Abstract
Aim:
A tissue-equivalent bolus of sufficient thickness is used to overcome build up effect to the chest wall region of postmastectomy radiotherapy (PMRT) patients with tangential technique till Radiation Therapy Oncology Group (RTOG) Grade 2 (dry desquamation) skin reaction is observed. The aim of this study is to optimize surface dose delivered to chest wall in three-dimensional radiotherapy using EBT3 film.
Materials and Methods:
Measurements were conducted with calibrated EBT3 films with thorax phantom under “open beam, Superflab gel (0.5 cm) and brass bolus conditions to check correlation against TPS planned doses. Eighty-two patients who received 50 Gy in 25# were randomly assigned to Group A (Superflab 0.5 cm gel bolus for first 15 fractions followed by no bolus in remaining 10 fractions), Group B or Group C (Superflab 0.5 cm gel or single layer brass bolus, respectively, till reaching RTOG Grade 2 skin toxicity).
Results:
Phantom measured and TPS calculated surface doses were within − 5.5%, 4.7%, and 8.6% under open beam, 0.5 cm gel, and single layer of brass bolus applications, respectively. The overall surface doses (OSD) were 80.1% ±2.9% (n = 28), 92.6% ±4.6% (n = 28), and 87.4% ±4.7% (n = 26) in Group A, B, and C, respectively. At the end of treatment, 7 out of 28; 13 out of 28; and 9 out of 26 patients developed Grade 2 skin toxicity having the OSD value of 83.0% ±1.6% (n = 7); 93.7% ±3.2% (n = 13); and 89.9% ±5.6% (n = 9) in Groups A, B, and C, respectively. At the 20th–23rd fraction, 2 out of 7; 6 out of 13; and 4 out of 9 patients in Groups A, B, and C developed a Grade 2 skin toxicity, while the remaining patients in each group developed at the end of treatment.
Conclusions:
Our objective to estimate the occurrence of optimal dose limit for bolus applications in PMRT could be achieved using clinical EBT3 film dosimetry. This study ensured correct dose to scar area to protect cosmetic effects. This may also serve as quality assurance on optimal dose delivery for expected local control in these patients.
Keywords: Brass bolus, chest wall, fractionation, postmastectomy radiotherapy, skin toxicity, surface dose
INTRODUCTION
Breast cancer is the most common malignancy among Indian females with an age-adjusted rate as high as 25.8 per 100,000 women and mortality of 12.7 per 100,000 women.[1] In the current era, one-third of patients diagnosed with invasive breast cancer with locally advanced tumors will undergo mastectomy.[2,3] Postmastectomy radiotherapy (PMRT) with megavoltage (MV) photon beam with a dose of 50 Gy in 25 fractions enhances local control and survival by substantially reducing chest wall recurrence from 21% to 7.8%.[4,5,6] In an earlier study in PMRT, it was highlighted that chest wall failure when the delivered dose did not achieve brisk erythema or moist desquamation on the treated skin.[7] This necessitates sufficient radiation dose delivery to chest wall.[8] Furthermore, the radiation-induced side effects onto the skin shall be minimal. Therefore, delivery of required dose in PMRT to the chest wall remains a challenge.[9,10]
Skin-sparing effect of the megavoltage beam may give rise to inadequate dose to the anterior aspect of the chest wall (target), extending up to the skin. Suitable thickness of tissue-equivalent bolus is kept on the skin during tangential irradiations for adequate dose buildup for a few initial fractions till clinically observable moderate-to-brisk skin erythema appears. According to the Radiation Therapy Oncology Group (RTOG)[11] criterion, a Grade 2 skin reaction (dry desquamation) is recommended as a surrogate marker for the optimum dose to the chest wall.[12,13,14] The surface dose is of great relevance in PMRT because of its impact on acute/late skin toxicity, cosmetic outcome, and local control. Rigid/semi-rigid wax/gel bolus sheets introduce irregularity in skin contact and lack of conformity to the chest wall surface doses.[15] Commercially available brass mesh bolus provides more chest wall conformity and has become popular in a few departments, and its benefits and surface dose estimates were highlighted in many reports.[16,17,18,19,20,21]
Chest wall dose estimates are carried out using thermoluminescent dosimeters (TLD), metal oxide semiconductor field effect transistors, optically stimulated luminescent dosimeters, radiochromic EBT2/EBT3 films in open and bolus chest wall techniques, namely, three-dimensional radiotherapy (3DCRT), intensity-modulated radiotherapy (IMRT), volumetric arc radiotherapy, helical tomotherapy, etc. Film dosimetry with cut pieces of radiochromic films is preferred because of convenience in handling, dose evaluation, and reproducibility of results. To avoid overdosing to chest wall, skin bolus application is stopped after completion of part of the total course of treatment, to avoid over dosage toxicity to skin. In this regard, the optimum application of bolus both in terms of material and duration of application for conventional fractionation needs to be evaluated.
The dosimetric characterization of the use of brass mesh bolus, methods of surface dose estimates, and standardization of phantom techniques were reported.[20,22] The present study has objective to prospectively examine and quantify the surface dose to the chest wall using radiochromic EBT3 films in patients undergoing 3DCRT with 6 MV photon beam to standardize protocol for optimal skin dose to the chest wall.
