Abstract
Background:
Postmenopausal women with isolated osteoporosis at the 1/3 radius (1/3RO) present a therapeutic dilemma. Little is known about whether these patients have generalized skeletal fragility, and whether this finding warrants treatment. The aim of this study was to investigate the biochemical and microarchitectural phenotype of women with 1/3RO compared to women with classic postmenopausal osteoporosis by DXA at the spine and hip (PMO), and controls without osteoporosis at any site.
Methods:
This cross-sectional study enrolled 266 postmenopausal women, who were grouped according to densitometric pattern. Subjects had serum biochemistries, areal BMD (aBMD) measured by DXA, trabecular and cortical vBMD, microarchitecture, and stiffness by high resolution peripheral QCT (HR-pQCT, voxel size ~82 μm) of the distal radius and tibia.
Results:
Mean age was 68±7 years. DXA T-Scores reflected study design. By HR-pQCT, 1/3RO had abnormalities at both radius and tibia compared to controls: lower total, cortical and trabecular vBMD, cortical thickness and trabecular number, higher trabecular separation and heterogeneity, and lower whole bone stiffness. In contrast, the magnitude and pattern of abnormalities in vBMD, microarchitecture and stiffness in 1/3RO were similar to those in PMO; the difference compared to controls was similar among the two groups. Serum calcium, creatinine, parathyroid hormone, 25-hydroxyvitamin D, and 24-hour urine calcium did not differ.
Conclusions:
Although aBMD appeared relatively preserved at the spine and hip by DXA, women with 1/3RO had significant microarchitectural and biomechanical deficits comparable to those in women with typical PMO. Further study is required to guide treatment decisions in this population.
Keywords: Osteoporosis, high resolution peripheral QCT, DXA, postmenopausal, microarchitecture
Introduction
Approximately 34 million Americans have osteoporosis and more than two million fragility fractures occur each year (1). Over 15 billion dollars are spent annually on health care costs related to osteoporosis and fragility fractures (1-3), which result in considerable morbidity and increased mortality (1, 4-9). While there are several available pharmacologic therapies that effectively lower fracture risk, identification of those patients at high risk for fracture remains a challenge. Treatment decisions are relatively straightforward in patients with osteoporosis at the spine and hip by DXA (10). However, the management of patients who have osteoporosis only at the 1/3 radius site is less clear. These patients present a therapeutic dilemma for several reasons. Little is known about fracture risk in patients who have isolated osteoporosis at the radius or whether isolated osteoporosis at this site reflects more widespread skeletal fragility. Moreover, data is lacking regarding the efficacy of anti-fracture treatment in these patients.
In this study, we investigated the biochemical and microarchitectural phenotype of women with isolated osteoporosis at the 1/3 radius using high resolution peripheral computed tomography (HR-pQCT). We compared the biochemical and structural characteristics of these women to those of women with classic postmenopausal osteoporosis by DXA at central sites, the spine and hip, and to postmenopausal controls without osteoporosis at any site. We hypothesized that women with isolated osteoporosis at the 1/3 radius have low volumetric bone mineral density (vBMD) and abnormal microarchitecture, with predominantly cortical bone deficits. We further hypothesized that microarchitectural abnormalities are less pronounced in these women compared with women who have osteoporosis at the spine and hip.
Methods
Patients
Postmenopausal women, over age 60 or more than 10 years postmenopause, were recruited at Columbia University Medical Center (CUMC; New York, NY) or Helen Hayes Hospital (HHH; West Haverstraw, NY) by advertisement, self- or physician referral. Potential subjects were excluded if they had a history of abnormal mineral metabolism (e.g., primary hyperparathyroidism, osteomalacia), endocrinopathy (e.g., untreated hyperthyroidism, Cushing's syndrome, prolactinoma), celiac or other gastrointestinal diseases, malignancy (except for skin cancer), and drug exposures that could affect bone metabolism (e.g., glucocorticoids, anticonvulsants, anticoagulants, methotrexate, aromatase inhibitors, thiazolidinediones, strontium). Women with stage 4 or 5 chronic kidney disease were excluded. Women who had ever used teriparatide, denosumab, or who had taken bisphosphonates for more than one year were excluded. Women using hormone replacement therapy or raloxifene were not excluded. At the study visit, past medical history, reproductive history, and medication use were assessed. A physical exam was performed including height by Harpenden stadiometer and weight, and body mass index (BMI) was calculated. All subjects provided written informed consent and the Institutional Review Board of Columbia University Medical Center approved this study.
