Abstract
The susceptibilities of 25 clinical isolates of various Aspergillus species (Aspergillus fumigatus, A. flavus, A. terreus, A. ustus, and A. nidulans) to itraconazole (ITC) and amphotericin B (AMB) were determined using the standard proposed by NCCLS for antifungal susceptibility testing of filamentous fungi, a modification of this method using spectrophotometric readings, and a colorimetric method using the tetrazolium salt 2,3-bis {2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-hydroxide} (XTT). Five MIC end points for ITC (MIC-0, no visible growth or ≤5% the growth control value [GC]; MIC-1, slight growth or 6 to 25% the GC; MIC-2, prominent reduction in growth or 26 to 50% the GC; MIC-3, slight reduction in growth or 51 to 75% the GC; and MIC-4, no reduction in growth or 76 to 100% the GC) and one for AMB (MIC-0) were determined visually by four observers and spectrophotometrically. The intraexperimental (between the observers) and interexperimental (between the experiments) levels of agreement of the NCCLS and XTT methods exceeded 95% for MIC-0 of AMB and MIC-0 and MIC-1 of ITC. The MIC-2 of ITC showed lower reproducibility, although spectrophotometric reading and/or incubation for 48 h increased the interexperimental reproducibility from 85 to >93%. Between visual and spectrophotometric readings, high levels of agreement were found for AMB (≈97%) and MIC-1 (≈92%) and MIC-2 (≈88%) of ITC. Poor agreement was found for MIC-0 of ITC (51% after 24 h), since the spectrophotometric readings resulted in higher MIC-0 values than the visual readings. The agreement was increased to 98% by shifting the threshold level of MIC-0 from 5 to 10% relative optical density and by establishing an optical density of greater than 0.1 for the GC as the validation criterion. No statistically significant differences were found between the NCCLS method and the XTT method, with the levels of agreement exceeding 97% for MIC-0 of AMB and 83% for MIC-0, MIC-1, and MIC-2 of ITC. The XTT method and spectrophotometric readings can increase the sensitivity and the precision, respectively, of in vitro susceptibility testing of Aspergillus species.
Opportunistic systemic mycoses caused by filamentous fungi occur more frequently now than before partly as a consequence of the larger numbers of individuals receiving more potent immunosuppressive therapy (1). The pathogens most frequently encountered belong to the genus Aspergillus, with Aspergillus fumigatus being responsible for over 90% of invasive infections in humans (10). Amphotericin B (AMB) and itraconazole (ITC) are the only licensed antifungal drugs available for treatment (4), although their efficacy is limited, perhaps partly because of drug resistance. Therefore, testing of the susceptibility of these pathogens may contribute to the management of patients. Such testing requires standardized and reproducible techniques (6, 7). Broth microdilution methods have been adopted, since they are less expensive and cumbersome than macrodilution methods and yield reproducible results (2, 5, 18). Fungal growth is assessed either visually by grading turbidity (16) or spectrophotometrically by measuring optical density (OD) (3, 12). Alternatively, biomass can be determined colorimetrically using indicator substances that are reduced to colored products by viable microorganisms. Thus, fungal biomass is estimated by the metabolic activity of the fungus (7, 9, 13).
The hyphae and nonhomogeneous growth of filamentous fungi, together with a trailing effect of fungistatic drugs, complicate the determination of MICs and do not allow for precise quantification of fungal growth (13). Visual assessment of fungal growth lacks objectivity and precision (13, 19), and the accuracy of spectrophotometric readings may be hampered by clumps of mycelia (11, 15). Colorimetric methods may be an alternative, since precise quantification of hyphal growth is achieved and clear-cut end points can be generated (8). Tetrazolium salts have been used as colorimetric indicators, since fungi convert them to colored formazan derivatives, which can be quantified (18). The tetrazolium salt 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) has been used for antifungal susceptibility testing of yeasts and has recently been shown to be useful for determining MICs for filamentous fungi (13, 14). However, the usefulness of this assay is limited, since dissolution of the formazan derivative is achieved in organic solvents and relatively large numbers of fungi are required (11, 15).
2,3-Bis {2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-hydroxide} (XTT) is a new yellow tetrazolium salt which is converted by mitochondrial dehydrogenases of viable fungi to an orange formazan product (8, 18, 24). Unlike MTT-derived formazan, the product of XTT conversion is a water-soluble formazan, which obviates the need for solubilization steps (15, 21, 22). The XTT assay has been used for testing of the susceptibility of yeasts to antifungal agents and has yielded a high level of agreement with the NCCLS method for various Candida species and Cryptococcus neoformans (8). The same assay has been used to determine MICs for a small collection of filamentous fungi and has resulted in reproducible and comparable results relative to those obtained with the NCCLS method (23). A recently developed XTT assay for Aspergillus species resulted in well-defined dose-response curves for antifungal drugs (13a).
In this study, 25 clinical isolates of five Aspergillus species were tested three times against AMB and ITC using the method proposed by NCCLS (M38-P), and five MIC end points were determined visually by four observers. Given the problems encountered with the visual observation and spectrophotometric assessment of the hyphal growth of filamentous fungi, the results of M38-P were compared with a modification of this method using spectrophotometric readings at 405 nm. Furthermore, in order to facilitate the determination of MICs for filamentous fungi, the activities of antifungal drugs were determined by a colorimetric method that uses the dye XTT as described previously (13a). The intraexperimental (between the observers) and interexperimental (between the experiments) agreements of the methods as well as the agreements between the NCCLS and colorimetric methods and between visual and spectrophotometric readings were calculated.
MATERIALS AND METHODS
Test isolates.
