Abstract
Synthetic cathinones, known as “bath salts” on the illicit drug market, pose a significant public health concern. 3,4-Methylenedioxypyrovalerone (MDPV), one of several popular constituents of illicit bath salts, produces similar pharmacological actions to cocaine, albeit with greater potency and efficacy. The present study sought to characterize behavioral and neurochemical effects of repeated exposure to MDPV alone and in combination with cocaine. Male Sprague-Dawley rats were randomly assigned to one the following four treatments, administered once daily for seven days: 1 mg/kg MDPV, 5 mg/kg cocaine, 1 mg/kg MDPV + 5 mg/kg cocaine, or saline. Locomotor activity was assessed for one hour immediately before and one hour immediately after injections on days 1 and 6. Brains were harvested 20 minutes after the final injection on day 7 and brain tissue punches were obtained to determine monoamine content within the anterior striatum, medial prefrontal cortex, and nucleus accumbens using High-Performance Liquid Chromatography (HPLC). Drug-induced increases in horizontal activity were significantly greater on treatment day 6 compared to treatment day 1 in all three drug treatment groups in comparison to the saline control group. MDPV produced significantly higher increases in activity compared to either saline or cocaine, although concurrent treatment with MDPV and cocaine produced sub-additive effects. Neurochemical analyses provided no evidence of alterations in total monoamine content following repeated administration of MDPV, cocaine, or the MDPV+COC mixture. Further investigations targeting possible changes in DA receptor sensitivity following repeated exposure to MDPV may help elucidate the mechanistic changes responsible for MDPV-induced behavioral sensitization.
Keywords: MDPV, cocaine, locomotor activity, behavioral sensitization, monoamines, dopamine, serotonin, medial prefrontal cortex, striatum, nucleus accumbens, rats
1. Introduction
Synthetic cathinones, casually known as “bath salts” on the illicit drug market, pose significant health and public safety risks. Methylenedioxypyrovalerone (MDPV) is one of several commonly abused synthetic cathinones (German et al., 2014). Users report MDPV’s effects to be similar to the classic psychostimulant cocaine (Spiller et al., 2011) and may supplement cocaine use with MDPV, leading to fatal overdoses (Murray et al., 2012).
Several recent preclinical studies have characterized the neurochemical and behavioral effects of MDPV. It is a potent reinforcer in self-administration assays (Aarde et al., 2013; Watterson et al., 2014; Schindler et al., 2016) and it produces conditioned place preference at a range of doses (1.0 – 3.2 mg/kg) (King et al., 2015) in rats and in mice (Karlsson et al., 2014). Analysis of extracellular dopamine content in the NAc of rats administered cocaine or MDPV revealed that MDPV is approximately 10-fold more potent than cocaine (Baumann et al., 2013) with longer lasting effects on DAT (Cameron et al., 2013). MDPV also produces weak inhibition at SERT and the norepinephrine transporter (NET) (Glennon and Young, 2016) in a manner similar to cocaine (Sora et al., 2001).
Enhanced locomotor stimulant effects have been established with MDPV (0.5 mg/kg) administered repeatedly for seven days (Berquist II et al., 2016) and with 1.0 mg/kg when administered in five, 48-hour intervals (Watterson et al., 2016). Locomotor sensitization reflects enhancements in the stimulus effects produced by repeated exposure to psychostimulants (Robinson and Berridge., 1993) and may contribute to stronger learned associations between environmental cues and drug injections (Pierce and Kalivas, 1997). These enhancements are mediated by dopaminergic neurons in the NAc and striatum (Henry et al., 1998) and glutamate receptor trafficking in the NAc (Boudreau and Wolf, 2005).
The aim of the current study was to extend the characterization of enhanced locomotor responses and the neurochemical effects of MDPV, when administered concurrently with a low dose of cocaine. No current research is available describing the potential additive effects of these two psychostimulant drugs on monoamine release or locomotor sensitization. Additionally, no research has been published describing the neurochemical effects of MDPV after repeated exposure and data describing enhancements to its locomotor effects is limited by dose (Berquist II et al., 2016) or protocol (Watterson et al., 2016). In the study by Berquist et al., (2016), rats injected daily with 0.5 mg/kg MDPV displayed significantly elevated locomotor responses when activity was compared between days 1 and 7. Watterson et al., (2016) reported a lack of enhanced increases in locomotor activity following repeated daily treatments with 1.0 mg/kg MDPV for five days, but did report enhanced locomotor activation when similar treatments were spaced apart by 48 hours. Additionally, responses were measured on a circular apparatus with quarter-turns as the independent variable in the study by Watterson et al. (2016).
