Abstract
Coronavirus disease-19 (COVID-19) is a global pandemic that is caused by COVID-19 virus, which was initially identified in December 2019 in Wuhan, China. Vaccination is one of the most effective public health interventions, and soon after the Pfizer/BioNTech (BNT162b2) vaccine became available late in 2020, it began to be actively used to fight against COVID-19. Since then, cases of vaccine-associated immune-mediated diseases (IMDs) have been reported. There have been few cases of IMD flare-ups or onset after COVID-19 vaccine administration, and emerging IMDs may be identified over next few years after high use of this vaccine. To this day, few cases of newly diagnosed systemic lupus erythematosus (SLE) following COVID-19 vaccine exposure were reported. Herein, we present the case of a patient diagnosed with SLE, acute pancreatitis, and vasculitic skin rash on the extremities 1 week after the first dose of the Pfizer-BioNTech COVID-19 vaccine.
|
Key Point • COVID-19 Vaccine induced Systemic Lupus Erythematosus. |
Keywords: COVID-19, Pfizer/BioNTech, Systemic lupus erythematosus, Vaccination
Introduction
In December 2019, a new RNA virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was isolated in clusters of pneumonia cases in Wuhan, China, and this virus was named coronavirus disease-19 (COVID-19) [1]. This highly infectious, rapidly transmitted disease with a high mortality rate was declared a pandemic by the World Health Organization in March 2020 [2]. On December 2020, the US Food and Drug Administration (FDA) approved using the Pfizer-BioNTech mRNA COVID-19 vaccine to help overcome this pandemic [3]. This vaccine’s safety profile was reassuring, but emerging concerns about cross-reactivity with human tissue may have contributed to the development of vaccine-associated immune-mediated diseases (IMDs) such as vaccine-induced immune thrombotic thrombocytopenia, autoimmune liver disease, IgA nephropathy, rheumatoid arthritis, and systemic lupus erythematosus (SLE) [4, 5]. SLE is an autoimmune disease that appears to be associated with some vaccine administration; a recent investigational cross-sectional study (the international VACOLUP study) that examined vaccine tolerability in SLE patients showed reassuring signals regarding vaccine tolerability with a minimal risk of a disease flare-up [6]. Another observational study that examines the side effect and flares risk after Pfizer-BioNTech mRNA COVID-19 vaccination showed mild side effects of vaccination and minimal risk for flare [7]. To date, new SLE cases after COVID-19 vaccines have occurred in three patients [8–10]. Herein, we describe another unique and rare presentation of a newly developed SLE case following the Pfizer-BioNTech COVID-19 vaccine.
Case report
Clinical information
A 22-year-old single Saudi female university student who had no known chronic medical illnesses presented to the emergency department at King Khaled Hospital in the Kingdom of Saudi Arabia with epigastric abdominal pain that radiated to her back and was associated with nausea, vomiting, and erythematous non-blanching maculopapular rashes over her extremities 1 week after receiving the first dose of the Pfizer-BioNTech COVID-19 vaccine (Fig. 1). She had no other complaints such as fever, joint pain, oral ulcers, hair loss, or recent illnesses. She had no history of gall stones or renal stones, and she denied exposure to alcohol and medications. She had no family history of autoimmune diseases or similar conditions. Clinical examination results indicated that she was conscious, alert, and oriented with mild distress due to her abdominal pain. Her vital signs were normal with a temperature of 37 °C, pulse of 80 beats per minute, and blood pressure of 118/75 mmHg. Skin examination showed erythematous non-blanchable papules over her extremities including hands, legs, and ears with observed hyperpigmentation, no joint swelling or tenderness, no nasal or mouth ulcers, and no lymph node enlargement as well as an abdomen that was soft and lax with mild epigastric tenderness with no observed organ enlargement. Cardiopulmonary and neurological examination results were unremarkable.
Fig. 1.