MATERIALS AND METHODS
Linear accelerator
An “Elekta Compact” (Elekta AB, Stockholm, Sweden), linear accelerator (linac) with 6 MV photon mode, with 40 pairs MLC having1 cm leaf thickness at iso-center is used in this study. Output is calibrated to a reference output of 1 cGy/MU at isocenter for a field size of 10 cm × 10 cm at 350 MU/min as per Technical Report Series 398 protocol.[23]
Calibration of EBT3 detectors
Radiochromic EBT3 film dosimetric films (International Specialty Products, Wayne, New Jersey, USA) were used for surface dose measurements. Ten 2 cm × 2 cm film strips were irradiated at 10 cm depth, in a 20 cm thick water equivalent slab phantom (ρ =1.045 g/cm3) (SP34, IBA Dosimetry GmbH, Germany) with 30 cm × 30 cm × 1 cm sheets, with source to phantom surface at 90 cm, for a field size 10 cm × 10 cm. Film doses (0.0 to 4.0 Gy at different increments) were correlated to measured doses with Farmer-type chamber (FC65, IBA Dosimetry GmbH, Germany). The measured net optical density (OD) using Epson 11000XL (Epson America Inc., Long Beach, CA, USA) was expressed against the unexposed film as the logarithmic value of the ratio of mean pixel value unexposed versus exposed film. The calibration curve with third order polynomial, interpolating radiation dose against net OD was used for calculating unknown radiation dose. Our earlier report[22] outlined dose estimates using EBT3 film in terms of dose-OD calibrations, with regular shaped phantom and prototype chest wall acrylic phantom.
Surface dose measurements to the chest wall region with elliptical thorax phantom
A locally fabricated acrylic elliptical shaped thorax phantom having outer dimensions of 31 cm (length) ×21 cm (height) ×30 cm (width) (ρ =1.03 g/cc) comprising materials simulating the lung and spine with cork (ρ =0.24 g/cc) and Teflon (ρ =1.62 g/cc), respectively, was used to measure surface dose to the chest wall region using EBT3 film. To estimate the surface dose with film across the chest wall region, four locations of size 2 cm × 2 cm were selected toward the left side of the phantom and marked with a radio-opaque marker to determine the location of the film. Computed tomography (CT) scan images of phantom with 0.5 cm slice spacing were transferred to the “Focalsim contouring station” (M/s Elekta Ltd., Crawley, UK). Five reference points at 1.0 mm below the surface contour at each film location in the images on the CT slices were marked. CMS XiO® (Elekta Ltd, Crawly, UK) version 4.80.02 treatment planning system (TPS) for dose calculations using superposition algorithm. A three-dimensional conformal radiotherapy (3DCRT) plan with a three-field (two tangential and an anterior oblique) technique was generated with a dose prescription of 50 Gy in 25 # normalized to the 100% isodose with a minimum target coverage of 95% as per the institutional protocol that is being followed for patients undergoing PMRT.
Field-in-field technique followed by the application of wedge (as and when required) was done to reduce the hot spots in the treatment region to improve dose coverage around the target. Six 3DCRT plans were generated by placing a virtual bolus of thickness 0.0 cm (no bolus), 0.2 cm, and 0.5 cm across the target area over the phantom chest wall. The 0.2 cm virtual bolus was chosen based on the water equivalent thickness of a single layer of brass mesh bolus as described in the literature.[21,22]
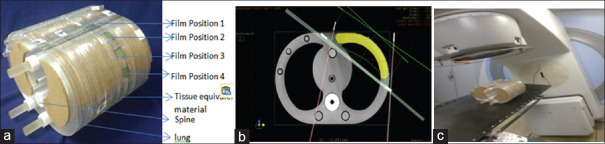
The mean dose obtained from the five interest points against each film location in the respective 3DCRT plan was noted as the TPS calculated surface dose. Before the execution of each treatment plan, EBT3 film strips were placed on the right side phantom surface at defined locations. Respective 3DCRT treatment plans were executed under linac using no bolus; by placing the Superflab gel with thicknesses of 0.5 cm, and single layer brass bolus around the target region of the chest wall (note that the film strips are under the bolus). Figure 1a-c shows the placement of film strips at four marked locations toward the left side of the chest wall of the phantom; representative CT image of a 3DCRT plan in TPS; followed by the plan execution under linac. To investigate and account for calculation, interfractional, and treatment delivery uncertainties, three different sets of measurements for each plan were obtained.
Figure 1.
(a) Film detectors at four different locations toward the left side of chest wall, (b) representative computed tomography image of a three-dimensional radiotherapy plan in treatment planning system and (c) plan execution under linac
Surface dose measurements in postmastectomy radiotherapy patients
This study is a prospective randomized trial with patients having institute's ethical committee approval (No. IEC KMC MLR 07-17/154). Histologically confirmed breast cancer receiving PMRT for conventional treatment fractionation to the chest wall was eligible for this study. All patients enrolled in the study were randomized into three groups (A, B, and C) using an accepted “stratified block randomization” method.[24] Based on fractionations treated with bolus applications, these three groups are shown in Table 1. A total of 82 patients who are planned for PMRT during the period January 2018 to December 2020 are enrolled in this study.
Table 1.