Repeated administration of cocaine has been shown to produce robust locomotor sensitization at doses of 15 and 30 mg/kg (Kalivas and Duffy., 1993), diminished increases in medial prefrontal cortex (mPFC) DA after repeated, systemic injections (Sorg et al., 1997), and increases in NAc DA (Weiss et al., 1992). However, few studies have investigated the behavioral effects of repeated exposure to lower cocaine doses. Rats administered 5.0 mg/kg of cocaine display significant place preference (Gong et al., 1997) and develop sensitized locomotor responses after repeated administration (Drouin et al., 2002). In consideration of the likelihood that MDPV and cocaine are commonly abused concurrently, the aim of this preclinical study was to determine the behavioral and neurochemical effects of repeated concurrent exposure to MDPV (1.0 mg/kg) and cocaine (5.0 mg/kg).
2. Methods
2.1. Subjects:
Thirty-six male Sprague-Dawley rats (Charles River) weighing between 250g–300g were pair-housed in a temperature (20 °C) and humidity (50%) controlled vivarium maintained on a 12:12 light/dark cycle. Food and water were provided ad libitum in polycarbonate cages consisting of corncob bedding (Harlan Teklad, Conrad, Iowa). Experimantal procedures were conducted during the light phase of the light/dark cycle. All procedures were reviewed and approved by the Western Michigan University Institutional Animal Care and Use Committee and were in accordance with the Guide for the Care and use of Laboratory Animals (National Research Council, 2010).
2.2. Apparatus:
Six custom built acrylic chambers (40.5 × 40.5 × 40.5 cm) were each housed within an Accuscan automated activity monitoring system equipped with infrared emitters and detectors and connected to a microprocessor with associated Versamax® software to analyze beam breaks (Accuscan Instruments, Inc., Columbus, OH, USA).
2.3. Drugs:
Methylenedioxypyrovalerone-hydrochloride (MDPV) and cocaine-hydrochloride (COC) were provided by the National Institute on Drug Abuse Drug Control Supply Program (Bethesda, Maryland). Drugs were dissolved in 0.9% bacteriostatic sodium chloride and administered via intraperitoneal injection at 1 ml/kg volume. The MDPV + COC mixture was dissolved in the same solution. Drug doses were calculated based on the weights of the salts.
2.4. Locomotor Screening Procedures:
Rats were randomly assigned to receive one of the following treatments once per day for seven consecutive days: saline, 1 mg/kg MDPV, 5 mg/kg COC, 1 mg/kg MDPV + 5 mg/kg COC. Injections were administered over the seven day period between 10:00 a.m. and 4:00 p.m. Injection time was consistent each day for individual animals and varied among the six cohorts (see below). On treatment days 1 and 6, rats were habituated to the behavioral test apparatus for 60 min, injected and then placed immediately back into the test apparatus. Activity was monitored during the 60 min habituation period and during the 60 min post-injection period. White noise was generated at 70 dB to mask extraneous environmental noise. Animals were tested in six cohorts of six animals, with at least three treatment groups represented in each cohort. Test chambers were cleaned with a 35% isopropyl alcohol between cohorts. On treatment days 2 through 5, rats were injected and immediately placed back in their home cages.
2.5. Tissue Processing and Neurochemical Analysis:
On day 7, brains were harvested immediately following rapid decapitation 20 minutes after injections. Brains were sliced in 2 mm sections in a chilled stainless steel rat brain matrix and slices were frozen on glass slides mounted on dry ice. Using a 1.5 mm biopsy punch, bilateral tissue punches were obtained from the medial prefrontal cortex, anterior striatum, and nucleus accumbens (see figure 1) in accordance with the Paxinos and Watson Rat Brain Atlas (Paxinos and Watson, 2007). Tissue punches were placed into microcentrifuge tubes and frozen and for later neurochemical analysis.
Figure 1.
Dissection Locations. Paxinos and Watson (2007) images of the brain regions in which punches were obtained.
Prior to analysis, 100 uL of 0.2 N HCLO4 was added to each sample vial and tissues were sonically disrupted and spun at 12,045 × g for 10 minutes in a centrifuge maintained at 4°C. A 50 uL aliquot of supernatant was removed from each sample and monoamine analysis was performed on a Dionex Ultimate 3000 HPLC system (Thermoscientific, Waltham, MA), equipped with an autosampler maintained at 4°C, a 100 uL sample loop, and a C18-RP (2uL diameter) column maintained at 25°C. TEST Mobile Phase (Thermoscientific, Waltham, MA) containing acetonitrile, phosphate buffer, and an ion-pairing reagent was utilized and coulometric electrochemical detection was achieved with a dual electrode cell set at −175 mV (reference) and 300 mV (working). Monoamine levels (i.e., dopamine, serotonin, and their metabolites) were expressed as ng neurochemical / mg tissue weight.