Twenty-five clinical isolates of Aspergillus species (from our private collection) were selected for testing and included 5 isolates each of the following species: A. fumigatus (AZN5161, AZN5241, AZG7, AZN8248, and AZN8244), A. flavus (AZN137, AZN510, AZN2865, AZN4094, and AZN4132), A. nidulans (AZN2867, AZN8033, AZN8236, AZN8933, and AZN4606), A. terreus (AZN286, AZN515, AZN2868, AZN7320, and AZN9152), and A. ustus (AZN677, AZN2725, AZN6989, AZN9420, and AZN7843). Candida parapsilosis (ATCC 22019) and C. krusei (ATCC 6258) were used for quality control.
Isolates were cultured twice on Sabouraud glucose agar at 30°C for 5 to 7 days. All isolates were tested on three different days. Conidia of the isolates were obtained from fresh cultures each time.
Medium.
RPMI 1640 medium (with l-glutamine but without bicarbonate) (GIBCO BRL, Life Technologies, Woerden, The Netherlands) buffered to pH 7.0 with 0.165 M 3-N-morpholinopropanesulfonic acid (MOPS) (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was used as the assay medium.
Inoculum.
Conidia were collected with a cotton swab and suspended in sterile saline containing 0.05% Tween 20. After heavy particles were allowed to settle, the turbidity of the supernatants was measured spectrophotometrically (Spectronic 20D; Milton Roy, Rochester, N.Y.) at 530 nm, and the transmission was adjusted to 80 to 82% to yield an initial inoculum of 1 × 106 to 5 × 106 CFU/ml. Each suspension was diluted 1:50 in medium to obtain twice the desired inoculum. The inoculum size was confirmed by plating of serial dilutions on Sabouraud glucose agar plates, with final inocula ranging from 1 × 104 to 5 × 104 CFU/ml.
Antifungal susceptibility testing.
The broth microdilution method of the NCCLS (M38-P) (16) was performed using 96-well flat-bottom microtitration trays. ITC (Janssen-Cilag, Beerse, Belgium) and AMB (Bristol-Myers Squibb, Woerden, The Netherlands) were dissolved in dimethyl sulfoxide and diluted serially in medium to yield final concentrations of 0.015 to 16 mg of AMB/liter and 0.03 to 32 mg of ITC/liter in a final volume of 200 μl after the inoculation. A drug-free growth control that contained 0.5% dimethyl sulfoxide in medium was included. Trays were kept at −70°C until the day of testing.
Incubation and MIC determination.
After the microtitration trays were defrosted, they were inoculated with 100 μl of inoculum. The trays were agitated for 10 s and incubated at 37°C for 48 h. The MICs of the antifungal drugs were determined after 24 and 48 h of incubation by two methods (the NCCLS and XTT methods) and by two modes of reading (visual and spectrophotometric) as described below.
(i) NCCLS method. (a) Visual reading (NCCLSvis).
Fungal growth was assessed visually by four different observers with the aid of a concave mirror and graded according to NCCLS guidelines as follows: 4, no reduction in growth; 3, slight reduction in growth; 2, prominent reduction in growth; 1, slight growth; and 0, absence of visual growth compared with the growth in the drug-free well. Only score 0 was recorded for AMB. Four MIC end points (MIC-0, MIC-1, MIC-2, and MIC-3) were determined as the lowest drug concentrations showing growth scaled to the corresponding score (0, 1, 2, and 3, respectively). MIC-4 was determined as the highest drug concentration showing growth scaled to score 4.
(b) Spectrophotometric reading (NCCLSsp).
After the MICs were determined visually, the OD of each well was measured at 405 nm with a spectrophotometer (Rosys Anthos ht3; Anthos Labtec Instruments GmbH, Salzburg, Austria). After subtraction of the ODs of the blank, which consisted of noninoculated wells that had been incubated together with the inoculated wells, from the ODs of the inoculated wells, the percentage of growth for each well was correlated with the relative OD estimated by the following equation: (OD405 of wells that contained the drug/OD405 of the drug-free well) × 100. Relative ODs after rounding were grouped in five levels: 4, 76 to 100% relative OD; 3, 51 to 75% relative OD; 2, 26 to 50% relative OD; 1, 6 to 25% relative OD; and 0, equal to or less than 5% relative OD. Five MIC end points were determined for ITC based on the levels of relative ODs. MIC-0, MIC-1, MIC-2, and MIC-3 were determined as the lowest drug concentrations with relative ODs of levels 0, 1, 2, and 3, respectively. MIC-4 was determined as the highest drug concentration with a relative OD of level 4. Only MIC-0 was determined for AMB.
Once these determinations were completed, the trays were returned to the incubator for another 24 h and read again visually and spectrophotometrically.
(ii) XTT method.
The MICs were also determined colorimetrically using XTT (Sigma-Aldrich Chemie). Microtitration trays were prepared and incubated for 24 and 48 h at 37°C according to NCCLS guidelines as described above. Then, 50 μl of saline containing 1 mg of XTT/ml and 20.2 μg of menadione (Sigma-Aldrich Chemie)/ml was added to each well in order to obtain final concentrations of 200 μg of XTT/ml and 4.3 μg of MEN/ml (25 μM). MEN was first dissolved in acetone at a concentration of 430.5 μg/ml and then diluted 1/10 in saline. Incubation was continued at 37°C for 2 h in the dark to allow conversion of XTT to its formazan derivative. The OD was measured at 450 nm, after shaking, with a microtitration tray spectrophotometer reader. The color was assessed first visually by the same four observers and graded according to NCCLS guidelines (XTTvis) and then spectrophotometerically based on relative ODs at 450 nm and grouped in five levels as described above (XTTsp). Five MIC end points were determined for ITC as described above. For AMB, only MIC-0 was determined.
Study design.