Description of three groups on postmastectomy radiotherapy (50 Gy/25 Fr) and type of applied bolus
| Description | Groups | ||
|---|---|---|---|
|
| |||
| A | B | C | |
| Type of bolus | Superflab gel | Superflab gel | Brass mesh |
| Density (g/cm3) | 1.02 | 1.02 | 8.5 |
| Dimensions (cm) | 35×35 | 35×35 | 45×45 |
| Physical/tissue equivalent thickness (mm) | 5/5 | 5/5 | 0.12/2 |
| Bolus applied on treatment fractions | 15 fractions with bolus; 10 fractions open beam | From start of treatment till the observation of Grade 2 skin toxicity (RTOG grading) | |
RTOG: Radiation therapy oncology group
Table 2 describes the patient-specific variables in three Groups A, B, and C. In Group A, there were 28 patients (left side, 15; right side, 13) with a mean age of 53 years and mean body mass index (BMI) of 23.8. In Group B, there were 28 patients (left side, 16; right side, 12) with a mean age 50 years and mean BMI of 22.3. In Group C, there were 26 patients (left side, 19; right side, 7) with a mean age of 49 years and mean BMI of 22.8 years.
Table 2.
Patient-specific parameters in three (A, B, and C) Groups
| Classifications | Group | ||
|---|---|---|---|
|
| |||
| A | B | C | |
| Number of patients (n) | 28 | 28 | 26 |
| Site of treatment | |||
| Left (n) | 13 | 16 | 19 |
| Right (n) | 15 | 12 | 7 |
| Age (years), range (mean) | 29-69 (53) | 32-73 (50) | 24-75 (49) |
| Height (m), range (mean) | 1.4-1.9 (1.5) | 1.4-1.6 (1.5) | 1.3-1.7 (1.5) |
| Weight (kg), range (mean) | 43-80 (56.9) | 35-86 (51.0) | 29-65 (52.0) |
| BMI, range (mean) | 16.2-31.2 (23.8) | 14.6-33.6 (22.3) | 13.6-35.6 (22.8) |
BMI: Body mass index
All patients were immobilized in the supine position using Vac-Loc and wing board, with both arms maximally abducted. Target volumes as appropriate were drawn CT images in the contouring station with European Society for Radiotherapy and Oncology consensus guideline for early stage breast cancer elective radiation therapy.[25] Bilateral lungs, spinal cord, and heart were considered as organs at risk (OAR). Two plans were generated (a) without bolus and (b) with virtual bolus on the chest wall for (3DCRT) 3-field technique (two tangential and added anterior oblique beams). Suitable weighted anterior oblique beam helps to improve conformity of dose to the target volume. This serves as single entity encompassing both chest wall and nodal regions obviating the need for separate isocenters, at the same time not exceeding tolerance limits of normal structures (bilateral lungs and heart). The planning process was same as described for the phantom.
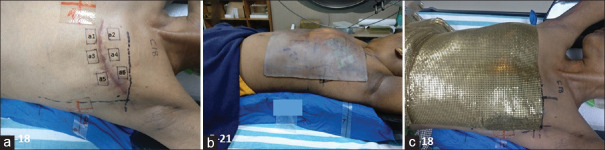
Six positions across the surgical scar (3 above and 3 below the scar) of the chest wall skin were marked as a1,…., a6 for skin dose estimates with EBT3 film. Figure 2a-c shows locations of EBT3 films on the skin and presence of Superflab gel bolus/brass mesh bolus. As indicated earlier, we always used the mesh bolus as a single layer only.
Figure 2.
(a) Six (a1, a2, a3, a4, a5, and a6) marked locations (2 cm × 2 cm) across the surgical scar on the left side of the chest wall for surface dose measurements for EBT3 films, (b) patient with Superflab 0.5 cm gel bolus in position on the right side chest wall, (c) Brass mesh bolus (single layer) across the chest wall region (film strips below bolus for clinical dosimetry). Patient's randomization number could be seen indicating study group
The treatment plan was executed under linac with selected bolus in all A, B, and C groups along with film detectors. At least 3–4 measurements (with/without bolus) were carried out for surface dose. Measured surface dose with/without bolus was defined as the mean surface dose measured against each marked location of the film and noted as “b“/”c,” respectively. Overall surface dose (OSD) for the entire course of conventional treatment to an individual patient is calculated using the below equation [Annexure 1 at end of the report indicates the application of our method [e.g. patient no. A14] described below].
OSD = r (With bolus) + s (Without bolus)…………….(1)
where r = Total surface dose obtained with bolus fractions
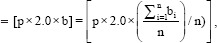
s = Total surface dose obtained without bolus fractions
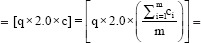
p = The number of bolus fractions.
bi=Mean surface dose with bolus obtained fromfilms at all six locations
n =The number of measurements taken with bolus.
b =Mean surface dose from all measurements with bolus.
r =Total surface dose obtained with bolus fractions.
q =The number of no-bolus fractions.
ci=Mean surface dose without bolus obtained from films at all six locations
(a1, a2, a3, a4, a5, and a6) obtained during “i”th measurement. i.e.,.
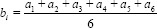
m =The number of measurements taken without bolus
c =Mean surface dose from all measurements without bolus
s =Total surface dose obtained without bolus fractions.
Every patient in three groups had their surface doses measured with the bolus, and all patients in Group A had their surface doses measured without bolus. During the treatment period, all patients were treated daily for 5 days per week and were reviewed for grading of skin toxicity as per RTOG guidelines at the end of every five fractions, for first three weeks, and daily till the development of Grade 2 skin toxicity. The bolus was discontinued once the patient develops Grade 2 skin toxicity.
RESULTS
Surface dose estimates with elliptical thorax phantom
It is observed that mean EBT3 measured surface doses showed an agreement with TPS calculated within 10% limits [Table 3]. The maximum difference between the calculated and measured mean surface dose was − 5.5%, 4.7%, and 8.6%, respectively, under no bolus, 0.5 cm gel bolus, and brass bolus. Mann–Whitney statistical test compared the calculated and measured surface dose under no bolus, gel, and brass bolus conditions. There is no significant difference observed between the calculated and the measured values P > 0.05 as seen in last column of Table 3], confirming good agreement between them.
