Abstract
Oncolytic virotherapy (OVT) is a novel type of immunotherapy that induces anti-tumor responses through selective self-replication within cancer cells and oncolytic virus (OV)-mediated immunostimulation. Notably, talimogene laherparepvec (T-Vec) developed by the Amgen company in 2015, is the first FDA-approved OV product to be administered via intratumoral injection and has been the most successful OVT treatment. However, the systemic administration of OVs still faces huge challenges, including in vivo pre-existing neutralizing antibodies and poor targeting delivery efficacy. Recently, state-of-the-art progress has been made in the development of systemic delivery of OVs, which demonstrates a promising step toward broadening the scope of cancer immunotherapy and improving the clinical efficacy of OV delivery. Herein, this review describes the general characteristics of OVs, focusing on the action mechanisms of OVs as well as the advantages and disadvantages of OVT. The emerging multiple systemic administration approaches of OVs are summarized in the past five years. In addition, the combination treatments between OVT and traditional therapies (chemotherapy, thermotherapy, immunotherapy, and radiotherapy, etc.) are highlighted. Last but not least, the future prospects and challenges of OVT are also discussed, with the aim of facilitating medical researchers to extensively apply the OVT in the cancer therapy.
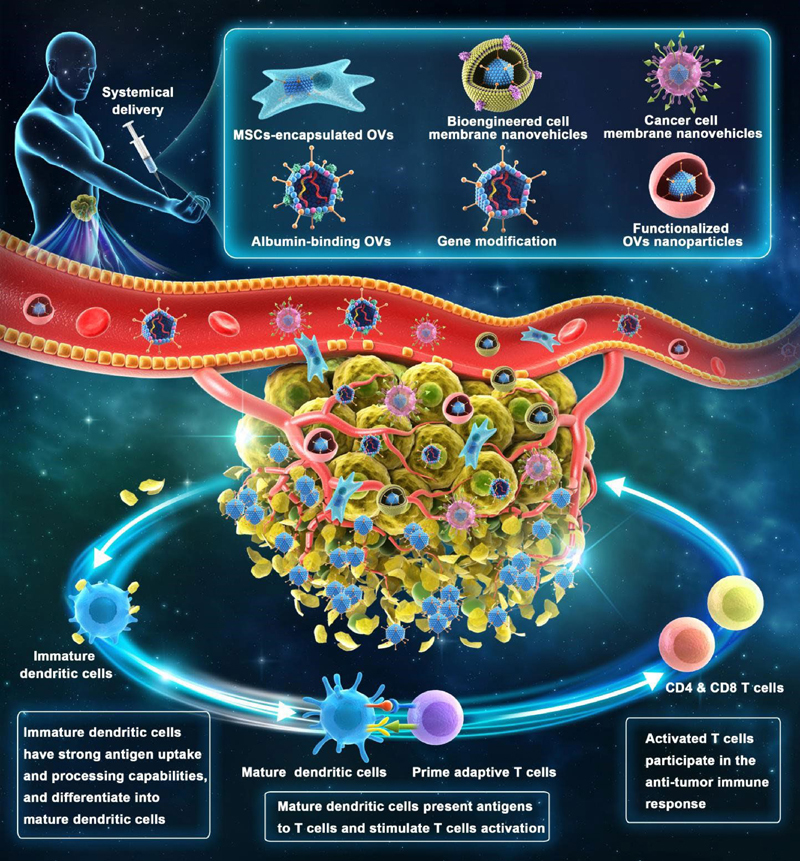
Keywords: oncolytic virotherapy, oncolytic viruses, talimogene laherparepvec, systemic administration, combination treatments
Acknowledgments
This work was supported by the National Key R&D Program of China (No. 2019YFC1316104), the National Natural Science Foundation of China (Nos. 81871960, 82073368, and 82073777), Liaoning Revitalization Talents Program (Nos. XLYC2007071 and XLYC1808017), China Postdoctoral Science Foundation (No. 2020M680986), and General Project of Liaoning Provincial Department of Education (No. JKZ0927).
Footnotes
Weiyue Ban and Jianhuan Guan contributed equally to this work.
Contributor Information
Mengchi Sun, Email: smc_1990@aliyun.com.
Funan Liu, Email: lfn540@126.com.
Jin Sun, Email: sunjin@syphu.edu.cn.
