Abstract
The progressive research into the nanoscale level upgrades the higher end modernized evolution with every field of science, engineering, and technology. Silver nanoparticles and their broader range of application from nanoelectronics to nano-drug delivery systems drive the futuristic direction of nanoengineering and technology in contemporary days. In this review, the green synthesis of silver nanoparticles is the cornerstone of interest over physical and chemical methods owing to its remarkable biocompatibility and idiosyncratic property engineering. The abundant primary and secondary plant metabolites collectively as multifarious phytochemicals which are more peculiar in the composition from root hair to aerial apex through various interspecies and intraspecies, capable of reduction, and capping with the synthesis of silver nanoparticles. Furthermore, the process by which intracellular, extracellular biological macromolecules of the microbiota reduce with the synthesis of silver nanoparticles from the precursor molecule is also discussed. Viruses are one of the predominant infectious agents that gets faster resistance to the antiviral therapies of traditional generations of medicine. We discuss the various stages of virus targeting of cells and viral target through drugs. Antiviral potential of silver nanoparticles against different classes and families of the past and their considerable candidate for up-to-the-minute need of complete addressing of the fulminant and opportunistic global pandemic of this millennium SARS-CoV2, illustrated through recent silver-based formulations under development and approval for countering the pandemic situation.
Graphical abstract
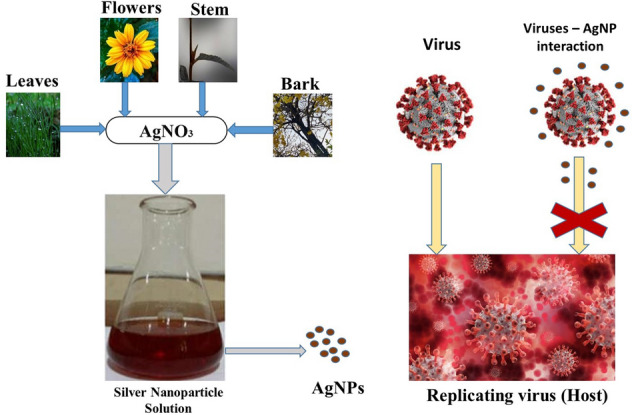
Keywords: Phytochemicals, Green synthesis, Nanosilver, Viral spectra, COVID
Introduction
‘Nano’ scale that refers to the one-billionth of a meter. Nanotechnology is a multi-disciplinary stream that emphasizes the purposeful design of manipulation of matter at the scale of atomic level utilizing the existing approaches, techniques, and types of equipment available with conventional and modern science and engineering. Nanoparticles do focus on particles that exist in the range of 1–100 nm [1]. Enhancement or acquisition of new characteristics at the nanoscale level compared to the bulk properties gained more interest with research on this avenue within the past 2 decades. Higher ratio of surface area to volume at the nanoscale level and the shift in the laws of physics at the nanometric level are the two important attributes that contribute to effective catalytic activity to various multi-disciplinary applications [2].
Approaches of nanoparticle synthesis include a top–down (TD) approach that encompasses the disintegrative breakdown of bulk materials into finer grain sizes of nanoscale. Synthesis methods such as mechanical milling, laser ablation, and sputtering follow the TD approach. The alternative approach of synthesis encloses gradual consecutive integration of atoms/molecules at various smaller scales that leads to the ‘nucleation’ site formation followed by agglomeration around the nucleation site engenders nanoparticle formation. Spray pyrolysis, sol–gel method & green synthesis methods, etc. are some of the BU route-based nanoparticle synthesis approaches [3].
Various methods for the synthesis of nanoparticles include physical, chemical, and biological methods with their own pros and cons for each. Physical methods utilize higher mechanical energy, high radiation, high temperature, and greater sized apparatus for the synthesis. Grain size control and less manual power are remarkable advantages, whereas parameter optimization and toxicity are notable demerits. Chemical methods involve the usage of chemical reducing and capping agents of organic and inorganic species; sometimes, the same reagents being both. Simple to process and control over scale-up are highlightable merits, whereas environmental unfriendly, lesser biocompatibility are notable demerits. To address the backlogs of physical and chemical methods, shift to biological methods of synthesis enters the research avenue. Environment-friendly, no application of higher temperature, pressure, heat, energy, most supporting biocompatibility, devoid of toxic chemicals, easier handling, and scale-up are all that makes biological synthesis more fascinating than any other [3–5]. Preference of water over any other organic solvents as the major solvent and thereby the greater colloidal stability attainment of the nanoparticle product is the unique property on green synthesis and the fact that water is the most biocompatible solvent is found to be reflected with the application part [6].
Silver nanoparticles: ‘the unique’
Among the widely explored metallic nanoparticles, silver nanoparticles (AgNPs) have the continuity of being used for centuries in human civilizational history due to their very unique and specific physical, biological, electronic, catalytic, surface, and chemical properties. The strongest biocidal properties against biota of microbial range from bacteria, viruses, fungi, algae to higher nematodes, and helminths. It also possesses non-toxicity toward animal cells and compatibility to human cell lines provides numerous biological product applications. Colloidal stability of AgNPs makes them suitable as preservatives in cosmetics and medicated products, optical plasma-resonance scattering property makes a bio-labeling candidate and sensor, imaging applications, anti-inflammation property-driven wound-healing engineering, surface coating property enhanced paints, reusable catalytic property over the degradation of different classes of dyes, anti-thrombogenic and hemodynamic properties utilized cardiac valves and stents, implants with anti-platelet property and stimulation of vascular endothelial growth factor (VEGF) that promotes angiogenesis, the process of new blood vessel formation and endothelial vasodilation property-driven anti-hypertensive implant, peculiar AgNPs mechanical properties such as elastic modulus and flexural strength improvising of acrylic resin-based removable dental dentures against opportunistic oral pathogens, anti-adhesion and anti-infective property-driven orthodontic brackets against dental caries, metabolonomics intervention and perturbation property with nucleotides, photosynthesis and photorespiration processes, anti-microbial properties, anti-static properties, electrically conducting, and most importantly self-cleaning property. Electro-conductive fibers help to protect from radiation emitted by electronics. Self-cleaning property resists the deepening of stains and dirt from the point of incidence. Nano-functional fibers are used to produce odor-free undergarments, socks and stockings and research over the face masks coated with silver nanoparticles used during the COVID-19 pandemic is contemporary anti-microbial property example [7–20].
Green synthesis of silver nanoparticles
These AgNPs shall be synthesized through various routes out of which biological routes again gain importance due to aforesaid attributes of the produced nanoparticles. The biological route shall be further taken as phyto-mediated, microbe-mediated, and other molecular templates of broader category—inorganic, organic, metals, polysaccharides, proteins & miscellaneous chemical reagents, etc. [21].
Phyto-mediated synthesis of nanoparticles has its own spectrum of source that includes extracts of leaves [22, 23], bark [24, 25], stem [26, 27], latex [28, 29], fruit [23, 30, 31], flower [32–35], root [36–38], seed [39, 40], and tuber [41, 42]. Different parts of the different plants have their own varying concentration of reductase enzyme that reduces the metal nitrate solution into the nanosized metal particles. Plenty of systems with single reducing agents, dual-reducing agents exist, whereas also a single source of an enzyme that also catalyzes hybrid formation and directs to nanocomposite hybrid system exists [43].
Leave-mediated synthesis
Leaves are rich source of a larger number of phytochemicals that includes tannins, flavonoids, saponins, alkaloids [44], phlobatannins, carbohydrates, glycosides, terpenoids, anthraquinones [45, 46], coumarines, proteins, emodins [47], anthocyanins [48], xanthoproteins, triterpenoidal sapogenis [49] steroids, phenol, and essential oils. Minerals such as sodium, calcium, iron, phosphorous, magnesium, potassium, and zinc are found in traceable quantity that does serves as the inorganic cofactors for enzymes present in the plants. Essential oils of the leaves can be general and species-specific constituents between which volatile compounds are of greater considerable proportion. Citrus plant leaves possess citreol, burneol, t-Muurolol, humulene, viridiflorol, geranial, Myrcenol, nerol, valencene, dextro-carvone, linalool, etc., [50] whereas cinnamon species have alcohol [2-nitro-ethanol, glycerin, cinnamyl alcohol, 1-methoxy-2-propanol], aldehyde [t-cinnamaldehyde, o-methoxy-cinnamaldehyde, benzylide nemalonaldehyde], alkane [dodecane], carboxylic acid [acetic acid], ester[Isopropyl acetate, ethyl formate], ether [1,1-diethoxy-ethane], and ketonic [coumarine] compounds in the essential oils [51]. All the components shall have a significant to least contributions in the process of phyto (leaf)-mediated nanoparticle synthesis. Table 1 is the list with representative examples of leaves used for the synthesis of silver nanoparticles.
Table 1.
Representative examples of leaves used for synthesis of silver nanoparticles
| Name of the plant | Nanoparticle size (nm) | Nanoparticle shape | References |
|---|---|---|---|
| A. indica (neem) | 20 | Triangular | [52] |
| Actaea racemosa (Black bugbane) | 3–9 | Spherical | [53] |
| Aegle marmelos (Vilvam) | 14–28 | Spherical | [54] |
| Aloe sp., | 5 | Spherical | [53] |
| Aloe vera | 70–192 | Spherical | [55] |
| Aloe vera | 10–30 | Spherical | [56] |
| Alternanthera dentata (Purple Joyweed) | 10–80 | Spherical | [57] |
| Amaranthus gangeticus (Elephant head) | 11–15 | Spherical | [58] |
| Anisomeles indica—Indian Catmint | 18–35 | Spherical | [59] |
| Annona squamosa (Sugar apple) | 200–500 | Irregularly spherical | [60] |
| Anthemis atropatana (plant) extract | 10–80 | Spherical | [61] |
| Arbutus Unedo (Strawberry) | 20–30 | Spherical | [62] |
| Argemone mexicana | 10–50 | Cubic, hexagonal | [63] |
| Artemisia turcomanica (Wormwood) | 4–42 | Spherical | [64] |
| Banana leaves | 50 | Spherical | [52] |
| Berberis vulgaris (Barberry) | 40 | Spherical | [65] |
| black pepper leaf | 5–50 | Spherical | [66] |
| Boerhaavia diffusa (Mookarati saarai) | 24–25 | Spherical | [67] |
| Buddleja globosa | 2–5 | Spherical | [68] |
| Butea monosperma—(Palash teak) | 10–100 | Spherical, triangular, hexagonal | [69] |
| Cadaba indica lam (Viluthi leaf) | 30–60 | Spherical | [70] |
| Carica papaya | 10–50 | Cubical | [71] |
| Carica papaya | 50–250 | Spherical | [72] |
| Carob leaf extract | 5–40 | Spherical | [73] |
| Cassia Roxburghii (Ceylon senna) | 57–95 | Spherical, triangular, truncated triangular, decahedral | [74] |
| Chamomile (a tea plant) | 20–70 | Spherical | [75] |
| Chrysanthemum indicum(Saamanthi) | 38–72 | Spherical | [76] |
| Citrullus colocynthis (Kumatti) | 1–60 | Spherical | [77] |
| Coleus aromaticus | 25–27 | Spherical | [78] |
| Coleus aromaticus—Mexican Mint | 20–30 | Spherical | [79] |
| Commelina benghalensis | 13–51 | Spherical | [80] |
| Crocus Haussknechtii Bois | 16 | Spherical | [81] |
| Cycas circinalis, | 13–51 | Spherical | [80] |
| Cycas Leaf (Panai Peyarani) | 2–6 | Spherical | [82] |
| Cynodon dactylon (Arugampul) | 25–60 | Spherical | [56] |
| Datura metel (Oomaththai) | 16–40 | Spherical | [83] |
| Diopyros kaki | 32 | Spherical | [84] |
| Eclipta leaf | 2–6 | Spherical | [82] |
| Eucalyptus | 4–60 | Spherical | [85] |
| Eucalyptus angophoroides | 3–15 | Spherical | [53] |
| Eucalyptus chapmaniana | 60 | – | [86] |
| Eucalyptus globulus | 1.9–25 | Spherical, oval | [87] |
| Eucalyptus leucoxylon | 50 | Spherical | [88] |
| Eucalyptus oleosa | 14–26 | Spherical | [89] |
| Ferocactus Echidne (Mexican Cactus) | 20–60 | Elliptical | [90] |
| Ficus amplissima | 13–51 | Spherical | [80] |
| Ficus benghalensis (Banyan) | 16 | Spherical | [91] |
| Fraxinus excelsior | 25–40 | Spherical | [92] |
| Galega officinalis (Professor weed) | 23–220 | Spherical | [93] |
| Ginkgo biloba | 32 | Spherical | [84] |
| Glaucium corniculatum | 45–53 | Spherical | [94] |
| Green and Black tea leaves | 10–20 | Spherical | [95] |
| Green tea | 6–8.5 | Spherical | [96] |
| Green tea leaves | 25–75 | Spherical | [97] |
| Hamamelis virginiana Leaf (American Witch hazel) | 8–35 | Spherical | [98] |
| Heritiera fomes | 20–100 | – | [99] |
| Hydrilla verticillata | – | Spherical | [100] |
| Iresine herbstii (Chicken Gizzard) | 44–64 | Spherical | [101] |
| Ixora coccinea leaves (Jungle Geranium) | 13–57 | Spherical | [102] |
| Justicia glauca (thavasi murungai) | 10–20 | Spherical | [103] |
| Lantana camara (Unni Chedi) | 20–34 | Spherical | [104] |
| Leptadenia reticulata (Palaikkodi) | 50–70 | Spherical | [105] |
| Lippia nodiflora | 13–51 | Spherical | [80] |
| Lonerica japonica | 20–60 | Spherical, hexagonal | [106] |
| Lysiloma acapulcensis (Legume Plant) | 1.2–62 | Spherical | [107] |
| M. pudica—Thottal sinungi (Mimosaceae) | 20–60 | Spherical | [108] |
| Magnolia grandiflora | 32 | Spherical | [84] |
| Mangosteen leaf | 6–57 | Spherical | [109] |
| Mentha piperita (Peppermint) | 20–50 | Spherical | [110] |
| Mimusops elengi Leaf (Spanish Cherry) | 55–83 | Spherical | [111] |
| Moringa oleifera—Drumstick tree | 9–11 | Spherical | [112] |
| Mulberry Leaves | 20–40 | Spherical | [113] |
| Murraya koenigii (Kari Vembu) | 20–35 | Spherical | [114] |
| Murraya Koenigii Leaf (Kari vembu) | 10–20 | Spherical | [115] |
| Mussaenda glabrata | 11–51 | Spherical | [116] |
| Myrica esculenta (Box berry) | 45–80 | Spherical | [117] |
| Nelumbo nucifera (Yellow Lotus) | 25–80 | Spherical, triangle, decahedral | [118] |
| Nicotiana tobaccum | 7–9 | Irregularly spherical | [119] |
| O.sanctum (tulsi) | 50 | cuboidal | [52] |
| O. tenuiflorum (black tulsi) | 20 | Hexagonal, pentagonal | [52] |
| Ocimum sanctum | 40–50 | Spherical | [120] |
| Ocimum gratissimum | 17 | Cuboidal | [121] |
| Ocimum sanctum | 6–110 | Triangular | [122] |
| Ocimum Sanctum (Tulsi) | 11–17 | Spherical | [123] |
| ocimum sp., | 3–20 | Spherical | [124] |
| Olive leaf | 20–25 | Spherical | [125] |
| Origanum heracleoticum | 30–40 | Spherical | [126] |
| Osmanthus Fragrans (Olive Variety) | 2–30 | Spherical | [127] |
| Padina tetrastromatica | 10–100 | Spherical | [128] |
| Paederia foetida (Gandha Prasarini) | 4–15 | Spherical | [129] |
| Parkia speciose (Bitter bean/Avara Paruppu) | 26–39 | Spherical | [130] |
| Parthenium leaf | 30–80 | Irregular | [131] |
| Pedalium murex (Yanai Nerunjil) | 20–50 | Spherical | [132] |
| Pine roxburghii | 32 | Spherical | [84] |
| Pineapple leaf | 7080 | Spherical | [133] |
| Piper nigrum | 7–50 | Spherical | [134] |
| Piper nigrum | 5–50 | Spherical | [68] |
| Platanus orientalis | 32 | Spherical | [84] |
| Plukenetia volubilis(Ankaaravalli) | 4–25 | Spherical | [135] |
| Portulaca oleracea (Tharai keerai) | 15–40 | Spherical | [56] |
| Prangos ferulacea (Medicinal plant) | 10–20 | Spherical | [136] |
| Prunus japonica—Japan bush cherry tree | 24–26 | Spherical | [137] |
| Prunus persica | 40–98 | Spherical | [138] |
| Rhizophora mucronata (Mangrove plant) | 4–26 | Spherical | [139] |
| Rosa rugosa (Rose) | 10–35 | Triangular, hexagonal | [140] |
| Rosmarinus officinalis (Rosemary) | 10–33 | Spherical | [141] |
| Salvia spinosa (Mint family) | 19–125 | Spherical | [142] |
| Salvinia molesta | 1–35 | Spherical | [143] |
| Saraca indica (Ashoka tree) | 51–230 | Spherical | [144] |
| Securinega leucopyrus (Plant) | 11–20 | Spherical, oval | [145] |
| Sesbania grandiflora (Agaththi) | 10–25 | Spherical | [146] |
| Sida acuta—Arivaalmanai Poondu | 20–60 | Triangular, pentagonal, hexagonal | [147] |
| Skimmia laureola (Ornamental Shrub) | 38–46 | Spherical, hexagonal | [148] |
| Sonneratia apetala | 20–100 | – | [99] |
| Strychnos potatorum (Thethan Kottai) | 20–62 | Cubical, hexagonal | [149] |
| Taxus baccata (English Yew) | 75–91 | Spherical | [150] |
| Tea leaf | 20–90 | Spherical | [151] |
| Tecomella undulata | 32–46 | Spherical | [22] |
| Terminalia arjuna (Marudha maram) | 10–50 | Spherical | [152] |
| Terminalia arjuna (Marudha maram) | 8–16 | Irregular-shaped | [153] |
| Terminalia chebula | 10–30 | Spherical | [154] |
| Terrestrial fern—Gleichenia Pectinata | 4–10 | Spherical | [155] |
| Thymbra spicata (Plant) | 20–50 | Spherical | [156] |
| Vitex Negundo Leaf (Vellai nochchi) | 10–30 | Cubical | [157] |
| Water hyacinth | 3–10 | Spherical | [158] |
| Wheatgrass | 21–32 | Spherical | [159] |
| Ziziphora tenuior (Turkey herb) | 8–40 | Spherical | [160] |
Stem, bark, and latex-mediated synthesis
Stem, bark, and latex of the plants are also utilized as the source of nanoparticle synthesis and its composition ranges with a wide number of constituents alkaloids, flavonoids, tannins, saponins, cardiac glycosides, glycosides, proteins, carbohydrates, steroids, reducing sugars, anthracene glycosides, resins, triterpenes, procyanadines, anthraquinone [161–165], fraxidin, fraxetin, scoparone, 3-acetylaleuritolic acid, beta-sitosterol, and sitosterone [166], etc. were the actual secondary metabolites of various biochemical cycles and some are growth steroids that assist in the regulation of growth and development of the plant assists phyto (stem, bark, and latex)-mediated nanoparticle synthesis. Gums and resins from bark, stem, and latex are also used for NP synthesis. Table 2 is the list with representative examples of stem, bark, and latex used for the synthesis of silver nanoparticles.
Table 2.
Representative examples of bark, stem, and latex used for synthesis of silver nanoparticles
| Name of the plant | Part of the plant | Nanoparticle size (nm) and shape | References |
|---|---|---|---|
| Afzelia quanzensis (Lucky bean tree) | Bark | 10–80, spherical | [167] |
| Butea monosperma (Palash teak) | Bark | 18–50, spherical | [168] |
| Cochlospermum gossypium (gum Plant) | Stem | 3–56, spherical | [169] |
| Euphorbia milii (Kireeda kalli) | Latex | 10–50, spherical | [170] |
| Euphorbia tirucalli | Latex | 20–30, spherical, cubical | [171] |
| Ficus benghalensis (Banyan tree) | Bark | 68–74, spherical | [172] |
| Garlic clove | Stem | 4–22, spherical | [173] |
| Gum Arabic | Latex | 10–50, spherical | [174] |
| Gum ghatti (Anogeissus latifolia) | Stem | 11–52, spherical | [175] |
| Hevea brasiliensis | Latex | 2–100, spherical | [176] |
| Jatropha curcas (Barbodos nut) | Latex | 10–20, irregularly spherical | [177] |
| Picrasama. quassinoids | Bark | 17.5–66.5, spherical | [178] |
| Pinus eldarica (Pine tree) | Bark | 10–40, spherical | [179] |
| Piper nigrum | Stem | 9–30, spherical | [134] |
| Prosopis juliflora (Mexican tree) | Bark | 10–50, spherical | [180] |
| Salacia chinensis (Pon Korandi) | Bark | 100–200, spherical | [181] |
| Salvadora persica | Bark | 2–100, spherical | [182] |
| Seidlitzia rosmarinus (Desert plant) | Stem | 16, spherical | [183] |
| Terminalia cuneata (Kadukkai) | Bark | 25–50, spherical | [184] |
| Thevetia peruviana (Persian nut) | Latex | 10–30, spherical | [185] |
Fruit-mediated synthesis
Fruits are another phyto-source of nanoparticle synthesis. Peels, pulps, and complete fruit can be used for reduction. Usually, they have polyphenols, minerals, vitamins—tocopherols and organic acids (linoleic acid, ascorbic acid, citric acid, etc.), triterpenoids, tannins, carotenoids, phenolics, and flavonoids (rutin, myricetin, luteolin, quercetin, apigenin, and kaempferol). Constituents include moisture, sugars (sucrose, fructose, and glucose), protein, fatty acid [total saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA)], ash contents, and energy contents [186–188]. The same constituents which are metabolic precursors and building blocks of the fruit cell wall that correspond to the texture of the fruit are found to a huge extent in seeds extracts of the fruits in addition to steroids [189]. Table 3 is the list with representative examples of fruits used for synthesis of silver nanoparticles.