Table 3.
Comparison EBT3 measured and calculated surface doses for no bolus, gel, and brass bolus (in chest wall phantom)
| Bolus type (thickness) | Film position | TPS calculated surface dose (%) | Mean±SD (%) | EBT3 mean surface dose (%) | Mean±SD (%) | P |
|---|---|---|---|---|---|---|
| No bolus (0.0 cm) | 1 | 54.8 | 58.2±8.5 | 51.3 | 52.7±7.9 | 0.15 |
| 2 | 70.9 | 63.7 | ||||
| 3 | 53.3 | 50.7 | ||||
| 4 | 53.8 | 44.9 | ||||
| Superflab gel (0.5 cm) | 1 | 98.7 | 98.4±4.8 | 102.4 | 103.1±4.6 | 0.25 |
| 2 | 104.8 | 109.6 | ||||
| 3 | 97.0 | 102.0 | ||||
| 4 | 93.3 | 98.5 | ||||
| Single layer brass mesh (0.12 cm) | 1 | 81.3 | 80.7±6.1 | 86.3 | 88.9±6.5 | 0.08 |
| 2 | 89.0 | 97.5 | ||||
| 3 | 77.6 | 89.4 | ||||
| 4 | 75.0 | 82.2 |
SD: Standard deviation, TPS: Treatment planning system
Surface doses measurements in postmastectomy radiotherapy patients
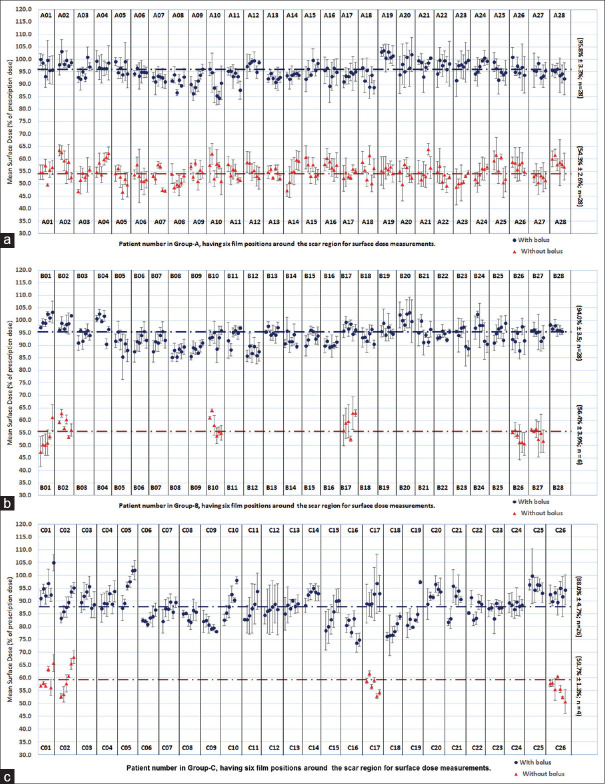
Measured mean surface dose without and with bolus applications (at six positions) estimated in treatment Groups A, B, and C shown in Figure 3a-c, respectively. The standard deviation in dose estimates is also highlighted. Due to the development of a Grade 2 (RTOG) skin reaction, the application of bolus was discontinued in six patients belongs to Group B (B01, B02, B10, B17, B26, and B27) and four patients in group C (C01, C02, C17, and C26), and to continue the measurements without bolus [Figure 3b and c]. As observed from these figures, the measured mean surface dose with and without bolus in Group A, B, and C was 95.8%±3.3%, 54.3%±2.6%; 94.0%±3.5%, 56.4%±3.9%; and 88.0%±4.7%, 59.7%±1.3%, respectively. This was shown as a horizontal dotted line against bolus/without bolus application.
Figure 3.
Mean surface dose (with standard deviation) measured without and with bolus application at six locations around the scar region against each patient during the course of conventional treatment in (a) Group A of postmastectomy radiotherapy patients (n = 28), (b) Group B of postmastectomy radiotherapy patients (n = 28), and (c) Group C of postmastectomy radiotherapy patients (n = 26)
The calculated OSD values for all groups of patients and are tabulated in Table 4. Accordingly, the OSD values were 80.1% ±2.9% (n = 28); 92.6% ±4.6% (n = 28); and 87.4% ±4.7% (n = 26); respectively, in A, B, and C groups. Data show the normal distribution where parametric test analysis of variance was adopted to compare the OSD and shows a significant difference between three groups (P < 0.001) as shown in Table 4. The post hoc comparison between B and A, B and C, C and A shows that overall there was a significant difference as shown in Table 5. At the end of treatment, 29 patients out of 82 developed Grade 2 skin toxicity, with 7 (25%) in Group A, 13 (46.4%) in Group B, and 9 (34.6%) in Group C. Their OSD value were 83.0% ±1.6% (n = 7); 93.7% ±3.2% (n = 13); and 89.9% ±5.6% (n = 9), respectively.
Table 4.
Overall surface dose around the chest wall in A, B and C groups of patients calculated as per the formula 1
| Groups | n | Mean±SD (%) | 95% CI | P* |
|---|---|---|---|---|
| A | 28 | 80.1±2.9 | 73-85 | <0.001 |
| B | 28 | 92.6±4.6 | 87-99 | |
| C | 26 | 87.4±4.7 | 77-96 |
*Calculated from ANOVA. SD: Standard deviation, n: Number of patients, CI: Confidence interval, ANOVA: Analysis of variance
Table 5.
