Abstract
We have developed and evaluated a new method to quantify human immunodeficiency virus type 2 (HIV-2) proviral DNA based on LightCycler real-time PCR. The assay has a detection limit of 5 copies/105 peripheral blood mononuclear cells (PBMC) and is insensitive to HIV-2 strain variability: HIV-2 subtypes A and B are both recognized and quantified. The intra- and interassay coefficients of variation range from 16 to 40% for high provirus concentrations (5 × 105 copies) and from 41 to 39% for low concentrations (5 copies). We used this method to compare the proviral DNA load and viral RNA load in plasma with clinical and immunological status for 29 patients infected by HIV-2 (subtype A in 17 and subtype B in 12). The proviral load (median, 201 copies/105 PBMC) was similar to that reported for HIV-1 infection. The median proviral loads did not correlate with the CD4+ cell count categories and were as follows for CD4+ cell counts of >400, 200 to 400, and <200 cells/mm3, respectively: 121 copies/105 PBMC (n = 8; range, <5 to 712 copies/105 PBMC); 114 copies/105 PBMC (n = 9; range, <5 to 1,907 copies/105 PBMC); and 285 copies/105 PBMC (n = 12; range, 53 to 2,524 copies/105 PBMC). Proviral load did not correlate with plasma HIV-2 RNA positivity. As HIV-2 is considered to replicate less efficiently than HIV-1, these high proviral loads might be explained by the proliferation of infected cells.
Human immunodeficiency virus type 2 (HIV-2) was first isolated in 1986 from peripheral blood mononuclear cells (PBMC) from patients in the Cape Verde Islands and Guinea-Bissau (7). Heterosexual and vertical HIV-2 transmission rates are lower than those of HIV-1 (4, 21, 24). HIV-2-infected patients exhibit a longer clinical latency period and progress more slowly toward AIDS (17, 22). The origins of these viruses have been clearly linked to cross-species transmission events between mangabeys (Cercocebus atys, small West African monkeys) and humans (23). Six HIV-2 subtypes have so far been described (6, 14). Only HIV-2 subtypes A and B are prevalent, the others being considered self-limiting infections at the epidemiological level. HIV-2 infection is mostly confined to West Africa (12).
The CD4+ cell count correlates negatively with clinical status in both HIV-2 and HIV-1 infections (3, 33). In contrast, HIV-2 is more difficult to detect in plasma than HIV-1 (3, 28). This fact has led us and others to consider HIV-2 poorly replicative, at least early in the infection (3, 12, 28, 33). This factor could explain the epidemiological and clinical differences between HIV-2 and HIV-1 infections. The reasons for this poorer fitness of HIV-2 in humans is unclear. The animal counterpart of HIV-2, SIVsm (infecting mangabeys), is highly replicative in its natural host, with high cellular and plasma viral loads, but is nonpathogenic (30). The reason for this lack of replicative robustness remains unclear.
For HIV-1 infection, it has been demonstrated that proviral load correlates with disease progression (32). Previous quantitative assessments of HIV-2 proviral load have proposed, as for HIV-1, an inverse correlation with the CD4+ cell count and clinical outcome (2, 27, 31). However, recent reports have suggested that HIV-2 proviral load does not correlate with the CD4+ cell count (15, 29).
The development of new quantitative methods using real-time PCR technology has led us to evaluate those methods for proviral HIV-2 DNA quantitation in 29 HIV-2-infected patients living in France. We also tested plasma RNA detection and compared the findings with immunological and clinical status.
MATERIALS AND METHODS
Study population and sample collection.
The study group consisted of 29 patients (17 males and 12 females) who had been enrolled since 1991 in the French National HIV-2 Cohort. These patients originated from various countries in West Africa, North Africa, and Europe. The infecting strains had been previously sequenced in the env region: 17 were subtype A, and 12 were subtype B (11). The epidemiological and clinical characteristics of the patients are summarized in Table 1.
TABLE 1.