2.6. Data Analysis:
Horizontal beam breaks were parsed into 5-minute intervals and treatment group means (±S.E.M) were plotted for graphic analysis to compare activity levels among the four treatment groups during the 60 min habituation period and the 60 min post-injection period on day 1 and day 6. Total horizontal beam breaks were calculated for each 60 min post-injection period and treatment group means (±S.E.M) were plotted for day 1 and day 6. These post-injection sums were statistically analyzed using a mixed model two-factor (treatment group × test day) analysis of variance (ANOVA). When a statistically significant interaction was present, simple main effects were computed to determine significant individual group differences between days 1 and 6 using Holm-Sidak adjustments. Two separate one-factor ANOVAs were also computed to determine differences among treatment groups on day 1 or on day 6. In the case of a significant treatment effect, Bonferroni multiple comparisons were performed. Total monoamine concentrations (ng/mg) for DA, 5-HT, and NE were plotted as treatment group means (±S.E.M). These data were analyzed using separate one-way ANOVAs for each brain region of interest (STR, NAc, mPFC) and each analyte. GraphPad Prism Version 7.0 software (La Jolla, CA, USA) was used for all statistical analysis and graphical representation of the data.
3. Results
3.1. Locmotor Activity Assessment:
All three drug-treated groups exhibited higher peak activity and higher total activity on day 6 compared to day 1, whereas saline-treated animals displayed similar levels of activity on day 1 and day 6. Figure 2 displays horizontal activity counts parsed into 5 min intervals during the one hour habituation period and the subsequent one hour post-injection period. On day 1, MDPV produced peak activity levels within 10 min that were nearly five times the increase produced by saline injections and two times the increase produced by cocaine injections. Interestingly, the time to peak activity was delayed and the increase in activity was prolonged by concurrent treatment with MDPV and COC on day 1, compared to the effects of each drug administered alone. However, after daily exposure to this mixture for six days, peak increases in horizontal activity occurred within 10 min and the time course of activity mirrored that of MDPV or COC administered alone.