Mean difference of overall surface dose among the A, B, and C groups of patients
| Groups | Mean difference (%) | 95% CI | P* |
|---|---|---|---|
| B and A | 12.64 | 9.96-15.32 | <0.001 |
| B and C | 5.28 | 2.60-7.97 | |
| C and A | 7.36 | 4.67-10.04 |
*Calculated from ANOVA. CI: Confidence interval, ANOVA: Analysis of variance
Among these 29 patients, 2 out of 7; 6 out of 13, and 4 out of 9 patients in Groups A, B, and C developed Grade 2 skin toxicity during the treatment at 20th–23rd fraction, whereas the remaining patients in the respective group developed Grade 2 skin reaction at the end of treatment. Figure 4 represents the skin toxicity grading (as per RTOG scoring criteria) observed at the end of every five fractions during treatment in A, B, and C groups.
Figure 4.
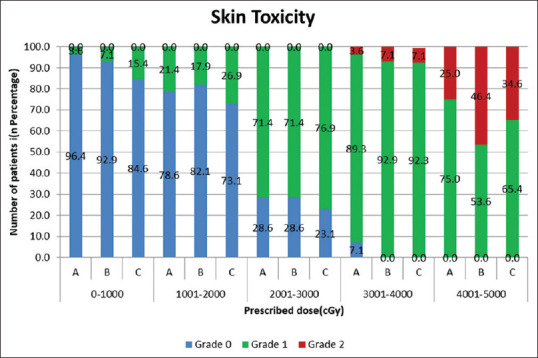
Skin toxicity grading (as per Radiation Therapy Oncology Group scoring criteria) observed at the end of every five fractions during treatment of patients undergoing PMRT in A, B, and C groups
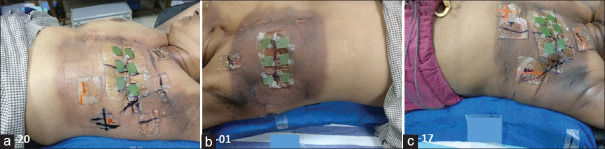
Figure 5a-c shows PMRT patient in groups A, B, and C developed grade 2 (RTOG) skin reactions at the 20th, 22nd, and 23rd fractions of treatment respectively and film strips placed across surgical scar for surface dose measurement without bolus.
Figure 5.
(a-c) shows postmastectomy radiotherapy patient belongs to Groups A, B, and C developed Grade 2 (Radiation Therapy Oncology Group) skin reactions at the 20th, 22nd, and 23rd fractions of treatment, respectively, and film strips placed across surgical scar for surface dose measurement without bolus for subsequent fractions. Number at bottom left corner indicates the patient randomization number given against the respective study group
DISCUSSION
This paper outlined the clinical dosimetry method for achieving optimal surface dose delivery in a prospective study to the chest wall. Agreement in measured doses from phantom study prompted us to undertake a prospective randomized study in PMRT dose delivery with 0.5 cm gel bolus and brass mesh bolus with 3DCRT using 6 MV photon beam to achieve Gade 2 skin reaction, with optimal bolus applications. The OSD values obtained with phantom and in patients under applications of no bolus, Superflab 0.5 cm gel, and single layer brass mesh bolus in the current study and published literature are shown in Table 6. All of the published literatures listed in Table 6 were carried out using a standard fractionation schedule, which was also used in this study. Estimated doses in the present work were in good agreement, with the range of doses reported by various authors; minor differences could be due to phantom designs, and the type of detectors, and their sensitivity in measuring surface doses.
Table 6.
Obtained overall surface dose values in the present study compared with published values
| Bolus type/thickness | Study type | OSD (%) range (mean±SD; n) | |
|---|---|---|---|
|
| |||
| Present work (%) a | Literature (%) | ||
| No bolus | Phantom | 44.9–63.1 (52.7±7.9) | 40.0–72.0 |
| Manger et al.[17]a, e | |||
| 56.1–71.2 (65.6±7.8) | |||
| Bahreyni et al.[25]a, f | |||
| 45.0–65.0 | |||
| Almberg et al.[26]a, g | |||
| 64.2±2.8 | |||
| Fiedler et al.[32]a, h | |||
| Patients | 48.0–59.0 (53.9±2.9; n=38) (Group A, B and C)$ | 47.2–58.1 (51.7±6.6) | |
| Rudat et al.[27]a | |||
| 0.5 cm gel bolus | Phantom | 77.1–91.2 (82.9±6.3) | Mean 81.6 |
| Fischbach et al.[28]b, f | |||
| Patient | 73.3–84.7 (79.2±3.2; n=28) (Group A) | 77.8–107.6 (94.3±12.4) | |
| Singh et al.[29]b | |||
| 0.5 cm gel bolus | Phantom | 98.5–109.6 (103.1±4.6) | 85.0–109.0 |
| Manger et al.[17]a, e | |||
| 99.7±3.9 | |||
| Fiedler et al.[32]a, h | |||
| Patient | 87.4–99.0 (93.5±3.1; n=22) (Group B) | 85.3–103.5 (90.1±3.2; n=19) | |
| Wake et al.[30]c | |||
| Brass mesh bolus | Phantom | 82.2–97.5 (88.9±6.5) | 75.0–110 |
| Manger et al.[17]a, e | |||
| 96.4±4.6 | |||
| Fiedler et al.[32]a, h | |||
| Patient | 76.7–96.0 (87.3±5.2; n=22) (Group C) | 81.0–122.0 (99.0±10.0; n=16) | |
| Healy et al.[18]b, d | |||
n = number of patients. SD: Standard deviation. OSD: Overall surface dose. $Patients treated under no bolus application in Group A, B and C. #Superflab bolus is applied to 60% of the treatment fractions, followed by nil bolus in the remaining course of treatment. ##Application of Superflab 0.5 cm bolus till the completion of treatment. ### Application of single layer brass bolus till the completion of treatment. aRadiochromic EBT3 film.bThermoluminescent Dosimeter (TLD), cOptically Stimulated Luminescent Dosimeter (OSLD), dMetal Oxide Semiconductor Field Effect Transistor (MOSFET), eCIRS heterogeneous IMRT thorax phantom, fRANDO Phantom, gAnthropomorphic female thorax phantom, hAnthropomorphic thorax phantom
When compared to the nil bolus condition, the increment in surface dose with either gel/brass bolus can be attributed to corpuscular radiations (low energy photons and scattered electrons) from the bolus material. We observed that under brass bolus application, the reason for overestimated surface dose (about 8.2%) against TPS, may be because our brass bolus was not configured in TPS by measurements. A water equivalent thickness (0.2 cm) as virtual bolus thickness had been used during 3DCRT plan generation.