A multifactorial panel of data was generated based on five parameters studied, i.e., modes of reading, MIC end points, drugs, species, and incubation periods, in order to test the reproducibility of and the agreement between the NCCLS and XTT methods. Data from five readings (four observers and spectrophotometer), five MIC end points, two drugs, five species, five strains, two incubation periods, and three independent experiments were obtained for both methods. Four comparisons were done in order to find the levels of absolute and relative agreements within the observers and the experiments (intra- and interexperimental agreements, respectively), between the visual and the spectrophotometric readings for each method, and between the NCCLS and the XTT methods.
Analysis of results.
The levels of agreement within the four observers and the three experiments as well as between the two methods and between the two modes of reading were calculated for each species-drug-MIC end point-incubation period combination. In order to approximate a normal distribution for statistical analysis, the drug concentrations were transformed by logarithmic transformation to log2 and the percentages of agreement were transformed by angular transformation (with the transformed value being the arcsine of the square root of the percentage). After the transformation, MIC end points were analyzed by two-way analysis of variance (ANOVA), which was applied to each drug-MIC end point-incubation period combination. P values of less than 0.05 were considered significant. Any systematic differences between the methods and the species were controlled by the interaction factor of two-way ANOVA. A P value of less than 0.05 indicated that the differences between the two methods are not the same for each species and therefore that the differences between the methods are species dependent. Discrepancies were analyzed by Fisher's exact test. The transformed percentages of agreement were used in order to estimate variations between the experiments, the species, and the strains and to analyze differences in intra- and interexperimental agreements between all MIC end points after 24 and 48 h by repeated-measures ANOVA. The 95% confidence intervals (95% CI) of the percentages of agreement were calculated using the Wald equation for proportions (Prism software; GraphPad: Software, Inc., San Diego, Calif.). The high and low off-scale MICs were included in the analysis by conversion to the next higher and lower drug concentrations, respectively.
(i) Intraexperimental agreement.
For the visual readings (NCCLSvis and XTTvis), the percentage of absolute or relative agreement between the four observers for each strain was defined as the proportion of the MIC end points which were identical or which belonged to the largest subset of four observations with a range of which not more than 3 dilutions, respectively. The levels of agreement between the four visual readings were calculated for each species and experiment, and the total percentage of agreement for all species and experiments was reported for each drug and MIC end point for the NCCLSvis and XTTvis methods after 24 and 48 h of incubation.
(ii) Interexperimental agreement.
For each method and mode of reading, the percentage of absolute and relative agreements between the three experiments was defined as the proportion of the MIC end points determined by the four observers or the spectrophotometer which were identical or within 1 dilution of the median, respectively. The levels of agreement between the three experiments were calculated for each species, and the total percentage of agreement for all species was reported for each drug and MIC end point for the NCCLSvis, NCCLSsp, XTTvis, and XTTsp methods after 24 and 48 h of incubation.
(iii) Agreement between visual and spectrophotometric readings.
For each method, the percentage of agreement between the visual and spectrophotometric readings was calculated as the proportion of the MIC end points determined visually by the four observers which fell within 1 dilution of the corresponding MIC end points determined spectrophotometrically. The total percentage of agreement for the three experiments and all species between NCCLSvis and NCCLSsp as well as between XTTvis and XTTsp was reported for each drug and MIC end point after 24 and 48 h of incubation, and the approximate 95% CI of percentages of agreement were calculated as described above.
(iv) Agreement between NCCLS and XTT methods.
For each strain, the percentage of agreement between the NCCLS and XTT methods based on visual and spectrophotometric readings of each MIC end point was calculated as the proportion of the MIC end points determined by the NCCLS method, visually or spectrophotometrically, which fell within 1 dilution of the corresponding MIC end points of the XTT method, determined visually or spectrophotometrically, respectively. The total percentage of agreement between NCCLSvis and XTTvis and between NCCLSsp and XTTsp for the three experiments was reported for each drug and MIC end point after 24 and 48 h of incubation, and the approximate 95% CI of percentages of agreement were calculated as described above.
RESULTS
MICs.
The Aspergillus strains tested in this study had various susceptibilities to AMB and ITC, with MICs ranging between 0.125 and 4 mg/liter for AMB and 0.015 to >32 mg/liter for ITC. Most of the MICs of ITC were less than 1 mg/liter, except for those for two strains of A. fumigatus (Table 1). For A. ustus strains, despite the fact that the MIC-2 values were less than 1 mg/liter, the MIC-0 values were higher than 2 mg/liter and up to >32 mg/liter (data not shown). The geometric means of the MICs of AMB and for ITC obtained by all methods after 24 and 48 h of incubation were similar, as shown in Table 1. Differences were observed with A. fumigatus and ITC, for which the geometric mean of the MICs obtained by NCCLSvis after 24 h was slightly higher than the corresponding value obtained by the other methods (2.4 and 1.4 mg/liter, respectively), and with A. ustus and ITC, for which the geometric means of the MICs obtained by NCCLSvis and NCCLSsp after 48 h were lower than the values obtained by the corresponding XTT methods (1.5 and 0.9 mg/liter for NCCLSvis and NCCLSsp and 3.2 and 2.7 mg/liter for XTTvis and XTTsp, respectively).
TABLE 1.
