PURPOSE
More than 80% of cervical cancer cases and deaths occur in low- and middle-income countries. Here, we analyze a large geographically extensive cross-sectional data set from the Western rural highlands of Guatemala. Our objective is to better characterize weak points in care along the cervical cancer care continuum and investigate sociodemographic and clinical correlates of loss to follow-up.
METHODS
We conducted a retrospective review of electronic health records data from July 21, 2015, through December 10, 2020 for a cytology-based screening and cervical cancer treatment program. We used a care cascade analysis to characterize the progression of individuals through screening, confirmatory testing, and treatment. We examined demographic and clinical factors correlated with screening and loss to follow-up using multivariate logistic regression.
RESULTS
A total of 8,872 individuals were included in the analysis. Five thousand nine hundred thirteen cervical cancer screenings were conducted. 4.1% of all screening tests were abnormal, including 0.61% cervical intraepithelial neoplasia or overt cervical cancer. Care cascade analysis showed that 67% of eligible women accepted screening. Of those requiring confirmatory testing or treatment, 73% completed recommended follow-up. In adjusted multivariable analysis, prior history of sexual transmitted infection, prior experience with cervical cancer screening, older age, and current contraceptive use were associated with accepting screening. Age and contraceptive use were also associated with retention in care after a positive first screen.
CONCLUSION
In a large rural Guatemalan retrospective cohort, a care continuum analysis showed that both declining the opportunity to receive cervical cancer screening as well as declining confirmatory testing after a first positive screen were both important weak points along the care continuum. These data support the need for comprehensive and culturally appropriate initiatives to improve screening uptake and retention in care.
INTRODUCTION
More than 80% of cervical cancer cases and deaths occur in low- and middle-income countries (LMICs), and in many low-income countries, cervical cancer remains the main cause of cancer death in women.1 In most high-income countries, programs to screen for cervical cancer and to coordinate follow-up testing and treatment have dramatically reduced the cervical cancer burden through sustained decades-long efforts. Screening programs have been less effective in LMICs, however, because of limited technical and financial capacity for screening, but also because of structural barriers that limit access to screening and impede retention in care for minority, rural, or impoverished populations.2,3
CONTEXT
Key Objective
How well does the cervical cancer care system function in rural Guatemala?
Knowledge Generated
Sixty-seven percent of eligible women received cervical cancer screening. Seventy-three percent of women with a positive screen received recommended follow-up treatment.
Relevance
Improving both uptake of screening through more effective public health messaging, and strategies for retaining individuals in care after a positive screen are both needed to for quality cervical cancer care in Guatemala.
Guatemala is a populous upper-middle-income country in Central America. Nevertheless, Guatemala has some of the worst health disparities for rural and Indigenous populations in all Latin America despite the country's economic status. For these reasons, cervical cancer remains a pressing public health concern. It is the second most common cancer in women in Guatemala, and the most common in women of childbearing age.1
Most efforts to improve cervical cancer care in Guatemala have focused on improved access to and uptake of screening. These efforts are especially justified by nationally representative Demographic Health Survey (DHS) data sets of women of childbearing age showing large disparities in screening rates for rural and Indigenous women.4 Recent efforts to use human papillomavirus (HPV)-based testing and self-sampling have shown very promising results.5,6 Most importantly, the Scale-Up Project screened more than 90,000 women and successfully provided confirmatory testing for 84% of HPV-positive screens.7 An important caveat of the Scale-Up Project, however, is that it was research-based and conducted primarily in central Guatemala in periurban settings using a center-based model. Its successes may therefore not be directly applicable in more remote areas of the country and with less financial inputs.
Recently, we have used a continuum-of-care approach to analyze data from a small cervical cancer screening and care program in rural Guatemala. An important finding from this work is that, although improving access to primary screening for rural Indigenous women is paramount, loss to follow-up (LTFU) at the confirmatory testing and ongoing treatment stages are equally of concern.8 Others have confirmed this concern, documenting 65% LTFU for confirmed cases of cervical cancer.9 Taken together, these findings emphasize the need for a structured framework to monitor the multiple transition points in cervical cancer care. To extend this work, here we analyze a large geographically extensive cross-sectional data set from the Western rural highlands of Guatemala using a continuum-of-care approach. Our objective is to better characterize weak points in care and investigate sociodemographic and clinical correlates of not accepting primary screening as well as LTFU.