Vasculitic skin rash on the patient’s upper and lower extremities
Diagnostic assessment
The laboratory test results were as follows: complete blood count (CBC) showed leukopenia (mainly lymphopenia) and white blood cell (WBC) levels were 1300/µL (normal range 4500–11,000/μL); hemolytic anemia (hemoglobin [Hb%], 9.6 mg/dL, normal range 13.5–17.5 mg/dL); a positive direct Coombs test; reticulocytes were 3.5% (normal range 0.5–2.5%) with high LDH level 308 U/L (normal range 140–280 U/L); and thrombocytopenia (platelet [PLT] count, 36,000/µL; normal range, 150,000–450,000/µL). Liver enzyme levels were increased (aspartate aminotransferase [AST], 301 IU/L, normal range 9–32 IU/L; and alanine aminotransferase [ALT], 81 IU/L, normal range 19–25 IU/L) and indirect bilirubin level was 2.1 mg/dL (normal range 0.2–0.8 mg/dL), pancreatic enzyme levels were mildly increased (amylase, 181 U/L, normal range 30–110 U/L; and lipase, 185 U/L, normal range 10–140 U/L), while creatinine levels and urinalysis results were normal. Inflammatory marker results were as follows: erythrocyte sedimentation rate (ESR) was increased (65 mm/h, normal range 0–20 mm/h), while the C-reactive protein (CRP) and ferritin levels were normal. Additionally, hypocomplementemia was noted (complement 3 [C3], 0.096, normal range 0.8–1.6 g/dL; and C4, 0.067, normal range 1.3–7.5 g/dL).
Immunological test results were as follows: immunofluorescence anti-nuclear antibody (ANA) results were positive; there was a homogenous pattern with a titer of 1/1200; and double strand deoxyribonucleic acid (dsDNA) results were positive with a titer of 1:160. Other antibody test results including rheumatoid factor (RF), anti-smooth muscle (ASMA), anti-smith, anti-histone, and cyclic citrullinated peptide (CCP) antibodies were negative. Blood cultures and transthoracic echocardiography results were negative. COVID-19, hepatitis B and C, and human immunodeficiency virus polymerase chain reaction (PCR) results were also negative. Peripheral blood film results showed no abnormal cells, and bone marrow aspirate results showed maturation arrest in the myeloid series and a decrease in the erythroid and megakaryocytes series.
A contrasted abdominal computerized tomography (CT) scan showed a slightly bulky pancreas with a loss of normal lobulation, which is suggestive of autoimmune pancreatitis (Fig. 2) [11]. Based on the 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus, our patient met the SLE diagnostic criteria, which include positive ANA as entry criterion, leukopenia, autoimmune hemolytic anemia, thrombocytopenia, positive anti-dsDNA, and low complement C4 and C3 levels [12]. According to the American College of Gastroenterology guideline: management of acute pancreatitis, she is diagnosed with acute pancreatitis based on the presence of the classical presentation of acute pancreatitis and supported by radiological findings that featured with an autoimmune picture despite her mild elevation of pancreatic enzymes and absence of IgG4 level [13]. Conjunction of both acute pancreatitis and SLE tends us toward considering it as a highly possible mild autoimmune pancreatitis and were treated accordingly.
Fig. 2.
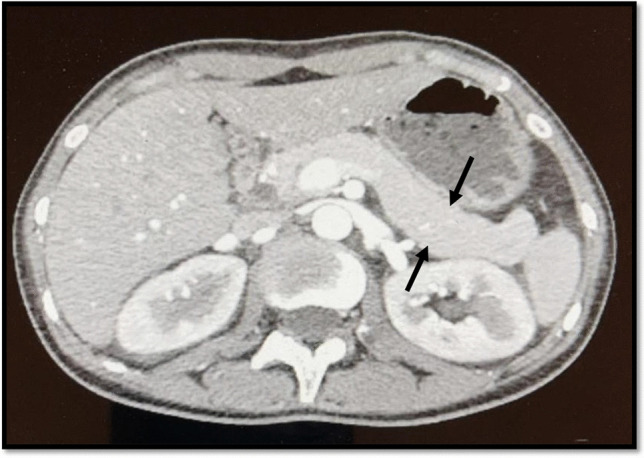
Abdominal CT with contrast showing a slightly bulky (edematous) pancreas with loss of normal lobulation, which is suggestive of autoimmune pancreatitis
Therapeutic intervention
The patient received an intravenous infusion of methylprednisone 500 mg for 3 days followed by a tapering regimen of oral prednisone with a starting dose of 40 mg daily intended to be tapered after 4 weeks in addition to 200 mg hydroxychloroquine and 50 mg azathioprine daily.
