Abstract
BACKGROUND AND OBJECTIVES:
Early obesity treatment seems to be the most effective, but few treatments exist. In this study we examine the effectiveness of a parent-only treatment program with and without booster sessions (Booster or No Booster) focusing on parenting practices and standard treatment (ST).
METHODS:
Families of children 4 to 6 years of age with obesity were recruited from 68 child care centers in Stockholm County and randomly assigned to a parent-only program (10 weeks) with or without boosters (9 months) or to ST. Treatment effects on primary outcomes (BMI z score) and secondary outcomes (BMI and waist circumference) during a 12-month period were examined with linear mixed models. The influence of sociodemographic factors was examined by 3-way interactions. The clinically significant change in BMI z score (−0.5) was assessed with risk ratios.
RESULTS:
A total of 174 children (mean age: 5.3 years [SD = 0.8]; BMI z score: 3.0 [SD = 0.6], 56% girls) and their parents (60% foreign background; 39% university degree) were included in the analysis (Booster, n = 44; No Booster, n = 43; ST, n = 87). After 12 months, children in the parent-only treatment had a greater reduction in their BMI z score (0.30; 95% confidence interval [CI]: −0.45 to −0.15) compared with ST (0.07; 95% CI: −0.19 to 0.05). Comparing all 3 groups, improvements in weight status were only seen for the Booster group (−0.54; 95% CI: −0.77 to −0.30). The Booster group was 4.8 times (95% CI: 2.4 to 9.6) more likely to reach a clinically significant reduction of ≥0.5 of the BMI z score compared with ST.
CONCLUSION
A parent-only treatment with boosters outperformed standard care for obesity in preschoolers.
The prevalence of obesity in preschoolers is still on the rise.1 Although treatment outcomes seem particularly promising when started early,2-5 the effectiveness of interventions as early as preschool age (up to 6 years of age) is not well known.6 The Cochrane Institute included 7 randomized controlled trials (RCTs) to evaluate treatment of preschoolers with obesity.7 However, different outcome measures, sample size, and attrition make conclusions for effective interventions difficult. Given the significant role of parents in young children’s lives, we created a treatment program grounded in evidence-based parenting practices8-12 to test if a focus on parenting would be especially suitable for this age group, as suggested by others.6,13,14 To improve clinical relevance, the program was developed and conducted in close collaboration with the health care system.15
In this article, we report results of the More and Less (ML) study, in which 2 obesity treatment approaches were examined: parent-only group treatment with follow-up booster sessions (Booster) or without follow-up booster sessions (No Booster) focusing on evidence-based parenting practices, and standard treatment (ST) focusing on lifestyle changes (parent and child). In addition, we examined the influence of sociodemographic factors on treatment results and the clinical significance of the results. We hypothesized that the parent-only treatment would be more effective than ST in reducing child BMI z score (primary outcome) with additional effects from booster sessions.
METHODS
Trial Design
The ML study is a parallel open-label RCT evaluating the effects of 2 treatment approaches for obesity in 4- to 6-year-old children for 12 months (parent-only [Booster and No Booster]) and ST. The study was approved by the ethics committee in Stockholm, Sweden on November 16th, 2011 (dnr: 2011/1329-31/4), and the trial protocol is available in Supplemental Information. The following amendments were made to the original study protocol: To minimize differences between the 3 groups, we decided to randomize to the booster follow-up from the start instead of after the parent program (approval No. 2012/1104-32; August 11, 2012). For practical reasons, we developed the parent-only program on the basis of Keeping Foster and Kin Parents Supported and Trained (KEEP) instead of the parent program the Parent Management Training -Oregon Model (PMTO) because the KEEP team had more flexibility to offer supervision (approval No. 2012/2005-32; November 23, 2012). Additionally, we expanded the age span to include 6-year-old children to be able to offer the program to more families. Finally, we added a 6-month follow-up post baseline to better evaluate the treatment program (approval No. 2013/486-32; March 12, 2013). The trial design was extensively described previously.15
Participants
Families were primarily recruited through child health care centers (primary care) between March 2012 and March 2016 but were also recruited from outpatient pediatric clinics and from school health care offices in Stockholm County, Sweden. Some families were self-referred; these families heard about the study from health care professionals, through advertisements in a local newspaper, or on bulletin boards in public areas. Parents were informed about the study if they were eligible for participation (see below). When parents consented, a nurse sent a notice of interest to the research group together with the child’s growth chart. Researchers provided written and oral information about the study before the family agreed to participate. Written informed consent was obtained from parents on behalf of their children.
Families were eligible for participation if the child was 4 to 6 years old, was diagnosed with obesity according to international recommendations,16,17 and had no other chronic diseases or developmental problems likely to influence child weight and height. In addition, parents had to be able to communicate in Swedish well enough to complete questionnaires and participate in treatment conducted in Swedish.15 Eligible families were randomly assigned (1:1:2) to Booster, No Booster, and ST by using an electronic randomization program with permuted blocks. The randomization sequence was maintained by the study statistician to ensure concealment. On the basis of power calculations, 75 children were needed in each treatment (parent-only and ST adjusted for dropout) to detect a difference of 0.3 BMI z score (0.5 SD) with 85% power at 12 months’ follow-up. Data were collected from March 2012 to October 2017.