Table 3.
Representative examples of fruits used for synthesis of silver nanoparticles
| Name of the plant | Nanoparticle size (nm) | Nanoparticle shape | References |
|---|---|---|---|
| Apple extract | 24–36 | Spherical | [190] |
| Averrhoa bilimbi Fruit (Cucumber) | 50–150 | Hexagonal, rhomboidal | [191] |
| Banana peel | 21–25 | Spherical | [192] |
| Bitter apple (citrullus colocynthis) | 20–80 | Spherical | [193] |
| Brucea javanica (Ayurvedic plant) | 24–58 | Spherical | [194] |
| Capuli cherry | 40–100 | Spherical | [195] |
| Carica papaya | 25–50 | Cubical | [71] |
| Citrullus lanatus (Watermelon) | 17–20 | Spherical | [196] |
| Coccinia grandis (kowai guard) | – | Spherical | [197] |
| Coconut | 7080 | Cubical | [198] |
| Cordia dichotoma (Naru valli) | 2–60 | Spherical | [199] |
| Crataegus douglasii (hawthorn) | 40–60 | Spherical | [200] |
| Dillenia Indica (Uvaa thaekku) | 40–100 | – | [201] |
| Emblica Officinalis fruit (Nellikaai) | 10–70 | Spherical | [202] |
| European black elderberry | 20–80 | Spherical | [203] |
| Feronia elephantum (Vilaam palam) | 20–60 | Triangular, pentagonal, hexagonal | [204] |
| Gmelina arborea (Kumil) | 8–32 | Spherical | [205] |
| Green carambola (star fruit) | 8–19 | Spherical | [206] |
| Kigelia africana fruit (Mara suraikkai) | 10 | Spherical | [207] |
| Locust bean gum (LBG) | 16–28 | Irregularly spherical | [208] |
| M. balbisiana (Banana) | 20 | Spherical, pentagonal | [52] |
| Malus domestica fruit (Apple) | 20 | Spherical | [209] |
| Oak fruit hull (Jaft) | 40 | Spherical | [210] |
| Orange peel | 1–15 | Spherical | [211] |
| Peels of Punica Granatum (Pomegranate) | 4–7 | Spherical | [212] |
| Phyllanthus emblica (Nelli- gooseberry) | – | Spherical | [197] |
| Pine cone | 20–100 | Triangular, hexagonal | [213] |
| Solanum xanthocarpum | 4–18 | Spherical | [214] |
| Tamarind fruit | 6–8 | Spherical | [215] |
| Terminalia chebula (kadukkai) | 25 | Spherical | [184] |
| Terminalia chebula fruit (Kadukkai) | 20–50 | Spherical, triangular | [216] |
| Ananas comosus | 10–300 | Sharp corners | [217] |
| Citrus sinensis | 10–300 | Spherical | [217] |
| Trachyspermum ammi (Omam) | 60–87 | Spherical | [218] |
Flower-mediated synthesis
Phyto-constituents of flower extracts are found to contain flavonoids, tannins, phlobatannins, cardiac glycosides, alkaloids and triterpenes, saponins, anthraquinone, phenol, protein and amino acids, carbohydrates, oil, fats & resins, coumarine, phytosterol, gums, and mucilages [219–223]. Table 4 is the list with representative examples of flowers used for synthesis of silver nanoparticles.
Table 4.
Representative examples of flowers used for synthesis of silver nanoparticles
| Name of the plant | Nanoparticle size (nm) | Nanoparticle shape | References |
|---|---|---|---|
| Achillea biebersteinii (Yarrow) | 5–35 | Spherical | [224] |
| Calotropis gigantea | 50 | Spherical | [225] |
| Calendula officinalis | 5–10 | Spherical | [226] |
| Cassia auriculata Flower (Pea family) | 10–35 | Spherical | [227] |
| Chrysanthemum morifolium (Saamanthi) | 20–50 | Spherical | [228] |
| Cinnamon zeylanicum (Lavangam pattai) | 31–40 | Spherical | [229] |
| Crocus sativus | 10–25 | Spherical | [81] |
| Crocus sativus L (Kunguma Poo) | 12–20 | Spherical | [230] |
| Fritillaria flower | 5–10 | Spherical | [231] |
| Hibiscus rosa-sinensis | 5–14 | Spherical | [232] |
| Inflorescence of Cocos nucifera (Coconut) | 22 | Spherical | [233] |
| Marigold flower | 10–90 | Spherical, Hexagonal | [234] |
| Nyctanthes arbor-tristis (Night flowering Jasmine) | 5–20 | Spherical, oval | [235] |
| Piper nigrum (Black Pepper) | 1–29 | Spherical | [236] |
| Rosa damascena petals (Damask rose) | 74–94 | Spherical | [237] |
| Syzygium aromaticum (Clove) | 20–149 | Spherical | [238] |
| Tithonia diversifolia (Mexican Sunflower) | 10–26 | Spherical | [239] |
Root-mediated synthesis
Root system constantly serves as the transport hub for water and dissolved minerals to all the aerial parts of the plants and exploitation of these roots as phyto-source for metallic nanoparticle synthesis includes products of tubers in the list. Steroids, saponins, alkaloids, glycosides, flavonoids, tannins, traces of myricetin, cholesterol and beta sitosterol, carbohydrates, phenol, anthraquinone, ellagic acid, coumarine, and phytosterol [240–243]. Table 5 is the list with representative examples of roots and tubers used for synthesis of silver nanoparticles.
Table 5.
Representative examples of roots and tubers used for synthesis of silver nanoparticles
| Name of the plant | Nanoparticle size (nm) | Nanoparticle shape | References |
|---|---|---|---|
| Berberis vulgaris (Barberry) | 30–70 | Spherical | [244] |
| Beetroot extract | 10–15 | Spherical | [245] |
| Cassia toral (Senna tora) | 20–100 | Spherical | [246] |
| Cibotium barometz root | 6–23 | Spherical | [247] |
| Curcuma longa tuber (Turmeric) | 4–9 | Spherical | [248] |
| Delphinium denudatum (Ayurvedic—Nirbasi) | 2–85 | Spherical | [249] |
| Diospyros Paniculata—(karunthuvarai) | 8–10 | Spherical | [250] |
| Diospyros Sylvatica (Forest Ebony) | 10–40 | Spherical | [251] |
| Garlic | 3–12 | Spherical | [252] |
| Garlic and turmeric extracts | 6–8.5 | Spherical | [96] |
| Garlic extract | 4–20 | Spherical | [253] |
| Nepeta leucophylla (White leaved catmint) | 40–100 | Spherical | [254] |
| Parthenium hysterophorus root | - | Spherical | [255] |
| Phytolacca Decandra (PokeWeed) | 91 | Spherical | [256] |
| Rheum palmatum (Rhubarb plant) | 11–210 | Spherical, hexagonal | [257] |
| Root of Zingiber officinale | 10–20 | Spherical | [258] |
| Thalictrum foliolosum | 15–30 | Spherical | [259] |
| Zingiber officinale | 10–20 | Spherical | [260] |
Seed-mediated synthesis
Seeds serve as the germination hub for any plantlet at favorable conditions for growth and development. They have constituents such as moisture, fat, protein, carbohydrate, fiber, minerals like calcium, phosphorus, magnesium, sodium, potassium, zinc, and many other minerals with varying concentrations corresponding to the needs of that particular species. Saponins, tannins, triterpenoids glycosides, and alkaloids are also present in the seeds [261]. Table 6 shows the list with representative examples of seeds used for synthesis of silver nanoparticles.
Table 6.
Representative examples of seed extract used for synthesis of silver nanoparticles
| Name of the plant | Nanoparticle size (nm) | Nanoparticle shape | References |
|---|---|---|---|
| Artocarpus heterophyllus (Jackfruit) | 3–25 | Spherical | [262] |
| Brassica nigra | 41 | – | [263] |
| Coffea arabica | 20–30 | Spherical, ellipsoidal | [264] |
| Ducrosia Anethifolia (oil plant) | 4–42.13 | Spherical | [265] |
| Embelia ribes (False black Pepper) | 5–35 | Spherical | [266] |
| Grape seed extract | 54.8 | Spherical | [267] |
| Jatropha curcas | 15–50 | Spherical | [268] |
| Macrotyloma Uniflorum (Horse gram) | 12–17 | Spherical | [269] |
| Nyctanthes arbor-tristis (Night Jasmine) | 50–80 | Spherical | [270] |
| Papaver somniferum (Kasa kasa) | 60–87 | Spherical | [218] |
| Pistacia atlantica | 10–50 | Spherical | [271] |
| Seeds of acranythes aspera (Naaiyuruvi) | 5–50 | Spherical | [272] |
| Sinapis arvensis seed (Wild mustard) | 1–35 | Spherical | [273] |
| Tectona grandis (Teak/thaekku) | 10–30 | Spherical | [274] |
| Trifolium resupinatum (Persian Clover) | 5–10 | Spherical | [275] |
Microbe-mediated synthesis
Microbes and metal interaction were greatly explored already in the discipline of environmental biotechnology through bioremediation, biomineralization, and bioleaching. Microbe-mediated synthesis of metallic nanoparticles (MNPs) includes prokaryotic bacteria, eukaryotic fungi, and some viral particles that in turn takes place either intracellular or extracellular. Interaction of positive metal ions in the solution with the negatively charged cell wall facilitates the transportation of ions to intracellular space and further reduction by the cellular enzyme system produces metallic nanoparticles which shall further diffuse out of the cell is the mechanism of intracellular microbe-mediated green synthesis of MNPs. Experiments that tend to chemical treatment of cell wall charge alteration show more favorable NP synthesis that proves the interaction of cell wall charge and cellular transportation in this process. The alternate synthesis mechanism includes nitrate-reductase enzymes of the microbes that reduce the metal ions extracellularly [276]. Tables 7, 8, and 9 show the list with representative examples of bacteria, fungi, and algae used for synthesis of silver nanoparticles.
Table 7.
Representative examples of Bacterial strains used for synthesis of silver nanoparticles
| Name of the bacteria | Nanoparticle size (nm) | Nanoparticle shape | References |
|---|---|---|---|
| Acetobacter xylinum | 40–60 | Spherical | [277] |
| Actinobacteria Rhodococcus | 5–30 | Spherical | [278] |
| Anabaena doliolum (Cyanobacteria) | 10–50 | Spherical | [279] |
| Bacillus amyloliquefaciens | 5–24 | Triangular | [280] |
| Bacillus methylotrophicus | 10–30 | Spherical | [281] |
| Bacillus safensis | 5–30 | Spherical | [282] |
| Escherichia coli | 65 | Spherical | [283] |
| E. fergusonii (Bacteria) | 10–80 | Spherical | [284] |
| Mutant Bacillus licheniformis | 10–30 | Spherical | [285] |
| Proteus mirabilis strain (Bacteria) | 10–20 | Spherical | [286] |
| Spore crystal of Bacillus thuringiensis | 10–20 | Cubical, hexagonal | [287] |
| Weissella oryzae (Bacteria) | 10–30 | Spherical | [288] |
Table 8.
Representative examples of fungal strains used for synthesis of silver nanoparticles
| Name of the fungi | Nanoparticle size (nm) | Nanoparticle shape | References |
|---|---|---|---|
| Aspergillus niger (Fungus) | 5–26 | Spherical | [289] |
| Aspergillus terreus | 1–20 | Spherical | [290] |
| Candida albicans (Fungus) | 5–10 | Spherical | [291] |
| Fusarium oxysporum | 15–84 | Spherical | [292] |
| Fusarium solani (Fungus) | 5–35 | Spherical | [293] |
| Macrophomina Phaseolina (Fungus) | 5–40 | Spherical | [294] |
| Metarhizium Anisopliae (Fungus) | 28–38 | Rod shaped | [295] |
| Mushroom Fungus Schizophyllum | 51–99 | Spherical | [296] |
| Penicillium citrinum | 90–120 | Spherical | [297] |
| Penicillium duclauxii | 3–32 | Spherical | [298] |
| Penicillium purpurogenum | 8–10 | Spherical | [299] |
| Phoma glomerata (Fungus) | 19–65 | Spherical | [300] |
| Sclerotinia sclerotiorum (Fungus) | 25–30 | Spherical | [301] |
| Trichoderma harzianum | 51.10 | Irregularly Spherical | [302] |
| Trichoderma viride | 1–50 | Spherical | [303] |
Table 9.
Representative examples of algal strains used for synthesis of silver nanoparticles
| Name of the algae | Nanoparticle size (nm) | Nanoparticle shape | References |
|---|---|---|---|
| Boiled Algae (Desmosus sp.,) | 3–6 | Spherical | [304] |
| Caulerpa racemosa | 5–25 | Spherical | [305] |
| Chaetomorpha linum (Macroalga) | 3–44 | Cubical | [306] |
| Chlorella vulgaris | 15–47 | Spherical | [307] |
| Colpomenia sinuosa | 16 | Spherical | [308] |
| Jania rubins | 7 | Spherical | [309] |
| Nostoc linckia (Algae) | 5–60 | Spherical | [310] |
| Pterocladia capillacae | 7 | Spherical | [309] |
| Raw algae (Desmosus sp.,) | 4–8 | Spherical | [304] |
| Sargassum Wightii Grevilli (Marine Alga) | 8–27 | Spherical | [311] |
| Spyridia fusiformis (Marine red alga) | 5–50 | Spherical | [312] |
| Turbinaria conoides (Marine brown seaweed) | 96 | Spherical | [313] |
| Ulvan Algae | 3–36 | Spherical | [314] |
Miscellaneous agent-mediated synthesis
Apart from the phyto-mediated and microbe-mediated routes, the macromolecules such as carbohydrates, organic acids, proteins, and other miscellaneous chemicals are also used in the reduction and capping of silver nanoparticles. Table 10 gives the list with representative examples of macromolecules that have been employed as reducing agents for silver nanoparticle synthesis.
Table 10.
Representative examples of other miscellaneous used for synthesis of silver nanoparticles
| Name of the sources | Nanoparticle size (nm) | Nanoparticle shape | References |
|---|---|---|---|
|
2,4-pentanedionate Ag (I) |
15–36 | Spherical | [315] |
| Arabic gum | 10–30 | Irregular shaped | [316] |
| Ascorbic acid | 29–82 | Spherical | [317] |
| Ascorbic acid and starch | 17–30 | Truncated triangle | [318] |
| Bacterial cellulose | 50–70 | Spherical | [319] |
| B-cyclodextrin grafted with poly acrylic acid [BCD-g-PAA] | 3–22 | Spherical | [320] |
| Casein hydrolytic peptides | 5–15 | Spherical | [321] |
| Chitosan | 5–15 | Spherical | [322] |
| Chitosan | 20–75 | Spherical | [323] |
| Chitosan/PEG | 5–19 | Spherical | [324] |
| Chondroitin 4-sulfate sodium salt | 50–77 | Spherical | [325] |
| Cocos nucifera coir extract (Coconut tree) | 21–25 | Spherical | [326] |
| Citrate | 7 | Spherical | [327] |
| Dextrose | 4–23 | Spherical | [328] |
| Gallic acid | 12–21 | Spherical | [329] |
| Ganoderma applanatum mushroom | 133 | Spherical | [330] |
| Gelatin | 3–14 | Spherical | [331] |
| Gelatin nanoshells | 4.1–6.9 | Spherical | [332] |
| Geraniol | 1–10 | Spherical | [333] |
| Glucose | 30–80 | Irregularly spherical | [334] |
| Glucose | 10–20 | Spherical | [335] |
| Glucose, gelatin | 5–20 | Spherical | [336] |
| Glutathione | 5–10 | Spherical | [337] |
| Graphene | 14–17 | Spherical | [338] |
| Honey | 4–6 | Spherical | [339] |
| Hyaluronan | 5–20 | Spherical | [340] |
| Hydroxypropyl-β-cyclodextrin | 2–5 | Spherical | [341] |
| Lentinus edodes (Edible mushroom) | 50–100 | Walnut | [342] |
| Local honey | 16–25 | Spherical | [343] |
| Maltose | 53–72 | Spherical | [344] |
| Malva parviflora (Cheeseweed) | 19–25 | Spherical | [345] |
| Mushroom Pleurotus florida | 1–3 | Spherical | [346] |
| Mushroom Extract of Pleurotus giganteus | 2–20 | Spherical | [347] |
| Mussel-inspired dopamine (GO-Dopa) | 5–8 | Irregularly spherical | [348] |
| Panicum virgatum (Switchgrass) | 20–40 | Spherical, rod-like, triangular, pentagonal, hexagonal | [349] |
| Pine honey | 21–31 | Spherical | [350] |
| Poly(acrylamide) | 2–5 | Cubical | [351] |
| rGO, MWCNT | 30–50 | Spherical | [352] |
| Ribose sugars, SDS | 7–17 | Spherical | [353] |
| Salmalia malabarica gum | 5–9 | Spherical | [354] |
| Seaweed Urospora sp. | 20–30 | Spherical | [355] |
| Sodium alginate | 12–18 | Spherical | [356] |
| Sodium citrate | 20–25 | Rhombical, hexagonal | [357] |
| Sodium tricitrate | 15–24 | Spherical | [358] |
| Spider cobweb | 3–50 | Spherical | [359] |
| Starch | 20–50 | Spherical | [360] |
| Sucrose | 1–11 | Spherical | [344] |
| Tannic acid | 28–47 | Spherical | [361] |
| Tannic acid | 3.3–22.1 | Spherical | [362] |
| Tannic acid | 7 | Spherical | [327] |
| Thyme honey | 21–31 | Spherical | [350] |
| Trisodium citrate | 32–53 | Spherical | [363] |
Antimicrobial activity of silver nanoparticles and nanocomposites
Antibacterial activity
Silver has always been widely preferred to treat various diseases; it is used as an antiseptic and anti-microbial against Gram-positive and Gram-negative bacteria. Although the highly antibacterial effect of AgNPs has been widely described, silver-based nanocomposites also have gained more attention in many different areas, including antibacterial applications. Generally, the nanocomposite material supports the extended release of silver nanoparticles by adhering to either large-sized or small-sized surface of support materials and thereby increases the anti-microbial activity [364]. The interaction of NPs with polymers not only makes the nanoparticles more compatible with polymer matrix, but also change their properties. The use of polymers in functionalization provides a large surface area and mechanical strength of nanoparticles, which transfers into increased durability and extended use. Moreover, it limits unintended release of nanoparticles into the environment and thereby preventing its loss and aggregation. Among the support materials investigated (Table 11), small-sized SiO2 NPs are cheap and release high quantity of AgNps per unit volume [365].
Table 11.
Details of silver nanocomposites support material and their antibacterial activity
| Name of the support material | Antimicrobial activity | MIC (µg/ml) | References |
|---|---|---|---|
| Graphene oxide | Multidrug-resistant E. coli strains | 4 | [367] |
| Chitosan | Botrytis cinerea | 125 | [368] |
| Silica | Escherichia coli ATCC 2732 | 62.5 | [369] |
| Silica | Klebsiella pneumoniae ATCC 4352 | 62.5 | [369] |
| Silica | Pseudomonas fluorescens LME 2333 | 62.5 | [369] |
| Silica | Salmonella enterica serovar Enteritidis D1 | 62.5 | [369] |
| Silica |
Salmonella enterica serovar Typhimurium DB 7155 |
62.5 | [369] |
| Silica | Enterococcus faecalis ATCC 19433 | 62.5 | [369] |
| Silica | Bacillus cereus ATCC 14579 | 250 | [369] |
| Silica | Listeria monocytogenes Scott A | 500 | [369] |
| Silica | Staphylococcus aureus ATCC 29213 | 250 | [369] |
| Silica | Candida albicans ATCC 10259 | 125 | [369] |
| Silica | Aspergillus niger ATCC 9642 | 2000 | [369] |
| Silica | Escherichia coli ATCC25922 | 7.8 | [370] |
| Silica | Escherichia coli | 100 | [371] |
| Silica | Staphylococcus aureus | 150 | [371] |
| Magnetic silica | Escherichia coli | 15,625 | [372] |
| Magnetic silica | Staphylococcus aureus | 3125 | [372] |
| Mesoporous silica particles | Escherichia coli | 12.5 | [373] |
| Mesoporous silica particles | Staphylococcus aureus | 25 | [373] |
| Mesoporous silica particles | Escherichia coli | 75 | [374] |
| Mesoporous silica particles | Staphylococcus aureus | 75 | [374] |
| TiO2 | Escherichia coli | 200–250 | [375] |
| Chitosan | Staphylococcus aureus | 50–100 | [376] |
| Chitosan | Escherichia coli (CICC 21524) | 32 | [376] |
| Chitosan | Salmonella choleraesuis (CICC 21493) | 64 | [376] |
| Chitosan | Staphylococcus aureus (CICC 10384) | 64 | [376] |
| Chitosan | Vegetative cells of Bacillus subtilis (CGMCC 1.1377) | 32 | [376] |
| Carboxymethyl-cellulose | Enterococcus faecalis | 60 | [377] |
| Diatomite | Staphylococcus aureus | 11.6 | [378] |
| Diatomite | Klebsiella pneumoniae | 232 | [378] |
| SiO2 | Escherichia coli | 195 | [379] |
| SiO2 | Staphylococcus aureus | 390 | [379] |
| SiO2 | Escherichia coli | 10 | [380] |
| SiO2 | Staphylococcus aureus | 4 | [380] |
| SiO2 | Aspergillus niger | 0.13 | [381] |
| SiO2 (irradiation) | Aspergillus niger | 0.06 | [381] |
Antiviral activity
Viruses: infection and targeting
Viruses are the minuscule obligate microbes that infect all form of lives ranging from bacterial pathogens to humans where generation of energy, synthesis and assembly of replication, and other factors for central dogma take place within the host making avail of the host cell machineries for the above process. The gene core material shall be either single or double stranded, ribonucleic acid (RNA), or deoxy-ribo nucleic acid (DNA) encapsulated with proteins made the layer of capsomeric subunits assembly to form either helical or spherical sphere [381].
