend any particular products.
Availability of data and material
Researchers can request our study group for permission to use these data by contacting Y.Y. (yamaday@nibiohn.go.jp).
Declarations
Conflict of interest
There are no conflicts of interests, other than those reported in funding, by any of the authors of the article.
Code availability
Not applicable.
Ethics approval
This study was conducted according to the guidelines laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Ethical approval of this study was obtained from the Research Ethics Committee of Kyoto Prefectural University of Medicine (RBMR-E-363), the National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN-76-2), and Kyoto University of Advanced Science (No. 20-1).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Contributor Information
Daiki Watanabe, Email: d2watanabe@nibiohn.go.jp.
Kayo Kurotani, Email: k-kurotani@swu.ac.jp.
Tsukasa Yoshida, Email: t-yoshida@nibiohn.go.jp.
Hinako Nanri, Email: hnanri@nibiohn.go.jp.
Yuya Watanabe, Email: yu15-watanabe@my-zaidan.or.jp.
Heiwa Date, Email: heiwa.date@biwako.shiga-u.ac.jp.
Aya Itoi, Email: aitoi@kwjc.kobe-wu.ac.jp.
Chiho Goto, Email: goto.chiho@nagoya-bunri.ac.jp.
Kazuko Ishikawa-Takata, Email: kt207460@nodai.ac.jp.
Misaka Kimura, Email: kimura.misaka@kuas.ac.jp.
Motohiko Miyachi, Email: cardiovascular0327@mac.com.
Yosuke Yamada, Email: yamaday@nibiohn.go.jp.
References
- 1.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 3.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y, Kim J, Han ES, Ryu M, Cho Y, Chae S. Frailty and body mass index as predictors of 3-year mortality in older adults living in the community. Gerontology. 2014;60(6):475–482. doi: 10.1159/000362330. [DOI] [PubMed] [Google Scholar]
- 5.Zaslavsky O, Zelber-Sagi S, Gray SL, LaCroix AZ, Brunner RL, Wallace RB, O'Sullivan MJ, Cochrane B, Woods NF. Comparison of frailty phenotypes for prediction of mortality, incident falls, and hip fracture in older women. J Am Geriatr Soc. 2016;64(9):1858–1862. doi: 10.1111/jgs.14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G, Papaioannou A, Thabane L, Levine MAH, Ioannidis G, Wong AKO, Lau A, Adachi JD. Modifying the phenotypic frailty model in predicting risk of major osteoporotic fracture in the elderly. J Am Med Dir Assoc. 2017;18(5):414–419. doi: 10.1016/j.jamda.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Yamada M, Arai H. Predictive value of frailty scores for healthy life expectancy in community-dwelling older Japanese adults. J Am Med Dir Assoc. 2015;16(11):1002e1007–1002e1011. doi: 10.1016/j.jamda.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi Y, Kitamura A, Nofuji Y, Ishizaki T, Seino S, Yokoyama Y, Shinozaki T, Murayama H, Mitsutake S, Amano H, Nishi M, Matsuyama Y, Fujiwara Y, Shinkai S. Association of trajectories of higher-level functional capacity with mortality and medical and long-term care costs among community-dwelling older Japanese. J Gerontol A Biol Sci Med Sci. 2019;74(2):211–218. doi: 10.1093/gerona/gly024. [DOI] [PubMed] [Google Scholar]
- 9.Asghari G, Mirmiran P, Yuzbashian E, Azizi F. A systematic review of diet quality indices in relation to obesity. Br J Nutr. 2017;117(8):1055–1065. doi: 10.1017/S0007114517000915. [DOI] [PubMed] [Google Scholar]
- 10.Burggraf C, Teuber R, Brosig S, Meier T. Review of a priori dietary quality indices in relation to their construction criteria. Nutr Rev. 2018;76(10):747–764. doi: 10.1093/nutrit/nuy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirt A, Collins CE. Diet quality—what is it and does it matter? Public Health Nutr. 2009;12(12):2473–2492. doi: 10.1017/S136898000900531X. [DOI] [PubMed] [Google Scholar]