In the current study, the OSD values obtained with phantom and in patients (n = 38 from Group A, B, and C) were 52.7% and 53.9%, respectively, under no bolus applications. This is because of the build-up effect of 6 MV photon beam. This implies that PMRT without bolus would result in under-dosage of the chest wall surface. In the present study, the OSD values with phantom and patients (Group A; n = 28) were 82.9% and 79.2%, respectively, under the application of gel bolus for 60% of treatment fractions (n = 15). This is in agreement with a mean surface dose of 81.6% measured with TLD on a RANDO phantom by Fischbach et al.[30] The surface dose measured was in the range 77.8%–107.6% with a mean of 83.0% reported by other groups.[31]
When the gel bolus was applied for the complete course of treatment, our results showed 6 patients out of 22 (in group B) developed Grade 2 skin toxicity before the end of treatment, when the dose estimated was 103.1% and 93.5%. These results were in agreement for similar bolus treatments.[17,32] In another group, gel bolus thickness of 0.5 cm was applied for PMRT and they reported Grade 2 acute skin toxicity (RTOG) was observed in (37%).[33]
Under the application of brass mesh bolus, the OSD observed in our study was 88.9% and 87.3% with phantom and in patients (n = 22 from Group C); the remaining four patients developed Grade 2 skin toxicity during the course of treatment. The outcome of surface dose measurements from the present study under the application of brass bolus for the full course of treatment is almost in agreement with earlier reports, in the similar percentage doses.[17,18]
Concerning the Grade 2 (RTOG) skin toxicity in brass bolus patients (Group C), around 35% of patients (n = 9 out of 26) developed Grade 2 skin toxicity (RTOG). Their mean OSD value was 89.9%. The remaining 17 patients were well within Grade 1 toxicity limits. According to Healy et al.[18] reported similar observation that once the brisk skin reaction was observed at a median value of 84% of the surface dose, they discontinued the application of brass bolus. In our study, the application of brass mesh bolus was discontinued at 20th fraction in patient's C01, C02, 23rd fraction in a patient C17 and 21st fraction in patient C26 since they developed Grade 2 skin toxicity (cumulated target dose was around 40–46 Gy). Al-Rahbi et al.[20] reported similar Grade 2 reactions between the 18th and 23rd fractions (in the range 79.5% to 84.9% with the mean skin dose of 81.9%).
It is widely followed practice to enhance skin dose, by keeping bolus material on the chest wall, and prevent skin dose increase by removing bolus after desired number of fractionations. There may be differences in the exact fraction at which skin reaction sets in, based on the texture of skin for different nationals, other host factors in addition to photon spectrum, bolus chemical compensations, and other specifications; in addition to the standard deviation in various detectors. Hence, there was a need in our department to exactly understand the skin response for our setup and patients' population.
When comparing the dose and the number of patients developed Grade 2 skin toxicity, Group B outperformed Groups A and C. Therefore, we recommend the regimen of treatment given in Group B to be considered. As there was no statistical significance difference Table 5 between Groups B and C (about 3.8%), considering the conformity of the brass mesh bolus over a three-dimensional convex irregular surface of the chest wall of the breast, the treatment given in Group C has an edge over conventional gel bolus.
CONCLUSIONS
In this study, both phantom results and large group of patient's study documented the total surface doses. As clinical dosimetry ongoing basis is not feasible due to many constraints, the present database will help in optimal Superflab and single layer brass bolus applications to achieve desired surface doses in PMRT situations. Group B situation confirms adequate scar dose to await for Grade 2 skin toxicity and remove bolus later. This skin toxicity study enables necessary quality assurance on correct optimal dose delivery to ensure local control expected in follow up. Our results may be associated with biologically effective doses if different fractionation protocols are adopted, as we expressed surface doses in percentage of total dose to PTV. This study can be further extended to the application of multiple layer brass mesh bolus applications if necessary.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors thankfully acknowledge the patients, medical dosimetrist, undergraduate students and college administration for their support.
ANNEXURE
Annexure 1.
