Abstract
Polydatin, one of the natural active small molecules, was commonly applied in protecting and treating liver disorders in preclinical studies. Oxidative stress plays vital roles in liver injury caused by various factors, such as alcohol, viral infections, dietary components, drugs, and other chemical reagents. It is reported that oxidative stress might be one of the main reasons in the progressive development of alcohol liver diseases (ALDs), nonalcoholic liver diseases (NAFLDs), liver injury, fibrosis, hepatic failure (HF), and hepatocellular carcinoma (HCC). In this paper, we comprehensively summarized the pharmacological effects and potential molecular mechanisms of polydatin for protecting and treating liver disorders via regulation of oxidative stress. According to the previous studies, polydatin is a versatile natural compound and exerts significantly protective and curative effects on oxidative stress-associated liver diseases via various molecular mechanisms, including amelioration of liver function and insulin resistance, inhibition of proinflammatory cytokines, lipid accumulation, endoplasmic reticulum stress and autophagy, regulation of PI3K/Akt/mTOR, and activation of hepatic stellate cells (HSCs), as well as increase of antioxidant enzymes (such as catalase (CAT), glutathione peroxidase (GPx), glutathione (GSH), superoxide dismutase (SOD), glutathione reductase (GR), and heme oxygenase-1 (HO-1)). In addition, polydatin acts as a free radical scavenger against reactive oxygen species (ROS) by its phenolic and ethylenic bond structure. However, further clinical investigations are still needed to explore the comprehensive molecular mechanisms and confirm the clinical treatment effect of polydatin in liver diseases related to regulation of oxidative stress.
1. Introduction
Increasing epidemic investigations have suggested that liver diseases remain one of the leading causes of deaths globally, and millions of people are suffering from acute or chronic liver disorders nowadays [1]. Currently, the morbidity of metabolic liver diseases including nonalcoholic fatty liver disease (NAFLD) and alcohol liver disease (ALD) are rising rapidly due to the continuous improving living standards, and it is reported that more than 10% of the world population were affected by liver diseases. Furthermore, the NAFLD and ALD are the very serious factors for ultimately leading to more cases of end-stage liver diseases, including hepatic failure (HF), cirrhosis, and hepatocellular carcinoma (HCC) [2].
Oxidative stress is a state due to the imbalance of free radicals and antioxidative enzymes. The imbalance tends to be oxidized, which leads to inflammatory infiltration of neutrophils, increasing the secretion of proteases and large amounts of oxidative intermediate products. Oxidative stress is produced by the excessive free radicals in the body which is considered as one of the most important factors to aging and various diseases. Redox state constitutes a necessary background of multiple liver diseases [3]. Oxidative stress is an important factor for development of liver diseases, especially in chronic liver diseases [4], and oxidative stress-associated liver diseases could also result in kidney injury and brain impairment [5, 6]. Reactive oxygen species (ROS), a highly reactive species of free radical, plays dual roles in living systems [7]. At physiological concentration, ROS plays essential roles in physiological process such as gene expression, signal transduction, and redox regulation. However, during some pathological conditions, the excessive ROS production has harmful effects for human body, such as damages of proteins, DNA, and lipids [8]. In addition, many etiological factors associated with liver disease are commonly highly productive under excessive ROS. It is reported that mitochondrial ROS levels could be highly increased by ROS, reactive nitrogen species (RNS), and excessive alcohol consumption in hepatocytes [9, 10]. Oxidative stress might be one of the main reasons in the progressive development of alcohol liver diseases (ALDs), nonalcoholic liver diseases (NAFLDs), liver injury, fibrosis, hepatic failure (HF), and hepatocellular carcinoma (HCC). Detection redox biomarkers may help diagnose liver diseases. For example, Świderska et al. found that advanced glycation end products (AGEs), an oxidative damage product, maybe a potential biomarker in NAFLD diagnostics [11].
Accumulating researches have shown that natural activity compounds such as paeoniflorin, taraxasterol, and oxymatrine possess versatile advantages for treating liver diseases with low toxicity and reliable pharmacological activities. Therefore, in the Europe and United States, approximately 65% of patients would like to use herbal medicines to treat liver diseases [12–15]. Polydatin (3,5,4-trihydroxystilbene-3-O-β-D-glucopyranoside, PD), commonly isolated from the roots of Polygonum cuspidatum, can be also obtained from many dietary supplements like grapes, peanuts, cocoa products, hop flowers (Humulus lupulus), and other plants (Figure 1 and Table 1) [16–21]. Preclinical trials revealed that PD has various pharmacological activities, such as anti-inflammatory [22–24], antiapoptotic [25], antitumor [26], lipid-lowering [27], and cardiovascular protection effects [28–30], especially exhibited strengthened pharmacological activities in antioxidant. The antioxidant activities were involved in immune system, osteoarthritis, endometriosis, pain, and intestinal inflammation and reported to be effective in treating liver disorders [31–35]. In the present review, we summarized and discussed the versatile effects of PD against liver diseases via regulation of oxidative stress.
