PURPOSE
The global pediatric oncology clinical research landscape, particularly in Central and South America, Africa, and Asia, which bear the highest burden of global childhood cancer cases, is less characterized in the literature. Review of how existing pediatric cancer clinical trial groups internationally have been formed and how their research goals have been pursued is critical for building global collaborative research and data-sharing efforts, in line with the WHO Global Initiative for Childhood Cancer.
METHODS
A narrative literature review of collaborative groups performing pediatric cancer clinical research in each continent was conducted. An inventory of research groups was assembled and reviewed by current pediatric cancer regional and continental leaders. Each group was narratively described with identification of common structural and research themes among consortia.
RESULTS
There is wide variability in the structure, history, and goals of pediatric cancer clinical trial collaborative groups internationally. Several continental regions have longstanding endogenously-formed clinical trial groups that have developed and published numerous adapted treatment regimens to improve outcomes, whereas other regions have consortia focused on developing foundational database registry infrastructure supported by large multinational organizations or twinning relationships.
CONCLUSION
There cannot be a one-size-fits-all approach to increasing collaboration between international pediatric cancer clinical trial groups, as this requires a nuanced understanding of local stakeholders and resources necessary to form partnerships. Needs assessments, performed either by local consortia or in conjunction with international partners, have generated productive clinical trial infrastructure. To achieve the goals of the Global Initiative for Childhood Cancer, global partnerships must be sufficiently granular to account for the distinct needs of each collaborating group and should incorporate grassroots approaches, robust twinning relationships, and implementation science.
INTRODUCTION
There are an estimated 397,000 annual cases of childhood cancer globally, with a higher burden in low-resource nations.1 Outcomes for children with cancer are widely variable on the international stage, with overall survival rates for common childhood cancers such as acute lymphoblastic leukemia (ALL) ranging from more than 90% in many high-resource nations to as low as 50% in some countries.2 In 2018, the WHO announced the Global Initiative for Childhood Cancer, with the goal of achieving a 60% survival rate for all children with cancer by 2030 by mobilizing numerous regional and national stakeholders.3,4 To achieve these goals, WHO developed the CureAll framework, which highlights the importance of establishing roadmaps for the treatment of childhood cancer, including the design of adapted treatment regimens for resource-limited health systems and the development of the data collection infrastructure and implementation models required to demonstrate the efficacy of those regimens in clinical trials.5,6 Collaborative research through cooperative groups and multinational consortia has been a significant pathway for improved outcomes in pediatric oncology since the founding of the first pediatric cancer clinical trial group by the US National Cancer Institute (NCI) in 1955.7-9 National and regional groups have since coalesced into several large international groups, including the Children's Oncology Group (COG) and tumor-specific study groups formed under the umbrella of the Société Internationale d'Oncologie Pédiatrique (SIOP).8,10
CONTEXT
Key Objective
To narratively describe pediatric cancer clinical trial groups on the international stage, with the goal of identifying the structure and function of these consortia, as well as the clinical data sources they collect, to reveal opportunities for collaborative efforts within these regions.
Knowledge Generated
There is an exceptional variety in pediatric oncology collaborative groups across continents with respect to clinical trial design and execution. Childhood cancer care is improved in regions with collaborative pediatric cancer groups, regardless of income status, in which implementation science, database registry creation, and clinical trials can be incorporated into standardized cancer treatment.
Relevance
This review suggests that a one-size-fits-all approach to increasing collaboration between international pediatric cancer clinical trial groups is not possible, with a nuanced understanding of local stakeholders and needs necessary to form partnerships.
However, the clinical research landscape outside of these large and well-established groups—and in the regions of Central and South America, Africa, and Asia, which bear the highest burden of global childhood cancer cases—is less characterized in the literature. Better understanding of the clinical data sources that currently exist, how these data were collected, and how existing pediatric cancer clinical research groups in these regions have been formed and for which purposes is critical for building and evaluating collaborative efforts within these regions to achieve the WHO initiative's goal. Furthermore, given the distinct epidemiology of patients with childhood cancer and the unique resources available to pediatric oncologists across regions, a one-size-fits-all approach to performing clinical research, executing clinical trials, and collecting data is likely not possible or appropriate.7 As such, the interfaces between large consortia and smaller regional or national clinical trial groups must be customized to account for a variety of organizational structures and the particular logistical realities and local research priorities of the countries that they serve.
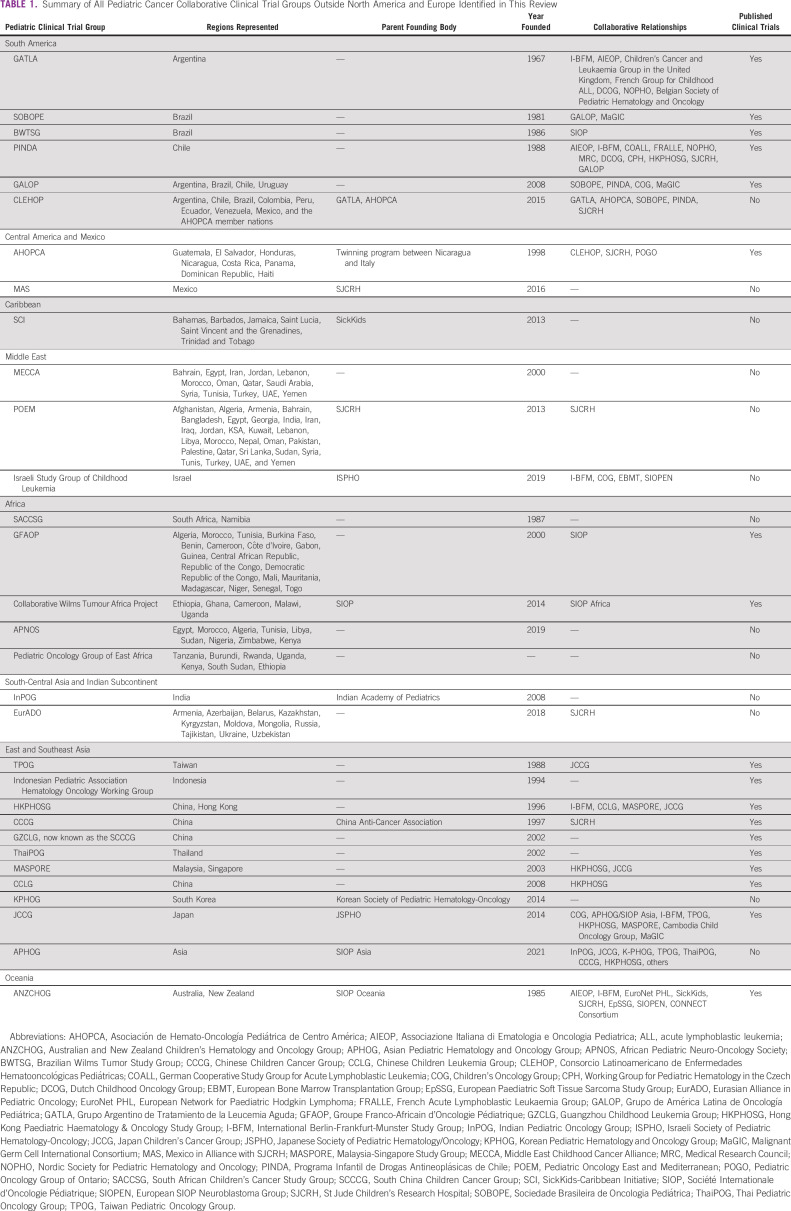
The aim of this narrative review is to describe how international pediatric cancer clinical trial groups are currently structured and for which purposes, with the goal of identifying sources of existing and future pediatric cancer data and common characteristics of established consortia to inform efforts such as the WHO Global Initiative for Childhood Cancer. To establish a reference point, we begin with a summary of the large pediatric cancer clinical trial groups in North America and Europe. We then narratively summarize the current pediatric cancer clinical trial collaborative groups in other regions. After reviewing the literature for groups performing pediatric cancer clinical research in each continent, we assembled an inventory of groups for review by our coauthors, current pediatric cancer regional and continental leaders. After coauthor consultation, a final list was established (Table 1), and each group was narratively described with identification of common themes among consortia.
TABLE 1.
Summary of All Pediatric Cancer Collaborative Clinical Trial Groups Outside North America and Europe Identified in This Review
METHODS
Pediatric Oncology Clinical Trial Groups in North America and Europe
United States.
COG was formed through a formal merger of four pre-existing cooperative groups in 2000 in the United States and now includes more than 200 institutions in the United States, Canada, Saudi Arabia, Australia, and New Zealand.8,9,11,12 COG has published more than 1,000 manuscripts since its inception and has designed many therapeutic regimens widely used today.8 COG is one of the six clinical trial groups designated by the NCI's National Clinical Trials Network, and COG's organizational structure includes multidisciplinary disease-specific research committees that develop clinical trials to be executed at member institutions.11
Europe.
SIOP was founded in 1969 by a group of pediatricians in Europe, with its first clinical trial of nephroblastoma launched in 1971.10 Initially based mainly in Europe, SIOP included pediatric oncologists from low-resource nations from its outset and began wider incorporation when it formally launched the Pediatric Oncology in Developing Countries (PODC) committee in 1990, now called the SIOP Global Health Network.10,13 Around this time, SIOP created continental branches with elected presidents and independent governance structures to meet the individual needs of their constituent nations.10 For example, SIOP Europe (SIOPE) was founded in 1998 and became an independent legal entity in 2007, now representing 35 European nations with membership through the national pediatric oncology society of each country and collaborating with more than 20 separate disease-specific clinical trial groups.14-16 Other continental branches have distinct governance structures, as will be discussed in this review. Although SIOP historically gave its name to clinical trials executed by the academic consortia of its members in the 1970s and 1980s, changes in European clinical trial legislation defining sponsor responsibilities together with financial constraints require that clinical trials are now run by national or continental cooperative groups, such as through the SIOPE Clinical Research Council's Clinical Trials Groups.10,16
Governance.