Infection of viruses has unique stages in the process of viral replication into the host cell starting with attachment to host cell, accumulation of viral load and penetration, the release of viral nucleic acid, processing of nucleic acid as replicative template form and its entry into the host cell nucleus, viral genome replication, transcription and translation of the replicated viral nucleic acid, assembly and release of virions, attachment to the closer proximal cells, and repetition of the cycle [382].
Therapeutic targeting shall be with any one of the above steps and sometimes combinatorial drug targeting two or more steps of the viral load increase. Targeting component shall be fusion inhibitors, channel blocking compounds, transcription blocking compounds, nucleotide polymerase inhibitor, reverse transcriptase and helicase inhibitors, protease and virion assembly inhibitors, neuraminidase inhibitors, and combination from any of the above [383].
Nanosilver: the most unique antiviral
Silver nanoparticles have efficacious anti-microbial properties, which have been taken advantage of for addressing the evolving hyper virulence spikes of different families of viruses during different times. Silver nanoparticles with its exceptional surface area and binding properties exhibit antiviral attributes through interaction either at the binding stage of virus with the host cell (viral entry inhibition) or interference with the viral genome expression cycle inside the cell (virucidal). The out of the ordinary porosity property of silver nanoparticles facilitates the movement and interaction of different other smaller molecules and particles with the viral factor and cellular factors of the viral genome [384, 385].
Silver nanoparticles have a different mechanism of action and activity against viruses such as the affinity of binding to glycoprotein-120, strong competitive binding of cell attachment with the viral strain, interference and inhibitory blocking of viral binding and penetration, viral DNA interaction and inactivation of the viral strain before entry into the host cell, etc. The mechanism for antiviral property of silver metallic nanoparticles with respect to virus entry inhibition includes interaction of metal ions with the host cell-binding surface glycoproteins of the virus and inhibition of the host–virus physical attachment. The denaturation of the protein coat of the virus by irreversible modification of the integrity of the coat frame through reduction of the disulfide bonds and hence diminish the infectivity of the viral residues. The silver nanoparticles are capable of targeting the genetic material of the virus irrespective of the nature of genetic material (DNA, RNA) and their type of strand (single, double). Due to their natural affinity with the phosphate groups of the nucleic acid interacts with the disassembled viral nucleic acid and cellular replication factors thereby preventing the viral replication and or propagation taking place within the host cell and hence block further progeny or virion expression [384–390].
Antiviral spectra of silver nanoparticles
Silver nanoparticles possess a diverse extent of interactive mechanism with every family and classes of virus. Human immunodeficiency virus (HIV), herpes virus, influenza virus, coxsackie, and dengue virus including a range of enveloped, non-enveloped viruses to RNA- and DNA-based virus titer against varying concentrations of silver nanoparticles were studied, and with fold reduction virucidal activity against all the viral classes, the enveloped and positive sense RNA viruses have greater reduction than non-enveloped and negative sense RNA viruses [391, 392].
Lara et al. substantiated the activity of silver nanoparticles against HIV in both the cell-free and cell-associated forms, and found to reduce many fold of the viral gp-120 interaction, accumulative fusion, and virulent factor infectivity with the CD-4 cell receptor of the host cell. With the interaction hypothesis, the AgNPs also tends to denature and weaken the disulfide regions of CD-4-binding domain present in the gp-120 of the viral cell-surface receptor which was reflected with the multi-fold reduced fusion and infectivity making it a suitable candidate for early stage and post-entry target [385].
The novel SARS-CoV2, a member of the family of coronaviridae being the enveloped, single-stranded RNA virus shall be tackled and targeted using silver nanoparticle on the basis of previous works done against epidemic and pandemic of the long past to later past that includes H5N1, H1N1 influenza A to foot and mouth disease of cattle and potato virus Y, and tomato mosaic virus of plants. Reduction in disease severity and viral infection with inhibitory action on localized effects on the host cell was promising to justify the selection of AgNPs as potential candidate for SARS-CoV. AgNPs have a greater enhanced virucidal effect against lettuce infecting tomato bushy stunt virus [TBSV] and also graphene-based silver nanocomposite contributes for absolute suppression of the disease against sun hemp rosetta virus [SHRV] in the plant culture system as potted plants exposed to the viral load sprayed [287, 393, 394].
Feline coronavirus (FCoV) and infectious bursal disease virus (IBDV) were systematically targeted using graphene oxide—silver nanocomposite and the inhibition route were found to be hydrophobic and electrostatic interaction between the aromatic GO plane and lipids. Dipolar bonds between thiol residues and Ag+ ions were another assisting inhibitory route. For non-enveloped viruses, there will be the absence of the hydrophobic interaction, thereby the stronger dipolar (coordinate covalent) bond directs the extent of inhibition [395]. Other composites of silver nanoparticles includes tannic acid, poly vinyl chloride, chitosan as second constituent along with silver that were acted against herpes simplex virus type 2 (HSV-2), human immunodeficiency virus, and H1N1 influenza virus, respectively, follows interference with attachment, membrane receptor channel binding and interaction with the genetic material of the virus upon uncoating [396].
Different results show that the AgNPs’ interaction with gp-120 was found to be size dependent and nanoparticles of 1–10 nm size were able to bind with extra-ordinary activity of inhibition and also involved with reduction of reverse transcription inhibition, so that the transformation of the viral RNA into cDNA gets inhibited and thus the viral load replicative steps and infectivity [397].
Respiratory syncytial infections of viral origin are a peril to humankind by making the infected individuals vulnerable to other range of infections, i.e., serving as a comorbidity to different other diseases. Silver nanoparticles and also its composite exploration as an antiviral agent to such respiratory infections are promising with past to recent present. Silver nanoparticles reduced using ascorbic acid with different weight percentages, capping of graphene oxide (GO) over the silver nanospheres, and silver nanoparticles bound to thiol-group functionalized GO were tested in vivo against coronavirus OC43 and Influenza A virus resulted with mild infectivity inhibition under certain conditions in ascorbic acid reduced AgNP and inhibition only at undiluted level in thiolated samples. Rapid viability and infectivity reduction in intact GO-capped Ag nanospheres observed were promoted by stabilization of bonds with steric hindrance of the composite. Interestingly, the plaque forming ability inhibition of the viruses was found with undiluted (100% concentrated) to diluted to 1% concentration of GO-capped-Ag-nanospheres as there is a synergistic effect between GO-AgNP against enveloped viruses that is independent of carrier solvent in the experiment. Five minute treatment to the viral load in prior infecting to the cell lines rapidly reduces the infectivity. Similar synergistic effect was also observed with the AgNPs–chitosan composites which is higher than the individual activities of them against the infection. Various assays that are useful to find the antiviral activity of the silver nanoparticles include proliferation assay, plaque forming unit assay, cell viability assay, real-time quantification polymerase chain reaction, western blot, cytotoxicity assays and pseudo virus entry assay, indirect immuno fluorescent assay, etc. [398–401].
Silver nanoparticles with their incredible antiviral attributes on monosystem also possess the property of agglomeration due to their tremendous surface energy when present as a single entity in the colloidal solution. Once after the agglomeration the increased grain size diminishes the properties of silver nanoparticles, i.e., reduced stability and activity. Various methodologies have been developed to address the agglomeration of colloidal AgNPs through the process of capping from green components to different inert molecules. The capping agent usually interacts with the external surface of the mono-nanoparticles and thereby reduces the aggregation. Polymers, inert macromolecules, resins and gums, plant extracts, and other capping agents influence the steric and electrostatic stabilization and enhance the activity. The following table (Table 12) comprises representative examples of capping agents with silver nanoparticles and their mode of action against various families of virus [402–405].
Table 12.
Representative example of different capping agents and spectra of virus treated with silver nanoparticles:
| S.No | Type of virus | Family | Capping agent | Size (nm) | Concentration of AgNP | Time of study | Mode of action | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Human immunodeficiency virus—1 | Retroviridae | Polyvinyl pyrrolidone | 30–50 | 0.44 mg/ml (± 0.3) | 48 h | Inhibition through impeding with gp120-CD4 interaction | [385] |
| 2 | Human Immunodeficiency Virus—1 | Retroviridae | Polyurethane | 30–60 | Ag-NPs-coated PUC (1 cm2) | 72 h | Direct transfer of silver ions from oxidized NPs to viral membrane proteins gp120 and gp41 | [406] |
| 3 | Herpes simplex virus—1 (HSV-1 and HSV-2) | Herpesviridae | 48 h | |||||
| 4 | Herpes simplex virus—1 (HSV-1 and HSV-2) | Herpesviridae | – | 4–23 | 10 mg/ml | 72 h | Irreversible inactivation of virions | [407] |
| 5 | Human parainfluenza virus (HPIV-3) | Paramyxoviridae | 5 mg/ml | 48 h | ||||
| 6 | H1N1 Influenza A virus | Orthomyxoviridae | Chitosan | 3.5–12.9 | 100 µg /mg of chitosan | 7 days | Spatial restriction of binding between virions and AgNP/Ch Matrix | [408] |
| 7 | Transmissible gastroenteritis coronavirus | Coronaviridae | Polyoxyethylene Glycerol Trioleate | 10–20 | 3.125–12.5 (µg/ml) | 48 h | Depolarization of host cell’s mitochondrial membrane protein and induction of apoptosis cascade | [409] |
| 8 | Tomato Bushy Stunt Virus | Tombusviridae | Graphene oxide | 30–50 | – | – | spatial distribution of the interacting ligand/receptor molecules between coat proteins of the virus and infected cell receptors | [393] |
| 9 | Respiratory Syncytial Virus | Pneumovirinae | Curcumin | 11–12 | 0.008, 0.015, 0.03, 0.06, 0.12 nM | – | Reduction of cytopathic effects and inactivation of RSV before its entry into the host cell | [410] |
| 10 | Feline coronavirus | Coronaviridae | Graphene oxide | 1–25 | 0.1 mg/ml | 96 h | Negatively charged GO adsorbs to the positively charged lipid membrane and disrupts its integrity | [411] |
| 11 | Infectious bursal disease virus | Birnaviridae | Graphene oxide | 1–0.125 mg/ml | 96 h | Conjugation between the sulfur group of viral protein and silver nanoparticle on GO surface | ||
| 12 | Severe acquired respiratory syndrome—Coronavirus 2 | Coronaviridae | Silicon dioxide | 65 | Approximately 50 ppm | 2–10 min | High oxidizing ROS production led damage to the virus | [412] |
| 13 | Feline calicivirus | Coronaviridae | Poly(tannic acid) | 10.61 ± 1.54 | 20 mm × 20 mm | 72 h | Direct binding of the silver nanoparticles to viral envelope glycoproteins, thereby inhibiting viral penetration into the host cell | [413] |
| 14 | Influenza virus | Orthomyxoviridae |
Silver nanoparticles in SARS-CoV-2 therapy
Silver nanoparticles have their application in a very broader spectrum among which the latest utilization is against the destructive core global pandemic of this millennium, novel coronavirus, severe acute respiratory syndrome-coronavirus 2 (SARS-CoV2), the seventh coronavirus till date from the first virus identified in 1960, which is the one with highest infectivity rate and the fatality rate among the others from the same class [401]. Coronavirinae the subfamily of coronaviridae viral family which is been composed of four genera of viruses such as α-genera, β-genera, γ-genera, and δ-genera among which the alpha and beta genera are so far reported to be infectious to highly infectious against humans, whereas the gamma and delta are targeted to avian species. Around 79% of the similarity with gene sequence of SARS-CoV2 are conserved with SARS-CoV reported earlier and 50% identical sequence with middle-east respiratory syndrome related coronavirus (MERS-CoV). The MERS and SARS-CoV2 binding to cell surface is a remarkable feature of difference among which earlier one binds to dipeptidyl peptidase receptor-4 and the later one to angiotensin-converting enzyme-2 receptor. Such unique non-conserved region and properties make the novel SARS-CoV2 more infectious than any other coronaviridae viruses and thus given the name ‘novel’ coronavirus [414–417].
One among the promising candidatures for the preventive recommendations, treatment has unique position for AgNPs. As AgNPs have been previously reported counter activity against wide spectrum of pneumonia-like zoonotic, acute respiratory viruses, they shall be utilized along the drugs or therapy in combination as well as the single bioactive compound with the therapeutic compound.
The exact sequential mechanism of virus and AgNP interaction have different conceptual hypothesis (Fig. 1) from mimicking as cell-surface receptor to innate immunity activation. Intervention with cell-surface receptor binding and thereby inhibiting the attachment of the virus to the ACE receptor cells. By the attachment of AgNPs to the viral genome inhibits the viral replication inside the host like paramyxoviridae viruses, influenza viruses, retroviridae viruses, and hepatitis B virus. The pH of airway epithelium might become more acidic due the decrease in pH by the release of silver ions, which makes the environment more difficult for the virus to sustain. Ag+ ions have the ability to interact and inhibit the respiratory enzymes of the virus and their potential interference with the viral nucleic acid was already demonstrated against wider spectrum of viruses in the past [394, 396, 418]. The In vitro study on Vero E6 cells infected with a fixed amount of SARS-CoV-2 virus revealed that the concentration of AgNPs between 1 and 10 ppm inhibited the SARS-CoV-2 viral infection by inhibiting the viral entry by disrupting viral integrity [400]. In another In vitro study on SARS-CoV-2 infection in cultured cells showed that a reduction of about 80% cells at a concentration of 0.03% [419]. Nanoparticle composite hybrids of silver, zinc, and copper exhibited vast antiviral property against HIV and other similar enveloped viruses. Capped silver nanoparticles are found to inhibit the negative riboxy nucleic acid strand synthesis of PEDV, another member of the corona virus family. Moreover, the innate immune response induction by the nanocomposites focuses on elimination of the probability of viral progeny development [420].
Fig. 1.
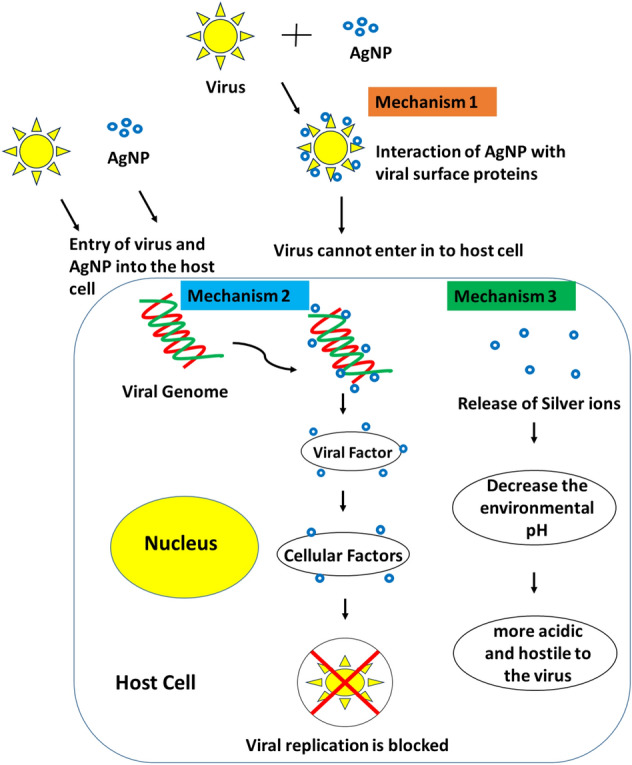
Possible antiviral mechanism of silver nanoparticles
Formulations on the way to store
A provisional patent filed formulation of Quickgun Lifesciences, India has cepharanthine (CEP), a potent inhibitor drug against the virus in screening, loaded in combinatorial with biosilver. This CEP-biosilver oral spray formulation is about to direct a double-targeting of glycoproteins present in the pathogenic virus among which the phyto-derived inhibitor CEP inhibits the replication through targeting the corona virus glycoprotein and AgNPs usually targets the glycoprotein knobs of viruses. In PEGylated form as dry powder, the silver nanoparticles are formulated to deliver in either single dose or multiple dose inhalers. With further research, this drug therapy shall be proven and considered as a potentially safe drug, as AgNPs are with extra-ordinary biocompatible characteristics, but are cytotoxic and apoptotic to cancer and other abnormal cells [421].
Imbed biosciences Inc., a Madison, Wisconsin-based pain killer and wound-healing formulation firm, is working on the integration of the pre-approved microlyte matrix wound-healing complex with the antiviral silver nanoparticles. They are bound with the viral particles and found to interact and freeze the mechanism through which viral particles and human cells interact. In a preliminary research carried out by the Virology Research Institute, London, the AgNPs synthesized by the company, is found to be either -cidic (kill) or -static (inactivate) with 99.9% of the SARS-CoV2. These controlled lab results are really hopeful to take on into the preliminary clinical trials and follow-of-human trials. The product is planned to be delivered in a nasal spray formulation once it clears the levels of pharmaceutical trials [422].
A consortium of companies that include ApIfilm, Braskem, Nanox, and the UFSCar (Brazil) and Jaume I of Castellón (Spain) universities developed and licensed technology of PVC polymer films used in food packaging, coated with silver and silica nanoparticles, is successfully found to inactivate the novel coronavirus. Different time bound direct exposure of novel SARS-CoV2 virus upon the film was carried out and after which the viral particles were made to infect African monkey kidney cell lines, called as vero cells. The infection, virulence and replication rate before and after exposure to films and films without silver and silica coatings were carried out and comparison studies were done. The amplification of viral materials by PCR shows about 99.84% and almost 100% reduction in the viral genetic materials, after exposure time of 2 and 15 min, respectively. A highly satisfactory performance of stretch-wrap wrapping material for perishable food and other grocery items is more about to explore and the exact mechanism studies shall open up more avenues of improvised strategies to tackle the novel SARS-CoV2 [423].
Recent research includes incorporative application of the AgNPs’ coating and dispersion in train cargos, air filters, handles of subways, handrails of elevators and to surgical face masks, medical devices like personal protection equipment kits, and the list of consumables extends.
Pros and cons of faster human trials of silver nanoparticles
Several advantages of utilizing AgNPs as candidature to SARS-CoV2 newer virus variants with faster clinical trials than usual include greater probability of effective virucidal properties to similar respiratory syncytial disease causing viruses, rapid activation of the host’s innate immunity response and cascade system, greater stability, biocompatibility and easy to control over coating process, diverse choice of conjugation and hybrid therapy as they are encapsulated nanocarrier themselves, synergistic property with improved efficacy and reduced level of resistance, and availability of valid, standard, optimized, controlled, and commendable property engineering technologies [424–426].
Concerns range from availability of nanoparticle precursor, activity variation with respect to the source of nanoparticle synthesized, non-optimized and unstandardized procedure of surface coatings, pharmacodynamics and pharmacokinetic studies of the antiviral candidatures, and no application restrictions framework—MRI exposure to metallic nanoparticle-coated mask leads to face burn that WHO advised lately. Lack of a proper standard disposal protocol of silver incorporated products shall be an eco-system pressure created on the natural microbiota of the environment [400]. The selection of the capping agent that provides prolonged stability to the silver nanoparticles coating from a wide range of such preceding successful components should be appropriately chosen with the trials. However, eventual addressing of all such cons and standardizing the protocols for prolonged activity retainment on the coated surfaces shall take the integrated research to tackle SARS-CoV2 for the very next level.
Conclusion
Silver nanoparticles, the prominent aspiring and promising candidate against the multitude of applications, have been narrowed toward its antiviral spectra attributes with the review. Green synthesis of silver nanoparticles through phyto-mediated route is found to be more promising due to its simplicity of conduction and presence of versatile natural plant-based compounds such as polyphenols to alkaloids, etc., provides the combined arena for synthesis that covers nanoparticles of varying sizes and morphologies as the outcome. Tailoring and scale-up of the plant-mediated route has a higher edge and ease of convenience compared to the microbe-mediated and other macromolecule-mediated methods of synthesis.