Areal bone mineral density (aBMD) and spine radiographs
Areal BMD was measured by DXA (QDR-4500, Hologic Inc., Marlborough, MA at CUMC; Lunar Prodigy, GE, Madison, WI at HHH) at the lumbar spine (LS: L1-4), total hip (TH), femoral neck (FN), and 1/3 radius (1/3R). Lumbar vertebrae with significant deformity, osteosclerosis, osteophytes or degenerative disease were excluded from the analysis. T-scores compared subjects with young-normal populations of the same race and sex, as provided by the manufacturer. Spine radiographs were performed at the study visit to evaluate prevalent vertebral fractures. Vertebral fracture severity was determined using the semi-quantitative method of Genant et al.(11). Women were classified into groups by densitometric pattern after DXA data had been obtained, specifically as: isolated osteoporosis at the 1/3 radius (1/3RO), postmenopausal osteoporosis at the hip and/or spine (PMO), and controls without osteoporosis at any site. Women with osteoporosis by DXA at the spine or hip (TH or FN) were classified as PMO regardless of wrist BMD. Among this group, 39% had osteoporosis only at the spine, 30% only at the hip, 31% at both the spine and hip. In addition, 40% had osteoporosis at the wrist as well as at the spine or hip.
HR-pQCT and Image-Based μFEA of the distal radius and tibia
HR-pQCT (XtremeCT1, voxel size 82 μm, Scanco Medical AG, Brüttisellen, Switzerland) was performed at CUMC. The non-dominant forearm and ipsilateral tibia (or non-fractured arm or leg in subjects with prior wrist or ankle fracture) was immobilized in a carbon fiber shell. Scans were performed as we have described in prior publications (12-17). Briefly, the region of interest was defined on a scout film by manual placement of a reference line at the endplate of the radius or tibia; with the first slice 9.5 mm and 22.5 mm proximal to the reference line at the radius and tibia, respectively. A stack of 110 parallel CT slices was acquired at the distal end of both sites using an effective energy of 40 keV, image matrix size 1024 x 1024, with a nominal voxel size of 82 μm. This provided a 3D image of approximately 9 mm in the axial direction. Attenuation data were converted to equivalent hydroxyapatite (HA) densities. The European Forearm Phantom was scanned daily for quality control. All scans were acquired by the same technician. HR-pQCT data were used to calculate whole bone stiffness, a measure of bone's resistance to force using finite element analysis. The analysis methods have been described, validated (18-20), and applied in several clinical studies (21-28). Bone tissue was modeled as an isotropic, linearly elastic material with a Young’s modulus (Es) of 15 GPa and a Poisson’s ratio of 0.3 (29). A uniaxial displacement equaling 1% of the bone segment height was applied perpendicularly to the distal surface of the radius or tibia while the proximal surface was imposed with zero displacement along the same direction. Both ends of the tibia were allowed to expand freely in the transverse plane. The total reaction force was calculated from the linear μFE analysis, and the axial stiffness was calculated as the reaction force divided by the imposed displacement.
Biochemistries
Fasting morning serum was collected from all subjects. Serum was archived at −80 degrees C and analyzed in one batch after all visits were completed. Laboratory assays were performed in the Core Laboratory of the CUMC Clinical and Translational Research Center. Serum calcium, albumin, and creatinine were measured using automated techniques. Serum 25-hydroxyvitamin D2 and D3 were measured by Ultra-performance Liquid Chromatography combined with tandem mass spectrometry (UPLC-MS/MS) using a 1290 UPLC and a 6410 Tandem Mass Spectrometer (Agilent, Santa Clara, CA). Inter-assay coefficient of variation (CV) was 2.9% for 25OHD2 and 5.4% for 25OHD3. Intact parathyroid hormone (iPTH) was measured by chemiluminescent immunoassay (CLIA, Siemens Healthcare Diagnostics, Deerfield, IL; CV 8.3%). Serum C-terminal telopeptide of type 1 collagen (CTX) was measured by ELISA (Immunodiagnostics Systems, Scottsdale AZ; CV <10%). Serum osteocalcin was measured by ELISA (Immunodiagnostic Systems, Scottsdale, Arizona; CV 2.7%). Serum bone alkaline phosphatase was measured by ELISA (Quidel Corp, Sand Diego, CA; CV 7.6%).
Statistical Methods
Analyses were conducted with STATA version 9.0 (Stata Corp, College Station, Texas) and SAS version 9.1 (SAS Institute Inc., Cary, North Carolina). Two-sided p values < 0.05 were considered to indicate statistical significance. Normality testing (Kolmogorov-Smirnov) was performed and variables that were not normally distributed were logarithmically transformed prior to group comparisons. Differences among the three groups were assessed by ANOVA with Scheffe test for multiple comparisons. Comparison of HR-pQCT parameters among groups after adjustment for age and BMI was performed using ANCOVA. Mean percent difference and the standard error of the difference between each osteoporosis group (1/3RO and PMO) and controls was calculated using multiple T-tests.
Results
Study Subjects
This study enrolled 266 postmenopausal women. Characteristics of the subjects in the three groups are detailed in Table 1. The majority of women were White Non-Hispanic (78%). The mean age was 68 ± 7 years. Controls were younger than the other two groups. Mean BMI was in the normal or overweight range for all groups, and was highest among controls. Tobacco and alcohol use were similar. There was no difference in use of calcium and vitamin D supplements or in mean intake between groups. Less than 10% of women were currently using HRT; use was higher among controls. Approximately half of the women in the overall cohort (56%) had a history of fragility fracture or a prevalent vertebral fracture by spine radiographs. There was no difference in overall clinical fracture history between the three groups. The prevalence of extremity fractures (wrist, ankle or humerus) appeared higher in patients with 1/3RO but the difference was not significant between groups. Vertebral fracture prevalence appeared higher in patients with PMO but was not significantly different between groups. Among patients with a vertebral fracture, there was no difference according to group in either the presence of multiple vertebral fractures or fracture severity.