Three days later, the patient’s abdominal pain and nausea were resolved and skin rashes were stabilized. Repeated labs showed improvement in the CBC, amylase, lipase, and liver profile results (Table 1). She was discharged home 1 week after admission. The patient was assessed at a follow-up clinic 2 weeks later using introductory laboratory, complement, and protein creatinine ratio tests. Her skin rashes were completely resolved and her laboratory tests showed normal CBC, C3, and C4 results and unremarkable urinalysis results.
Table 1.
Pre and post-treatment laboratory results showing an improvement after treatment
| Investigation | Pre-treatment results | Post-treatment results (3 days later) | Reference range |
|---|---|---|---|
| White blood cells | 1300/µL | 5580/µL | 4500–11,000/μL |
| Hemoglobin | 9.6 mg/dL | 10.5 mg/dL | 13.5–17.5 mg/dL |
| Platelet count | 36,000/µL | 84,000/µL | 150,000–450,000/µL |
| Alanine aminotransferase | 81 IU/L | 10 IU/L | 19–25 IU/L |
| Aspartate aminotransferase | 301 IU/L | 16 IU/L | 9–32 IU/L |
| Amylase | 181 U/L | 121 U/L | 30–110 U/L |
| Lipase | 185 U/L | 64 U/L | 10–140 U/L |
Literature review
We performed a systematic English literature search in Pubmed/Medline, Embase, Cochrane, and Google Scholar databases search using the key words including “systemic lupus erythematosus,” “SLE,” “lupus nephritis,” “COVID-19 vaccine,” “SARS-CoV2,” and “Vaccine induced systemic lupus erythematosus” to identify all available articles published online between December 2019 and January 2, 2022. And we systematically searched the reference sections of these articles for further references. All retrieved articles were screened by the title and abstract, and eligible ones were selected for full-text review. Case reports of patients with newly diagnosed SLE following any type of COVID-19 vaccination were selected for inclusion. Review articles with no clinical case reports were excluded. Publications were excluded if they did not fulfill the above criteria or were not available in English. We reviewed 3 reports and we summarized their demographics, clinical presentation, vaccine type, timing of symptoms onset following vaccine exposure, laboratory findings, and received treatment regimen (Table 2).
Table 2.
Summary of published cases of COVID-19-induced SLE
| Authors | Age in years and sex | Clinical presentation | Vaccine type | Appearing of symptoms after vaccine exposure | Investigations result | Therapeutic intervention | Serology result |
|---|---|---|---|---|---|---|---|
| Nune et al. [8] | 24 male | Polyarthralgia Fever Fatigue Oral ulcer Lymphadenopathy | Pfizer-BioNTech SARS-CoV-2 vaccine | 14 days after 2nd dose | Lymphopenia High CRP | GC, MTX | Positive ANA and anti-dsDNA low complements (C3&4) levels |
| Patil and Patil [9] | 22 female | Polyarthralgia Fever Skin rash Bipedal edema Lymphadenopathy Mild hepatomegaly | AstraZeneca COVID-19 vaccine | 10 days after the 2nd dose | Thrombocytopenia Anemia Positive Direct Coombs test High ESR Proteinuria (300 mg/24 h) | GC, HCQ, MMF | Positive ANA and anti-dsDNA |
| Zavala-Miranda et al. [10] | 23 female | Nephrotic syndrome Hair loss | AstraZeneca COVID-19 vaccine | 7 days after the 1st dose | Lymphopenia Proteinuria (12.6 g/24 h) Renal biopsy showed class V lupus nephritis | GC, MMF, HCQ, Diuretics | Positive ANA and anti-dsDNA low complements (C3&4) levels |
| Current case | 22 female | Skin rash Acute pancreatitis | Pfizer-BioNTech SARS-CoV-2 vaccine | 7 days after the 1st dose | Lymphopenia Anemia Thrombocytopenia Positive Direct Coombs test High amylase and lipase High ESR | GC, HCQ, AZA | Positive ANA and anti-dsDNA low complements (C3&4) levels |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ANA, antinuclear antibodies; Anti-dsDNA, anti-double-stranded DNA antibody; GC, glucocorticoids; MTX, methotrexate; HCQ, hydroxychloroquine; MMF, mycophenolate mofetil; AZA, azathioprine
Discussion
Twenty-seven cases of IMDs after COVID-19 vaccination were presented in May 2021 by Watad et al. Most of these patients had pre-exciting rheumatic diseases. None of the new IMDs was SLE, and the average time for new IMD symptoms to develop after vaccine exposure was 4 days [4]. In our case, the development of new vasculitic rashes on the extremities with hematological abnormalities including leukopenia, hemolytic anemia, thrombocytopenia, and positive immunological tests including ANA and anti-dsDNA soon after the first Pfizer/BioNTech vaccine dose suggests the possibility of an autoimmune response rather than a preexisting SLE flare-up.