Silver nanoparticles’ activity against the virus is dependent on various factors such as the size and concentration of the nanoparticles, enveloped and non-enveloped coat of virus, nature of genetic material (DNA /RNA), sensing of strand (positive/negative sense strands), agglomeration, etc. Binding with the glycoproteins to formation of affinity interactions and denaturation of the bonds of viral surface, there are conglomerated routes through which the inactivation and the disintegration of viral strain takes place at different targeting points such as during entry and post-entry. Several SARS-CoV2 formulations utilize silver as a core targeting compound or with combinatorial drugs as hybrids. The proven antiviral property strongly suggests the usage of silver nanoparticles with appropriate capping agent coatings and also with composites in surface sterilization to therapeutic targeting. A newer avenue of being a composite component of targeted drug delivery system emulsions to being the core component of drug composite, multiple actions of silver nanoparticles against existing and still evolving viruses would be a more fascinating research and development area of the near to far future.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare no conflict of interest in preparing this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
C. Karthik and K. A. Punnaivalavan are equal contributors.
References
- 1.Mousavi SM, Hashemi SA, Ghasemi Y, Atapour A, Amani AM, Savar Dashtaki A, Arjmand O. Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif. Cells. Nanomed. Biotechnol. 2018 doi: 10.1080/21691401.2018.1517769. [DOI] [PubMed] [Google Scholar]
- 2.Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019 doi: 10.1039/c8ra08982e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res. 2016 doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG. Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019 doi: 10.1016/j.jddst.2019.101174. [DOI] [Google Scholar]
- 5.Kaabipour S, Hemmati S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021 doi: 10.3762/bjnano.12.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park Y. New paradigm shift for the green synthesis of antibacterial silver nanoparticles utilizing plant extracts. Toxicol. Res. 2014 doi: 10.5487/TR.2014.30.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy S, Das TK. Plant mediated green synthesis of silver nanoparticles—a review. Int J Plant Biol Res. 2015;3(3):1044. [Google Scholar]
- 8.Rauwel P, Küünal S, Ferdov S, Rauwel E. A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv. Mater. Sci. Eng. 2015 doi: 10.1155/2015/68274. [DOI] [Google Scholar]
- 9.El-Aassar MR, Ibrahim OM, Fouda MM, El-Beheri NG, Agwa MM. Wound healing of nanofiber comprising polygalacturonic/hyaluronic acid embedded silver nanoparticles: in-vitro and in-vivo studies. Carbohydr. Polym. 2020 doi: 10.1016/j.carbpol.2020.116175. [DOI] [PubMed] [Google Scholar]
- 10.Ghareeb RY, Alfy H, Fahmy AA, Ali HM, Abdelsalam NR. Utilization of cladophora glomerata extract nanoparticles as eco-nematicide and enhancing the defense responses of tomato plants infected by Meloidogyne javanica. Sci. Rep. 2020 doi: 10.1038/s41598-020-77005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goel V, Kaur P, Singla LD, Choudhury D. Biomedical evaluation of lansium parasiticum extract-protected silver nanoparticles against haemonchus contortus, a parasitic worm. Front. Mol. Biosci. 2020 doi: 10.3389/fmolb.2020.595646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokura S, Handa O, Takagi T, Ishikawa T, Naito Y, Yoshikawa T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomedicine. 2010 doi: 10.1016/j.nano.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010 doi: 10.1002/wnan.103. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson LJ, White RJ, Chipman JK. Silver and nanoparticles of silver in wound dressings: a review of efficacy and safety. J. Wound. Care. 2011 doi: 10.12968/jowc.2011.20.11.543. [DOI] [PubMed] [Google Scholar]
- 15.Bogireddy NKR, Kumar HAK, Mandal BK. Biofabricated silver nanoparticles as green catalyst in the degradation of different textile dyes. J. Environ. Chem. Eng. 2016 doi: 10.1016/j.jece.2015.11.004. [DOI] [Google Scholar]
- 16.Talapko J, Matijević T, Juzbašić M, Antolović-Požgain A, Škrlec I. Antibacterial activity of silver and its application in dentistry. Cardiology and dermatology. Microorganisms. 2020 doi: 10.3390/microorganisms8091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacali C, Baldea I, Moldovan M, Carpa R, Olteanu DE, Filip GA, Badea F. Flexural strength, biocompatibility, and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin. Oral. Investig. 2019 doi: 10.1007/s00784-019-03133-2. [DOI] [PubMed] [Google Scholar]
- 18.Dong Y, Ye H, Liu Y, Xu L, Wu Z, Hu X, Wu G. pH dependent silver nanoparticles releasing titanium implant: a novel therapeutic approach to control peri-implant infection. Colloids Surf. B. 2017 doi: 10.1016/j.colsurfb.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Mahmud R, Nabi F. Application of nanotechnology in the field of textile. IOSR J. Polymer. Text. Eng. 2017 doi: 10.9790/019X-04010106. [DOI] [Google Scholar]
- 20.Liu W, Majumdar S, Li W, Keller AA, Slaveykova VI. Metabolomics for early detection of stress in freshwater alga poterioochromona s malhamensis exposed to silver nanoparticles. Sci. Rep. 2020 doi: 10.1038/s41598-020-77521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung IM, Park I, Seung-Hyun K, Thiruvengadam M, Rajakumar G. Plant-mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res. Lett. 2016 doi: 10.1186/s11671-016-1257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhuri SK, Chandela S, Malodia L. Plant mediated green synthesis of silver nanoparticles using tecomella undulata leaf extract and their characterization. Nano Biomed. Eng. 2016 doi: 10.5101/nbe.v8i1.p1-8. [DOI] [Google Scholar]
- 23.Chahardooli M, Khodadadi E, Khodadadi E. Green synthesis of silver nanoparticles using oak leaf and fruit extracts (Quercus) and its antibacterial activity against plant pathogenic bacteria. Int. J. Biosci. 2014 doi: 10.12692/ijb/4.3.97-103. [DOI] [Google Scholar]
- 24.Shetty P, Supraja N, Garud M, Prasad TNVKV. Synthesis, characterization and antimicrobial activity of Alstonia scholaris bark-extract-mediated silver nanoparticles. J. Nanostruct. Chem. 2014 doi: 10.1007/s40097-014-0132-z. [DOI] [Google Scholar]
- 25.Ankanna STNVKVP, Prasad TNVKV, Elumalai EK, Savithramma N. Production of biogenic silver nanoparticles using Boswellia ovalifoliolata stem bark. Digest. J. Nanomater. Biostruct. 2010;5(2):369–372. [Google Scholar]
- 26.Aina DA, Owolo O, Lateef A, Aina FO, Hakeem AS, Adeoye-Isijola M, Okon V, Asafa TB, Elegbede JA, Olukanni OD, Adediji I. Biomedical applications of chasmanthera dependens stem extract mediated silver nanoparticles as antimicrobial, antioxidant, anticoagulant, thrombolytic, and larvicidal agents. Karbala Int J. Mod. Sci. 2019;5(2):2. doi: 10.33640/2405-609X.1018. [DOI] [Google Scholar]
- 27.Balachandar R, Gurumoorthy P, Karmegam N, Barabadi H, Subbaiya R, Anand K, Boomi P, Saravanan M. Plant-mediated synthesis, characterization and bactericidal potential of emerging silver nanoparticles using stem extract of phyllanthus pinnatus: a recent advance in phytonanotechnology. J. Clust. Sci. 2019;30(6):1481–1488. doi: 10.1007/s10876-019-01591-y. [DOI] [Google Scholar]
- 28.Borase HP, Patil CD, Suryawanshi RK, Patil SV. Ficus carica latex-mediated synthesis of silver nanoparticles and its application as a chemophotoprotective agent. Appl. Biochem. Biotech. 2013 doi: 10.1007/s12010-013-0385-x. [DOI] [PubMed] [Google Scholar]
- 29.Thakore SI, Nagar PS, Jadeja RN, Thounaojam M, Devkar RV, Rathore PS. Sapota fruit latex mediated synthesis of Ag, Cu mono and bimetallic nanoparticles and their in vitro toxicity studies. Arab. J. Chem. 2019 doi: 10.1016/j.arabjc.2014.12.042. [DOI] [Google Scholar]
- 30.Ojemaye MO, Okoh SO, Okoh AI. Silver nanoparticles (AgNPs) facilitated by plant parts of Crataegus ambigua Becker AK extracts and their antibacterial, antioxidant and antimalarial activities. Green. Chem. Lett. Rev. 2020 doi: 10.1080/17518253.2020.1861344. [DOI] [Google Scholar]
- 31.Nithya Deva Krupa A, Raghavan V. Biosynthesis of silver nanoparticles using Aegle marmelos (Bael) fruit extract and its application to prevent adhesion of bacteria: a strategy to control microfouling. Bioinorg. Chem. Appl. 2014 doi: 10.1155/2014/949538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittal AK, Kaler A, Banerjee UC. Free radical scavenging and antioxidant activity of silver nanoparticles synthesized from flower extract of rhododendron dauricum. Nano Biomed. Eng. 2012 doi: 10.5101/nbe.v4i3.p118-124. [DOI] [Google Scholar]
- 33.Saygi KO, Cacan E. Antioxidant and cytotoxic activities of silver nanoparticles synthesized using Tilia cordata flowers extract. Mat. Today. Comm. 2021 doi: 10.1016/j.mtcomm.2021.102316. [DOI] [Google Scholar]
- 34.Ingarsal N, Kasthuri V, Vinothkanna A, Ananth S. Woodfordia fruticosa flower extract mediated silver nanoparticles and its prodigious potential as antioxidant, antibacterial and photocatalyst. Ann. Rom. Soc. Cell Biol. 2021;25:3022–3037. [Google Scholar]
- 35.Jeyasundari J, Praba PS, Jacob YBA, Rajendran S, Kaleeswari K. Green synthesis and characterization of silver nanoparticles using mimusops elengi flower extract and its synergistic antimicrobial potential. Am. Chem. Sci. J. 2016 doi: 10.9734/ACSJ/2016/23161. [DOI] [Google Scholar]
- 36.Rashmi V, Sanjay KR. Green synthesis, characterisation and bioactivity of plant-mediated silver nanoparticles using decalepis hamiltonii root extract. IET Nanobiotechnol. 2017 doi: 10.1049/iet-nbt.2016.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukunthan KS, Balaji S. Silver nanoparticles shoot up from the root of Daucus carrota (L.) Int. J. Green Nanotech. 2012 doi: 10.1080/19430892.2012.654745. [DOI] [Google Scholar]
- 38.Scherer MD, Sposito JC, Falco WF, Grisolia AB, Andrade LH, Lima SM, Machado G, Nascimento VA, Gonçalves DA, Wender H, Oliveira SL. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of allium cepa roots: a close analysis of particle size dependence. Sci. Tot. Env. 2019;660:459–467. doi: 10.1016/j.scitotenv.2018.12.444. [DOI] [PubMed] [Google Scholar]
- 39.Teerasong S, Jinnarak A, Chaneam S, Wilairat P, Nacapricha D. Poly (vinyl alcohol) capped silver nanoparticles for antioxidant assay based on seed-mediated nanoparticle growth. Talanta. 2017 doi: 10.1016/j.talanta.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Ma J, Guo X, Ge H, Tian G, Zhang Q. Seed-mediated photodeposition route to Ag-decorated SiO2@ TiO2 microspheres with ideal core-shell structure and enhanced photocatalytic activity. Appl. Surf. Sci. 2018 doi: 10.1016/j.apsusc.2017.11.020. [DOI] [Google Scholar]
- 41.Aravinthan A, Govarthanan M, Selvam K, Praburaman L, Selvankumar T, Balamurugan R, Kamala-Kannan S, Kim JH. Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and rat splenocyte cytotoxic effects. Int. J. Nanomed. 2015 doi: 10.2147/IJN.S79106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shameli K, Ahmad MB, Zamanian A, Sangpour P, Shabanzadeh P, Abdollahi Y, Zargar M. Green biosynthesis of silver nanoparticles using curcuma longa tuber powder. Int. J. Nanomed. 2012 doi: 10.2147/IJN.S36786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammadalinejhad S, Almasi H, Esmaiili M. Simultaneous green synthesis and in-situ impregnation of silver nanoparticles into organic nanofibers by Lythrum salicaria extract: morphological, thermal, antimicrobial and release properties. Mater. Sci. Eng. C. 2019 doi: 10.1016/j.msec.2019.110115. [DOI] [PubMed] [Google Scholar]
- 44.Wintola OA, Afolayan A. P hytochemical constituents and antioxidant activities of the whole leaf extract of aloe ferox mill. J. Pharmacogn. Mag. 2011 doi: 10.4103/0973-1296.90414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Njoku OV, Obi C. Phytochemical constituents of some selected medicinal plants. Afr. J. Pure Appl. Chem. 2009;3:228–233. [Google Scholar]
- 46.Wadood A, Ghufran M, Jamal SB, Naeem M, Khan A, Ghaffar R. Phytochemical analysis of medicinal plants occurring in local area of mardan. Biochem Anal Biochem. 2013 doi: 10.4172/2161-1009.1000144. [DOI] [Google Scholar]
- 47.Yadav M, Chatterji S, Gupta SK, Watal G. Preliminary phytochemical screening of six medicinal plants used in traditional medicine. Int J Pharm Pharm Sci. 2014;6:539–542. [Google Scholar]
- 48.Sawant RS, Godghate AG. Preliminary phytochemical analysis of leaves of tridax procumbens Linn. Int. J. Environ. Sci. Technol. 2013;2:388–394. [Google Scholar]
- 49.Marimuthu J, Aparna JS, Jeeva S, Sukumaran S, Anantham B. Preliminary phytochemical studies on the methanolic flower extracts of some selected medicinal plants from India. Asian Pac J Trop Biomed. 2012;2:S79–S82. doi: 10.1016/S2221-1691(12)60134-8. [DOI] [Google Scholar]
- 50.Kpenyong CE, Akpan EE, Daniel NE. Phytochemical constituents, therapeutic applications and toxicological profile of cymbopogon citratus stapf (DC) leaf extract. J. Pharmacogn. Phytochem. 2014;3:133–141. [Google Scholar]
- 51.Wang R, Wang R, Yang B. Extraction of essential oils from five cinnamon leaves and identification of their volatile compound compositions. Innov. Food Sci. Emerg. Technol. 2008 doi: 10.1016/j.ifset.2008.12.002. [DOI] [Google Scholar]
- 52.Banerjee P, Satapathy M, Mukhopahayay A, Das P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 2014 doi: 10.1186/s40643-014-0003-y. [DOI] [Google Scholar]
- 53.Okafor F, Janen A, Kukhtareva T, Edwards V, Curley M. Green synthesis of silver nanoparticles, their characterization, application and antibacterial activity. Int. J. Environ. Res. Public Health. 2013 doi: 10.3390/ijerph10105221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao KJ, Paria S. Green synthesis of silver nanoparticles from aqueous Aegle marmelos leaf extract. Mater. Res. Bull. 2013 doi: 10.1016/j.materresbull.2012.11.035. [DOI] [Google Scholar]
- 55.Tippayawat P, Phromviyo N, Boueroy P, Chompoosor A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ. 2016 doi: 10.7717/peerj.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abalkhil TA, Alharbi SA, Salmen SH, Wainwright M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotechnol. Biotechnol. Equip. 2017 doi: 10.1080/13102818.2016.1267594. [DOI] [Google Scholar]
- 57.Kumar DA, Palanichamy V, Roopan SM. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014 doi: 10.1016/j.saa.2014.02.058. [DOI] [PubMed] [Google Scholar]
- 58.Kolya H, Maiti P, Pandey A, Tripathy T. Green synthesis of silver nanoparticles with antimicrobial and azo dye (Congo red) degradation properties using Amaranthus gangeticus Linn leaf extract. J. Anal. Sci. Technol. 2015 doi: 10.1186/s40543-015-0074-1. [DOI] [Google Scholar]
- 59.Govindarajan M, Rajeswary M, Veerakumar K, Muthukumaran U, Hoti SL, Benelli G. Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp. Parasitol. 2016 doi: 10.1016/j.exppara.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 60.Arjunan NK, Murugan K, Rejeeth C, Madhiyazhagan P, Barnard DR. Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vector Borne Zoonotic Dis. 2012 doi: 10.1089/vbz.2011.0661. [DOI] [PubMed] [Google Scholar]
- 61.Dehghanizade S, Arasteh J, Mirzaie A. Green synthesis of silver nanoparticles using anthemis atropatana extract: characterization and in vitro biological activities. Artif. Cells Nanomed. Biotechnol. 2018 doi: 10.1080/21691401.2017.1304402. [DOI] [PubMed] [Google Scholar]
- 62.Kouvaris P, Delimitis A, Zaspalis V, Papadopoulos D, Tsipas SA, Michailidis N. Green synthesis and characterization of silver nanoparticles produced using arbutus unedo leaf extract. Mater. Lett. 2012 doi: 10.1016/j.matlet.2012.02.025. [DOI] [Google Scholar]
- 63.Singh A, Jain D, Upadhyay MK, Khandelwal N, Verma HN. Green synthesis of silver nanoparticles using argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Bios. 2010;5:483–489. [Google Scholar]
- 64.Mousavi B, Tafvizi F, Zaker Bostanabad S. Green synthesis of silver nanoparticles using artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS) Artif. Cells Nanomed. Biotechnol. 2018 doi: 10.1080/21691401.2018.1430697. [DOI] [PubMed] [Google Scholar]
- 65.Safipour Afshar A, Saeid NF. Evaluation of the cytotoxic activity of biosynthesized silver nanoparticles using berberis vulgaris leaf extract. Jentashapir J. Cell. Mol. Biol. 2021 doi: 10.5812/jjcmb.112437. [DOI] [Google Scholar]
- 66.Augustine R, Kalarikkal N, Thomas S. A facile and rapid method for the black pepper leaf mediated green synthesis of silver nanoparticles and the antimicrobial study. Appl. Nanosci. 2014 doi: 10.1007/s13204-013-0260-7. [DOI] [Google Scholar]
- 67.Kumar PV, Pammi SVN, Kollu P, Satyanarayana KVV, Shameem U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti bacterial activity. Ind. Crops Prod. 2014 doi: 10.1016/j.indcrop.2013.10.0. [DOI] [Google Scholar]
- 68.Carmona ER, Benito N, Plaza T, Recio-Sánchez G. Green synthesis of silver nanoparticles by using leaf extracts from the endemic Buddleja globosa hope. Green Chem. Lett. Rev. 2017 doi: 10.1080/17518253.2017.1360400. [DOI] [Google Scholar]
- 69.Patra S, Mukherjee S, Barui AK, Ganguly A, Sreedhar B, Patra CR. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C. 2015 doi: 10.1016/j.msec.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 70.Kalimuthu K, Panneerselvam C, Murugan K, Hwang JS. Green synthesis of silver nanoparticles using Cadaba indica lam leaf extract and its larvicidal and pupicidal activity against anopheles stephensi and Culex quinquefasciatus. J. Entomol. Acarol. Res. 2013 doi: 10.4081/jear.2013.e11. [DOI] [Google Scholar]
- 71.Jain D, Daima HK, Kachhwaha S, Kothari SL. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Dig. J. Nanomater. Biostruct. 2009;4:557–563. [Google Scholar]
- 72.Banala RR, Nagati VB, Karnati PR. Green synthesis and characterization of Carica papaya leaf extract coated silver nanoparticles through X-ray diffraction, electron microscopy and evaluation of bactericidal properties. Saudi J. Biol. Sci. 2015 doi: 10.1016/j.sjbs.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Awwad AM, Salem NM, Abdeen AO. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int. J. Ind. Chem. 2013 doi: 10.1186/2228-5547-4-29. [DOI] [Google Scholar]
- 74.Muthukumaran U, Govindarajan M, Rajeswary M. Green synthesis of silver nanoparticles from Cassia roxburghii—a most potent power for mosquito control. Parasitol. Res. 2015 doi: 10.1007/s00436-015-4677-7. [DOI] [PubMed] [Google Scholar]
- 75.Parlinska-Wojtan M, Kus-Liskiewicz M, Depciuch J, Sadik O. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using camomile terpenoids as a combined reducing and capping agent. Bioproc. Biosyst. Eng. 2016 doi: 10.1007/s00449-016-1599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arokiyaraj S, Arasu MV, Vincent S, Prakash NU, Choi SH, Oh YK, Kim KH. Rapid green synthesis of silver nanoparticles from chrysanthemum indicum L and its antibacterial and cytotoxic effects: an in vitro study. Int. J. Nanomed. 2014 doi: 10.2147/IJN.S53546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Satyavani K, Gurudeeban S, Ramanathan T, Balasubramanian T. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J. Nanobiotechnol. 2011 doi: 10.1186/1477-3155-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Narayanan KB, Sakthivel N. Extracellular synthesis of silver nanoparticles using the leaf extract of Coleus amboinicus Lour. Mater. Res. Bull. 2011 doi: 10.1016/j.materresbull.2011.05.041. [DOI] [Google Scholar]
- 79.Vanaja M, Annadurai G. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl. Nanosci. 2013 doi: 10.1007/s13204-012-0121-9. [DOI] [Google Scholar]
- 80.Johnson I, Prabu H. Green synthesis and characterization of silver nanoparticles by leaf extracts of cycas circinalis, ficus amplissima, commelina benghalensis and lippia nodiflora. J. Int. Nano. Lett. 2015 doi: 10.1007/s40089-014-0136-1. [DOI] [Google Scholar]
- 81.Mosaviniya M, Kikhavani T, Tanzifi M, Yaraki MT, Tajbakhsh P, Lajevardi A. Facile green synthesis of silver nanoparticles using crocus haussknechtii bois bulb extract: catalytic activity and antibacterial properties. Colloids Interface Sci. Commun. 2019 doi: 10.1016/j.colcom.2019.100211. [DOI] [Google Scholar]
- 82.Jha AK, Prasad K, Kumar V, Prasad K. Biosynthesis of silver nanoparticles using eclipta leaf. Biotechnol. Prog. 2009 doi: 10.1002/btpr.233. [DOI] [PubMed] [Google Scholar]