- 12.Talegawkar SA, Bandinelli S, Bandeen-Roche K, Chen P, Milaneschi Y, Tanaka T, Semba RD, Guralnik JM, Ferrucci L. A higher adherence to a Mediterranean-style diet is inversely associated with the development of frailty in community-dwelling elderly men and women. J Nutr. 2012;142(12):2161–2166. doi: 10.3945/jn.112.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Garcia E, Hagan KA, Fung TT, Hu FB, Rodriguez-Artalejo F. Mediterranean diet and risk of frailty syndrome among women with type 2 diabetes. Am J Clin Nutr. 2018;107(5):763–771. doi: 10.1093/ajcn/nqy026. [DOI] [PubMed] [Google Scholar]
- 14.Ward RE, Orkaby AR, Chen J, Hshieh TT, Driver JA, Gaziano JM, Djousse L. Association between diet quality and frailty prevalence in the physicians' health study. J Am Geriatr Soc. 2020;68(4):770–776. doi: 10.1111/jgs.16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hengeveld LM, Wijnhoven HAH, Olthof MR, Brouwer IA, Simonsick EM, Kritchevsky SB, Houston DK, Newman AB, Visser M. Prospective associations of diet quality with incident frailty in older adults: the health, aging, and body composition study. J Am Geriatr Soc. 2019 doi: 10.1111/jgs.16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshiike N, Hayashi F, Takemi Y, Mizoguchi K, Seino F. A new food guide in Japan: the Japanese Food Guide Spinning Top. Nutr Rev. 2007;65(4):149–154. doi: 10.1111/j.1753-4887.2007.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 17.Kurotani K, Akter S, Kashino I, Goto A, Mizoue T, Noda M, Sasazuki S, Sawada N, Tsugane S, Japan Public Health Center based Prospective Study G Quality of diet and mortality among Japanese men and women: Japan Public Health Center based prospective study. BMJ. 2016;352:i1209. doi: 10.1136/bmj.i1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dent E, Lien C, Lim WS, Wong WC, Wong CH, Ng TP, Woo J, Dong B, de la Vega S, Hua Poi PJ, Kamaruzzaman SBB, Won C, Chen LK, Rockwood K, Arai H, Rodriguez-Manas L, Cao L, Cesari M, Chan P, Leung E, Landi F, Fried LP, Morley JE, Vellas B, Flicker L. The Asia-Pacific clinical practice guidelines for the management of frailty. J Am Med Dir Assoc. 2017;18(7):564–575. doi: 10.1016/j.jamda.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Hewitt J, Carter B, Vilches-Moraga A, Quinn TJ, Braude P, Verduri A, Pearce L, Stechman M, Short R, Price A, Collins JT, Bruce E, Einarsson A, Rickard F, Mitchell E, Holloway M, Hesford J, Barlow-Pay F, Clini E, Myint PK, Moug SJ, McCarthy K, Collaborators CS. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada Y, Nanri H, Watanabe Y, Yoshida T, Yokoyama K, Itoi A, Date H, Yamaguchi M, Miyake M, Yamagata E, Tamiya H, Nishimura M, Fujibayashi M, Ebine N, Yoshida M, Kikutani T, Yoshimura E, Ishikawa-Takata K, Yamada M, Nakaya T, Yoshinaka Y, Fujiwara Y, Arai H, Kimura M. Prevalence of frailty assessed by Fried and Kihon checklist indexes in a prospective cohort study: design and demographics of the Kyoto-kameoka longitudinal study. J Am Med Dir Assoc. 2017;18(8):733e737–733e715. doi: 10.1016/j.jamda.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Kimura M, Group KS. Objectively measured daily step counts and prevalence of frailty in 3,616 older adults. J Am Geriatr Soc. 2020;68(10):2310–2318. doi: 10.1111/jgs.16655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Kimura M, Kyoto-Kameoka Study G A U-shaped relationship between the prevalence of frailty and body mass index in community-dwelling Japanese older adults: The Kyoto-Kameoka study. J Clin Med. 2020 doi: 10.3390/jcm9051367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe D, Yoshida T, Nanri H, Watanabe Y, Date H, Itoi A, Goto C, Ishikawa-Takata K, Sagayama H, Ebine N, Kobayashi H, Kimura M, Yamada Y, for Kyoto-Kameoka S, Association between the prevalence of frailty and doubly labeled water-calibrated energy intake among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2021;76(5):876–884. doi: 10.1093/gerona/glaa133. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe D, Nanri H, Yoshida T, Yamaguchi M, Sugita M, Nozawa Y, Okabe Y, Itoi A, Goto C, Yamada Y, Ishikawa-Takata K, Kobayashi H, Kimura M, Kyoto-Kameoka Study Group KS Validation of energy and nutrition intake in Japanese elderly individuals estimated based on a short food frequency questionnaire compared against a 7-day dietary record: the Kyoto-Kameoka study. Nutrients. 2019 doi: 10.3390/nu11030688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe D, Nanri H, Sagayama H, Yoshida T, Itoi A, Yamaguchi M, Yokoyama K, Watanabe Y, Goto C, Ebine N, Higaki Y, Ishikawa-Takata K, Kimura M, Yamada Y, Kyoto-Kameoka Study G Estimation of energy intake by a food frequency questionnaire: calibration and validation with the doubly labeled water method in Japanese older people. Nutrients. 2019 doi: 10.3390/nu11071546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe D, Yoshida T, Yokoyama K, Yoshinaka Y, Watanabe Y, Kikutani T, Yoshida M, Yamada Y, Kimura M, Kyoto-Kameoka Study G Association between mixing ability of masticatory functions measured using color-changing chewing gum and frailty among Japanese older adults: the Kyoto-Kameoka study. Int J Environ Res Public Health. 2020 doi: 10.3390/ijerph17124555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe D, Kurotani K, Yoshida T, Nanri H, Watanabe Y, Date H, Itoi A, Goto C, Ishikawa-Takata K, Kikutani T, Yoshida M, Fujita H, Yamada Y, Kimura M, Kyoto-Kameoka Study G Adherence to the food-based Japanese dietary guidelines and prevalence of poor oral health-related quality of life among older Japanese adults in the Kyoto-Kameoka study. Br J Nutr. 2021 doi: 10.1017/S0007114521003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachat C, Hawwash D, Ocke MC, Berg C, Forsum E, Hornell A, Larsson C, Sonestedt E, Wirfalt E, Akesson A, Kolsteren P, Byrnes G, De Keyzer W, Van Camp J, Cade JE, Slimani N, Cevallos M, Egger M, Huybrechts I. Strengthening the reporting of observational studies in epidemiology-nutritional epidemiology (STROBE-nut): an extension of the STROBE statement. PLoS Med. 2016;13(6):e1002036. doi: 10.1371/journal.pmed.1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imaeda N, Goto C, Sasakabe T, Mikami H, Oze I, Hosono A, Naito M, Miyagawa N, Ozaki E, Ikezaki H, Nanri H, Nakahata NT, Kamano SK, Kuriki K, Yaguchi YT, Kayama T, Kurihara A, Harada S, Wakai K. Reproducibility and validity of food group intake in a short food frequency questionnaire for the middle-aged Japanese population. Environ Health Prev Med. 2021;26(1):28. doi: 10.1186/s12199-021-00951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokudome S, Goto C, Imaeda N, Tokudome Y, Ikeda M, Maki S. Development of a data-based short food frequency questionnaire for assessing nutrient intake by middle-aged Japanese. Asian Pac J Cancer Prev. 2004;5(1):40–43. [PubMed] [Google Scholar]
- 31.Tokudome Y, Goto C, Imaeda N, Hasegawa T, Kato R, Hirose K, Tajima K, Tokudome S. Relative validity of a short food frequency questionnaire for assessing nutrient intake versus three-day weighed diet records in middle-aged Japanese. J Epidemiol. 2005;15(4):135–145. doi: 10.2188/jea.15.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe D, Yoshida T, Yoshimura E, Nanri H, Goto C, Ishikawa-Takata K, Ebine N, Fujita H, Kimura M, Yamada Y, Kyoto-Kameoka Study G Doubly labelled water-calibration approach attenuates the underestimation of energy intake calculated from self-reported dietary assessment data in Japanese older adults. Public Health Nutr. 2021 doi: 10.1017/S1368980021003785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Council for Science and Technology; Ministry of Education, Culture, Sports, Science and Technology, Japan . Standard tables of food composition in Japan, fifth revised and enlarged edition. National Printing Bureau: Tokyo; 2001. [Google Scholar]