Table 1.
Characteristics of the study population
| 1/3 RO N=27 |
PMO N=81 |
Control N=158 |
P-value | |
|---|---|---|---|---|
| Age (years) | 70 ± 8 | 71 ± 8a | 67 ± 6 | <0.01 |
| Race – Caucasian (%) | 81 | 75 | 79 | 0.58 |
| BMI (kg/m2) | 26 ± 4a | 24 ± 4a | 28 ± 6 | <0.001 |
| History of fragility fracture (clinical + radiographic %) | 63 | 58 | 54 | 0.62 |
| Wrist Fracture (%) | 30 | 26 | 18 | 0.18 |
| Humerus fracture (%) | 7 | 4 | 3 | 0.54 |
| Ankle fracture (%) | 15 | 10 | 11 | 0.77 |
| Any extremity fracture (%) | 48 | 37 | 30 | 0.16 |
| Vertebral fracture (%) | 11 | 21 | 13 | 0.27 |
| Family history of osteoporosis (%) | 55 | 47 | 47 | 0.76 |
| Tobacco use – | ||||
| Never (%) | 61 | 56 | 42 | 0.06 |
| Former (%) | 36 | 40 | 52 | |
| Current (%) | 3 | 4 | 5 | |
| Alcohol use (beverages per day) | 1 ± 1 | 1 ± 1 | 1 ± 1 | 0.71 |
| Calcium supplements – total daily dose (mg) | 732 ± 489 | 658 ± 540 | 600 ± 578 | 0.53 |
| Vitamin D supplements – total daily dose (IU) | 864 ± 300 | 952 ± 176 | 880 ± 125 | 0.94 |
| HRT – Past (%) | 48 | 34a | 49 | 0.05 |
| HRT - Current (%) | 0 | 1a | 9 | 0.02 |
| Raloxifene (%) | 4 | 6 | 2 | 0.14 |
| Thyroxine (%) | 8 | 21 | 18 | 0.76 |
Data shown as mean ± SD. Abbreviations: 1/3RO (isolated osteoporosis at the 1/3 radius by DXA), PMO (osteoporosis at the spine or hip by DXA), HRT (Hormone Replacement Therapy).
statistically different from controls
Biochemical Data
Calciotropic hormones and bone turnover markers are detailed in Table 2. Calcium and intact parathyroid hormone (iPTH) were in the normal range and did not differ between groups. Kidney function assessed by creatinine and estimated Glomerular Filtration Rate (eGFR) calculated by MDRD (30), was in the normal range and similar between groups. Urinary calcium excretion, measured as 24 hour urine calcium/creatinine ratio was also similar. Serum 25OHD levels were above 20 ng/ml in all groups. Although 25OHD was numerically lower in the 1/3RO group compared to controls and PMO this difference was not significant. Bone formation markers, serum osteocalcin and bone specific alkaline phosphatase were higher in 1/3RO. Bone resorption, assessed by serum CTX was also higher in 1/3RO.
Table 2.
Biochemical Data
| 1/3RO | PMO | Control | P-value | |
|---|---|---|---|---|
| Serum Calcium (8.6-10.2 mg/dL) | 9.4 ± 0.5 | 9.5 ± 0.3 | 9.5 ± 0.4 | 0.80 |
| Creatinine (0.5-1.2 mg/dL) | 0.9 ± 0.3 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.29 |
| Estimated GFR (ml/min) | 74 ± 26 | 77 ± 17 | 75 ± 20 | 0.80 |
| iPTH (11-67 pg/mL) | 41 ± 16 | 47 ± 26 | 46 ± 27 | 0.57 |
| 24 hour urine calcium (mg/g Cr) | 119 ± 57 | 155 ± 78 | 158 ± 87 | 0.23 |
| 25OHD (20-50 ng/ml) | 27 ± 8 | 37 ± 16 | 36 ± 12 | 0.07 |
| Osteocalcin (8.4-33.9 ng/mL) | 25 ± 11a | 21 ± 8 | 18 ± 8 | <0.001 |
| BSAP (11.5-29.6 U/L) | 29 ± 14 | 27 ± 8 | 24 ± 10 | <0.03 |
| CTX (0.11-0.74 ng/mL) | 0.6 ± 0.2a | 0.4 ± 0.2 | 0.4 ± 0.2 | <0.02 |
Data shown as mean ± SD with normal ranges in parentheses. Abbreviations: 1/3RO (isolated osteoporosis at the 1/3 radius by DXA), PMO (osteoporosis at the spine or hip by DXA), GFR (glomerular filtration rate), iPTH (intact parathyroid hormone), BSAP (bone specific alkaline phosphatase), CTX (C-telopeptide).
statistically different from controls in pairwise comparison after an omnibus F-test is significance at the 0.05 level.