Epidemiological, clinical, and virological characteristics of the 29 HIV-2-infected patients
| Patient | Age (yr) | Sexa | Geographic origin | Subtype | Stage | Treatmentb | CD4+ cell count (106 cells/liter) | Cell culture resultc | RNA detectionc | DNA viral load (copies)/105 PBMC |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | M | Mali | A | C | AZT | 54 | + | + | 199 |
| 2 | 41 | M | Mali | A | C | AZT-3TC | 24 | + | + | 2,524 |
| 3 | 46 | F | Mali | A | C | DDC | 25 | + | + | 1,287 |
| 4 | 43 | M | France | A | C | AZT | 31 | + | + | 369 |
| 5 | 42 | M | Burkina Faso | A | A | AZT-3TC | 37 | + | + | 2,342 |
| 6 | 45 | M | Ivory Coast | B | A | D4T-3TC-IDV | 83 | + | + | 69 |
| 7 | 47 | M | Senegal | B | C | AZT | 120 | + | + | 161 |
| 8 | 63 | M | France | B | A | AZT-3TC-IDV | 13 | − | − | 53 |
| 9 | 39 | M | Ivory Coast | B | A | AZT-DDI | 153 | + | + | 80 |
| 10 | 32 | F | Martinique | B | A | AZT | 178 | + | + | 791 |
| 11 | 57 | F | France | A | A | AZT | 189 | + | + | 457 |
| 12 | 31 | M | Guinea-Bissau | A | A | DDC | 199 | + | + | 201 |
| 13 | 43 | F | Senegal | A | A | NT | 210 | + | − | 1,907 |
| 14 | 25 | F | Ivory Coast | A | A | NT | 240 | + | − | 1,730 |
| 15 | 36 | F | Cape Verde Islands | A | A | NT | 333 | − | − | <5 |
| 16 | 45 | M | Mali | B | C | AZT | 283 | + | − | 92 |
| 17 | 57 | M | France | B | A | NT | 328 | − | − | <5 |
| 18 | 64 | F | Cape Verde Islands | A | A | NT | 339 | + | + | 354 |
| 19 | 27 | F | Ivory Coast | B | A | NT | 347 | + | − | 5 |
| 20 | 35 | M | Guinea-Bissau | A | A | NT | 375 | + | + | 709 |
| 21 | 39 | M | Morocco | A | A | NT | 391 | + | − | 114 |
| 22 | 37 | M | Mali | A | A | NT | 540 | − | + | <5 |
| 23 | 34 | F | Ivory Coast | A | A | NT | 644 | − | − | 223 |
| 24 | 41 | M | France | A | A | AZT | 474 | − | − | <5 |
| 25 | 33 | F | Ivory Coast | B | A | NT | 799 | − | − | 712 |
| 26 | 33 | F | Senegal | A | A | NT | 832 | + | + | 334 |
| 27 | 62 | F | France | B | A | NT | 418 | + | + | 390 |
| 28 | 27 | M | Ivory Coast | B | A | NT | 413 | + | + | 19 |
| 29 | 55 | M | Congo | B | A | NT | 483 | ND | − | <5 |
M, male; F, female.
AZT, zidovudine; 3TC, lamivudine; DDC, dideoxycytidine; IDV, indinavir; NT, no treatment; DDI, didanosine; D4T, stavudine.
+, positive; −, negative; ND, not done.
According to Centers for Disease Control and Prevention criteria (5), 23 patients were in stage A and 6 were in stage C. Eight patients had CD4+ cell counts above 400 × 106/liter (median, 511; range, 413 to 832), 9 had counts between 200 × 106 and 400 × 106/liter (333; 210 to 391), and 12 had counts below 200 × 106/liter (68; 13 to 199). Fourteen patients were receiving antiretroviral treatment at enrollment in this study (one drug for 9, two drugs for 3, and three drugs for 2).
Sample preparation and DNA extraction.
Whole blood was collected in EDTA-containing Vacutainer tubes, and PBMC were isolated by Ficoll-Hypaque density gradient centrifugation. Plasma was clarified and stored at −80°C until viral RNA assays were done. DNA was extracted using a QIAamp DNA mini kit (QiAgen) and quantified spectrophotometrically. Cellular and plasma viral culturing was performed as previously described (33).
Preparation of HIV-2 DNA and RNA assay standards.
