Abstract
Cisplatin-based adjuvant chemotherapy remains the standard of care for patients with resected stage II or III non–small-cell lung cancer. However, biomarker-informed clinical trials are starting to push the management of early-stage lung cancer beyond cytotoxic chemotherapy. This review explores recent and ongoing studies focused on improving cytotoxic chemotherapy and incorporating targeted and immunotherapies in the management of early-stage, resectable lung cancer. Adjuvant osimertinib for patients with EGFR-mutant tumors, preoperative chemoimmunotherapy, and adjuvant immunotherapy could improve outcomes for selected patients with resectable lung cancer, and ongoing or planned studies leveraging biomarkers, immunotherapy, and targeted therapy may further improve survival. We also discuss the unique barriers associated with clinical trials of early-stage lung cancer and the need for innovative trial designs to overcome these challenges.
BACKGROUND
The appropriate curative management of early-stage lung cancer is dependent upon multidisciplinary evaluation. First and foremost are the determinations of clinical stage, technical resectability of the tumor and involved lymph nodes, and medical operability or fitness of the patient for the required procedure to attain a complete surgical resection. In this multidisciplinary evaluation, the role of systemic therapy is largely driven by tumor stage. Stage for stage, survival is worse when clinical stage is used compared with pathologic stage, likely because of upstaging in a subset of patients at surgery.1 This staging reality should be considered when setting patient expectations about systemic therapy.
CONTEXT
Key Objective
What are the current and likely future best approaches for the perioperative treatment of early-stage lung cancer and what barriers must be overcome to continue progress?
Knowledge Generated
Although cytotoxic chemotherapy remains the current standard of care for most patients with resected early-stage lung cancer, new approaches that incorporate biomarkers, immunotherapy, and targeted therapy are in development. Adjuvant osimertinib improves disease-free survival for patients with EGFR-mutant non–small-cell lung cancer and should be offered to eligible patients. Preoperative chemoimmunotherapy induces pathologic complete response in a quarter of patients and may convert marginally resectable tumors requiring pneumonectomy to lobectomy. Further progress is on the horizon, but hurdles remain.
Relevance
Innovative trial design holds the potential to usher in new therapies in early-stage lung cancer that will further improve outcomes for patients with this disease.
STANDARDS OF CARE FOR PERIOPERATIVE CYTOTOXIC THERAPIES
In addition to complete surgical resection, level 1 evidence demonstrates a survival advantage for adjuvant cisplatin-doublet chemotherapy. Trial-level data were pooled in the Lung Adjuvant Cisplatin Evaluation, demonstrating a 5.8% improvement in disease-free survival (DFS) and a 5.4% improvement in overall survival (OS) at 5 years.2 These studies were largely performed in an era before positron emission tomography scan staging and enrolled patients across stages IA-III; however, subsets showed possible harm in stage IA and greater benefit of chemotherapy with increasing stage. To better clarify the role of adjuvant chemotherapy in stage I disease, a study of adjuvant carboplatin plus paclitaxel was performed in patients with resected stage IB disease. Of note, stage IB in the sixth edition staging included all lymph node–negative solitary tumors > 3 cm. Although no overall benefit of adjuvant carboplatin and paclitaxel was observed in the intention-to-treat (ITT) population with stage I non–small-cell lung cancer (NSCLC), patients with tumors ≥ 4 cm were demonstrated to have a survival advantage.3 Despite this being an unpowered subset analysis, the observation has driven clinical care for the past decade where adjuvant cytotoxic chemotherapy is recommended for patients with tumors ≥ 4 cm and/or those with involved lymph nodes.4 This tumor size threshold was further substantiated in a post hoc analysis of JBR-10, the North American Intergroup Study of adjuvant cisplatin and vinorelbine, in which the hazard ratio (HR) for OS was 0.66 for patients with tumors ≥ 4 cm, whereas no benefit was demonstrated in patients with tumors < 4 cm (HR 1.73).5 To apply this finding today in the current eighth edition TNM staging, the population considered most appropriate for adjuvant therapy is now stage IIA (T2bN0) or greater.6
Neoadjuvant therapy hit a barrier when the earlier readouts of the individual adjuvant trials put a stop to the concurrently enrolling neoadjuvant trials. The most robust neoadjuvant data are in meta-analysis form, pooling 15 studies of neoadjuvant cisplatin-based therapy versus surgery alone, with an identical finding of 5% improvement in 5-year OS.7 Side by side, the Kaplan-Meier curves of neoadjuvant and adjuvant chemotherapy appear nearly identical. The decision for neoadjuvant or adjuvant cytotoxic chemotherapy varies tremendously by region, institution, stage, and disease management team.