- 83.Kesharwani J, Yoon KY, Hwang J, Rai M. Phytofabrication of silver nanoparticles by leaf extract of datura metel: hypothetical mechanism involved in synthesis. J. Bionanosci. 2009 doi: 10.1166/jbns.2009.1008. [DOI] [Google Scholar]
- 84.Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009 doi: 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- 85.Mo YY, Tang YK, Wang SY, Lin JM, Zhang HB, Luo DY. Green synthesis of silver nanoparticles using eucalyptus leaf extract. Mater. Lett. 2015 doi: 10.1016/j.matlet.2015.01.004. [DOI] [Google Scholar]
- 86.Sulaiman GM, Mohammed WH, Marzoog TR, Al-Amiery AAA, Kadhum AAH, Mohamad AB. Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles using eucalyptus chapmaniana leaves extract. Asian Pac. J. Trop. Biomed. 2013;3:58–63. doi: 10.1016/S2221-1691(13)60024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ali K, Ahmed B, Dwivedi S, Saquib Q, Al-Khedhairy AA, Musarrat J. Microwave accelerated green synthesis of stable silver nanoparticles with Eucalyptus globulus leaf extract and their antibacterial and antibiofilm activity on clinical isolates. PLoS ONE. 2015 doi: 10.1371/journal.pone.0131178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rahimi-Nasrabadi M, Pourmortazavi SM, Shandiz SAS, Ahmadi F, Batooli H. Green synthesis of silver nanoparticles using Eucalyptus leucoxylon leaves extract and evaluating the antioxidant activities of extract. Nat. Prod. Res. 2014 doi: 10.1080/14786419.2014.918124. [DOI] [PubMed] [Google Scholar]
- 89.Pourmortazavi SM, Taghdiri M, Makari V, Rahimi-Nasrabadi M. Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of eucalyptus oleosa. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015 doi: 10.1016/j.saa.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 90.Shah AT, Din MI, Bashir S, Qadir MA, Rashid F. Green synthesis and characterization of silver nanoparticles using ferocactus echidne extract as a reducing agent. Anal. Lett. 2015 doi: 10.1080/00032719.2014.974057. [DOI] [Google Scholar]
- 91.Ulug B, Turkdemir MH, Cicek A, Mete A. Green synthesis of silver nanoparticles using aqueous solution of ficus benghalensis leaf extract and characterization of their antibacterial activity. Spectrochim. Acta A Mol. Biomol. 2015 doi: 10.1016/j.saa.2014.06.142. [DOI] [Google Scholar]
- 92.Parveen M, Ahmad F, Malla AM, Azaz S. Microwave-assisted green synthesis of silver nanoparticles from fraxinus excelsior leaf extract and its antioxidant assay. Appl. Nanosci. 2016 doi: 10.1007/s13204-015-0433-7. [DOI] [Google Scholar]
- 93.Manosalva N, Tortella G, Diez MC, Schalchli H, Seabra AB, Durán N, Rubilar O. Green synthesis of silver nanoparticles: effect of synthesis reaction parameters on antimicrobial activity. World J. Microbiol. Biotechnol. 2019 doi: 10.1007/s11274-019-2664-3. [DOI] [PubMed] [Google Scholar]
- 94.Allafchian AR, Jalali SAH, Aghaei F, Farhang HR. Green synthesis of silver nanoparticles using glaucium corniculatum (L.) curtis extract and evaluation of its antibacterial activity. IET Nanobiotechnol. 2018 doi: 10.1049/iet-nbt.2017.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asghar MA, Zahir E, Shahid SM, Khan MN, Asghar MA, Iqbal J, Walker G. Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT. 2018 doi: 10.1016/j.lwt.2017.12.009. [DOI] [Google Scholar]
- 96.Selvan DA, Mahendiran D, Kumar RS, Rahiman AK. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: phytochemical, antioxidant and in vitro cytotoxicity studies. J. Photochem. Photobiol. B. 2018 doi: 10.1016/j.jphotobiol.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 97.Nakhjavani M, Nikkhah V, Sarafraz MM, Shoja S, Sarafraz M. Green synthesis of silver nanoparticles using green tea leaves: experimental study on the morphological, rheological and antibacterial behaviour. Heat Mass Transf. 2017 doi: 10.1007/s00231-017-2065-9. [DOI] [Google Scholar]
- 98.Rostami-Vartooni A, Nasrollahzadeh M, Alizadeh M. Green synthesis of perlite supported silver nanoparticles using Hamamelis virginiana leaf extract and investigation of its catalytic activity for the reduction of 4-nitrophenol and congo red. J. Alloys Compd. 2016 doi: 10.1016/j.jallcom.2016.04.008. [DOI] [Google Scholar]
- 99.Thatoi P, Kerry RG, Gouda S, Das G, Pramanik K, Thatoi H, Patra JK. Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species, heritiera fomes and sonneratia apetala and investigation of their biomedical applications. J. Photochem. Photobiol. B. 2016 doi: 10.1016/j.jphotobiol.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 100.Karthik C, Caroline DG, Dhanam Priya M, Pandi Prabha S. Synthesis, characterization of Ag-SiO2 nanocomposite and its application in food packaging. J Inorg Organomet Polym. 2021 doi: 10.1007/s10904-020-01853-7. [DOI] [Google Scholar]
- 101.Dipankar C, Murugan S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from iresine herbstii leaf aqueous extracts. Colloids Surf. B. 2012 doi: 10.1016/j.colsurfb.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 102.Karuppiah M, Rajmohan R. Green synthesis of silver nanoparticles using ixora coccinea leaves extract. Mater. Lett. 2013 doi: 10.1016/j.matlet.2013.01.087. [DOI] [Google Scholar]
- 103.Emmanuel R, Palanisamy S, Chen SM, Chelladurai K, Padmavathy S, Saravanan M, Al-Hemaid FM. Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Mater. Sci. Eng. C. 2015 doi: 10.1016/j.msec.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 104.Ajitha B, Reddy YAK, Reddy PS. Green synthesis and characterization of silver nanoparticles using lantana camara leaf extract. Mater. Sci. Eng. C. 2015 doi: 10.1016/j.msec.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 105.Swamy MK, Sudipta KM, Jayanta K, Balasubramanya S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from leptadenia reticulata leaf extract. Appl. Nanosci. 2015 doi: 10.1007/s13204-014-0293-6. [DOI] [Google Scholar]
- 106.Balan K, Qing W, Wang Y, Liu X, Palvannan T, Wang Y, Zhang Y. Antidiabetic activity of silver nanoparticles from green synthesis using lonicera japonica leaf extract. RSC Adv. 2016 doi: 10.1039/c5ra24391b. [DOI] [Google Scholar]
- 107.Garibo D, Borbón-Nuñez HA, de León JND, Mendoza EG, Estrada I, Toledano-Magaña Y, Susarrey-Arce A. Green synthesis of silver nanoparticles using lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020 doi: 10.1038/s41598-020-69606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marimuthu S, Rahuman AA, Rajakumar G, Santhoshkumar T, Kirthi AV, Jayaseelan C, Kamaraj C. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol. Res. 2011 doi: 10.1007/s00436-010-2212-4. [DOI] [PubMed] [Google Scholar]
- 109.Veerasamy R, Xin TZ, Gunasagaran S, Xian TFW, Yang EFC, Jeyakumar N, Dhanaraj SA. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011 doi: 10.1016/j.jscs.2010.06.004. [DOI] [Google Scholar]
- 110.Erci F, Cakir-Koc R, Isildak I. Green synthesis of silver nanoparticles using Thymbra spicata L. var. spicata (zahter) aqueous leaf extract and evaluation of their morphology-dependent antibacterial and cytotoxic activity. Artif. Cells Nanomed. Biotechnol. 2018 doi: 10.1080/21691401.2017.1415917. [DOI] [PubMed] [Google Scholar]
- 111.Prakash P, Gnanaprakasam P, Emmanuel R, Arokiyaraj S, Saravanan M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B. 2013 doi: 10.1016/j.colsurfb.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 112.Moodley JS, Krishna SBN, Pillay K, Govender P. Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci.-Nanosci. 2018 doi: 10.1088/2043-6254/aaabb2. [DOI] [Google Scholar]
- 113.Awwad AM, Salem NM. Green synthesis of silver nanoparticles by mulberry leaves extract. Nanosci. Nanotechnol. 2012 doi: 10.5923/j.nn.20120204.06. [DOI] [Google Scholar]
- 114.Suganya A, Murugan K, Kovendan K, Kumar PM, Hwang JS. Green synthesis of silver nanoparticles using Murraya koenigii leaf extract against Anopheles stephensi and Aedes aegypti. Parasitol. Res. 2013 doi: 10.1007/s00436-012-3269-z. [DOI] [PubMed] [Google Scholar]
- 115.Philip D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys. E Low Dimens. Syst. 2010 doi: 10.1016/j.physe.2009.11.081. [DOI] [Google Scholar]
- 116.Francis S, Joseph S, Koshy EP, Mathew B. Green synthesis and characterization of gold and silver nanoparticles using Mussaenda glabrata leaf extract and their environmental applications to dye degradation. Environ. Sci. Pollut. Res. 2017 doi: 10.1007/s11356-017-9329-2. [DOI] [PubMed] [Google Scholar]
- 117.Phanjom P, Zoremi E, Mazumder J, Saha M, Baruah SB. Green synthesis of silver nanoparticles using leaf extract of Myrica esculenta. Int J NanoSci Nanotechnol. 2012;3:73–79. [Google Scholar]
- 118.Santhoshkumar T, Rahuman AA, Rajakumar G, Marimuthu S, Bagavan A, Jayaseelan C, Kamaraj C. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol. Res. 2011 doi: 10.1007/s00436-010-2115-4. [DOI] [PubMed] [Google Scholar]
- 119.Prasad KS, Pathak D, Patel A, Dalwadi P, Prasad R, Patel P, Selvaraj K. Biogenic synthesis of silver nanoparticles using Nicotiana tobaccum leaf extract and study of their antibacterial effect. Afr. J. Biotechnol. 2011 doi: 10.5897/AJB11.394. [DOI] [Google Scholar]
- 120.Rout Y, Behera S, Ojha AK, Nayak PL. Green synthesis of silver nanoparticles using Ocimum sanctum (Tulashi) and study of their antibacterial and antifungal activities. J. Microbiol. Antimicrob. 2012 doi: 10.5897/JMA11.060. [DOI] [Google Scholar]
- 121.Karthik C, Suresh S, Mirulalini S, Kavitha S. FTIR approach of green synthesized silver nanoparticles by Ocimum sanctum and Ocimum gratissimum on mung bean seeds. Inorg. Nano-Met. Chem. 2020 doi: 10.1080/24701556.2020.1723025. [DOI] [Google Scholar]
- 122.Rao YS, Kotakadi VS, Prasad TNVKV, Reddy AV, Gopal DS. Green synthesis and spectral characterization of silver nanoparticles from lakshmi tulasi (Ocimum sanctum) leaf extract. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2013 doi: 10.1016/j.saa.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 123.Jain S, Mehata MS. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 2017 doi: 10.1038/s41598-017-15724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mallikarjuna K, Narasimha G, Dillip GR, Praveen B, Shreedhar B, Lakshmi CS, Raju BDP. Green synthesis of silver nanoparticles using ocimum leaf extract and their characterization. Dig. J. Nanomater. Biostruct. 2011;6:181–186. [Google Scholar]
- 125.Khalil MM, Ismail EH, El-Baghdady KZ, Mohamed D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014 doi: 10.1016/j.arabjc.2013.04.007. [DOI] [Google Scholar]
- 126.Rajendran R, Ganesan N, Balu SKA, Thandavamorthy P, Thiruvengadam D. Green synthesis, characterization, antimicrobial and cytotoxic effects of silver nanoparticles using Origanum heracleoticum L. leaf extract. Int J Pharm Pharm Sci. 2015;7:288–293. [Google Scholar]
- 127.Dong C, Zhang X, Cai H, Cao C. Green synthesis of biocompatible silver nanoparticles mediated by Osmanthus fragrans extract in aqueous solution. Optik. 2016 doi: 10.1016/j.ijleo.2016.08.055. [DOI] [Google Scholar]
- 128.Jegadeeswaran P, Shivaraj R, Venckatesh R. Green synthesis of silver nanoparticles from extract of Padina tetrastromatica leaf. Dig. J. Nanomater. Biostructures. 2012;7:991–998. [Google Scholar]
- 129.Mollick MMR, Bhowmick B, Maity D, Mondal D, Bain MK, Bankura K, Chattopadhyay D. Green synthesis of silver nanoparticles using Paederia foetida L. leaf extract and assessment of their antimicrobial activities. Int. J. Green Nanotechnol. 2012 doi: 10.1080/19430892.2012.706103. [DOI] [Google Scholar]
- 130.Ravichandran V, Vasanthi S, Shalini S, Shah SAA, Tripathy M, Paliwal N. Green synthesis, characterization, antibacterial, antioxidant and photocatalytic activity of Parkia speciosa leaves extract mediated silver nanoparticles. Results. Phys. 2019 doi: 10.1016/j.rinp.2019.102565. [DOI] [Google Scholar]
- 131.Parashar V, Parashar R, Sharma B, Pandey AC. Parthenium leaf extract mediated synthesis of silver nanoparticles: a novel approach towards weed utilization. Dig. J. Nanomater. Biostructures. 2009;4:45–50. [Google Scholar]
- 132.Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016 doi: 10.1007/s13204-015-0449-z. [DOI] [Google Scholar]
- 133.Emeka EE, Ojiefoh OC, Aleruchi C, Hassan LA, Christiana OM, Rebecca M, Temitope AE. Evaluation of antibacterial activities of silver nanoparticles green-synthesized using pineapple leaf (Ananas comosus) Micron. 2014 doi: 10.1016/j.micron.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 134.Paulkumar K, Gnanajobitha G, Vanaja M, Rajeshkumar S, Malarkodi C, Pandian K, Annadurai G. Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci. World. J. 2014 doi: 10.1155/2014/829894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kumar B, Smita K, Cumbal L, Debut A. Synthesis of silver nanoparticles using Sacha inchi (Plukenetia volubilis L.) leaf extracts. Saudi J. Biol. Sci. 2014 doi: 10.1016/j.sjbs.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Habibi B, Hadilou H, Mollaei S, Yazdinezhad A. Green synthesis of silver nanoparticles using the aqueous extract of Prangos ferulaceae leaves. Int. J. Nano Dimens. 2017 doi: 10.22034/ijnd.2017.24954. [DOI] [Google Scholar]
- 137.Saravanakumar A, Peng MM, Ganesh M, Jayaprakash J, Mohankumar M, Jang HT. Low-cost and eco-friendly green synthesis of silver nanoparticles using Prunus japonica (Rosaceae) leaf extract and their antibacterial, antioxidant properties. Artif. Cells. Nanomed. Biotechnol. 2017 doi: 10.1080/21691401.2016.1203795. [DOI] [PubMed] [Google Scholar]
- 138.Kumar R, Ghoshal G, Jain A, Goyal M. Rapid green synthesis of silver nanoparticles (AgNPs) using (Prunus persica) plants extract: exploring its antimicrobial and catalytic activities. J Nanomed Nanotechnol. 2017 doi: 10.4172/2157-7439.1000452. [DOI] [Google Scholar]
- 139.Umashankari J, Inbakandan D, Ajithkumar TT, Balasubramanian T. Mangrove plant, Rhizophora mucronata (Lamk, 1804) mediated one pot green synthesis of silver nanoparticles and its antibacterial activity against aquatic pathogens. Aquat. Biosyst. 2012 doi: 10.1186/2046-9063-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dubey SP, Lahtinen M, Sillanpää M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf. A Physicochem. Eng. Asp. 2010 doi: 10.1016/j.colsurfa.2010.04.023. [DOI] [Google Scholar]
- 141.Ghaedi M, Yousefinejad M, Safarpoor M, Khafri HZ, Purkait MK. Rosmarinus officinalis leaf extract mediated green synthesis of silver nanoparticles and investigation of its antimicrobial properties. J Ind Eng Chem. 2015 doi: 10.1016/j.jiec.2015.06.020. [DOI] [Google Scholar]
- 142.Pirtarighat S, Ghannadnia M, Baghshahi S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J Nanostruct. Chem. 2019 doi: 10.1007/s40097-018-0291-4. [DOI] [Google Scholar]
- 143.Verma DK, Hasan SH, Banik RM. Photo-catalyzed and phyto-mediated rapid green synthesis of silver nanoparticles using herbal extract of Salvinia molesta and its antimicrobial efficacy. J. Photochem. Photobiol. B. 2016 doi: 10.1016/j.jphotobiol.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 144.Perugu S, Nagati V, Bhanoori M. Green synthesis of silver nanoparticles using leaf extract of medicinally potent plant Saraca indica: a novel study. Appl. Nanosci. 2016 doi: 10.1007/s13204-015-0486-7. [DOI] [Google Scholar]
- 145.Donda MR, Kudle KR, Alwala J, Miryala A, Sreedhar B, Rudra MP. Synthesis of silver nanoparticles using extracts of Securinega leucopyrus and evaluation of its antibacterial activity. Int. J. Curr. Sci. 2013;7:1–8. [Google Scholar]
- 146.Das J, Das MP, Velusamy P. Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim. Acta A Mol. Biomol. 2013 doi: 10.1016/j.saa.2012.11.075. [DOI] [PubMed] [Google Scholar]
- 147.Veerakumar K, Govindarajan M, Rajeswary M. Green synthesis of silver nanoparticles using Sida acuta (Malvaceae) leaf extract against Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti (Diptera: Culicidae) Parasitol. Res. 2013 doi: 10.1007/s00436-013-3598-6. [DOI] [PubMed] [Google Scholar]
- 148.Ahmed MJ, Murtaza G, Mehmood A, Bhatti TM. Green synthesis of silver nanoparticles using leaves extract of Skimmia laureola: characterization and antibacterial activity. Mater. Lett. 2015 doi: 10.1016/j.matlet.2015.03.143. [DOI] [Google Scholar]
- 149.Kagithoju S, Godishala V, Nanna RS. Eco-friendly and green synthesis of silver nanoparticles using leaf extract of Strychnos potatorum Linn. F. and their bactericidal activities. 3 Biotech. 2015 doi: 10.1007/s13205-014-0272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kajani AA, Bordbar AK, Esfahani SHZ, Khosropour AR, Razmjou A. Green synthesis of anisotropic silver nanoparticles with potent anticancer activity using Taxus baccata extract. RSC Adv. 2014 doi: 10.1039/c4ra08758e. [DOI] [Google Scholar]
- 151.Sun Q, Cai X, Li J, Zheng M, Chen Z, Yu CP. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2014 doi: 10.1016/j.colsurfa.2013.12.065. [DOI] [Google Scholar]
- 152.Raj S, Singh H, Trivedi R, Soni V. Biogenic synthesis of AgNPs employing Terminalia arjuna leaf extract and its efficacy towards catalytic degradation of organic dyes. Sci. Rep. 2020 doi: 10.1038/s41598-020-66851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ahmed S, Ikram S. Silver nanoparticles: one pot green synthesis using Terminalia arjuna extract for biological application. J. Nanomed. Nanotechnol. 2015 doi: 10.4172/2157-7439.1000309. [DOI] [Google Scholar]
- 154.Espenti CS, Rao KK, Rao KM. Bio-synthesis and characterization of silver nanoparticles using Terminalia chebula leaf extract and evaluation of its antimicrobial potential. Mater. Lett. 2016 doi: 10.1016/j.matlet.2016.03.106. [DOI] [Google Scholar]
- 155.Femi-Adepoju AG, Dada AO, Otun KO, Adepoju AO, Fatoba OP. Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia Pectinata (Willd.) C. Presl.: characterization and antimicrobial studies. Heliyon. 2019 doi: 10.1016/j.heliyon.2019.e01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Veisi H, Azizi S, Mohammadi P. Green synthesis of the silver nanoparticles mediated by Thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. J. Clean. Prod. 2018 doi: 10.1016/j.jclepro.2017.09.265. [DOI] [Google Scholar]
- 157.Zargar M, Hamid AA, Bakar FA, Shamsudin MN, Shameli K, Jahanshiri F, Farahani F. Green synthesis of the silver nanoparticles mediated by Thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. Molecules. 2011 doi: 10.3390/molecules16086667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mochochoko T, Oluwafemi OS, Jumbam DN, Songca P. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Carbohydr. Polym. 2013 doi: 10.1016/j.carbpol.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kouhbanani MAJ, Beheshtkhoo N, Fotoohiardakani G, Hosseini-Nave H, Taghizadeh S, Amani AM. A biogenic approach for green synthesis of silver nanoparticles using extract of Foeniculum vulgare and its activity against Staphylococcus aureus and Escherichia coli. J. Environ. Treat. 2019;7(1):142–149. [Google Scholar]
- 160.Sadeghi B, Gholamhoseinpoor F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015 doi: 10.1016/j.saa.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 161.Tarh JE, Iroegbu CU. In-vitro anti-bacterial activity of extracts of euphorbia abyssinica (desert candle) stem-bark and latex. J. Adv Microbiol. 2017 doi: 10.9734/JAMB/2017/32277. [DOI] [Google Scholar]
- 162.Kawo A, Mustapha A, Abdullahi B, Rogo L, Gaiya Z, Kumurya A. Phytochemical properties and antibacterial activities of the leaf and latex extracts of calotropis procera. Bayero J. Pure Appl. Sci. 2009;2:34–40. [Google Scholar]
- 163.Aliba MO, Ndukwe IG, Ibrahim H. Isolation and characterization of Β-sitosterol from methanol extracts of the stem bark of large-leaved rock fig (Ficus abutilifolia Miq) J. Appl. Sci. Environ. Manag. 2018 doi: 10.4314/jasem.v22i10.19. [DOI] [Google Scholar]
- 164.Atawodi SE, Atawodi JC, Idakwo GA, Pfundstein B, Haubner R, Wurtele G, Owen RW. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of moringa oleifera lam. J. Med. Food. 2010 doi: 10.1089/jmf.2009.0057. [DOI] [PubMed] [Google Scholar]
- 165.Itoandon EE, Olatope SOA, Shobowale OO. Phytochemical and antimicrobial evaluation of aqueous and organic extracts of calotropis procera ait leaf and latex. Niger. Food J. 2012;30(2):51–56. doi: 10.1016/S0189-7241(15)30035-7. [DOI] [Google Scholar]