DNA templates were derived from a PCR-amplified region of the gag gene of HIV-2 strain ROD. HIV-2 ROD DNA in fresh PBMC was amplified by nested PCR with a first round of long PCR. The LTR1-5′–LTR2-3′ primer pair was used for the first amplification step as previously described (10). Nested PCR was done using the following primers containing enzyme restriction sequences at their 5′ extremities: QC1Bam, 5′-GATTGCGGATCCGTGGGAGAATGGGCGCGAGA-3′, and GAG OG AS1 Hind, 5′-CTGCATAAGCTTGCCTTCTGAGAGTGCCTGAAATCC-3′ (Genset, Paris, France). The resulting PCR product was cloned into vector pGEM3Z (Promega, Madison, Wis.). Plasmids were purified on columns with a Qiagen kit and quantified by A260 measurements. To control the validity of our primers, the amplified product was sequenced.
RNA transcripts were obtained from the linearized templates by using a Riboprobe in vitro transcription system according to the manufacturer's instructions (Promega). RNA was quantified spectrophotometrically at 260 nm. Transcripts were diluted to 106 copies/μl, divided into aliquots, and stored at −80°C. Subsequent dilutions were made in a solution containing carrier tRNA (from Escherichia coli; 30 μg/ml; Sigma, St. Louis, Mo.).
Plasma RNA detection.
Viral RNA was extracted from 1 ml of plasma and ultracentrifuged for 1 h at 4°C using an HCV specimen preparation kit (Roche Diagnostics Systems). RNA was amplified by a one-step procedure using a Titan one-tube RT-PCR kit (Roche) with primers located in the gag region (GAG OG S1 and GAG OG AS1). Amplified products were subjected to nested PCR with primers GAG OG S2 and GAG OG AS2 as previously described (16, 19). The efficiency and sensitivity of qualitative reverse transcription-PCR for detection of viral RNA isolated from plasma were determined by using a transcript standard diluted in 200 μl of RPMI medium to obtain 5,000, 1,000, 500, and 250 copies (seven replicates each). The standard was extracted and processed as described above. The sensitivities of reverse transcription-PCR were 100% at 5,000, 1,000, and 500 copies/ml and 66% at 250 copies/ml.
PCR primers and labeled hybridization probe.
In order to avoid major mismatches due to HIV-2 variability, the primers and probe were designed using specific software from TibmolBiol (Berlin, Germany), on the basis of all HIV-2 subtype A and B sequences so far published, according to the Los Alamos National Laboratory database (26). Primers used for amplification were located in the highly conserved gag region: U3, 5′-GGGAGATGGGCGCGAGA-3′, and L140, 5′-TCCAACAGGCTCTCTGCTAATCC-3′. Probe S65GAG2 had the sequence 5′-R-TAGGTTACGGCCCGGCGGAAAGA-Q-3′, where reporter R indicates a 6-carboxyfluorescein group and quencher Q indicates a 6-carboxytetramethylrhodamine group conjugated through a linker arm nucleotide as described previously (35). The specificity of the primers was evaluated with reference samples (11).
Proviral load quantitation.
Real-time quantitative PCR was performed using an LC Fast Start DNA master mix hybridization probe kit (Roche Molecular Diagnostics). Each PCR mixture (20-μl total volume) contained the following: 2 μl of DNA master mix, 2.4 μl of 25 mM MgCl2, primers U3 and L140 (0.8 μM each), TaqMan probe S65 (100 nM), and 20 μl of H2O qsp. Cycling parameters were as follows: denaturation for 8 min at 95°C, followed by 45 cycles of 10 at 95°C and 40 at 60°C. For each run, a standard curve was generated from purified HIV-2 gag plasmid ranging from 5 × 105 to 5 copies. Fresh dilutions of the control were prepared with salmon sperm DNA (100 ng/ml) before each experiment from a stock stored at −20°C. Five hundred nanograms of DNA from each patient was analyzed. Results were expressed in copies per 105 PBMC.
Reproducibility of DNA quantitation with the LightCycler system.
To assess the inter- and intra-assay reproducibilities of the LightCycler system, we first analyzed two- and fivefold differences in the starting copy number using 25,000 to 50,000 and 5,000 to 25,000 copies per capillary (12 replicates each). Calculations were determined with the cycle threshold (CT). We also tested 10-fold differences in copy number with a broader concentration range: from 5 to 50 copies (10 replicates each), 500 to 5,000 copies (6 replicates), and 50,000 to 500,000 copies (4 replicates). The last experiment was used to assess the interassay reproducibility (10 runs at each point from 5 to 5 × 105 copies).