References
- [1].Advani S J, Chung S M, Yan S Y, Gillespie G Y, Markert J M, Whitley R J, Roizman B, Weichselbaum R R. Replication-competent, nonneuroinvasive genetically engineered herpes virus is highly effective in the treatment of therapy-resistant experimental human tumors. Cancer Res. 1999;59:2055–2058. [PubMed] [Google Scholar]
- [2].Skoetz N, Will A, Monsef I, Brillant C, Engert A, von Tresckow B. Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst. Rev. 2017;5:Cd007941. doi: 10.1002/14651858.CD007941.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Watanabe D, Goshima F. Oncolytic virotherapy by HSV. Adv. Exp. Med. Biol. 2018;1045:63–84. doi: 10.1007/978-981-10-7230-7_4. [DOI] [PubMed] [Google Scholar]
- [4].Masoud S J, Hu J B, Beasley G M, Stewart J H, IV, Mosca P J. Efficacy of talimogene laherparepvec (T-VEC) therapy in patients with in-transit melanoma metastasis decreases with increasing lesion size. Ann. Surg. Oncol. 2019;26:4633–4641. doi: 10.1245/s10434-019-07691-3. [DOI] [PubMed] [Google Scholar]
- [5].Bai Y, Hui P, Du X Y, Su X. Updates to the antitumor mechanism of oncolytic virus. Thorac. Cancer. 2019;10:1031–1035. doi: 10.1111/1759-7714.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee P, Gujar S. Potentiating prostate cancer immunotherapy with oncolytic viruses. Nat. Rev. Urol. 2018;15:235–250. doi: 10.1038/nrurol.2018.10. [DOI] [PubMed] [Google Scholar]
- [7].Lv P, Liu X, Chen X M, Liu C, Zhang Y, Chu C C, Wang J Q, Wang X Y, Chen X Y, Liu G. Genetically engineered cell membrane nanovesicles for oncolytic adenovirus delivery: A versatile platform for cancer virotherapy. Nano Lett. 2019;19:2993–3001. doi: 10.1021/acs.nanolett.9b00145. [DOI] [PubMed] [Google Scholar]
- [8].Bommareddy P K, Shettigar M, Kaufman H L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018;18:498–513. doi: 10.1038/s41577-018-0014-6. [DOI] [PubMed] [Google Scholar]
- [9].Smith J S, Xu Z L, Tian J, Palmer D J, Ng P, Byrnes A P. The role of endosomal escape and mitogen-activated protein kinases in adenoviral activation of the innate immune response. PLoS One. 2011;6:e26755. doi: 10.1371/journal.pone.0026755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Waddington S N, McVey J H, Bhella D, Parker A L, Barker K, Atoda H, Pink R, Buckley S M K, Greig J A, Denby L, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- [11].Atasheva S, Yao J, Shayakhmetov D M. Innate immunity to adenovirus: Lessons from mice. FEBS Lett. 2019;593:3461–3483. doi: 10.1002/1873-3468.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Taipale K, Liikanen I, Juhila J, Turkki R, Tähtinen S, Kankainen M, Vassilev L, Ristimäki A, Koski A, Kanerva A, et al. Chronic activation of innate immunity correlates with poor prognosis in cancer patients treated with oncolytic adenovirus. Mol. Ther. 2016;24:175–183. doi: 10.1038/mt.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brown M C, Holl E K, Boczkowski D, Dobrikova E, Mosaheb M, Chandramohan V, Bigner D D, Gromeier M, Nair S K. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci. Transl. Med. 2017;9:eaan4220. doi: 10.1126/scitranslmed.aan4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guo G, Gong K, Wohlfeld B, Hatanpaa K J, Zhao D W, Habib A A. Ligand-independent EGFR signaling. Cancer Res. 2015;75:3436–3441. doi: 10.1158/0008-5472.CAN-15-0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ilkow C S, Marguerie M, Batenchuk C, Mayer J, Ben Neriah D, Cousineau S, Falls T, Jennings V A, Boileau M, Bellamy D, et al. Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nat. Med. 2015;21:530–536. doi: 10.1038/nm.3848. [DOI] [PubMed] [Google Scholar]
- [16].Arulanandam R, Batenchuk C, Angarita F A, Ottolino-Perry K, Cousineau S, Mottashed A, Burgess E, Falls T J, De Silva N, Tsang J, et al. VEGF-mediated induction of PRD1-BF1/blimp1 expression sensitizes tumor vasculature to oncolytic virus infection. Cancer Cell. 2015;28:210–224. doi: 10.1016/j.ccell.2015.06.009. [DOI] [PubMed] [Google Scholar]
- [17].Shmulevitz M, Marcato P, Lee P W K. Unshackling the links between reovirus oncolysis, Ras signaling, translational control, and cancer. Oncogene. 2005;24:7720–7728. doi: 10.1038/sj.onc.1209041. [DOI] [PubMed] [Google Scholar]
- [18].Martin N T, Bell J C. Oncolytic virus combination therapy: Killing one bird with two stones. Mol. Ther. 2018;26:1414–1422. doi: 10.1016/j.ymthe.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chiappinelli K B, Strissel P L, Desrichard A, Li H L, Henke C, Akman B, Hein A, Rote N S, Cope L M, Snyder A, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell. 2015;162:974–986. doi: 10.1016/j.cell.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kaufman H L, Kohlhapp F J, Zloza A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gujar S, Pol J G, Kim Y, Lee P W, Kroemer G. Antitumor benefits of antiviral immunity: An underappreciated aspect of oncolytic virotherapies. Trends Immunol. 2018;39:209–221. doi: 10.1016/j.it.2017.11.006. [DOI] [PubMed] [Google Scholar]