Treatment Approaches and Settings
Parent-Only Treatment
The ML program was developed in collaboration with the KEEP program developers,8-12 with conceptual and cultural adaptations designed to fit the Swedish population of parents of preschoolers with obesity. The key concept of KEEP is to support parents in positive parenting practices (eg, encouragement and limit-setting strategies) to improve parent-child communication.8,10,12 Content regarding healthy food habits and physical activity was also included. In addition, inspired by the PMTO, techniques to regulate emotional control were added.18,19 Initially, the ML program consisted of 12 weekly 90-minute sessions with parents only. On the basis of program evaluation forms and interviews with participants, the program was condensed to 10 sessions; however, the content was maintained (Supplemental Table 3).
Program group leaders were 5 registered dieticians. Before the program, they received training by the KEEP developers, who also provided weekly supervision on videotaped sessions for program certification and fidelity.
Each session introduced 1 parenting practice. Role plays and home practice assignments were used to facilitate better comprehension of the presented material. The meetings were held in health care facilities. Both parents were invited to participate, and child care was provided. If parents missed a session, material was sent home and key parts were reviewed over the phone by 1 of the group leaders.
Families and the group leaders were blinded to Booster or No Booster group allocation until after the parent program. The booster sessions consisted of 30-minute phone calls (maximum 7) every 4 to 6 weeks for 9 months. During the booster sessions, a group leader encouraged the parents to maintain healthy habits and provided support for challenges by reminding them about the strategies covered during the program.
ST
ST was offered in 14 outpatient pediatric clinics and was based on the action plan for childhood obesity in Stockholm County.20 Parents and children attended treatment together. The treatment focused on healthy food choices and active lifestyle habits. The families were offered ≥4 individual visits of ~30 minutes over 12 months; the first and the last visits were with a pediatrician, and the remaining visits were with a pediatric nurse. Some children were referred to dieticians and/or physiotherapists. The treatment setup varied between clinics; thus, each clinic filled out a protocol at every visit documenting what treatment the family had attended and on how many occasions.
Measures and Outcomes
At baseline, parents completed sociodemographic questionnaires (see Table 1). Child weight, height, and waist circumference (WC) were measured at baseline and at 3, 6, and 12 months post baseline by child health care professionals. Calibrated instruments were used. All measures were repeated 3 times, and mean values were derived. Child weight was measured to the nearest 0.1 kg with the child wearing underwear, and child height was measured to the nearest 0.1 cm by using a fixed stadiometer and the child’s WC to the nearest 0.1 cm in between the lower rib and the iliac crest by using nonextensible tape.
TABLE 1.
Sample Characteristics of Children and Parents in the ML Study
| Variable | n a | Mean (SD), N = 174 | Parent-Only Treatment | ST (n = 87) | ||
|---|---|---|---|---|---|---|
| Booster (n = 44) | No Booster (n = 43) |
Both Groups (n = 87) |
||||
| Child | ||||||
| Age, y | 174 | 5.3 (0.8) | 5.2 (0.8) | 5.2 (0.9) | 5.2 (0.8) | 5.3 (0.7) |
| Sex (girl), n (%) | 174 | 98 (56.3) | 19 (43.2) | 23 (53.2) | 42 (48.3) | 56 (64.4) |
| First born, n (%) | 147 | 72 (49.0) | 15 (41.7) | 21 (51.2) | 36 (46.8) | 36 (51.4) |
| Live with both parents, n (%) | 143 | 113 (79.0) | 25 (78.1) | 31 (81.6) | 56 (80.0) | 57 (78.1) |
| BMI z score | 174 | 3.0 (0.6) | 3.0 (0.5) | 3.1 (0.7) | 3.0 (0.6) | 2.9 (0.6) |
| BMI | 174 | 21.4 (1.8) | 21.4 (1.5) | 21.9 (2.3) | 21.6 (1.9) | 21.3 (1.7) |
| WC | 135 | 66.7 (5.9) | 65.1 (4.3) | 67.6 (6.5) | 66.4 (5.6) | 66.9 (6.2) |
| Mother | ||||||
| Age, y | 139 | 36.6 (5.5) | 38.1 (5.1) | 36.1 (5.4) | 37.1 (5.3) | 36.3 (5.7) |
| BMI | 141 | 28.1 (5.7) | 28.2 (6.0) | 29.1 (6.5) | 28.7 (6.2) | 27.6 (5.1) |
| Wt category, n (%) | 141 | |||||
| Normal wt | 43 (30.5) | 9 (28.1) | 12 (33.3) | 21 (30.9) | 22 (30.1) | |
| Overweight | 53 (37.6) | 12 (37.5) | 11 (30.6) | 23 (33.8) | 30 (41.1) | |
| Obese | 45 (31.9) | 11 (34.4) | 13 (36.1) | 24 (35.3) | 21 (28.8) | |
| Foreign origin, n (%) | 145 | 89 (61.4) | 21 (63.6) | 21 (56.8) | 42 (60.0) | 47 (62.7) |