Areal BMD
As expected according to our study design, mean T-Scores were lowest in PMO at all central sites compared to both controls and 1/3RO, lumbar spine (LS: −2.6 ± 1.1), total hip (TH: −2.0 ± 0.6) and femoral neck (FN: −2.4 ± 0.6). At the 1/3 radius, mean T-Score was −3.1 (± 0.5) among 1/3RO but higher and above the osteoporosis threshold in PMO (−2.3 ± 1.3). Values for 1/3RO fell within the osteopenic range at all other sites, the LS (−1.2 ± 1.0), TH (−1.5 ± 0.6), and FN (−1.9 ± 0.4). Controls had average T-Scores in the normal range at the LS (−0.8 ± 1.2), TH (0.8 ± 0.9) and 1/3R (−0.7 ± 1.0), and in the osteopenic range at the FN (−1.4 ± 0.8).
Volumetric BMD, Microarchitecture and Stiffness
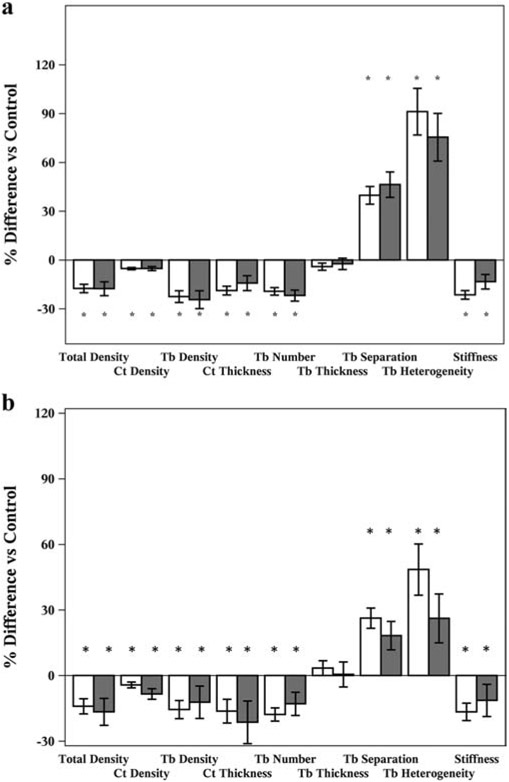
Bone size, vBMD, cortical and trabecular microarchitecture were assessed by HR-pQCT (Figure 1A and 1B). Raw data are detailed in Table 3. Compared to controls, 1/3RO had substantial abnormalities in cortical and trabecular bone at both radius and tibia. At the radius, 1/3RO had lower total vBMD (−18%; p<0.001), cortical vBMD (−5%; p<0.01) and trabecular vBMD (−24%; p<0.001). They had lower cortical thickness (−14%; p<0.01), lower trabecular number (−22%; p<0.001), greater trabecular separation (+46%; p<0.05), and greater heterogeneity (+75%; p<0.05). Abnormalities were observed in vBMD and microarchitecture in women with 1/3RO at the tibia as well; compared to controls, 1/3RO had lower total vBMD (−16%; p<0.001), cortical vBMD (−6%; p <0.01) and trabecular vBMD (−16%; p <0.001). They had lower cortical thickness (−15%; p<0.05), lower trabecular number (−16%; p<0.001), greater trabecular separation (+24%; p<0.001), and greater heterogeneity (+49%; p<0.01). Trabecular thickness did not differ significantly at either site.
Figure 1.
A. Percent differences +/− SEM in vBMD, microarchitecture and whole bone stiffness at the radius compared to controls for women with 1/3RO (white bars) and PMO (grey bars) * P-value<0.05.
B. Percent differences +/− SEM in vBMD, microarchitecture and whole bone stiffness at the tibia compared to controls for women with 1/3RO (white bars) and PMO (grey bars). * P-value<0.05.
Table 3.
