Abstract
Vibrio parahaemolyticus is a halophilic bacterium frequently involved in human outbreaks of seafood-associated gastroenteritis. For epidemiological purposes, different molecular typing methods, such as pulsed-field gel electrophoresis (PFGE) or ribotyping, have been developed for this pathogen; however, these methods are mostly labor-intensive and time-consuming. In this work, we designed and evaluated three rapid PCR typing methods for this pathogen using primers designed on the basis of the following specific sequences: conserved ribosomal gene spacer sequence (RS), repetitive extragenic palindromic sequence (REP), and enterobacterial repetitive intergenic consensus sequence (ERIC). Typing patterns and clustering analysis indicated that these methods apparently differentiated V. parahaemolyticus strains from reference strains of interspecific Escherichia coli, V. cholerae, and V. vulnificus and were also valuable in subspecies typing of this pathogen. Forty domestic strains of V. parahaemolyticus, representing a wide range of PFGE patterns, were grouped into 15, 27, and 27 patterns, with discrimination indexes of 0.91, 0.97, and 0.98, by RS-, REP-, and ERIC-PCR, respectively. The discriminative abilities of these PCR methods closely approached or even exceeded those of PFGE and ribotyping. REP-PCR is preferable to ERIC-PCR because of the greater reproducibility of its fingerprints, while RS-PCR may be a practical method because it generates fewer amplification bands and patterns than the alternatives.
Vibrio parahaemolyticus is a halophilic gram-negative bacterium that causes acute gastroenteritis in humans. Food poisoning caused by this pathogen is generally associated with the consumption of contaminated seafood; this organism is a crucial food-borne pathogen in Taiwan, Japan, and other coastal countries with high rates of seafood consumption (17). Clinical manifestations include diarrhea, abdominal cramps, nausea, vomiting, headache, fever, and chills, with incubation periods ranging from 4 to 96 h (4, 9).
Isolates of V. parahaemolyticus can be differentiated by serotyping, and 13 O groups and 71 K types have been identified (7). Serotyping is generally unable to differentiate all isolates originating from different regions or sources. Reliable molecular methods for strain typing would significantly aid epidemiological investigations. Recently, several molecular methods were developed for the subspecies typing of V. parahaemolyticus, namely, pulsed-field gel electrophoresis (PFGE) (33), ribotyping (29), and random amplified polymorphic DNA (RAPD) analysis (30). The PFGE method using SfiI digestion is reliable, achieves high discrimination efficiency, and has been applied to typing of V. parahaemolyticus strains in many situations, such as the first pandemic O3:K6 strains (32), food poisoning outbreaks (28), environmental strains from seafood (31), and nosocomial outbreaks (12). However, the whole process takes several days to complete. Compared with PFGE, RAPD analysis has the merits of being less labor-intensive and faster to complete (30). Nevertheless, RAPD analysis, or the arbitrarily primed PCR method, which is based on short oligonucleotide primers, is impaired by lower discrimination efficiency (16, 30) and is complicated by variations in band intensity and the lack of reproducibility of certain minor bands (21).
By using a 22-mer primer specific for the enterobacterial repetitive intergenic consensus sequence (ERIC), Marshall et al. found that ERIC-PCR is useful for evaluating genetic and epidemiological relationships among V. parahaemolyticus strains (14). Besides ERIC-PCR, PCR methods based on the highly conserved ribosomal gene spacer sequence (RS) and the 38-bp repetitive extragenic palindromic sequence (REP) in Enterobacteriaceae and other bacteria have been used for the typing of pathogenic bacteria (25, 26). To develop a reliable rapid subspecies typing method for V. parahaemolyticus, the application of these three PCR methods (RS-, REP-, and ERIC-PCR) for typing 41 strains representing different PFGE patterns was evaluated.
MATERIALS AND METHODS
Bacterial strains.
Forty strains of V. parahaemolyticus isolated from outbreaks in Taiwan during 1993 and 1994 and representing different PFGE patterns were analyzed here (28). Clinical strain ST550, O4:K13 and Kanagawa phenomenon positive and originating from Japan, was used as a reference strain (34). Escherichia coli JM109, V. cholerae 569B, and V. vulnificus CCRC12905 were used as interspecies reference strains. These bacterial cultures were stored at −80°C in tryptic soy broth (Difco Laboratories, Detroit, Mich.) containing 20% glycerol with no supplementary NaCl for E. coli and 3% NaCl for the Vibrio cultures. The Vibrio stock cultures were incubated in tryptic soy broth–3% NaCl at 37°C, agitated at 160 rpm for about 16 h, and streaked on tryptic soy agar–3% NaCl. The E. coli stock was cultured in Luria-Bertani broth medium (Difco) at 37°C, shaken at 160 rpm for about 16 h, and streaked on Luria-Bertani medium with 1.5% agar.
Preparation of genomic DNA.
Colonies on agar plates were picked, and their genomic DNA was isolated by the small-scale preparation method of Sambrook et al. (20), suspended in 10 mM Tris hydrochloride buffer–1 mM EDTA (pH 7.5), and stored at −20°C until required.
PCR primers.