The governance structures of COG and SIOPE have changed significantly to accomplish organizational missions although they share several important similarities that have been important for their continuous operations. In both COG and SIOPE, supervisory organizations are separate from their clinical trial infrastructure: COG is the pediatric cancer clinical trial arm for the NCI Cancer Therapy Evaluation Program, and SIOPE acts as a coordinating society working in partnership with myriad disease-specific partner organizations. Both COG and SIOPE use a decentralized system for clinical trial execution, where patients are treated at local institutions using centralized processes and protocols.17 COG's clinical trials are designed within the organization itself, and COG works directly with institutions to execute trials.11 The regulatory framework for conducting clinical trials in Europe is highly complex, which has made it difficult for not-for-profit organizations such as SIOPE to take on the legal, administrative, and financial responsibilities to coordinate multinational clinical trials. In Europe, clinical trials have been successfully delivered under the auspices of independent clinical trial groups, which have collaborated with a range of associated academic institutions that address the legal and administrative burdens for individual trials.
Although COG and SIOPE have brought major advances to pediatric oncology in their respective regions, < 15% of patients with childhood cancer globally have access to cooperative trials from these groups, as shown in Figure 1, which depicts the percentage of global childhood cancer cases in each continental region on the basis of 2020 WHO Cancer Data.18 As such, there are significant opportunities for collaboration among pediatric cancer clinical trial groups outside of COG and SIOPE to enable interoperability and comparison across the remaining 85% of childhood cancer cases throughout the world and to work toward the data-oriented aims of the WHO Global Initiative for Childhood Cancer CureAll framework. In the following section, we describe these international pediatric cancer groups outside North America and Europe, which are summarized in Table 1.
FIG 1.
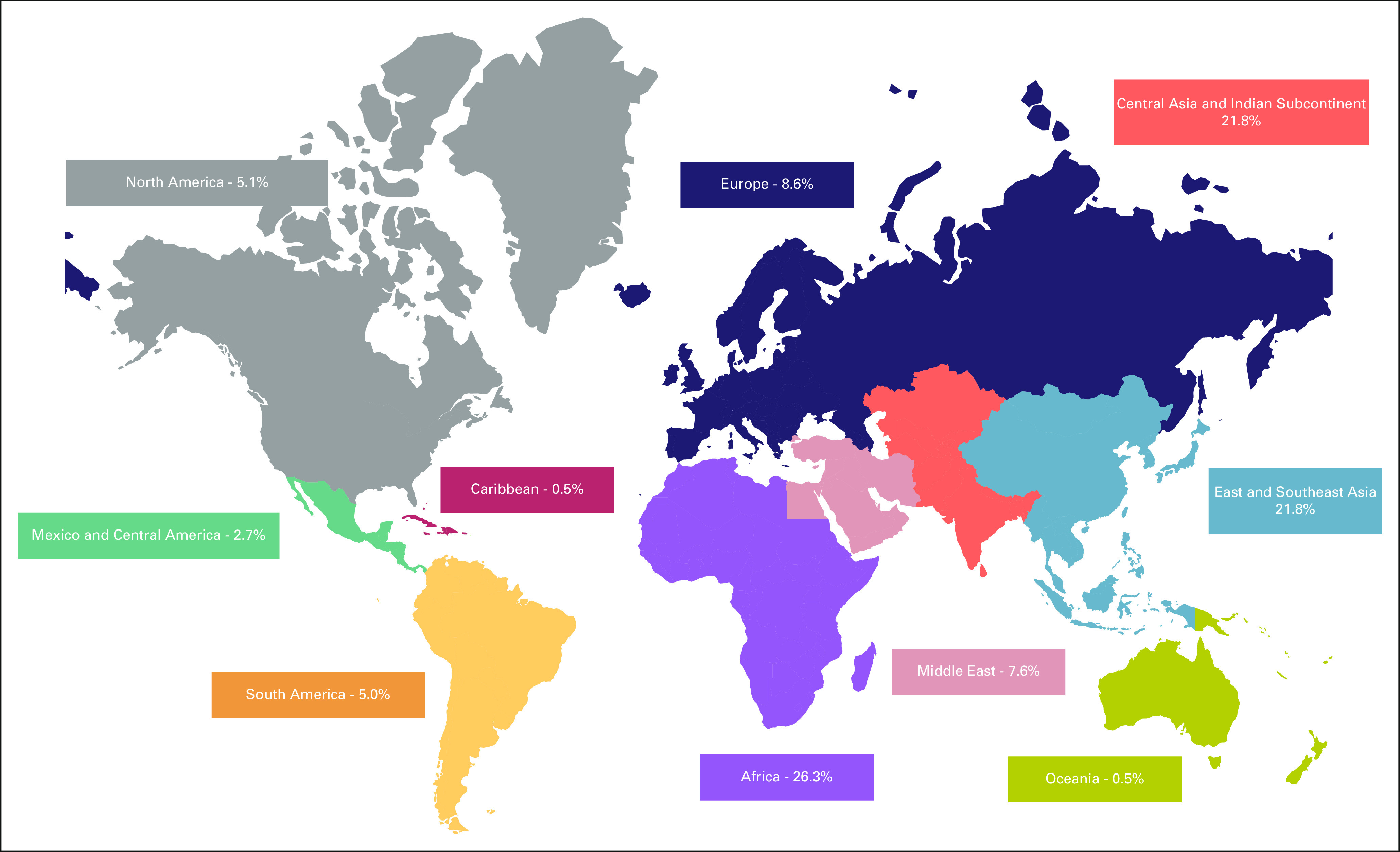
Percent of global childhood cancer cases in each continental region.
Pediatric Oncology Clinical Trial Collaborative Groups by Region
South America, Central America, the Caribbean, and Mexico.
Central America and South America have had numerous well-established pediatric cancer collaborative groups since the 1960s. Although survival rates vary widely across the continent, factors such as late diagnosis, treatment refusal, and treatment abandonment, which are rare in high-income countries, make accurate assessments of survival rates challenging.2,19-24 Mexico and the Caribbean have historically lacked access to clinical infrastructure for the treatment of childhood cancer, with low survival rates.25,26
South America
One of the oldest pediatric cancer clinical trial groups on the continent is the Grupo Argentino de Tratamiento de la Leucemia Aguda (GATLA), founded in 1967 in Argentina to develop treatment protocols for childhood leukemia.27 In 1977, GATLA published the results of several protocols for pediatric ALL and acute myeloid leukemia, with significant improvements in survival seen after nationwide implementation of standardized protocols.28,29 GATLA has also published extensively on Hodgkin lymphoma (HL), with over 46 years of ongoing clinical trials that have improved survival.30-32 GATLA has developed multiple international collaborative relationships, including codevelopment of protocols with the International Berlin-Frankfurt-Munster (I-BFM) group33,34 and the International Consortium for Childhood Acute Promyelocytic Leukemia.35 In 2015, GATLA assisted in the founding of the multinational clinical trial consortium Consorcio Latinoamericano de Enfermedades Hematooncológicas Pediátricas (CLEHOP),36-38 which has created several of its own collaborative clinical trial protocols.39,40
Brazil has been a major contributor to pediatric oncology through the Sociedade Brasileira de Oncologia Pediátrica (SOBOPE) founded in 1981.41 SOBOPE opened its first leukemia clinical trial in 1980, managed by one of its disease-specific clinical trial groups, the Brazilian Cooperative Group for Treatment of Childhood Acute Lymphocytic Leukemia.42,43 Since then, SOBOPE has overseen the development of numerous novel treatment protocols in multiple cancer types.42,44,45 Similar to SIOPE, SOBOPE facilitates collaboration between several disease-specific clinical trial groups and does not itself execute trials. For example, SOBOPE facilitated a collaborative relationship with the Grupo de América Latina de Oncología Pediátrica (GALOP) on an Ewing sarcoma (EWS) protocol.44,46 Furthermore, SOBOPE's germ cell tumor (GCT) clinical trial arm, the Brazilian Childhood GCT Study Group, joined the Malignant Germ Cell International Consortium in 2014.47,48 The Brazilian Wilms Tumor Study Group was founded in 1986 and conducted several of its own clinical trials before joining the SIOP Renal Tumor Study Group in 2001 to contribute patients to the randomized SIOP-2001 study.49,50
Chile also has a long history of executing collaborative clinical trials through the Programa Infantil de Drogas Antineoplásicas de Chile (PINDA), founded in 1988.51 PINDA has developed numerous protocols for multiple tumor types52-55 with extensive international collaboration, including with I-BFM56 and St Jude Children's Research Hospital (SJCRH).57
GALOP was founded in 2008 in Uruguay with the support of COG to execute pediatric cancer clinical trials in Brazil, Argentina, Uruguay, and Chile.7,58,59 The initial clinical trial protocol around which GALOP organized was a protocol on unilateral retinoblastoma, the results of which were published in 2018 after a decade of organizational and financial challenges.60 Protocols for the treatment of sarcoma, retinoblastoma, and GCT have been developed by GALOP in collaboration with PINDA60 and SOBOPE.61
The SIOP continental branch for Latin America, the Sociedad Latinoamericana de Oncología Pediátrica (SLAOP), was founded in 1979 by a group of pediatric oncologists in Uruguay independent of SIOP and was subsequently accepted as SIOP's functioning continental branch. Similar to SIOPE, SLAOP does not execute clinical trials but rather coordinates efforts between 17 countries through meetings and evidence-based guidelines for pediatric cancer care.62,63 The pediatric cancer clinical trial landscape in South America is defined by several longstanding national groups that have endogenously generated their own adapted treatment regimens on the basis of availability of local resources with the development of robust centralized infrastructure, as well as both continental and international collaborative data-sharing relationships, to study clinical trial data and publish their outcomes.