ATTEMPTS TO PERSONALIZE AND IMPROVE CYTOTOXIC CHEMOTHERAPY
There have been a few large efforts to improve upon adjuvant cisplatin-based chemotherapy alone. The MAGRIT study evaluated the recombinant MAGE-A3 vaccine in patients with tumors that express MAGE-A3; however, this study showed no improvement in DFS and development was terminated.8 E1505 was a large, randomized phase III study adding bevacizumab during and after chemotherapy for a year; it showed no improvement in DFS or OS.9 A European study prescribed personalized adjuvant therapy on the basis of tumor mRNA expression of ERCC1 and TS, thought to predict tumor resistance to cisplatin and pemetrexed, respectively. The study showed better tolerability of non–cisplatin-based regimens but no recurrence-free survival or OS differences.10
The modern reality is that the drugs combined with cisplatin in the landmark adjuvant studies, namely vinorelbine, mitomycin, vindesine, and etoposide, are considered minimally active in the treatment of advanced NSCLC and are rarely used.11 There have been no phase III studies in the United States powered to compare more modern cytotoxic agents with the historic drugs. A randomized phase II study of cisplatin plus vinorelbine versus cisplatin plus pemetrexed did not demonstrate an efficacy difference in regimens.12 A phase III study in Japan comparing the same treatments showed improved tolerability but no superiority of the pemetrexed-based regimen.13 E1505 allowed physician choice of a variety cytotoxic agents to be combined with cisplatin. Although each regimen appeared comparable with cisplatin plus vinorelbine as the reference regimen, this was not a powered analysis.9
Another therapeutic dilemma is encountered regularly in the clinic: all level 1 evidence for adjuvant cytotoxic therapy is with a cisplatin-based doublet; however, cisplatin is a drug that may be dangerous to administer to the elderly and those with comorbidities—the majority of the lung cancer population. Data for carboplatin-based therapy are limited. The aforementioned study of adjuvant carboplatin plus paclitaxel in resected node-negative NSCLC gives precedent for a carboplatin-based regimen.3 In combination with pemetrexed, a randomized phase II study of cisplatin versus carboplatin was done for feasibility; however, no efficacy data are available.14 Therefore, we remain dependent on consensus guidelines that enable the use of carboplatin-based regimens for patients in whom cisplatin poses undue risk of harm.4,15
MOVING BEYOND CYTOTOXIC CHEMOTHERAPY IN THE PRE- AND POSTOPERATIVE SETTING
Targeted Therapy
For the purposes of this discussion, targeted therapies are considered anticancer drugs designed to inhibit the protein products of activated oncogenes or their resultant pathways. The testing for driver oncogenes and prescription of targeted therapies has been a standard of care in the treatment of advanced NSCLC for more than a decade.4 The use of biomarker-matched targeted therapies has been singularly credited for the improvement in population-level lung cancer–specific mortality observed between 2013 and 2016.16 Incorporation of these therapeutic advances in the treatment of resectable NSCLC has significantly lagged, not for lack of interest but because of both the absence of routine predictive biomarker testing in early-stage disease and the length of trials historically designed with OS primary end points.
In the adjuvant setting, retrospective and small single-arm studies demonstrate that adjuvant epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) improved outcomes compared with historical controls.17,18 In China, randomized phase II19 and subsequent phase III studies compared gefitinib with cisplatin and vinorelbine chemotherapy in patients with resected stage III-N2 EGFR-mutant NSCLC. This study met its primary end point of DFS but was not powered for OS and did not show an OS advantage.20 This study has been criticized for the lack of standardized preoperative staging and withholding standard-of-care chemotherapy in the gefitinib arm. In Japan, a study of adjuvant gefitinib versus cisplatin plus vinorelbine in resected stage II and III EGFR-mutant NSCLC failed to meet its DFS end point.21 In the United States, phase III studies of targeted therapies against EGFR and anaplastic lymphoma kinase (ALK) were launched in 2014 as part of the National Cancer Institute's ALCHEMIST portfolio of trials.22 These studies were designed in the gold standard fashion; TKI was prescribed after standard-of-care chemotherapy in phase III randomized placebo-controlled studies with OS primary end points. However, the ALCHEMIST trials were hindered by slow trial accrual. The fast pace of drug design has also led to the availability of better tolerated and more effective TKIs. In 2020, the US Food and Drug Administration granted the first approval to a drug in the perioperative space. On the basis of the phase III ADAURA study, after standard-of-care adjuvant chemotherapy, the EGFR TKI osimertinib was approved for a duration of 3 years. When compared with placebo, osimertinib in patients with resected EGFR-mutant tumors ≥ 3 cm or with involved lymph nodes improved DFS with a HR of 0.20 (P < .0010).23 The ADAURA OS data are a secondary end point and years away from maturity. A similar industry-sponsored adjuvant trial of alectinib versus chemotherapy (NCT03456076) is enrolling internationally; however, results are likely years away.
The challenges of low mutation incidence and lengthy time for biomarker testing have posed barriers to preoperative TKI studies; therefore, few trials of biomarker-matched neoadjuvant targeted therapies have been completed and published. Early studies enrolled on the basis of clinical characteristics and later matched biomarkers.24 Other single-arm EGFR TKI studies have been completed in China where the incidence of EGFR mutation is relatively high; however, only a single randomized study is published. The EMERGING-CTONG 1103 study was a randomized phase II study of erlotinib versus cisplatin plus gemcitabine in patients with stage IIIA-N2 EGFR-mutant NSCLC, with a primary end point of radiographic response rate.25 This study also prescribed a year of adjuvant erlotinib, and although no clinical differences were seen between arms, data presented at the time of recurrence showed that regardless of treatment arm, the majority of patients responded to standard-of-care postprogression treatment with an EGFR TKI.26
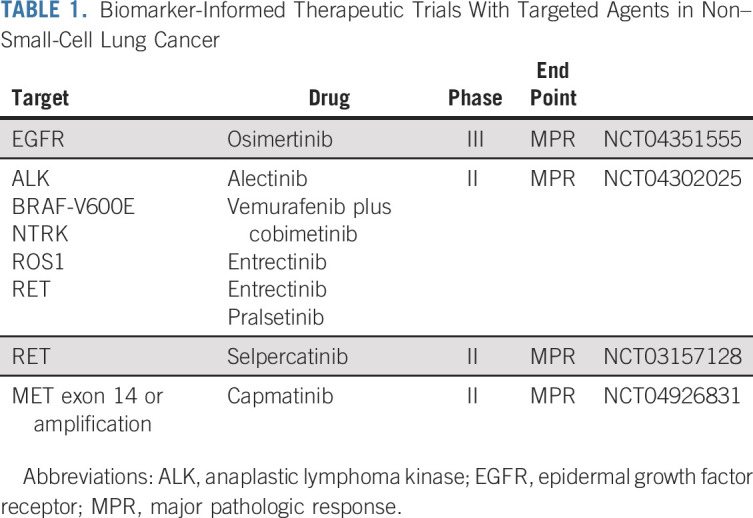
There are current efforts geared at moving targeted therapy into the neoadjuvant setting. The Lung Cancer Research Foundation–supported LEADER study will enroll patients with early-stage lung cancer appropriate for resection and perform centralized plasma and tumor genotyping. This study's predictive biomarker panel is acceptable for enrollment in matched therapeutic trials, such as those listed in Table 1. A global phase III study also enrolling patients with resectable EGFR-mutant NSCLC is evaluating osimertinib versus chemotherapy versus the combination (NCT04351555).