- 166.Minh TN, Xuan TD, Tran HD, Van TM, Andriana Y, Khanh TD, Ahmad A. Isolation and purification of bioactive compounds from the stem bark of jatropha podagrica. Molecules. 2019 doi: 10.3390/molecules24050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moyo M, Gomba M, Nharingo T. Afzelia quanzensis bark extract for green synthesis of silver nanoparticles and study of their antibacterial activity. Int. J. Ind. Chem. 2015 doi: 10.1007/s40090-015-0055-7. [DOI] [Google Scholar]
- 168.Pattanayak S, Mollick MMR, Maity D, Chakraborty S, Dash SK, Chattopadhyay S, Chakraborty M. Butea monosperma bark extract mediated green synthesis of silver nanoparticles: characterization and biomedical applications. J. Saudi Chem. Soc. 2017 doi: 10.1016/j.jscs.2015.11.004. [DOI] [Google Scholar]
- 169.Kora AJ, Sashidhar RB, Arunachalam J. Gum kondagogu (Cochlospermum gossypium): a template for the green synthesis and stabilization of silver nanoparticles with antibacterial application. Carbohydr. Polym. 2010 doi: 10.1016/j.carbpol.2010.05.034. [DOI] [Google Scholar]
- 170.De Matos RA, da Silva Cordeiro T, Samad RE, Vieira ND, Jr, Courrol LC. Green synthesis of stable silver nanoparticles using Euphorbia milii latex. Colloids Surf. A Physicochem. Eng. Asp. 2011 doi: 10.1016/j.colsurfa.2011.08.040. [DOI] [Google Scholar]
- 171.Kalaiselvi D, Mohankumar A, Shanmugam G, Nivitha S, Sundararaj P. Green synthesis of silver nanoparticles using latex extract of Euphorbia tirucalli: a novel approach for the management of root knot nematode Meloidogyne incognita. Crop Prot. 2019 doi: 10.1016/j.cropro.2018.11.020. [DOI] [Google Scholar]
- 172.Nayak D, Ashe S, Rauta PR, Kumari M, Nayak B. Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. 2016 doi: 10.1016/j.msec.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 173.Ahamed M, Khan MM, Siddiqui MKJ, AlSalhi MS, Alrokayan SA. Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2011 doi: 10.1016/j.physe.2011.02.014. [DOI] [Google Scholar]
- 174.Medina-Ramirez I, Bashir S, Luo Z, Liu JL. Green synthesis and characterization of polymer-stabilized silver nanoparticles. Colloids Surf. B. 2009 doi: 10.1016/j.colsurfb.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 175.Kora AJ, Beedu SR, Jayaraman A. Size-controlled green synthesis of silver nanoparticles mediated by gum ghatti (Anogeissus latifolia) and its biological activity. Org. Med. Chem. Lett. 2012 doi: 10.1186/2191-2858-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Guidelli EJ, Ramos AP, Zaniquelli MED, Baffa O. Green synthesis of colloidal silver nanoparticles using natural rubber latex extracted from Hevea brasiliensis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011 doi: 10.1016/j.saa.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 177.Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009 doi: 10.1016/j.colsurfa.2009.02.008. [DOI] [Google Scholar]
- 178.Sreekanth TVM, Jung MJ, Eom IY. Green synthesis of silver nanoparticles, decorated on graphene oxide nanosheets and their catalytic activity. Appl. Surf. Sci. 2016 doi: 10.1016/j.apsusc.2015.11.146. [DOI] [Google Scholar]
- 179.Iravani S, Zolfaghari B. Green synthesis of silver nanoparticles using Pinus eldarica bark extract. BioMed. Res. Int. 2013 doi: 10.1155/2013/639725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Arya G, Kumari RM, Gupta N, Kumar A, Chandra R, Nimesh S. Green synthesis of silver nanoparticles using Prosopis juliflora bark extract: reaction optimization, antimicrobial and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018 doi: 10.1080/21691401.2017.1354302. [DOI] [PubMed] [Google Scholar]
- 181.Jadhav K, Dhamecha D, Dalvi B, Patil M. Green synthesis of silver nanoparticles using Salacia chinensis: characterization and its antibacterial activity. Part. Sci. Technol. 2015 doi: 10.1080/02726351.2014.1003628. [DOI] [Google Scholar]
- 182.Miri A, Dorani N, Darroudi M, Sarani M. Green synthesis of silver nanoparticles using Salvadora persica L. and its antibacterial activity. Cell. Mol. Biol. 2016 doi: 10.14715/cmb/2016.62.9.8. [DOI] [PubMed] [Google Scholar]
- 183.Aladpoosh R, Montazer M, Samadi N. In situ green synthesis of silver nanoparticles on cotton fabric using Seidlitzia rosmarinus ashes. Cellulose. 2014 doi: 10.1007/s10570-014-0369-1. [DOI] [Google Scholar]
- 184.Edison TNJI, Lee YR, Sethuraman MG. Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow-12 dye. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016 doi: 10.1016/j.saa.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 185.Rupiasih NN, Aher A, Gosavi S, Vidyasagar PB. Green synthesis of silver nanoparticles using latex extract of Thevetia peruviana: a novel approach towards poisonous plant utilization. J. Phys. Conf. Ser. 2013;423:12–032. doi: 10.1088/1742-6596/423/1/012032. [DOI] [Google Scholar]
- 186.Barros L, Carvalho AM, Morais JS, Ferreira IC. Strawberry-tree, blackthorn and rose fruits: detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010 doi: 10.1016/j.foodchem.2009.10.016. [DOI] [Google Scholar]
- 187.Fiorentino A, D’Abrosca B, Pacifico S, Mastellone C, Scognamiglio M, Monaco P. Identification and assessment of antioxidant capacity of phytochemicals from kiwi fruits. J. Agric. Food Chem. 2009 doi: 10.1021/jf900210z. [DOI] [PubMed] [Google Scholar]
- 188.Kubola J, Siriamornpun S. Phytochemicals and antioxidant activity of different fruit fractions (peel., pulp., aril and seed) of Thai gac (Momordica cochinchinensis Spreng) Food Chem. 2011 doi: 10.1016/j.foodchem.2011.01.115. [DOI] [PubMed] [Google Scholar]
- 189.Bhandary SK, Kumari S, Bhat VS, Sharmila KP, Bekal MP. preliminary phytochemical screening of various extracts of punica granatum peel: whole fruit and seeds. J Health Sci. 2012;2:35–38. [Google Scholar]
- 190.Ali ZA, Yahya R, Sekaran SD, Puteh R. Green synthesis of silver nanoparticles using apple extract and its antibacterial properties. Adv. Mater. Sci. Eng. 2016 doi: 10.1155/2016/4102196. [DOI] [Google Scholar]
- 191.Isaac RS, Sakthivel G, Murthy CH. Green synthesis of gold and silver nanoparticles using Averrhoa bilimbi fruit extract. J. Nanotechnol. 2013 doi: 10.1155/2013/906592. [DOI] [Google Scholar]
- 192.Ibrahim HM. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015 doi: 10.1016/j.jrras.2015.01.007. [DOI] [Google Scholar]
- 193.Sathityavani K, Ramanathan T, Gurudeeban S. Green synthesis of silver nanoparticles by using stem derived callus extract of bitter apple (Citrullus colocynthis) Dig. J. Nanomater. Biostruct. 2011;63:1019–1024. [Google Scholar]
- 194.Notriawan D, Angasa E, Suharto TE, Hendri J, Nishina Y. Green synthesis of silver nanoparticles using aqueous rinds extract of Brucea javanica (L.) Merr at ambient temperature. Mater. Lett. 2013 doi: 10.1016/j.matlet.2013.01.114. [DOI] [Google Scholar]
- 195.Kumar B, Angulo Y, Smita K, Cumbal L, Debut A. Capuli cherry-mediated green synthesis of silver nanoparticles under white solar and blue LED light. Particuology. 2016 doi: 10.1016/j.partic.2015.05.005. [DOI] [Google Scholar]
- 196.Ndikau M, Noah NM, Andala DM, Masika E. Green synthesis and characterization of silver nanoparticles using Citrullus lanatus fruit rind extract. Int. J. Anal. Chem. 2017 doi: 10.1155/2017/8108504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nayagam V, Gabriel M, Palanisamy K. Green synthesis of silver nanoparticles mediated by Coccinia grandis and Phyllanthus emblica: a comparative comprehension. Appl. Nanosci. 2018 doi: 10.1007/s13204-018-0739-3. [DOI] [Google Scholar]
- 198.Elumalai EK, Kayalvizhi K, Silvan S. Coconut water assisted green synthesis of silver nanoparticles. J. Pharm. Bioall. Sci. 2014 doi: 10.4103/0975-7406.142953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bharathi D, Vasantharaj S, Bhuvaneshwari V. Green synthesis of silver nanoparticles using Cordia dichotoma fruit extract and its enhanced antibacterial, anti-biofilm and photo catalytic activity. Mater. Res. Express. 2018 doi: 10.1088/2053-1591/aac2ef. [DOI] [Google Scholar]
- 200.Ghaffari-Moghaddam M, Hadi-Dabanlou R. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Crataegus douglasii fruit extract. J. Ind. Eng. Chem. 2014 doi: 10.1016/j.jiec.2013.09.005. [DOI] [Google Scholar]
- 201.Singh S, Saikia JP, Buragohain AK. A novel ‘green’synthesis of colloidal silver nanoparticles (SNP) using Dillenia indica fruit extract. Colloids Surf. B. 2013 doi: 10.1016/j.colsurfb.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 202.Ramesh PS, Kokila T, Geetha D. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015 doi: 10.1016/j.saa.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 203.David L, Moldovan B, Vulcu A, Olenic L, Perde-Schrepler M, Fischer-Fodor E, Filip GA. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf. B. 2014 doi: 10.1016/j.colsurfb.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 204.Veerakumar K, Govindarajan M, Rajeswary M, Muthukumaran U. Low-cost and eco-friendly green synthesis of silver nanoparticles using Feronia elephantum (Rutaceae) against Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti (Diptera: Culicidae) J Parasitol Res. 2014 doi: 10.1007/s00436-014-3823-y. [DOI] [PubMed] [Google Scholar]
- 205.Saha J, Begum A, Mukherjee A, Kumar S. A novel green synthesis of silver nanoparticles and their catalytic action in reduction of Methylene Blue dye. Sustain. Environ. Res. 2017 doi: 10.1016/j.serj.2017.04.003. [DOI] [Google Scholar]
- 206.Chowdhury IH, Ghosh S, Roy M, Naskar MK. Green synthesis of water-dispersible silver nanoparticles at room temperature using green carambola (star fruit) extract. J. Solgel. Sci Technol. 2015 doi: 10.1007/s10971-014-3515-1. [DOI] [Google Scholar]
- 207.Ashishie PB, Anyama CA, Ayi AA, Oseghale CO, Adesuji ET, Labulo AH. Green synthesis of silver monometallic and copper-silver bimetallic nanoparticles using Kigelia africana fruit extract and evaluation of their antimicrobial activities. Int. J. Phys. Sci. 2018 doi: 10.5897/IJPS2017.4689. [DOI] [Google Scholar]
- 208.Tagad CK, Dugasani SR, Aiyer R, Park S, Kulkarni A, Sabharwal S. Green synthesis of silver nanoparticles and their application for the development of optical fiber based hydrogen peroxide sensor. Sens. Actuators B Chem. 2013 doi: 10.1016/j.snb.2013.03.106. [DOI] [Google Scholar]
- 209.Roy K, Sarkar CK, Ghosh CK. Green synthesis of silver nanoparticles using fruit extract of Malus domestica and study of its antimicrobial activity. Dig. J. Nanomater. Biostruct. 2014;93:1137–1147. [Google Scholar]
- 210.Heydari R, Rashidipour M. Green synthesis of silver nanoparticles using extract of oak fruit hull (Jaft): synthesis and in vitro cytotoxic effect on MCF-7 cells. Int. J. Breast Cancer. 2015 doi: 10.1155/2015/846743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kahrilas GA, Wally LM, Fredrick SJ, Hiskey M, Prieto AL, Owens JE. Microwave-assisted green synthesis of silver nanoparticles using orange peel extract. ACS Sustain. Chem. 2014 doi: 10.1021/sc4003664. [DOI] [Google Scholar]
- 212.Ahmad N, Sharma S, Rai R. Rapid green synthesis of silver and gold nanoparticles using peels of Punica granatum. Adv. Mater. Lett. 2012 doi: 10.5185/amlett.2012.5357. [DOI] [Google Scholar]
- 213.Velmurugan P, Lee SM, Iydroose M, Lee KJ, Oh BT. Pine cone-mediated green synthesis of silver nanoparticles and their antibacterial activity against agricultural pathogens. Appl. Microbiol. Biotechnol. 2013 doi: 10.1007/s00253-012-3892-8. [DOI] [PubMed] [Google Scholar]
- 214.Amin M, Anwar F, Janjua MRSA, Iqbal MA, Rashid U. Green synthesis of silver nanoparticles through reduction with solanum xanthocarpum l berry extract: characterization, antimicrobial and urease inhibitory activities against helicobacter pylori. Int. J. Mol. Sci. 2012 doi: 10.3390/ijms13089923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jayaprakash N, Vijaya JJ, Kaviyarasu K, Kombaiah K, Kennedy LJ, Ramalingam RJ, Al-Lohedan HA. Green synthesis of Ag nanoparticles using Tamarind fruit extract for the antibacterial studies. J. Photochem. Photobiol. 2017 doi: 10.1016/j.jphotobiol.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 216.Kumar KM, Sinha M, Mandal BK, Ghosh AR, Kumar KS, Reddy PS. Green synthesis of silver nanoparticles using Terminalia chebula extract at room temperature and their antimicrobial studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012 doi: 10.1016/j.saa.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 217.Hyllested JE, Palanco ME, Hagen N, Mogensen KB, Kneipp K. Green preparation and spectroscopic characterization of plasmonic silver nanoparticles using fruits as reducing agents. Beilstein J. Nanotechnol. 2015 doi: 10.3762/bjnano.6.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vijayaraghavan K, Nalini SK, Prakash NU, Madhankumar D. Biomimetic synthesis of silver nanoparticles by aqueous extract of Syzygium aromaticum. Mater. Lett. 2012 doi: 10.1016/j.matlet.2012.01.083. [DOI] [Google Scholar]
- 219.Moteriya P, Satasiya R, Chanda S. Preliminary phytochemical and anti-bacterial studies on flowers and pods of Albizia lebbeck (Benth) J. Pharmacogn. Phytochem. 2015;3:112–120. [Google Scholar]
- 220.Padamanabhan V, Ganapathy M, Evanjelene VK. In vitro total phenolics, flavonoids contents, antioxidant and antimicrobial activites of various solvent extracts from the medicinal plant physalis minima linn. Int. J. Adv. Res. Technol. 2013;3:541–544. [Google Scholar]
- 221.Subrhamanian H, Suriyamoorthy P, Kanakasabapathi D. Phytochemical screening and HPTLC fingerprinting analysis of ethanolic extract of Erythrina variegata L. flowers. Int J Pharm Pharm Sci. 2016;8:210–217. [Google Scholar]
- 222.Joselin J, Brintha TSS, Florence AR, Jeeva S. Screening of select ornamental flowers of the family apocynaceae for phytochemical constituents. Asian Pac J Trop Dis. 2012;2:S260–S264. doi: 10.1016/S2222-1808(12)60162-5. [DOI] [Google Scholar]
- 223.Syed YH, Khan M, Bhuvaneshwari J, Ansari JA. Chromatographic profiling of ellagic acid in Woodfordia fruticosa flowers and their gastroprotective potential in ethanol-induced ulcers in rats. J Pharm Biosci. 2013;1:134–140. doi: 10.4103/0974-8490.178649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Baharara J, Namvar F, Ramezani T, Hosseini N, Mohamad R. Green synthesis of silver nanoparticles using achillea biebersteinii flower extract and its anti-angiogenic properties in the rat aortic ring model. Molecules. 2014 doi: 10.3390/molecules19044624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Karthik C, Anand K, Leecanro M, Preethy KR. Phytosynthesis of silver nanoparticles using Calotropis gigantea flower extract and its antibacterial activity. J. Nanosci. NanoEng. Appl. 2019;9:53–60. [Google Scholar]
- 226.Baghizadeh A, Ranjbar S, Gupta VK, Asif M, Pourseyedi S, Karimi MJ, Mohammadinejad R. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J. Mol. Liq. 2015 doi: 10.1016/j.molliq.2015.03.029. [DOI] [Google Scholar]
- 227.Muthu K, Priya S. Green synthesis, characterization and catalytic activity of silver nanoparticles using Cassia auriculata flower extract separated fraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017 doi: 10.1016/j.saa.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 228.He Y, Du Z, Lv H, Jia Q, Tang Z, Zheng X, Zhao F. Green synthesis of silver nanoparticles by Chrysanthemum morifolium Ramat extract and their application in clinical ultrasound gel. Int. J. Nanomedicine. 2009 doi: 10.2147/IJN.S43289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B. 2009 doi: 10.1016/j.colsurfb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 230.Bagherzade G, Tavakoli MM, Namaei MH. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac J Trop. 2017 doi: 10.1016/j.apjtb.2016.12.014. [DOI] [Google Scholar]
- 231.Hemmati S, Rashtiani A, Zangeneh MM, Mohammadi P, Zangeneh A, Veisi H. Green synthesis and characterization of silver nanoparticles using fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron. 2019 doi: 10.1016/j.poly.2018.10.049. [DOI] [Google Scholar]
- 232.Philip D, Unni C, Aromal SA, Vidhu VK. Murraya koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011 doi: 10.1016/j.saa.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 233.Mariselvam R, Ranjitsingh AJA, Nanthini AUR, Kalirajan K, Padmalatha C, Selvakumar P. Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (Family: Arecaceae) for enhanced antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014 doi: 10.1016/j.saa.2014.03.066. [DOI] [PubMed] [Google Scholar]
- 234.Padalia H, Moteriya P, Chanda S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab. J. Chem. 2015 doi: 10.1016/j.arabjc.2014.11.015. [DOI] [Google Scholar]
- 235.Gogoi N, Babu PJ, Mahanta C, Bora U. Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities. Mater. Sci. Eng. C. 2015 doi: 10.1016/j.msec.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 236.Mohapatra B, Kuriakose S, Mohapatra S. Rapid green synthesis of silver nanoparticles and nanorods using Piper nigrum extract. J. Alloys Compd. 2015 doi: 10.1016/j.jallcom.2015.02.206. [DOI] [Google Scholar]
- 237.Venkatesan B, Subramanian V, Tumala A, Vellaichamy E. Rapid synthesis of biocompatible silver nanoparticles using aqueous extract of Rosa damascena petals and evaluation of their anticancer activity. Asian Pac. J. Trop. Med. 2014 doi: 10.1016/S1995-76451460249-2. [DOI] [PubMed] [Google Scholar]
- 238.Vijayaraghavan K, Nalini SK, Prakash NU, Madhankumar D. One step green synthesis of silver nano/microparticles using extracts of Trachyspermum ammi and Papaver somniferum. Colloids Surf. B. 2012 doi: 10.1016/j.colsurfb.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 239.Dada AO, Inyinbor AA, Idu EI, Bello OM, Oluyori AP, Adelani-Akande TA, Dada O. Effect of operational parameters, characterization and antibacterial studies of green synthesis of silver nanoparticles using Tithonia diversifolia. PeerJ. 2018 doi: 10.7717/peerj.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Majaz Q, Nazim S, Siraj S, Zameeruddin M, Khan R. Phytochemical analysis of methanolic extract of roots of kalanchoe pinnata by HPLC and GCMS. Asian J. Res. Chem. 2011;4:655–658. [Google Scholar]
- 241.El-Mahmood AM, Doughari JH. Phytochemical screening and antibacterial evaluation of the leaf and root extracts of Cassia alata Linn. Afr. J. Pharm. Pharmacol. 2008;2:124–129. [Google Scholar]
- 242.Nono RN, Barboni L, Teponno RB, Quassinti L, Bramucci M, Vitali LA, Tapondjou ALS. Antimicrobial, antioxidant, anti-inflammatory activities and phytoconstituents of extracts from the roots of Dissotis thollonii Cogn (Melastomataceae) Afr. J. Bot. 2014 doi: 10.1016/j.sajb.2014.03.009. [DOI] [Google Scholar]
- 243.Sood H, Kumar Y, Gupta VK, Arora DS. Scientific validation of the antimicrobial and antiproliferative potential of Berberis aristata DC root bark, its phytoconstituents and their biosafety. AMB Express. 2019 doi: 10.1186/s13568-019-0868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Behravan M, Panahi AH, Naghizadeh A, Ziaee M, Mahdavi R, Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019 doi: 10.1016/j.ijbiomac.2018.11.101. [DOI] [PubMed] [Google Scholar]
- 245.Bindhu MR, Umadevi M. Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015 doi: 10.1016/j.saa.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 246.Shaikh R, Zainuddin Syed I, Bhende P. Green synthesis of silver nanoparticles using root extracts of Cassia toral L. and its antimicrobial activities. Asian J. Green Chem. 2019 doi: 10.22034/ajgc.2018.132083.1073. [DOI] [Google Scholar]
- 247.Wang D, Markus J, Wang C, Kim YJ, Mathiyalagan R, Aceituno VC, Yang DC. Green synthesis of gold and silver nanoparticles using aqueous extract of Cibotium barometz root. Artif. Cells Nanomed. Biotechnol. 2017 doi: 10.1080/21691401.2016.1260580. [DOI] [PubMed] [Google Scholar]
- 248.Shameli K, Bin Ahmad M, Jaffar Al-Mulla EA, Ibrahim NA, Shabanzadeh P, Rustaiyan A, Zidan M. Green biosynthesis of silver nanoparticles using callicarpa maingayi stem bark extraction. Molecules. 2012 doi: 10.3390/molecules17078506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Suresh G, Gunasekar PH, Kokila D, Prabhu D, Dinesh D, Ravichandran N, Siva GV. Green synthesis of silver nanoparticles using Delphinium denudatum root extract exhibits antibacterial and mosquito larvicidal activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;127:61–66. doi: 10.1016/j.saa.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 250.Rao NH, Lakshmidevi N, Pammi SVN, Kollu P, Ganapaty S, Lakshmi P. Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater. Sci. Eng. C. 2016 doi: 10.1016/j.msec.2016.01.072. [DOI] [PubMed] [Google Scholar]