Figure 1.
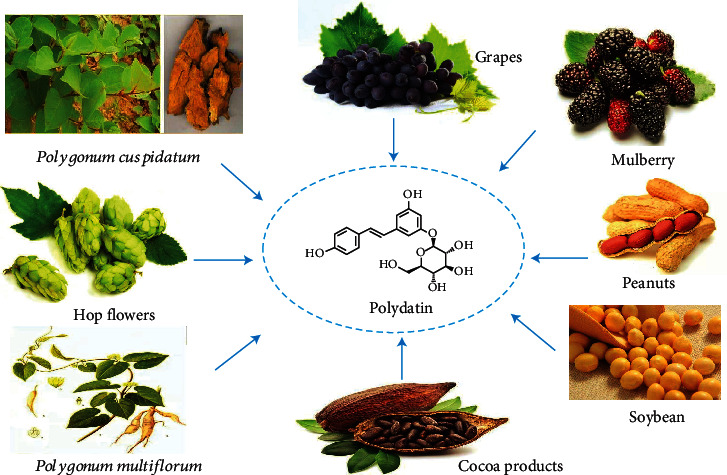
The plant sources of PD.
Table 1.
The contents of PD in different plants.
2. Effects of Polydatin on Liver Diseases via Regulating Oxidative Stress
By collecting relevant literatures in the past decades regarding the protection and treatment of PD against liver diseases, it has been found that PD has marked effects on liver-related conditions, including nonalcoholic fatty liver disease (NAFLD), alcohol liver disease (ALD), liver fibrosis, and HCC. The specific molecular mechanisms of protective and therapeutic effects of PD are concluded in Figure 2 and Table 2.
Figure 2.
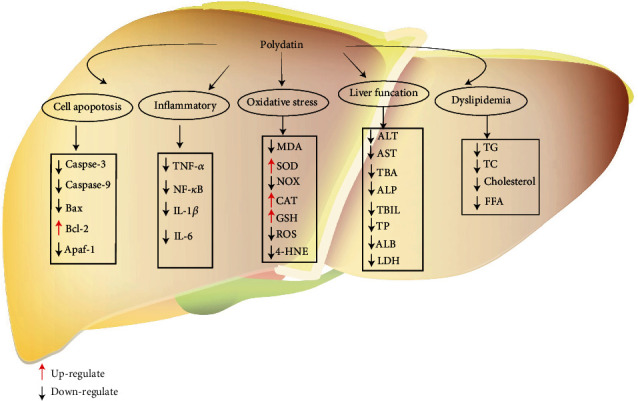
Cellular and molecular mechanisms of PD in the prevention of oxidative stress induced liver diseases. Bax: BCL-2-associated; Bcl-2: B-cell lymphoma-2; MDA: malondialdehyde; SOD: superoxide dismutase; NOX: nicotinamide adenine dinucleotide phosphate oxidative; CAT: catalase; GSH: glutathione; ROS: reactive oxygen species; 4-HNE: 4-hydroxynonenal; TNF-α: tumor necrosis factor-α; NF-κB: nuclear factor kappa B; IL-1β: interleukin-1β; IL-6: interleukin-6; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TBA: total bile acid; TBIL: total bilirubin; ALP: alkaline phosphatase; ALB: albumin; LDH: lactate dehydrogenase; TG: triglyceride; TC: total cholesterol; FFA: free fatty acid.
Table 2.