Three sets of amplification oligonucleotide primers were synthesized. For RS-PCR, a pair of 15-mer primers (L1, 5′-CAA GGC ATC CAC CGT-3′, and G1, 5′-GAA GTC GTA ACA AGG-3′) was designed on the basis of the spacer sequences of 16S and 23S ribosomal DNAs (8). For REP-PCR, the primers contained multiple nucleotides at ambiguous positions in the consensus REP. The following pair of 18-mer primers was used for REP-PCR: REP-1D, 5′-NNN RCG YCG NCA TCM GGC-3′, and REP-2D, 5′-RCG YCT TAT CMG GCC TAC-3′, where M is A or C, R is A or G, Y is C or T, and N is any nucleotide (25). For ERIC-PCR, a pair of 22-mer primers (ERIC1R, 5′-ATG TAA GCT CCT GGG GAT TCA C-3′, and ERIC2, 5′-AAG TAA GTG ACT GGG GTG AGC G-3′) was designed on the basis of the core repeated sequence of ERIC (27).
Amplification conditions.
Optimized PCR conditions were developed to produce reproducible fingerprints for V. parahaemolyticus strains. V. parahaemolyticus strain ST550 was used as a reference strain in every PCR experiment and was resolved in every electrophoresis gel, while the PCR assays were repeated three times with other V. parahaemolyticus strains to ensure reproducibility. PCR amplifications were conducted with a buffer (50 mM KCl, 1.5 mM MgCl2, 10 mM Tris HCl [pH 8.8], 1% Triton X-100) containing 200 μM each dATP, dCTP, dGTP, and dTTP, 50 pmol of primers, and 100 ng of template DNA in a final volume of 50 μl. Amplification was performed with a thermal cycler, Personal Cycler 20 (Biometra Biomedizinische Analytik Gmbh, Gottingen, Germany). All manipulations were conducted using dedicated DNA-free pipettes in a sterile field to minimize contamination risk. The reaction mixture was overlaid with a drop of sterile mineral oil and incubated in the thermal cycler at 95°C for 7 min. Then, 1.0 U of DyNAZyme II thermostable DNA polymerase (Finnzymes Oy, Espoo, Finland) was added, and the mixture was amplified at different temperature settings. RS-PCR was performed via denaturation at 90oC for 30 s, annealing at 55oC for 1 min, and extension at 70oC for 5 min; REP-PCR was performed via denaturation at 90oC for 30 s, annealing at 45oC for 1 min, and extension at 65oC for 5 min; and ERIC-PCR was performed via denaturation at 90oC for 30 s, annealing at 52oC for 1 min, and extension at 70oC for 5 min. Following 30 reaction cycles, all the reaction mixtures were further incubated at 70oC for an additional 10 min.
Gel electrophoresis.
Following PCR, 10 μl of the reaction mixture was mixed with 2 μl of loading buffer (20). The mixture was electrophoresed in a horizontal 2% agarose gel (10 by 15 cm) in Tris-borate buffer at 100 V for 30 min. The process was continued at 75 V until the bromophenol blue tracking dye approached the front of the running gel. The amplified DNA bands were visualized following ethidium bromide staining and photographed under UV light. A mixture of lambda DNA digested with HindIII and φX174 DNA digested with HaeIII (Finnzymes) was used to mark molecular masses.
Similarities among patterns.
The size of each band was determined via Stratascan 7000 densitometry with one-dimensional analysis software (Stratagene, La Jolla, Calif.). Data were coded as 0 (negative) or 1 (positive). Following the method described by Martin-Kearley et al. (15), hierarchical cluster analysis was performed using the average linkage method with the squared Euclidean distance measure. The dendrogram was produced using the program SPSS for Windows, Release 6.0 (SPSS Inc., Chicago, Ill.) (15, 30). Finally, the discriminative abilities of different typing methods were calculated using the method of Hunter and Gaston (6).
RESULTS
The 40 domestic strains of V. parahaemolyticus used here have been previously examined and grouped into 22, 20, or 8 patterns through PFGE, ribotyping, or the RAPD method, respectively (Table 1). Strains with differences of one or more amplification bands were differentiated into different patterns here.