Figure 2.
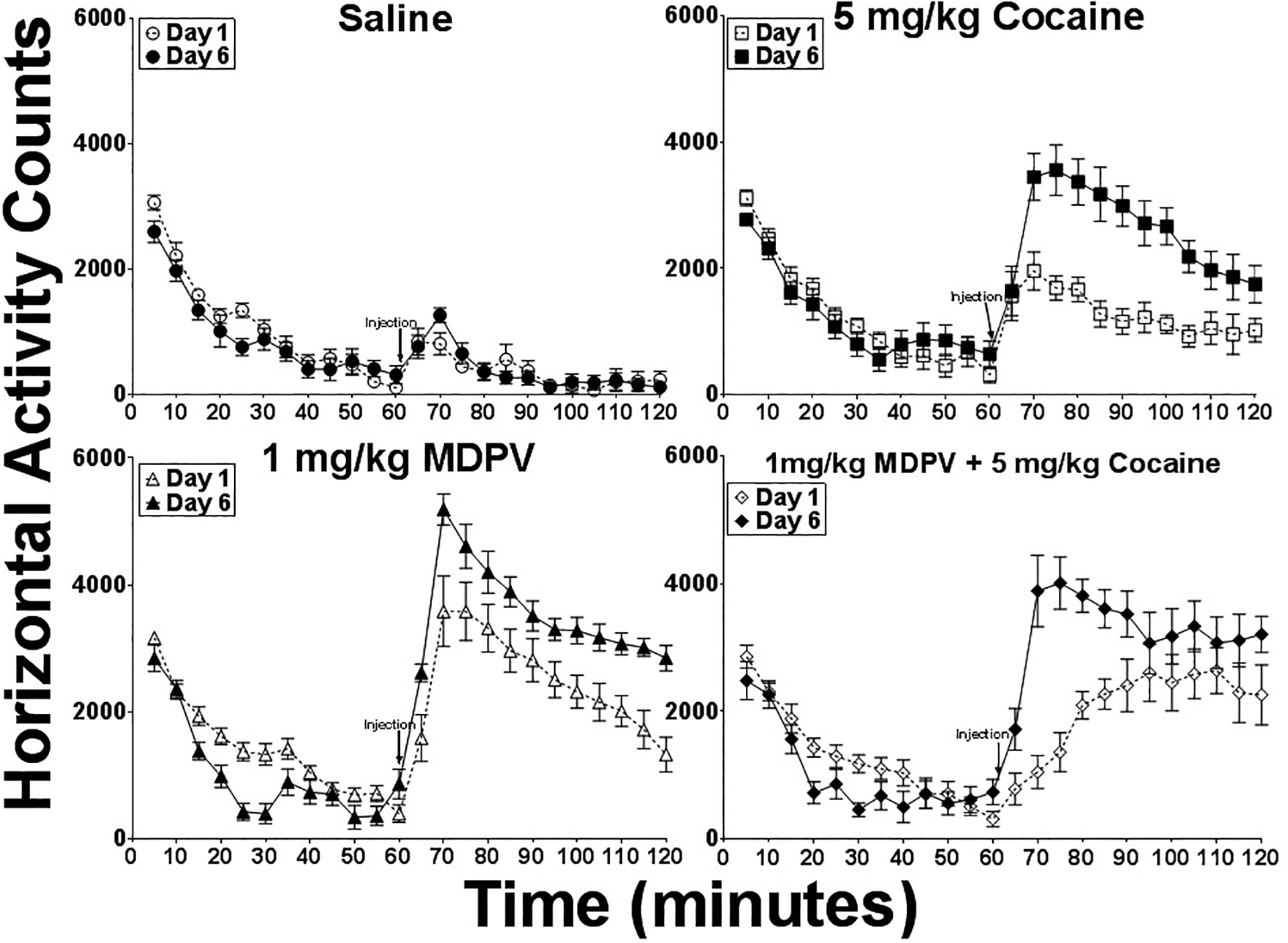
Locomotor Activity Over Time. Horizontal activity counts for one hour immediately before and one hour immediately after injections on day 1 and day 6. Injections were administered at 60 min. Each data point represents group means (+/− SEM) at each 5-min interval [n=8–9].
Figure 3 depicts the total horizontal activity counts during the 60 min post-injection period, expressed as averages (± S.E.M.) for each treatment group on day 1 and day 6. Results from the two-factor repeated measures ANOVA revealed significant main effects of treatment (F [3, 30] = 52.88, P< .001) and test day (F [1,30] = 30.21, P<.001). A significant interaction (treatment × test day) (F [3,30] = 3.2, P<.05) was obtained and simple main effects were computed for each treatment condition. Analysis of simple effects of treatment group by test day revealed significant increases in locomotor activity between day 1 and day 6 in MDPV 1 mg/kg (F = 11.15, μ1 − μ6 = −12796), MDPV 1 mg/kg + COC 5 mg/kg (F= 14.75, μ1 − μ6 = −14715), and COC 5 mg/kg (F = 14.96, μ1 − μ6 = −15715). Percent change values were calculated to determine the magnitude of the change in locomotor response (MDPV 1.0 mg/kg = 43%, MDPV 1.0 mg/kg + COC 5.0 mg/kg = 59%, COC 5.0 mg/kg = 100%) between day 1 and 6. A one-factor ANOVA on day 1 activity confirmed a significant effect of treatment (F [3,29] = 15.37, P< .001). Bonferroni multiple comparisons indicated significant differences in activity between MDPV 1.0 mg/kg and COC 5 mg/kg (μ1 − μ2 = 14344, P<.01), between MDPV 1.0 mg/kg and Saline (μ1 − μ2 = 25403, P<.0001), and between MDPV 1.0 mg/kg + COC 5 mg/kg and Saline (μ1 − μ6 = 20271, P<.001). A similar analysis comparing activity among treatment groups on day 6 revealed a significant effect of treatment among groups (F [3,30] = 36.68, P<.0001). Multiple comparison tests found significant differences in day 6 activity among the following groups: MDPV 1.0 mg/kg vs. COC 5 mg/kg (μ1 − μ2 = 11425, P<.05), MDPV 1.0 mg/kg vs. Saline (μ1 − μ2 = 38132, P<.0001), MDPV 1.0 mg/kg + COC 5 mg/kg vs. Saline (μ1 − μ6 = 34920, P<.0001), and COC 5 mg/kg vs. Saline (μ1 − μ6 = 26707, P<.0001).
Figure 3.