- 34.Oba S, Nagata C, Nakamura K, Fujii K, Kawachi T, Takatsuka N, Shimizu H. Diet based on the Japanese Food Guide Spinning Top and subsequent mortality among men and women in a general Japanese population. J Am Diet Assoc. 2009;109(9):1540–1547. doi: 10.1016/j.jada.2009.06.367. [DOI] [PubMed] [Google Scholar]
- 35.Satake S, Shimokata H, Senda K, Kondo I, Toba K. Validity of total Kihon checklist score for predicting the incidence of 3-year dependency and mortality in a community-dwelling older population. J Am Med Dir Assoc. 2017;18(6):552e551–552e556. doi: 10.1016/j.jamda.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Ambagtsheer RC, Visvanathan R, Dent E, Yu S, Schultz TJ, Beilby J. Commonly used screening instruments to identify frailty among community-dwelling older people in a general practice (primary care) setting: a study of diagnostic test accuracy. J Gerontol A Biol Sci Med Sci. 2020;75(6):1134–1142. doi: 10.1093/gerona/glz260. [DOI] [PubMed] [Google Scholar]
- 37.van Buuren S, Groothuis-Oudshoorn K. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 38.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 39.Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107–1117. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- 40.Lin SM, Aliberti MJR, Fortes-Filho SQ, Melo JA, Aprahamian I, Suemoto CK, Jacob Filho W. Comparison of 3 frailty instruments in a geriatric acute care setting in a low-middle income country. J Am Med Dir Assoc. 2018;19(4):310e313–314e313. doi: 10.1016/j.jamda.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 42.Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56(5):898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc. 2010;58(2):318–323. doi: 10.1111/j.1532-5415.2009.02667.x. [DOI] [PubMed] [Google Scholar]
- 44.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartali B, Frongillo EA, Bandinelli S, Lauretani F, Semba RD, Fried LP, Ferrucci L. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61(6):589–593. doi: 10.1093/gerona/61.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balboa-Castillo T, Struijk EA, Lopez-Garcia E, Banegas JR, Rodriguez-Artalejo F, Guallar-Castillon P. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing. 2018;47(6):872–879. doi: 10.1093/ageing/afy105. [DOI] [PubMed] [Google Scholar]
- 47.Collaborators GBDD. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okubo H, Murakami K, Sasaki S. Monetary value of self-reported diets and associations with sociodemographic characteristics and dietary intake among Japanese adults: analysis of nationally representative surveys. Public Health Nutr. 2016;19(18):3306–3318. doi: 10.1017/S1368980016001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joanna M, Blodgett KR, Theou O. Changes in the severity and lethality of age-related health deficit accumulation in the USA between 1999 and 2018: a population-based cohort study. Lancet Healthy Longev. 2021;2(2):e96–104. doi: 10.1016/S2666-7568(20)30059-3. [DOI] [PubMed] [Google Scholar]
- 50.Buckland G, Agudo A, Travier N, Huerta JM, Cirera L, Tormo MJ, Navarro C, Chirlaque MD, Moreno-Iribas C, Ardanaz E, Barricarte A, Etxeberria J, Marin P, Quiros JR, Redondo ML, Larranaga N, Amiano P, Dorronsoro M, Arriola L, Basterretxea M, Sanchez MJ, Molina E, Gonzalez CA. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Br J Nutr. 2011;106(10):1581–1591. doi: 10.1017/S0007114511002078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers can request our study group for permission to use these data by contacting Y.Y. (yamaday@nibiohn.go.jp).