TABLE 1.
Subspecies typing patterns determined for different strains of V. parahaemolyticus by different molecular methodsa
| Pattern determined by:
|
Strain | Location of isolation | Date of isolation (yr/mo/day) | Serotype | |||||
|---|---|---|---|---|---|---|---|---|---|
| RS-PCR | ERIC-PCR | REP-PCR | PFGEb | Ribotypingb | RAPD analysisb | ||||
| A1 | K | P | D3 | A7 | C5 | 677 | Taipei | 1994/6/30 | K8 |
| A1 | K | P | D3 | A7 | C5 | 679 | Peng-Hu | 1994/6/30 | K8 |
| A1 | K | P | D3 | A7 | C5 | 680 | Peng-Hu | 1994/6/30 | K8 |
| A2 | F1 | F2 | B2 | E5 | B1 | 323 | Miao-Li | Unknown | ND |
| A2 | G1 | E | A5 | E2 | D1 | 166 | Taichung | 1992/10/5 | K29 |
| A2 | G2 | D2 | A5 | G2 | C3 | 168 | Taipei | 1992/9/28 | ND |
| A2 | H1 | D1 | A4 | F1 | D2 | 302 | Miao-Li | 1993/6/14 | K29 |
| A2 | I | G | A6 | F2 | C3 | 197 | Kaohsiung | 1992/10/22 | ND |
| A2 | J | C1 | E1 | C3 | C5 | 283 | Kaohsiung | 1993/7/4 | K3 |
| A3 | B3 | I2 | ND | ND | ND | ST550 | Japan | Unknown | K13 |
| A3 | G3 | I1 | G2 | E1 | B2 | 134 | Kaohsiung | 1992/9/20 | ND |
| A3 | L | P | D3 | A7 | C5 | 690 | Peng-Hu | 1994/6/30 | ND |
| A3 | N | Q | C4 | B2 | E1 | 757 | Tai-nan | 1994/12/13 | K68 |
| A3 | N | Q | C4 | B2 | E1 | 758 | Tai-nan | 1994/12/13 | K68 |
| A3 | N | Q | C4 | B2 | E1 | 759 | Tai-nan | 1994/12/13 | K68 |
| A3 | P | P | B3 | A2 | C5 | 742 | Ping-Tung | 1994/10/15 | K8 |
| A4 | Q | N | E2 | F1 | D2 | 554 | Taichung | 1993/10/2 | K29 |
| B | A1 | L1 | O1 | C2 | B1 | 402 | Tai-nan | 1993/7/28 | K12 |
| B | A1 | L3 | E3 | A5 | B1 | 355 | Taipei | 1993/6/27 | ND |
| B | A2 | L1 | O1 | C2 | B1 | 403 | Tai-nan | 1993/7/28 | K12 |
| B | M | N | A6 | F1 | D2 | 415 | Chia-Yi | 1993/7/29 | K29 |
| B | O1 | M | C3 | A1 | E1 | 418 | Chia-Yi | 1993/7/29 | ND |
| B | O2 | M | C5 | G1 | E1 | 436 | Yun-lin | 1993/8/3 | K60 |
| C | H1 | D1 | A4 | F1 | D2 | 304 | Miao-Li | 1993/6/14 | K29 |
| C | H1 | N | A6 | F1 | D2 | 314 | Miao-Li | 1993/6/14 | K29 |
| D1 | F2 | A2 | H1 | E3 | C5 | 325 | Miao-Li | Unknown | ND |
| D1 | P | R | H3 | E3 | E1 | 718 | Tao-Yuan | 1994/9/16 | K41 |
| D1 | P | R | H1 | E3 | E1 | 720 | Tao-Yuan | 1994/9/16 | K41 |
| D2 | C1 | A1 | H1 | E3 | E1 | 199 | Taipei | 1992/10/2 | K41 |
| E | A1 | H | H1 | I | B2 | 182 | Tai-Tung | 1992/10/16 | ND |
| F | E | J | B2 | H1 | C5 | 272 | Kaohsiung | 1993/1/4 | K6 |
| G | D | F1 | C2 | A2 | C3 | 145 | Kaohsiung | 1992/9/26 | ND |
| H1 | B1 | L2 | B1 | A3 | C3 | 364 | Kaohsiung | 1993/7/11 | ND |
| H1 | B2 | K2 | B1 | A3 | C3 | 626 | Kaohsiung | 1994/4/15 | ND |
| H1 | C2 | K1 | G3 | D2 | C3 | 473 | Kaohsiung | 1993/8/10 | K15 |
| H1 | C2 | K1 | B1 | A3 | C3 | 474 | Kaohsiung | 1993/8/10 | K15 |
| H1 | C2 | K1 | B1 | A3 | C3 | 487 | Kaohsiung | 1993/8/10 | K15 |
| H1 | C3 | O | J | G2 | E1 | 434 | Yun-lin | 1993/8/3 | K60 |
| H2 | G1 | S | F3 | E1 | C3 | 135 | Kaohsiung | 1992/9/26 | ND |
| I | ND | B | B1 | A3 | C4 | 702 | Kaohsiung | 1994/7/30 | ND |
| J | H2 | C2 | K | C1 | C3 | 308 | Miao-Li | 1993/6/14 | ND |
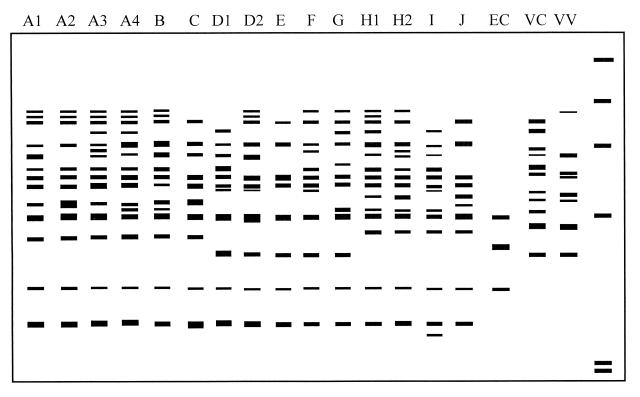
In RS-PCR, 8 to 15 amplified bands with sizes of between 330 and 1,000 bp were found in the V. parahaemolyticus strains. Bands ranging from 350 to 720 bp could be easily observed on the electrophoresis gel. Specifically, six amplified bands with molecular sizes of 350, 420, 610, 720, 750, and 870 bp were common in all strains (Fig. 1A), and two amplified bands (350 and 720 bp) occurred in all V. parahaemolyticus strains but not in E. coli, V. cholerae, and V. vulnificus. All 41 V. parahaemolyticus strains were grouped into 15 patterns, with A3 (17.1% of the total number of strains) being the predominant pattern (Fig. 2 and Table 2).