TABLE 1.
Biomarker-Informed Therapeutic Trials With Targeted Agents in Non–Small-Cell Lung Cancer
Immunotherapy
Adjuvant programmed death-ligand 1 or programmed cell death protein 1 blockade.
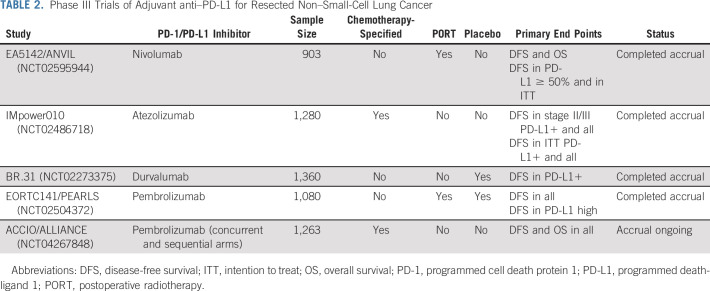
Several large phase III trials of adjuvant programmed death-ligand 1 (PD-L1) or programmed cell death protein 1 (PD-1) blockade in patients with resected NSCLC staged IB with tumors ≥ 4 cm to IIIA (seventh edition TNM staging) have completed accrual while one ongoing study also includes concurrent adjuvant chemotherapy with anti–PD-1 (Table 2). The IMpower010 study has recently reported results of its hierarchical primary end points of DFS. The study enrolled patients after resection and prescribed cisplatin-based adjuvant chemotherapy. Patients without progression after chemotherapy were randomly assigned 1:1 to receive 16 cycles of 1,200 mg intravenous atezolizumab every 3 weeks or best supportive care. At the time of presentation, the end points that had crossed a significance boundary included DFS in PD-L1+ (defined as PD-L1 expression in ≥ 1% of tumor cells) stage II-III where atezolizumab improved DFS with a HR of 0.66 (P = .004) and DFS in all stage II-III (irrespective of PD-L1 expression) with a HR of 0.79 (P = .02); however, in the preplanned subset of patients with tumors without PD-L1, there was no DFS benefit from adjuvant atezolizumab. Additional analyses are ongoing.27
TABLE 2.
Phase III Trials of Adjuvant anti–PD-L1 for Resected Non–Small-Cell Lung Cancer
Neoadjuvant immunotherapy.
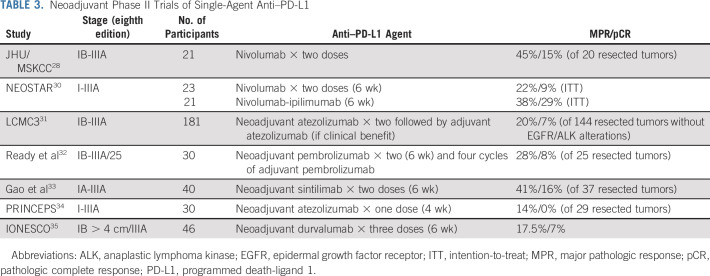
With the rapid development and approval of new therapies for advanced lung cancer over the past 10 years, interest has reawakened in neoadjuvant clinical trials. In a 2018 single-arm clinical trial, 21 patients with resectable stage IB-IIIA NSCLC received two doses of neoadjuvant nivolumab followed by standard surgery and adjuvant therapy.28 Despite the short course of therapy, nine of 21 resected tumors underwent a major pathologic response (MPR, defined as ≤ 10% residual viable tumor), including two pathologic complete responses (pCR, defined as no residual viable tumor cells), and in-depth correlative studies performed highlighted the potential for neoadjuvant clinical trials to act as a platform for correlative science.29 Subsequently, a series of larger single-arm phase II neoadjuvant trials of single-agent PD-L1 or PD-1 blockade or combination anti–PD-1 and anti–cytotoxic T-cell lymphocyte-4 blockade (Table 3) and combination chemotherapy with PD-L1 or PD-1 blockade phase II trials have been reported.
TABLE 3.
Neoadjuvant Phase II Trials of Single-Agent Anti–PD-L1
Several groups have reported single-arm phase II studies consisting of short courses (4-6 weeks) of anti–PD-L1 or anti–PD-1 therapy before surgery, then standard adjuvant chemotherapy and in some cases further adjuvant immunotherapy (Table 3).36,37 One study, NEOSTAR, randomly assigned patients to receive either neoadjuvant nivolumab alone or in combination with ipilimumab, whereas another has examined the combination of neoadjuvant durvalumab with subablative stereotactic radiation.30,38
In trial reports to date, neoadjuvant anti–PD-L1 or anti–PD-1 therapy has been well-tolerated with no significant delays to surgery or unexpected surgical complications.31 MPR rates after neoadjuvant anti–PD-L1 or anti–PD-1 monotherapy have ranged from 14% to 45%; for context, the median MPR rate reported after neoadjuvant chemotherapy is approximately 15%-20%.29 In the largest phase II study reported, the LCMC3 trial, patients with eighth edition stage IB-IIIB resectable NSCLC received two doses of preoperative atezolizumab; patients who had clinical benefit were permitted to receive adjuvant atezolizumab for up to 1 year postoperatively.31 In the primary efficacy population of 144 patients without EGFR or ALK alterations, 20% (95% CI, 14 to 28) of tumors demonstrated MPR and 7% (95% CI, 3 to 12) pCR. There was an association between PD-L1 positivity and MPR. There was also a trend toward greater pathologic response in tumors with higher tumor mutation burden.