Susceptibilities of five strains of five Aspergillus spp. to AMB and ITC based on MIC-0 and MIC-2, respectively, after 24 and 48 h of incubation
| Drug | Time (h) | Species | Geometric mean (range) MIC, in mg/liter, determined by the following method:
|
|||
|---|---|---|---|---|---|---|
| NCCLSvis | NCCLSsp | XTTvis | XTTsp | |||
| AMB | 24 | A. fumigatus | 0.68 (0.125–4) | 0.63 (0.5–2) | 0.63 (0.25–2) | 0.63 (0.5–2) |
| A. flavus | 1.19 (0.5–4) | 1.20 (0.5–2) | 1.11 (0.5–2) | 1.15 (0.5–2) | ||
| A. nidulans | 0.74 (0.25–4) | 0.72 (0.25–2) | 0.68 (0.25–2) | 0.66 (0.25–1) | ||
| A. terreus | 1.11 (0.5–4) | 0.95 (0.5–4) | 1.07 (0.5–2) | 1.10 (0.5–2) | ||
| A. ustus | 1.11 (0.5–2) | 0.88 (0.5–2) | 0.92 (0.5–4) | 1.12 (0.5–2) | ||
| 48 | A. fumigatus | 1.50 (1–4) | 1.26 (1–4) | 1.14 (0.5–4) | 1.15 (0.5–4) | |
| A. flavus | 1.64 (1–4) | 1.38 (1–2) | 1.57 (1–4) | 1.45 (1–2) | ||
| A. nidulans | 1.10 (0.25–4) | 0.87 (0.25–4) | 0.97 (0.25–4) | 0.95 (0.25–4) | ||
| A. terreus | 2.89 (2–4) | 2.09 (2–4) | 2.64 (2–4) | 2.52 (2–4) | ||
| A. ustus | 1.91 (1–4) | 1.36 (1–2) | 1.53 (1–4) | 1.47 (1–2) | ||
| ITC | 24 | A. fumigatus | 2.39 (0.015–>32) | 1.15 (0.125–>32) | 1.36 (0.125–>32) | 1.38 (0.125–>32) |
| A. flavus | 0.14 (0.063–0.25) | 0.10 (0.063–0.125) | 0.14 (0.0125–0.5) | 0.14 (0.063–0.25) | ||
| A. nidulans | 0.13 (0.031–0.5) | 0.13 (0.063–0.25) | 0.13 (0.062–0.5) | 0.10 (0.062–0.5) | ||
| A. terreus | 0.09 (0.031–0.25) | 0.09 (0.063–0.125) | 0.12 (0.062–0.5) | 0.11 (0.063–0.125) | ||
| A. ustus | 0.40 (0.062–1) | 0.35 (0.063–1) | 0.54 (0.062–1) | 0.46 (0.063–1) | ||
| 48 | A. fumigatus | 2.89 (0.125–>32) | 2.41 (0.125–>32) | 2.96 (0.125–>32) | 2.10 (0.063–>32) | |
| A. flavus | 0.19 (0.125–0.5) | 0.12 (0.063–0.25) | 0.26 (0.125–1) | 0.24 (0.125–0.5) | ||
| A. nidulans | 0.16 (0.062–0.25) | 0.20 (0.063–2) | 0.17 (0.031–0.5) | 0.15 (0.031–0.5) | ||
| A. terreus | 0.15 (0.062–0.5) | 0.14 (0.063–0.25) | 0.29 (0.062–0.5) | 0.23 (0.125–0.5) | ||
| A. ustus | 1.48 (0.125–>32) | 0.89 (0.5–1) | 3.21 (0.125–>32) | 2.72 (0.25–>32) | ||
(i) Intraexperimental agreement.
For AMB, the absolute agreement between the four observers was higher with the XTTvis method (96%) than with the NCCLSvis method (91%) after 24 and 48 h (Table 2). The lowest level of agreement was found for A. ustus with the NCCLSvis method (75%); the agreement was higher with the XTTvis method (>88%). For ITC, the absolute agreement was higher for MIC-0 and MIC-1 with both methods (>76%) than for the other MIC end points. Based on MIC-0 and MIC-1, A. ustus (67%) and A. fumigatus (98%) showed the lowest and the highest levels of absolute agreement, respectively, among the tested species. The coefficient of variation of intraexperimental absolute agreement was always less than 10% among the experiments as well as among the species for both the NCCLSvis and the XTTvis methods. No statistically significant differences were found between the percentages of agreement of the NCCLSvis and XTTvis methods after 24 and 48 h of incubation.
TABLE 2.
Overall absolute and relative (within 0 and 1 dilutions, respectively) agreements within the four observers and the three experiments for all tested Aspergillus isolates
| Agreement | Groupa | Drug | Growth levelb | Overall absolute (relative) % agreement for the indicated method after the following h of incubation:
|
|||
|---|---|---|---|---|---|---|---|
| 24
|
48
|
||||||
| NCCLS | XTT | NCCLS | XTT | ||||
| Intraexperimental | 1 | ITC | 0 | 82.9 (99.1) | 90.3 (97.7) | 89.7 (99.4) | 92.5 (99.5) |
| 1 | 83.2 (98.6) | 77.6 (99.1) | 76.1 (99.5) | 82.0 (98.8) | |||
| 2 | 69.0 (88.8) | 66.9 (87.6) | 69.8 (95.3) | 72.0 (94.9) | |||
| 3 | 62.4 (80.2) | 59.1 (73.6) | 65.5 (78.2) | 65.0 (86.7) | |||
| 4 | 60.8 (71.7) | 63.2 (71.8) | 64.1 (77.0) | 63.5 (70.3) | |||
| AMB | 0 | 91.0 (99.8) | 96.1 (99.9) | 90.6 (100) | 95.8 (100) | ||
| Interexperimental | 2 | ITC | 0 | 78.3 (97.6) | 75.3 (95.6) | 83.7 (99.4) | 85.6 (99.3) |
| 1 | 69.6 (95.5) | 74.5 (96.6) | 78.3 (98.9) | 77.0 (98.6) | |||
| 2 | 65.8 (85.7) | 64.6 (85.3) | 71.5 (94.3) | 68.8 (93.3) | |||
| 3 | 62.8 (82.2) | 57.7 (75.9) | 67.7 (82.5) | 69.1 (87.8) | |||
| 4 | 61.9 (73.8) | 58.5 (68.4) | 66.0 (79.3) | 66.8 (77.2) | |||
| AMB | 0 | 81.7 (99.0) | 87.9 (99.7) | 78.1 (99.7) | 83.1 (99.7) | ||
| 3 | ITC | 0 | 54.7 (76.2) | 64.4 (90.9) | 75.4 (91.6) | 87.3 (99.4) | |
| 1 | 71.2 (99.7) | 74.8 (95.7) | 84.7 (97.6) | 81.0 (97.2) | |||
| 2 | 65.6 (96.2) | 63.4 (93.1) | 72.3 (99.7) | 67.0 (89.6) | |||
| 3 | 58.8 (71.4) | 56.0 (80.2) | 62.9 (89.6) | 49.3 (88.9) | |||
| 4 | 61.7 (64.3) | 61.7 (67.0) | 59.5 (64.8) | 56.1 (78.2) | |||
| AMB | 0 | 78.9 (99.7) | 88.2 (100) | 92.4 (100) | 89.6 (100) | ||
Groups 1 and 2, NCCLSvis and XTTvis, 95% CI limits of absolute agreement ranged from ±3.6 to ±5.6%; group 3, NCCLSsp and XTTsp, 95% CI limits of absolute agreement ranged from ±2.6 to ±11.3%.