METHODS
Program Description
Institutionally, the data analyzed are drawn from a joint care initiative between Maya Health Alliance, one of Guatemala's largest primary care organizations, and Friendship Bridge, one of Guatemala's largest microfinance institutions. As described in detail elsewhere, since 2015, Maya Health Alliance and Friendship Bridge have collaborated to provide health screenings and primary care services, including cytology-based cervical cancer screening and treatment, to Friendship Bridge's approximately 20,000 clients.10 Services are free of charge to clients in good loan standing and are financed by loan revenue. Clients are distributed throughout rural municipalities in nine provinces (Fig 1).
FIG 1.
Geographic distribution of cross-sectional cervical cancer care cohort. The individuals included in data analysis come from gray-shaded provinces.
The program is largely nurse-driven with physician oversight. Mobile cervical cancer screening is provided in clients' homes. Cytology specimens are analyzed by reference laboratories at the Instituto de Cancerología (INCAN) or Asociación Quetzalteca Contra el Cancer (ASCAN). Clinical management algorithms are outlined in the Data Supplement. Cytology laboratories in Guatemala do not follow international reporting guidelines. In particular, reporting on the degree of inflammation observed on the cytologic preparation is common and requires special consideration as it may represent a sexually transmitted infection (STI), which may obscure early neoplastic changes.11 Cases of severe inflammation are therefore empirically treated for STIs before repeat cytologic testing (Data Supplement). Women who require colposcopy; excisional, thermocoagulation, or cryotherapy; or definitive treatment for cervical cancer are referred to INCAN. Patients with possible neoplastic cells, or who have severe inflammation that persists after STI treatment, are referred to INCAN. Patient navigators from Maya Health Alliance assist with referrals and transitions in care.12 Clinical data are maintained in a cloud-based electronic health record (EHR, OpenMRS13), which forms the basis for the data analysis presented here.
Participants and Data Extraction
We used an automated structured query language-based search sequence to extract all available data (Data Supplement) on cervical cancer screening and treatment as well as basic sociodemographic and clinical covariates from the EHR. Data were extracted on all females receiving care from the Friendship Bridge-Maya Health Alliance program who were eligible for cervical cancer screening services (defined according to clinical algorithms as age 21-65 years, see the Data Supplement). Data were extracted from the start of the clinical program, July 21, 2015, through December 10, 2020. To determine clinical course and treatment completion, all clinical charts with a screening finding requiring confirmatory testing or treatment (severe inflammation, carcinoma in situ, and overt cancer) were manually audited to verify clinical treatments and retention in care versus LTFU by the first author (A.G.) with resolution of inconsistencies by the last author (P.R.).
Outcomes and Data Analysis
Statistical analysis was done using Stata 16.0 (College Station, TX). For baseline characteristics, percentages are given for categorical variables, and median and interquartile range (IQR) for continuous variables.
Care continuum analysis followed our previously reported approach and is outlined in Figure 2.8 Eligible for cervical cancer screening was defined as all female individuals age 21-65 years with no concurrent history of active cervical cancer. Received cervical cancer screening was any eligible individual with a cervical cancer screening examination documented in the EHR. Received screening results was any screening examination with discussion or delivery of screening results documented. Needing confirmatory testing or treatment was defined as a screening examination with any finding of overt cancer, carcinoma in situ, or severe inflammation. In the case of severe inflammation, received testing or treatment was defined as completion of a full cycle of empiric treatment for STI followed by repeat cytologic screening (Data Supplement). In the case of overt cancer or carcinoma in situ, received testing or treatment was defined as completion of confirmatory tests and indicated treatment according to the institution's clinical protocols (Data Supplement). Lost to follow-up was defined as any individual needing confirmatory testing or treatment and not receiving it.
FIG 2.
Visualization of the cervical cancer screening and care continuum.