RESULTS
Reproducibility of the LightCycler system for HIV-2 DNA quantitation.
DNA concentrations of 5,000, 25,000, and 50,000 copies were distinguished with high confidence. The coefficients of variation (CVs) ranged between 0.31 and 0.56% for the CT and between 7.5 and 12.9% for the copy number (Table 2). At a high virus concentration (mean, 534,340 copies per 105 PBMC), the intra-assay CV was 16%. At a mean copy number of 586,692 per 105 PBMC, the interassay CV was 40%. At the lowest concentration (five copies), the intra-assay CV was 41% and the interassay CV was 39%. Intra- and interassay CVs are summarized in Table 3.
TABLE 2.
CVs for quantitation of standards of 5,000, 25,000, and 50,000 copies
| Copies | % CV for:
|
|
|---|---|---|
| CT | Copy no. | |
| 5,000 | 0.5 | 11.8 |
| 25,000 | 0.31 | 7.5 |
| 50,000 | 0.56 | 12.9 |
TABLE 3.
Intra- and interassay precision of the LightCycler system
| Copies | Reproducibility
|
|||
|---|---|---|---|---|
| Intra-assay
|
Interassay
|
|||
| % CV | Meana | % CV | Meana | |
| 5 | 41 | 11 | 39 | 9 |
| 50 | 31 | 82 | 35 | 44 |
| 500 | 34 | 727 | 39 | 430 |
| 5,000 | 11.8 | 2,363 | 32 | 3,951 |
| 50,000 | 12.9 | 39,880 | 30 | 44,559 |
| 5,000,000 | 16.3 | 534,340 | 40 | 586,692 |
Values are in copy numbers per milliliter.
Sensitivity of the LightCycler system for HIV-2 DNA quantitation.
The sensitivities of the assay on the basis of repeated testing (eight replicates) of the lower concentrations of our plasmid standard were 100% at 5 copies, 62% at 2.5 copies, and 75% at 1 copy. Consequently, we set the detection limit of the assay at five copies.
HIV-2 proviral DNA detection according to subtype.
DNA viral load, expressed as copies per 105 PBMC, was determined for the 29 HIV-2-infected patients. DNA was detected in 24 (83%) of the 29 patients and was consistently below five copies in the other 5 patients. The lack of HIV-2 provirus amplification was not linked to the subtype, three subtype A and two subtype B samples being below the cutoff. This result indicates that the selected primers and probes cover a wide range of HIV-2 diversity.
HIV-2 proviral load is high whatever the CD4+ cell count.
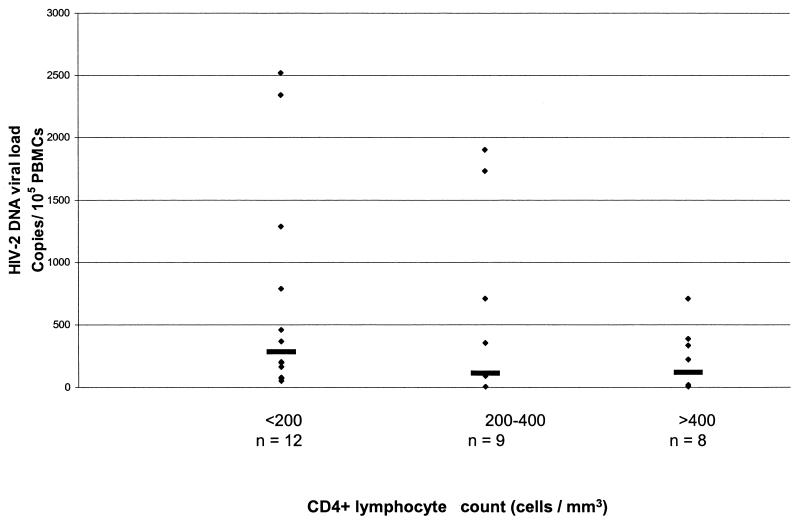
Levels of proviral DNA ranged from 5 to 2,524 copies per 105 PBMC, with a median of 201 copies/105 PBMC. These values are similar to those previously reported for both HIV-2 and HIV-1 (1, 2, 8, 15, 27, 29, 31). DNA viral load in these 29 patients was then examined according to the following CD4+ cell count categories: <200/mm3 (all these patients being on antiretroviral therapy), 200 to 400/mm3, and >400/mm3. The respective median proviral loads per 105 cells were 285 (12; 53 to 2,524 [n, range]), 114 (9; <5 to 1,907), and 121 (8; <5 to 712). This trend toward increasing proviral load according to CD4+ cell count was not significant. All the samples with negative provirus amplification were from patients with CD4+ cell counts above 328 × 106 cells/liter. Viral load did not differ significantly among these three categories of CD4+ cell count (χ2 = 3.071; P < 0.3; Kruskal-Wallis test) (Fig. 1).