Central America
In Central America, the dominant pediatric cancer clinical trial consortium is the Asociación de Hemato-Oncología Pediátrica de Centro América (AHOPCA), which was founded in 1998 by participants of the Monza International School of Pediatric Hematology-Oncology. Monza International School of Pediatric Hematology-Oncology was created by the lead Italian pediatric oncologist who had initiated a twinning program between hospitals in Managua (Nicaragua) and Monza (Italy) 1986.64 The group executed their first clinical trial protocol for pediatric HL in 1999, which found inferior survival outcomes because of significant abandonment of therapy65 and spurred the development of shorter and less intensive treatment protocols to promote adherence.32,66 AHOPCA has published multiple clinical trial results67,68 and is a model pediatric cancer clinical trial consortium that leverages international support and existing treatment regimens to enhance access to care across Central America.64 Central to the operations of AHOPCA at first was the Pediatric Oncology Network Database (POND4Kids), a comprehensive data management system developed by collaborating partner SJCRH, which enabled centralized capture of clinical trial outcomes data.64 Since POND4Kids was discontinued, SJCRH now offers St Jude Global Childhood Cancer Analytics Resource and Epidemiological Surveillance System (SJCARES).69 As is recommended by the CureAll framework, dedicated financial, data management, and administrative support by the SJCRH Department of Global Pediatric Medicine and international expertise from the United States and Europe70,71 have been instrumental in enabling AHOPCA to conduct centralized research within Central America.64
Mexico
Mexico is historically under-represented in the pediatric clinical trials landscape, with an overall childhood cancer survival rate of 50%.72 In 2017, the Mexican Association of Pediatric Oncology/Hematology declared a need to build research infrastructure across Mexico to address this unmet need.73 Although individual institutions in Mexico have executed existing treatment regimens,74 there is no collaborative consortium in Mexico to execute trials nationwide. Several twinning programs have been developed, including the Cross-Border Neuro-Oncology Program between Rady Children's Hospital in San Diego and Tijuana, Mexico.75 The Mexico in Alliance with SJCRH program, established in 2016 as a scalable alliance-building model that includes 25 Mexican institutions, developed a risk-adapted treatment protocol for ALL that has demonstrated preliminary improvements in treatment-related mortality.76,77 With the introduction of several collaborative groups within Mexico, including the Grupo Cooperativo de Investigación en Oncología Pediátrica (GCIOP)78 and the Grupo Mexicano de Retinoblastoma (RtbMex),79 there may be new opportunities for international partnership to execute pediatric clinical trials in Mexico.
Caribbean
Although Haiti and the Dominican Republic are members of AHOPCA, creation of pediatric cancer clinical trials in other Caribbean countries is lacking. The SickKids-Caribbean Initiative, founded in 2013 by the Hospital for Sick Children in partnership with Caribbean institutions, conducted a retrospective study of children treated from 2011 to 2015 and found a 2-year overall survival of 55%,26 which led to implementation of a standardized protocol for the treatment of ALL.80,81 There are barriers to clinical trial implementation across the region, including limited infrastructure, treatment abandonment, and lack of national childhood cancer plans.82
Middle East.
Five-year survival rates in the Middle East have been estimated to be 50%-60%,83 with the recent emergence of several cooperative groups and twinning programs aimed at improving childhood cancer survival in the region.
The largest collaborative group in the Middle East is the Pediatric Oncology East and Mediterranean (POEM) Group, founded in 2013 in collaboration with SJCRH.84 POEM currently represents 119 facilities in 28 countries across the Middle East, North Africa, and Western and South Asia and has focused primarily on case discussions, pediatric oncology training opportunities for physicians and nurses, and building hospital-based cancer registries with the eventual goal of creating national cancer data registries for the region.84 Preliminary epidemiologic research from POEM has described survival rates at major pediatric cancer hospitals in the region, with lack of established registries and disjointed referral networks identified as significant barriers to pediatric cancer care.85 POEM's large geographic network is a strength and has enabled other groups to conduct research on pediatric cancer care in the region.86 Although POEM is engaged primarily in resource building and foundational epidemiologic research at this time, there are prospective clinical trials on ALL and retinoblastoma currently under development.84
Several twinning initiatives with institutions in the Middle East have resulted in the development of clinical trial protocols. These include a relationship between the Children's Welfare Teaching Hospital in Iraq and Sapienza University in Rome, resulting in a treatment protocol for childhood acute promyelocytic leukemia,87 and a collaboration between Hadassah University Medical Center in Israel and Memorial Sloan Kettering Cancer Center on an EWS protocol.88 Other twinning initiatives in the region have focused on assistance with the diagnosis and management of pediatric cancers rather than trial protocol design.89,90
The Middle East Childhood Cancer Alliance was established in 2000 and represents 16 countries in the Middle East and North Africa.7 Similar to POEM, it has published epidemiologic research, including a large prospective registry of pediatric ALL,91 but has not yet pursued any clinical trial work.
In 2019, the Israeli Study Group of Childhood Leukemia, a disease-specific group under the Israeli Society of Pediatric Hematology-Oncology, published a multicenter retrospective review on treatment outcomes in pediatric ALL.92 The group participates in international group protocols and also builds disease-specific registries.93
Overall, the Middle East faces considerable barriers to pediatric cancer clinical trial development and execution, including lack of standardized approaches to diagnosis, wide variations in health care infrastructure, financial barriers, and geopolitical instability, which lead to delays in diagnosis, treatment abandonment, and ultimately poorer outcomes.84,94 However, twinning initiatives with large specialized centers throughout the region have resulted in promising improvements in pediatric cancer care,94 which highlights the utility of twinning relationships in promoting the foundational pillars of the CureAll framework.
Africa.
Africa bears a disproportionate burden of childhood cancer globally, as 41% of its total population is under age 15 years.95 Although the African continent contains 16.7% of the world's population, it represents 26.3% of global pediatric cancer cases (Fig 1). In recognition of the scarcity of childhood cancer registries across the continent,96 contemporary simulation-based analyses have demonstrated disparities in 5-year survival across the continent, from 8.1% in Eastern Africa to as high as 30.3% in Northern Africa and 52.1% in South Africa.83,97,98 Access to specialized pediatric oncology care is a significant driver of poor outcomes, with 14 of 54 countries having no full-time pediatric oncologists and only 57% of childhood cancer cases estimated to be formally diagnosed.1,99 However, it is encouraging that 23 of 54 countries in Africa have reported active pediatric cancer clinical research programs, five countries have fellowship programs with research training, and several clinical trial cooperative groups have emerged.99
The Groupe Franco-Africain d'Oncologie Pédiatrique (GFAOP) is one of the most established pediatric clinical trial groups on the continent, founded in 2000 by establishing specialized childhood cancer pilot units in 18 French-speaking African countries.100,101 The GFAOP has executed and published multiple prospective clinical trials, including several trials in which treatment protocols developed in Europe were modified by the group for implementation in resource-poor regions.102 For example, implementation of the SIOP 2001 protocol for Wilms tumor demonstrated one of the highest survival rates in sub-Saharan Africa.103,104 The GFAOP has asserted its commitment to leveraging multidisciplinary and multicenter support to construct the necessary infrastructure for future trials, and a third prospective multicenter study of a novel adapted treatment regimen was recently published.105
Several other cooperative groups emerged from the SIOP Africa Continental Conference in Cairo, Egypt, in 2019, including the Pediatric Oncology Group of East Africa (whose formalization has been delayed because of the COVID-19 pandemic; Dr J. Balagadde Kambugu, Uganda Cancer Institute, Personal communication (e-mail), November 2021),99 the South African Children's Cancer Study Group,106 the African Pediatric Neuro-Oncology Society,107 and the aforementioned POEM Group, which includes the Middle East and North Africa.99,107 The South African Children's Cancer Study Group was founded to coordinate pediatric cancer care and plays a strong advocacy role in the region,106,108 with ongoing collaborative trials for retinoblastoma, HL, GCT, neuroblastoma, and nutrition in childhood cancer. There are numerous twinning initiatives throughout Africa aiming to build foundational infrastructure,99,109,110 including twinning between the Moroccan Society of Pediatric Oncology and Hematology and SJCRH, which resulted in the execution of a prospective HL treatment protocol.111
SIOP Africa, the continental arm of SIOP, has sought to unify the pediatric cancer clinical trial groups on the African continent to improve collaborative research efforts. To this end, SIOP provided funding to the Collaborative Wilms Tumor Africa Project, a multicenter prospective clinical trial initiated in 2014 to execute an adapted treatment regimen for Wilms tumor.112-114 Preliminary data have shown increased 2-year survival and significant reductions in treatment abandonment and treatment-related mortality.115 The Collaborative has added a supportive care clinical research program, Supportive Care for Children with Cancer in Africa, as part of the larger Collaborative African Network of Clinical Care and Research for Childhood Cancer (CANCaRe Africa) to investigate treatment abandonment and develop locally-appropriate treatment guidelines.116-118
Barriers to childhood cancer treatment across Africa include late clinical presentation, lack of access to specialty pediatric oncology care, treatment abandonment, lack of supportive care, and lack of access to clinical trials.99 However, local leadership, design of resource-conscious adapted treatment regimens, and prioritization of stepwise infrastructure building, as accomplished by GFAOP and others and as advocated by the CureAll framework, have already resulted in improved childhood cancer survival.
South-Central Asia and Indian Subcontinent.
The five-year childhood cancer survival across South-Central Asia is estimated to be 31.3%83 although the overall burden of childhood cancer is poorly described because of a lack of childhood cancer registries.119 The region historically suffers from a scarcity of specialized pediatric oncology facilities although there have been large collaborative clinical trial efforts centered primarily in India at large pediatric cancer centers.120
The Indian Pediatric Oncology Group (InPOG) was founded in 2008 to create a platform for multicenter cooperative group research across India.120 After several years of building clinical trial infrastructure, InPOG started recruiting pediatric patients for its first collaborative trials in 2015.120,121 A preliminary report from the InPOG HL trial described the process of creating a risk-stratified treatment protocol that could be feasibly executed at 27 participating centers across India and the challenges of implementing a multicenter clinical trial for the first time.122 InPOG is planning two additional prospective HL clinical trials122 and has also opened a multicenter randomized trial in ALL that is currently enrolling.123 InPOG has also collaborated with the Indian Pediatric Oncology Initiative to create the India Pediatric Oncology Database, a web-based database to facilitate hospital-based cancer registries and clinical trial research.7,124,125
There is a long history of twinning in India, with the first collaborative relationship between the Cancer Institute in Chennai and the US NCI in the 1980s leading to the seminal MCP-841 pediatric ALL protocol, which tripled survival rates.120,125 The Nepali-Norwegian EWS Study, a twinning relationship between the B.P. Koirala Memorial Cancer Hospital in Nepal and Oslo University Hospital in Norway, executed the first prospective cancer clinical trial in Nepal using an adapted treatment regimen for EWS.126 Although the study had a significant number of patients lost to follow-up, twinning demonstrated that international collaboration could improve patient outcomes.126
The Eurasian Alliance in Pediatric Oncology, which was founded in 2018 in collaboration with SJCRH, includes 19 hospitals in Armenia, Azerbaijan, Belarus, Kazakhstan, Kyrgyzstan, Moldova, Mongolia, Russia, Tajikistan, Ukraine, and Uzbekistan.127 Eurasian Alliance in Pediatric Oncology published a recent study on the integration of palliative care into pediatric oncology and has also developed coursework for the training of pediatric oncology nurses.127,128
Although there are barriers to execution of pediatric cancer clinical trials in South-Central Asia, including high rates of treatment abandonment and delays in diagnosis,129,130 robust initiatives over the past decade to build databases and implementation infrastructure as highlighted by the CureAll framework have produced collaborative trials across the subcontinent.