In the phase II NEOSTAR study, patients with resectable stage I-IIIA NSCLC were randomly assigned to receive either three cycles of neoadjuvant nivolumab or the same regimen with one dose of ipilimumab.30 The nivolumab plus ipilimumab arm met the prespecified primary end point threshold of six or more MPRs in 21 patients, achieving a 38% MPR rate (8 of 21), whereas the MPR rate was lower in the nivolumab arm (5 of 21; 24%). When compared with nivolumab monotherapy, the combination of nivolumab plus ipilimumab led to increased pCR (9% v 29%), less viable tumor in resections (median 50% v 9%), and greater frequencies of effector, tissue-resident memory, and effector memory T cells.
Clinical trials of neoadjuvant anti–PD-L1 or anti–PD-1 with chemotherapy.
Two single-arm trials were among the first to explore the combination of standard platinum-doublet chemotherapy with anti–PD-L1 or anti–PD-1 before surgery for resectable NSCLC. A multicenter phase II study of atezolizumab plus carboplatin and nab-paclitaxel for up to four cycles enrolled 30 patients with resectable stage IB-IIIA NSCLC and a history of smoking39; 77% had stage IIIA disease and 87% underwent a complete tumor resection. MPR was demonstrated in 57% (95% CI, 37 to 75) of tumors and surgical resection was not compromised by neoadjuvant therapy.
The NADIM study enrolled patients with resectable stage IIIA NSCLC who received three cycles of nivolumab with carboplatin and paclitaxel followed by resection; 1 year of nivolumab was administered after surgery.40 Among 46 enrolled patients, 41 (89%) underwent resection. The primary end point, 2-year progression-free survival in all patients who received induction therapy, was 77% (95% CI, 60 to 88). The MPR rate was 83% (95% CI, 68 to 93), including 63% (95% CI, 62 to 91) pCR. There were no fatal events or delays to surgery.
The phase III CheckMate 816 trial compared three cycles of neoadjuvant nivolumab plus platinum-doublet chemotherapy with the control arm of chemotherapy alone.41,42 The primary end points were pCR and event-free survival (EFS), each assessed in the ITT population. pCR was defined as no residual cancer cells in the resected primary tumor and lymph nodes and was evaluated by a blinded independent pathology review committee. The study enrolled 358 patients with clinical stage IB (primary tumor ≥ 4 cm), II, or IIIA NSCLC (seventh edition staging). Patients were randomly assigned 1:1, stratified by stage, PD-L1 status, and histology. In the ITT population, nivolumab plus chemotherapy increased the pCR rate to 24% compared with 2% with chemotherapy (odds ratio [OR], 14; P < .0001). MPR (37% v 9%; OR, 5.7) was also increased with the addition of neoadjuvant nivolumab to chemotherapy. The pCR benefit was consistent across subgroups, including histology, stage, PD-L1 status, and tumor mutation burden.
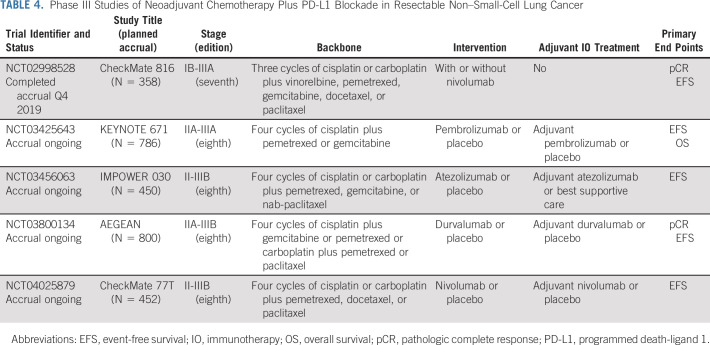
The results of CheckMate 816 are reassuring both in terms of toxicity and impact on surgery. Overall and grade 3 to 4 treatment-emergent adverse event rates were similar in both arms (nivolumab-chemotherapy 82% and 34%, respectively; chemotherapy alone 89% and 37%, respectively) and rates of immune-mediated toxicity were low in the nivolumab-chemotherapy arm with only two low-grade cases of pneumonitis. Patients who received nivolumab and chemotherapy also had higher rates of lung-sparing surgery. Complete resection rates were also higher in the combination arm and comparable with other neoadjuvant studies. Although follow-up for EFS is ongoing, the results for pCR in CheckMate 816 are encouraging, particularly given the absence of increased toxicity or delays to surgery. Several other phase III neoadjuvant chemotherapy plus PD-L1 or PD-1 blockade studies are ongoing (Table 4).
TABLE 4.
Phase III Studies of Neoadjuvant Chemotherapy Plus PD-L1 Blockade in Resectable Non–Small-Cell Lung Cancer
ANTICIPATING CHANGES TO THE STANDARDS OF CARE
The data presented above on adjuvant osimertinib, preoperative chemoimmunotherapy, and adjuvant immunotherapy are certain to change the standards of care in the management of early-stage lung cancer; however, each study has pending data. Critics of the ADAURA study await the OS data, although the study was not powered for OS as a primary end point. The CheckMate 816 study awaits EFS follow-up and what we anticipate being the first prospective data to show a correlation between pCR and EFS. Finally, we await the mature data on the benefit of adjuvant atezolizumab in lymph node–negative tumors 4-5 cm in size, the OS data, and analysis of benefit in the population with tumors that express PD-L1 in 1%-49% of cells.
Although there are pending data that may refine these new standards of care, ADAURA and IMpower010 define new standards of care and lead to an immediate need to move comprehensive biomarker testing earlier in the time line of the management of all patients with NSCLC. To appropriately manage our patients with lung cancer, process changes must be made to ensure timely biopsy and appropriate tissue stewardship to enable sufficient material remains to test for PD-L1 expression by immunohistochemistry and oncogene driver mutations by next-generation sequencing.