| University degree, n (%) | 143 | 58 (40.6) | 14 (42.4) | 15 (41.7) | 29 (42.0) | 29 (39.2) |
| Income level (SEK per mo), n (%) | 137 | |||||
| <10 000 | 26 (19.0) | 4 (13.3) | 7 (20.6) | 11 (17.2) | 15 (20.5) | |
| 10 000 < 20 000 | 59 (43.1) | 11 (36.7) | 13 (38.2) | 24 (37.5) | 35 (47.9) | |
| 20 000 < 30 000 | 40 (29.2) | 12 (40.0) | 9 (26.5) | 21 (32.8) | 19 (26.0) | |
| 30 000 < 40 000 | 10 (7.3) | 3 (10.0) | 4 (11.8) | 7 (10.9) | 3 (4.1) | |
| 40 000 < 50 000 | 1 (0.7) | — | — | — | 1 (1.4) | |
| >50 000 | 1 (0.7) | — | 1 (2.9) | 1 (1.6) | — | |
| Father | ||||||
| Age, y | 124 | 39.8 (7.1) | 43.1 (7.9) | 38.7 (7.4) | 40.7 (7.9) | 38.9 (6.3) |
| BMI | 126 | 29.5 (4.5) | 29.1 (4.2) | 30.0 (4.6) | 29.5 (4.4) | 29.3 (4.5) |
| Wt category, n (%) | 126 | |||||
| Normal wt | 14 (11.1) | 5 (16.1) | 2 (6.5) | 7 (11.3) | 7 (10.9) | |
| Overweight | 65 (51.6) | 17 (54.8) | 16 (51.6) | 33 (53.2) | 32 (50.0) | |
| Obese | 47 (37.3) | 9 (29.0) | 13 (41.9) | 22 (35.5) | 25 (39.1) | |
| Foreign origin, n (%) | 130 | 75 (57.7) | 17 (54.8) | 21 (63.6) | 38 (59.4) | 37 (56.1) |
| University degree, n (%) | 128 | 49 (38.3) | 11 (36.7) | 12 (37.5) | 23 (37.1) | 26 (39.4) |
| Income level (SEK per mo), n (%) | 123 | |||||
| <10 000 | 14 (11.4) | 3 (10.7) | 4 (13.8) | 7 (12.3) | 7 (10.6) | |
| 10 000 < 20 000 | 31 (25.2) | 8 (28.6) | 6 (20.7) | 14 (24.6) | 17 (25.8) | |
| 20 000 < 30 000 | 56 (45.5) | 11 (39.3) | 13 (44.8) | 24 (42.1) | 32 (48.5) | |
| 30 000 < 40 000 | 15 (12.2) | 3 (10.7) | 4 (13.8) | 7 (12.3) | 8 (12.1) | |
| 40 000 < 50 000 | 3 (2.4) | 1 (3.6) | 1 (3.4) | 2 (3.5) | 1 (1.5) | |
| >50 000 | 4 (3.3) | 2 (7.1) | 1 (3.4) | 3 (5.3) | 1 (1.5) | |
Inclusion measures were used for children who never attended the baseline assessments: 9 Booster, 5 No Booster, and 8 ST. Parents were classified as normal weight, overweight, or obese according to the World Health Organization’s cutoff criteria for BMI. Foreign origin was defined as parent and both grandparents born in other country than Sweden or parent born in Sweden and grandparents born abroad. The mean monthly income level in 2015 was 33 600 SEK (men) and 26 400 SEK (women). SEK, Swedish kronor; —, not applicable.
Total number of responders; this varied equally between groups.
The primary outcome of the study was child BMI z score derived from age- and sex-specific reference data.16 The secondary outcomes were child BMI and WC and the clinical significance of the results (ie, a reduction in BMI z score using the cutoffs ≥0.25 and ≥0.5 after 3, 6, and 12 months). A reduction of ≥0.25 in BMI z score has been associated with improvements of the components included in the metabolic syndrome in both prepubertal and pubertal children, and a reduction of ≥0.5 doubled the effect.21-23
Statistical Analysis
Intention-to-treat analysis using linear mixed models were used to examine the difference in treatment effects on primary (BMI z score) and secondary outcomes (BMI, WC) at 3, 6, and 12 months follow-up. The main models included the following variables: time (months), treatment group (defined as parent-only and ST [primary aim] and Booster, No Booster, and ST [secondary aim]), and interaction (group by time). Random intercept and a random slope for time were used. Missing values on baseline covariates and on follow-up values were imputed simultaneously by using multiple imputation with chained equations (m [number of imputations] = 40).24 Three-way interactions with BMI z score as a dependent variable were used to evaluate if the treatment effect was moderated by any of the baseline covariates (ie, child age, sex, first born or not, living with both parents or not, parental BMI, foreign background, income, and education level). Differences between those that did and did not attend baseline and any follow-up measurements were analyzed by using independent sample t tests and χ2 tests. Risk ratios (RRs) were calculated to compare the clinical significance of treatment effect (ie, reduction of BMI z score ≥0.25 and ≥0.5). In sensitivity analyses, we generated mixed models as described above on our primary outcome but considered time as a factor, thus not assuming a linear trend. We additionally investigated the possible effect of missing not at random (MNAR) using δ adjustment,25 adding a BMI z score value to all imputations for Booster but not the other 2 groups. A value of P < .05 was considered statistically significant. Stata 13.1 (Stata Corp, College Station, TX) was used for our primary analyses, and SPSS Statistics 23 was used for descriptive analyses (IBM SPSS Statistics, IBM Corporation).