Raw Values of vBMD and Microarchitecture by HR-pQCT
| HR-pQCT Parameter | Radius | Tibia | ||||
|---|---|---|---|---|---|---|
| 1/3RO | PMO | Control | 1/3RO | PMO | Control | |
| Total Area (cm2) | 235 ± 7 | 234 ± 3 | 234 ± 3 | 693 ± 21 | 645 ± 12 | 683 ± 9 |
| Cortical Area (cm2) | 41 ± 2 | 38 ± 1 | 48 ± 1 | 78 ± 5 | 74 ± 3 | 91 ± 2 |
| Trabecular Area (cm2) | 196 ± 8 | 183 ± 5 | 186 ± 3 | 615 ± 22 | 571 ± 12 | 593 ± 10 |
| Total Density (mgHA/cm3) | 244 ± 12 | 244 ± 7 | 295 ± 5 | 205 ± 9 | 209 ± 5 | 245 ± 4 |
| Cortical Density (mgHA/cm3) | 810 ± 13 | 810 ± 8 | 854 ± 5 | 742 ± 13 | 746 ± 8 | 786 ± 6 |
| Cortical Thickness (mm) | 0.62 ± 0.30 | 0.59 ± 0.02 | 0.73 ± 0.01 | 0.74 ± 0.05 | 0.73 ± 0.03 | 0.87 ± 0.02 |
| Trabecular Density (mgHA/cm3) | 98 ± 7 | 100 ± 4 | 129 ± 3 | 147 ± 3 | 123 ± 4 | 147 ± 3 |
| Bone Volume Fraction (BV/TV %) | 0.08 ± 0.01 | 0.08 ± 0.00 | 0.11 ± 0.00 | 0.10 ± 0.01 | 0.10 ± 0.00 | 0.12 ± 0.00 |
| Trabecular Number (1/mm) | 1.41 ± 0.07 | 1.45 ± 0.04 | 1.80 ± 0.03 | 1.52 ± 0.06 | 1.51 ± 0.03 | 1.81 ± 0.03 |
| Trabecular Thickness (mm) | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.00 |
| Trabecular Separation (mm) | 0.75 ± 0.05 | 0.72 ± 0.03 | 0.52 ± 0.01 | 0.62 ± 0.02 | 0.62 ± 0.01 | 0.50 ± 0.01 |
| Trabecular Heterogeneity (mm) | 0.43 ± 0.05 | 0.47 ±0.03 | 0.24 ± 0.01 | 0.34 ± 0.03 | 0.34 ± 0.02 | 0.23 ± 0.01 |
| Stiffness (N/mm) | 27643 ± 1354 | 25059 ± 792 | 31888 ± 612 | 82840 ± 3625 | 78886 ± 2052 | 95195 ± 1597 |
Mean ± SEM
As expected, vBMD and microarchitecture were worse in PMO compared with controls as well. Specifically, at the radius, PMO had lower total vBMD (−17%; p<0.001), trabecular vBMD (−22%; p<0.001), and cortical vBMD (−5%; p<0.001). They had lower cortical thickness (−19%; p<0.001) and trabecular number (−19%; p<0.001), greater trabecular separation (+40%; p<0.001), and greater heterogeneity (+91%; p<0.001). At the tibia, PMO had lower total vBMD (−15%; p<0.001), trabecular vBMD (−16%; p<0.001), and cortical vBMD (−5%; p<0.001) compared to controls. They had lower cortical thickness (−17%; p<0.001) and trabecular number (−16%; p<0.001), greater trabecular separation (+24%; p<0.001), and greater heterogeneity (+49%; p<0.001).
Interestingly, the extent of abnormalities in vBMD and microarchitecture were similar among 1/3RO and PMO. There were no significant differences between these groups in any cortical or trabecular parameter. Both groups had substantial deficits at both the radius and the tibia. When compared with controls, the same parameters were significantly worse in 1/3RO and PMO and differences from controls were of similar magnitude.
Biomechanical properties of bone were worse in both groups compared to controls. At the radius, whole bone stiffness was lower in both 1/3RO (−13%; p<0.01) and PMO (−21%; p<0.001) compared to controls. At the tibia, stiffness was lower in 1/3RO (−13%, p<0.01) and PMO (−17%, p<0.001). As observed with vBMD and microarchitecture, stiffness did not differ between women with 1/3RO and PMO.
Differences between groups were further compared after adjustment for age and BMI. The previously observed significant differences in vBMD, microarchitecture, and stiffness between the 1/3RO and the other groups remained statistically significant after adjustment.
Volumetric BMD and microarchitecture in women with 1/3RO with and without a history of fracture were compared. While no parameter was significantly different between the groups, radial total and trabecular vBMD tended to be lower among fracture subjects (p=0.06 and p=0.07 respectively).
Discussion
In this study, we investigated the microarchitectural and biochemical phenotype of women with isolated osteoporosis at the 1/3 radius by DXA compared to women who manifested the more typical pattern of osteoporosis at the spine and hip, and controls without osteoporosis. We found that women with 1/3RO had substantial abnormalities in vBMD, microarchitecture, and stiffness compared to controls. Significantly, the deficits documented by HR-pQCT at the radius and tibia in women with 1/3RO mirrored those detected in women with central osteoporosis at the spine and hip. These results suggest that the finding of isolated osteoporosis at the wrist by DXA is indicative of deteriorated microarchitecture and compromised biomechanical properties of bone similar to those seen in women with central osteoporosis at the spine and hip.
As the 1/3 radius is a predominantly cortical site, we hypothesized that women with 1/3RO would have cortical abnormalities compared to controls. In support of this, we found that they had lower cortical vBMD and thinner cortices. However, interestingly, they had substantial trabecular deficits as well, with lower trabecular vBMD, and fewer, more widely and irregularly spaced trabeculae at the radius. Moreover, the trabecular deficits were more marked than the cortical deficits. It could be postulated that small bone size might contribute to the finding of 1/3RO as 2-dimensional DXA measurements are artifactually lower in patients with smaller bones (31), however, we observed that bone size measured as cross-sectional area by HR-pQCT was not smaller among women with 1/3RO compared to controls or PMO.