- [22].Gujar S A, Pan D, Marcato P, Garant K A, Lee P W K. Oncolytic virus-initiated protective immunity against prostate cancer. Mol. Ther. 2011;19:797–804. doi: 10.1038/mt.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lucas T, Abraham D, Untergasser G, Zins K, Hofer E, Gunsilius E, Aharinejad S. Adenoviral-mediated endothelial precursor cell delivery of soluble cd115 suppresses human prostate cancer xenograft growth in mice. Stem Cells. 2009;27:2342–2352. doi: 10.1002/stem.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mogensen T H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3:e955691. doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Getts D R, Chastain E M L, Terry R L, Miller S D. Virus infection, antiviral immunity, and autoimmunity. Immunol. Rev. 2013;255:197–209. doi: 10.1111/imr.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dörner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. doi: 10.1016/j.immuni.2007.09.002. [DOI] [PubMed] [Google Scholar]
- [28].Bhattacharya P, Budnick I, Singh M, Thiruppathi M, Alharshawi K, Elshabrawy H, Holterman M J, Prabhakar B S. Dual role of GM-CSF as a pro-inflammatory and a regulatory cytokine: Implications for immune therapy. J. Interferon Cytokine Res. 2015;35:585–599. doi: 10.1089/jir.2014.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tähtinen S, Blattner C, Vähä-Koskela M, Saha D, Siurala M, Parviainen S, Utikal J, Kanerva A, Umansky V, Hemminki A. T-cell therapy enabling adenoviruses coding for IL2 and TNFα induce systemic immunomodulation in mice with spontaneous melanoma. J. Immunother. 2016;39:343–354. doi: 10.1097/CJI.0000000000000144. [DOI] [PubMed] [Google Scholar]
- [30].Passer B J, Cheema T, Wu S, Wu C L, Rabkin S D, Martuza R L. Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models. Cancer Gene Ther. 2013;20:17–24. doi: 10.1038/cgt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zamarin D, Wolchok J D. Potentiation of immunomodulatory antibody therapy with oncolytic viruses for treatment of cancer. Mol. Ther. Oncolytics. 2014;1:14004. doi: 10.1038/mto.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Choi J W, Lee Y S, Yun C O, Kim S W. Polymeric oncolytic adenovirus for cancer gene therapy. J. Control. Release. 2015;219:181–191. doi: 10.1016/j.jconrel.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Andtbacka R H I, Amatruda T, Nemunaitis J, Zager J S, Walker J, Chesney J A, Liu K T, Hsu C P, Pickett C A, Mehnert J M. Biodistribution, shedding, and transmissibility of the oncolytic virus talimogene laherparepvec in patients with melanoma. EBioMedicine. 2019;47:89–97. doi: 10.1016/j.ebiom.2019.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hirooka Y, Kasuya H, Ishikawa T, Kawashima H, Ohno E, Villalobos I B, Naoe Y, Ichinose T, Koyama N, Tanaka M, et al. A phase I clinical trial of EUS-guided intratumoral injection of the oncolytic virus, HF10 for unresectable locally advanced pancreatic cancer. BMC Cancer. 2018;18:596. doi: 10.1186/s12885-018-4453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mahalingam D, Wilkinson G A, Eng K H, Fields P, Raber P, Moseley J L, Cheetham K, Coffey M, Nuovo G, Kalinski P, et al. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: A phase Ib study. Clin. Cancer Res. 2020;26:71–81. doi: 10.1158/1078-0432.CCR-19-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Packiriswamy N, Upreti D, Zhou Y M, Khan R, Miller A, Diaz R M, Rooney C M, Dispenzieri A, Peng K W, Russell S J. Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma. Leukemia. 2020;34:3310–3322. doi: 10.1038/s41375-020-0828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].García M, Moreno R, Gil-Martin M, Cascallò M, de Olza M O, Cuadra C, Piulats J M, Navarro V, Domenech M, Alemany R, et al. A phase 1 trial of oncolytic adenovirus ICOVIR-5 administered intravenously to cutaneous and uveal melanoma patients. Hum. Gene Ther. 2019;30:352–364. doi: 10.1089/hum.2018.107. [DOI] [PubMed] [Google Scholar]
- [38].Müller L M E, Holmes M, Michael J L, Scott G B, West E J, Scott K J, Parrish C, Hall K, Stäble S, Jennings V A, et al. Plasmacytoid dendritic cells orchestrate innate and adaptive antitumor immunity induced by oncolytic coxsackievirus A21. J. Immunother. Cancer. 2019;7:164. doi: 10.1186/s40425-019-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Annels N E, Mansfield D, Arif M, Ballesteros-Merino C, Simpson G R, Denyer M, Sandhu S S, Melcher A A, Harrington K J, Davies B, et al. Phase I trial of an ICAM-1-targeted immunotherapeutic-coxsackievirus A21 (CVA21) as an oncolytic agent against non muscle-invasive bladder cancer. Clin. Cancer Res. 2019;25:5818–5831. doi: 10.1158/1078-0432.CCR-18-4022. [DOI] [PubMed] [Google Scholar]
- [40].Kurokawa C, Iankov I D, Anderson S K, Aderca I, Leontovich A A, Maurer M J, Oberg A L, Schroeder M A, Giannini C, Greiner S M, et al. Constitutive interferon pathway activation in tumors as an efficacy determinant following oncolytic virotherapy. J. Natl. Cancer Inst. 2018;110:1123–1132. doi: 10.1093/jnci/djy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lauer U M, Schell M, Beil J, Berchtold S, Koppenhöfer U, Glatzle J, Königsrainer A, Möhle R, Nann D, Fend F, et al. Phase I study of oncolytic vaccinia virus GL-ONC1 in patients with peritoneal carcinomatosis. Clin. Cancer Res. 2018;24:4388–4398. doi: 10.1158/1078-0432.CCR-18-0244. [DOI] [PubMed] [Google Scholar]