Unmet Needs
Platinum-doublet chemotherapy improves survival for otherwise healthy patients with resectable stage II or IIIA NSCLC, and a similar degree of benefit is seen whether it is given before or after surgical resection.7,43 Distinct potential benefits and challenges are associated with adjuvant and neoadjuvant approaches. More patients start and complete planned chemotherapy when given preoperatively and neoadjuvant therapy allows time for pulmonary prehabilitation. Additionally, with the new wave of neoadjuvant phase III trials now underway, pathologic response is emerging as a potential surrogate end point to assess benefit from neoadjuvant therapy.43-45 Conversely, the adjuvant approach avoids the potential for complications from neoadjuvant therapy, enables a longer duration of adjuvant systemic therapy, and allows time for postoperative recovery.46 Unfortunately, most of the ongoing phase III chemoimmunotherapy and TKI trials are studying perioperative therapy with combined agents preoperatively and adjuvant immunotherapy or TKI alone and will therefore be unable to definitively address questions about the absolute benefit of systemic therapy relative to its timing before or after complete surgical resection.
Additional questions remain about the duration of therapy. CheckMate 816 was a neoadjuvant-only study, whereas all the other ongoing adjuvant immunotherapy studies and perioperative studies include a year of adjuvant checkpoint inhibitor therapy. Is this year necessary? Is it too little or too much? Should this decision be based on pathologic responses? And where should we draw the cutoff for sufficient neoadjuvant response rates that would suggest adjuvant benefit? There are similar questions about the duration of adjuvant TKI therapy. On the basis of observed relapses after TKI discontinuation in earlier studies, are the 3 years of osimertinib enough or should patients continue in a maintenance setting indefinitely? Most studies have been designed with a relatively arbitrary duration of therapy, leaving many questions for future studies to address.
Additionally, we beg the question of cisplatin's role in the curative multimodal management of lung cancer. To date, all level 1 evidence for perioperative cytotoxic chemotherapy is cisplatin-based. However, the benefit of osimertinib was seen with or without chemotherapy.23 Similarly, the pCR rate with chemoimmunotherapy in CheckMate 816 was numerically higher when nivolumab was combined with carboplatin plus paclitaxel compared with cisplatin with pemetrexed or gemcitabine.41 When many of our patients cannot receive cisplatin-based therapy, the question remains if the ones fit for cisplatin should be exposed to its toxicities or is carboplatin-based therapy comparable. Finally, is there a better way to select patients for perioperative therapy than stage alone? This essential issue will be thoroughly covered in the article on circulating tumor (ct)DNA in this issue.
Trial Design
The following discussion puts into context the trials discussed above and proposes rationale for future trials on the basis of surrogate primary end points that require fewer patients and have the potential to bring drugs to the curative setting faster. To reach this goal, we discuss validation of the following: when can EFS/DFS be translated into OS, and when can a pathologic end point such as MPR or pCR be used as the primary end point in randomized phase III trials. Furthermore, when the biomarker is not binary, how do we discern which subset of patients truly benefits, such as ranges of PD-L1 expression?
SURROGATE END POINTS
After neoadjuvant therapy and surgery, clinical end points of EFS, DFS, and OS take years of clinical follow-up. Earlier trial readouts such as MPR (defined as ≤ 10% residual viable tumor in the resection specimen), pCR (defined as no residual viable tumor cells in the resection specimen), and nodal downstaging have been considered as potential surrogate end points. Nodal downstaging is of limited clinical utility as it is relevant only to patients with pathologically proven nodal metastases before treatment. Historically, pCR was likewise of limited applicability because of its rarity when using available systemic agents. This has been recently confirmed in CheckMate 816 where the pCR rate with standard-of-care chemotherapy was only 2%.41 MPR is seen at a more clinically relevant frequency29,47; however, many debate its reproducibility and studies of such are ongoing. Additional techniques such as artificial intelligence are being investigated as tools to reliably assess pathologic specimens after neoadjuvant therapy.48
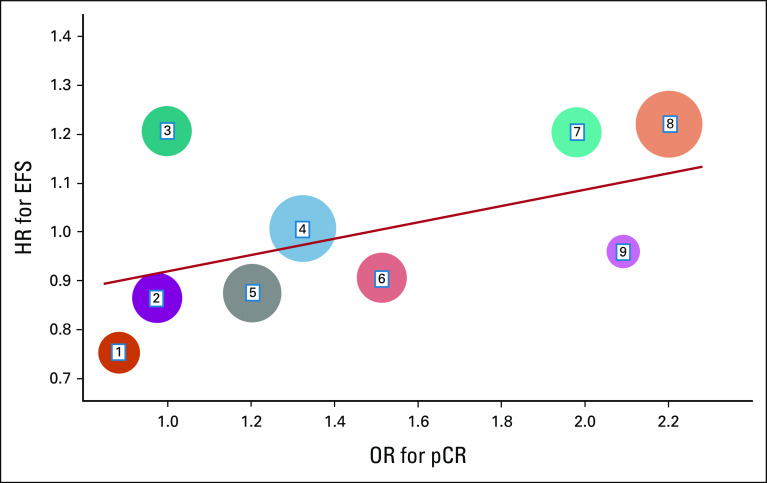
To prove a variable is a surrogate end point of the true end point, we need to confirm two associations: the individual-level association (I-association) and the treatment-level association (T-association).49 The I-association is the association between the surrogate end point and the true end point, which is independent of treatment effect. To validate the T-association, trial-level data are needed to correlate the treatment effect of the candidate surrogate end point (OR) with the same treatment effect of the true end point (HR). In Figure 1, we illustrate an example—the correlation between the OR for pCR and the HR for EFS across multiple studies. The size of each circle represents the corresponding sample size and a general linear model depicts the correlation between the OR for pCR and the HR for EFS. In this hypothetical example, the statistically significant result from the linear model association in Figure 1 could be used as evidence that pCR is a valid surrogate end point of EFS. To date, we have seen excellent trial-level associations of MPR and pCR with EFS in single-arm studies; it is the collated data from randomized studies we need to move the field forward, as has been done in platform studies in other diseases.50,51
FIG 1.
T-association between the OR for pCR and the HR for EFS. Each circle represents a study, and the size of each circle represents the corresponding sample size. EFS, event-free survival; HR, hazard ratio; OR, odds ratio; pCR, pathologic complete response.