Growth levels: 0, no visible growth (≤5% that of growth control); 1 slight growth (6 to 25% that of growth control); 2, prominent reduction of growth (26 to 50% that of growth control); 3, slight reduction of growth (51 to 75% that of growth control); 4, no reduction of growth (76 to 100% of growth control).
(ii) Interexperimental agreement.
For AMB, the absolute agreement between the experiments was slightly higher for the XTTvis method than for the NCCLSvis method and was lower after 48 h of incubation than after 24 h for both methods (Table 2). When spectrophotometric readings were used, the interexperimental agreement for the XTTsp and NCCLSsp methods was higher after 48 h of incubation than after 24 h. For ITC, the reproducibility of the NCCLSvis and XTTvis methods was high (>70%) with MIC-0 and MIC-1 and was always higher after 48 h. For these MIC end points, the lowest and highest levels of absolute agreement among the different species were found with A. ustus (64%) and A. fumigatus (93%), respectively. For the NCCLSsp and XTTsp methods, the highest reproducibility was found for MIC-1 (71–85%) with both methods after 24 and 48 h, with the exception of the XTTsp method after 48 h, for which the MIC-0 showed the highest reproducibility (87%). The reproducibility of MIC-0 with the NCCLSsp method was very low after 24 h (55%) but improved after 48 h (75%) and with the XTTsp method (87%). The coefficient of variation of interexperimental absolute agreement among the species was always less than 13% for visual readings, with an average coefficient of variation of 10%; for the spectrophotometric readings, the coefficient of variation was always less than 19%, with an average coefficient of variation of 13% for both methods. Unlike that of the spectrophotometric readings, the relative agreement of the visual readings increased statistically significantly after 48 h for both methods (P < 0.05).
(iii) Agreement between visual and spectrophotometric readings.
For AMB, the relative agreements between the NCCLSvis and NCCLSsp methods as well as between the XTTvis and XTTsp methods were high after 24 and 48 h (>96 and >99%, respectively) (Table 3). For ITC, the levels of agreement between the XTTvis and XTTsp methods for MIC-0, MIC-1, and MIC-2 ranged from 83 to 95% after 24 and 48 h of incubation. Between the NCCLSvis and NCCLSsp methods, the agreements were higher for MIC-1 and MIC-2 after 24 h (93 and 88%, respectively) and 48 h (92 and 87%, respectively). Very low agreement was found for MIC-0 after 24 h (51%; P < 0.01), but it increased after 48 h (82%). Statistically significant differences were found for MIC-3 (P < 0.01) and MIC-4 (P < 0.05) of ITC after 24 h with the NCCLS methods and for MIC-3 after 24 h (P < 0.05) and MIC-4 after 48 h (P < 0.01) of ITC with the XTT methods. No statistically significant systematic differences between the two methods and the species for both drugs after 24 and 48 h of incubation for all MIC end points were found (P > 0.15). For MIC-0 of ITC after 24 h of incubation, in 42 (56%) out of 75 comparisons (three experiments, five species, and five strains), the MIC-0 values of the NCCLSsp method were higher than those of the NCCLSvis method, and only six comparisons (8%) were the opposite. Furthermore, out of 35 comparisons in which differences of higher than 1 dilution were observed, the MIC-0 values of the spectrophotometric method were higher than those of the NCCLS method in 33 and vice versa in 2.
TABLE 3.
Overall relative (within 1 dilution) agreements and 95% CI limits between the visual and spectrophotometric readings and between the NCCLS and XTT methods
| Time (h) | Drug | Growth levela | % Agreement ± 95% CI for the following comparisons:
|
|||
|---|---|---|---|---|---|---|
| Visual vs spectrophotometric readings
|
NCCLS vs XTT methods
|
|||||
| NCCLSvis vs NCCLSspb | XTTvis vs XTTspb | NCCLSvis vs XTTvisb | NCCLSsp vs XTTspc | |||
| 24 | ITC | 0 | 51.4 ± 5.7d | 90.8 ± 3.3 | 88.0 ± 3.7 | 60.0 ± 11.1e |
| 1 | 92.8 ± 3.0 | 91.1 ± 3.3 | 92.7 ± 3.0 | 94.7 ± 5.1 | ||
| 2 | 87.7 ± 3.8 | 86.0 ± 4.0 | 86.8 ± 3.9 | 82.7 ± 8.6 | ||
| 3 | 67.8 ± 5.4e | 75.4 ± 4.9e | 79.8 ± 4.6e | 73.3 ± 10.0e | ||
| 4 | 68.1 ± 22.6d | 67.9 ± 5.3 | 68.3 ± 5.4 | 73.3 ± 10.0 | ||
| AMB | 0 | 96.7 ± 2.0 | 99.3 ± 0.9 | 96.7 ± 2.0 | 98.7 ± 2.5 | |
| 48 | ITC | 0 | 81.5 ± 4.4 | 95.3 ± 2.4 | 94.0 ± 2.7 | 84.0 ± 8.3 |
| 1 | 91.6 ± 3.1 | 90.9 ± 3.3 | 85.1 ± 4.1 | 77.3 ± 9.5 | ||
| 2 | 87.2 ± 3.8 | 83.2 ± 4.2 | 82.8 ± 4.3 | 73.3 ± 10.0 | ||
| 3 | 72.5 ± 5.1 | 69.5 ± 5.2 | 78.0 ± 4.7 | 54.7 ± 11.3 | ||
| 4 | 58.1 ± 5.6 | 67.5 ± 5.5d | 69.6 ± 5.2 | 48.0 ± 11.3 | ||
| AMB | 0 | 98.7 ± 1.3 | 100.0 ± 0.0 | 97.0 ± 1.9 | 98.7 ± 2.6 | |
See Table 2, footnote b.