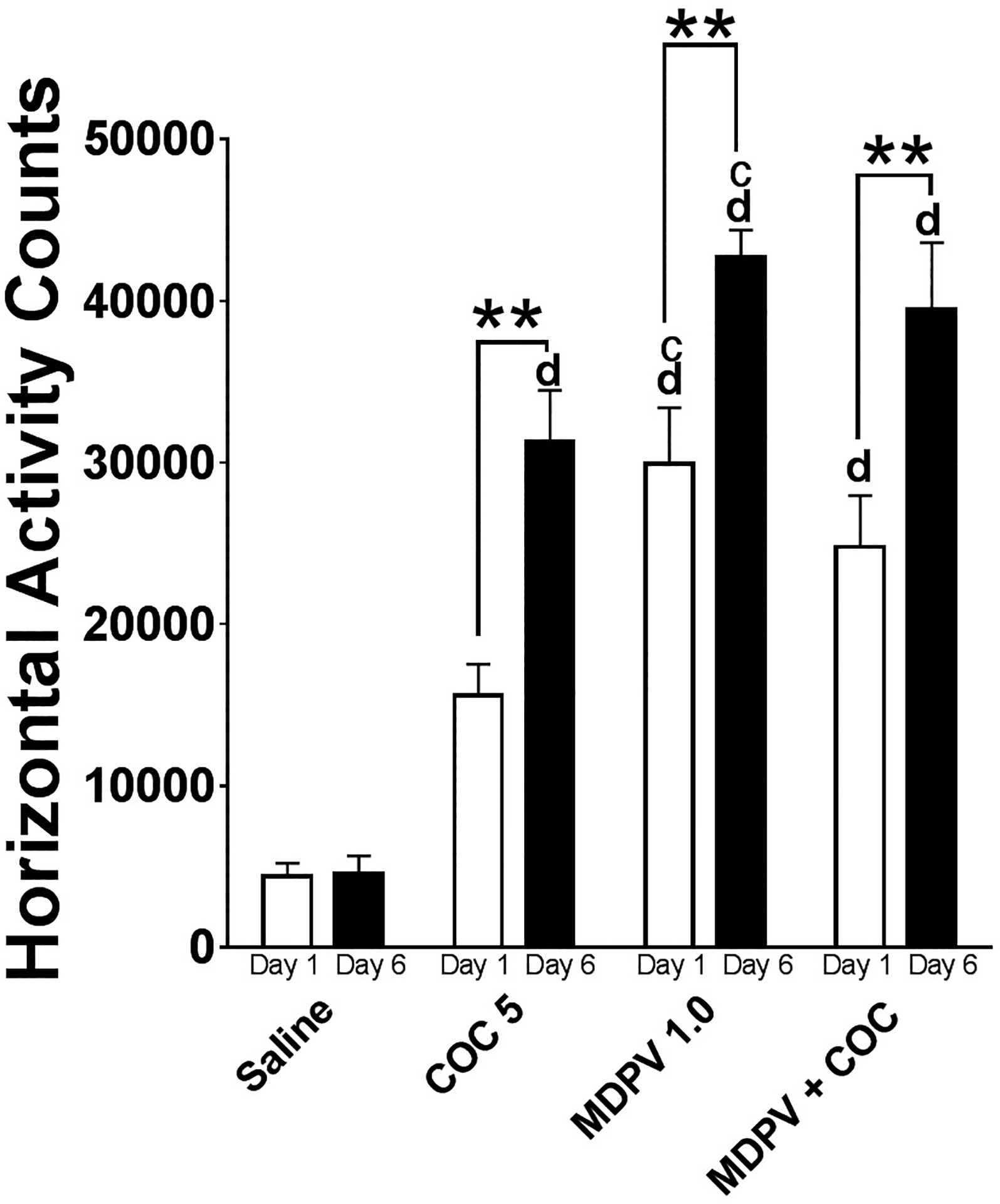
Total Locomotor Activity. Total horizontal activity counts for the one hour post-injection interval (61–120 min) for each treatment group on Day 1 and Day 6. Bars represent a treatment group means (± SEM). Asterisks (*) represent significant differences within groups (Day 1 vs. Day 6). Letters indicate statistically significant differences from COC 5 (c) or Saline (d).
3.2. Monoamine Quantification:
Figure 4 displays average monoamine concentrations across treatments in the STR, NAc, and mPFC. Nine separate one-factor ANOVAs were computed on each brain region of interest to compare monoamine concentrations (DA, 5HT, and NE) among treatment conditions. Results from these analyses revealed no significant effects of drug treatment on monoamine levels in any of the brain regions analyzed.
Figure 4.