- [42].Pascual-Pasto G, Bazan-Peregrino M, Olaciregui N G, Restrepo-Perdomo C A, Mato-Berciano A, Ottaviani D, Weber K, Correa G, Paco S, Vila-Ubach M, et al. Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Sci. Transl. Med. 2019;11:eaat9321. doi: 10.1126/scitranslmed.aat9321. [DOI] [PubMed] [Google Scholar]
- [43].Lang F F, Conrad C, Gomez-Manzano C, Yung W K A, Sawaya R, Weinberg J S, Prabhu S S, Rao G, Fuller G N, Aldape K D, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: Replication and immunotherapeutic effects in recurrent malignant glioma. J. Clin. Oncol. 2018;36:1419–1427. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Streby K A, Currier M A, Triplet M, Ott K, Dishman D J, Vaughan M R, Ranalli M A, Setty B, Skeens M A, Whiteside S, et al. First-in-human intravenous seprehvir in young cancer patients: A phase 1 clinical trial. Mol. Ther. 2019;27:1930–1938. doi: 10.1016/j.ymthe.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jonker D J, Tang P A, Kennecke H, Welch S A, Cripps M C, Asmis T, Chalchal H, Tomiak A, Lim H, Ko Y J, et al. A randomized phase II study of FOLFOX6/bevacizumab with or without pelareorep in patients with metastatic colorectal cancer: IND. 210, a Canadian cancer trials group trial. Clin. Colorectal Cancer. 2018;17:231–239.e7. doi: 10.1016/j.clcc.2018.03.001. [DOI] [PubMed] [Google Scholar]
- [46].Machiels J P, Salazar R, Rottey S, Duran I, Dirix L, Geboes K, Wilkinson-Blanc C, Pover G, Alvis S, Champion B, et al. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE) J. Immunother. Cancer. 2019;7:20. doi: 10.1186/s40425-019-0510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Packiam V T, Lamm D L, Barocas D A, Trainer A, Fand B, Davis R L, III, Clark W, Kroeger M, Dumbadze I, Chamie K, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol. Oncol.:Semin. Orig. Investig. 2018;36:440–447. doi: 10.1016/j.urolonc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- [48].Parakrama R, Fogel E, Chandy C, Augustine T, Coffey M, Tesfa L, Goel S, Maitra R. Immune characterization of metastatic colorectal cancer patients post reovirus administration. BMC Cancer. 2020;20:569. doi: 10.1186/s12885-020-07038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bradbury P A, Morris D G, Nicholas G, Tu D S, Tehfe M, Goffin J R, Shepherd F A, Gregg R W, Rothenstein J, Lee C, et al. Canadian cancer trials group (CCTG) IND211: A randomized trial of pelareorep (Reolysin) in patients with previously treated advanced or metastatic non-small cell lung cancer receiving standard salvage therapy. Lung Cancer. 2018;120:142–148. doi: 10.1016/j.lungcan.2018.03.005. [DOI] [PubMed] [Google Scholar]
- [50].Reid E G, Looney D, Maldarelli F, Noy A, Henry D, Aboulafia D, Ramos J C, Sparano J, Ambinder R F, Lee J, et al. Safety and efficacy of an oncolytic viral strategy using bortezomib with ICE/R in relapsed/refractory HIV-positive lymphomas. Blood Adv. 2018;2:3618–3626. doi: 10.1182/bloodadvances.2018022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schenk E L, Mandrekar S J, Dy G K, Aubry M C, Tan A D, Dakhil S R, Sachs B A, Nieva J J, Bertino E, Hann C L, et al. A randomized double-blind phase II study of the Seneca valley virus (NTX-010) versus placebo for patients with extensive-stage SCLC (ES SCLC) who were stable or responding after at least four cycles of platinum-based chemotherapy: North central cancer treatment group (Alliance) N0923 study. J. Thorac. Oncol. 2020;15:110–119. doi: 10.1016/j.jtho.2019.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kiyohara E, Tanemura A, Nishioka M, Yamada M, Tanaka A, Yokomi A, Saito A, Sakura K, Nakajima T, Myoui A, et al. Intratumoral injection of hemagglutinating virus of Japan-envelope vector yielded an antitumor effect for advanced melanoma: A phase I/IIa clinical study. Cancer Immunol., Immunother. 2020;69:1131–1140. doi: 10.1007/s00262-020-02509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fujita K, Kato T, Hatano K, Kawashima A, Ujike T, Uemura M, Imamura R, Okihara K, Ukimura O, Miki T, et al. Intratumoral and s. c. injection of inactivated hemagglutinating virus of Japan envelope (GEN0101) in metastatic castration-resistant prostate cancer. Cancer Sci. 2020;111:1692–1698. doi: 10.1111/cas.14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bernstein V, Ellard S L, Dent S F, Tu D, Mates M, Dhesy-Thind S K, Panasci L, Gelmon K A, Salim M, Song X, et al. A randomized phase II study of weekly paclitaxel with or without pelareorep in patients with metastatic breast cancer: Final analysis of Canadian cancer trials group IND. 213. Breast Cancer Res. Treat. 2018;167:485–493. doi: 10.1007/s10549-017-4538-4. [DOI] [PubMed] [Google Scholar]