RESULTS
In total, 68 child health care centers referred children to the study, and 336 children were screened for eligibility; 177 children were enrolled in the study (Fig 1). Three children were excluded post randomization as a result of receiving a diagnosis that affected the child’s physical development. Thus, 174 children qualified for the intention-to-treat analysis. See Table 1 for participants’ characteristics.
FIGURE 1.
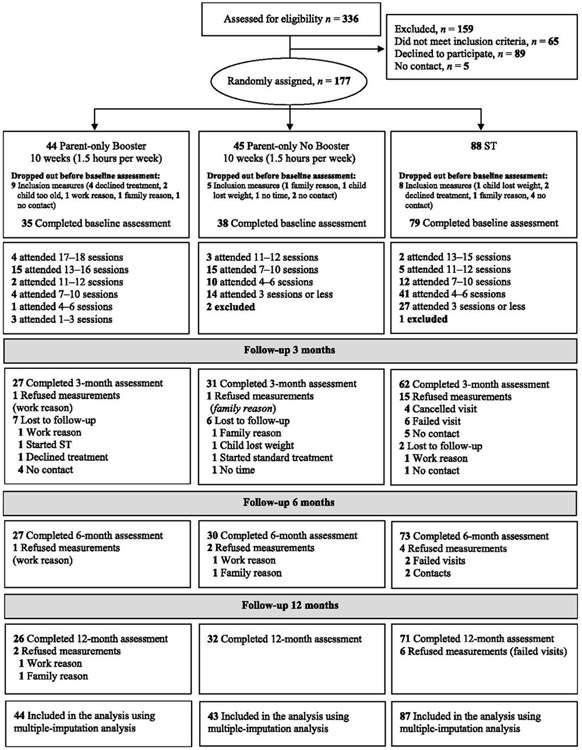
Study participant flowchart.
In total, 21% of the children were lost to follow-up (ie, no follow-up assessment): 30% in the parent-only (36% Booster, 25% No Booster) and 11% in the ST (P = .003) groups. Many dropped out before baseline assessments (Booster 21%, No Booster 11%, ST 9%). The most common reasons cited for dropout were parents’ work schedules or family situation. The only difference between those who remained in the study and those lost to follow-up with baseline measures (n = 8) was a higher BMI for fathers (P < .001) that remained in the study. No adverse events were reported.
For No Booster, the mean number of attended group sessions was 6.5 (SD = 3.1). The mean number of attended group sessions for the Booster group was 7.9 (SD = 3.1); with the addition of booster phone calls, the number of visits were 9.2 (SD = 3.6) at 6 months and 12.3 (SD = 4.7) at 12 months. For ST, the mean number of attended visits was 2.5 (SD = 1.5; range: 1–11) at 3 months, 3.8 (SD = 2.2; range: 1–13) at 6 months, and 5.5 (SD = 2.9, range: 1–15) at 12 months. After 12 months, between 1 and 4 failed visits were reported for 38% of ST families, and between 1 and 7 visits were cancelled by 70% of the families. Attendance differed only significantly after 6 and 12 months between Booster and No Booster (data not shown). There was no significant difference between attended visits between No Booster and ST at 12 months (P = .08).
Effects of Treatment
The children in the parent-only treatment had a greater reduction in their BMI z score at all time points compared with ST (−0.30; 95% confidence interval [CI]: −0.45 to −0.15) after 12 months, compared with no significant decrease for ST (Fig 2, Supplemental Table 4). No significant changes were seen in BMI or WC except for significant increases at all time points for ST. See Supplemental Table 5 for the coefficients for the group by time interaction effect.
FIGURE 2.
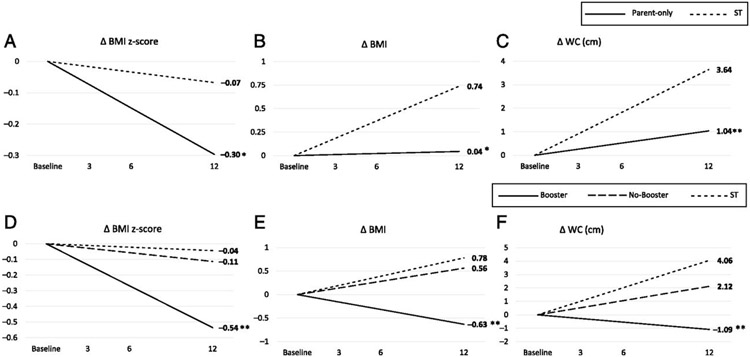
Mean difference change over time in primary (BMI z score) and secondary (BMI and WC) outcomes comparing obesity treatments over 12 months. A, Change in BMI z score. Shown are a parent-only treatment and ST. B, Change in BMI. Shown are a parent-only treatment and ST. C, Change in WC (centimeters). Shown are a parent-only treatment and ST. D, Change in BMI z score. Shown are a parent-only treatment (Booster and No Booster) and ST. E, Change in BMI. Shown are a parent-only treatment (Booster and No Booster) and ST. F, Change in WC (centimeters). Shown are a parent-only treatment (Booster and No Booster) and ST. *P < .05, **P < .01 (group difference, with ST as a reference).