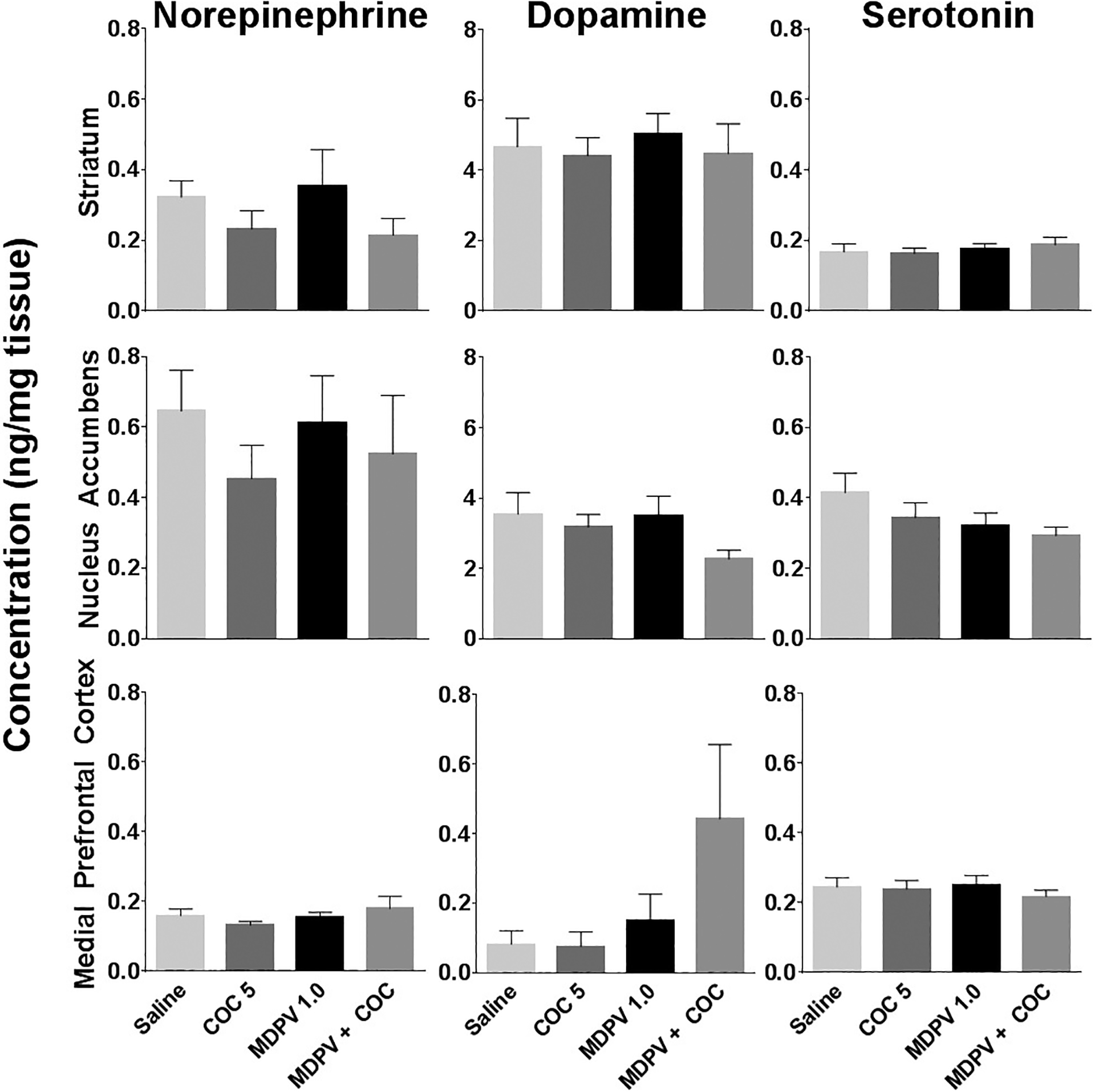
High-Pressured Liquid Chromatography Results. Monoamine concentrations in the striatum, nucleus accumbens, and medial prefrontal cortex tissues in rats euthanized 20 min after the last of seven daily injections of saline, 5 mg/kg cocaine, 1 mg/kg MDPV, or 1 mg/kg MDPV + 5 mg/kg cocaine. Tissue concentrations are expressed as ng/mg wet tissue weight.
4. Discussion
Current literature is limited regarding the effects of repeated MDPV administration on locomotor activity. Results of the present study extend previous findings (Berquist et al., 2016) that a low dose of MDPV produces enhanced locomotor responses after repeated dosing. Furthermore, this effect was demonstrated in animals receiving concurrent treatment with MDPV and cocaine. This is not surprising given that both cocaine and MDPV produced significant increases in activity when administered alone. Although MDPV produced the highest increases in overall activity on both test days, the percentage increase from day 1 to day 6 was greater in the cocaine-treated animals (100%) and the MDPV + COC-treated animals (59%) compared to those treated with MDPV alone (43%). The lower percentage increase by MDPV alone is apparently due to the higher activity levels observed on day 1 in this treatment group.