East and Southeast Asia.
Five-year childhood cancer survival rates vary widely across Asia, from 28.8% in Southeast Asia to 53.8% in East Asia.83 Although individual regions have developed their own autonomous clinical trial networks, there is not yet a single coordinating clinical trial body in the region, with SIOP Asia focusing efforts on this task. Similar to SIOPE, SIOP Asia facilitates clinical trials through a separate corpus, the Asian Pediatric Hematology and Oncology Group (APHOG), which was founded in 2021.131 Several clinical trials are under discussion by APHOG although lack of funding has hindered further development.131
Japan has adopted a similar clinical trial model to SIOPE, with the Japanese Society of Pediatric Hematology/Oncology acting as the coordinating academic society for the Japan Children's Cancer Group (JCCG), which designs and executes clinical trials through disease-specific subgroups.131 The JCCG has published numerous prospective clinical trials of novel therapeutic regimens132-134 and has established relationships with COG and European cooperative groups.135 Since its unification in 2014, the JCCG has also extended its clinical trial network to other countries in Southeast Asia, including the Pediatric Hepatic International Tumor Trial and an Asia-wide trial for children with ALL and Down syndrome.135
Although China historically has poor childhood cancer outcomes because of widespread lack of health insurance, policy changes in the past decade to provide governmental funding for all children with ALL have galvanized pediatric oncology care.7 There are two separate national clinical trial groups in China: the Chinese Children Cancer Group (CCCG), founded in 1997 by the China Anti-Cancer Association, and the Chinese Children Leukemia Group (CCLG).136 The CCLG initiated the first prospective multicenter pediatric oncology study in China in 2008 by enrolling patients to a modified I-BFM–based ALL protocol,137 followed by several additional prospective trials.136 In 2015, the CCCG, in conjunction with SJCRH, executed a multicenter ALL study with initial results reported in 2020.138 Several regional clinical trial groups in China have also produced their own clinical trials, including the Guangzhou Childhood Leukemia Group, which initiated their first pediatric ALL study in 2002 and eventually grew into the South China Children Cancer Group in 2016 with 22 participating centers and multiple trials.136 However, these groups operate separately, and there is not currently a single organization that oversees and coordinates clinical trial activities throughout China.136
Both Malaysia and Singapore have established international collaborations in pediatric oncology, with Malaysia developing its own Dutch-based adapted treatment regimen for ALL in 1995 and Singapore collaborating with Hong Kong to develop an I-BFM–based protocol in 1997.139 In 2003, Malaysia and Singapore formed the Malaysia-Singapore Study Group (MASPORE) to collaboratively design a risk-stratified ALL protocol, which demonstrated survival rates comparable with high-income nations.139 Because of relatively high treatment-related mortality rates in certain groups, MASPORE developed new protocols in 2010 and 2020 with the goal of deintensifying therapy.139
Several regional clinical trial groups throughout Southeast Asia have published prospective trials, particularly in ALL: the Thai Pediatric Oncology Group,140,141 the Indonesian Pediatric Hematology and Oncology Working Group,142 the Taiwan Pediatric Oncology Group,143 the Hong Kong Pediatric Hematology & Oncology Study Group,144 and the Korean Pediatric Hematology and Oncology Group.145 Myanmar, a particularly at-risk country in the region with nearly 90% of its patients with pediatric cancer unable to receive care, has developed twinning relationships with SJCRH and the National University Hospital in Singapore to help build foundational infrastructure.146,147
Although inability to afford care, treatment abandonment, and a lack of pediatric-trained subspecialists are major barriers throughout East and Southeast Asia,136,148 pediatric cancer clinical trials are ongoing in siloed cooperative groups that may benefit from an overarching body to enable intergroup collaboration, as suggested in the linked policies and governance pillar of CureAll.
Oceania.
Australia and New Zealand are high-income countries that are represented by both COG and the Australian and New Zealand Children's Hematology and Oncology Group (ANZCHOG), the clinical trial arm of SIOP Oceania founded in 1985.149 ANZCHOG was initially formed as a collaborative clinical group for investigator-initiated clinical trials; after the formation of COG, all children's cancer centers in Australia and New Zealand elected to join COG and there was no longer a need for regional investigator-initiated clinical trials. However, by 2005, many treatment centers wished to develop early-phase clinical trial programs not available through COG, which led to the development of the Australasian Children's Cancer Trials group hosted by ANZCHOG. This group has enabled multicenter participation in several trials, including the EuroNet HL PHL-C2 and European FarRMS rhabdomyosarcoma trials.
Oceania includes 14 low- and middle-income countries in Pacific Polynesia and the Melanesian country of Papua New Guinea, and there are barriers to pediatric oncology care delivery for many of these geographically-isolated island nations.149 With the support of Australia and New Zealand, both Fiji and Papua New Guinea have established child cancer programs but do not yet have the capacity to participate in clinical trials. However, the National Child Cancer Network, founded in New Zealand in 2010, established a Pacific Island Working Group, which facilitates twinning relationships with Fiji, Tonga, Samoa, and Vanuatu to develop resource-stratified treatment protocols on the basis of SIOP PODC guidelines.150-152 These nations have a great need for access to resource-stratified clinical protocols per CureAll and the infrastructure to implement sustained data capture into population-based child cancer registries.
RESULTS
The published results from the cooperative groups described above suggest that childhood cancer care is improved in regions with collaborative pediatric cancer groups, regardless of income status, in which implementation science, database registry creation, and clinical trials can be incorporated into standardized cancer treatment. Development of the infrastructure, organizational culture, and supportive care required for cooperative clinical trial execution may also further improve patient outcomes although infrastructure building requires sustained longitudinal investment by local stakeholders and parent advocacy groups. This narrative review reveals a wide variability in the structure, history, and goals of pediatric cancer clinical trial collaborative groups internationally, with several common models and potential avenues to facilitate further global collaboration identified.
Several collaborative groups in this review demonstrated improved outcomes with the implementation of adapted treatment regimens, particularly in resource-poor areas with historically poor survival rates. The GFAOP is a model of this improvement in outcomes, with its endogenous formation within Africa demonstrating that engaging local stakeholders in the creation of infrastructure and protocols is a critical component of forming sustainable collaborative groups. The creation of regional pediatric cancer units by the GFAOP is a cornerstone of the group's research productivity and is evidence that careful mapping of available cancer care resources may mitigate some of the challenges of operating in resource-poor areas.7,99 As such, supervisory cooperative groups should ensure that stakeholders are integrated into all aspects of collaborative projects, with lessons learned from the challenges of GALOP, which was mentored by COG and encountered considerable local organizational challenges in the execution of its first trial60 or the challenges of the SIOP-APHOG partnership to unify groups across Asia.131 These stand in contrast to groups like MASPORE and AHOPCA, which have executed numerous trials through a comprehensive understanding of how to mobilize local resources while exploiting international expertise and support. A nuanced understanding of the differing states of pediatric cancer care in each region contributes to successful clinical research implementation; for example, in CANCaRe Africa and AHOPCA's research efforts, treatment abandonment was notable and subsequent trials focused on its reduction, whereas efforts in Myanmar and Cambodia have focused on adequate pediatric cancer training for physicians, nurses, and supportive staff.
A common theme identified in this review is the lack of overarching coordinating bodies for clinical research in many of these regions, in contrast to the clinical trial cooperative groups in Europe. The absence of supervisory groups has not inhibited collaborative research, as evidenced by numerous collaborative clinical trial efforts beginning in the 1980s and 1990s across South America and in several countries in East Asia. However, the presence of siloed research efforts in these regions, such as the CCCG, CCLG, and South China Children Cancer Group in China, suggests that there may be a role for organizations such as SIOP's continental groups to streamline research execution in the future. It is important to note that the establishment of cooperative groups in the absence of supervisory organizations may be driven by the high human development index (a statistic composite index of life expectancy, education, and per-capita income indicators) and availability of resources in these countries,153 in addition to the history of autonomous and endogenous formation of their collaborative groups. As such, the independent governance model of these groups may not be feasible in countries with lower resource availability, with supervisory groups needing to play a more active role in creating clinical research opportunities and facilitating collaboration. This is in line with the objectives of the CureAll framework, which calls for situational analysis of each region as the first step in its implementation approach before strategic planning and the creation of cooperative relationships.
There is still a role for the partnership of large multinational groups like COG and SIOP in international clinical research efforts, with this review identifying twinning as a prominent means of creating pediatric clinical trial networks, particularly in more resource-poor areas and for specific disease groups. Many twinning relationships were based on the development of a single adapted treatment regimen, which enabled subsequent infrastructure building and the formation of a new cooperative group. A landmark example is the Collaborative Wilms Tumour Africa Project, which was supported by SIOP around a single multicenter clinical trial, or the COG-sponsored GALOP unilateral retinoblastoma protocol. Endogenous groups, such as InPOG and MASPORE, have also used this approach of developing a single initial protocol to champion local groups and develop regional networks. As a proof of concept, SJCRH established a twinning relationship with a member hospital of PINDA in Chile in 2000 to implement a complex frontline osteosarcoma protocol.57 On the basis of their experience, they offered a series of recommendations on twinning, including partnering with institutions with robust and adequately funded public health systems and availability of high-quality medical and supportive care services. Interestingly, the lack of a clinical trial data infrastructure at the participating hospital in Chile was identified as a primary weakness that delayed compliance and required training of data managers and research managers.57 SIOP has recently published twinning guidelines that advocate for the use of needs assessments to identify institutional and local stakeholders and to ensure that they are actively involved in all decision making.154 It is important to note that twinning initiatives need not only be focused on clinical trial development, as building foundational cancer databases and registries, such as the experience of SJCRH-sponsored POEM in the Middle East or Mexico in Alliance with SJCRH in Mexico, is a necessary prelude to clinical trial design and execution. Database-building efforts, such as SJCARES or the Pediatric Cancer Data Commons,69,155 are highlighted by the CureAll framework as instrumental to demonstrating improved outcomes that may result from these twinning partnerships.