Examining the effects of treatment intensity and length by comparing all 3 groups, improvements in BMI z score were only seen for the Booster group (Fig 2, Supplemental Table 6). No significant changes were seen for BMI, but for WC, significant increases were seen for the No Booster and ST groups. See Supplemental Table 7 for the coefficients for the group by time interaction effect.
Effects of Sociodemographics
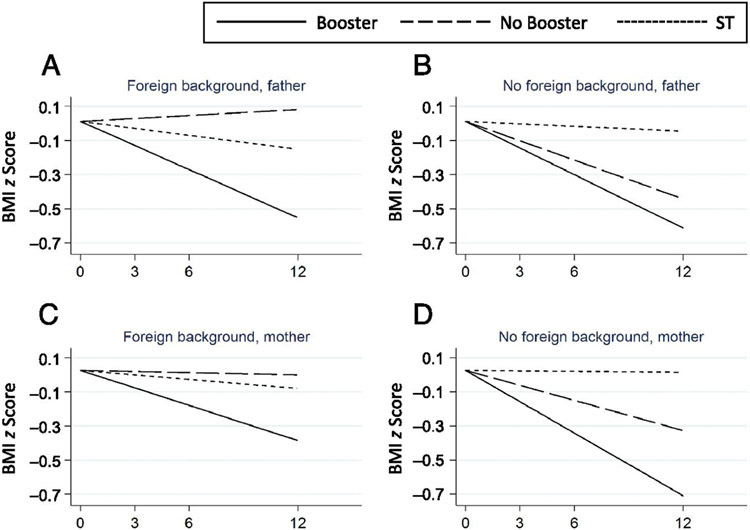
The only sociodemographic factor that moderated the effect of treatment was father’s foreign background (0.05 [95% CI: −0.01 to −0.08]; P = .006), but only for No Booster (Fig 3). Although the effect of the No Booster treatment was on par with the Booster treatment of children with Swedish fathers, it was inefficient for children with fathers with a foreign background. For results for other covariates, see Supplemental Table 8.
FIGURE 3.
A, Three-way interactions examining the effects of the father (foreign background) on BMI z score over 12 months. Three-way interactions examining the effects of the father (no foreign background) on BMI z score over 12 months. C, Three-way interactions examining the effects of the mother (foreign background) on BMI z score over 12 months. D, Three-way interactions examining the effects of mother (no foreign background on BMI z score over 12 months. The father’s foreign background affected the result, but only in the No Booster group (P = .006). Regarding the mother’s foreign background, the difference was not significant. Foreign background was defined as a parent and grandparents born abroad or a parent born in Sweden and grandparents born abroad.
Clinical Significance
The probability of reaching a 0.25 decrease in BMI z score was higher for the parent groups compared with ST, with the largest difference seen after 12 months: Booster, 3.3 (95% CI: 2.0 to 5.6); No Booster, 2.3 (95% CI: 1.3 to 3.9). Regarding the 0.5 decrease, the Booster group was 4.8 (2.4 to 9.6) times more likely to reach this level compared with ST after 12 months (Table 2).
TABLE 2.
The Clinical Significance of Treatment Results Expressed as RRs for Decrease in BMI z Score Using the Cutoffs 0.25 and 0.5
| Group | Decrease in BMI z Score ≥0.25, RR (95% CI) |
Decrease in BMI z Score ≥0.5, RR (95% CI)> |
|---|---|---|
| 3-mo follow-up | ||
| Booster | 1.4 (1.2 to 1.5)** | 1.5 (1.2 to 1.8)** |
| No Booster | 1.2 (1.1 to 1.4)* | 1.1 (0.9 to 1.4) |
| 6-mo follow-up | ||
| Booster | 1.8 (1.4 to 2.4)** | 2.2 (1.6 to 3.1)** |
| No Booster | 1.5 (1.1 to 2.0)* | 1.3 (0.8 to 2.0) |
| 12-mo follow-up | ||
| Booster | 3.3 (2.0 to 5.6)** | 4.8 (2.4 to 9.6)** |
| No Booster | 2.3 (1.3 to 3.9)* | 1.6 (0.6 to 4.0) |
ST was used as a reference in all calculations.
P < .01
P < .001.
The sensitivity analysis using the same models as above with time as a factor and not assuming a linear trend did not alter the results (Supplemental Fig 4). Applying δ adjustment, the “tipping point” (the point at which Booster was no longer statistically significantly better than ST) occurred when adding a BMI z score of 0.7 to the imputed values for the Booster group (Supplemental Table 9).
DISCUSSION
We found that an intensive parent-only treatment focused on evidence-based parenting practices was superior to ST in treating obesity in preschoolers, with strongest effects for the Booster group. After 12 months, the Booster group was nearly 5 times more likely to reach a clinically significant decrease in BMI z score of 0.5 as compared with ST.