A total of 300 comparisons.
A total of 75 comparisons.
The P value (obtained by a two-way ANOVA of log2 MIC end points derived by the two methods) was <0.01.
The P value was <0.05.
In order to study further the low levels of agreement between the NCCLSvis and NCCLSsp methods for MIC-0 of ITC after 24 h, the relative ODs of the NCCLSsp method which corresponded to the MIC-0 values of the NCCLSvis method were associated with the ODs of their growth controls for all 75 comparisons in a cross-sectional study. Various contingency tables were constructed based on different cutoff values for relative ODs of MIC-0 (5 and 10%) and ODs of the growth control as validation criteria (0, 0.05, and 0.1). The strongest association was found when a relative OD of 10% as a cutoff for MIC-0 was combined with an OD of 0.1 for the growth control as a validation criterion (P < 0.0005). The Spearman correlation coefficient between these two parameters was −0.35 (P = 0.029). Using the 10% relative OD as a cutoff for MIC-0 instead of 5% and an OD of 0.1 for the growth control as an evaluation criterion, the discrepancy between the NCCLSvis and NCCLSsp methods was reduced 10 times (from 28 to 2.7%) and the levels of agreement reached 98%. However, 27% of MIC-0 values of the NCCLSsp method will be in agreement with those of the NCCLSvis method, although the ODs of their growth controls could be less than 0.1. The levels of agreement between the XTTvis and XTTsp methods, especially for MIC-0, were higher after 24 and 48 h (91 and 95, respectively).
(iv) Agreement between NCCLS and XTT methods.
For AMB, the levels of agreement between the NCCLSvis and XTTvis methods as well as between the NCCLSsp and XTTsp methods were higher than 97% (Table 3). For ITC, the agreement between the NCCLSvis and XTTvis methods was higher than 83% for MIC-0, MIC-1, and MIC-2, with MIC-1 showing the highest agreement after 24 h (93%) and MIC-0 doing so after 48 h (94%). Among the species, A. ustus showed the lowest level of agreement (82%). The levels of agreement between the NCCLSsp and XTTsp methods were lower with MIC-1 after 24 h and higher with MIC-0 after 48 h (95 and 84%, respectively). Statistically significant differences were found for MIC-3 of ITC after 24 h between the NCCLSvis and XTTvis methods as well as between the NCCLSsp and XTTsp methods (P < 0.05). The statistically significant differences for MIC-0 of ITC after 24 h between the NCCLSsp and XTTsp methods (P < 0.05) were due to the erroneous higher MICs obtained by the NCCLSsp method. The discrepancies were reduced when the above-mentioned validation criteria were applied for the determination of MIC-0 by the NCCLSsp method. No statistically significant systematic differences between the two methods and the species were obtained for both drugs after 24 and 48 h of incubation for all MIC end points (P > 0.25).
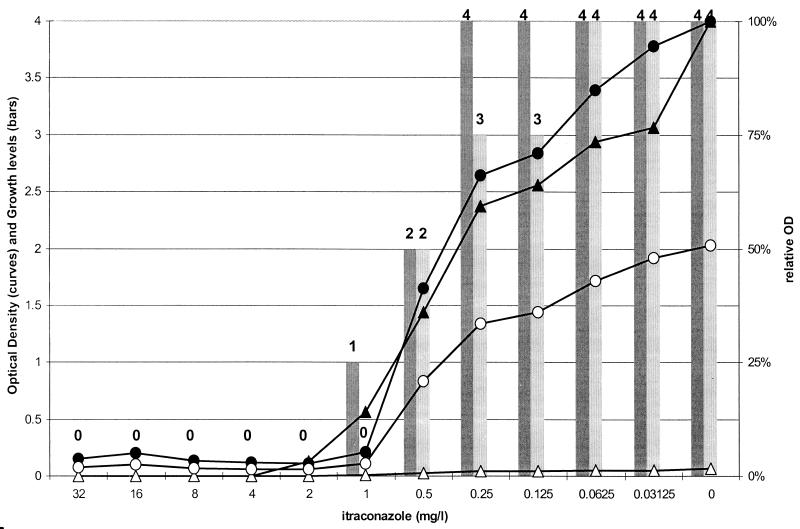
A representative graph is shown in Fig. 1, with the results of NCCLSvis, NCCLSsp, XTTvis, and XTTsp for an A. ustus strain and ITC. Growth at an OD at 405 nm of 0.047 with the NCCLSsp method corresponded to formazan production of 2.0 OD units at 450 nm with the XTTsp method.
FIG. 1.
Results of susceptibility testing of an A. ustus strain against ITC in the NCCLS and XTT methods, with data determined visually and spectophotometrically, after 24 h of incubation. The bars represent the growth levels obtained in the NCCLSvis (dark bars) and XTTvis (light bars) methods for each concentration on a scale of 0 (absence of growth or color) to 4 (no reduction of growth or color compared with the data for the drug-free control). The curves with the circles represent the OD at 450 nm (open symbols) and the relative OD (percentage) (closed symbols) obtained by the XTTsp method. The curves with the triangles represent the OD at 405 nm (open symbols) and the relative OD (percentage) (closed symbols) obtained by the NCCLSsp method.