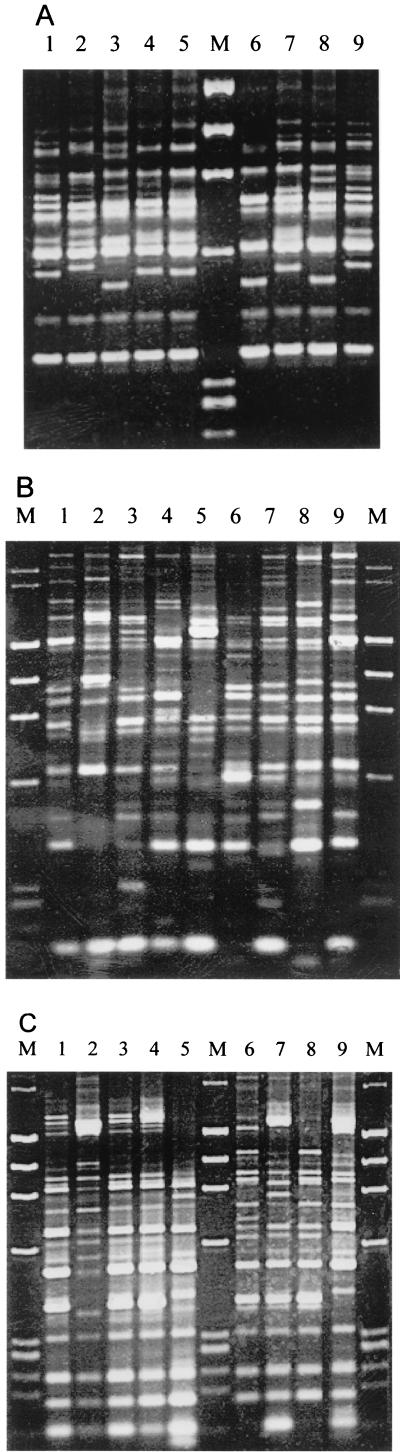
FIG. 1.
Amplification fingerprints of some V. parahaemolyticus strains with RS-, REP-, and ERIC-PCR methods. (A) RS-PCR. Lanes: 1, strain 134 (pattern A3); 2, strain 135 (pattern H2); 3, strain 145 (pattern G); 4, strain 166 (pattern A2); 5, strain 168 (pattern A2); 6, strain 182 (pattern E); 7, strain 197 (pattern A2); 8, strain 199 (pattern D2); 9, strain ST550 (pattern A3); M, molecular size markers (from top to bottom, 1,353, 1,078, 872, 603, 310, and 281 bp). (B) REP-PCR. Lanes: 1, strain 134 (pattern I1); 2, strain 135 (pattern S); 3, strain 145 (pattern F1); 4, strain 166 (pattern E); 5, strain 168 (pattern D2); 6, strain 182 (pattern H); 7, strain 197 (pattern G); 8, strain 199 (pattern A1); 9, strain ST550 (pattern I2); M, molecular size markers (from top to bottom, 2,322, 2,027, 1,353, 1,078, 872, 603, 310, 281, and 271 bp). (C) ERIC-PCR. Lanes: 1, strain 355 (pattern A1); 2, strain 364 (pattern B1); 3, strain 402 (pattern A1); 4, strain 403 (pattern A2); 5, strain 415 (pattern M); 6, strain 418 (pattern O1); 7, strain 434 (pattern C3); 8, strain 436 (pattern O2); 9, strain ST550 (pattern B3); M, molecular size markers (from top to bottom, 2,027, 1,353, 1,078, 872, 603, 310, 281, and 271 bp).
FIG. 2.
Diagram of amplification patterns of V. parahaemolyticus with RS-PCR. EC, E. coli; VC, V. cholerae; VV, V. vulnificus. The rightmost lane contains the molecular size markers described in the legend to Fig. 1A.
TABLE 2.
Comparison of different molecular methods used in the subspecies typing of V. parahaemolyticus
| Method | No. of:
|
% of strains with the predominant pattern | Discrimination indexa | |
|---|---|---|---|---|
| Strains | Patterns | |||
| PFGE | 40 | 22 | 12.5 | 0.96 |
| Ribotyping | 40 | 20 | 12.5 | 0.95 |
| RAPD | 40 | 8 | 25.0 | 0.84 |
| RS-PCR | 41 | 15 | 17.1 | 0.91 |
| REP-PCR | 41 | 27 | 12.2 | 0.97 |
| ERIC-PCR | 40 | 27 | 7.5 | 0.98 |
Simpson's index of diversity (14).
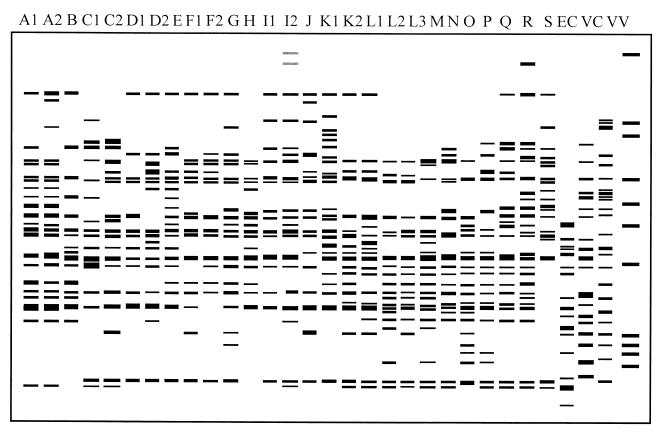
In REP-PCR, between 13 and 26 amplified bands ranging in size from 160 to 3,000 bp were discernible in the V. parahaemolyticus strains. Several amplified bands with molecular sizes of 200, 470, 500, 600, 640, 805, and 1,355 bp were common in most strains, while only the 805-bp band was present in all V. parahaemolyticus strains (Fig. 1B). The V. parahaemolyticus strains were grouped into 27 patterns, with P (12.2% of the total number of strains) being the predominant one (Fig. 3 and Table 2).
FIG. 3.
Diagram of amplification patterns of V. parahaemolyticus with REP-PCR. EC, E. coli; VC, V. cholerae; VV, V. vulnificus. The rightmost lane contains the molecular size markers described in the legend to Fig. 1B.