It is important to contrast the governance models of COG and SIOPE with other cooperative groups identified in this review, as both groups sequester their clinical trial operations in separate bodies. Although several groups use a similar structure, such as in Japan with the Japanese Society of Pediatric Hematology/Oncology acting as the coordinating academic society for the JCCG or SIOP Oceania with ANZCHOG as the clinical trial partner, many groups house all their operations under one organization, which may be necessary to ensure that all group activities are coordinated. This has important implications for collaboration, as the example of APHOG demonstrates that designation of a separate clinical trial arm may be superfluous in some regions where it may be preferable to work directly with individual groups.
There are intrinsic limitations to this narrative review, namely, bias introduced by a nonsystematic review of the literature to identify groups and the results of their clinical trials. Furthermore, assessment of the veracity or accuracy of the results from published clinical trials by groups included in this review was not possible. In addition, nuances of the internal operations and governance structures of the clinical trial groups summarized in this review cannot necessarily be captured through a review of the literature although authorship by regional leaders provides this necessary context.
DISCUSSION
In conclusion, it is clear that there cannot be a one-size-fits-all approach to increasing collaboration between international pediatric cancer clinical trial groups, with a nuanced understanding of local stakeholders and needs necessary to form partnerships. In many regions, these needs assessments have been performed by endogenously formed groups, such as the GFAOP and groups in South America, which highlights the importance of situational analysis and data gathering as the first step of the CureAll implementation approach. Twinning initiatives by individual institutions or coordinated through the SIOP Global Health Network (formerly PODC) have generated productive clinical trial infrastructure using the same approach, with AHOPCA and the SIOP collaborative Wilms tumor CANCaRe studies in Africa as landmark examples, because of enhanced local stakeholder mobilization and engagement. In addition, the design of adapted treatment regimens is ultimately more feasible when local stakeholders are integrated into the clinical trial process.99,156 The ongoing SIOP mapping program is critical in identifying the needs of each individual region, with the recent publication of SIOP Africa's mapping survey as a prime example.99 Finally, the creation of database registry infrastructure is paramount, as the ability to link local cancer registries with national and international data commons is necessary to assess not only the efficacy of clinical trials but also the success of partnerships and the CureAll framework itself. To achieve the goals of the Global Initiative for Childhood Cancer, global partnerships must be sufficiently granular to account for the distinct needs of each collaborating group and each region, and incorporation of grassroots approaches, promotion of robust twinning relationships, and integration of implementation science are likely to help facilitate the mission to improve cancer outcomes even in less-resourced countries.
ACKNOWLEDGMENT
We thank Caitlin Pike for her editorial assistance.
Pamela Kearns
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, AstraZeneca
Research Funding: Pfizer (Inst), Bayer (Inst)
A. Lindsay Frazier
Stock and Other Ownership Interests: Decibel Therapeutics
Consulting or Advisory Role: Decibel Therapeutics
Samuel L. Volchenboum
Stock and Other Ownership Interests: Litmus Health
Consulting or Advisory Role: Accordant
Travel, Accommodations, Expenses: Sanford Health
No other potential conflicts of interest were reported.
SUPPORT
The Pediatric Cancer Data Commons was supported in part by the Children's Research Foundation, The Comer Development Board, The Leukemia & Lymphoma Society, The Andrew McDonough B + Foundation, Mr Daniel Tierney, Neuroblastoma Children's Cancer Society, Rally Foundation for Childhood Cancer Research, Sammy's Superheroes, St Baldrick's Foundation, The Matthew Bittker Foundation, a gift made in memory of Payton O'Brien, Team Bright Side, and the Children's Cancer Research Fund.
AUTHOR CONTRIBUTIONS
Conception and design: Ajay Major, Monica Palese, Anthony James, Laila Hessissen, Jennifer Geel, A. Lindsay Frazier, Carlos Rodriguez-Galindo, Samuel L. Volchenboum
Financial support: Samuel L. Volchenboum
Administrative support: Monica Palese, Samuel L. Volchenboum
Collection and assembly of data: Ajay Major, Monica Palese, Ebru Ermis, Anthony James, Federico Antillon Klussmann, Rashmi Dalvi, Pamela Kearns, Akira Nakagawara, Samuel L. Volchenboum
Data analysis and interpretation: Ajay Major, Monica Palese, Anthony James, Milena Villarroel, Federico Antillon Klussmann, Laila Hessissen, Muhammad Saghir Khan, Rashmi Dalvi, Michael Sullivan, A. Lindsay Frazier, Kathy Pritchard-Jones, Akira Nakagawara, Samuel L. Volchenboum
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Pamela Kearns
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, AstraZeneca
Research Funding: Pfizer (Inst), Bayer (Inst)
A. Lindsay Frazier
Stock and Other Ownership Interests: Decibel Therapeutics
Consulting or Advisory Role: Decibel Therapeutics
Samuel L. Volchenboum
Stock and Other Ownership Interests: Litmus Health
Consulting or Advisory Role: Accordant
Travel, Accommodations, Expenses: Sanford Health
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ward ZJ, Yeh JM, Bhakta N, et al. : Estimating the total incidence of global childhood cancer: A simulation-based analysis. Lancet Oncol 20:483-493, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Matsuda T, Di Carlo V, et al. : Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391:1023-1075, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piñeros M, Mery L, Soerjomataram I, et al. : Scaling up the surveillance of childhood cancer: A global roadmap. JNCI J Natl Cancer Inst 113:9-15, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Global Initiative for Childhood Cancer : St. Jude Children's Research Hospital. https://www.stjude.org/global/collaborating-to-cure/global-initiative.html
- 5.WHO Launches New Tools to Help Countries Build Effective Childhood Cancer Programmes. World Health Organization, 2021. https://www.who.int/news/item/15-02-2021-who-launches-new-tools-to-help-countries-build-effective-childhood-cancer-programmes [PMC free article] [PubMed] [Google Scholar]
- 6.Pamphlet: CURE All Framework. Pan American Health Association, 2021. https://www.paho.org/en/node/78537 [Google Scholar]
- 7.Rodriguez-Galindo C, Friedrich P, Alcasabas P, et al. : Toward the cure of all children with cancer through collaborative efforts: Pediatric oncology as a global challenge. J Clin Oncol 33:3065-3073, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Leary M, Krailo M, Anderson JR, et al. : Progress in childhood cancer: 50 years of research collaboration, a report from the Children's Oncology Group. Semin Oncol 35:484-493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleyer WA: The U.S. pediatric cancer clinical trials programmes: International implications and the way forward. Eur J Cancer 33:1439-1447, 1997 [DOI] [PubMed] [Google Scholar]
- 10.The SIOP story: An informal history of the International Society of Pediatric Oncology. Pediatr Blood Cancer 63:S5-S42, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Withycombe JS, Alonzo TA, Wilkins-Sanchez MA, et al. : The Children's Oncology Group: Organizational structure, membership, and institutional characteristics. J Pediatr Oncol Nurs 36:24-34, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locations. Children's Oncology Group. https://www.childrensoncologygroup.org/index.php/locations/
- 13.Arora RS, Challinor JM, Howard SC, et al. : Improving care for children with cancer in low- and middle-income countries—A SIOP PODC Initiative: SIOP PODC Initiative. Pediatr Blood Cancer 63:387-391, 2016 [DOI] [PubMed] [Google Scholar]
- 14.About us: SIOP Europe. https://siope.eu/about-siope/ [Google Scholar]
- 15.Membership: SIOP Europe. https://siope.eu/about-siope/members/ [Google Scholar]
- 16.European Clinical Trial Groups : SIOP Europe. https://siope.eu/european-research-and-standards/clinical-research-council/siopecrc/european-clinical-study-groups/ [Google Scholar]
- 17.Moore TB, McCabe ERB: National collaborative study groups: Structure, benefits gained and potential for rare genetic diseases. Genet Med 8:793-796, 2006 [DOI] [PubMed] [Google Scholar]
- 18.WHO Cancer Country Profiles. World Health Organization. https://www.who.int/cancer/country-profiles/en/ [Google Scholar]
- 19.Denburg A, Wilson MG, Johnson SI, et al. : Advancing the development of national childhood cancer care strategies in Latin America. J Cancer Policy 12:7-15, 2017 [Google Scholar]
- 20.Allemani C, Weir HK, Carreira H, et al. : Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 385:977-1010, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valsecchi MG, Tognoni G, Bonilla M, et al. : Clinical epidemiology of childhood cancer in Central America and Caribbean countries. Ann Oncol 15:680-685, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Fedorovsky JM, Cuervo LG, Luciani S: Pediatric cancer registries in Latin America: The case of Argentina's pediatric cancer registry. Rev Panam Salud Publica 41:e152, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatenoud L, Bertuccio P, Bosetti C, et al. : Childhood cancer mortality in America, Asia, and Oceania, 1970 through 2007. Cancer 116:5063-5074, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Bernasconi DP, Antolini L, Rossi E, et al. : A causal inference approach to compare leukaemia treatment outcome in the absence of randomization and with dependent censoring. Int J Epidemiol:dyab150, 2021 [DOI] [PubMed] [Google Scholar]