- [55].Chesney J, Puzanov I, Collichio F, Singh P, Milhem M M, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 2018;36:1658–1667. doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tejada S, Alonso M, Patiño A, Fueyo J, Gomez-Manzano C, Diez-Valle R. Phase I trial of DNX-2401 for diffuse intrinsic pontine glioma newly diagnosed in pediatric patients. Neurosurgery. 2018;83:1050–1056. doi: 10.1093/neuros/nyx507. [DOI] [PubMed] [Google Scholar]
- [57].Chesney J, Awasthi S, Curti B, Hutchins L, Linette G, Triozzi P, Tan M C B, Brown R E, Nemunaitis J, Whitman E, et al. Phase IIIb safety results from an expanded-access protocol of talimogene laherparepvec for patients with unresected, stage IIIB-IVM1c melanoma. Melanoma Res. 2018;28:44–51. doi: 10.1097/CMR.0000000000000399. [DOI] [PubMed] [Google Scholar]
- [58].Macedo N, Miller D M, Haq R, Kaufman H L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer. 2020;8:e001486. doi: 10.1136/jitc-2020-001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zheng M J, Huang J H, Tong A P, Yang H. Oncolytic viruses for cancer therapy: Barriers and recent advances. Mol. Ther. Oncolytics. 2019;15:234–247. doi: 10.1016/j.omto.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Smith E, Breznik J, Lichty B D. Strategies to enhance viral penetration of solid tumors. Hum. Gene Ther. 2011;22:1053–1060. doi: 10.1089/hum.2010.227. [DOI] [PubMed] [Google Scholar]
- [61].Roy D G, Bell J C. Cell carriers for oncolytic viruses: Current challenges and future directions. Oncolytic Virother. 2013;2:47–56. doi: 10.2147/OV.S36623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bridle B W, Stephenson K B, Boudreau J E, Koshy S, Kazdhan N, Pullenayegum E, Brunellière J, Bramson J L, Lichty B D, Wan Y H. Potentiating cancer immunotherapy using an oncolytic virus. Mol. Ther. 2010;18:1430–1439. doi: 10.1038/mt.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hill C, Carlisle R. Achieving systemic delivery of oncolytic viruses. Expert Opin. Drug Deliv. 2019;16:607–620. doi: 10.1080/17425247.2019.1617269. [DOI] [PubMed] [Google Scholar]
- [64].Guimarães-Camboa N, Cattaneo P, Sun Y F, Moore-Morris T, Gu Y S, Dalton N D, Rockenstein E, Masliah E, Peterson K L, Stallcup W B, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20:345–359.e5. doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hadryś A, Sochanik A, McFadden G, Jazowiecka-Rakus J. Mesenchymal stem cells as carriers for systemic delivery of oncolytic viruses. Eur. J. Pharmacol. 2020;874:172991. doi: 10.1016/j.ejphar.2020.172991. [DOI] [PubMed] [Google Scholar]
- [66].Abdallah B M, Kassem M. The use of mesenchymal (skeletal) stem cells for treatment of degenerative diseases: Current status and future perspectives. J. Cell. Physiol. 2009;218:9–12. doi: 10.1002/jcp.21572. [DOI] [PubMed] [Google Scholar]
- [67].Cheng X F, Zhang G Y, Zhang L, Hu Y, Zhang K, Sun X J, Zhao C Q, Li H, Li Y M, Zhao J. Mesenchymal stem cells deliver exogenous mir-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J. Cell. Mol. Med. 2018;22:261–276. doi: 10.1111/jcmm.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wang B, Yao K, Huuskes B M, Shen H H, Zhuang J L, Godson C, Brennan E P, Wilkinson-Berka J L, Wise A F, Ricardo S D. Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol. Ther. 2016;24:1290–1301. doi: 10.1038/mt.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Na Y J, Nam J P, Hong J, Oh E, Shin H C, Kim H S, Kim S W, Yun C O. Systemic administration of human mesenchymal stromal cells infected with polymer-coated oncolytic adenovirus induces efficient pancreatic tumor homing and infiltration. J. Control. Release. 2019;305:75–88. doi: 10.1016/j.jconrel.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Dapkute D, Steponkiene S, Bulotiene D, Saulite L, Riekstina U, Rotomskis R. Skin-derived mesenchymal stem cells as quantum dot vehicles to tumors. Int. J. Nanomedicine. 2017;12:8129–8142. doi: 10.2147/IJN.S143367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Liu Y, Ye T, Maynard J, Akbulut H, Deisseroth A. Engineering conditionally replication-competent adenoviral vectors carrying the cytosine deaminase gene increases the infectivity and therapeutic effect for breast cancer gene therapy. Cancer Gene Ther. 2006;13:346–356. doi: 10.1038/sj.cgt.7700906. [DOI] [PubMed] [Google Scholar]
- [72].Long G V, Trefzer U, Davies M A, Kefford R F, Ascierto P A, Chapman P B, Puzanov I, Hauschild A, Robert C, Algazi A, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- [73].Harrington K J, Puzanov I, Hecht J R, Hodi F S, Szabo Z, Murugappan S, Kaufman H L. Clinical development of talimogene laherparepvec (T-VEC): A modified herpes simplex virus type-1-derived oncolytic immunotherapy. Expert Rev. Anticancer Ther. 2015;15:1389–1403. doi: 10.1586/14737140.2015.1115725. [DOI] [PubMed] [Google Scholar]