We reported here the first case to report a possible association between the Pfizer/BioNTech vaccine and the development of SLE with autoimmune pancreatitis. However, our search found three other possible cases of COVID-19-induced SLE (Table 2). New development of SLE with musculoskeletal features and lymphadenitis after a COVID-19 vaccine was reported by Nune et al. in one Caucasian man who was diagnosed based on the presence of polyarthritis, oral ulcers, leukopenia, lymphopenia, strongly positive ANA and anti-dsDNA results, and low complement C4 and C3 levels [8]. The other case reported by Patil and Patil also shares the same clinical manifestation despite a different type of received vaccine [9]. Renal involvement in term of biopsy proven class V lupus nephritis following COVID-19 vaccination had been also reported by Zavala-Miranda et al. [10]. All reported cases developed in a young age (22–24 year) and three out of four cases (75%) were females.
Many factors may provide unique challenges in SLE patients’ response to COVID-19 vaccines; lymphopenia and thrombocytopenia are the most prevalent hematological manifestations in SLE, which have also been well documented as adverse effects of vaccines [14]. There is also an overlap between SLE-specific criteria and vaccine-induced autoimmunity reactions such as hematological and clinical manifestations including vasculitis and autoimmune pancreatitis [15].
Acute pancreatitis as a side effect after Pfizer/BioNTech vaccine exposure has only been reported in a few vaccine recipients, and none of these cases were reported as autoimmune pancreatitis. Since the extensive long-term post-marketing surveillance that will elaborate upon rare side effects is not available, we proposed that the development of autoimmune pancreatitis in our patient was a SLE-related manifestation rather than a post-vaccine exposure side effect which coincidently occurs with newly manifested SLE [15].
The VACOLUP study suggested that COVID-19 vaccination was well tolerated in patients with SLE, and it showed only a minimal risk of flare-up (3%), which typically occurred with predominant musculoskeletal symptoms and fatigue [6]. Exposure to the Pfizer/BioNTech vaccine in our case may contribute to either a new SLE or a pre-clinical SLE flare-up.
The potential pathophysiology of which the mRNA Pfizer/BioNTech vaccine can induce autoimmune disease like SLE could be related to the vaccine adjuvants that trigger the NLR pyrin domain containing 3 (NLRP3) inflammasome which plays a major role in innate and adaptive immune system [5].
Despite the huge number of exposed populations to the COVID-19 vaccine and the rarity of developing SLE in the proper age group following vaccine exposure, the causatively or flare-up-associated risk secondary to vaccines exposure and development of SLE clinical manifestations increases concern about the safety of such vaccines in pre-diagnosed and diagnosed SLE patients. However, this case does not necessarily demonstrate a cause-effect relationship between the COVID-19 vaccines and SLE and future clinical trials and registry data may be required to determine this association.
Conclusion
Vaccination campaigns are increasing as the demand for a rapid resolution of the COVID-19 pandemic also increases. Although cases of SLE following the vaccines exposure are rare, we emphasize the importance of clinical vigilance for unusual clinical SLE presentations such as autoimmune pancreatitis.