- 251.Pethakamsetty L, Kothapenta K, Nammi HR, Ruddaraju LK, Kollu P, Yoon SG, Pammi SVN. Green synthesis, characterization and antimicrobial activity of silver nanoparticles using methanolic root extracts of Diospyros sylvatica. J. Environ. Sci. 2017 doi: 10.1016/j.jes.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 252.Rastogi L, Arunachalam J. Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential. J. Mater. Chem. Phys. 2011 doi: 10.1016/j.matchemphys.2011.04.068. [DOI] [Google Scholar]
- 253.Von White G, Kerscher P, Brown RM, Morella JD, McAllister W, Dean D, Kitchens CL. Green synthesis of robust, biocompatible silver nanoparticles using garlic extract. J. Nanomater. 2012 doi: 10.1155/2012/730746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Singh J, Dhaliwal AS. Novel green synthesis and characterization of the antioxidant activity of silver nanoparticles prepared from nepeta leucophylla root extract. Anal. Lett. 2014 doi: 10.1080/00032719.2018.1454936. [DOI] [Google Scholar]
- 255.Mondal NK, Chowdhury A, Dey U, Mukhopadhya P, Chatterjee S, Das K, Datta JK. Green synthesis of silver nanoparticles and its application for mosquito control. Asian Pac. J. Trop. Dis. 2014 doi: 10.1016/S2222-18081460440-0. [DOI] [Google Scholar]
- 256.Bhattacharyya SS, Das J, Das S, Samadder A, Das D, De A, Khuda-Bhuksh AR. Rapid green synthesis of silver nanoparticles from silver nitrate by a homeopathic mother tincture phytolacca decandra. Chin. J. Int. Med. 2012 doi: 10.3736/jcim20120510. [DOI] [PubMed] [Google Scholar]
- 257.Arokiyaraj S, Vincent S, Saravanan M, Lee Y, Oh YK, Kim KH. Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 2017 doi: 10.3109/21691401.2016.1160403. [DOI] [PubMed] [Google Scholar]
- 258.Vijaya JJ, Jayaprakash N, Kombaiah K, Kaviyarasu K, Kennedy LJ, Ramalingam RJ, Maaza M. Bioreduction potentials of dried root of Zingiber officinale for a simple green synthesis of silver nanoparticles: antibacterial studies. J. Photochem. Photobiol. B. 2017 doi: 10.1016/j.jphotobiol.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 259.Hazarika SN, Gupta K, Shamin KNAM, Bhardwaj P, Boruah R, Yadav KK, Namsa ND. One-pot facile green synthesis of biocidal silver nanoparticles. Mater. Res. Express. 2016 doi: 10.1088/2053-1591/3/7/075401. [DOI] [Google Scholar]
- 260.Velmurugan P, Anbalagan K, Manosathyadevan M, Lee KJ, Cho M, Lee SM, Oh BT. Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioprocess. Biosyst. Eng. 2014 doi: 10.1007/s00449-014-1169-6. [DOI] [PubMed] [Google Scholar]
- 261.Tabiri B, Agbenorhevi JK, Wireko-Manu FD, Ompouma EI. Watermelon seeds as food: Nutrient composition, phytochemicals and antioxidant activity. Int. J. Nutr. Food Sci. 2016 doi: 10.11648/j.ijnfs.20160502.18. [DOI] [Google Scholar]
- 262.Jagtap UB, Bapat VA. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam seed extract and its antibacterial activity. Ind. Crops. Prod. 2013 doi: 10.1016/j.indcrop.2013.01.019. [DOI] [Google Scholar]
- 263.Pandit R. Green synthesis of silver nanoparticles from seed extract of Brassica nigra and its antibacterial activity. Nusantara Bioscie. 2015 doi: 10.13057/nusbiosci/n070103. [DOI] [Google Scholar]
- 264.Dhand V, Soumya L, Bharadwaj S, Chakra S, Bhatt D, Sreedhar BL. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C. 2016 doi: 10.1016/j.msec.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 265.Kouhbanani MAJ, Beheshtkhoo N, Nasirmoghadas P, Yazdanpanah S, Zomorodianc K, Taghizadeh S, Amani AM. Green synthesis of spherical silver nanoparticles using Ducrosia anethifolia aqueous extract and its antibacterial activity. J. Environ. Treat. Tech. 2019;73:61–466. [Google Scholar]
- 266.Dhayalan M, Denison MIJ, Krishnan K. In vitro antioxidant, antimicrobial, cytotoxic potential of gold and silver nanoparticles prepared using Embelia ribes. Nat. Prod. Res. 2017 doi: 10.1080/14786419.2016.1166499. [DOI] [PubMed] [Google Scholar]
- 267.Ping Y, Zhang J, Xing T, Chen G, Tao R, Choo KH. Green synthesis of silver nanoparticles using grape seed extract and their application for reductive catalysis of direct orange 26. J Ind Eng Chem. 2018 doi: 10.1016/j.jiec.2017.09.009. [DOI] [Google Scholar]
- 268.Bar, H., Bhui, D.K., Sahoo, G.P., Sarkar, P., Pyne, S., Misra, A.:Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf. A Physicochem Eng Asp6. (2009). doi: 10.1016/j.colsurfa.2009.07.021
- 269.Vidhu VK, Aromal SA, Philip D. Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011 doi: 10.1016/j.saa.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 270.Basu S, Maji P, Ganguly J. Rapid green synthesis of silver nanoparticles by aqueous extract of seeds of nyctanthes arbor-tristis. J. Appl. Nanosci. 2016 doi: 10.1007/s13204-015-0407-9. [DOI] [Google Scholar]
- 271.Sadeghi B, Rostami A, Momeni SS. Facile green synthesis of silver nanoparticles using seed aqueous extract of Pistacia atlantica and its antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015 doi: 10.1016/j.saa.2014.05.078. [DOI] [PubMed] [Google Scholar]
- 272.Vijayaraj R, Kumar KN, Mani P, Senthil J, Kumar GD, Jayaseelan T. Green synthesis of silver nanoparticles from ethanolic seed extract of Acranythes aspera (Linn) and its anti-inflammatory activities. Int J Pharm Ther. 2016;7:42–48. [Google Scholar]
- 273.Khatami M, Nejad MS, Salari S, Almani PGN. Plant-mediated green synthesis of silver nanoparticles using Trifolium resupinatum seed exudate and their antifungal efficacy on Neofusicoccum parvum and Rhizoctonia solani. IET Nanobiotechnol. 2016 doi: 10.1049/iet-nbt.2015.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rautela A, Rani J, Das MD. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019 doi: 10.1186/s40543-018-0163-z. [DOI] [Google Scholar]
- 275.Khatami M, Pourseyedi S, Khatami M, Hamidi H, Zaeifi M, Soltani L. Synthesis of silver nanoparticles using seed exudates of Sinapis arvensis as a novel bioresource, and evaluation of their antifungal activity bioresour. Bioprocess. 2015 doi: 10.1186/s40643-015-0043-y. [DOI] [Google Scholar]
- 276.Das RK, Pachapur VL, Lonappan L, Naghdi M, Pulicharla R, Maiti S, Brar SK. Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017 doi: 10.1007/s41204-017-0029-4. [DOI] [Google Scholar]
- 277.Li Z, Wang L, Chen S, Feng C, Chen S, Yin N, Xu Y. Facilely green synthesis of silver nanoparticles into bacterial cellulose. Cellulose. 2015 doi: 10.1007/s10570-014-0487-9. [DOI] [Google Scholar]
- 278.Otari SV, Patil RM, Nadaf NH, Ghosh SJ, Pawar SH. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater. Lett. 2012 doi: 10.1016/j.matlet.2011.12.109. [DOI] [Google Scholar]
- 279.Singh G, Babele PK, Shahi SK, Sinha RP, Tyagi MB, Kumar A. Green synthesis of silver nanoparticles using cell extracts of Anabaena doliolum and screening of its antibacterial and antitumor activity. J. Microbiol. Biotechnol. 2014 doi: 10.4014/jmb.1405.05003. [DOI] [PubMed] [Google Scholar]
- 280.Wei X, Luo M, Li W, Yang L, Liang X, Xu L, Liu H. Synthesis of silver nanoparticles by solar irradiation of cell-free Bacillus amyloliquefaciens extracts and AgNO3. Bioresour. Technol. 2012 doi: 10.1016/j.biortech.2011.09.118. [DOI] [PubMed] [Google Scholar]
- 281.Wang C, Kim YJ, Singh P, Mathiyalagan R, Jin Y, Yang DC. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2016 doi: 10.3109/21691401.2015.1011805. [DOI] [PubMed] [Google Scholar]
- 282.Lateef A, Adelere IA, Gueguim-Kana EB, Asafa TB, Beukes LS. Green synthesis of silver nanoparticles using keratinase obtained from a strain of Bacillus safensis LAU 13. Int. Nano Lett. 2015 doi: 10.1007/s40089-014-0133-4. [DOI] [Google Scholar]
- 283.Kokilavani R, Karthik C. Comparative study on the biosynthesis and characterization of silver nanoparticles by E. coli using LB and M9 media and their anitimicrobial application. Int. J. Curr. Res. Life. Sci. 2018;7:2745–2749. [Google Scholar]
- 284.Gurunathan S, Han JW, Dayem AA, Eppakayala V, Park JH, Cho SG, Kim JH. Green synthesis of anisotropic silver nanoparticles and its potential cytotoxicity in human breast cancer cells (MCF-7) J Ind Eng Chem. 2013 doi: 10.1016/j.jiec.2013.01.029. [DOI] [Google Scholar]
- 285.Momin B, Rahman S, Jha N, Annapure US. Valorization of mutant Bacillus licheniformis M09 supernatant for green synthesis of silver nanoparticles: photocatalytic dye degradation, antibacterial activity, and cytotoxicity. Bioprocess Biosyst. Eng. 2019 doi: 10.1007/s00449-018-2057-2. [DOI] [PubMed] [Google Scholar]
- 286.Samadi N, Golkaran D, Eslamifar A, Jamalifar H, Fazeli MR, Mohseni FA. Intra/extracellular biosynthesis of silver nanoparticles by an autochthonous strain of proteus mirabilis isolated fromphotographic waste. J. Biomed. Nanotechnol. 2009 doi: 10.1166/jbn.2009.1029. [DOI] [PubMed] [Google Scholar]
- 287.Jain D, Kothari SL. Green synthesis of silver nanoparticles and their application in plant virus inhibition. J. Mycol. Plant. Pathol. 2014;441:21–24. [Google Scholar]
- 288.Otari SV, Patil RM, Nadaf NH, Ghosh SJ, Pawar SH. Green synthesis of silver nanoparticles by microorganism using organic pollutant: its antimicrobial and catalytic application. Environ. Sci. Pollut. Res. 2014 doi: 10.3109/21691401.2015.1064937. [DOI] [PubMed] [Google Scholar]
- 289.Vala AK, Chudasama B, Patel RJ. Green synthesis of silver nanoparticles using marine-derived fungus Aspergillus niger. Micro. Nano. Letters. 2012 doi: 10.1049/mnl.2012.0403. [DOI] [Google Scholar]
- 290.Li G, He D, Qian Y, Guan B, Gao S, Cui Y, Wang L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 2012 doi: 10.3390/ijms13010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Banasiuk R, Krychowiak M, Swigon D, Tomaszewicz W, Michalak A, Chylewska A, Krolicka A. Carnivorous plants used for green synthesis of silver nanoparticles with broad-spectrum antimicrobial activity. Arab. J. Chem. 2020 doi: 10.1016/j.arabjc.2017.11.013. [DOI] [Google Scholar]
- 292.Ghaseminezhad SM, Hamedi S, Shojaosadati SA. Green synthesis of silver nanoparticles by a novel method: comparative study of their properties. Carbohydr. Polym. 2012 doi: 10.1016/j.carbpol.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 293.Ingle A, Rai M, Gade A, Bawaskar M. Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. J. Nanoparticle Res. 2009 doi: 10.1007/s11051-008-9573-y. [DOI] [Google Scholar]
- 294.Chowdhury S, Basu A, Kundu S. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Res. Lett. 2014 doi: 10.1186/1556-276X-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Amerasan D, Nataraj T, Murugan K, Panneerselvam C, Madhiyazhagan P, Nicoletti M, Benelli GJ. Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies giles (diptera: culicidae) Pest Sci. 2016 doi: 10.1007/s10340-015-0675-x. [DOI] [Google Scholar]
- 296.Arun G, Eyini M, Gunasekaran P. Green synthesis of silver nanoparticles using the mushroom fungus schizophyllum commune and its biomedical applications. Biotechnol. Bioprocess Eng. 2014 doi: 10.1007/s12257-014-0071-z. [DOI] [Google Scholar]
- 297.Honary S, Barabadi H, Gharaei-Fathabad E, Naghibi F. Green synthesis of silver nanoparticles induced by the fungus penicillium citrinum. Trop J Pharm Res. 2013 doi: 10.4314/tjpr.v12i1.2. [DOI] [Google Scholar]
- 298.Almaary KS, Sayed SR, Abd-Elkader OH, Dawoud TM, El Orabi NF, Elgorban AM. Complete green synthesis of silver-nanoparticles applying seed-borne penicillium duclauxii. Saudi J. Biol. Sci. 2020 doi: 10.1016/j.sjbs.2019.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nayak RR, Pradhan N, Behera D, Pradhan KM, Mishra S, Sukla LB, Mishra BK. Green synthesis of silver nanoparticle by penicillium purpurogenum NPMF: the process and optimization. J. Nanoparticle Res. 2011 doi: 10.1007/s11051-010-0208-8. [DOI] [Google Scholar]
- 300.Gade A, Gaikwad S, Duran N, Rai M. Green synthesis of silver nanoparticles by phoma glomerata. Micron. 2014 doi: 10.1016/j.micron.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 301.Saxena J, Sharma PK, Sharma MM, Singh AL. Process optimization for green synthesis of silver nanoparticles by sclerotinia sclerotiorum MTCC 8785 and evaluation of its antibacterial properties. Springerplus. 2016 doi: 10.1186/s40064-016-2558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ahluwalia V, Kumar J, Sisodia R, Shakil NA, Walia S. Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and klebsiella pneumonia. Ind. Crops Prod. 2014 doi: 10.1016/j.indcrop.2014.01.026. [DOI] [Google Scholar]
- 303.Elgorban AM, Al-Rahmah AN, Sayed SR, Hirad A, Mostafa AAF, Bahkali AH. Antimicrobial activity and green synthesis of silver nanoparticles using trichoderma viride. Biotechnol. Biotechnol. Equip. 2016 doi: 10.1080/13102818.2015.1133255. [DOI] [Google Scholar]
- 304.Öztürk BY. Intracellular and extracellular green synthesis of silver nanoparticles using desmodesmus sp: their antibacterial and antifungal effects". Caryologia. Int. J. Cytol. Cytosyst. Cytogenet. Caryol. 2019 doi: 10.13128/cayologia-249. [DOI] [Google Scholar]
- 305.Kathiraven T, Sundaramanickam A, Shanmugam N, Balasubramanian T. Green synthesis of silver nanoparticles using marine algae caulerpa racemosa and their antibacterial activity against some human pathogens. Appl. Nanosci. 2015 doi: 10.1007/s13204-014-0341-2. [DOI] [Google Scholar]
- 306.Kannan RRR, Arumugam R, Ramya D, Manivannan K, Anantharaman P. Green synthesis of silver nanoparticles using marine macroalga chaetomorpha linum. Appl. Nanosci. 2013 doi: 10.1007/s13204-012-0125-5. [DOI] [Google Scholar]
- 307.Annamalai J, Nallamuthu T. Green synthesis of silver nanoparticles: characterization and determination of antibacterial potency. Appl. Nanosci. 2016 doi: 10.1007/s13204-015-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.El-Rafie HM, El-Rafie M, Zahran MK. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr. Polym. 2013 doi: 10.1016/j.carbpol.2013.03.071. [DOI] [PubMed] [Google Scholar]
- 309.Sinha SN, Paul D, Halder N, Sengupta D, Patra SK. Green synthesis of silver nanoparticles using fresh water green alga pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Appl. Nanosci. 2015 doi: 10.1007/s13204-014-0366-6. [DOI] [Google Scholar]
- 310.Vanlalveni C, Rajkumari K, Biswas A, Adhikari PP, Lalfakzuala R, Rokhum L. Green synthesis of silver nanoparticles using nostoc linckia and its antimicrobial activity: a novel biological approach. BioNanoScience. 2018 doi: 10.1007/s12668-018-0520-9. [DOI] [Google Scholar]
- 311.Govindaraju K, Kiruthiga V, Kumar VG, Singaravelu G. Extracellular synthesis of silver nanoparticles by a marine alga, sargassum wightii grevilli and their antibacterial effects. J. Nanosci. Nanotechnol. 2009 doi: 10.1166/jnn.2009.1199. [DOI] [PubMed] [Google Scholar]
- 312.Murugesan S, Bhuvaneswari S, Sivamurugan V. Green synthesis, characterization of silver nanoparticles of a marine red alga spyridia fusiformis and their antibacterial activity. Int. J. Pharm. Pharm. Sci. 2017 doi: 10.22159/ijpps.2017v9i5.17105. [DOI] [Google Scholar]
- 313.Rajeshkumar S, Kannan C, Annadurai G. Green synthesis of silver nanoparticles using marine brown algae turbinaria conoides and its antibacterial activity. Int. J. Pharma Bio Sci. 2012;3:502–510. [Google Scholar]
- 314.Massironi A, Morelli A, Grassi L, Puppi D, Braccini S, Maisetta G, Chiellini F. Ulvan as novel reducing and stabilizing agent from renewable algal biomass: application to green synthesis of silver nanoparticles. Carbohydr. Polym. 2019 doi: 10.1016/j.carbpol.2018.09.066. [DOI] [PubMed] [Google Scholar]
- 315.Giuffrida S, Ventimiglia G, Sortino S. Straightforward green synthesis of “naked” aqueous silver nanoparticles. Chem. Commun. 2009 doi: 10.1039/b907075c. [DOI] [PubMed] [Google Scholar]
- 316.Balantrapu K, Goia DV. Silver nanoparticles for printable electronics and biological applications. J. Mater. Res. 2009 doi: 10.1557/JMR.2009.0336. [DOI] [Google Scholar]
- 317.Malassis L, Dreyfus R, Murphy RJ, Hough LA, Donnio B, Murray CB. One-step green synthesis of gold and silver nanoparticles with ascorbic acid and their versatile surface post-functionalization. RSC Adv. 2016 doi: 10.1039/x0xx00000x. [DOI] [Google Scholar]
- 318.Khan Z, Singh T, Hussain JI, Obaid AY, Al-Thabaiti SA, El-Mossalamy EH. Starch-directed green synthesis, characterization and morphology of silver nanoparticles. Colloids Surf. B. 2013 doi: 10.1016/j.colsurfb.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 319.Morena AG, Roncero MB, Valenzuela SV, Valls C, Vidal T, Pastor FJ, Diaz P, Martínez J. Laccase/TEMPO-mediated bacterial cellulose functionalization: production of paper-silver nanoparticles composite with antimicrobial activity. Cellulose. 2019 doi: 10.1007/s10570-019-02678-5. [DOI] [Google Scholar]
- 320.Hebeish A, El-Shafei A, Sharaf S, Zaghloul S. Novel precursors for green synthesis and application of silver nanoparticles in the realm of cotton finishing. Carbohydr. Polym. 2011 doi: 10.1016/j.carbpol.2010.12.032. [DOI] [Google Scholar]
- 321.Ghodake G, Lim SR, Lee DS. Casein hydrolytic peptides mediated green synthesis of antibacterial silver nanoparticles. Colloids Surf. B. 2013 doi: 10.1016/j.colsurfb.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 322.Venkatesham M, Ayodhya D, Madhusudhan A, Babu NV, Veerabhadram G. A novel green one-step synthesis of silver nanoparticles using chitosan: catalytic activity and antimicrobial studies. Appl. Nanosci. 2014 doi: 10.1007/s13204-012-0180-y. [DOI] [Google Scholar]
- 323.Wongpreecha J, Polpanich D, Suteewong T, Kaewsaneha C, Tangboriboonrat P. One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr. Polym. 2018 doi: 10.1016/j.carbpol.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 324.Ahmad MB, Tay MY, Shameli K, Hussein MZ, Lim JJ. Green synthesis and characterization of silver/chitosan/polyethylene glycol nanocomposites without any reducing agent. Int. J. Mol. Sci. 2011 doi: 10.3390/ijms12084872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Cheng KM, Hung YW, Chen CC, Liu CC, Young JJ. Green synthesis of chondroitin sulfate-capped silver nanoparticles: characterization and surface modification. Carbohydr. Polym. 2014 doi: 10.1016/j.carbpol.2014.03.053. [DOI] [PubMed] [Google Scholar]
- 326.Roopan SM, Madhumitha G, Rahuman AA, Kamaraj C, Bharathi A, Surendra TV. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using cocos nucifera coir extract and its larvicidal activity. Ind. Crops Prod. 2013 doi: 10.1016/j.indcrop.2012.08.013. [DOI] [Google Scholar]
- 327.Ristig S, Chernousova S, Meyer-Zaika W, Epple M. Synthesis, characterization and in vitro effects of 7 nm alloyed silver–gold nanoparticles. Beilstein J. Nanotechnol. 2015 doi: 10.3762/bjnano.6.124375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Mohan S, Oluwafemi OS, George SC, Jayachandran VP, Lewu FB, Songca SP, Thomas S. Completely green synthesis of dextrose reduced silver nanoparticles, its antimicrobial and sensing properties. Carbohydr. Polym. 2014 doi: 10.1016/j.carbpol.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 329.Li D, Liu Z, Yuan Y, Liu Y, Niu F. Green synthesis of gallic acid-coated silver nanoparticles with high antimicrobial activity and low cytotoxicity to normal cells. Process Biochem. 2015 doi: 10.1016/j.procbio.2015.01.002. [DOI] [Google Scholar]
- 330.Mohanta YK, Singdevsachan SK, Parida UK, Panda SK, Mohanta TK, Bae H. Green synthesis and antimicrobial activity of silver nanoparticles using wild medicinal mushroom Ganoderma applanatum (Pers) Pat from Similipal Biosphere Reserve, Odisha India. IET Nanobiotechnol. 2016 doi: 10.1049/iet-nbt.2015.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Darroudi M, Ahmad MB, Abdullah AH, Ibrahim NA. Green synthesis and characterization of gelatin-based and sugar-reduced silver nanoparticles. Int. J. Nanomed. 2011 doi: 10.2147/IJN.S16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Pourjavadi A, Soleyman R. Novel silver nano-wedges for killing microorganisms. J. Nanopart. Res. 2011 doi: 10.1007/s11051-011-0428-6. [DOI] [Google Scholar]
- 333.Safaepour M, Shahverdi AR, Shahverdi HR, Khorramizadeh MR, Gohari AR. Green synthesis of small silver nanoparticles using geraniol and its cytotoxicity against fibrosarcoma-wehi 164. Avicenna J. Med. Biotechnol. 2009;1(2):111. [PMC free article] [PubMed] [Google Scholar]