Microarchitectural differences between women with 1/3RO and controls were apparent at both radius and tibia. Although the site assessed by HR-pQCT is distal to the 1/3 radius site by DXA, the finding that patients who have lower values at the DXA 1/3 radius site have microarchitectural abnormalities at the radius is not unexpected. More surprising were the number and extent of abnormalities that were also observed at the tibia. This observation suggests that the microarchitectural deficits are generalized, at least throughout the peripheral skeleton, rather than limited to the 1/3 radius. While we observed differences at both radius and tibia, a limitation of our study is that we did not use modalities other than DXA to evaluate central sites. Other studies have shown that peripheral HR-pQCT measurements correlate with central QCT measurements in young women with osteoporosis (32). Future studies using higher-order imaging techniques at the spine and hip will help to elucidate how pervasive skeletal abnormalities are in the postmenopausal population.
While vBMD and microarchitecture were similar between women with 1/3RO and PMO, forearm DXA measurements were higher in the women with PMO. Work from our group and others has shown that peripheral HR-pQCT measurements are predictive of fragility at central sites (14, 17, 26). Peripheral DXA, in contrast, may not be as sensitive to these abnormalities. It is well established that low aBMD at the hip and spine are highly correlated with fracture risk (33-37). Current guidelines recommend that central DXA be used primarily as the reference of osteoporosis for postmenopausal women, as they are more reliable than peripheral measures (36). However, there are many instances when peripheral measures may be necessary. In the older population, spine measurements are often artifactually high because of degenerative disease. In the growing population of patients who have had orthopedic procedures involving hardware at the spine and the hip, these sites may not be evaluable. As a result, in some patients only wrist measurements are available. Studies have shown that aBMD at the wrist does correlate with aBMD at the spine and the hip (38). In the NORA trial, patients with osteoporosis at peripheral sites, including the wrist, had a higher rate of fracture at both the wrist and hip compared to patients with higher aBMD at these peripheral sites (39). Our results suggest that wrist DXA measurements provide valuable information even when central measurements are available, as they allow for identification of patients with microarchitectural abnormalities who might otherwise be missed if only spine and hip values are considered.
Subjects were classified in this study based upon densitometric pattern alone, and there were subjects with a history of fragility fracture included in each of the groups. Overall fracture prevalence was similar between the groups. As might be expected, a greater proportion of women with 1/3RO had fractures of the extremities, and more women in the PMO group had vertebral fractures. However, these differences were not significant, possibly due to our small number of woman with 1/3RO. This pattern of differences in fracture suggests that 1/3RO may represent a specific entity of peripheral skeletal abnormalities, with a particular susceptibility to extremity fractures. Further, larger studies are needed to confirm these observations.
We investigated biochemical differences between the groups to determine whether there were differences in calciotropic hormones or bone turnover markers that might provide an underlying mechanism for the observed phenotype. Bone turnover was elevated in the 1/3RO women compared to the other groups which may have contributed to the microarchitectural abnormalities observed. No underlying metabolic abnormalities were observed to explain this increase in turnover or the structural abnormalities in 1/3RO. Although women with overt hyperparathyroidism and known renal disease were excluded from this study, we hypothesized that more subtle elevations in PTH, secondary to vitamin D deficiency, inadequate calcium intake, or mild chronic kidney disease, might contribute to cortical bone loss and 1/3RO, however we did not find this to be the case. We found that 25OHD levels were sufficient in the majority of subjects (>20 ng/ml). While values were numerically lower among 1/3RO, they did not differ significantly between groups. Calcium intake was similar between groups and 24-hour urine calcium was normal in these women, making it unlikely that inadequate calcium intake or a renal calcium leak played a role in the development of 1/3RO. Renal function, assessed by creatinine and MDRD did not differ. These results suggest that factors other than these biochemistries are responsible for the densitometric and microarchitectural abnormalities seen in women with 1/3RO. Genetic factors could play an important role.
Our study is limited by its cross-sectional design and the relatively small population of women with 1/3RO. We were unable to investigate the relationship between the structural abnormalities observed and prediction of fractures at peripheral or central sites. Studies from our group and others have shown that vBMD, microarchitecture and stiffness can discriminate fracture status in multiple populations. Recently HR-pQCT measurements predicted fracture in a longitudinal study (40). Whether the abnormalities that we detected in our cohort with isolated 1/3RO are directly related to fracture risk is an important topic for future work. Whether the higher remodeling rate observed in the 1/3RO contributed to the microarchitectural abnormalities cannot be determined in our cross-sectional study but is an important question to be addressed in future prospective work. There were differences in some demographic factors, age and BMI between our groups. While we did adjust for these differences in our analyses, it is possible that multiple factors may have contributed to the observed differences in microarchitecture. Further, although all HR-pQCT measurements were performed on one machine, DXA measurements were performed at two sites, one using a Hologic (CUMC) and the other a Lunar (HHH) system. We used T-Scores to reduce the confounding introduced by this variability (41) however, some disparities likely still existed and 1/3 radius measurements may be more variable between manufacturers than other sites. Finally, we enrolled women on the basis of aBMD and therefore women with fractures were included in each group of our cohort. Although the presence of a fragility fracture should prompt consideration of treatment regardless of aBMD, in practice treatment is often not initiated. Our subjects were not treated and many, despite their fracture history had never been told that they had osteoporosis, underscoring the reliance of many practitioners on DXA for assessment of bone health.