To examine sociodemographic and clinical factors correlated with cervical cancer screening (eligible individuals who were v were not screened) and lost to follow-up, we conducted multivariate and hierarchical multivariate logistic regression, with and without random effects to control for clustering at the provincial level. The fixed-effects components of the multivariable models were constructed by including available variables likely associated with the outcome of interest as determined by the team's clinical expertise and review of the literature and excluding variables with strong collinearity or very low dispersion. Age was categorized (< 30, 30-45, and > 45 years), as this improved model fit. Serial likelihood ratio tests were then used to remove variables, producing the most parsimonious model, and goodness of fit was assessed using the Hosmer-Lemeshow test.
Ethics
The study was approved by the Maya Health Alliance institutional review board (WK 2017 006). A waiver of informed consent was granted for abstraction of EHR data.
RESULTS
Figure 3 gives an overview of the chart review and record extraction process. Overall 9,422 unique individuals were identified through chart review, of whom 550 were excluded for being outside the eligible age range for cervical cancer screening of 21-65 years. Therefore, a total of 8,872 individuals were included in the analysis.
FIG 3.
Flow diagram of record extraction and data analysis process.
Sociodemographic and Clinical Characteristics of Population
Basic characteristics of the total population of women eligible for cervical cancer screening in the cohort are given in Table 1. The median age was 37.2 years (IQR, 29.1-46.7 years), the median number of pregnancies was four (IQR, 2-7), and 22.3% were postmenopausal. The overall proportion who had never used contraception was high (40.6), as was the proportion who reported never screening for cervical cancer (37.3%). The cohort was well distributed through the Western highlands of Guatemala (Fig 1) but concentrated in the three provinces of Chimaltenango, Quiche, and Sololá, representing 68% of all individuals.
TABLE 1.
Sociodemographic and Clinical Characteristics of a Cohort of Rural Guatemalan Women Eligible for Cervical Cancer Screening
All individuals in the cohort were offered routine cervical cancer screening as part of their preventative health care package, with 5,913 (66.6%) accepting screening. Most sociodemographic and clinical characteristics of the population of individuals accepting screening differed significantly from those not accepting screening (Table 1). For example, those not screened were younger (median age 35.3 [IQR, 27.6-46.1] v 38.0 [30.0-47.0] years, P < .001), more likely to be never-users of contraception (56.5% v 32.5%, P < .001), and less likely to have received previous screening (47.1% v 70.3%, P < .001). There were also geographic differences; for example, the province of Chimaltenango account for relatively fewer and Quiche relatively more refusals.
Results of Cervical Cancer Screening
Table 2 gives the results from cervical cancer screening. In total, 5,726 results were available from 5,913 screenings (96.8% of all examinations). Of this, 4.1% reported any abnormality requiring confirmatory testing or treatment, of which the bulk were severe inflammation (3.4%), and the remainder were cervical intraepithelial neoplasia (CIN) or cancer (0.61%).
TABLE 2.
The Results of Cervical Cancer Screening

Description of Cervical Cancer Care Cascade and LTFU
Cascade analysis showed three weak points along the care continuum. First, of the 8,872 screen eligible women, only 5,913 (67%) accepted screening (Table 1, Fig 4). Next, of those who underwent screening, a small proportion (3%) did not have test results documented in the electronic medical record, likely representing a combination of misplaced or misprocessed specimens and faulty documentation. Finally, among the 232 individuals (4% of all abnormal findings) needing confirmatory testing or treatment, 73% completed recommended follow-up (see the Data Supplement for details of follow-up). Sociodemographic and clinical characteristics of individuals requiring confirmatory testing or treatment who completed treatment versus were lost to follow-up are given in Table 3, with few noted differences. In particular, there was no difference in the frequency of LTFU on the basis of initial screening test result and indicated treatment (severe inflammation v CIN or cervical cancer).
FIG 4.
Cervical cancer care continuum outcomes for screen-eligible women.
TABLE 3.
Sociodemographic and Clinical Characteristics of Individuals Completing Confirmatory Testing/Treatment Versus Lost to Follow-Up After a Positive Cervical Cancer Screen
Manual chart review of LTFU cases showed that virtually all cases were because of the patient declining further care at the confirmatory testing stage (Fig 4), with fear of the health care system or lack of family support as the common reasons. In fact, only one case out of 25 with CIN or cancer who agreed to confirmatory testing and treatment subsequently discontinued care, despite lengthy and complex courses in some cases.