Calculation sheet for obtaining overall surface dose measured with EBT3 film across scar during the full course of conventional fractionation according to formula (1) in a patient belonging to Group A with randomization number A14
| Parameter | Measured surface dose with bolus | Measured surface dose without bolus | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | ||
| Film position | a1 | 93.0 | 94.8 | 92.8 | 48.8 | 44.6 | 48.6 |
| a2 | 92.7 | 87.8 | 95.8 | 57.6 | 45.7 | 49.0 | |
| a3 | 98.0 | 92.1 | 91.0 | 60.1 | 50.6 | 53.8 | |
| a4 | 92.8 | 94.8 | 94.9 | 61.1 | 50.7 | 53.3 | |
| a5 | 92.8 | 94.6 | 95.8 | 63.9 | 56.7 | 58.3 | |
| a6 | 94.2 | 92.0 | 94.6 | 63.3 | 55.9 | 58.1 | |
| Calculation parameters | Mean = 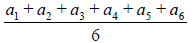
|
93.9 (b1) | 92.7 (b2) | 94.1 (b3) | 59.1 (c1) | 50.7 (c2) | 53.5 (c3) |
| Overall mean surface dose (%) from all measurements | b = 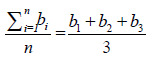 = 93.6 = 93.6 |
c = 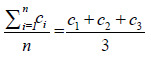 = 54.4 = 54.4 |
|||||
| Number of fractions | P=15 | q=10 | |||||
| Total surface dose (Gy) =r | r = p × 2.0×b = 15×2.0×0.936=28.08 | s = q × 2.0×c = 10×2.0×0.544=10.88 | |||||
| OSD (Gy) “t”=sum of total surface dose obtained with and without bolus=r + s | t = r + s=28.08+10.88=38.96 | ||||||
| OSD (%)= |
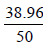 ˣ100 = 77.92 ˣ100 = 77.92 |
||||||
OSD: Overall surface dose
REFERENCES
- 1.Malvia S, Bagadi SA, Dubey US, Saxena S. Epidemiology of breast cancer in Indian women. Asia Pac J Clin Oncol. 2017;13:289–95. doi: 10.1111/ajco.12661. [DOI] [PubMed] [Google Scholar]
- 2.Gaudette LA, Gao RN, Spence A, Shi F, Johansen H, Olivotto IA. Declining use of mastectomy for invasive breast cancer in Canada, 1981-2000. Can J Public Health. 2004;95:336–40. doi: 10.1007/BF03405141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazovich D, Solomon CC, Thomas DB, Moe RE, White E. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early stage invasive breast carcinoma. Cancer. 1999;86:628–37. [PubMed] [Google Scholar]
- 4.Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–55. doi: 10.1056/NEJM199710023371401. doi:10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 5.Whelan TJ, Julian J, Wright J, Jadad AR, Levine ML. Does locoregional radiation therapy improve survival in breast cancer.A meta-analysis? J Clin Oncol. 2000;18:1220–9. doi: 10.1200/JCO.2000.18.6.1220. [DOI] [PubMed] [Google Scholar]
- 6.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 7.Thoms WW, Jr, McNeese MD, Fletcher GH, Buzdar AU, Singletary SE, Oswald MJ. Multimodal treatment for inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 1989;17:739–45. doi: 10.1016/0360-3016(89)90060-6. [DOI] [PubMed] [Google Scholar]
- 8.Taylor ME, Perez CA, Mortimer JE, Levitt SH, Leumwananonthachai N, Wahab SH. Breast: Locally advanced (T3 and T4) and recurrent tumors. In: Perez CA, Brady LW, Halperin EC, Schmidth-Ullrich RK, editors. Principles and Practices of Radiation Oncology. 4th ed. Philadelphia, USA: Lippincott Williams and Wilkins Press; 2004. pp. 1502–53. [Google Scholar]
- 9.Cho BC, Hurkmans CW, Damen EM, Zijp LJ, Mijnheer BJ. Intensity modulated versus non-intensity modulated radiotherapy in the treatment of the left breast and upper internal mammary lymph node chain: A comparative planning study. Radiother Oncol. 2002;62:127–36. doi: 10.1016/s0167-8140(01)00472-8. [DOI] [PubMed] [Google Scholar]
- 10.Sonnik D, Selvaraj RN, Faul C, Gerszten K, Heron DE, King GC. Treatment techniques for 3D conformal radiation to breast and chest wall including the internal mammary chain. Med Dosim. 2007;32:7–12. doi: 10.1016/j.meddos.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 12.Hsu SH, Roberson PL, Chen Y, Marsh RB, Pierce LJ, Moran JM. Assessment of skin dose for breast chest wall radiotherapy as a function of bolus material. Phys Med Biol. 2008;53:2593–606. doi: 10.1088/0031-9155/53/10/010. [DOI] [PubMed] [Google Scholar]
- 13.Fessenden P, Palos BB, Karzmark CJ. Dosimetry for tangential chest wall irradiation. Radiology. 1978;128:485–9. doi: 10.1148/128.2.485. [DOI] [PubMed] [Google Scholar]
- 14.Chung JB, Lee JW, Suh TS, Lee DH, Choe BY, Kim YS, et al. Dosimetric characteristics of standard and micro MOSFET dosimeters as in-vivo dosimeter for clinical electron beam. J Korean Phys Soc. 2009;55:2566–70. [Google Scholar]