- [74].Du W, Seah I, Bougazzoul O, Choi G, Meeth K, Bosenberg M W, Wakimoto H, Fisher D, Shah K. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. P. Natl. Acad. Sci. USA. 2017;114:E6157–E6165. doi: 10.1073/pnas.1700363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yang F J, Wu L N, Xu W X, Liu Y, Zhen L M, Ning G, Song J, Jiao Q, Zheng Y Y, Chen T T, et al. Diverse effects of the NTCP p. Ser267Phe variant on disease progression during chronic HBV infection and on HBV pres1 variability. Front. Cell. Infect. Microbiol. 2019;9:18. doi: 10.3389/fcimb.2019.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cui S X, Zhang H L, Xu W F, Qu X J. 13F-1, a novel 5-fluorouracil prodrug containing an Asn-Gly-Arg (NO2) COOCH3 tripeptide, inhibits human colonic carcinoma growth by targeting Aminopeptidase N (APN/CD13) Eur. J. Pharmacol. 2014;734:50–59. doi: 10.1016/j.ejphar.2014.04.001. [DOI] [PubMed] [Google Scholar]
- [77].Fusciello M, Fontana F, Tähtinen S, Capasso C, Feola S, Martins B, Chiaro J, Peltonen K, Ylösmäki L, Ylösmäki E, et al. Artificially cloaked viral nanovaccine for cancer immunotherapy. Nat. Commun. 2019;10:5747. doi: 10.1038/s41467-019-13744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rojas L A, Condezo G N, Moreno R, Fajardo C A, Arias-Badia M, San Martín C, Alemany R. Albumin-binding adenoviruses circumvent pre-existing neutralizing antibodies upon systemic delivery. J. Control. Release. 2016;237:78–88. doi: 10.1016/j.jconrel.2016.07.004. [DOI] [PubMed] [Google Scholar]
- [79].Mato-Berciano A, Morgado S, Maliandi M V, Farrera-Sal M, Gimenez-Alejandre M, Ginestà M M, Moreno R, Torres-Manjon S, Moreno P, Arias-Badia M, et al. Oncolytic adenovirus with hyaluronidase activity that evades neutralizing antibodies: VCN-11. J. Control. Release. 2021;332:517–528. doi: 10.1016/j.jconrel.2021.02.035. [DOI] [PubMed] [Google Scholar]
- [80].Guedan S, Rojas J J, Gros A, Mercade E, Cascallo M, Alemany R. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol. Ther. 2010;18:1275–1283. doi: 10.1038/mt.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Schmid M, Ernst P, Honegger A, Suomalainen M, Zimmermann M, Braun L, Stauffer S, Thom C, Dreier B, Eibauer M, et al. Adenoviral vector with shield and adapter increases tumor specificity and escapes liver and immune control. Nat. Commun. 2018;9:450. doi: 10.1038/s41467-017-02707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rangaswamy U S, Cotter C R, Cheng X, Jin H, Chen Z. CD55 is a key complement regulatory protein that counteracts complement-mediated inactivation of Newcastle Disease Virus. J. Gen. Virol. 2016;97:1765–1770. doi: 10.1099/jgv.0.000498. [DOI] [PubMed] [Google Scholar]
- [83].Brunetti-Pierri N, Palmer D J, Beaudet A L, Carey K D, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- [84].Beebe D P, Cooper N R. Neutralization of vesicular stomatitis virus (VSV) by human complement requires a natural IgM antibody present in human serum. J. Immunol. 1981;126:1562–1568. doi: 10.4049/jimmunol.126.4.1562. [DOI] [PubMed] [Google Scholar]
- [85].Tesfay M Z, Ammayappan A, Federspiel M J, Barber G N, Stojdl D, Peng K W, Russell S J. Vesiculovirus neutralization by natural IgM and complement. J. Virol. 2014;88:6148–6157. doi: 10.1128/JVI.00074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Atasheva S, Emerson C C, Yao J, Young C, Stewart P L, Shayakhmetov D M. Systemic cancer therapy with engineered adenovirus that evades innate immunity. Sci. Transl. Med. 2020;12:eabc6659. doi: 10.1126/scitranslmed.abc6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bradley R R, Lynch D M, Iampietro M J, Borducchi E N, Barouch D H. Adenovirus serotype 5 neutralizing antibodies target both hexon and fiber following vaccination and natural infection. J. Virol. 2012;86:625–629. doi: 10.1128/JVI.06254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bradley R R, Maxfield L F, Lynch D M, Iampietro M J, Borducchi E N, Barouch D H. Adenovirus serotype 5-specific neutralizing antibodies target multiple hexon hypervariable regions. J. Virol. 2012;86:1267–1272. doi: 10.1128/JVI.06165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pipperger L, Koske I, Wild N, Müllauer B, Krenn D, Stoiber H, Wollmann G, Kimpel J, von Laer D, Bánki Z. Xenoantigen-dependent complement-mediated neutralization of lymphocytic choriomeningitis virus glycoprotein-pseudotyped vesicular stomatitis virus in human serum. J. Virol. 2019;93:e00567–19. doi: 10.1128/JVI.00567-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Howard F, Muthana M. Designer nanocarriers for navigating the systemic delivery of oncolytic viruses. Nanomedicine. 2020;15:93–110. doi: 10.2217/nnm-2019-0323. [DOI] [PubMed] [Google Scholar]
- [91].Huang L L, Li X, Zhang J F, Zhao Q R, Zhang M J, Liu A A, Pang D W, Xie H Y. MnCaCs-biomineralized oncolytic virus for bimodal imaging-guided and synergistically enhanced anticancer therapy. Nano Lett. 2019;19:8002–8009. doi: 10.1021/acs.nanolett.9b03193. [DOI] [PubMed] [Google Scholar]