DISCUSSION
The selection of an endpoint for MIC determination of antifungal drugs is an important factor of antifungal susceptibility testing of filamentous fungi which may increase the variability of these tests (5). This parameter is crucial especially for fungistatic drugs like ITC since partial inhibition of the growth due to the delayed action of the drug (trailing phenomenon) may result in limited growth over a range of drug concentrations which may elevate the MIC-0. Therefore, MIC-2 (prominent reduction of growth) was chosen for this drug (20). However, in previous studies MIC-1 (slight growth or 75% reduction of growth) showed higher interlaboratory agreement and similar interexperimental agreement relative to MIC-2 (6, 12). In the present study, high levels of intra- and interexperimental agreement were found for MIC-0 and MIC-1 (>95%) as was found in our previous study (13). For MIC-2, the levels of intra- and interexperimental agreement were lower after 24 h (89 and 86%, respectively) although they increased after 48 h of incubation (95 and 94%, respectively). The interexperimental agreement for MIC-2 was improved when the spectrophotometric reading was used (96% after 24 h and 99% after 48 h). Similar results were obtained when XTT was used.
For fungicidal drugs such as AMB, growth ceases abruptly after exposure to the drug which results in clear-cut end points (20). Therefore, the MIC-0 was chosen as the end point. In this study, in agreement with previous studies, high levels of inter- and intraexperimental agreement (>98%) were found after 24 and 48 h of incubation. Using the dye XTT, the absolute inter- and intraagreements of visual determination were further increased.
It is assumed that spectrophotometric estimation of the growth of filamentous fungi is inaccurate because of nonhomogeneity (4, 5). However, in previous studies, high levels of agreement were found between visual and spectrophotometric readings at 405 and 570 nm for MIC-1 and MIC-2 for ITC and MIC-0 for AMB (3, 12). The same results were obtained in this study, since the levels of agreement for MIC-1 and MIC-2 were 92 and 87%, respectively, for ITC and 97% for AMB with MIC-0. However, the levels of agreement between visual and spectrophotometric readings at 405 nm for MIC-0 of ITC were very low, especially after 24 h (51%; P < 0.01). In most cases, the MIC-0 values based on spectrophotometric readings were higher than those of visual readings. Further analysis of the discrepancies showed that this disagreement depended on the cutoff of relative OD as well as the OD of the growth control (P < 0.0005). In a previous study, an OD of 0.15 for the growth control was chosen as validation criterion for spectrophotometric readings of susceptibility testing of Aspergillus species although no evidence for the choice of threshold level was provided (17). Using a threshold level of 10% relative OD for MIC-0 and as a validation criterion an OD of the growth control of greater than 0.1, the discrepancies were reduced 10-fold and the agreement between visual and spectrophotometric readings was increased to 98%.
Spectrophotometric readings are in general more precise and reproducible than visual reading. Since the hyphal growth of Aspergillus species does not seem to present an obstacle, spectrophotometry can be used to determine the susceptibility of these fungi to antifungal drugs resulting in more precise quantification of hyphal growth than is attained by a visual reading. However, further optimization is required. The presence of phenol red in RPMI 1640 medium may pose problems as it might lower the sensitivity of spectrophotometric readings. The absorbance of this medium is very high at 405 nm (OD, 0.15 to 0.2), a wavelength at which hyphae have the highest absorbance. At lower wavelengths, the background absorbance is decreased (OD at 630 nm, 0.06), but the absorbance of hyphae is also decreased (unpublished observations). Thus, due to high background absorbance, limited fungal growth is not detectable by spectrophotometer. Studies using RPMI 1640 medium without phenol red for in vitro susceptibility testing show identical results compared with the standard medium (23). Since phenol red was originally used to allow contamination of the medium to be detected, in antifungal susceptibility testing it serves no useful purpose; therefore, phenol red should be omitted.
Although spectrophotometric reading resulted in higher accuracy and reproducibility, lack in sensitivity was observed since growth higher than 0.1 OD is required for precise hyphal quantification. The sensitivity of spectrophotometric readings was increased with the colorimetric method, since ODs of up to 2.0 were achieved compared with 0.047 using the noncolorimetric method. In a previous study, a colorimetric method based on the dye XTT was applied for antifungal susceptibility testing of various yeasts against different azoles and flucytosine, resulting in high levels of agreement with the standard NCCLS method (8). XTT was applied for the first time for filamentous fungi by Sugar and Liu (23), who found lower MICs compared with the NCCLS method. These discrepancies between the two methods may be due to the different MIC end points that were chosen, namely, the MIC-0 defined as the first well showing no growth for the NCCLS method and a decrease in OD of greater than 50% for the XTT method (23). Variation in the colorimetric method may be caused by the absence of a step that stops the conversion, since extraction steps are not necessary. Since high levels of agreements were found between the spectrophotometric and the visual readings of the XTT method, the colorimetric method can be automated by using spectrophotometric readings. However, wells containing hyphae may interfere with OD measurements, since at 450 nm, where the formazan derivative is absorbed, hyphae also show high absorbance. These problems could be overcome by removing the formazan derivative from the wells where the fungi are growing, although it will increase the time required to generate results.
Strains for which MICs of antifungal agents are high were detectable with the colorimetric method, in some cases 24 h earlier than it was possible with visual reading. Furthermore, it was possible to distinguish between metabolically active hyphae and dead hyphae, which would both produce turbidity, which would be seen as growth when examined visually, possibly resulting in a trailing effect. Besides helping to alleviate this problem, the colorimetric method should allow detection of small amounts of growth, more precise quantification, and earlier MIC determination than is possible with the noncolorimetric method.