Effects of polydatin in the protection and treatment of oxidative stress-associated liver diseases.
| Liver disease type | Experimental model | Dose and formulation | Duration of treatment | References | ||
|---|---|---|---|---|---|---|
| Alcohol liver diseases | Animals | Male Wistar rats | Ethanol/7 mL/kg/every 12 h/(i.g.) | 25, 50, and 100 mg/kg/day/(i.g.) | Pretreatment for 7 days | [41] |
| Hepatic steatosis | Animals | Zebrafish strain | Ethanol/350 mM (2% EtOH)/32 h at 28.5°C | 6.25, 12.5, 25 μg/mL | 48 h | [42] |
| Acute liver injury | Animals | C57BL/6 male mice | Ethanol/50%/10 mL/kg/oral/2 days | 50 and 100 mg/kg/day (i.g.) | Pretreatment for 8 days | [46] |
| Nonalcohol fatty liver | Animals | Male Sprague Dawley rats | High-fat diet/12 weeks | 0.3%/day (i.g.) | 12 weeks | [55] |
| Nonalcohol fatty liver | Animals | Male Sprague Dawley rats | Fructose-induced/drinking 10% Fructose/6 weeks |
7.5, 15, 30 mg/kg (i.g.) | 7 weeks | [56] |
| Cells | BRL-3A/HepG2 | 4.5 mg/mL glucose/12 h | 10, 20, and 40 μM | 24 h | ||
| Nonalcoholic steatohepatitis | Animals | C57BL/6 male mice | Methionine-choline deficient diet/4 weeks | 5 mg/kg (i.p.) | 4 weeks | [59] |
| Cells | HepG2 cells | 250 μM palmitic acid/24 h | 5, 10, and 20 μM | 24 h | ||
| Nonalcohol fatty liver | Animals | Male Sprague Dawley rats | High-fat diet/16 weeks | 30, 90 mg/kg/day/(i.g.) | 8 weeks | [60] |
| Nonalcohol fatty liver | Animals | Male C57/BL6 mice | High-fat diet/14 weeks | 100 mg/kg/day/(i.g.) | 4 weeks | [61] |
| NASH | Animals | C57Bl/KsJ-db/db (db/db) mice | Methionine-choline deficient/4 weeks | 100 mg/kg/(i.g.) | Every other day for 4 weeks | [63] |
| Cells | L02 cells | Palmitic acid/60 μg/mL/24 h | 24 μM | 24 h | ||
| Liver injury | Animals | Male ICR mice | APAP/220 mg. kg−1/i.p. | 25, 50, and 100 mg/kg/day/(i.g.) | Pretreatment for 7 days | [66] |
| Liver injury | Animals | Male Wistar albino rats | Cis/7 mg/kg/i.p. | 25, 50, and 100 mg/kg/day/(i.g.) | Pretreatment for 10 days | [67] |
| Liver injury | Animals | Male ICR mice | Sulfur mustard/40 mg/kg/i.p. | 100, 200, and 400 mg/kg/day | 7 days | [70] |
| Cells | L02 cells | Sulfur mustard/50 μM/30 min | 50 μM | 24 h | ||
| Liver injury | Animals | Male ICR mice | CCl4/5 μL/kg/i.p. | 25, 50, and 100 mg/kg/day/(i.g.) | Pretreatment for 5 days | [72] |
| Liver injury | Animals | Male Wistar albino rats | As/100 mg/L/drinking | 50, 100, and 200 mg/kg/day/(i.g.) | 60 days | [75] |
| Liver injury | Animals | Male Wistar albino rats | Cadmium chloride/5 mg/kg/gastric gavage/4 weeks | 120 mg/kg/day/(i.g.) | 4 weeks | [77] |
| Fulminant hepatic failure | Animals | Balblc mice | LPS (50 μg/kg) and D − GaIN (700 mg/kg)/i.p. | 10, 30, 100 mg/kg/day/i.p. | Pretreatment for 1 h | [84] |
| Liver injury | Animals | Male C57BL/6 mice | ANIT/60 mg/kg/48 h (i.g.) | 40, 60, and 80 mg/kg/day/(i.g.) | Pretreatment for 7 days | [90] |
| Liver fibrosis | Animals | C57BL/6 mice | CCl4/5 ml/kg/i.p./twice a week for 6 weeks | 5 mg/kg/(i.p.) | 3 and 6 weeks | [91] |
| Liver fibrosis | Animals | C57BL/6 mice | CCl4/50 μL/kg/i.p./twice a week for 6 weeks | 5 mg/kg/(i.p.) | 6 weeks | [93] |
| Cells | LX − 2 cells | PDGF − BB/10 ng/mL | 10 μM | 24 h | ||
| Liver fibrosis | Animals | Male Sprague Dawley rats | Fructose/10%/6 weeks/(i.g.) | 7.5, 15, and 30 mg/kg/(i.g.) | 11 weeks | [99] |
| Cells | BRL − 3A cells | Fructose/5 mM | 10, 20, and 40 μM | 6, 12, 24 h | ||
| Hepatocellular carcinoma | Animals | Male BALB/c nude mice | HepG2 cells/5 × 106/subcutaneous injection/120 mm3 | 25, 50, and 100 mg/kg/100 μL (i.p.) | 20 days | [104] |
| Cells | HepG2and SMMC − 7721 | 1, 3, 10, 30, and 100 mM | 48 h | |||
2.1. Polydatin and Alcoholic Liver Diseases