FIG. 1.
HIV-2 proviral load according to CD4+ cell count category. Horizontal lines represent median values in each category. Samples below the limit of detection were given a value of four copies/105 PBMC. The number of samples in each category is shown. There is no correlation among the three groups (χ2 = 3.071; P < 0.3; Kruskal-Wallis test).
HIV-2 proviral load does not influence plasma RNA detection.
There was no significant relationship between the level of HIV-2 proviral load and the rate of plasma RNA detection (U = 61; P < 0.07, Mann-Whitney test). All but one of the patients with a CD4+ cell count below 200 × 106/liter were positive for plasma RNA; the remaining patient was receiving antiretroviral tritherapy (Table 4). A correlation was found between positive cell culture and viral DNA load (U = 26; P < 0.02; Mann-Whitney test).
TABLE 4.
Relationships among the CD4+ cell count, HIV-2 proviral load, cell culture positivity, and plasma RNA positivity
| CD4+ cell count (no./mm3) | No. of patients tested | Median (range)
|
No. positive/no. tested (%) for:
|
||
|---|---|---|---|---|---|
| CD4+ cell count, 106/liter | Viral DNA load, copies/105 PBMC | Cell culture | Plasma RNA | ||
| <200 | 12a | 68 (13–199) | 285 (53–2,524) | 11/12 (92) | 11/12 (92) |
| 200–400 | 9b | 333 (210–391) | 114 (<5–1,907) | 2/9 (22) | 7/9 (78) |
| >400 | 8b | 511 (413–832) | 121 (<5–712) | 3/7 (43) | 4/8 (50) |
Eleven patients were treated.
One patient was treated.
DISCUSSION
We evaluated a real-time PCR assay with the LightCycler system for HIV-2 proviral DNA quantitation in clinical specimens. The LightCycler system is simple and rapid and offers a standardized approach to HIV-2 DNA quantitation. It also avoids false-positive results due to PCR contamination.
In our experiments, the reproducibility of the system was similar to that of commercial viral load kits (9, 25). The high sensitivity of our assay (detection limit, 5 DNA copies/105 cells) must be emphasized, as it provides ample opportunities for further clinical studies. Given the importance of HIV-2 genetic diversity, we first tried to select conserved and efficient segments of the viral genome to use as primers. During a previous evaluation (data not shown) with different gag primers (U5, 5′-GAGAATGGGCGCGAGAAACT-3′, and L130, 5′-AATTCATTCGCTGCCCACAC-3′), we successfully amplified all the A subtypes but failed to amplify any B subtypes. This result convinced us of the underlying importance of primer and probe design in genome-based detection assays. Previous studies of HIV-2 quantitation were performed in West Africa, where only subtype A circulates (1, 2, 31). In contrast, subtype B is frequent in HIV-2-infected patients living in France (11) and probably in other Western countries as well (18), creating the need to assess a large panel of representative HIV-2 strains.
The levels of HIV-2 proviral DNA found in the 29 patients tested here were high and similar to those observed in studies of HIV-2 subtype A infections (1, 2, 15). We found no significant differences in the amount of proviral DNA according to subtype. Likewise, we found no correlation between proviral DNA level and CD4+ cell count or viral RNA positivity. These results for DNA quantitation are similar to those recently reported by Popper et al. (29) and Gomes et al. (15), although other studies have shown a correlation between these parameters (2, 27, 31). Popper et al. also reported a lack of correlation between levels of viral RNA and proviral DNA (29).
