Abstract
Introduction:
Core needle biopsies of solid masses in children are a minimally invasive technique. It guides to a definitive diagnosis and facilitates management.
Aims and Objectives:
To determine the accuracy, sensitivity, and specificity of core needle biopsies in diagnosing pediatric solid masses.
Materials and Methods:
A retrospective analysis of 430 children, who underwent core needle biopsy for solid masses between January 2007 and December 2016 at CMC Vellore, was done.
Results:
Retroperitoneal and intra-abdominal masses constituted 66% of cases. Real-time image guidance was used in 44% of cases. An accurate diagnosis was obtained in 93.6% of cases, while results did not correlate with the final diagnosis in 3.4%. Three percent had inadequate or necrotic tissue. None of the children had postprocedure complications.
Conclusion:
Core needle biopsies serve as good diagnostic modality, with minimal risks, in making a conclusive diagnosis and deciding on the line of management.
KEYWORDS: Core needle biopsy, image-guided biopsy, solid malignancies
INTRODUCTION
Pediatric extracranial solid malignancies account for more than 30% of all childhood malignancies.[1] Imaging modalities such as ultrasound and computed tomography (CT) may provide clues to suggest a diagnosis but are not conclusive. The need to make a histological diagnosis is paramount as tumor-specific treatment can be instituted and over-treatment of benign tumors can be avoided. Pathological diagnosis can be obtained by open biopsies, fine-needle aspiration cytology (FNAC), or core needle biopsy. Open biopsy requires a general anesthetic, are painful, and may upgrade the tumor resulting in a delayed and protracted course of treatment. Conversely, FNAC often provides inadequate tissue samples. However, core needle biopsy done under short general anesthesia provides a more than adequate sample for histopathological and other ancillary studies such as immunohistochemistry and cytogenetical analysis without the morbidity associated with an open biopsy.[2,3,4] This study was performed to determine the diagnostic accuracy of percutaneous core needle biopsies for pediatric solid extracranial tumors and to assess the procedure-related complications.
MATERIALS AND METHODS
A retrospective analysis was done on 430 children who underwent core needle biopsies in the Department of Pediatric Surgery at Christian Medical College, Vellore, between 2007 and 2016. The study was approved by the Institutional Research Board and the Ethics Committee (IRB no: 11614 dated 31/10/2018). Patients who had biopsy elsewhere but underwent definitive treatment with us were excluded from the study. Clinical data relating to the biopsy procedure, anesthesia, complications, and follow-up were obtained from the electronic medical records. The core biopsy and the final histopathology reports obtained from the records were compared for diagnostic accuracy.
Patients underwent basic investigations, including a coagulation profile and full blood count before the biopsy. Based on the imaging findings, core needle biopsies were planned either under intravenous sedation or general anesthesia. It was obtained under real-time image guidance Ultrasonography (USG)/CT) or after a preprocedure radiological marking indicating the different directions and distance the needle needs to traverse for optimal tissue procurement in easily palpable and accessible extremity and retroperitoneal masses. The need for real-time guidance was determined primarily by the location, accessibility, palpability, homogeneity of the mass and the presence of any major organ, neural or vascular structure either in the needle path or in close proximity. A16-22 G core cut needle or core biopsy gun with co-axial introducers for image-guided biopsies was used. A minimum of four passes were made, and the tissue was collected in formalin. The posterolateral approach was used in almost all retroperitoneal tumors to avoid peritoneal contamination and in intraperitoneal tumors to prevent bowel injury. Postprocedure, patients were monitored for the changes in heart rate, blood pressure, temperature, and respiratory rate overnight and were discharged the next morning.
Descriptive statistical analyses were employed where appropriate, continuous data were summarized using mean and standard deviation, and categorical data using counts and percentages. The analysis was done using the IBM SPSS Statistics for Windows, Version 21.0 (IBM Co., Armonk, NY, USA), and P < 0.05 was considered statistically significant.
RESULTS
Four hundred and thirty seven biopsies were done on 430 children in the study period. The mean age was 45 months (range: 2 months to 15 years) with the majority being girls (M:F = 1:1.85). Biopsies were mostly performed for intra-peritoneal/retroperitoneal (66%) and mediastinal masses (15%). The remaining was constituted by head, neck, and extremity masses. Real-time image guidance, either ultrasound or CT, was used for 194 biopsies in 188 patients (43.7%), and preprocedure radiological marking in the remaining 243 biopsies in 242 patients (56.3%) [Table 1].
Table 1.