- 25.Health System Strengthening for Childhood Cancer in the Caribbean. Pan American Health Organization, 2020. https://iris.paho.org/handle/10665.2/51940 [Google Scholar]
- 26.Gibson TN, Beeput S, Gaspard J, et al. : Baseline characteristics and outcomes of children with cancer in the English-speaking Caribbean: A multinational retrospective cohort. Pediatr Blood Cancer 65:e27298, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Our History. GATLA. https://www.gatla.com.ar/index.php/en/about-us/41-historia-3 [Google Scholar]
- 28.Pavlovsky S, Muriel FS: Long-term survival in acute leukemia in Argentina: A study of 78 cases. Cancer 40:1402-1409, 1977 [DOI] [PubMed] [Google Scholar]
- 29.Deana A, Moran L, Fynn A: Results of protocol GATLA 8-AMLP ´07. Clinical challenges in de novo pediatric acute myeloid leukemia. Hematología 23:82-91, 2019 [Google Scholar]
- 30.Pavlovsky S: Treatment options in early stages of Hodgkin's lymphoma, high cure rate with lower short and long-term toxicity. Hematology 10:3-5, 2005. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 31.Veron D, Streitenberger P, García M, et al. : Pediatric Hodgkin lymphoma through clinical trials in Argentina in the past 46 years: The GATLA experience. Klin Pädiatr 226, 2014 [Google Scholar]
- 32.Mauz-Körholz C, Metzger ML, Kelly KM, et al. : Pediatric Hodgkin lymphoma. J Clin Oncol 33:2975-2985, 2015 [DOI] [PubMed] [Google Scholar]
- 33.ALL IC GATLA 2010/ALL IC BFM 2009. GATLA. https://www.gatla.com.ar/images/Protocolos/LLAP2010.pdf [Google Scholar]
- 34.Soria M, Ferraro C, Moran L, et al. : Acute lymphoblastic leukemia, analysis of minimal residual disease. Hematología 24:80-90, 2020 [Google Scholar]
- 35.Testi AM, Pession A, Diverio D, et al. : Risk-adapted treatment of acute promyelocytic leukemia: Results from the International Consortium for Childhood APL. Blood 132:405-412, 2018 [DOI] [PubMed] [Google Scholar]
- 36.History. CLEHOP. https://www.clehop.org/history [Google Scholar]
- 37.Freigeiro D, Arancibia A, Dibar E, et al. : Is it possible to work together in Latin America? Creation of a Latin American Consortium (CLEHOP). Klin Pädiatr 226, 2014 [Google Scholar]
- 38.Gonzalez-Ramella O, Freigeiro D, Castellanos ME, et al. : Joining forces for children with cancer in Latin America. Lancet Oncol 17:701-703, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Veron D, Castellanos M, Arancibia A, et al. : Is it possible to work together in Latin America? The Latin American Consortium (CLEHOP) experience with Hodgkin lymphoma (HL). Klin Pädiatr 232:98, 2020 [Google Scholar]
- 40.Freigeiro D: Pediatric acute promyelocytic leukemia (APL) in Latin American children. Is it possible to work together? The CLEHOP initiative. https://www.ercongressi.it/slides-apl-2017/APL-25-09-2017/20D-Freigeiro.pdf
- 41.Who We Are. SOBOPE. http://sobope.org.br/apex/f?p=106:4 [Google Scholar]
- 42.Childhood and Adolescent Cancer in Brazil. Nacional Cancer Institute (INCA), 2009. https://www.inca.gov.br/sites/ufu.sti.inca.local/files/media/document/childhood-adolescent-cancer-2009.pdf [Google Scholar]
- 43.Brandalise S, Odone V, Pereira W, et al. : Treatment results of three consecutive Brazilian cooperative childhood ALL protocols: GBTLI-80, GBTLI-82 and -85. ALL Brazilian Group. Leukemia 7:S142-S145, 1993. (suppl 2) [PubMed] [Google Scholar]
- 44.de Castro Junior CG, Macedo CRPD: Brazilian Society of Pediatric Oncology—SOBOPE: 30 years of history, a lot in the present, full of the future. Rev Bras Hematol E Hemoter 33:326-327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brandalise SR, Pinheiro VR, Aguiar SS, et al. : Benefits of the intermittent use of 6-mercaptopurine and methotrexate in maintenance treatment for low-risk acute lymphoblastic leukemia in children: Randomized trial from the Brazilian Childhood Cooperative Group—Protocol ALL-99. J Clin Oncol 28:1911-1918, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Brunetto AL, Castillo LA, Petrilli AS, et al. : Carboplatin in the treatment of Ewing sarcoma: Results of the first Brazilian Collaborative Study Group for Ewing Sarcoma Family Tumors-EWING1: Carboplatin in the treatment of Ewing sarcoma. Pediatr Blood Cancer 62:1747-1753, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Lopes LF, Macedo CRP, Pontes EM, et al. : Cisplatin and etoposide in childhood germ cell tumor: Brazilian Pediatric Oncology Society Protocol GCT-91. J Clin Oncol 27:1297-1303, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Bleyer A: Magic still needed for germ cell tumor research, especially in adolescents and young adults. J Oncol Pract 15:445-446, 2019 [DOI] [PubMed] [Google Scholar]
- 49.De Camargo B, Melaragno R, Silva NSE, et al. : Phase II study of carboplatin as a single drug for relapsed Wilms' tumor: Experience of the Brazilian Wilms' tumor study group. Med Pediatr Oncol 22:258-260, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Viana LS, Silva NdeP, Balmant NV, et al. : Challenges on participation in a cooperative group of childhood renal tumors in Brasil. Rev Assoc Médica Bras 66:284-289, 2020 [DOI] [PubMed] [Google Scholar]
- 51.Historia de PINDA. PINDA. http://www.pindachile.cl/historia-de-pinda/ [Google Scholar]
- 52.Quintana J, Advis P, Becker A, et al. : Acute myelogenous leukemia in Chile PINDA protocols 87 and 92 results. Leukemia 19:2143-2146, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Campbell M, Salgado C, Quintana J, et al. : Improved outcome for acute lymphoblastic leukemia in children of a developing country: Results of the Chilean National Trial PINDA 87. Med Pediatr Oncol 33:88-94, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Joannon P, Becker A, Kabalan P, et al. : Results of therapy for Wilms tumor and other malignant kidney tumors: A report from the Chilean Pediatric National Cancer Program (PINDA). J Pediatr Hematol Oncol 38:372-377, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Concha E, Cordova M, Becker A, et al. : Result of therapy for Hodgkin lymphoma (HL): A report from the Chilean pediatric National Cancer Program (PINDA). Klin Pädiatr 232:95, 2020 [Google Scholar]
- 56.Biondi A, Schrappe M, De Lorenzo P, et al. : Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): A randomised, open-label, intergroup study. Lancet Oncol 13:936-945, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivera GK, Quintana J, Villarroel M, et al. : Transfer of complex frontline anticancer therapy to a developing country: The St. Jude osteosarcoma experience in Chile. Pediatr Blood Cancer 50:1143-1146, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Villarroel CM, Chantada GL: Tumores Raros en Niños y adolescentes. Rev Médica Clínica Las Condes 26:495-502, 2015 [Google Scholar]
- 59.Barr R: Developments in paediatric care in Latin America. Lancet Oncol 16:1401-1403, 2015 [DOI] [PubMed] [Google Scholar]
- 60.Pérez V, Sampor C, Rey G, et al. : Treatment of nonmetastatic unilateral retinoblastoma in children. JAMA Ophthalmol 136:747-752, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Protocolo TCG-GALOP-2017. Brazilian Association of Pediatric Surgery. https://cipe.org.br/novo/wp-content/uploads/2020/05/PROTOCOLO-TCG.GALOP-Versao-Espanhol-19.06.18.pdf [Google Scholar]
- 62.Sobre SLAOP. SLAOP. https://slaop.org/sobre-slaop/ [Google Scholar]
- 63.About SIOP Latin America/SLAOP. SIOP. https://siop-online.org/sp_cb/latin-america/ [Google Scholar]
- 64.Barr RD, Klussmann FA, Baez F, et al. : Asociación de Hemato-Oncología Pediátrica de Centro América (AHOPCA): A model for sustainable development in pediatric oncology: AHOPCA Model Twinning Program. Pediatr Blood Cancer 61:345-354, 2014 [DOI] [PubMed] [Google Scholar]
- 65.Castellanos EM, Barrantes JC, Báez LF, et al. : A chemotherapy only therapeutic approach to pediatric Hodgkin lymphoma: AHOPCA LH 1999: Treatment of Hodgkin lymphoma in AHOPCA. Pediatr Blood Cancer 61:997-1002, 2014 [DOI] [PubMed] [Google Scholar]
- 66.Castellanos EM, Metzger M, Baez LF, et al. : A risk-adapted, response-based therapeutic regimen using a modified stanford V approach for the treatment of children with high risk Hodgkin lymphoma, AHOPCA LH 2004, a therapeutic regimen from the Central America and Dominican Republic Association of Pediatric Oncology. Blood 114:718, 2009 [Google Scholar]
- 67.Peña‐Hernandez A, Ortiz R, Garrido C, et al. : Outcome of pediatric non‐Hodgkin lymphoma in Central America: A report of the Association of Pediatric Hematology Oncology of Central America (AHOPCA). Pediatr Blood Cancer 66:e27621, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luna-Fineman S, Chantada G, Alejos A, et al. : Delayed enucleation with neoadjuvant chemotherapy in advanced intraocular unilateral retinoblastoma: AHOPCA II, a prospective, multi-institutional protocol in Central America. J Clin Oncol 37:2875-2882, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.SJCARES Registry. St. Jude Children's Research Hospital. https://www.stjude.org/global/sjcares/registry.html#348c826f6bc64f2cbe30c3d0f56323024d7d3f97e1cefa4a936287ef039cfa27=3 [Google Scholar]
- 70.St. Jude Children's Research Hospital expands international reach with St. Jude Global. St. Jude Children's Research Hospital. 2018. https://www.stjude.org/media-resources/news-releases/2018-medicine-science-news/st-jude-expands-international-reach-with-st-jude-global.html