The lack of enhanced locomotor stimulation by the MDPV+COC mixture compared to MDPV alone was an unexpected finding, and suggests cocaine may actually attenuate the acute effects of MDPV. Of particular interest, the pattern of activity changes following injections varied among treatment groups. MDPV+COC produced lower peak activity, but more sustained increases in activity compared to MDPV alone. If activity was monitored for a longer post-injection time period, the MDPV+COC mixture may actually produce higher levels of total activity due to a more sustained increase in activity. Additional studies are required to evaluate this possibility.
A mechanistic explanation for the current behavioral findings remains to be determined. The possibility that cocaine interferes with MDPV transport into the brain and/or competes with MDPV for DAT binding sites could be investigated. Previous literature indicates MDPV as a more potent blocker than cocaine with longer lasting effects (Baumann et al., 2013), but no research has described the effects at DAT when both drugs are administered concurrently.
No significant differences were obtained from measurements of whole tissue monoamine levels in the NAc, STR, or mPFC of animals administered MDPV, cocaine, or the mixture. Repeated, binge exposure to cocaine (20 mg/kg 3x daily and 10 mg/kg 2x daily) has been shown to decrease DA levels in the NAc as long as 14 days after a final injection (Puig et al., 2012; Imperato et al., 1992). However, research also suggests DA levels in the NAc increase after repeated administration to cocaine (Weiss et al., 1992). These cited studies utilized more precise methodologies to measure dynamic changes in DA through microdialysis procedures, whereas the present study determined static, whole tissue concentrations. With this, it is not surprising that the dose administered in the present study, almost four times as low as reported in the cited literature, did not produce significant alterations in whole tissue DA levels when administered once daily for seven days. Lack of evidence for altered DA levels, despite clear evidence for the induction of locomotor sensitization following repeated exposure to low doses of cocaine and MDPV, begs the question of dopamine’s involvement in this process. Research suggests glutamatergic involvement in the induction of behavioral sensitization to cocaine (Boudreau and Wolf., 2005) through the increased trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) in the NAc. Perhaps AMPA receptor trafficking is a potential mechanism for the induction of sensitization by MDPV. Further quantification of this trafficking, and glutamate expression, in rats administered low doses of MDPV and/or cocaine is necessary to test this hypothesis.
As described previously, MDPV acts in a similar manner to cocaine through its effects on DAT. These similarities at DAT may lead to alterations in DA that are akin to decreases seen after repeated cocaine exposure and may help describe the null results presented herein. No current research details DA concentrations in whole tissue following repeated administration of MDPV. After single injections (Schindler et al., 2016 & Baumann et., 2013), MDPV has been shown to increase extracellular DA. However, these studies may have demonstrated decreases in DA, or conflicting results, had the injections been given repeatedly or if monoamines were analyzed in whole tissue. Interestingly, although highly speculative given non-significant effects, average levels of DA in the NAc of animals given both MDPV and cocaine show close to a 1 ng/mg decrease when compared to the other treatment groups. Given the variability of whole tissue monoamine analyses, more statistical power through the inclusion of more animals may be necessary to obtain significant drug effects between groups. If the hypothesis is supported that cocaine and MDPV produce decreases in DA following repeated exposure, both drugs given concurrently may display additive decreases in DA concentrations in brain areas associated with reward processing. It is important to note, however, that the studies reporting decreases in extracellular DA administered cocaine more than once daily and provided a withdrawal period after the last injection (Puig et al., 2012; Imperato et al., 1992)
MDPV demonstrates binding affinity for NE and 5-HT transporters (Baumann et al., 2013) but does not produce significant elevations in extracellular 5-HT concentrations following low dose infusions (Schindler et al., 2016). The results of the present study extend these findings by providing evidence for uninterrupted 5-HT concentrations after 7 days of repeated exposure in brain areas of reward processing and provide new evidence for uninterrupted changes in the mPFC. Further research is necessary to characterize the effects of MDPV on reward-related pathways and the impact of repeated exposure to the drug. Previous findings suggest repeated cocaine treatment (15 mg/kg, once per day for 5 days) attenuates mPFC DA increases after a systemic challenge (Sorg et al., 1997). MDPV may produce similar results in assays more sensitive to extracellular expression, given the similarities between cocaine and MDPV’s effects on DAT. Future endeavors should examine a wider range of doses of MDPV and cocaine, when given concurrently, to better characterize neurochemical alterations at doses that may be rewarding or neurotoxic.