Location of the tumor and mode of biopsy
| Site of biopsy | Ultrasound guided | Computed tomography guided | Without real time image guidance | Total, n (%) |
|---|---|---|---|---|
| Head/neck | 31 | 31 (7.09) | ||
| Extremity | 3 | 34 | 37 (8.47) | |
| Liver | 19 | 2 | 22 | 43 (9.84) |
| Chest wall/mediastinum | 18 | 31 | 15 | 64 (14.65) |
| Pelvis | 13 | 3 | 6 | 22 (5.03) |
| Retroperitoneum (incl kidneys) | 77 | 19 | 131 | 227 (51.95) |
| Paravertebral | 3 | 6 | 4 | 13 (2.97) |
| Total | 133 | 61 | 243 | 437 (100.00) |
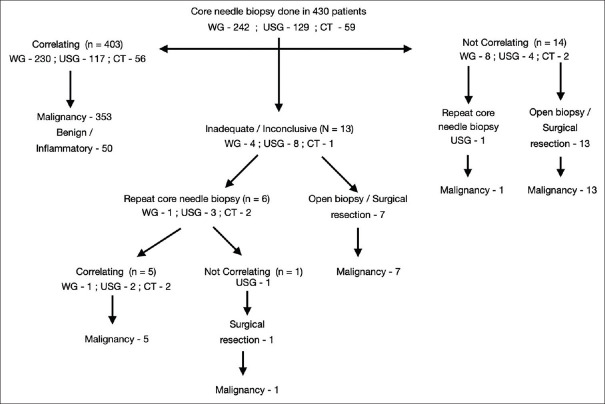
The tissue sample was inadequate or necrotic in 13 (3%) patients. Of the 13, six underwent a repeat core biopsy while seven underwent a surgical excision. A conclusive core biopsy histopathological report was obtained in 424, of which 409 correlated with the final surgical specimen histopathological diagnosis [Figure 1]. Of the 15 core biopsies with an incorrect diagnosis, seven had ganglioneuroblastoma, which was initially reported as ganglioneuroma, and one initially reported as lipoma turned out to be a lipoblastoma. Two biopsies of Wilms' tumor and one each of rhabdomyosarcoma, Ewing's sarcoma, and mucoepidermoid carcinoma, had atypical elements in their biopsy but definite malignancy could not be conclusively proven. One case of lymphoma and another case of thymic carcinoma was reported as chronic inflammatory lesions. This was attributed to core biopsies being taken from the nonrepresentative areas. Whether non usage of real-time imaging was seen to have an impact on incorrect diagnosis was studied and was found not significant.
Figure 1.
Flowchart showing the number of core needle biopsies done and the results (WG: Without real time image guidance, USG: Ultrasound guided, CT: Computed Tomography guided)
The most common diagnoses were Wilms' tumor and neuroblastoma. Others include hepatoblastoma, rhabdomyosarcoma and other high-grade sarcomas, germ cell tumors, lymphomas, and benign entities such as teratomas, infective, and inflammatory masses. The diagnostic yield of core needle biopsy for various malignant tumors is summarized in Table 2. Core needle biopsy was found to have a high yield for most tumors, with only neuroblastoma having a value <90%. The sensitivity of core needle biopsy in detecting malignancies was 92.8%, and specificity was 100% indicating that no case was wrongly diagnosed as malignancy. The diagnostic accuracy of core needle biopsy was 93.6% [Table 3].
Table 2.
Pathological diagnoses of malignant tumours with number diagnosed by core needle biopsy
| Diagnosis | Total cases | Number diagnosed by CNB | Diagnostic yield |
|---|---|---|---|
| Wilms' tumor | 90 | 88 | 97.78 |
| Neuroblastoma | 79 | 71 | 89.87 |
| Liver malignancies | 34 | 33 | 97.06 |
| Other renal malign | 23 | 22 | 95.65 |
| Sarcomas | 57 | 55 | 96.49 |
| Lymphoma | 40 | 37 | 92.50 |
| Germ cell tumor | 18 | 17 | 94.44 |
| Other malign | 39 | 36 | 92.31 |
| Total | 380 | 359 | 94.47 |
This includes seven patients, where initial core needle biopsy was inconclusive but repeat core needle biopsy confirmed the diagnosis. CNB: Core needle biopsy
Table 3.
Sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy
| Final diagnosis |
SEN | SPE | PPV | NPV | DA | ||
|---|---|---|---|---|---|---|---|
| Malignant | Nonmalignant | ||||||
| Ultrasound guided | |||||||
| Malignant | 107 | 0 | 89.17 | 100 | 100 | 50.00 | 90.23 |
| Nonmalignant | 13 | 13 | |||||
| Computed tomography guided | |||||||
| Malignant | 47 | 0 | 94.00 | 100 | 100 | 78.57 | 95.08 |
| Nonmalignant | 3 | 11 | |||||
| Without image guidance | |||||||
| Malignant | 205 | 0 | 94.47 | 100 | 100 | 68.42 | 95.06 |
| Nonmalignant | 12 | 26 | |||||
| Total | |||||||
| Malignant | 359 | 0 | 92.76 | 100 | 100 | 64.10 | 93.59 |
| Nonmalignant | 28 | 50 | |||||
SEN: Sensitivity, SPE: Specificity, PPV: Positive predictive value, NPV: Negative predictive value, DA: Diagnostic accuracy
We also analyzed whether various factors such as tumor characteristics (malignant\benign), location of the tumor, and the mode of biopsy had any influence on the diagnostic accuracy. The diagnostic yield for benign and malignant pathologies was at 100% and 95%, respectively, and it was nearly 100% with head, neck, and extremity tumors and around 92% for other site tumors. None of the above-mentioned entities were found to be having a statistically significant bearing on the diagnostic accuracy. Furthermore, the diagnostic accuracy for real-time image-guided biopsies were compared to biopsies done with preprocedure radiological marking and were found to be similar (P value-0.1734). There were no reported or recorded instances of complications in our study, either with the procedure or anesthesia.
DISCUSSION
Pretreatment tissue sampling in pediatric extra cranial solid tumors helps determine the malignant potential of the tumor, the need for preoperative chemotherapy, plan for limited or radical surgery, and confirm prognostic factors.[5] Core needle biopsy as an instrument to obtain pretherapy tissue samples has been found to be almost as effective as open biopsy without the associated morbidity and provides more tissue for analysis than FNAC.
The two important parameters that are to be assessed in determining whether a particular mode of obtaining tissue for biopsy is effective are by studying the “adequacy” and “accuracy.” The diagnostic accuracy of core needle biopsy in various studies have ranged from 84% to 98%.[6] A study by Blondiaux et al. conducted over 26 years, and 396 biopsies showed that the diagnostic accuracy increased from 85% to 93% from the initial decade to the latter one.[7] This was attributed to advances in the pathological techniques whereby immune-histochemical markers and gene fusion studies helped in obtaining a definitive diagnosis even in limited samples. In our study, the diagnostic accuracy was 93.6%, sensitivity 92.8%, and specificity 100%. The accuracy and sensitivity in our study was found to be slightly lower when compared to recent studies conducted by Ilivitzki et al. (sensitivity of 97% and specificity of 100%) and Wang et al. (sensitivity of 98.8%).[8,9] This may be attributed to the fact that, unlike other studies, we have included the “inadequate samples” also while calculating the overall accuracy as their exclusion could result in a spuriously high value. If inadequate samples are excluded, the diagnostic accuracy and the sensitivity of our study increase to 96% and 95%, respectively.
“Adequacy” of the tissue sample does not depend on the quantity of tissue but on whether a meaningful pathological examination can be performed with the sample obtained. A systematic review of core needle biopsies in children conducted by Sebire and Roebuck which included 13 studies with 698 biopsies showed that adequate tissue was obtained in almost 95% of cases.[6] In our study, adequate tissue for the pathological examination was obtained in 97% of cases, with the major problem in getting adequate tissue being tumor necrosis. Studies have mentioned a higher level of fibrosis and necrosis being associated with discordant diagnosis. Parsons et al. have recommended the assessment of tissue adequacy by touch imprint or concurrent fine needle aspiration when performing core needle biopsies on lesions with substantial necrosis on imaging.[10]
Studies by Acord and Shaikh and Blondiaux et al. focussed on factors that might affect diagnostic accuracy,[7,11] namely the anatomical location, pathological diagnosis, size of the mass, type of image guidance, number of passes, and gauge of the needle used. It was found that none of these factors had any bearing on the diagnostic accuracy except for the number of passes. Furthermore, operator dependency was found to be low as the returns were the same irrespective of the person who performed the biopsy. We were able to demonstrate similar results in our study with no single factor, significantly altering the diagnostic rate.