- 71.Valsecchi MG, Steliarova-Foucher E: Cancer registration in developing countries: Luxury or necessity? Lancet Oncol 9:159-167, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Comportamiento Epidemiológico del Cáncer en menores de 18 años. México 2008-2014. Secretaria de Salud. http://censia.salud.gob.mx/contenidos/descargas/cancer/20160601_Boletin-2014_SEDP12sep16_4.pdf [Google Scholar]
- 73.Rodriguez-Romo L, Olaya Vargas A, Gupta S, et al. : Delivery of pediatric cancer care in Mexico: A national survey. J Glob Oncol 4:1-12, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiménez-Hernández E, Jaimes-Reyes EZ, Arellano-Galindo J, et al. : Survival of Mexican children with acute lymphoblastic leukaemia under treatment with the protocol from the Dana-Farber Cancer Institute 00-01. Biomed Res Int 2015:1-9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aristizabal P, Burns LP, Kumar NV, et al. : Improving pediatric neuro-oncology survival disparities in the United States–Mexico border region: A cross-border initiative between San Diego, California, and Tijuana, Mexico. JCO Glob Oncol 6:1791-1802, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzalez-Montalvo P, Romo H, Vega-Vega L: Collaborative risk-adapted treatment for pediatric acute lymphoblastic leukemia in Mexico. Blood 130:2116, 2017. (suppl 1) [Google Scholar]
- 77.Friedrich P, Echeandia N, Romo-Rubio H: V108 SIOP19-1254 scaling-up effective interventions in global pediatric oncology: Mexico in alliance with St Jude, a breakthrough model. Pediatr Blood Cancer 66, 2019. (suppl 4) [Google Scholar]
- 78.López-Facundo NA, Velasco-Hidalgo L, Arreguín-González FE, et al. : Rare malignant tumors in Mexican pediatric patients: A Cooperative Pediatric Oncology Research Group report. J Rare Disord Diagn Ther 5:3, 2019 [Google Scholar]
- 79.Garza-Garza LA, Ruiz-Lozano RE, Rebolledo-Méndez G, et al. : Challenge of retinoblastoma in Mexico in 2020: Perspectives and solutions. J Ophthalmol 2020:1-6, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reece-Mills M, Alexis C, Allen U, et al. : SickKids-Caribbean Initiative: Collaborating to improve the diagnosis and care of children with cancer and serious blood disorders in the Caribbean. Blood Adv 1:84-85, 2017. (suppl) [Google Scholar]
- 81.SickKids Centre for Global Child Health (C-GCH) 2019-2020 Annual Report. SickKids. https://www.sickkids.ca/contentassets/c325125a57d64a94bd7b2abbf4e1c344/c-gch-2019-20-summary-report.pdf [Google Scholar]
- 82.Denburg A, Cuadrado C, Alexis C, et al. : Improving childhood cancer care in Latin America and the Caribbean: A PAHO Childhood Cancer Working Group position statement. Lancet Oncol 18:709-711, 2017 [DOI] [PubMed] [Google Scholar]
- 83.Ward ZJ, Yeh JM, Bhakta N, et al. : Global childhood cancer survival estimates and priority-setting: A simulation-based analysis. Lancet Oncol 20:972-983, 2019 [DOI] [PubMed] [Google Scholar]
- 84.Saab R, Belgaumi A, Muwakkit S, et al. : The Pediatric Oncology East and Mediterranean (POEM) group—A regional collaborative platform for childhood cancer healthcare professionals. Pediatr Hematol Oncol J 5:3-6, 2020 [Google Scholar]
- 85.Basbous M, Al-Jadiry M, Belgaumi A, et al. : Childhood cancer care in the Middle East, North Africa, and West/Central Asia: A snapshot across five countries from the POEM network. Cancer Epidemiol 71:101727, 2021 [DOI] [PubMed] [Google Scholar]
- 86.Burges M, Qaddoumi I, Brennan RC, et al. : Assessment of retinoblastoma capacity in the Middle East, North Africa, and West Asia region. JCO Glob Oncol 6:1531-1539, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abbass Al-Hadad S, Faisal Al-Jadiry M, Fadhil Al-Darraji A, et al. : Reality of pediatric cancer in Iraq. J Pediatr Hematol Oncol 33:S154-S156, 2011. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 88.Adler RL: Reinventing Hadassah Ein Kerem's pediatric oncology department. Jerusalem Post. 2019. https://www.jpost.com/in-jerusalem/reinventing-hadassahs-pediatric-oncology-department-576129 [Google Scholar]
- 89.Merabi Z, Boulos F, Santiago T, et al. : Pediatric cancer pathology review from a single institution: Neuropathology expert opinion is essential for accurate diagnosis of pediatric brain tumors. Pediatr Blood Cancer 65:e26709, 2018 [DOI] [PubMed] [Google Scholar]
- 90.Qaddoumi I, Nawaiseh I, Mehyar M, et al. : Team management, twinning, and telemedicine in retinoblastoma: A 3-tier approach implemented in the first eye salvage program in Jordan: Twinning in retinoblastoma. Pediatr Blood Cancer 51:241-244, 2008 [DOI] [PubMed] [Google Scholar]
- 91.Al-Mulla NA, Chandra P, Khattab M, et al. : Childhood acute lymphoblastic leukemia in the Middle East and neighboring countries: A prospective multi-institutional international collaborative study (CALLME1) by the Middle East Childhood Cancer Alliance (MECCA): Middle East Childhood Lymphoblastic Leukemia. Pediatr Blood Cancer 61:1403-1410, 2014 [DOI] [PubMed] [Google Scholar]
- 92.Elitzur S, Arad‐Cohen N, Barzilai‐Birenboim S, et al. : Blinatumomab as a bridge to further therapy in cases of overwhelming toxicity in pediatric B‐cell precursor acute lymphoblastic leukemia: Report from the Israeli Study Group of Childhood Leukemia. Pediatr Blood Cancer 66:e27898, 2019 [DOI] [PubMed] [Google Scholar]
- 93.Yaniv I: Pediatric oncology palliative care in Israel. J Pediatr Hematol Oncol 34:S32-S35, 2012. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 94.Sultan I: Pediatric oncology in the Arab world, in Laher I. (ed): Handbook of Healthcare in the Arab World. Cham, Switzerland, Springer International Publishing, 2020, pp 1-25 [Google Scholar]
- 95.International Data: Africa. Population Reference Bureau (PRB). https://www.prb.org/international/geography/africa [Google Scholar]
- 96.Parkin DM, Youlden DR, Chitsike I, et al. : Stage at diagnosis and survival by stage for the leading childhood cancers in three populations of sub‐Saharan Africa. Int J Cancer 148:2685-2691, 2021 [DOI] [PubMed] [Google Scholar]
- 97.Joko‐Fru WY, Parkin DM, Borok M, et al. : Survival from childhood cancers in Eastern Africa: A population‐based registry study. Int J Cancer 143:2409-2415, 2018 [DOI] [PubMed] [Google Scholar]
- 98.Stones DK, De Bruin GP, Esterhuizen TM, et al. : Childhood cancer survival rates in two South African units. S Afr Med J 104:501-504, 2014 [DOI] [PubMed] [Google Scholar]
- 99.van Heerden J, Zaghloul M, Neven A, et al. : Pediatric oncology clinical trials and collaborative research in Africa: Current landscape and future perspectives. JCO Glob Oncol 6:1264-1275, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Les unités d'oncologie pédiatrique. GFAOP. https://www.gfaop.org/unites-oncologie-pediatrique/ [Google Scholar]
- 101.Hessissen L, Patte C, Martelli H, et al. : African School of Pediatric Oncology Initiative: Implementation of a Pediatric Oncology Diploma Program to address critical workforce shortages in French-speaking Africa. J Glob Oncol 5:1-12, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diagne Akonde FB, Togo B, Moreira C, et al. : Treatment of childhood Hodgkin lymphoma in sub-Saharan Africa: A report from the French-African Paediatric Oncology Group (GFAOP). South Afr J Child Health 14:155, 2020 [Google Scholar]
- 103.Yao AJJ, Moreira C, Traoré F, et al. : Treatment of Wilms tumor in sub-Saharan Africa: Results of the Second French African Pediatric Oncology Group study. J Glob Oncol 5:1-8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moreira C, Nachef MN, Ziamati S, et al. : Treatment of nephroblastoma in Africa: Results of the First French African Pediatric Oncology Group (GFAOP) study: Nephroblastoma in Africa. Pediatr Blood Cancer 58:37-42, 2012 [DOI] [PubMed] [Google Scholar]
- 105.Bouda GC, Traoré F, Couitchere L, et al. : Advanced burkitt lymphoma in sub-Saharan Africa Pediatric Units: Results of the third prospective multicenter study of the Groupe Franco-Africain d'Oncologie pédiatrique. J Glob Oncol 5:1-9, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Geel JA, Chirwa TC, Rowe B, et al. : Treatment outcomes of children with Hodgkin lymphoma between 2000 and 2010: First report by the South African Children's Cancer Study Group. Pediatr Blood Cancer 64:e26536, 2017 [DOI] [PubMed] [Google Scholar]
- 107.Khalek ER, Afungchwi GM, Beltagy ME, et al. : Highlights from the 13th African continental meeting of the International Society of Paediatric Oncology (SIOP), 6-9 March 2019, Cairo, Egypt. Ecancermedicalscience 13:932, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Heerden J, Geel J, Hendricks M, et al. : The evaluation of induction chemotherapy regimens for high-risk neuroblastoma in South African children. Pediatr Hematol Oncol 37:300-313, 2020 [DOI] [PubMed] [Google Scholar]
- 109.Hailu D, Adamu H, Fufa D, et al. : Training pediatric hematologists/oncologists for capacity building in Ethiopia. Blood 134:3423, 2019. (suppl 1) [Google Scholar]
- 110.Afungchwi GM, Kruger M, Wharin PD, et al. : The evolution of a hospital-based cancer registry in Northwest Cameroon from 2004 to 2015. J Trop Pediatr 67:fmaa038, 2021 [DOI] [PubMed] [Google Scholar]
- 111.Hessissen L, Khtar R, Madani A, et al. : Improving the prognosis of pediatric Hodgkin lymphoma in developing countries: A Moroccan Society of Pediatric Hematology and Oncology Study: Pediatric Hodgkin lymphoma in Morocco. Pediatr Blood Cancer 60:1464-1469, 2013 [DOI] [PubMed] [Google Scholar]
- 112.Paintsil V, David H, Kambugu J, et al. : The collaborative Wilms tumour Africa project; baseline evaluation of Wilms tumour treatment and outcome in eight institutes in sub-Saharan Africa. Eur J Cancer 51:84-91, 2015 [DOI] [PubMed] [Google Scholar]