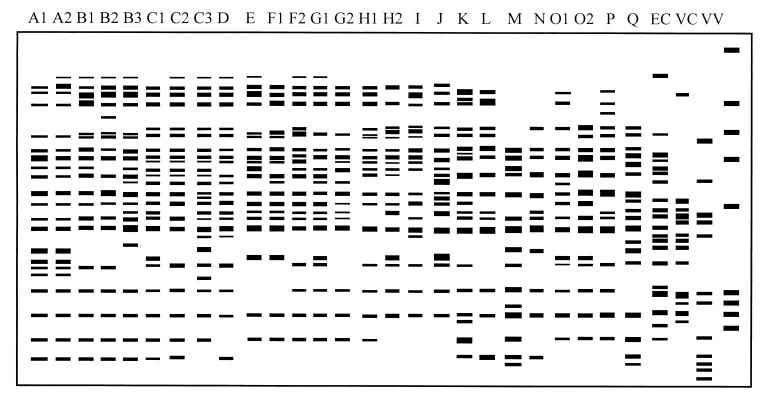
In ERIC-PCR, 12 to 25 amplified bands with sizes ranging between 160 and 1,690 bp were easily discernible in the V. parahaemolyticus strains. Several bands with molecular sizes of 270, 320, 520, 560, 660, 780, 900, 950, and 1,355 bp were common in most strains, while 270-, 520-, 660-, and 950-bp bands were present in all V. parahaemolyticus strains (Fig. 1C). The 39 domestic strains plus the reference strain from Japan were grouped into 27 patterns. Patterns A1, C2, H1, and P, each comprising three strains, were the most predominant patterns (7.5% of the total number of strains) (Fig. 4 and Table 2).
FIG. 4.
Diagram of amplification patterns of V. parahaemolyticus with ERIC-PCR. EC, E. coli; VC, V. cholerae; VV, V. vulnificus. The rightmost lane contains the molecular size markers described in the legend to Fig. 1C.
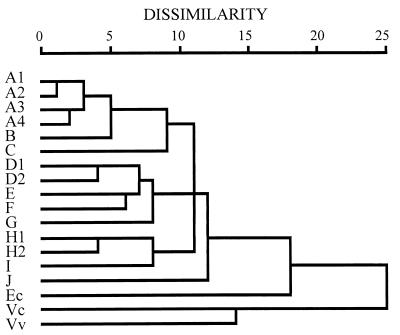
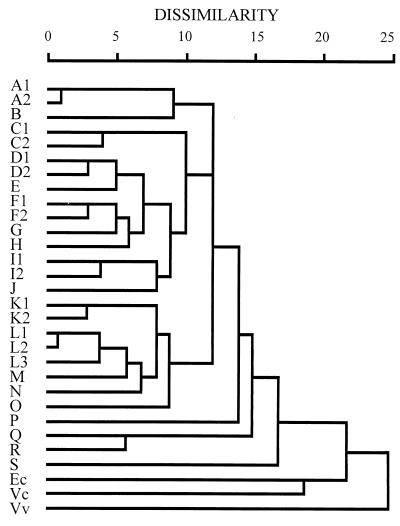
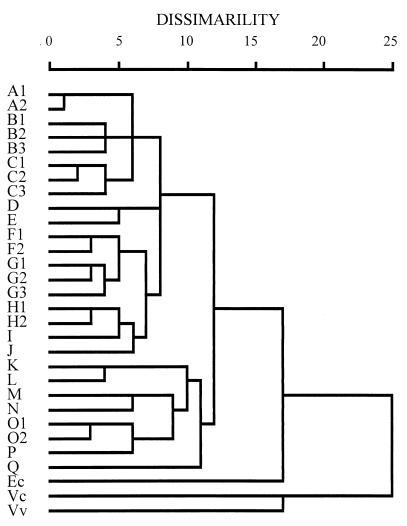
The clonal relationships among these V. parahaemolyticus strains were examined through cluster analysis of the PCR-generated patterns and are presented in dendrograms (Fig. 5, Fig. 6, and Fig. 7). Following cluster analysis, different patterns were arbitrarily classified into different types with strain dissimilarity values of 5 or more (33). Each type consisted of one to seven different patterns (Table 1). Compared with the interspecies reference strains, all the V. parahaemolyticus strains were closely related, according to analysis by the PCR methods; they differed significantly from the reference strains of E. coli, V. cholerae, and V. vulnificus, having dissimilarity values of 17 or more (Fig. 5 to 7). Strains of V. parahaemolyticus belonging to one or closely related PFGE patterns were generally grouped into closely related patterns by these PCR methods. Also, strains determined by one of these PCR methods to belong to strongly dissimilar patterns were generally noted as being dissimilar by the other two PCR methods (Fig. 5 to 7).
FIG. 5.
Dendrogram illustrating the clustering of amplification patterns of V. parahaemolyticus with RS-PCR. The dendrogram was produced using the squared Euclidean distance measure and average linkage clustering method with the program SPSS for Windows, Release 6.0. The dissimilarity units are arbitrary, being based on the sum of the squared Euclidian distance measure. Strains were arbitrarily grouped into different types. Letters at left designate the patterns.
FIG. 6.
Dendrogram illustrating the clustering of amplification patterns of V. parahaemolyticus with REP-PCR. See the legend to Fig. 5 for details.
FIG. 7.
Dendrogram illustrating the clustering of amplification patterns of V. parahaemolyticus with ERIC-PCR. See the legend to Fig. 5 for details.