Because of the low pathogenicity of HIV-2 compared to that of HIV-1, we were intrigued in find low HIV-2 proviral loads but relatively large amounts of HIV-2 DNA in our patients. A selection bias can be ruled out, as the majority of the patients were symptom free and the CD4+ cell count distribution was normal. In a community-based study, Ariyoshi et al. found similar high levels of HIV-2 DNA, regardless of the percentage of CD4+ cells (1). Although not statistically significant, we tended to find higher levels of HIV-2 DNA and a trend toward lower CD4+ cell counts with more advanced stages of infection. The effect of antiretroviral treatment in patients with CD4+ cell counts of <200/μl could explain the lack of correlation between proviral loads and CD4+ cell counts. Alternatively, our results could be due to a high rate of defective integrated virus. Low cellular pathogenicity of HIV-2 in the early stages of infection, together with the long duration of the symptom-free period, could explain the high proviral loads. As with human T-cell leukemia virus type 1 infection, where high proviral loads have been reported (13, 20, 34), normal proliferation of infected cells could explain the large number of PBMC harboring proviral DNA. This discordance between high proviral loads and inefficient replication of HIV-2 might help to explain the roles of viral replication and immune responses in HIV-2 disease.
ACKNOWLEDGMENT
This study was supported by French National Agency on AIDS Research (A.N.R.S.) grant 98015.
REFERENCES
- 1.Ariyoshi K, Berry N, Wilkins A, Ricard D, Aaby P, Naucler A, Ngom P T, Jobe O, Jaffar S, Dias F, Tedder R S, Whittle H A. Community-based study of human immunodeficiency virus type 2 provirus load in rural village in West Africa. J Infect Dis. 1996;173:245–248. doi: 10.1093/infdis/173.1.245. [DOI] [PubMed] [Google Scholar]
- 2.Berry N, Aryoshi K, Jobe O, Ngum P T, Corrah T, Wilkins A, Whittle H, Tedder R. HIV type 2 proviral load measured by quantitative polymerase chain reaction correlates with CD4+ lymphopenia in HIV type 2 infected individuals. AIDS Res Hum Retrovir. 1994;10:1031–1037. doi: 10.1089/aid.1994.10.1031. [DOI] [PubMed] [Google Scholar]
- 3.Berry N, Ariyoshi K, Jaffar S, Sabally S, Corrah T, Tedder R S, Whittle H A. Low peripheral blood viral HIV-2 RNA in individuals with high CD4+ percentage differenciates HIV-2 from HIV-1 infection. J Hum Virol. 1998;1:457–468. [PubMed] [Google Scholar]
- 4.Bruker G, Brun-Vézinet F, Rosenheim M, Rey M A, Katlama C, Gentilini M. HIV-2 infection in two homosexual men in France. Lancet. 1987;i:223. doi: 10.1016/s0140-6736(87)90043-2. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescent and adults. Morb Mortal Wkly Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 6.Chen Z, Luckay A, Sodora D L, Telfer P, Reed P, Gettie A, Kanu J M, Sadek R F, Yee J A, Ho D D, Zhang L, Marx P A. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of a distinct HIV-2 genetic subtype from the natural range of simian immunodeficiency virus-infected sooty mangabey. J Virol. 1997;71:3953–3960. doi: 10.1128/jvi.71.5.3953-3960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavel F, Guetard D, Brun-Vézinet F, Chamaret S, Rey M A, Santos-Ferreira M O, Laurent A G, Dauguet C, Katlama C, Rouzioux C, Klatzmann D, Champalimaud J L, Montanier L. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986;233:343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- 8.Clementi M, Menzo S, Bagnarelli P, Valenza A, Paolucci S, Sampaolesi R, Manzin A, Varaldo P E. Clinical use of quantitative molecular methods in studying human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 1996;9:135–147. doi: 10.1128/cmr.9.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coste J, Montes B, Reynes J, Peeters M, Segarra C, Vendrell J P, Delaporte E, Segondy M. Comparative evaluation of three assays for the quantitation of human immunodeficiency virus type 1 RNA in plasma. J Med Virol. 