- 113.Israëls T, Kambugu J, Kouya F, et al. : Clinical trials to improve childhood cancer care and survival in sub-Saharan Africa. Nat Rev Clin Oncol 10:599-604, 2013 [DOI] [PubMed] [Google Scholar]
- 114.Collaborative African Network for Childhood Cancer Care and Research (CANCaRe Africa). SIOP. https://siop-online.org/cancareafrica/ [DOI] [PubMed] [Google Scholar]
- 115.Israels T, Paintsil V, Nyirenda D, et al. : Improved outcome at end of treatment in the collaborative Wilms tumour Africa project. Pediatr Blood Cancer 65:e26945, 2018 [DOI] [PubMed] [Google Scholar]
- 116.Chitsike I, Paintsil V, Sung L, et al. : Working together to build a better future for children with cancer in Africa. JCO Glob Oncol 6:1076-1078, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chagaluka G, Afungchwi GM, Landman L, et al. : Treatment abandonment: A report from the collaborative African network for childhood cancer care and research—CANCaRe Africa. Pediatr Blood Cancer 68:e29367, 2018 [DOI] [PubMed] [Google Scholar]
- 118.Israels T, Afungchwi GM, Klootwijk L, et al. : Fever and neutropenia outcomes and areas for intervention: A report from SUCCOUR—Supportive Care for Children with Cancer in Africa. Pediatr Blood Cancer 68:e29224, 2021 [DOI] [PubMed] [Google Scholar]
- 119.Khasru AAM: Childhood cancer: A situation analysis and challenges, Bangladesh perspective. Bangladesh J Child Health 41:140-142, 2018 [Google Scholar]
- 120.Arora RS, Bakhshi S: Indian Pediatric Oncology Group (InPOG)—Collaborative research in India comes of age. Pediatr Hematol Oncol J 1:13-17, 2016 [Google Scholar]
- 121.Roy P, Narula G, Arora B, et al. : Implementation of risk adapted therapeutic strategy for childhood acute lymphoblastic leukaemia—Interim report of the pilot InPOG-ALL-15-01 study. Pediatr Hematol Oncol J 3:S19, 2018 [Google Scholar]
- 122.Arora RS, Mahajan A, Dinand V, et al. : InPOG-HL-15-01—Challenges and lessons learnt in setting up the first collaborative multicentre prospective clinical trial in childhood cancer in India. Pediatr Hematol Oncol J 5:166-170, 2020 [Google Scholar]
- 123.Sidhu J, Saha D, Roy P, et al. : Therapeutic drug monitoring informs asparaginase dose scheduling in the InPOG-ALL-15-01-ICiCLe-ALL-14 trial. Pediatr Hematol Oncol J 2:S2-S3, 2017 [Google Scholar]
- 124.India Pediatric Oncology Initiative : Jiv Daya Foundation. https://www.jivdayafound.org/pediatric-oncology [Google Scholar]
- 125.Arora RS, Arora B: Acute leukemia in children: A review of the current Indian data. South Asian J Cancer 5:155-160, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jha AK, Neupane P, Pradhan M, et al. : Ewing sarcoma in Nepal treated with combined chemotherapy and definitive radiotherapy. J Glob Oncol 5:1-10, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sullivan CE, Hans M, Yakimkova T, et al. : Survey identifies need for subspecialized pediatric hematology/oncology nursing education in nine Еurasian countries. Russ J Pediatr Hematol Oncol 7:138-144, 2020 [Google Scholar]
- 128.Ehrlich BS, Movsisyan N, Batmunkh T, et al. : Barriers to the early integration of palliative care in pediatric oncology in 11 Eurasian countries. Cancer 126:4984-4993, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hazarika M, Mishra R, Saikia BJ, et al. : Causes of treatment abandonment of pediatric cancer patients—Experience in a Regional Cancer Centre in North East India. Asian Pac J Cancer Prev 20:1133-1137, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siddiqui DEF, Ashraf MS, Iftikhar S, et al. : Predictors of treatment abandonment for patients with pediatric cancer at Indus Children Cancer Hospital, Karachi, Pakistan. Pediatr Blood Cancer 65:e26818, 2018 [DOI] [PubMed] [Google Scholar]
- 131.Nakagawara A: Asian Pediatric Hematology and Oncology Group (APHOG) and SIOP Asia: Two wheels of a cart. Pediatr Hematol Oncol J 5:140-144, 2020 [Google Scholar]
- 132.Hishiki T, Matsumoto K, Ohira M, et al. : Results of a phase II trial for high-risk neuroblastoma treatment protocol JN-H-07: A report from the Japan Childhood Cancer Group Neuroblastoma Committee (JNBSG). Int J Clin Oncol 23:965-973, 2018 [DOI] [PubMed] [Google Scholar]
- 133.Koshinaga T, Takimoto T, Oue T, et al. : Outcome of renal tumors registered in Japan Wilms tumor study-2 (JWiTS-2): A report from the Japan Children's Cancer Group (JCCG). Pediatr Blood Cancer 65:e27056, 2018 [DOI] [PubMed] [Google Scholar]
- 134.Tomizawa D, Miyamura T, Imamura T, et al. : A risk-stratified therapy for infants with acute lymphoblastic leukemia: A report from the JPLSG MLL-10 trial. Blood 136:1813-1823, 2020 [DOI] [PubMed] [Google Scholar]
- 135.Okamoto Y: Japan Children's Cancer Group: International collaborations and plans. Pediatr Hematol Oncol J 5:162-165, 2020 [Google Scholar]
- 136.Li C, Tang J, Zheng H, et al. : Treatment of childhood cancer in China: Current status and future direction. Pediatr Investig 4:153-156, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cui L, Li ZG, Chai YH, et al. : Outcome of children with newly diagnosed acute lymphoblastic leukemia treated with CCLG-ALL 2008: The first nation-wide prospective multicenter study in China. Am J Hematol 93:913-920, 2018 [DOI] [PubMed] [Google Scholar]
- 138.Shen S, Chen X, Cai J, et al. : Effect of dasatinib vs imatinib in the treatment of pediatric Philadelphia chromosome–positive acute lymphoblastic leukemia: A randomized clinical trial. JAMA Oncol 6:358-366, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ariffin H, Ab Rahman S, Leong SH, et al. : Malaysia-Singapore (MASPORE) leukaemia study group: From common history to successful collaboration. Pediatr Hematol Oncol J 5:11-16, 2020 [Google Scholar]
- 140.Rujkijyanont P, Photia A, Traivaree C, et al. : Clinical outcomes and prognostic factors to predict treatment response in high risk neuroblastoma patients receiving topotecan and cyclophosphamide containing induction regimen: A prospective multicenter study. BMC Cancer 19:961, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bunyatisai W, Jia-Mahasap B, Chitapanarux I: Treatment outcomes of acute lymphoblastic leukemia in both children and adults using the Thai Pediatric Oncology Group-based protocol at Chiang Mai University Hospital. J Thai Assn Radiat Oncol 25:12-28, 2019 [Google Scholar]
- 142.Widjajanto PH, Supriyadi E, Purwanto I, et al. : Dexamethasone versus prednisone in childhood acute lymphoblastic leukemia treatment: Results of the Indonesian randomized trial. J Cancer Ther 8:735-750, 2017 [Google Scholar]
- 143.Liang DC, Yang CP, Lin DT, et al. : Long-term results of Taiwan Pediatric Oncology Group studies 1997 and 2002 for childhood acute lymphoblastic leukemia. Leukemia 24:397-405, 2010 [DOI] [PubMed] [Google Scholar]
- 144.Leung AWK, Vincent L, Chiang AKS, et al. : Prognosis and outcome of relapsed acute lymphoblastic leukemia: A Hong Kong pediatric hematology and Oncology Study Group report: Treatment of relapsed childhood leukemia. Pediatr Blood Cancer 59:454-460, 2012 [DOI] [PubMed] [Google Scholar]
- 145.Lee JM, Choi JY, Hong KT, et al. : Clinical characteristics and treatment outcomes in children, adolescents, and young-adults with Hodgkin's lymphoma: A KPHOG lymphoma working-party, multicenter, retrospective study. J Korean Med Sci 35:e393, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Halbert J, Khaing AA: Overview of pediatric oncology and hematology in Myanmar. South Asian J Cancer 3:78-82, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hnin TM, Khaing AA, Kyaw YY, et al. : National capacity-building initiatives for childhood hematologic malignancies in Myanmar. Blood Adv 1:52-55, 2017. (suppl) [Google Scholar]
- 148.Othman MY, Blair S, Nah SA, et al. : Pediatric solid tumor care and multidisciplinary tumor boards in low- and middle-income countries in Southeast Asia. JCO Glob Oncol 6:1328-1345, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Oceania. SIOP. https://siop-online.org/sp_cb/oceania/ [Google Scholar]
- 150.Foliaki S, Bates C, Tukana I, et al. : Cancer control in the Pacific: A South Pacific collaborative approach. Cancer Epidemiol 50:193-198, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ekeroma A, Dyer R, Palafox N, et al. : Cancer management in the Pacific region: A report on innovation and good practice. Lancet Oncol 20:e493-e502, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Paediatric Cancer Protocols: Paediatric Society of Papua New Guinea. http://pngpaediatricsociety.org/treatment/paediatric-cancer-protocols/ [Google Scholar]
- 153.Magrath I, Steliarova-Foucher E, Epelman S, et al. : Paediatric cancer in low-income and middle-income countries. Lancet Oncol 14:e104-e116, 2013 [DOI] [PubMed] [Google Scholar]
- 154.Kanwar VS, Schwartz KR, Salifu N, et al. : The role of twinning in sustainable care for children with cancer: A TIPPing point? SIOP PODC working group on twinning, collaboration, and support. Pediatr Blood Cancer 67:e28667, 2020 [DOI] [PubMed] [Google Scholar]
- 155.Plana A, Furner B, Palese M, et al. : Pediatric cancer data commons: Federating and democratizing data for childhood cancer research. JCO Clin Cancer Inform 5:1034-1043, 2021 [DOI] [PubMed] [Google Scholar]
- 156.Howard SC, Davidson A, Luna-Fineman S, et al. : A framework to develop adapted treatment regimens to manage pediatric cancer in low- and middle-income countries: The Pediatric Oncology in Developing Countries (PODC) Committee of the International Pediatric Oncology Society (SIOP). Pediatr Blood Cancer 64:e26879, 2017 [DOI] [PubMed] [Google Scholar]