The clinical relevance of treatment is important given the relevance for metabolic and cardiovascular health.21-23 Such information is seldom reported in intervention studies, yet critical when interpreting results.26,27 Of note, the US Preventive Services Task Force recommends a BMI z score reduction of 0.15 to 0.25 to be clinically significant for many metabolic markers.27 We also included the higher cutoff of 0.5 because it has been shown to double the effect on the metabolic markers.23 In addition, our results on mean change in BMI z score are within and greater than the range reported on RCTs targeting preschool obesity in the latest Cochrane review7 in which the mean decrease in BMI z score ranged from 0.20 to 0.49 after 6 to 12 months. Our results are also of similar magnitude as those from parent-only treatment studies for children 5 to 11 years of age included in another Cochrane review.28 In this review, the largest treatment effects were obtained for 2 Australian programs similar to ML.29,30 However, none of the studies were specifically designed for preschoolers.29-35 We have found only 1 other study treating preschool obesity that also included parenting skills training.6 The results from that study are comparable to ours, supporting the focus on parenting skills as a potential mechanism for treatment success.
High intensity and longer duration have become 2 key determinants of effective interventions.27,36-39 Specifically, a minimum of 26 contact hours was found necessary by the US Preventive Services Task Force in studies with children ≥6 years of age.27 We also found increased contact and length to be deciding factors because the Booster group outperformed the other groups. However, in the ML study, the highest number of visits corresponded to 21 hours, suggesting that treatments in this young age group can be less intensive but still need follow-up visits. The difference in weight status between Booster and No Booster at 3 months is unexpected. However, we suggest that length of follow-up rather than intensity was more important. We examined the influence of several sociodemographic factors on treatment results, but none could explain the difference. We cannot rule out that the difference may be due to selection bias of variables not considered in this article. For example, we have yet to examine if the parent groups responded differently to the intervention of parent skills practice training. In addition, in future studies, we will examine the role of parent feeding practices, eating behaviors, and psychosocial health as possible underlying mechanisms for observed differences in weight status.
Parental education is the sociodemographic factor commonly associated with obesity in children.40 In our study, fathers’ foreign background was the only factor that played a role in treatment effect. However, this effect was only seen in the No Booster group, suggesting that parents of foreign background may need prolonged support in treatment.
Studies are often criticized for failing to appeal to those most in need and thus lacking generalizability.41 What sets the ML study apart from previous interventions29,31 is the large study population with sociodemographic variation in parental education and foreign background. A limitation in our study was the rate of children lost to follow-up among the families in the parent-only treatment (30%). A similar finding was seen in an Australian study in which 35% of patients were lost to follow-up in the intervention group.31 In the ML study, many families were lost before starting treatment, indicating that a structured parent-only program may not be suitable for all families, whereas ST offers more flexibility, as shown by the many cancelled visits in the ST. The multiple-imputation model assuming MNAR could be questioned. However, the sensitivity analyses revealed that missing values would need to be >0.7 BMI z score to make our results nonsignificant, which is unlikely. In addition, we did not examine the impact of session content being reviewed over the phone; however, this is an area of interest for future studies, including the ML program. Another limitation is lack of blinding of assessors who conducted measurements in the parent groups and who analyzed the outcomes. The ML program was tested in Sweden and may not be generalizable to other populations; however, the program is currently being implemented in Spain and Romania within the European Union–funded Science and Technology in Childhood Obesity Policy project.
CONCLUSIONS
In this RCT conducted in a diverse population, we report the results from a parent-only treatment program with and without boosters compared with ST. The parent-only program with boosters was superior in improving the children’s weight status at all time points. In addition, children in the parent-only group were most likely to achieve clinically significant reductions in BMI z score. Such clinical reductions are connected to improvements in metabolic health but rarely evaluated.27 This study bolsters evidence from previous research in which intensive and parent-only obesity treatments for young children are favored.
Supplementary Material
WHAT’S KNOWN ON THIS SUBJECT:
Although obesity among preschoolers is common and on the rise, few existing treatment programs, including standard care, have been properly evaluated. Early treatment should be directed to parents and be of high intensity to be effective.
WHAT THIS STUDY ADDS:
We show that parent-only obesity treatment, including parenting skills training with follow-up booster sessions (but not without), outperformed standard treatment regarding improvements in child weight status. Thus, for successful obesity treatment in preschoolers, only parents need to be involved.
ACKNOWLEDGMENTS
We thank all participating families, child health care and school nurses, and all personnel involved in the ST offered in the pediatric outpatient clinics. We also thank Lena Frenzel (pediatric nurse and child health care coordinator), Ola Eklund (pediatrician), Marie Johannesson (school health care physician), Nilüfer Kayihan Kuru (pediatrician), Sofia Ljung (former project coordinator), JP Davis (senior clinical trainer from OSLC Developments), and Stanley Ulijaszek (professor of Human Ecology, University of Oxford). We also thank Marion Forgatch (senior scientist from OSLC Developments) for her valuable comments in the early stages of the study and for allowing us to use the emotional regulation section from the PMTO in the ML program. Finally, we thank Christine Delisle Nyström for her wise comments on the article.