- [92].Almstätter I, Mykhaylyk O, Settles M, Altomonte J, Aichler M, Walch A, Rummeny E J, Ebert O, Plank C, Braren R. Characterization of magnetic viral complexes for targeted delivery in oncology. Theranostics. 2015;5:667–685. doi: 10.7150/thno.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Garofalo M, Bellato F, Magliocca S, Malfanti A, Kuryk L, Rinner B, Negro S, Salmaso S, Caliceti P, Mastrotto F. Polymer coated oncolytic adenovirus to selectively target hepatocellular carcinoma cells. Pharmaceutics. 2021;13:949. doi: 10.3390/pharmaceutics13070949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hill C, Grundy M, Bau L, Wallington S, Balkaran J, Ramos V, Fisher K, Seymour L, Coussios C, Carlisle R. Polymer stealthing and mucin-1 retargeting for enhanced pharmacokinetics of an oncolytic vaccinia virus. Mol. Ther. Oncolytics. 2021;21:47–61. doi: 10.1016/j.omto.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lou X Y, Chen Z C, He Z G, Sun M C, Sun J. Bacteria-mediated synergistic cancer therapy: Small microbiome has a big hope. Nano-Micro Lett. 2021;13:37. doi: 10.1007/s40820-020-00560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Harrington K J, Kong A, Mach N, Chesney J A, Fernandez B C, Rischin D, Cohen E E W, Radcliffe H S, Gumuscu B, Cheng J, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): A multicenter, phase 1b study. Clin. Cancer Res. 2020;26:5153–5161. doi: 10.1158/1078-0432.CCR-20-1170. [DOI] [PubMed] [Google Scholar]
- [97].Kelly C M, Antonescu C R, Bowler T, Munhoz R, Chi P, Dickson M A, Gounder M M, Keohan M L, Movva S, Dholakia R, et al. Objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with pembrolizumab: A phase 2 clinical trial. JAMA Oncol. 2020;6:402–408. doi: 10.1001/jamaoncol.2019.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sun L L, Funchain P, Song J M, Rayman P, Tannenbaum C, Ko J, Mcnamara M, Diaz-Montero C M, Gastman B. Talimogene laherparepvec combined with anti-PD-1 based immunotherapy for unresectable stage III-IV melanoma: A case series. J. Immunother. Cancer. 2018;6:36. doi: 10.1186/s40425-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka R H I, Michielin O, Olszanski A J, Malvehy J, Cebon J, Fernandez E, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves Anti-PD-1 immunotherapy. Cell. 2017;170:1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Soliman H, Hogue D, Han H, Mooney B, Costa R, Lee M C, Niell B, Williams A, Chau A, Falcon S, et al. A phase I trial of talimogene laherparepvec in combination with neoadjuvant chemotherapy for the treatment of nonmetastatic triple-negative breast cancer. Clin. Cancer Res. 2021;27:1012. doi: 10.1158/1078-0432.CCR-20-3105. [DOI] [PubMed] [Google Scholar]
- [101].Wang L, Ning J F, Wakimoto H, Wu S L, Wu C L, Humphrey M R, Rabkin S D, Martuza R L. Oncolytic herpes simplex virus, and PI3K inhibitor BKM120 synergize to promote killing of prostate cancer stem-like cells. Mol. Ther. Oncolytics. 2019;13:58–66. doi: 10.1016/j.omto.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Khuri F R, Nemunaitis J, Ganly I, Arseneau J, Tannock I F, Romel L, Gore M, Ironside J, MacDougall R H, Heise C, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat. Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- [103].Jovanović B, Mayer I A, Mayer E L, Abramson V G, Bardia A, Sanders M E, Kuba M G, Estrada M V, Beeler J S, Shaver T M, et al. A randomized phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus in patients with stage II/III triple-negative breast cancer (TNBC): Responses and long-term outcome correlated with increased frequency of DNA damage response gene mutations, TNBC subtype, AR status, and Ki67. Clin. Cancer Res. 2017;23:4035–4045. doi: 10.1158/1078-0432.CCR-16-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Matuszewska K, Santry L A, van Vloten J P, AuYeung A W K, Major P P, Lawler J, Wootton S K, Bridle B W, Petrik J. Combining vascular normalization with an oncolytic virus enhances immunotherapy in a preclinical model of advanced-stage ovarian cancer. Clin. Cancer Res. 2019;25:1624–1638. doi: 10.1158/1078-0432.CCR-18-0220. [DOI] [PubMed] [Google Scholar]
- [105].Saha D, Wakimoto H, Peters C W, Antoszczyk S J, Rabkin S D, Martuza R L. Combinatorial effects of VEGFR kinase inhibitor axitinib and oncolytic virotherapy in mouse and human glioblastoma stem-like cell models. Clin. Cancer Res. 2018;24:3409–3422. doi: 10.1158/1078-0432.CCR-17-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Park A K, Fong Y, Kim S I, Yang J, Murad J P, Lu J M, Jeang B, Chang W C, Chen N G, Thomas S H, et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 2020;12:eaaz1863. doi: 10.1126/scitranslmed.aaz1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Aalipour A, Le Boeuf F, Tang M, Murty S, Simonetta F, Lozano A X, Shaffer T M, Bell J C, Gambhir S S. Viral delivery of CAR targets to solid tumors enables effective cell therapy. Mol. Ther. Oncolytics. 2020;17:232–240. doi: 10.1016/j.omto.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Nishio N, Diaconu I, Liu H, Cerullo V, Caruana I, Hoyos V, Bouchier-Hayes L, Savoldo B, Dotti G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014;74:5195–5205. doi: 10.1158/0008-5472.CAN-14-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Blake Z, Marks D K, Gartrell R D, Hart T, Horton P, Cheng S K, Taback B, Horst B A, Saenger Y M. Complete intracranial response to talimogene laherparepvec (T-Vec), pembrolizumab and whole brain radiotherapy in a patient with melanoma brain metastases refractory to dual checkpointinhibition. J. Immunother. Cancer. 