Hypoxaemia in COVID-19 is primarily caused by disruption of the alveolocapillary barrier on inflammation and dysfunction of the endothelium.1 To date, antiviral or immune-modulatory treatment options have been thoroughly studied, yet there is no approved therapy targeting endothelial dysfunction. Imatinib is a tyrosine kinase inhibitor that attenuates vascular leakage under inflammatory conditions.2 In the CounterCOVID study, patients admitted to hospital with COVID-19 treated with imatinib had a shorter duration of invasive ventilation and shorter stay at the intensive care unit (ICU).3 Although a signal for reduced mortality was observed, a definite answer on mortality was precluded by correction for imbalances in patient characteristics at baseline and the short follow-up of 28 days. Here we report the 90-day outcomes of the CounterCOVID study and investigate the mechanisms underlying the clinical benefit of imatinib.
The study design and protocol were described previously.3 In brief, 385 eligible patients were randomly assigned (1:1) to either 400 mg imatinib once per day with a loading dose of 800 mg on the first day or an equivalent number of placebo tablets for 10 days. Electronic medical records and follow-up telephone calls were used to assess clinical endpoints during a 90-day follow-up. The 90-day clinical outcomes were available for all 385 patients (for patient characteristics, see appendix p 6). Time to mortality was compared using Cox regression analysis. Baseline imbalances (sex, obesity, diabetes, and cardiovascular disease) were used as adjustment factors.
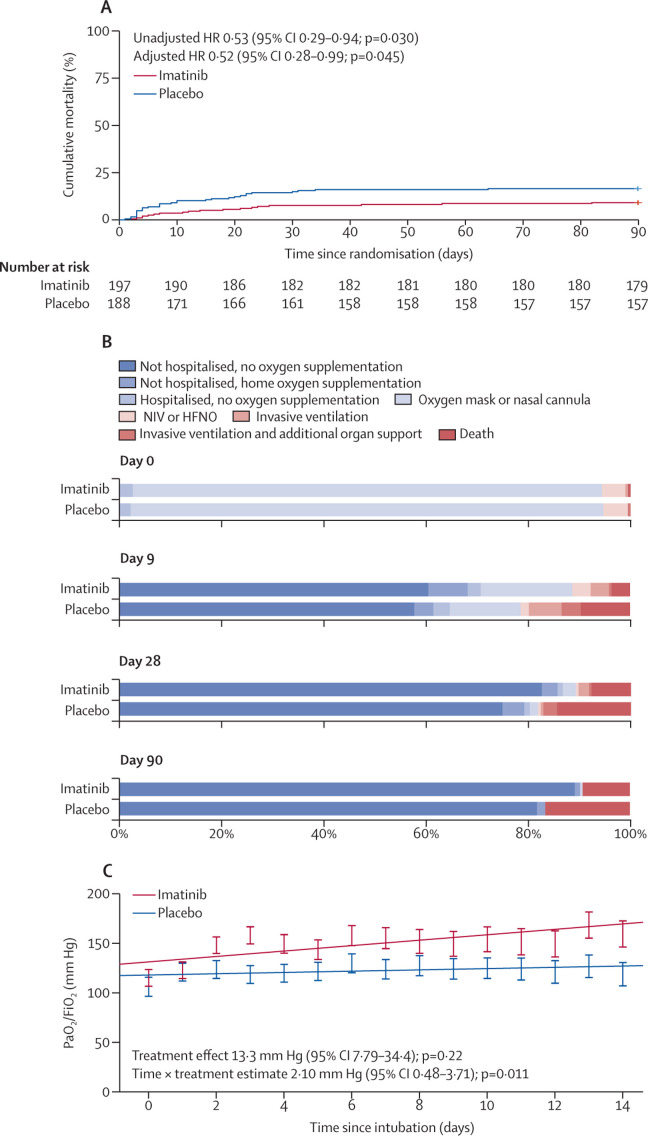
At day 90, 18 (9·1%) patients in the imatinib group and 31 (16·5%) patients in the placebo group had died. The unadjusted hazard ratio (HR) for mortality was 0·53 (95% CI 0·29–0·94) favouring the imatinib group (figure ). This result remained significant after adjusting for baseline imbalances (HR 0·52 [0·28–0·99]; appendix p 7). The median duration of invasive ventilation was 7 days (IQR 3–15) in the imatinib group versus 12 days (7–22) in the placebo group (appendix p 8). Patients in the ICU treated with imatinib had a median of 84 ventilator-free days (54–88), versus 64 ventilator-free days (0–84) in patients treated with placebo (appendix p 8). The median length of ICU admission was 9 days (5–15) in the imatinib group and 15 days (7–21) in the placebo group (appendix p 8).
Figure.