1996;50:293–302. doi: 10.1002/(SICI)1096-9071(199612)50:4<293::AID-JMV3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Damond F, Loussert-Ajaka I, Apetrei C, Descamps D, Souquière S, Leprêtre A, Matheron S, Brun-Vézinet F, Simon F. Highly sensitive method for amplification of human immunodeficiency virus type 2 DNA. J Clin Microbiol. 1998;36:809–811. doi: 10.1128/jcm.36.3.809-811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damond F, Apetrei C, Robertson D L, Souquière S, Leprêtre A, Matheron S, Brun-Vézinet F, Simon F. Variability of human immunodeficiency virus type 2 infecting patients living in France. Virology. 2001;280:19–30. doi: 10.1006/viro.2000.0685. [DOI] [PubMed] [Google Scholar]
- 12.De Cock K, Brun-Vézinet F. Epidemiology of HIV-2 infection. AIDS. 1989;3(Suppl. 1):S89–S95. doi: 10.1097/00002030-198901001-00013. [DOI] [PubMed] [Google Scholar]
- 13.Gabet S A, Mortreux F, Talarmin A, Plumelle Y, Leclercq I, Leroy A, gessain A, Clity E, Joubert M, Wattel E. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene. 2000;19:4954–4960. doi: 10.1038/sj.onc.1203870. [DOI] [PubMed] [Google Scholar]
- 14.Gao F, Yue L, Robertson D L, Hill S C, Hui H, Biggar R J, Neequaye A E, Whelan T M, Ho D D, Shaw G M, Sharp P M, Hahn B H. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J Virol. 1994;68:7433–7447. doi: 10.1128/jvi.68.11.7433-7447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes P, Taveira N C, Pereira J M, Antunes F, Ferreira M O, Lourenco M H. Quantitation of human immunodeficiency virus type 2 DNA in peripheral blood mononuclear cells by using a quantitative-competitive PCR assay. J Clin Microbiol. 1999;37:453–456. doi: 10.1128/jcm.37.2.453-456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grankvist O, Bredberg-Raden U, Gustafsson A, Albert J, Albino P, Andreasson P A, Naucler A, Biberfeld G, Wadell G. Improved detection of HIV-2 DNA in clinical samples using a nested primer based polymerase chain reaction. J Acquir Immune Defic Syndr Hum Retrovirol. 1992;5:286–293. [PubMed] [Google Scholar]
- 17.Kanki P J, Travers K U, Mboup S, Hsieh C C, Marlink R G, Guèye-Ndiaye A, Siby T, Thior I, Hernandezavila M, Sankale J L, Ndoye I, Essex M E. Slower heterosexual spread of HIV-2 than HIV-1. Lancet. 1994;343:943–946. doi: 10.1016/s0140-6736(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 18.Kühnel H, Von Briesen H, Dietrich U, Adamski M, Mix D, Biesert L, Kreutz R, Immelmann A, Henco K, Meichsner C, Andreesen R, Gelderblom H, Rübsamen-Waigmann H. Molecular cloning of two West African human immunodeficiency virus type 2 isolates that replicate well in macrophage: a Gambian isolate, from a patient with neurologic acquired immunodeficiency syndrome, and a highly divergent Ghanian isolate. Proc Natl Acad Sci USA. 1989;86:2383–2387. doi: 10.1073/pnas.86.7.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loussert-Ajaka I, Simon F, Farfara I, Descamps D, Collin G, Brun-Vézinet F. Detection of circulating human immunodeficiency virus type 2 in plasma by reverse transcription polymerase chain reaction. Res Virol. 1995;146:409–414. doi: 10.1016/0923-2516(96)80900-9. [DOI] [PubMed] [Google Scholar]
- 20.Manns A, Miley W J, Wilks R J, Morgan O S, Hanchard B, Wharfe G, Cranston B, Maloney E, Welles S L, Blattner W A, Waters D. Quantitative proviral DNA and antibody levels in the natural history of HTLV-1 infection. J Infect Dis. 1999;180:1487–1493. doi: 10.1086/315088. [DOI] [PubMed] [Google Scholar]
- 21.Marlink R. Lessons from the second virus, HIV-2. AIDS. 1996;10:689–699. doi: 10.1097/00002030-199606001-00002. [DOI] [PubMed] [Google Scholar]
- 22.Marlink R G, Ricard D, Mboup S, Kanki P J, Romet-Lemonne J L, Doye I N, Diop K, Simpson M A, Greco F, Chou M J, Degruttola V, Hsieh C C, Boye C, Barin F, Denis F, McLane M F, Essex M. Clinical, hematologic and immunologic cross-sectional evaluation of individuals exposed to human immunodeficiency virus type 2. AIDS Res Hum Retrovir. 1988;2:137–148. doi: 10.1089/aid.1988.4.137. [DOI] [PubMed] [Google Scholar]