4.1. Conclusion.
The current results suggest a low dose of MDPV produces robust increases in locomotor activity after repeated exposure that are matched when given in combination with low doses of cocaine. Furthermore, neither MDPV nor the MDPV + COC mixture produced significant alterations in monoamine systems related to reward and reward processing. Finally, this study extends the literature regarding behavioral and neurochemical effects of polysubstance abuse involving synthetic cathinones. Additional research is necessary to better characterize the behavioral and neurochemical effects of MDPV when given in combination with other common drugs of abuse.
Acknowledgements
This research was supported by a grant from the National Institutes of Health (R15DA038295). The National Institute on Drug Abuse drug control supply program provided several of the test compounds used in this study.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, & Taffe MA (2013). The novel recreational drug 3, 4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology, 71, 130–140. Chicago: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, … & Brandt SD (2013). Powerful cocaine-like actions of 3, 4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology, 38(4), 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquist MD, Traxler HK, Mahler AM, & Baker LE (2016). Sensitization to the locomotor stimulant effects of “bath salt” constituents, 4‐methylmethcathinone (4-MMC) and 3, 4-methylenedioxypyrovalerone (MDPV), in male Sprague-Dawley rats. Drug and alcohol dependence, 164, 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, & Wolf ME (2005). Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. Journal of Neuroscience, 25(40), 9144–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin C, Blanc G, Villégier AS, Glowinski J, & Tassin JP (2002). Critical role of α1‐adrenergic receptors in acute and sensitized locomotor effects of D‐amphetamine, cocaine, and GBR 12783: Influence of preexposure conditions and pharmacological characteristics. Synapse, 43(1), 51–61. [DOI] [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, & Hanson GR (2014). Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life sciences, 97(1), 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, & Young R (2016). Neurobiology of 3, 4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinovalerophenone (α-PVP). Brain research bulletin, 126, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Neill D, & Justice JB (1997). 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain research, 754(1), 103–112. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Hu XT, & White FJ (1998). Adaptations in the mesoaccumbens dopamine system resulting from repeated administration of dopamine D1 and D2 receptor-selective agonists: relevance to cocaine sensitization. Psychopharmacology, 140(2), 233–242. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mele A, Scrocco MG, & Puglisi-Allegra S (1992). Chronic cocaine alters limbic extracellular dopamine. Neurochemical basis for addiction. European journal of pharmacology, 212(2–3), 299–300. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, & Duffy P (1993). Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. Journal of Neuroscience, 13(1), 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, & Kugelberg FC (2014). Mephedrone, methylone and 3, 4‐methylenedioxypyrovalerone (MDPV) induce conditioned place preference in mice. Basic & clinical pharmacology & toxicology, 115(5), 411–416. [DOI] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, & Beuhler MC (2012). Death following recreational use of designer drug “bath salts” containing 3, 4-methylenedioxypyrovalerone (MDPV). Journal of Medical Toxicology, 8(1), 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos GWC 2007. The rat brain in stereotaxic coordinates. Burlington MA Elsevier Inc. [Google Scholar]
- Pierce RC, & Kalivas PW (1997). A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain research reviews, 25(2), 192–216. [DOI] [PubMed] [Google Scholar]
- Puig S, Noble F, & Benturquia N (2012). Short-and long-lasting behavioral and neurochemical adaptations: relationship with patterns of cocaine administration and expectation of drug effects in rats. Translational psychiatry, 2(10), e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain research reviews, 18(3), 247–291. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, & Baumann MH (2016). Reinforcing and neurochemical effects of the “bath salts” constituents 3, 4-methylenedioxypyrovalerone (MDPV) and 3, 4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology, 233(10), 1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, … & Uhl GR (2001). Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proceedings of the National Academy of Sciences, 98(9), 5300–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Davidson DL, Kalivas PW, & Prasad BM (1997). Repeated daily cocaine alters subsequent cocaine-induced increase of extracellular dopamine in the medial prefrontal cortex. Journal of Pharmacology and Experimental Therapeutics, 281(1), 54–61. [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, & Jansen J (2011). Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clinical toxicology, 49(6), 499–505. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, & Olive MF (2014). Potent rewarding and reinforcing effects of the synthetic cathinone 3, 4‐methylenedioxypyrovalerone (MDPV). Addiction biology, 19(2), 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Taylor SB, Nemirovsky NE, & Olive MF (2016). Sensitization to the motor stimulant effects of 3, 4-methylenedioxypyrovalerone (MDPV) and cross-sensitization to methamphetamine in rats. Journal of drug and alcohol research, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Paulus MP, Lorang MT, & Koob GF (1992). Increases in extracellular dopamine in the nucleus accumbens by cocaine are inversely related to basal levels: effects of acute and repeated administration. Journal of Neuroscience, 12(11), 4372–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]