In conclusion, we found that postmenopausal women with isolated osteoporosis at the 1/3 radius have low volumetric BMD, abnormal cortical and trabecular microarchitecture and low stiffness at both radius and tibia compared to controls. In addition, these women had similar deficits in volumetric density, microarchitecture and stiffness to those observed in women with osteoporosis at the spine and hip. Our results suggest that although aBMD appears relatively preserved at the spine and hip by DXA, women with 1/3RO have substantial microarchitectural and biomechanical deficits throughout their extremities. Further study is required investigate the extent of these abnormalities throughout the axial skeleton and to guide treatment decisions in this population.
Highlights.
Isolated osteoporosis at 1/3 radius (1/3RO) presents a therapeutic dilemma
Women with 1/3RO had microarchitectural and biomechanical deficits
The skeletal abnormalities in 1/3RO were similar to those in classic PMO
Acknowledgments
Funding:
This work was supported by NIH K23 DK084337 (Stein), NIH U01 AR055968 (Shane), and the Thomas L. Kempner and Katheryn C. Patterson Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nanes MS, Kallen CB 2014. Osteoporosis. Semin Nucl Med 44:439–450 [DOI] [PubMed] [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A 2007. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 22:465–475 [DOI] [PubMed] [Google Scholar]
- 3.Blume SW, Curtis JR 2011. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int 22:1835–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nih Consensus Development Panel on Osteoporosis Prevention D, Therapy 2001. Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–79511176917 [Google Scholar]
- 5.Curtis EM, Moon RJ, Harvey NC, Cooper C 2017. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone 104:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnell O, Kanis JA 2006. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733 [DOI] [PubMed] [Google Scholar]
- 7.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA 1999. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 353:878–882 [DOI] [PubMed] [Google Scholar]
- 8.Cummings SR, Cosman F, Eastell R, Reid IR, Mehta M, Lewiecki EM 2013. Goal-directed treatment of osteoporosis. J Bone Miner Res 28:433–438 [DOI] [PubMed] [Google Scholar]
- 9.Cummings SR, Cosman F, Lewiecki EM, Schousboe JT, Bauer DC, Black DM, Brown TD, Cheung AM, Cody K, Cooper C, Diez-Perez A, Eastell R, Hadji P, Hosoi T, Jan De Beur S, Kagan R, Kiel DP, Reid IR, Solomon DH, Randall S 2017. Goal-Directed Treatment for Osteoporosis: A Progress Report From the ASBMR-NOF Working Group on Goal-Directed Treatment for Osteoporosis. J Bone Miner Res 32:3–10 [DOI] [PubMed] [Google Scholar]
- 10.Kanis JA 2002. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936 [DOI] [PubMed] [Google Scholar]
- 11.Genant HK, Wu CY, van Kuijk C, Nevitt MC 1993. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148 [DOI] [PubMed] [Google Scholar]
- 12.Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, Zhang C, Yin PT, Cosman F, Nieves J, Guo XE, Shane E 2010. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res 25:2572–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen A, Dempster DW, Muller R, Guo XE, Nickolas TL, Liu XS, Zhang XH, Wirth AJ, van Lenthe GH, Kohler T, McMahon DJ, Zhou H, Rubin MR, Bilezikian JP, Lappe JM, Recker RR, Shane E 2010. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int 21:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein EM, Liu XS, Nickolas TL, Cohen A, McMahon DJ, Zhou B, Zhang C, Kamanda-Kosseh M, Cosman F, Nieves J, Guo XE, Shane E 2012. Microarchitectural abnormalities are more severe in postmenopausal women with vertebral compared to nonvertebral fractures. J Clin Endocrinol Metab 97:E1918–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, Zhang C, Cosman F, Nieves J, Greisberg J, Guo XE, Shane E 2011. Abnormal microarchitecture and stiffness in postmenopausal women with ankle fractures. J Clin Endocrinol Metab 96:2041–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein EM, Silva BC, Boutroy S, Zhou B, Wang J, Udesky J, Zhang C, McMahon DJ, Romano M, Dworakowski E, Costa AG, Cusano N, Irani D, Cremers S, Shane E, Guo XE, Bilezikian JP 2013. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J Bone Miner Res 28:1029–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein EM, Kepley A, Walker M, Nickolas TL, Nishiyama K, Zhou B, Liu XS, McMahon DJ, Zhang C, Boutroy S, Cosman F, Nieves J, Guo XE, Shane E 2014. Skeletal structure in postmenopausal women with osteopenia and fractures is characterized by abnormal trabecular plates and cortical thinning. J Bone Miner Res 29:1101–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laib A, Ruegsegger P 1999. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone 24:35–39 [DOI] [PubMed] [Google Scholar]