FUNDING:
Supported by the Swedish Research Council (2014-02404), Karolinska Institutet Doctoral Funds, the Swedish Society of Medicine, Vinnova (2011-3443), the Jerring Foundation, the Samariten Foundation for Paediatric Research, the Magnus Bergvall Foundation, the Ingrid and Fredrik Thuring Foundation, the Helge Ax:son Johnsons Foundation, the Her Royal Highness Crown Princess Lovisa’s Foundation, the Frimurare Barnhuset Foundation, the Pediatric Care Foundation, the Martin Rind Foundation, the Jane and Dan Olssons Foundation, the Clas Groschinsky Foundation, the Sigurd and Elsa Golje’s Memory Foundation, the iShizu Matsumurais Donation, the National Institute on Drug Abuse United States Public Health Service grant P50DA035763 from the Division of Epidemiology, Services, and Prevention Research.
ABBREVIATIONS
- CI
confidence interval
- KEEP
Keeping Foster and Kin Parents Supported and Trained
- ML
More and Less
- MNAR
missing not at random
- PMTO
Parent Management Training – Oregon Model
- RCT
randomized controlled trial
- RR
risk ratio
- ST
standard treatment
- WC
waist circumference
Footnotes
This trial has been registered at www.clinicaltrials.gov (identifier NCT01792531).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The More and Less parent program is based on the parent program Keeping Foster and Kin Parents Supported and Trained. Keeping Foster and Kin Parents Supported and Trained was developed by Dr Chamberlain at the Oregon Social Learning Center (Eugene, OR), who receives royalties as the model developer Dr Chamberlain was a consultant for the More and Less study during the completion of this work; the other authors have indicated they have no potential conflicts of interest to disclose.
Reprints Information about ordering reprints can be found online: http://www.aappublications.org/site/misc/reprints.xhtml
REFERENCES
- 1.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999-2016 [published correction appears in Pediatrics. 2018; 142(3):e20181916]. Pediatrics. 2018;141(3):e20173459. [DOI] [PubMed] [Google Scholar]
- 2.Reinehr T, Kleber M, Lass N, Toschke AM. Body mass index patterns over 5 y in obese children motivated to participate in a 1-y lifestyle intervention: age as a predictor of long-term success. Am J Clin Nutr. 2010;91(5):1165–1171 [DOI] [PubMed] [Google Scholar]
- 3.Danielsson P, Svensson V, Kowalski J, Nyberg G, Ekblom O, Marcus C. Importance of age for 3-year continuous behavioral obesity treatment success and dropout rate. Obes Facts. 2012;5(1):34–44 [DOI] [PubMed] [Google Scholar]
- 4.Danielsson P, Kowalski J, Ekblom Ö, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolesc Med. 2012;166(12):1103–1108 [DOI] [PubMed] [Google Scholar]
- 5.Wiegand S, Keller KM, Lob-Corzilius T, et al. Predicting weight loss and maintenance in overweight/obese pediatric patients. Horm Res Paediatr. 2014;82(6):380–387 [DOI] [PubMed] [Google Scholar]
- 6.Stark LJ, Spear Filigno S, Bolling C, et al. Clinic and home-based behavioral intervention for obesity in preschoolers: a randomized trial. J Pediatr. 2018;192:115.e1–121.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colquitt JL, Loveman E, O’Malley C, et al. Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in preschool children up to the age of 6 years. Cochrane Database Syst Rev. 2016;3:CD012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price JM, Chamberlain P, Landsverk J, Reid JB, Leve LD, Laurent H. Effects of a foster parent training intervention on placement changes of children in foster care. Child Maltreat. 2008;13(1):64–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchanan R, Chamberlain P, Price JM, Sprengelmeyer P. Examining the equivalence of fidelity over two generations of KEEP implementation: a preliminary analysis. Child Youth Serv Rev. 2013;35(1):188–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamberlain P, Price J, Leve LD, Laurent H, Landsverk JA, Reid JB. Prevention of behavior problems for children in foster care: outcomes and mediation effects. Prev Sci. 2008;9(1):17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamberlain P, Feldman SW, Wulczyn F, Saldana L, Forgatch M. Implementation and evaluation of linked parenting models in a large urban child welfare system. Child Abuse Negl. 2016;53:27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horwitz SM, Chamberlain P, Landsverk J, Mullican C. Improving the mental health of children in child welfare through the implementation of evidence-based parenting interventions. Adm Policy Ment Health. 2010;37 (1–2):27–39 [DOI] [PubMed] [Google Scholar]
- 13.Golan M, Weizman A, Apter A, Fainaru M. Parents as the exclusive agents of change in the treatment of childhood obesity. Am J Clin Nutr. 1998;67(6):1130–1135 [DOI] [PubMed] [Google Scholar]
- 14.Golan M, Kaufman V, Shahar DR. Childhood obesity treatment: targeting parents exclusively v. parents and children. Br J Nutr. 2006;95(5):1008–1015 [DOI] [PubMed] [Google Scholar]
- 15.Ek A, Chamberlain KL, Ejderhamn J, et al. The More and Less Study: a randomized controlled trial testing different approaches to treat obesity in preschoolers. BMC Public Health. 2015;15(1):735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole TJ, Lobstein T. Extended international (I0TF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284–294 [DOI] [PubMed] [Google Scholar]