Recent studies have also focussed on the adequacy of tissue for ancillary testing such as cytogenetics, fluorescence in situ hybridization, immunohistochemistry, and flow cytometry. Deeney et al. in a prospective study of open versus core needle biopsy for nonnephroblastic intra-abdominal tumours had suggested that adequate tissue was not available in the majority of cases.[12] However, Metz et al. and Parsons et al. have shown that adequate tissue for ancillary testing was available in 94%–100% of cases[5,10] which was similar to our study. The capability of percutaneous core needle biopsy in providing sufficient tissue for pretherapy testing has made groups like International Neuroblastoma Risk Group task force and SIOP/UKCCSG set forth recommendations allowing this procedure.[13]
Core needle biopsies are usually performed under real-time image guidance, even when the tumor is easily palpable. While ultrasound has been the preferred imaging modality, CT scan has been employed for inaccessible lesions or those surrounded by air-filled structures, such as mediastinal masses.[14,15] Image guidance ensures sampling of viable tumor while avoiding necrotic areas and injury to adjacent organs and major blood vessels.[16,17] However, the major problem that we have encountered has been in getting an early time slot for these real-time image-guided procedures when compared to the early slots obtained for preprocedure tumor marking where the different directions and distance the needle needs to traverse for optimal tissue procurement was determined. Homogeneity of the mass, accessibility, palpability and the presence of any major organ, neural or vascular structure either in the needle path or in close proximity were the factors that were considered in selecting cases for either methods. In our study, real-time image guidance was not used in 242 cases (56.3% of cases), and we found that the tissue yield and diagnostic accuracy was similar to patients where image guidance was used 98% and 95% versus 95% and 93%, respectively. The relatively lower diagnostic accuracy of ultrasound-guided biopsies compared to other two modalities can be attributed to a selection bias. USG guidance was predominantly used for small, inaccessible, or heterogeneous masses with necrosis while the majority of palpable, homogeneous masses underwent biopsy without real-time guidance.
Complications are rare after core needle biopsies with bleeding, pain, and infection being mentioned more often than pneumothorax and injury to an organ or vessels. While Wang et al. and Ilivitzki et al. have mentioned a complication rate of about 1%–3% with bleeding and wound infection being the only ones, there were no major complications in studies performed by Sebire et al. and Hussain et al.[6,8,9,18] Based on experimental models, needle tract recurrence used to be quoted quite often as a risk in these biopsies, with estimated frequencies of 1 in 625–1 in 8500 cases. However, needle tract recurrences in the clinical practise have been rare probably due to immunological factors and the effects of chemo-radiation. Although needle tract recurrence was reported in two cases of Wilms tumor by Lee et al. and Aslam et al.,[19,20] none of the recent studies have reported this.[6] We have seen a case of needle tract recurrence in a child with Wilms tumor whose biopsy was transperitoneal and done from elsewhere (excluded from the analysis). We have had no major complications in our study; however, minor complications may have been underestimated due to the retrospective nature of the study.
In our study, malignancy was not detected in 4% of the patients. In certain conditions like nodular ganglioneuroblastoma and lipoblastoma, the representative areas were not sampled, resulting in a false-negative diagnosis. We believe that core needle biopsy should be considered as an adjunct to clinical and imaging features in diagnosis, and that a negative core biopsy should not be a contra-indication for employing open biopsy or surgical excision in suspicious cases.
CONCLUSION
Our study shows that percutaneous core needle biopsy is rapid, cost-effective, reliable, safe, and can form an integral part in the diagnostic workup of pediatric extracranial solid malignancies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD. [Last accessed on 2021 Mar 02]. Available from: https://seer.cancer.gov/archive/csr/1975_2011/