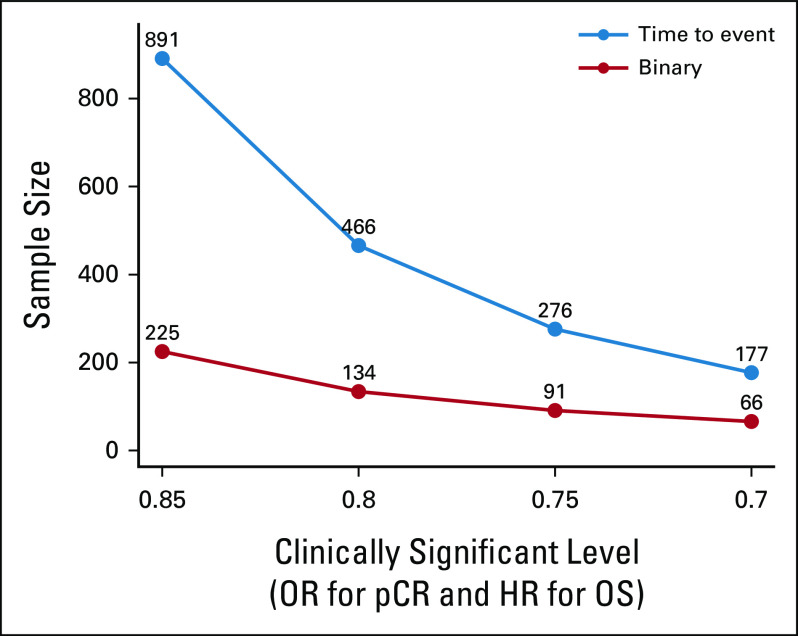
The benefits of using a surrogate end point in pivotal trials include the shorter study duration and smaller study sample size, as well as earlier trial readout. Consider the example of a neoadjuvant NSCLC study using pCR as the primary end point instead of EFS or OS. If one envisions a fixed time for enrollment and readout of pCR at the time of surgery, the study's primary end point would be available shortly after the last patient is resected, years before anticipated EFS data. In Figure 2, the drastic differences in sample size are presented when one considers differential target OR for pCR between intervention and control arms, using a standard phase III design. This decrease in sample size is essential for successful enrollment of studies in rare disease subsets.
FIG 2.
Sample size differences between a traditional time-to-event end point (PFS or OS; in blue) and a binary surrogate end point (PCR; in red) in a phase III study design with power of 80% and two-sided type I error 5%. HR, hazard ratio; OR, odds ratio; OS, overall survival; pCR, pathologic complete response; PFS, progression-free survival.
INTERPRETING BIOMARKERS
The major statistical challenges in analyzing immunotherapy trial time-to-event data include long survival curve tails and early crossover, which violate the proportional hazards assumption in the Cox model. The HR from the traditional Cox model is no longer an appropriate statistical measurement of the treatment effect; there are several statistical methods that have been developed to replace or correct the HR from the Cox model, including the cure model,52 restricted mean survival time model,53-55 and the recently developed Cox-TEL model.56 The reason that data contain long tails and early crossover is that the treatment benefit is mainly driven by the subset of true responders. Future trial designs should consider formally testing for the interaction effect between the biomarker subset and the treatment groups with adequate study power. Designs exist for four-arm biomarker-stratified design57 or modified three-arm biomarker-stratified design58 to confirm treatment effect in each subset. These designs are particularly relevant in the adjuvant setting where some patients are cured by surgery at the time of study enrollment and the duration of therapy is arbitrary.
In conclusion, for now, perioperative cisplatin-based adjuvant chemotherapy remains the standard of care for patients with resected stages II or III (eighth edition) NSCLC. Adjuvant immunotherapy is certain to become a standard of care, but the appropriate patient selection for this intervention requires more data. Adjuvant osimertinib provides a marked improvement in DFS for patients with resected EGFR-mutant NSCLC and should be offered to all patients with resected EGFR-mutant tumors stage IB (seventh edition) or greater. Preoperative chemoimmunotherapy induces pCR in a quarter of patients and appears to have the potential to convert marginally resectable tumors requiring pneumonectomy to lobectomy. These are major advances in the management of early-stage resectable NSCLC; however, these advances should not undermine ongoing efforts to bring targeted therapies to biomarker-matched populations of patients with early-stage disease or improve biomarker selection for immunotherapy. After all, the therapies and efforts that have already improved survival in advanced disease are also the most likely to improve the cure rates in early-stage resectable NSCLC when combined with surgery and chemotherapy. Finally, if we commit to the validation of surrogate pathologic end points, it will enable the opportunity for innovative trial designs to bring these effective systemic therapies to patients through smaller clinical trials with earlier trial readouts.
Jamie E. Chaft
Consulting or Advisory Role: Genentech/Roche, AstraZeneca/MedImmune, Merck, Bristol Myers Squibb, Flame Biosciences, Janssen Oncology, Guardant Health, Regeneron/Sanofi, Novartis
Research Funding: Genentech/Roche, Bristol Myers Squibb, AstraZeneca/MedImmune, Merck
Yu Shyr
Consulting or Advisory Role: Janssen Research & Development, Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, AstraZeneca
Patents, Royalties, Other Intellectual Property: Royalty for TNBCType for Insight Genetics. TNBCType is a Web-based subtyping tool for candidate TNBC samples using our gene expression metadata and classification method
Boris Sepesi
Consulting or Advisory Role: Bristol Myers Squibb/Medarex
Speakers' Bureau: AstraZeneca
Patrick M. Forde
Consulting or Advisory Role: AstraZeneca/MedImmune, Bristol Myers Squibb, Janssen, Daiichi Sankyo/UCB Japan¸ Amgen, ITeos Therapeutics, Mirati Therapeutics, Sanofi¸ Novartis
Research Funding: Bristol Myers Squibb, AstraZeneca/MedImmune, Kyowa Hakko Kirin, Novartis, Corvus Pharmaceuticals,
No other potential conflicts of interest were reported.