- 17.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forgatch MS, DeGarmo DS. Parenting through change: an effective prevention program for single mothers. J Consult Clin Psychol. 1999;67(5):711–724 [DOI] [PubMed] [Google Scholar]
- 19.DeGarmo DS, Patterson GR, Forgatch MS. How do outcomes in a specified parent training intervention maintain or wane over time? Prev Sci. 2004;5(2):73–89 [DOI] [PubMed] [Google Scholar]
- 20.The Health Care Administration, Stockholm County Council. The Action Program for Overweight and Obesity 2016-2020. Stockholm, Sweden: Stockholm Health Care Administration;2015 [Google Scholar]
- 21.Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89(5):419–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinehr T, Kleber M, Toschke AM. Lifestyle intervention in obese children is associated with a decrease of the metabolic syndrome prevalence. Atherosclerosis. 2009;207(1):174–180 [DOI] [PubMed] [Google Scholar]
- 23.Reinehr T, Lass N, Toschke C, Rothermel J, Lanzinger S, Holl RW. Which amount of BMI-SDS reduction is necessary to improve cardiovascular risk factors in overweight children? J Clin Endocrinol Metab. 2016;101(8):3171–3179 [DOI] [PubMed] [Google Scholar]
- 24.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399 [DOI] [PubMed] [Google Scholar]
- 25.Mallinckrodt C, Roger J, Chuang-Stein C, et al. Missing data: turning guidance into action. Stat Biopharm Res. 2013;5(4):369–382 [Google Scholar]
- 26.Magarey A, Mauch C, Mallan K, et al. Child dietary and eating behavior outcomes up to 3.5 years after an early feeding intervention: The NOURISH RCT. Obesity (Silver Spring). 2016;24(7):1537–1545 [DOI] [PubMed] [Google Scholar]
- 27.US Preventive Services Task Force, Grossman DC, Bibbins-Domingo K, Curry SJ, et al. Screening for obesity in children and adolescents: US preventive services task force recommendation statement. JAMA. 2017;317(23):2417–2426 [DOI] [PubMed] [Google Scholar]
- 28.Loveman E, Al-Khudairy L, Johnson RE, et al. Parent-only interventions for childhood overweight or obesity in children aged 5 to 11 years. Cochrane Database Syst Rev. 2015;(12):CD012008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golley RK, Magarey AM, Baur LA, Steinbeck KS, Daniels LA. Twelve-month effectiveness of a parent-led, family-focused weight-management program for prepubertal children: a randomized, controlled trial. Pediatrics. 2007;119(3):517–525 [DOI] [PubMed] [Google Scholar]
- 30.Magarey AM, Perry RA, Baur LA, et al. A parent-led family-focused treatment program for overweight children aged 5 to 9 years: the PEACH RCT. Pediatrics. 2011;127(2):214–222 [DOI] [PubMed] [Google Scholar]
- 31.West F, Sanders MR, Cleghorn GJ, Davies PS. Randomised clinical trial of a family-based lifestyle intervention for childhood obesity involving parents as the exclusive agents of change. Behav Res Ther. 2010;48(12):1170–1179 [DOI] [PubMed] [Google Scholar]
- 32.Moens E, Braet C. Training parents of overweight children in parenting skills: a 12-month evaluation. Behav Cogn Psychother. 2012;40(1):1–18 [DOI] [PubMed] [Google Scholar]
- 33.Boutelle KN, Cafri G, Crow SJ. Parent-only treatment for childhood obesity: a randomized controlled trial. Obesity (Silver Spring). 2011;19(3):574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boutelle KN, Braden A, Douglas JM, et al. Design of the FRESH study: a randomized controlled trial of a parent-only and parent-child family-based treatment for childhood obesity. Contemp Clin Trials. 2015;45(pt B):364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzeo SE, Kelly NR, Stern M, et al. Parent skills training to enhance weight loss in overweight children: evaluation of NOURISH. Eat Behav. 2014;15(2):225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen E, Mulkens S, Jansen A. Tackling childhood overweight: treating parents exclusively is effective. Int J Obes. 2011;35(4):501–509 [DOI] [PubMed] [Google Scholar]
- 37.Savoye M, Nowicka P, Shaw M, et al. Long-term results of an obesity program in an ethnically diverse pediatric population. Pediatrics. 2011;127(3):402–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilfley DE, Saelens BE, Stein RI, et al. Dose, content, and mediators of family-based treatment for childhood obesity: a multisite randomized clinical trial. JAMA Pediatr. 2017;171(12):1151–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duncanson K, Shrewsbury VA, Collins CE; The DiET-CO Consortium. Interim Report on the Effectiveness of Dietary Interventions for Children and Adolescents With Overweight and Obesity for the World Health Organization. Callaghan, Australia: University of Newcastle; 2017 [Google Scholar]
- 40.Bambra CL, Hillier FC, Cairns JM, et al. How Effective Are Interventions at Reducing Socioeconomic Inequalities in Obesity Among Children and Adults? Two Systematic Reviews. Southampton, United Kingdom: National Institute for Health Research Journals Library; 2015 [PubMed] [Google Scholar]
- 41.Summerbell CD, Ashton V, Campbell KJ, Edmunds L, Kelly S, Waters E. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2003;(3):CD001872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.