- 15.Anderson PR, Hanlon AL, Fowble BL, McNeeley SW, Freedman GM. Low complication rates are achievable after post-mastectomy breast reconstruction and radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:1080–7. doi: 10.1016/j.ijrobp.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Ordonez-Sanz C, Bowles S, Hirst A, MacDougall ND. A single plan solution to chest wall radiotherapy with bolus? Br J Radiol. 2014;87:20140035. doi: 10.1259/bjr.20140035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manger R, Paxton A, Cerviño L. Dosimetric assessment of brass mesh bolus for post-mastectomy photon radiotherapy. J Appl Clin Med Phys. 2016;17:86–96. doi: 10.1120/jacmp.v17i6.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Healy E, Anderson S, Cui J, Beckett L, Chen AM, Perks J, et al. Skin dose effects of postmastectomy chest wall radiation therapy using brass mesh as an alternative to tissue equivalent bolus. Pract Radiat Oncol. 2013;3:e45–53. doi: 10.1016/j.prro.2012.05.009. doi:10.1016/j.prro.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Richmond ND, Daniel JM, Whitbourn JR, Greenhalgh AD. Dosimetric characteristics of brass mesh as bolus under megavoltage photon irradiation. Br J Radiol. 2016;89:20150796. doi: 10.1259/bjr.20150796. [DOI] [PubMed] [Google Scholar]
- 20.Al-Rahbi ZS, Cutajar DL, Metcalfe P, Rosenfeld AB. Dosimetric effects of brass mesh bolus on skin dose and dose at depth for postmastectomy chest wall irradiation. Phys Med. 2018;54:84–93. doi: 10.1016/j.ejmp.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Andic F, Ors Y, Davutoglu R, Baz Cifci S, Ispir EB, Erturk ME. Evaluation of skin dose associated with different frequencies of bolus applications in post-mastectomy three-dimensional conformal radiotherapy. J Exp Clin Cancer Res. 2009;28:41. doi: 10.1186/1756-9966-28-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobo D, Banerjee S, Saxena PU, Ravichandran R, Srinivas C, Putha SK, et al. Clinical implementation of brass mesh bolus for chest wall postmastectomy radiotherapy and film dosimetry for surface dose estimates. J Cancer Res Ther. 2019;15:1042–50. doi: 10.4103/jcrt.JCRT_1034_17. [DOI] [PubMed] [Google Scholar]
- 23.IAEA. Vienna: International Atomic Energy Agency; 2000. Absorbed Dose Determination in External Beam Radiotherapy. Technical Report Series TRS 398. [Google Scholar]
- 24.Suresh K, Thomas SV, Suresh G. Design, data analysis and sampling techniques for clinical research. Ann Indian Acad Neurol. 2011;14:287–90. doi: 10.4103/0972-2327.91951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Bahreyni Toosi MT, Mohamadian N, Ghorbani M, Khorshidi F, Akbari F, Knaup C. Skin dosimetry in radiotherapy of breast cancer: A comparison between EBT and EBT3 radiochromic films. J Biomed Phys Eng. 2016;6:51–60. [PMC free article] [PubMed] [Google Scholar]
- 27.Almberg SS, Lindmo T, Frengen J. Superficial doses in breast cancer radiotherapy using conventional and IMRT techniques: A film-based phantom study. Radiother Oncol. 2011;100:259–64. doi: 10.1016/j.radonc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Fiedler DA, Hoffman S, Roeske JC, Hentz CL, Small W, Jr, Kang H. Dosimetric assessment of brass mesh bolus and transparent polymer-gel type bolus for commonly used breast treatment delivery techniques. Med Dosim. 2021;46:e1–6. doi: 10.1016/j.meddos.2021.01.001. doi: 10.1016/j.meddos. 2021.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Rudat V, Nour A, Alaradi AA, Mohamed A, Altuwaijri S. In vivo surface dose measurement using GafChromic film dosimetry in breast cancer radiotherapy: Comparison of 7-field IMRT, tangential IMRT and tangential 3D-CRT. Radiat Oncol. 2014;9:156. doi: 10.1186/1748-717X-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischbach M, Hälg RA, Hartmann M, Besserer J, Gruber G, Schneider U. Measurement of skin and target dose in post-mastectomy radiotherapy using 4 and 6 MV photon beams. Radiat Oncol. 2013;8:270. doi: 10.1186/1748-717X-8-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh R, Oinam AS, Trivedi G, Kainth HS, Shahi JS, Singh B, et al. A comparative study for surface dose evaluation in conventional treatment of carcinoma breast patients irradiated with Co-60 and 6 MV radiation beam. J Cancer Res Ther. 2019;15:1035–41. doi: 10.4103/jcrt.JCRT_789_17. doi:10.4103/jcrt.JCRT_789_17. [DOI] [PubMed] [Google Scholar]
- 32.Wake JR, Chen FQ, Ashworth S, Byth K, Wang W, Stuart KE, et al. Verification using in vivo optically stimulated luminescent dosimetry of the predicted skin surface dose in patients receiving post-mastectomy radiotherapy. Med Dosim. 2021;46:e10–4. doi: 10.1016/j.meddos.2020.10.001. doi: 10.1016/j.meddos.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Yap ML, Tieu M, Sappiatzer J, Panzarella T, Cuartero J, McCready D, et al. Outcomes in Patients Treated with Post-mastectomy Chest Wall Radiotherapy without the Routine Use of Bolus. Clin Oncol (R Coll Radiol) 2018;30:427–32. doi: 10.1016/j.clon.2018.03.005. doi: 10.1016/j.clon.2018.03.005. [DOI] [PubMed] [Google Scholar]