Multivariate Regressions for Receiving Screening or Retention in Care
Finally, we constructed multivariable models to examine adjusted factors associated with accepting cervical cancer screening or retention in care after a first positive screen (Table 4). For both models, an interaction term between age and contraceptive use was included to improve model fit. The final adjusted model for accepting cervical cancer screening also included a random-effects variable for province (patient location), which was statistically significant but overall of very small magnitude (intraclass correlation coefficient of 0.02 [95% CI, 0.004 to 0.05], explaining only 2% of the observed variance). The random-effects term for provincial variation in the retention-in-care model was not statistically significant. These terms are excluded from Table 4 for clarity.
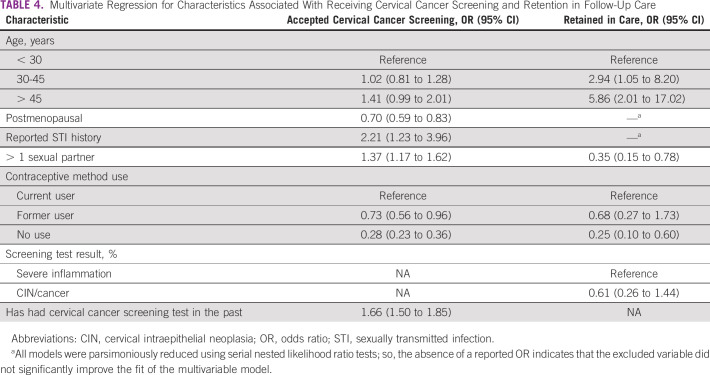
TABLE 4.
Multivariate Regression for Characteristics Associated With Receiving Cervical Cancer Screening and Retention in Follow-Up Care
Prior history of STI (odds ratio [OR], 2.21; 95% CI, 1.23 to 3.96) and prior experience with cervical cancer screening (OR, 1.66; 95% CI, 1.50 to 1.85) were strongly associated with accepting screening. Age > 45 years was also positively correlated. By contrast, postmenopausal status was associated with declining screening (OR, 0.70; 95% CI, 0.59 to 0.83), as was former (OR, 0.73; 95% CI, 0.56 to 0.96) or never use (OR, 0.28; 95% CI, 0.23 to 0.36) of contraceptives, compared with active ongoing use.
For LTFU, the final parsimonious model showed a similar but even stronger association with older age and retention in care. Never use of contraceptive methods was strongly associated with LTFU and not remaining engaged with care (OR, 0.24; 95% CI, 0.10 to 0.58). Type of screening diagnosis (severe inflammation v CIN or cancer) was not significant but was included in the final model because of its clinical importance.
DISCUSSION
In this paper, we use EHR data from a routine cervical cancer clinical program involving a cohort of women from rural, Indigenous areas of Guatemala to investigate the cervical cancer care continuum and factors associated with LTFU. Previously, we have shown in a small pilot study that a care cascade analysis can help highlight weak points in care delivery for cervical cancer.8 Here, we extend that work to a larger cohort of approximately 9,000 individuals from nine Guatemalan provinces.
Our findings are comparable to recently published analyses of the 2014-2015 nationally representative DHS.4 That survey reported an overall 64% cervical cancer screening rate nationally, diminishing to 57.5% among Indigenous women and 47.5% in rural areas. Here, we found an overall ever-screening rate of 54.3% (Table 1). Our population reported here is older than the DHS sample, which is restricted to women of childbearing age, but otherwise quite similar. Our EHR data set did not include any ethnicity variables like the DHS sample, but does include municipality of residence. Virtually all individuals in our cohort reside in municipalities known to be rural and majority Indigenous. These data are also in contrast to a recent reported prior screening rate of 70% in the HPV Scale-Up Project, which preferentially recruited in periurban areas of Guatemala, once again highlighting the rural-urban disparity.