2018;6:25. doi: 10.1186/s40425-018-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kieran M W, Goumnerova L, Manley P, Chi S N, Marcus K J, Manzanera A G, Polanco M L S, Guzik B W, Aguilar-Cordova E, Diaz-Montero C M, et al. Phase I study of genemediated cytotoxic immunotherapy with Adv-tk as adjuvant to surgery and radiation for pediatric malignant glioma and recurrent ependymoma. Neuro Oncol. 2019;21:537–546. doi: 10.1093/neuonc/noy202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Mao L J, Kan Y, Li B H, Ma S, Liu Y R, Yang D L, Yang C H. Combination therapy of prostate cancer by oncolytic adenovirus harboring interleukin 24 and ionizing radiation. Front. Oncol. 2020;10:421. doi: 10.3389/fonc.2020.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Roulstone V, Pedersen M, Kyula J, Mansfield D, Khan A A, McEntee G, Wilkinson M, Karapanagiotou E, Coffey M, Marais R, et al. BRAF- and MEK-targeted small molecule inhibitors exert enhanced antimelanoma effects in combination with oncolytic reovirus through ER stress. Mol. Ther. 2015;23:931–942. doi: 10.1038/mt.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kuryk L, Møller A S W, Garofalo M, Cerullo V, Pesonen S, Alemany R, Jaderberg M. Antitumor-specific T-cell responses induced by oncolytic adenovirus ONCOS-102 (Adv5/3-D24-GM-CSF) in peritoneal mesothelioma mouse model. J. Med. Virol. 2018;90:1669–1673. doi: 10.1002/jmv.25229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhang F, Le T, Wu X, Wang H, Zhang T, Meng Y F, Wei B J, Soriano S S, Willis P, Kolokythas O, et al. Intrabiliary RF heat-enhanced local chemotherapy of a cholangiocarcinoma cell line: Monitoring with dual-modality imaging-preclinical study. Radiology. 2014;270:400–408. doi: 10.1148/radiol.13130866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Bourgeois-Daigneault M C, Roy D G, Aitken A S, El Sayes N, Martin N T, Varette O, Falls T, St-Germain L E, Pelin A, Lichty B D, et al. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci. Transl. Med. 2018;10:eaao1641. doi: 10.1126/scitranslmed.aao1641. [DOI] [PubMed] [Google Scholar]
- [116].Song J J, Zhang F, Ji J S, Chen M J, Li Q, Weng Q Y, Gu S N, Kogut M J, Yang X M. Orthotopic hepatocellular carcinoma: Molecular imaging-monitored intratumoral hyperthermia-enhanced direct oncolytic virotherapy. Int. J. Hyperthermia. 2019;36:343–349. doi: 10.1080/02656736.2019.1569731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ngwa V M, Edwards D N, Philip M, Chen J. Microenvironmental metabolism regulates antitumor immunity. Cancer Res. 2019;79:4003–4008. doi: 10.1158/0008-5472.CAN-19-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Liu Z Q, Ravindranathan R, Kalinski P, Guo Z S, Bartlett D L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 2017;8:14754. doi: 10.1038/ncomms14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Topalian S L, Drake C G, Pardoll D M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Chen D S, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- [121].Lichty B D, Breitbach C J, Stojdl D F, Bell J C. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- [122].June C H, Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].O’Cathail S M, Pokrovska T D, Maughan T S, Fisher K D, Seymour L W, Hawkins M A. Combining oncolytic adenovirus with radiation—A paradigm for the future of radiosensitization. Front. Oncol. 2017;7:153. doi: 10.3389/fonc.2017.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Golden E B, Frances D, Pellicciotta I, Demaria S, Barcellos-Hoff M H, Formenti S C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ottolino-Perry K, Diallo J S, Lichty B D, Bell J C, McCart J A. Intelligent design: Combination therapy with oncolytic viruses. Mol. Ther. 2010;18:251–263. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Udayakumar T S, Betancourt D M, Ahmad A, Tao W S, Totiger T M, Patel M, Marples B, Barber G, Pollack A. Radiation attenuates prostate tumor antiviral responses to vesicular stomatitis virus containing IFNβ, resulting in pronounced antitumor systemic immune responses. Mol. Cancer Res. 2020;18:1232–1243. doi: 10.1158/1541-7786.MCR-19-0836. [DOI] [PubMed] [Google Scholar]
- [127].Vijayakumar G, Palese P, Goff P H. Oncolytic Newcastle disease virus expressing a checkpoint inhibitor as a radioenhancing agent for murine melanoma. EBioMedicine. 2019;49:96–105. doi: 10.1016/j.ebiom.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zhang H J, Wang F, Mao C J, Zhang Z C, Fu S J, Lu J Z, Zhai Z X, Li R J, Li S W, Rodriguez R, et al. Effect of combined treatment of radiation and tissue-specific recombinant oncolytic adenovirus on bladder cancer cells. Int. J. Radiat. Biol. 2017;93:174–183. doi: 10.1080/09553002.2017.1231942. [DOI] [PubMed] [Google Scholar]