- 2.Sklair-Levy M, Lebensart PD, Applbaum YH, Ramu N, Freeman A, Gozal D, et al. Percutaneous image-guided needle biopsy in children – Summary of our experience with 57 children. Pediatr Radiol. 2001;31:732–6. doi: 10.1007/s002470100533. [DOI] [PubMed] [Google Scholar]
- 3.Bain G, Bearcroft PW, Berman LH, Grant JW. The use of ultrasound-guided cutting-needle biopsy in paediatric neck masses. Eur Radiol. 2000;10:512–5. doi: 10.1007/s003300050086. [DOI] [PubMed] [Google Scholar]
- 4.Kilpatrick SE, Garvin AJ. Recent advances in the diagnosis of pediatric soft-tissue tumours. Med Pediatr Oncol. 1999;32:373–6. doi: 10.1002/(sici)1096-911x(199905)32:5<373::aid-mpo11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Metz T, Heider A, Vellody R, Jarboe MD, Gemmete JJ, Grove JJ, et al. Image-guided percuta- neous core needle biopsy of soft-tissue masses in the pediatric population. Pediatr Radiol. 2016;46:1173–8. doi: 10.1007/s00247-016-3571-5. [DOI] [PubMed] [Google Scholar]
- 6.Sebire NJ, Roebuck DJ. Pathological diagnosis of paediatric tumours from image-guided needle core biopsies: A systematic review. Pediatr Radiol. 2006;36:426–31. doi: 10.1007/s00247-006-0123-4. [DOI] [PubMed] [Google Scholar]
- 7.Blondiaux E, Laurent M, Audureau E, Boudjemaa S, Sileo C, Lenoir M, et al. Factors influencing the diagnostic yield and accuracy of image-guided percutaneous needle biopsy of pediatric tumours: Single-center audit of a 26-year experience. Pediatr Radiol. 2016;46:372–82. doi: 10.1007/s00247-015-3484-8. [DOI] [PubMed] [Google Scholar]
- 8.Ilivitzki A, Abugazala M, Arkovitz M, Benbarak A, Postovsky S, Arad-Cohen N, et al. Ultrasound-guided core biopsy as the primary tool for tissue diagnosis in pediatric oncology. J Pediatr Hematol Oncol. 2014;36:333–6. doi: 10.1097/MPH.0b013e31827e4c4d. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Li F, Liu J, Zhang S. Ultrasound-guided core needle biopsy in diagnosis of abdominal and pelvic neoplasm in pediatric patients. Pediatr Surg Int. 2014;30:31–7. doi: 10.1007/s00383-013-3427-0. [DOI] [PubMed] [Google Scholar]
- 10.Parsons LN, Vo N, Moe DC, Jarzembowski JA. Adequacy and accuracy of core biopsy in children: A radiologic/pathologic correlation study. Pediatr Dev Pathol. 2019;22:137–41. doi: 10.1177/1093526618809862. [DOI] [PubMed] [Google Scholar]
- 11.Acord M, Shaikh R. Predictors of diagnostic success in image-guided pediatric soft-tissue biopsies. Pediatr Radiol. 2015;45:1529–34. doi: 10.1007/s00247-015-3364-2. [DOI] [PubMed] [Google Scholar]
- 12.Deeney S, Stewart C, Treece AL, Black JO, Lovell MA, Garrington T, et al. Diagnostic utility of core needle biopsy versus open wedge biopsy for pediatric intraabdominal solid tumors: Results of a prospective clinical study. J Pediatr Surg. 2017;52:2042–6. doi: 10.1016/j.jpedsurg.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 13.Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG task force report. J Clin Oncol. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skoldenberg EG, Jakobson AA, Elvin A, Sandstedt B, Olsen L, Christofferson RH, et al. Diagnosing childhood tumours: A review of 147 cutting needle biopsies in 110 children. J Pediatr Surg. 2002;37:50–6. doi: 10.1053/jpsu.2002.29426. [DOI] [PubMed] [Google Scholar]
- 15.Hugosson CO, Nyman RS, Cappelen-Smith JM, Akhtar M, Hugosson C. Ultra-sound-guided biopsy of abdominal and pelvic lesions in children. A comparison between fine-needle aspiration and 1.2 mm-needle core biopsy. Pediatr Radiol. 1999;29:31–6. doi: 10.1007/s002470050529. [DOI] [PubMed] [Google Scholar]
- 16.Garrett KM, Fuller CE, Santana VM, Shochat SJ, Hoffer FA. Percutaneous biopsy of pediatric solid tumors. Cancer. 2005;104:644–52. doi: 10.1002/cncr.21193. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Mu J, Du P, Wang H, Mao Y, Xu Y, et al. Ultrasound-guided core needle biopsy in the diagnosis of neuroblastic tumours in children: A retrospective study on 83 cases. Pediatr Surg Int. 2017;33:347–53. doi: 10.1007/s00383-016-4037-4. [DOI] [PubMed] [Google Scholar]
- 18.Hussain HK, Kingston JE, Domizio P, Norton AJ, Reznek RH. Imaging-guided core biopsy for the diagnosis of malignant tumours in pediatric patients. AJR. 2021;176:43–7. doi: 10.2214/ajr.176.1.1760043. [DOI] [PubMed] [Google Scholar]
- 19.Aslam A, Foot AB, Spicer RD. Needle track recurrence after biopsy of non-metastatic Wilms tumour. Pediatr Surg Int. 1996;11:416–7. doi: 10.1007/BF00497834. [DOI] [PubMed] [Google Scholar]
- 20.Lee IS, Nguyen S, Shanberg AM. Needle tract seeding after percutaneous biopsy of Wilms' tumor. J Urol. 1995;153:1074–6. [PubMed] [Google Scholar]