In addition to sociodemographic variables such as ethnicity, wealth, and education, analyses of DHS data have also highlighted the associations between several sexual health variables and cervical cancer screening, such as positive associations with prior history of STI and number of lifetime sexual partners.4 Our analysis here confirms these findings for cervical cancer screening, but a similar pattern was not seen for retention in care after a positive cancer screen (Tables 1 and 4). We also find, for the first time in Guatemala, strong associations between contraceptive use and cervical cancer screening and care (Table 4). Multiple studies from other countries have demonstrated positive associations between cervical cancer screening and sexual health history and contraceptive usage factors; so, our results are concordant with those findings.14-18 Since these associations remained after multivariable adjustment, we hypothesize that individuals who use contraception are more likely to have more positive attitudes and opinions about preventative health care that also leads them to seek cervical cancer screening. Although this hypothesis requires further study, if confirmed through qualitative analysis, it may lead to important insights into what motivates individuals to seek care that could lead to more effective public health messaging.
The most important contribution of our study was to study the entire cervical cancer care continuum, which was facilitated by access to comprehensive EHR treatment outcome data. In our prior pilot work, we demonstrated that both acceptance of screening by screen-eligible women and retention in care for confirmatory testing or treatment are significant weak points along the care continuum (Fig 2).8 The former is a commonplace and well accepted as a major opportunity for improvement in most cervical cancer care studies from LMICs. Retention in care, however, has been much less well studied, although findings on LTFU in effectiveness trials of visual inspection and HPV-based screening and the subsequent advocacy for same-day see-and-treat paradigms are implicit recognition of the problem.19 The recent Central American HPV Scale-Up Project reported an overall LTFU rate of 28% after a positive screen, and Guatemala's largest cancer treatment hospital has reported an overall 65% LTFU rate for invasive cervical cancer.7,9 Here, in our cohort, we show a 67% screening acceptance rate, a 3% misplaced or misprocessed specimen rate, and a 27% LTFU rate for confirmatory testing or treatment following a positive screen (Fig 4). Importantly, LTFU rates did not vary by severity of cytologic diagnosis (severe inflammation v CIN v cervical cancer, Table 4) as we had previously hypothesized in our pilot study.8 Equally importantly, manual chart review showed that almost all LTFU was at the initial stage of hesitancy for patients to receive confirmatory testing, as retention in care after this initial stage was very high.
Our study has several limitations. First, it is a survey of women both engaged in preventative clinical care and receiving microfinance services. As such, the population may differ in important ways from other women in Guatemala who do not meet this profile. This weakness is balanced by the large size of the sample and geographic distribution including primary underserved rural and Indigenous regions of the country. Another weakness of the study is that several important clinical variables, such as prior cervical cancer screen testing and results, were self-reported; additionally, few sociodemographic variables (such as ethnicity) were available in the EHR for inclusion in the analysis. Another important limitation of this study is that it was conducted with the context of a cytology-based screening program, and therefore, the findings may not be generalizable to HPV-based programs. However, although Guatemala and many other peer countries are slowly transitioning to HPV-based screening, the bulk of individuals still receive cytology-based screening. Finally, the cytology findings reported here may not be directly comparable to other international studies, given that pathologists in Guatemala do not use international cytology reporting standards; nevertheless, the care transition points (Fig 2), which are the main subject of analysis here, should be comparable with other settings.