- 334.Li J, Kuang D, Feng Y, Zhang F, Xu Z, Liu M, Wang D. Green synthesis of silver nanoparticles–graphene oxide nanocomposite and its application in electrochemical sensing oftryptophan. Biosens. Bioelectron. 2013 doi: 10.1016/j.bios.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 335.Darroudi M, Ahmad MB, Abdullah AH, Ibrahim NA, Shameli K. Effect of accelerator in green synthesis of silver nanoparticles. Int. J. Mol. Sci. 2010 doi: 10.3390/ijms11103898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Darroudi M, Ahmad MB, Zamiri R, Zak AK, Abdullah AH, Ibrahim NA. Time-dependent effect in green synthesis of silver nanoparticles. Int. J. Nanomed. 2011 doi: 10.2147/IJN.S17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Baruwati B, Polshettiwar V, Varma RS. Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem. 2009 doi: 10.1039/b902184a. [DOI] [Google Scholar]
- 338.Tian Y, Wang F, Liu Y, Pang F, Zhang X. Green synthesis of silver nanoparticles on nitrogen-doped graphene for hydrogen peroxide detection. Electrochim. Acta. 2014 doi: 10.1016/j.electacta.2014.08.133. [DOI] [Google Scholar]
- 339.Philip D. Honey mediated green synthesis of silver nanoparticles. Spectrochim. Acta. Part A. 2010 doi: 10.1016/j.saa.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 340.Xia N, Cai Y, Jiang T, Yao J. Green synthesis of silver nanoparticles by chemical reduction with hyaluronan. Carbohydr. Polym. 2011 doi: 10.1016/j.carbpol.2011.05.053. [DOI] [Google Scholar]
- 341.Celebioglu A, Topuz F, Yildiz ZI, Uyar T. One-step green synthesis of antibacterial silver nanoparticles embedded in electrospun cyclodextrin nanofibers. Carbohydr. Polym. 2019 doi: 10.1016/j.carbpol.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 342.Lateef A, Adeeyo AO. Green synthesis and antibacterial activities of silver nanoparticles using extracellular laccase of lentinus edodes. Not. Sci. Biol. 2015 doi: 10.15835/nsb.7.4.9643. [DOI] [Google Scholar]
- 343.Haiza H, Azizan A, Mohidin AH, Halin DSC. Green synthesis of silver nanoparticles using local honey. Nano Hybrids. 2013 doi: 10.4028/www.scientific.net/NH.4.87. [DOI] [Google Scholar]
- 344.Filippo E, Serra A, Buccolieri A, Manno DJ. Green synthesis of silver nanoparticles with sucrose and maltose: morphological and structural characterization. Non-Cryst. Solids. 2010 doi: 10.1016/j.jnoncrysol.2009.11.021. [DOI] [Google Scholar]
- 345.Zayed MF, Eisa WH, Shabaka AA. Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim. Acta. Part A. 2012 doi: 10.1016/j.saa.2012.08.072. [DOI] [PubMed] [Google Scholar]
- 346.Sen IK, Mandal AK, Chakraborti S, Dey B, Chakraborty R, Islam SS. Green synthesis of silver nanoparticles using glucan from mushroom and study of antibacterial activity. Int. J. Biol. Macromol. 2013 doi: 10.1016/j.ijbiomac.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 347.Debnath G, Das P, Saha AK. Green synthesis of silver nanoparticles using mushroom extract of pleurotus giganteus: characterization antimicrobial, and α-amylase inhibitory activity. Bionanoscience. 2019 doi: 10.1007/s12668-019-00650-y. [DOI] [Google Scholar]
- 348.Jeon EK, Seo E, Lee E, Lee W, Um MK, Kim BS. Mussel-inspired green synthesis of silver nanoparticles on graphene oxide nanosheets for enhanced catalytic applications. Chem. Commun. 2013 doi: 10.1039/c3cc00115f. [DOI] [PubMed] [Google Scholar]
- 349.Mason C, Vivekanandhan S, Misra M, Mohanty AK. Switchgrass (Panicum virgatum) extract mediated green synthesis of silver nanoparticles. World J. Nano Sci. Eng. 2012 doi: 10.4236/wjnse.2012.22008. [DOI] [Google Scholar]
- 350.Siddiqui MN, Redhwi HH, Achilias DS, Kosmidou E, Vakalopoulou E, Ioannidou MD. Green synthesis of silver nanoparticles and study of their antimicrobial properties. J. Polym. Environ. 2018 doi: 10.1007/s10924-017-0962-0. [DOI] [Google Scholar]
- 351.Vimala K, Sivudu KS, Mohan YM, Sreedhar B, Raju KM. Controlled silver nanoparticles synthesis in semi-hydrogel networks of poly (acrylamide) and carbohydrates: a rational methodology for antibacterial application. Carbohydr. Polym. 2009 doi: 10.1016/j.carbpol.2008.08.009. [DOI] [Google Scholar]
- 352.Lorestani F, Shahnavaz Z, Mn P, Alias Y, Manan NS. One-step hydrothermal green synthesis of silver nanoparticle-carbon nanotube reduced-graphene oxide composite and its application as hydrogen peroxide sensor. Sens. Actuators. B. 2015 doi: 10.1016/j.snb.2014.11.074. [DOI] [Google Scholar]
- 353.Mallmann EJJ, Cunha FA, Castro BN, Maciel AM, Menezes EA, Fechine PB. Antifungal activity of silver nanoparticles obtained by green synthesis. Rev. Inst. Med. Trop. Sao Paulo. 2015 doi: 10.1590/S0036-46652015000200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Krishna IM, Reddy GB, Veerabhadram G, Madhusudhan A. Eco-friendly green synthesis of silver nanoparticles using Salmalia malabarica: synthesis, characterization, antimicrobial, and catalytic activity studies. Appl. Nanosci. 2016 doi: 10.1007/s13204-015-0479-6. [DOI] [Google Scholar]
- 355.Suriya J, Raja SB, Sekar V, Rajasekaran R. Biosynthesis of silver nanoparticles and its antibacterial activity using seaweed Urospora sp. Afr. J. Biotechnol. 2012 doi: 10.5897/AJB12.452. [DOI] [Google Scholar]
- 356.Balavandy SK, Shameli K, Abidin ZZ. Stirring time effect of silver nanoparticles prepared in glutathione mediated by green method. Int. J. of Elechem. Sci. 2015;10(1):486–497. doi: 10.1186/1752-153X-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yuan W, Gu Y, Li L. Green synthesis of graphene/Ag nanocomposites. Appl. Surf. Sci. 2012 doi: 10.1016/j.apsusc.2012.08.094. [DOI] [Google Scholar]
- 358.Thuc DT, Huy TQ, Hoang LH, Tien BC, Van Chung P, Thuy NT, Le AT. Antibacterial activity. Mater. Lett. 2016 doi: 10.1016/j.matlet.2016.06.008. [DOI] [Google Scholar]
- 359.Lateef A, Ojo SA, Azeez MA, Asafa TB, Yekeen TA, Akinboro A, Oladipo IC, Gueguim-Kana EB, Beukes LS. Cobweb as novel biomaterial for the green and eco-friendly synthesis of silver nanoparticles. Appl. Nanosci. 2016 doi: 10.1007/s13204-015-0492-9. [DOI] [Google Scholar]
- 360.Cheviron P, Gouanvé F, Espuche E. Green synthesis of colloid silver nanoparticles and resulting biodegradable starch/silver nanocomposites. Carbohydr. Polym. 2014 doi: 10.1016/j.carbpol.2014.02.059. [DOI] [PubMed] [Google Scholar]
- 361.Kim TY, Cha SH, Cho S, Park Y. Tannic acid-mediated green synthesis of antibacterial silver nanoparticles. Arch. Pharm. Res. 2016 doi: 10.1007/s12272-016-0718-8. [DOI] [PubMed] [Google Scholar]
- 362.Sivaraman SK, Elango I, Kumar S, Santhanam V. A green protocol room temperature synthesis of silver nanoparticles in seconds. Curr. Sci. 2009;97:105–1059. [Google Scholar]
- 363.Saxena A, Tripathi RM, Zafar F, Singh P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater. lett. 2012 doi: 10.1016/j.matlet.2011.09.038. [DOI] [Google Scholar]
- 364.Tang J, Chen Q, Xu L, Zhang S, Feng L, Cheng L, Xu H, Liu Z, Peng R. Graphene oxide–silver nanocomposite as a highly effective antibacterial agent with species-specific mechanisms. ACS Appl. Mater. Interfaces. 2013 doi: 10.1021/am4005495. [DOI] [PubMed] [Google Scholar]
- 365.Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M. Silver nanoparticles as potential antibacterial agents. Molecules. 2015 doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Chen Y, Wu W, Xu Z, Jiang C, Han S, Ruan J, Wang Y. Photothermal-assisted antibacterial application of graphene oxide-Ag nanocomposites against clinically isolated multi-drug resistant Escherichia coli. R. Soc. Open Sci. 2020 doi: 10.1098/rsos.192019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Moussa SH, Tayel AA, Alsohim AS, Abdallah RR. Botryticidal activity of nanosized silver-chitosan composite and its application for the control of gray mold in strawberry. J. food. Sci. 2013 doi: 10.1111/1750-3841.12247. [DOI] [PubMed] [Google Scholar]
- 368.Egger S, Lehmann RP, Height MJ, Loessner MJ, Schuppler M. Antimicrobial properties of a novel silver-silica nanocomposite material. Appl. Environ. Microbiol. 2009 doi: 10.1128/AEM.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Devi P, Patil SD, Jeevanandam P, Navani NK, Singla ML. Synthesis, characterization and bactericidal activity of silica/silver core–shell nanoparticles. J Mater Sci Mater Med. 2014 doi: 10.1007/s10856-014-5165-9. [DOI] [PubMed] [Google Scholar]
- 370.Adak D, Sarkar M, Maiti M, Tamang A, Mandal S, Chattopadhyay B. Anti-microbial efficiency of nano silver–silica modified geopolymer mortar for eco-friendly green construction technology. RSC Adv. 2015 doi: 10.1039/C5RA12776A. [DOI] [Google Scholar]
- 371.Zhang X, Niu H, Yan J, Cai Y. Immobilizing silver nanoparticles onto the surface of magnetic silica composite to prepare magnetic disinfectant with enhanced stability and antibacterial activity. Colloids Surf. A Physicochem. Eng. 2011 doi: 10.1016/j.colsurfa.2010.12.009. [DOI] [Google Scholar]
- 372.Lu MM, Wang QJ, Chang ZM, Wang Z, Zheng X, Shao D, Dong WF, Zhou YM. Synergistic bactericidal activity of chlorhexidine-loaded, silver-decorated mesoporous silica. J. Nanomed. . 2017 doi: 10.2147/IJN.S133846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Wang Y, Ding X, Chen Y, Guo M, Zhang Y, Guo X, Gu H. Antibiotic-loaded, silver core-embedded mesoporous silica nanovehicles as a synergistic antibacterial agent for the treatment of drug-resistant infections. Biomaterials. 2016 doi: 10.1016/j.biomaterials.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 374.Ye J, Cheng H, Li H, Yang Y, Zhang S, Rauf A, Zhao Q, Ning G. Highly synergistic antimicrobial activity of spherical and flower-like hierarchical titanium dioxide/silver composites. J. Colloid Interface Sci. 2017 doi: 10.1016/j.jcis.2017.05.111. [DOI] [PubMed] [Google Scholar]
- 375.Chen Q, Jiang H, Ye H, Li J, Huang J. Preparation, antibacterial, and antioxidant activities of silver/chitosan composites. J. Carbohydr. Chem. 2014 doi: 10.1080/07328303.2014.931962. [DOI] [Google Scholar]
- 376.Martínez-Rodríguez MDLÁ, Madla-Cruz E, Urrutia-Baca VH, de la Garza-Ramos MA, González-González VA, Garza-Navarro MA. Influence of polysaccharides molecular structure on the antibacterial activity and cytotoxicity of green synthesized composites based on silver nanoparticles and carboxymethyl-cellulose. Nanomaterials. 2020 doi: 10.3390/nano10061164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kubasheva Z, Sprynskyy M, Railean-Plugaru V, Pomastowski P, Ospanova A, Buszewski B. Synthesis and antibacterial activity of (AgCl, Ag) NPs/diatomite hybrid composite. Materials. 2020 doi: 10.3390/ma13153409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Xu K, Wang JX, Kang XL, Chen JF. Fabrication of antibacterial monodispersed Ag–SiO2 core–shell nanoparticles with high concentration. Mater. Lett. 2009 doi: 10.1016/j.matlet.2008.08.039. [DOI] [Google Scholar]
- 379.Suktha P, Lekpet K, Siwayaprahm P, Sawangphruk M. Enhanced mechanical properties and bactericidal activity of polypropylene nanocomposite with dual-function silica–silver core-shell nanoparticles. J. Appl. Polym. Sci. 2013 doi: 10.1002/APP.38649. [DOI] [Google Scholar]
- 380.Quaglia G, Ambrogi V, Pietrella D, Nocchetti M, Latterini L. Solid state photoreduction of silver on mesoporous silica to enhance antifungal activity. Nanomaterials. 2021 doi: 10.3390/nano11092340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Milovanovic M, Arsenijevic A, Milovanovic J, Kanjevac T, Arsenijevic N. Antimicrob. Nanoarchitecton. Elsevier. 2017 doi: 10.1016/B978-0-323-52733-0.00014-8. [DOI] [Google Scholar]
- 382.Gonçalves BC, Lopes Barbosa MG, Silva Olak AP, Belebecha Terezo N, Nishi L, Watanabe MA, Faccin-Galhardi LC. Antiviral therapies: advances and perspectives. Fundam. Clin. Pharmacol. 2020 doi: 10.1111/fcp.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Dutta R, Roy S, Datta C. Silver nanoparticle as antiviral agent and its uses. Nano Trends. 2020;22:28–34. [Google Scholar]
- 384.Rai M, Deshmukh SD, Ingle AP, Gupta IR, Galdiero M, Galdiero S. Plant–fungal interactions: what triggers the fungi to switch among lifestyles? Crit. Rev. Microbiol. 2016 doi: 10.3109/1040841X.2013.879849. [DOI] [PubMed] [Google Scholar]
- 385.Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla CJ. Mode of antiviral action of silver nanoparticles against HIV-1. NanoBiotechnology. 2010;8:1–10. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Gurunathan S, Qasim M, Choi Y, Do JT, Park C, Hong K, Song H. Antiviral potential of nanoparticles—can nanoparticles fight against coronaviruses? Nanomaterials. 2020 doi: 10.3390/nano10091645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Hashim AI, Al Falahy JA, Hussain SS, Nihad APD, Tektook K. Efficiency of silver nanoparticle against virus coronaviruses. Ann. Trop. Med. PH. 2020 doi: 10.36295/ASRO.2020.23938. [DOI] [Google Scholar]
- 388.Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M. Silver nanoparticles inhibit hepatitis B virus replication. Molecules. 2011 doi: 10.3390/molecules16108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Lu L, Sun RWY, Chen R, Hui CK, Ho CM, Luk JM, Che CM. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008 doi: 10.1177/135965350801300210. [DOI] [PubMed] [Google Scholar]
- 390.Naik K, Kowshik M. The silver lining: towards the responsible and limited usage of silver. J. Appl. Microbiol. 2017 doi: 10.1111/jam.13525. [DOI] [PubMed] [Google Scholar]
- 391.Imani SM, Ladouceur L, Marshall T, Maclachlan R, Soleymani L, Didar TF. Antimicrobial nanomaterials and coatings: current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano. 2020 doi: 10.1021/acsnano.0c05937. [DOI] [PubMed] [Google Scholar]
- 392.Hodek J, Zajícová V, Lovětinská-Šlamborová I, Stibor I, Müllerová J, Weber J. Protective hybrid coating containing silver, copper and zinc cations effective against human immunodeficiency virus and other enveloped viruses. BMC Microbiol. 2016 doi: 10.1186/s12866-016-0675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Elazzazy AM, Elbeshehy EK, Betiha MA. In vitro assessment of activity of graphene silver composite sheets against multidrug-resistant bacteria and tomato bushy stunt virus. Trop. J. Pharm. Res. 2017 doi: 10.4314/tjpr.v16i11.19. [DOI] [Google Scholar]
- 394.Vargas-Hernandez M, Macias-Bobadilla I, Guevara-Gonzalez RG, Rico-Garcia E, Ocampo-Velazquez RV, Avila-Juarez L, Torres-Pacheco I. Nanoparticles as potential antivirals in agriculture. Agriculture. 2020 doi: 10.3390/agriculture10100444. [DOI] [Google Scholar]
- 395.Palestino G, García-Silva I, González-Ortega O, Rosales-Mendoza S. Can nanotechnology help in the fight against COVID-19? Expert. Rev. Anti. Infect. Ther. 2020 doi: 10.1080/14787210.2020.1776115. [DOI] [PubMed] [Google Scholar]
- 396.Salleh A, Naomi R, Utami ND, Mohammad AW, Mahmoudi E, Mustafa N, Fauzi MB. The potential of silver nanoparticles for antiviral and antibacterial applications: a mechanism of action. Nanomaterials. 2020 doi: 10.3390/nano10081566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Chakravarty M, Vora A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2020 doi: 10.1007/s13346-020-00818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Crane MJ, Devine S, Jamieson AM. Graphene oxide/silver nanoparticle ink formulations rapidly inhibit influenza A virus and OC43 coronavirus infection in vitro. bioRxiv. 2021 doi: 10.1101/2021.02.25.432893. [DOI] [Google Scholar]
- 399.Das C, Paul SS, Saha A, Singh T, Saha A, Im J, Biswas G. Silver-based nanomaterials as therapeutic agents against coronaviruses: a review. Int. J. Nanomed. 2020 doi: 10.2147/IJN.S280976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Jeremiah SS, Miyakawa K, Morita T, Yamaoka Y, Ryo A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020 doi: 10.1016/j.bbrc.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Kaye M, Druce J, Tran T, Kostecki R, Chibo D, Morris J, Birch C. SARS–associated coronavirus replication in cell lines. Emerg. Infect. Dis. 2006 doi: 10.3201/eid1201.050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Restrepo CV, Villa CC. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: a review. Environ. Nanotechnol. 2021 doi: 10.1016/j.enmm.2021.100428. [DOI] [Google Scholar]
- 403.Hebeish A, Shaheen TI, El-Naggar ME. Solid state synthesis of starch-capped silver nanoparticles. Int. J. Biol. Macromol. 2016 doi: 10.1016/j.ijbiomac.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 404.Tanner EE, Tschulik K, Tahany R, Jurkschat K, Batchelor-McAuley C, Compton RG. Nanoparticle capping agent dynamics and electron transfer: polymer-gated oxidation of silver nanoparticles. J. Phys. Chem. C. 2015 doi: 10.1021/acs.jpcc.5b05789. [DOI] [Google Scholar]
- 405.Li CC, Chang SJ, Su FJ, Lin SW, Chou YC. Effects of capping agents on the dispersion of silver nanoparticles. Colloids Surf. A Physicochem. Eng. 2013 doi: 10.1016/j.colsurfa.2012.11.077. [DOI] [Google Scholar]
- 406.Fayaz AM, Ao Z, Girilal M, Chen L, Xiao X, Kalaichelvan PT, Yao X. Inactivation of microbial infectiousness by silver nanoparticles-coated condom: a new approach to inhibit HIV-and HSV-transmitted infection. Int. J. Nanomed. 2012 doi: 10.2147/IJN.S34973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Gaikwad S, Ingle A, Gade A, Rai M, Falanga A, Incoronato N, Galdiero M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013 doi: 10.2147/IJN.S50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Mori, Y., Ono, T., Miyahira, Y., Nguyen, V. Q., Matsui, T., & Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. (2013). Nanoscale Res. Lett. http://www.nanoscalereslett.com/content/8/1/93 (Accessed 29 Nov 2021) [DOI] [PMC free article] [PubMed]
- 409.Lv X, Wang P, Bai R, Cong Y, Suo S, Ren X, Chen C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2014.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Yang XX, Li CM, Huang CZ. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale. 2016 doi: 10.1039/C5NR07918G. [DOI] [PubMed] [Google Scholar]
- 411.Chen YN, Hsueh YH, Hsieh CT, Tzou DY, Chang PL. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int. J. Environ. Res. Public. Health. 2016 doi: 10.3390/ijerph13040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Assis M, Simoes LGP, Tremiliosi GC, Coelho D, Minozzi DT, Santos RI, Longo E. SiO2-Ag composite as a highly virucidal material: a roadmap that rapidly eliminates SARS-CoV-2. Nanomaterials. 2021 doi: 10.3390/nano11030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ali A, Hussain F, Attacha S, Kalsoom A, Qureshi WA, Shakeel M, Militky J, Tomkova B, Kremenakova D. Development of novel antimicrobial and antiviral green synthesized silver nanocomposites for the visual detection of Fe3+ ions. Nanomaterials. 2021 doi: 10.3390/nano11082076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Fan Y, Zhao K, Shi ZL, Zhou P. Bat coronaviruses in China. Viruses. 2019 doi: 10.3390/v110302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Zhang YZ, Holmes EC. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020 doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020 doi: 10.1038/s4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Sarkar DS. Silver nanoparticles with bronchodilators through nebulisation to treat COVID 19 patients. Curr. Med. Res. Opin. 2020 doi: 10.15520/jcmro.v3i04.276. [DOI] [Google Scholar]
- 419.Almanza-Reyes H, Moreno S, Plascencia-López I, Alvarado-Vera M, Patrón-Romero L, Borrego B, Reyes-Escamilla A, Valencia-Manzo D, Brun A, Pestryakov A, Bogdanchikova N. Evaluation of silver nanoparticles for the prevention of SARS-CoV-2 infection in health workers: in vitro and in vivo. PLoS ONE. 2021 doi: 10.1371/journal.pone.0256401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Medhi R, Srinoi P, Ngo N, Tran HV, Lee TR. Nanoparticle-based strategies to combat COVID-19. ACS Appl. Nano Mater. 2020 doi: 10.1021/acsanm.0c01978. [DOI] [PubMed] [Google Scholar]
- 421.Quickgun Lifesciences official site - https://www.medstartr.com/project/detail/201398-Biosilver-nanoparticles-with-payload-of-CEP (Accessed 12 Dec 2020)
- 422.Imbeb biosciences Official site - https://www.imbedbio.com/imbed-biosciences-chases-nasel-spray-to-combat-covid-19-spread/ (Accessed 12 Dec 2020)
- 423.R & I world by ruvid official site - https://ruvid.org/ri-world/9618-2/ (Accessed 10 Feb 2021).
- 424.Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016 doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Du T, Lu J, Liu L, Dong N, Fang L, Xiao S, Han H. Antiviral activity of graphene oxide–silver nanocomposites by preventing viral entry and activation of the antiviral innate immune response. ACS Appl. Bio Mater. 2018 doi: 10.1021/acsabm.8b00154. [DOI] [PubMed] [Google Scholar]
- 426.Malik P, Mukherjee TK. Recent advances in gold and silver nanoparticle based therapies for lung and breast cancers. Int. J. Pharm. 2020 doi: 10.1016/j.ijpharm.2018.10.048. [DOI] [PubMed] [Google Scholar]