Acknowledgements
The authors would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project. The authors also thank American Manuscript Editors (www.americanmanuscripteditors.com) for English language editing.
Author contribution
Alrashdi Mousa N: conceptualization. Alanazi Majed Saleh: conceptualization. Almoaqly Khalid: writing — original draft, visualization, project administration. Abdulrahman Khaled Alshaya: writing — original draft. Alrashdi Mousa N: writing — review & editing. Sultan Mahja Marzouq Alanazi: writing — review & editing. Alanazi Majed Saleh: writing — review & editing. Alrashdi Mousa N: supervision. Alrashdi Mousa N and Almoaqly Khalid did the literature review and drafted the manuscript. Almoaqly Khalid, Alrashdi Mousa N, Sultan Mahja Marzouq Alanazi, Alanazi Majed Saleh, and Abdulrahman Khaled Alshaya have critically revised and finalized the manuscript. All authors have approved the final version of the manuscript.
Declarations
Consent to participate
Informed consent was obtained from the patient for publication.
Disclosures
None.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30]. Lancet 395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed]
- 2.World Health Organization. Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. http://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed 12 Feb 2020
- 3.Emergency use authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus. Fact sheet for healthcare providers administering vaccine. https://www.fda.gov/media/144413/download. Accessed 23 Aug 2021
- 4.Watad A, De Marco G, Mahajna H et al (2021) Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel). 9(5):435. 10.3390/vaccines9050435 [DOI] [PMC free article] [PubMed]
- 5.Chen Y, Xu Z, Wang P, Li X-M, Shuai Z-W, Ye D-Q, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;00:1–16. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 6.Felten R, Kawka L, Dubois M, et al. Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study. Lancet Rheumatol. 2021;3(9):e613–e615. doi: 10.1016/S2665-9913(21)00221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.E Zavala-Flores J Salcedo-Matienzo A Quiroz-Alva et al (2021) Side effects and flares risk after SARS-CoV-2 vaccination in patients with systemic lupus erythematosus. Clin Rheumatol 10.1007/s10067-021-05980-5 [DOI] [PMC free article] [PubMed]
- 8.Nune A, Iyengar KP, Ish P, Varupula B, Musat CA, Sapkota HR. The emergence of new-onset SLE following SARS-CoV-2 vaccination. QJM. 2021;114(10):739–740. doi: 10.1093/qjmed/hcab229. [DOI] [PubMed] [Google Scholar]
- 9.Patil S, Patil A. Systemic lupus erythematosus after COVID-19 vaccination: a case report. J Cosmet Dermatol. 2021;20(10):3103–3104. doi: 10.1111/jocd.14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zavala-Miranda MF, González-Ibarra SG, Pérez-Arias AA, Uribe-Uribe NO, Mejia-Vilet JM. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. 2021;100(6):1340–1341. doi: 10.1016/j.kint.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chari ST, Takahashi N, Levy MJ, et al. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7(10):1097–1103. doi: 10.1016/j.cgh.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019;71(9):1400–1412. doi: 10.1002/art.40930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenner, S, Baillie J, DeWitt J, Vege, SS, FACG4 (2013) American College of Gastroenterology Guideline: Management of acute pancreatitis. Am J Gastroenterol 108(9):1400–1415. 10.1038/ajg.2013.218. Erratum in: Am J Gastroenterol (2014), 109(2):302. PMID: 23896955 [DOI] [PubMed]
- 14.Martin M, Guffroy A, Argemi X, Martin T. Lupus érythémateux systémique et lymphopénie: aspects cliniques et physiopathologiques [Systemic lupus erythematosus and lymphopenia: Clinical and pathophysiological features] Rev Med Interne. 2017;38(9):603–613. doi: 10.1016/j.revmed.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Parkash O, Sharko A, Farooqi A, Ying GW, Sura P (2021) Acute pancreatitis: a possible side effect of COVID-19 vaccine. Cureus 13(4):e14741. 10.7759/cureus.14741. PMID: 34084669; PMCID: PMC8163516 [DOI] [PMC free article] [PubMed]