In conclusion, we used a care continuum approach to analyze data from a large cervical cancer screening and treatment program in rural Guatemala. We found that both declining the opportunity to receive cervical cancer screening and declining confirmatory testing after a first positive screen were important weak points along the care continuum. Subsequent LTFU after confirmatory testing was minimal, with most individuals retained in care throughout their treatment course. These data support the findings of other researchers, who have emphasized the need for more comprehensive and culturally appropriate educational and outreach campaigns to improve screening uptake by rural and indigenous Guatemalan women.4,20 Given that in our data set, younger age and less prior health care utilization were associated with lower acceptance of screening, these efforts are especially a priority for these demographics. The findings of improved screening among contraceptive users suggest possible important individual-level differences in opinions about preventative health care. One strategy to improve screening uptake that we are currently exploring is to use a positive deviance approach, exploring the motivations and perspectives of these early adopters and translating these into new public health messages. In addition, our findings highlight the need to develop effective strategies for retention in care after a positive screen. In this regard, we believe that care navigation—which is widely used in higher-income settings for cancer care—may be an effective strategy. We have preliminary anecdotal experience using care navigation in rural Guatemala to help overcome the many financial, linguistic, cultural, and logistical barriers that women face when receiving cancer care, and we are in the process of evaluating this strategy more formally.12
AUTHOR CONTRIBUTIONS
Conception and design: Andrea Garcia, Ann Miller
Financial support: Ann Miller
Provision of study materials or patients: Michel Juarez, Ann Miller
Collection and assembly of data: Michel Juarez, Neftali Sacuj, Evelyn Tzurec, Ann Miller,
Data analysis and interpretation: Michel Juarez, Neftali Sacuj, Karen Larson, Ann Miller, Peter Rohloff
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Arbyn M, Weiderpass E, Bruni L, et al. : Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob Health 8:e191-e203, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R: Screening for cancer in low- and middle-income countries. Ann Glob Health 80:412-417, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Pimple SA, Mishra GA: Global strategies for cervical cancer prevention and screening. Minerva Ginecol 71:313-320, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Gottschlich A, Ochoa P, Rivera-Andrade A, et al. : Barriers to cervical cancer screening in Guatemala: A quantitative analysis using data from the Guatemala demographic and health surveys. Int J Public Health 65:217-226, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murchland AR, Gottschlich A, Bevilacqua K, et al. : HPV self-sampling acceptability in rural and indigenous communities in Guatemala: A cross-sectional study. BMJ Open 9:e029158, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottschlich A, Rivera-Andrade A, Bevilacqua K, et al. : Using self-collection HPV testing to increase engagement in cervical cancer screening programs in rural Guatemala: A longitudinal analysis. BMC Public Health 20:1406, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holme F, Jeronimo J, Maldonado F, et al. : Introduction of HPV testing for cervical cancer screening in Central America: The Scale-Up project. Prev Med 135:106076, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austad K, Chary A, Xocop SM, et al. : Barriers to cervical cancer screening and the cervical cancer care continuum in rural Guatemala: A mixed-method analysis. JCO Glob Oncol 4:1-10, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamorano AS, Barnoya J, Gharzouzi E, et al. : Treatment compliance as a major barrier to optimal cervical cancer treatment in Guatemala. JCO Glob Oncol 5:1-5, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colom M, Austad K, Sacuj N, et al. : Expanding access to primary healthcare for women through a microfinance institution: A case study from rural Guatemala. Healthc (Amst) 6:223-230, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Kelly BA, Black AS: The inflammatory cervical smear: A study in general practice. Br J Gen Pract 40:238-240, 1990 [PMC free article] [PubMed] [Google Scholar]
- 12.Flood D, Chary A, Austad K, et al. : Patient navigation and access to cancer care in Guatemala. JCO Glob Oncol 4:1-3, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.OpenMRS : www.openmrs.org
- 14.Ruddies F, Gizaw M, Teka B, et al. : Cervical cancer screening in rural Ethiopia: A cross- sectional knowledge, attitude and practice study. BMC Cancer 20:563, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilima, Puranik A, Shreenidhi SM, et al. : Spatial evaluation of prevalence, pattern and predictors of cervical cancer screening in India. Public Health 178:124-136, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Mignot S, Ringa V, Vigoureux S, et al. : Pap tests for cervical cancer screening test and contraception: Analysis of data from the CONSTANCES cohort study. BMC Cancer 19:317, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romli R, Shahabudin S, Saddki N, et al. : Cervical cancer and pap smear screening: Knowledge, attitude and practice among working women in northern state of Malaysia. Med J Malaysia 74:8-14, 2019 [PubMed] [Google Scholar]
- 18.Brandão M, Tulsidás S, Damasceno A, et al. : Cervical cancer screening uptake in women aged between 15 and 64 years in Mozambique. Eur J Cancer Prev 28:338-343, 2019 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization : Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Geneva, Switzerland, World Health Organization, 2020 [Google Scholar]
- 20.Arana-Chicas E, Gómez-Trillos S, Cartujano-Barrera F, et al. : Cancer prevention in indigenous communities from Guatemala: A needs assessment study. J Health Care Poor Underserved 31:1595-1611, 2020 [DOI] [PubMed] [Google Scholar]