- 23.Marx P A, Li Y, Lerche N W, Sutjipto S, Gettie A, Yee J A, Brotman B H, Prince A M, Hanson A, Webster R G, Desrosiers R C. Isolation of a simian immunodeficiency virus related to human immunodeficiency virus type 2 from a West African pet sooty mangabey. J Virol. 1991;65:4480–4485. doi: 10.1128/jvi.65.8.4480-4485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matheron S, Courpotin C, Simon F, Di Maria H, Balloul S, Bartzack S, Dormont D, Brun-Vézinet F, Saimot A G, Coulaud J P. Vertical transmission of HIV-2. Lancet. 1990;335:1103–1104. doi: 10.1016/0140-6736(90)92682-8. [DOI] [PubMed] [Google Scholar]
- 25.Murphy D G, Gonin P, Fauvel M. Reproducibility and performance of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1999;37:812–814. doi: 10.1128/jcm.37.3.812-814.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers G, Foley B, Mellors J W, Korber B, Jeang K T, Wain-Hobson S. Human retroviruses and AIDS 1996. A compilation and analysis of nucleic acid and amino-acid sequences. Los Alamos, N.Mex: Los Alamos National Laboratory; 1996. [Google Scholar]
- 27.Norrgren H, Marquina S, Leitner T, Aaby P, Melbye M, Poulsen A G, Larsen O, Dias F, Escanilla D, Andersson S, Albert J, Naucler A. HIV-2 genetic variation and DNA load in asymptomatic carriers and AIDS cases in Guinea-Bissau. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:31–38. doi: 10.1097/00042560-199709010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Popper S J, Sarr A D, Travers K U, Gueye-Ndiaye A, Mboup S, Essex M E, Kanki P J. Lower human immunodeficiency virus (HIV) type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J Infect Dis. 1999;180:1116–1121. doi: 10.1086/315010. [DOI] [PubMed] [Google Scholar]
- 29.Popper S J, Sarr A D, Gueye-Ndiaye A, Mboup S, Essex M E, Kanki P J. Low plasma human immunodeficiency virus type 2 viral load is independent of proviral load: low virus production in vivo. J Virol. 2000;74:1554–1557. doi: 10.1128/jvi.74.3.1554-1557.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rey-Cuillé M A, Berthier J L, Bomsel-Demontoy M C, Chaduc Y, Montagnier L, Hovanessian A G, Chakrabarti L A. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J Virol. 1998;72:3872–3886. doi: 10.1128/jvi.72.5.3872-3886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarr A D, Popper S, Thior I, Hamel D J, Sankale J L, Siby T, Marlink R, Essex M E, Mboup S, Kanki P J. Relation between HIV-2 proviral load and CD4+ lymphocyte count differs in monotypic and dual HIV infections. J Hum Virol. 1999;2:45–51. [PubMed] [Google Scholar]
- 32.Schechter M T, Neumann P W, Weaver M S, Montaner J S, Cassol S A, Le T N, Craib K J, O'Shaughnessy M V. Low HIV-1 proviral DNA burden detected by negative polymerase chain reaction in seropositive individuals correlates with slower disease progression. AIDS. 1991;5:373–379. doi: 10.1097/00002030-199104000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Simon F, Matheron S, Tamalet C, Loussert-Ajaka I, Bartzack S, Pépin J M, Dever C, Gamba E, Elbim C, Gastaut J A, Saimot A G, Brun-Vézinet F. Cellular and plasma viral load in patients infected with HIV-2. AIDS. 1993;7:1411–1417. doi: 10.1097/00002030-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Wattel E, Cavrois M, Gessain A, Wain-Hobson S. Clonal expansion of infected cells: a way of life for HTLV-1. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(Suppl. 1):S92–S99. doi: 10.1097/00042560-199600001-00016. [DOI] [PubMed] [Google Scholar]
- 35.Wittwer C T, Ririe K M, Andrew R V, David D A, Gundry R A, Balis U J. The LightCycler: a microvolume multisample fluorimeter with rapid temperature control. BioTechniques. 1997;22:176–181. doi: 10.2144/97221pf02. [DOI] [PubMed] [Google Scholar]