- 19.MacNeil JA, Boyd SK 2007. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys 29:1096–1105 [DOI] [PubMed] [Google Scholar]
- 20.Liu XS, Zhang XH, Sekhon KK, Adams MF, McMahon DJ, Bilezikian JP, Shane E, Guo XE 2010. High-resolution peripheral quantitative computed tomography can assess microstructural and mechanical properties of human distal tibial bone. J Bone Miner Res 25:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boutroy S, Bouxsein ML, Munoz F, Delmas PD 2005. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90:6508–6515 [DOI] [PubMed] [Google Scholar]
- 22.Melton LJ 3rd, Riggs BL, van Lenthe GH, Achenbach SJ, Muller R, Bouxsein ML, Amin S, Atkinson EJ, Khosla S 2007. Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. J Bone Miner Res 22:1442–1448 [DOI] [PubMed] [Google Scholar]
- 23.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD 2007. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res 22:425–433 [DOI] [PubMed] [Google Scholar]
- 24.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD 2008. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 23:392–399 [DOI] [PubMed] [Google Scholar]
- 25.Vico L, Zouch M, Amirouche A, Frere D, Laroche N, Koller B, Laib A, Thomas T, Alexandre C 2008. High-resolution pQCT analysis at the distal radius and tibia discriminates patients with recent wrist and femoral neck fractures. J Bone Miner Res 23:1741–1750 [DOI] [PubMed] [Google Scholar]
- 26.Sornay-Rendu E, Cabrera-Bravo JL, Boutroy S, Munoz F, Delmas PD 2009. Severity of vertebral fractures is associated with alterations of cortical architecture in postmenopausal women. J Bone Miner Res 24:737–743 [DOI] [PubMed] [Google Scholar]
- 27.Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S 2008. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 23:205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melton LJ 3rd, Christen D, Riggs BL, Achenbach SJ, Muller R, van Lenthe GH, Amin S, Atkinson EJ, Khosla S 2010. Assessing forearm fracture risk in postmenopausal women. Osteoporos Int 21:1161–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo XE, Goldstein SA 1997. Is trabecular bone tissue different from cortical bone tissue? Forma 12:185–196 [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470 [DOI] [PubMed] [Google Scholar]
- 31.Blake GM, Fogelman I 2008. How important are BMD accuracy errors for the clinical interpretation of DXA scans? J Bone Miner Res 23:457–462 [DOI] [PubMed] [Google Scholar]
- 32.Liu XS, Cohen A, Shane E, Yin PT, Stein EM, Rogers H, Kokolus SL, McMahon DJ, Lappe JM, Recker RR, Lang T, Guo XE 2010. Bone density, geometry, microstructure, and stiffness: Relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res 25:2229–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ 3rd, O'Neill T, Pols H, Reeve J, Silman A, Tenenhouse A 2005. Predictive value of BMD for hip and other fractures. J Bone Miner Res 20:1185–1194 [DOI] [PubMed] [Google Scholar]
- 34.Leslie WD, Tsang JF, Caetano PA, Lix LM, Manitoba Bone Density P 2007. Effectiveness of bone density measurement for predicting osteoporotic fractures in clinical practice. J Clin Endocrinol Metab 92:77–81 [DOI] [PubMed] [Google Scholar]
- 35.Kanis JA, Johansson H, Oden A, McCloskey EV 2009. Assessment of fracture risk. Eur J Radiol 71:392–397 [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Genant HK, Shepherd J, Zhao S, Mathur A, Fuerst TP, Cummings SR 2001. Classification of osteoporosis based on bone mineral densities. J Bone Miner Res 16:901–910 [DOI] [PubMed] [Google Scholar]
- 37.Leslie WD, Tsang JF, Lix LM 2008. Validation of ten-year fracture risk prediction: a clinical cohort study from the Manitoba Bone Density Program. Bone 43:667–671 [DOI] [PubMed] [Google Scholar]
- 38.Eftekhar-Sadat B, Ghavami M, Toopchizadeh V, Ghahvechi Akbari M 2016. Wrist bone mineral density utility in diagnosing hip osteoporosis in postmenopausal women. Ther Adv Endocrinol Metab 7:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM 2001. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 286:2815–2822 [DOI] [PubMed] [Google Scholar]
- 40.Samelson EJ, Broe KE, Xu H, Yang L, Boyd S, Biver E, Szulc P, Adachi J, Amin S, Atkinson E, Berger C, Burt L, Chapurlat R, Chevalley T, Ferrari S, Goltzman D, Hanley DA, Hannan MT, Khosla S, Liu CT, Lorentzon M, Mellstrom D, Merle B, Nethander M, Rizzoli R, Sornay-Rendu E, Van Rietbergen B, Sundh D, Wong AKO, Ohlsson C, Demissie S, Kiel DP, Bouxsein ML 2019. Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): a prospective study. Lancet Diabetes Endocrinol 7:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiebzak GM, Binkley N, Lewiecki EM, Miller PD 2007. Diagnostic agreement at the total hip using different DXA systems and the NHANES III database. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry 10:132–137 [DOI] [PubMed] [Google Scholar]