SUPPORT
Supported in part by NIH grants (P30 CA008748 to Memorial Sloan Kettering Cancer Center and P30 CA006973 to Johns Hopkins Kimmel Cancer Center).
AUTHOR CONTRIBUTIONS
Conception and design: Jamie E. Chaft, Yu Shyr, Patrick M. Forde
Collection and assembly of data: Jamie E. Chaft, Yu Shyr, Patrick M. Forde
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Preoperative and Postoperative Systemic Therapy for Operable Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jamie E. Chaft
Consulting or Advisory Role: Genentech/Roche, AstraZeneca/MedImmune, Merck, Bristol Myers Squibb, Flame Biosciences, Janssen Oncology, Guardant Health, Regeneron/Sanofi, Novartis
Research Funding: Genentech/Roche, Bristol Myers Squibb, AstraZeneca/MedImmune, Merck
Yu Shyr
Consulting or Advisory Role: Janssen Research & Development, Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, AstraZeneca
Patents, Royalties, Other Intellectual Property: Royalty for TNBCType for Insight Genetics. TNBCType is a Web-based subtyping tool for candidate TNBC samples using our gene expression metadata and classification method
Boris Sepesi
Consulting or Advisory Role: Bristol Myers Squibb/Medarex
Speakers' Bureau: AstraZeneca
Patrick M. Forde
Consulting or Advisory Role: AstraZeneca/MedImmune, Bristol Myers Squibb, Janssen, Daiichi Sankyo/UCB Japan¸ Amgen, ITeos Therapeutics, Mirati Therapeutics, Sanofi¸ Novartis
Research Funding: Bristol Myers Squibb, AstraZeneca/MedImmune, Kyowa Hakko Kirin, Novartis, Corvus Pharmaceuticals,
No other potential conflicts of interest were reported.
REFERENCES
- 1.Goldstraw P, Chansky K, Crowley J, et al. : The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 11:39-51, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, Tribodet H, Scagliotti GV, et al. : Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol 26:3552-3559, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Strauss GM, Herndon JE II, Maddaus MA, et al. : Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 26:5043-5051, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Non-Small Cell Lung Cancer, 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf [Google Scholar]
- 5.Butts CA, Ding K, Seymour L, et al. : Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: Updated survival analysis of JBR-10. J Clin Oncol 28:29-34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Detterbeck FC: The eighth edition TNM stage classification for lung cancer: What does it mean on main street? J Thorac Cardiovasc Surg 155:356-359, 2018 [DOI] [PubMed] [Google Scholar]
- 7.NSCLC Meta-analysis Collaborative Group : Preoperative chemotherapy for non-small-cell lung cancer: A systematic review and meta-analysis of individual participant data. Lancet 383:1561-1571, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vansteenkiste JF, Cho BC, Vanakesa T, et al. : Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17:822-835, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Wakelee HA, Dahlberg SE, Keller SM, et al. : Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): An open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 18:1610-1623, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novello S, Monica V, Serke M, et al. : PS01.04 International tailored chemotherapy adjuvant trial: ITACA trial. Final results. J Thorac Oncol 16:S58-S59, 2021 [Google Scholar]
- 11.Hess LM, Cui ZL, Li XI, et al. : Drug wastage and costs to the healthcare system in the care of patients with non-small cell lung cancer in the United States. J Med Econ 21:755-761, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Kreuter M, Vansteenkiste J, Fischer JR, et al. : Three-year follow-up of a randomized phase II trial on refinement of early-stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine (the TREAT study). J Thorac Oncol 11:85-93, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Kenmotsu H, Yamamoto N, Yamanaka T, et al. : Randomized phase III study of pemetrexed plus cisplatin versus vinorelbine plus cisplatin for completely resected stage II to IIIA nonsquamous non-small-cell lung cancer. J Clin Oncol 38:2187-2196, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Schmid-Bindert G, Engel-Riedel W, Reck M, et al. : A randomized phase 2 study of pemetrexed in combination with cisplatin or carboplatin as adjuvant chemotherapy in patients with completely resected stage IB or II non-small-cell lung cancer. Lung Cancer 90:397-404, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Kris MG, Gaspar LE, Chaft JE, et al. : Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario clinical practice guideline update. J Clin Oncol 35:2960-2974, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Howlader N, Forjaz G, Mooradian MJ, et al. : The effect of advances in lung-cancer treatment on population mortality. N Engl J Med 383:640-649, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janjigian YY, Park BJ, Zakowski MF, et al. : Impact on disease-free survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor EGFR mutations. J Thorac Oncol 6:569-575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pennell NA, Neal JW, Chaft JE, et al. : SELECT: A phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol 37:97-104, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue D, Xu S, Wang Q, et al. : Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): A randomised, open-label, phase 2 trial. Lancet Respir Med 6:863-873, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Zhong WZ, Wang Q, Mao WM, et al. : Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: Final overall survival analysis of CTONG1104 phase III trial. J Clin Oncol 39:713-722, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tada H, Mitsudomi T, Yamanaka T, et al. : Adjuvant gefitinib versus cisplatin/vinorelbine in Japanese patients with completely resected, EGFR-mutated, stage II-III non-small cell lung cancer (IMPACT, WJOG6410L): A randomized phase 3 trial. J Clin Oncol 39, 2021. (suppl; abstr 8501) [Google Scholar]
- 22.Govindan R, Mandrekar SJ, Gerber DE, et al. : ALCHEMIST trials: A golden opportunity to transform outcomes in early-stage non-small cell lung cancer. Clin Cancer Res 21:5439-5444, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu YL, Tsuboi M, He J, et al. : Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 383:1711-1723, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Rizvi NA, Rusch V, Pao W, et al. : Molecular characteristics predict clinical outcomes: Prospective trial correlating response to the EGFR tyrosine kinase inhibitor gefitinib with the presence of sensitizing mutations in the tyrosine binding domain of the EGFR gene. Clin Cancer Res 17:3500-3506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong WZ, Chen KN, Chen C, et al. : Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non-small-cell lung cancer (EMERGING-CTONG 1103): A randomized phase II study. J Clin Oncol 37:2235-2245, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Wu Y-L, Zhong W, Chen K-N, et al. : CTONG1103: Final overall survival analysis of the randomized phase 2 trial of erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non–small cell lung cancer. J Clin Oncol 39, 2021. (suppl; abstr 8502) [DOI] [PubMed] [Google Scholar]