DISCUSSION
Several molecular methods have been developed and assessed for the typing of V. parahaemolyticus. PFGE is the method favored in our laboratory, owing to its highly reproducible fingerprints and strong discriminative ability (12, 31, 32). However, the ability of this method may be impaired by a high proportion of nontypeable isolates (23%), owing to DNA degradation during endonuclease digestion or other steps (14). A DNA degradation problem has been encountered with some strains in our laboratory, but these strains were successfully typed by repeating the experiment (28). The cause of DNA degradation is unknown; however, careful processing to avoid shearing interference and the use of a suboptimal enzyme reaction temperature of 37°C (optimum temperature of 50°C for SfiI) may have reduced the level of DNA degradation in our study. Furthermore, difficulties were recently encountered with the typing of several strains collected from Japan and America in 2000, with the nontypeable rate reaching about 7% (unpublished data). Therefore, a combination of methods may be required to achieve the complete typing of different V. parahaemolyticus strains.
PCR typing methods using specific primers designed on the basis of the repeated and conserved sequences in bacteria and more stringent annealing conditions display more promising fingerprints than RAPD analysis (11). Spacer regions within the 16S and 23S genes in prokaryotic rRNA genetic loci exhibit significant length and sequence polymorphisms in different species and are flanked by highly conserved sequences (8). Multiple copies of these loci occur in bacteria (24). Therefore, amplification using primers designed on the basis of these flanking sequences will generate polymorphic fingerprints which can be used to distinguish bacterial strains at the species and subspecies levels (1, 2, 8). RS-PCR has been applied to typing of species from many genera, including Listeria, Staphylococcus, and Salmonella (8, 10), but had not yet been applied to V. parahaemolyticus. The 16S-23S rRNA intergenic spacer regions of V. parahaemolyticus contain different tRNA compositions, and similarities in the nucleotide sequences of the noncoding regions flanked by the tRNA genes have been noted (13).
REP-PCR and ERIC-PCR are both based on the presence of repetitive conserved sequences in bacteria. The REP-PCR method is based on the presence of 38-bp REPs in Enterobacteriaceae and other bacteria and has been applied to many species (14, 19, 25, 26). With REP-PCR, the fingerprinting profiles differentiate toxigenic V. cholerae O1 strains from nontoxigenic O1 and non-O1 strains, while ERIC-PCR further differentiates toxigenic O1 strains into El Tor and classical biotypes (22). This work is the first to apply the RS-PCR and REP-PCR methods to the typing of V. parahaemolyticus.
ERIC-PCR is the most widely adopted of the above three PCR typing methods and has been applied to the typing of many species, including V. cholerae (18, 23) and V. parahaemolyticus. Marshall et al. (14) examined 38 clinical strains of V. parahaemolyticus from outbreaks on Canada's Pacific coast and several environmental strains using ERIC-PCR, ribotyping, PFGE, and restriction fragment length polymorphism analysis of the genetic locus encoding the polar flagellum. Six ERIC-PCR patterns were identified by using a single primer for the amplification, and it was concluded that ERIC-PCR and ribotyping were useful for evaluating genetic and epidemiological relationships among V. parahaemolyticus strains (14).
All three PCR typing methods described here could differentiate V. parahaemolyticus from other species and effectively differentiate intraspecific strains. The V. parahaemolyticus strains examined here were deliberately selected to represent a variety of different patterns and have been typed using PFGE, ribotyping, and RAPD analysis. The discriminative ability of these PCR methods can thus be evaluated and compared with that of other published methods. PFGE, ribotyping, REP-PCR, and ERIC-PCR exhibited an excellent discrimination index of 0.95 or higher (Table 2). Based solely on the discrimination index (Simpson's index of diversity [14]), REP-PCR and ERIC-PCR will be selected as the two best rapid PCR typing methods for V. parahaemolyticus. However, REP-PCR could be the better of the two owing to its higher rate of reproducible fingerprints. In the current study, the PCR assays were repeated three times for each V. parahaemolyticus strain, and the reproducibility of the banding patterns was observed. In ERIC-PCR, some of the minor light amplification bands were inconsistent, thus complicating pattern differentiation. Among the three PCR methods, RS-PCR generated fewer amplification bands than REP-PCR and ERIC-PCR and thus fewer subspecies patterns and a slightly lower discrimination index (0.91) (Table 2). However, since the RS-PCR patterns were more easily discernible visually than the REP-PCR or ERIC-PCR patterns, they may be a practical method for routine use.
Although the discriminative ability of these PCR typing methods differed from 0.91 to 0.98, these methods are effective for typing strains from outbreaks. When the typing of strains in each outbreak is examined, the results obtained by these PCR methods mirrored those of the PFGE method for some outbreaks, although they differed slightly for other outbreaks. For example, the outbreak occurring in Miao-Li on 14 June 1993 was typed as A4, A4, K, and A6 by PFGE, A2, C, J, and C by RS-PCR, H1, H1, H2, and H1 by ERIC-PCR, and D1, D1, C2, and N by REP-PCR for strains 302, 304, 308, and 314, respectively (Table 1). In another example, the outbreak occurring in Peng-Hu on 30 June 1994 was typed as D3 by PFGE and P by REP-PCR but as A1 and A3 by RS-PCR and K and L by ERIC-PCR (Table 1). The use of a combination of these PCR methods could achieve even higher discriminative ability when fine and rapid typing is required.