The rate of mortality, clinical status over time, and the longitudinal course of the PaO2/FiO2 in invasively ventilated patients
(A) Kaplan Meier curve of the time to mortality. The hazard ratio and p value were calculated using Cox regression analysis and were adjusted for baseline imbalances (sex, diabetes, obesity [body-mass index >30 kg/m2], and cardiovascular disease; n=385). (B) Proportions of patients in each category over time according to an eight-point ordinal clinical scale (n=385). (C) The estimated longitudinal course of the ratio of PaO2/FiO2 during the first 14 days of invasive ventilation (n=56) calculated using a linear mixed model. Treatment, time, and time × treatment were entered as fixed effects, a random intercept on individual patient level was entered. The error bars indicate the SEs of the predicted values on each study day. FiO2=fraction of inspired. PaO2=partial pressure of oxygen. HR=hazard ratio. NIV=non-invasive ventilation. HFNO=high-flow nasal oxygen.
The clinical status at baseline, and days 9, 28, and 90 was assessed using an eight-point ordinal scale ranging from discharged without oxygen supplementation (1) to death (8) and was analysed using a linear mixed model (appendix pp 9–10; figure). During the 90-day follow-up, patients treated with imatinib had a more favourable clinical status with and without adjusting for baseline imbalances (adjusted estimate –0·56 [95% CI –0·99 to –0·13]).
In a subgroup of 56 patients who required invasive ventilation, the longitudinal course of oxygenation parameters and ventilatory conditions during the first 14 days of invasive ventilation were compared using a linear mixed model. Patients who received imatinib had a lower positive end-expiratory pressure (estimate –1·16 cm H2O [95% CI –2·28 to –0·04]), a lower mean airway pressure (−1·84 cm H2O [–3·65 to –0·04]), and a lower oxygenation index (−3·99 [–7·75 to –0·23]) indicating that patients treated with imatinib required less ventilatory support throughout the 14 days (appendix p 11). A significant interaction between time and treatment was observed in the fraction of inspired oxygen (FiO2; estimate –0·71 [–1·38 to –0·04]), and in the ratio between partial pressure of oxygen (PaO2) and FiO2 (figure; estimate 2·10 [0·48 to 3·71]), indicating a faster improvement in oxygenation status.
A lower mortality rate, better clinical status according to an eight-point ordinal scale, and shorter duration of invasive ventilation were observed in hospitalised patients with COVID-19 treated with imatinib during a 90-day follow-up. In general, invasively ventilated patients treated with imatinib had better ventilatory conditions and required less oxygen supplementation, indicating that imatinib treatment favourably influences oxygen uptake. A difference in the need for oxygen supplementation in this study was evident from changes in PaO2/FiO2 (reflecting efficiency of gas exchange) and oxygenation index (an index integrating gas exchange efficiency and degree of ventilatory support). Although immunomodulatory effects cannot be excluded, we speculate that the clinical benefit of imatinib in critically ill patients follows resolution of pulmonary oedema by attenuation of vascular leak. However, vascular leak was not quantified in this study, nor was the volume of extravascular lung water assessed. To evaluate the effect of imatinib on vascular leak, a randomised placebo-controlled trial evaluating the efficacy of imatinib in invasively ventilated COVID-19 patients started in March, 2021. The primary outcome of this study is the change in the extravascular lung water index (ClinicalTrials.gov, NCT04794088). Additionally, WHO and the Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia are in progress to test the efficacy of imatinib in COVID-19 (ClinicalTrials.gov, NCT05220280).4
In conclusion, longer follow-up of patients included in the CounterCOVID study substantiates a previous signal of improved survival in COVID-19 patients treated with imatinib. This improved survival is accompanied by a better clinical status according to an eight-point ordinal scale and a shorter duration of invasive ventilation. Other large, randomised trials are currently in progress to further establish the potential clinical benefit of imatinib in reversing pulmonary vascular leak.
JA and AVN are inventors on a patent (WO2012150857A1, 2011) covering protection against endothelial barrier dysfunction through inhibition of the tyrosine kinase Abl-related gene (Arg). All other authors have no competing interests. ED and JRS contributed equally. Members of the CounterCOVID study group are listed in the appendix (p 12). This project was funded with an unrestricted grant from the Amsterdam Medical Center Foundation, a bottom-up grant from NWO ZonMW (number 10430 01 201 0007), and from the Innovative Medicines Initiative 2 Joint Undertaking (number 101005142). The funding sources had no role in the design of the study, data collection, analysis, or in the decision to submit the paper for publication.
Supplementary Material
References
- 1.Smadja DM, Mentzer SJ, Fontenay M, et al. COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis. 2021;24:755–788. doi: 10.1007/s10456-021-09805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aman J, van Bezu J, Damanafshan A, et al. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation. 2012;126:2728–2738. doi: 10.1161/CIRCULATIONAHA.112.134304. [DOI] [PubMed] [Google Scholar]
- 3.Aman J, Duijvelaar E, Botros L, et al. Imatinib in patients with severe COVID-19: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Respir Med. 2021;9:957–968. doi: 10.1016/S2213-2600(21)00237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupferschmidt K. WHO relaunches global drug trial with three new candidates. Science. 2021;373:606–607. doi: 10.1126/science.373.6555.606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.