- 27.Wakelee HA, Altorki NK, Zhou C, et al. : IMpower010: Primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). J Clin Oncol 39, 2021. (suppl; abstr 8500) [Google Scholar]
- 28.Forde PM, Chaft JE, Smith KN, et al. : Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 378:1976-1986, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellmann MD, Chaft JE, William WN Jr, et al. : Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: Proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 15:e42-e50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cascone T, William WN Jr, Weissferdt A, et al. : Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat Med 27:504-514, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carbone D, Lee J, Kris M, et al. : OA06.06 Clinical/biomarker data for neoadjuvant atezolizumab in resectable stage IB-IIIB NSCLC: Primary analysis in the LCMC3 study. J Thorac Oncol 16:S115-S116, 2021 [Google Scholar]
- 32.Ready N, Tong B, Clarke J, et al. : P2.04-89 Neoadjuvant pembrolizumab in early stage non-small cell lung cancer (NSCLC): Toxicity, efficacy, and surgical outcomes. J Thorac Oncol 14:S745, 2019 [Google Scholar]
- 33.Gao S, Li N, Gao S, et al. : Neoadjuvant PD-1 inhibitor (sintilimab) in NSCLC. J Thorac Oncol 15:816-826, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Besse B, Adam J, Cozic N, et al. : 1215O-SC Neoadjuvant atezolizumab (A) for resectable non-small cell lung cancer (NSCLC): Results from the phase II PRINCEPS trial. Ann Oncol 31:S794-S795, 2020 [Google Scholar]
- 35.Wislez M, Mazieres J, Lavole A, et al. : 1214O Neoadjuvant durvalumab in resectable non-small cell lung cancer (NSCLC): Preliminary results from a multicenter study (IFCT-1601 IONESCO). Ann Oncol 31:S794, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cascone T, William WN, Weissferdt A, et al. : Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. J Clin Oncol 37, 2019. (suppl; abstr 8504) [Google Scholar]
- 37.Rothschild S, Zippelius A, Eboulet EI, et al. : SAKK 16/14: Anti-PD-L1 antibody durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small cell lung cancer (NSCLC)—A multicenter single-arm phase II trial. J Clin Oncol 38, 2020. (suppl; abstr 9016) [DOI] [PubMed] [Google Scholar]
- 38.Altorki NK, McGraw TE, Borczuk AC, et al. : Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: A single-centre, randomised phase 2 trial. Lancet Oncol 22:824-835, 2021 [DOI] [PubMed] [Google Scholar]
- 39.Shu CA, Gainor JF, Awad MM, et al. : Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21:786-795, 2020 [DOI] [PubMed] [Google Scholar]
- 40.Provencio M, Nadal E, Insa A, et al. : Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21:1413-1422, 2020 [DOI] [PubMed] [Google Scholar]
- 41.Forde PM, Spicer J, Lu S, et al. : Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as neoadjuvant treatment (tx) for resectable (IB-IIIA) non-small cell lung cancer (NSCLC) in the phase 3 CheckMate 816 trial. Cancer Res 81, 2021. (abstr CT003) [Google Scholar]
- 42.Spicer J, Wang C, Tanaka F, et al. : Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J Clin Oncol 39, 2021. (suppl; abstr 8503) [Google Scholar]
- 43.Felip E, Rosell R, Maestre JA, et al. : Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 28:3138-3145, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Travis WD, Dacic S, Wistuba I, et al. : IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol 15:709-740, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tenconi S, Mainini C, Rapicetta C, et al. : Rehabilitation for lung cancer patients undergoing surgery: Results of the PUREAIR randomized trial. Eur J Phys Rehabil Med 57:1002-1011, 2021 [DOI] [PubMed] [Google Scholar]
- 46.Rusch VW, Chaft J, Hellmann M: KEYNOTE-024: Unlocking a pathway to lung cancer cure? J Thorac Cardiovasc Surg 155:1777-1780, 2018 [DOI] [PubMed] [Google Scholar]
- 47.Pataer A, Kalhor N, Correa AM, et al. : Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 7:825-832, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dacic S, Travis WD, Giltnane JM, et al. : Artificial intelligence (AI)–powered pathologic response (PathR) assessment of resection specimens after neoadjuvant atezolizumab in patients with non-small cell lung cancer: Results from the LCMC3 study. J Clin Oncol 39, 2021. (suppl; abstr 106) [DOI] [PubMed] [Google Scholar]
- 49.Shyr Y, Shyr D: What constitutes a valid surrogate end point in cancer clinical trials? JAMA Oncol 6:1334-1335, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker CC, James ND, Brawley CD, et al. : Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 392:2353-2366, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yee D, DeMichele AM, Yau C, et al. : Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: Three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol 6:1355-1362, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farewell VT: The use of mixture models for the analysis of survival data with long-term survivors. Biometrics 38:1041-1046, 1982 [PubMed] [Google Scholar]
- 53.Royston P, Parmar MK: Restricted mean survival time: An alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 13:152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uno H, Claggett B, Tian L, et al. : Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol 32:2380-2385, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim DH, Uno H, Wei LJ: Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol 2:1179-1180, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu CY, Lin EP, Shyr Y: Development and evaluation of a method to correct misinterpretation of clinical trial results with long-term survival. JAMA Oncol 7:1041-1044, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Zhou J, Wang T, et al. : On enrichment strategies for biomarker stratified clinical trials. J Biopharm Stat 28:292-308, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kris MG, Johnson BE, Berry LD, et al. : Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 311:1998-2006, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]