The presence of the repeatable fingerprints in REP-PCR and ERIC-PCR suggested the presence of these repetitive consensus sequences (REP and ERIC) in V. parahaemolyticus. In another Vibrio species, V. cholerae, the presence of ERIC has been confirmed to be located near the hemolysin gene. Meanwhile, ERIC of V. cholerae is highly homologous with those found in Enterobacteriaceae. A previous study has speculated that a transpecific genetic exchange has affected a group of E. coli hemolysin genes and that ERIC has thus “hitchhiked” with the hemolysin gene (5). Besides the presence of ERIC, the possibility of fingerprints being formed by random amplification cannot be excluded, and Gillings and Holley (3) confirmed that ERIC-PCR fingerprints may be thus produced. Gillings and Holley performed PCR with ERIC primers using salmon and lambda DNA templates without ERIC, and the fingerprints were formed and changed according to different annealing conditions used in the PCR procedure (3).
In conclusion, RS-PCR, REP-PCR, and ERIC-PCR are suitable rapid typing methods for V. parahaemolyticus. All three methods have high discriminative ability, but REP-PCR is superior to ERIC-PCR owing to the better reproducibility of fingerprints produced with this method. Nevertheless, RS-PCR, with a slightly lower discriminative ability, may be a more practical method because fewer amplification bands and patterns are generated, simplifying review and interpretation of data.
ACKNOWLEDGMENT
We thank the Department of Health of the Republic of China for financially supporting this research under contract no. DOH90-TD-1075.
REFERENCES
- 1.Al-Saif N M, O'-Neill G L, Magee J T, Brazier J S, Duerden B I. PCR-ribotyping and pyrolysis mass spectrometry fingerprinting of environmental and hospital isolates of Clostridium difficile. J Med Microbiol. 1998;47:117–121. doi: 10.1099/00222615-47-2-117. [DOI] [PubMed] [Google Scholar]
- 2.Bidet P, Lalande V, Salauze B, Burghoffer B, Avesani V, Delmee M, Rossier A, Barbut F, Petit J C. Comparison of PCR-ribotyping, arbitrarily primed PCR, and pulsed-field gel electrophoresis for typing Clostridium difficile. J Clin Microbiol. 2000;38:2484–2487. doi: 10.1128/jcm.38.7.2484-2487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillings M, Holley M. Repetitive elemment PCR fingerprinting (rep-PCR) using enterobacterial repetitive intergenic consensus (ERIC) primers is not necessarily directed at ERIC elements. Lett Appl Microbiol. 1997;25:17–21. doi: 10.1046/j.1472-765x.1997.00162.x. [DOI] [PubMed] [Google Scholar]
- 4.Hill W E, Madden J M, McCardell B A, Shah D B, Jagow J A, Payne W L, Boutin B K. Food-borne enterotoxigenic Escherichia coli: detection and enumeration by DNA colony hybridization. Appl Environ Microbiol. 1983;45:1324–1330. doi: 10.1128/aem.45.4.1324-1330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulton C S J, Higgins C F, Sharp P M. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol. 1991;5:825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 6.Hunter P R, Gaston M A. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iguchi T, Kondo S, Hisatsune K. Vibrio parahaemolyticus O serotypes from O1 to O13 all produce R-type lipopolysaccharide: SDS-PAGE and compositional sugar analysis. FEMS Microbiol Lett. 1995;130:287–292. doi: 10.1111/j.1574-6968.1995.tb07733.x. [DOI] [PubMed] [Google Scholar]
- 8.Jensen M A, Webster J A, Straus N. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl Environ Microbiol. 1993;59:945–952. doi: 10.1128/aem.59.4.945-952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph S W, Colwell R R, Kaper J B. Vibrio parahaemolyticus and related halophilic vibrios. Crit Rev Microbiol. 1983;10:77–123. doi: 10.3109/10408418209113506. [DOI] [PubMed] [Google Scholar]
- 10.Lagatolla C, Dolzani L, Tonin E, Lavenia A, Di-Michele M, Tommasini T, Monti-Bragadin C. PCR ribotyping for characterizing Salmonella isolates of different serotypes. J Clin Microbiol. 1996;34:2440–2443. doi: 10.1128/jcm.34.10.2440-2443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu P Y F, Shi Z Y, Lau Y J, Hu B S, Shyr J M, Tsai W S, Lin Y H, Tseng C Y. Comparison of different PCR approaches for characterization of Burkholderia (Pseudomonas) cepacia isolates. J Clin Microbiol. 1995;33:3304–3307. doi: 10.1128/jcm.33.12.3304-3307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu P-L, Chang S-C, Pan H-J C M L, Luh K-T. Application of pulsed-field gel electrophoresis to the investigation of a nosocomial outbreak of Vibrio parahaemolyticus. J Microbiol Immunol Infect (Taipei) 2001;33:29–33. [PubMed] [Google Scholar]
- 13.Maeda T, Takada N, Furushita M, Shiba T. Structural variation in the 16S–23S rRNA intergenic spacers of Vibrio parahaemolyticus. FEMS Microbiol Lett. 2000;192:73–77. doi: 10.1111/j.1574-6968.2000.tb09361.x. [DOI] [PubMed] [Google Scholar]