In conclusion, for AMB high levels of agreement were always obtained. For ITC with both the NCCLS and the XTT methods, MIC-0 and MIC-1 showed the highest levels of intra- and interexperimental agreement. For MIC-2, similar levels of agreement were achieved either by prolonging the incubation period to 48 h or by using spectrophotometric reading after 24h. The spectrophotometric readings can be used for antifungal susceptibility testing of Aspergillus species, since high levels of agreement with the visual readings were achieved for MIC-0, MIC-1, and MIC-2. The low agreement was found between the visual readings and the spectrophotometric readings of the NCCLS method for MIC-0 of ICZ was improved by shifting the threshold level to 10% relative OD and establishing an OD of 0.1 for the growth control as a validation criterion for the spectrophotometric readings. The XTT method based on both visual and spectrophotometric readings used in this study showed high levels of agreement with the NCCLS method, and higher levels of sensitivity and precision were achieved. Therefore, the colorimetric method could be used as an alternative method for antifungal susceptibility testing of Aspergillus species, although further work is required to study the robustness of this colorimetric method.
ACKNOWLEDGMENTS
This work was supported by the European Commission Training and Mobility of Researchers grant FMRX-CT970145 to Joseph Meletiadis and by the Mycology Research Center of Nijmegen.
REFERENCES
- 1.Anaissie E. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin Infect Dis. 1992;14(Suppl. 1):S43–S53. doi: 10.1093/clinids/14.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 2.Cormican M G, Pfaller M A. Standardization of antifungal susceptibility testing. J Antimicrob Chemother. 1996;38:561–578. doi: 10.1093/jac/38.4.561. [DOI] [PubMed] [Google Scholar]
- 3.Dannaoui E, Persat F, Monier M F, Borel E, Piens M A, Picot S. Use of spectrophotometric reading for in vitro antifungal susceptibility testing of Aspergillus spp. Can J Microbiol. 1999;45:871–874. [PubMed] [Google Scholar]
- 4.Denning D W, Radford S A, Oakley K L, Hall L, Johnson E M, Warnock D W. Correlation between in vitro susceptibility testing to itraconazole and in vivo outcome of Aspergillus fumigatus infection. J Antimicrob Chemother. 1997;40:401–441. doi: 10.1093/jac/40.3.401. [DOI] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff A, Barchiesi F, Hazen K C, Martinez-Suarez J V, Scalise G. Standardization of antifungal susceptibility testing and clinical relevance. Med Mycol. 1998;36(Suppl. 1):68–78. [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M G, Walsh T J. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawser S P, Norris H, Jessup C J, Ghannoum M A. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998;36:1450–1452. doi: 10.1128/jcm.36.5.1450-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahn B, Martin E, Stueben A, Bhakdi S. Susceptibility testing of Candida albicans and Aspergillus species by a simple microtiter menadione-augmented 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay. J Clin Microbiol. 1995;33:661–667. doi: 10.1128/jcm.33.3.661-667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latge J P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levitz S M, Diamond R D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985;152:938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- 12.Llop C, Pujol I, Aguilar C, Sala J, Riba D, Guarro J. Comparison of three methods of determining MICs for filamentous fungi using different end-point criteria and incubation periods. Antimicrob Agents Chemother. 2000;44:239–242. doi: 10.1128/aac.44.2.239-242.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meletiadis J, Meis J F G M, Mouton J W, Donnelly J P, Verweij P E. Comparison of NCCLS and 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J Clin Microbiol. 2000;38:2949–2954. doi: 10.1128/jcm.38.8.2949-2954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Melatiadis J, Mouton J W, Meis J F G M, Bouman B A, Donnelly J P, Verweij P E Eurofung Network. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol. 2001;39:3402–3408. doi: 10.1128/JCM.39.9.3402-3408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meletiadis J, Mouton J W, Rodriguez-Tudela J L, Meis J F G M, Verweij P E. In vitro interaction of terbinafine with itraconazole against clinical isolates of Scedosporium prolificans. Antimicrob Agents Chemother. 2000;44:470–472. doi: 10.1128/aac.44.2.470-472.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meshulam T, Levitz S M, Christin L, Diamond R D. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT) J Infect Dis. 1995;172:1153–1156. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium forming filamentous fungi. Proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 17.Odds F C, Van den Bossche H. Antifungal activity of itraconazole compared with hydroxy-itraconazole in vitro. J Antimicrob Chemother. 2000;45:371–373. doi: 10.1093/jac/45.3.371. [DOI] [PubMed] [Google Scholar]
- 18.Paull D K, Shoemaker H, Boyd M R. The synthesis of XTT: a new tetrazolium reagent that is bioreducible to a water-soluble formazan. J Heterocycl Chem. 1998;25:911–914. [Google Scholar]
- 19.Pfaller M A, Rinaldi M G. Antifungal susceptibility testing. Current state of technology, limitations, and standardization. Infect Dis Clin North Am. 1993;7:435–444. [PubMed] [Google Scholar]
- 20.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roehm N W, Rodgers G H, Hatfield S M, Glasebrook A L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142:257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- 22.Seudiero D A, Shoemaker R H, Paull K D, Monks A, Tierney S, Nofziger T H, Currens M J, Seniff D, Boyd M R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 23.Sugar A M, Liu X. Comparison of three methods of antifungal susceptibility testing with the proposed NCCLS standard broth macrodilution assay: lack of effect of phenol red. Diagn Microbiol Infect Dis. 1995;21:129–133. doi: 10.1016/0732-8893(95)00067-k. [DOI] [PubMed] [Google Scholar]
- 24.Tellier R, Krajden M, Grigoriew G A, Campbell I. Innovative end-point determination system for antifungal susceptibility testing of yeasts. Antimicrob Agents Chemother. 1992;36:1619–1625. doi: 10.1128/aac.36.8.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]