- 14.Marshall S, Clark C G, Wang G, Mulvey M, Kelly M T, Johnson W M. Comparison of molecular methods for typing Vibrio parahaemolyticus. J Clin Microbiol. 1999;37:2473–2478. doi: 10.1128/jcm.37.8.2473-2478.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-Kearley J, Gow J A, Peloquin M, Greer C W. Numerical analysis and the application of random amplified polymorphic DNA polymerase chain reaction to the differentiation of Vibrio strains from a seasonally cold ocean. Can J Microbiol. 1994;40:446–455. doi: 10.1139/m94-073. [DOI] [PubMed] [Google Scholar]
- 16.Okuda J, Ishibashi M, Hayakawa E, Nishino T, Takeda Y, Mukhopadhyay A K, Garg S, Bhattacharya S K, Nair G B, Nishibuchi M. Emergence of a unique O3:K6 clone of Vibrio parahaemolyticus in Calcutta, India, and isolation of strains from the same clonal group from Southeast Asian travelers arriving in Japan. J Clin Microbiol. 1997;35:3150–3155. doi: 10.1128/jcm.35.12.3150-3155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan T M, Wang T K, Lee C L, Chien S W, Horng C B. Food-borne disease outbreaks due to bacteria in Taiwan, 1986 to 1995. J Clin Microbiol. 1997;35:1260–1262. doi: 10.1128/jcm.35.5.1260-1262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera I G, Chowdhury M A R, Huq A, Jacobs D, Martins M T, Colwell R R. Enterobacterial repetitive intergenic consensus sequences and the PCR to generate fingerprints of genomic DNAs from Vibrio cholerae O1, O139, and non-O1 strains. Appl Environ Microbiol. 1995;61:2898–2904. doi: 10.1128/aem.61.8.2898-2904.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez B M, Hamill R J, Houston E D, Georghiou P R, Clarridge J E, Regnery R L, Koehler J E. Genomic fingerprinting of Bartonella species by repetitive-element PCR for distinguishing species and isolates. J Clin Microbiol. 1995;33:1089–1093. doi: 10.1128/jcm.33.5.1089-1093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Samore M H, Kristjansson M, Venkataraman L, DeGirolami P C, Arbeit R D. Comparison of arbitrarily-primed polymerase chain reaction, restriction enzyme analysis and pulsed-field gel electrophoresis for typing Clostridium difficile. J Microbiol Methods. 1996;25:215–224. [Google Scholar]
- 22.Shangkuan Y H, Lin H C, Wang T M. Diversity of DNA sequences among Vibrio cholerae O1 and non-O1 isolates detected by whole-cell repetitive element sequence-based polymerase chain reaction. J Appl Microbiol. 1997;82:335–344. doi: 10.1046/j.1365-2672.1997.00365.x. [DOI] [PubMed] [Google Scholar]
- 23.Shangkuan Y H, Tsao C M, Lin H C. Comparison of Vibrio cholerae O1 isolates by polymerase chain reaction fingerprinting and ribotyping. J Med Microbiol. 1997;46:941–948. doi: 10.1099/00222615-46-11-941. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava A K, Schlessinger D. Mechanism and regulation of ribosomal RNA processing. Annu Rev Microbiol. 1990;44:105–129. doi: 10.1146/annurev.mi.44.100190.000541. [DOI] [PubMed] [Google Scholar]
- 25.Stern M J, Ames G F L, Smith N H, Robinson E C, Higgins C F. Repetitive extragenic palindromic sequences: a major component of the bacterial genome. Cell. 1984;37:1015–1026. doi: 10.1016/0092-8674(84)90436-7. [DOI] [PubMed] [Google Scholar]
- 26.Stubbs S L, Brazier J S, O'Neill G L, Duerden B I. PCR targeted to the 16S–23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J Clin Microbiol. 1999;37:461–463. doi: 10.1128/jcm.37.2.461-463.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequence in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong H C, Liu S-H, Ku L-W, Wang T K, Lee Y-S, Lee C-L, Kuo L-P, Shih D Y C. Characterization of Vibrio parahaemolyticus isolates obtained from Food Poisoning Outbreaks during 1992–1995 in Taiwan. J Food Prot. 2000;63:900–906. doi: 10.4315/0362-028x-63.7.900. [DOI] [PubMed] [Google Scholar]
- 29.Wong H C, Ho C Y, Kuo L P, Pan T M, Wang T K, Shih D Y C. Ribotyping of Vibrio parahaemolyticus isolates obtained from food poisoning outbreaks in Taiwan. Microbiol Immunol. 1999;43:631–636. doi: 10.1111/j.1348-0421.1999.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 30.Wong H C, Liu C C, Pan T-M, Wang T-K, Shih D Y C. Molecular typing of Vibrio parahaemolyticus isolates obtained from food poisoning outbreaks in Taiwan by random amplified polymorphic DNA analysis. J Clin Microbiol. 1999;37:1809–1812. doi: 10.1128/jcm.37.6.1809-1812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong H C, Liu S-H, Liu D P. Incidence of Highly Genetically Diversified Vibrio parahaemolyticus in Seafood Imported from Asian countries. Int J Food Microbiol. 1999;52:181–188. doi: 10.1016/s0168-1605(99)00143-9. [DOI] [PubMed] [Google Scholar]
- 32.Wong H C, Liu S-H, Wang T-K, Lee C L, Chiou C S, Liu D P, Nishibuchi M, Lee B-K. Characterization of Vibrio parahaemolyticus O3:K6 from Asia. Appl Environ Microbiol. 2000;66:3981–3986. doi: 10.1128/aem.66.9.3981-3986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong H C, Lu K-T, Pan T-M, Lee C-L, Shih D Y C. Subspecies typing of Vibrio parahaemolyticus by pulsed-field gel electrophoresis. J Clin Microbiol. 1996;34:1535–1539. doi: 10.1128/jcm.34.6.1535-1539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong H C, Peng P Y, Lan S L. Effect of heat shock on the thermotolerance and protein composition in Vibrio parahaemolyticus. Infect Immun. 1998;66:3066–3071. doi: 10.1128/iai.66.7.3066-3071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]