In the past 30 years, alcohol consumption has a dramatically increased tendency due to the booming economy in China, leading to the highly incidence of alcohol liver disease (ALD) at 4.5% [36]. Nowadays, ALD has become one of the leading causes of chronic diseases and death in the world. Therefore, previous researchers paid much attention to ALD due to the molecular mechanism of ALD not completely clear [37]. ALD covers a wide spectrum of histological features, ranging from lipid accumulation in liver cells (fatty liver or steatosis) with minimal parenchymal damage to more severe liver injury, including steatohepatitis fibrosis/cirrhosis [38]. It was widely accepted that ALD pathogens are related to lipid accumulation, oxidative stress, inflammation, and mitochondrial dysfunction (Figure 3). Among the pathogenesis of ALD, oxidative stress and inflammation were considered as the fundamental mechanisms [39]. Alcohol-induced liver damage is related to excessive production of ROS and the presence of oxidative stress in liver cells. About 90% of alcohol was metabolized in the liver. Some metabolic enzymes in the liver, including alcohol dehydrogenase (ADH) and cytochrome P4502E1 (CYP2E1), converted alcohol to acetaldehyde. Then, acetaldehyde was oxidized to acetate by aldehyde dehydrogenase (ALDH) and converted to carbon dioxide through the citric acid cycle [40]. The main resource of ROS in the liver is related to the cytochrome P450 enzymes. Using the liver-injured male rats induced by ethanol, pretreatment with PD could improve the liver injury via suppressing oxidative stress by upregulation of ADH and ALDH and downregulating CYP2E1 [41]. Hepatic steatosis model was established in zebrafish induced by ethanol larvae, and it is found that PD treatment could improve ethanol metabolism by decreasing the gene expressions of CYP2Y3 and CYP3A [42]. Furthermore, the capacity of alcohol could stimulate the production of free radicals, which impaired liver antioxidant defense capability and greatly promoted the oxidative stress damage of ALD [38]. PD could improve the activities of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and upregulated nuclear factor erythroid 2-related factor 2 (Nrf2) and its target gene heme oxygenase-1 (HO-1) [41]. Besides, oxidative stress might contribute to the pathogenesis of alcoholic steatosis through actions on transcription factors regulating mitochondrial injury, lipid metabolism, DNA damage, and endoplasmic reticulum (ER) stress. PD treatment could decrease the mRNA levels of fatty acid synthase (FASN), HMGCRa, and HMGCRb to attenuate hepatic fat accumulation [42]. Moreover, DNA damage and ER stress play a key role in the disruption of lipid homeostasis, metabolism, and liver function [43]. PD could improve ethanol-induced DNA damage and ER stress by decreasing the mRNA levels of C/EBP homologous protein (CHOP) and growth arrest and DNA damage-inducible gene, 45αa (GADD45αa) in zebrafish larvae [42]. Acetaldehyde is a vital metabolite of alcohol and ROS, which can stimulate the secretion of matrix metalloproteinase (MMP). MMP promotes the degradation of extracellular matrix (ECM) components while distorting liver tissue structure [45]. Pretreatment with PD at the doses 50 and 100 mg/kg, respectively, can significantly prevent the rise in MMP activities in the liver tissue [46]. Furthermore, mitochondria are highly sensitive to oxidative stress. ROS accumulation in the mitochondrial membrane will deplete the mitochondrial complexes and cause mitochondrial dysfunctions including deterioration of respiratory enzymes, enhanced mitochondrial stress, and loss of functioning in mitochondria. Mitochondrial dysfunctions may eventually lead to apoptosis or necrotic cell death in liver tissue [44, 47]. Pretreatment with PD could ameliorate the activities of redox and mitochondrial respiratory enzyme, such as succinate dehydrogenase, NADH dehydrogenase, and cytochrome c (Cyt-C) oxidase. It is reported that PD could restore the mitochondrial respiratory complexes and ameliorate their functioning, providing evidence for its hepatoprotective potential through the mitochondrial oxidative stress inhibitory activity [46].
Figure 3.
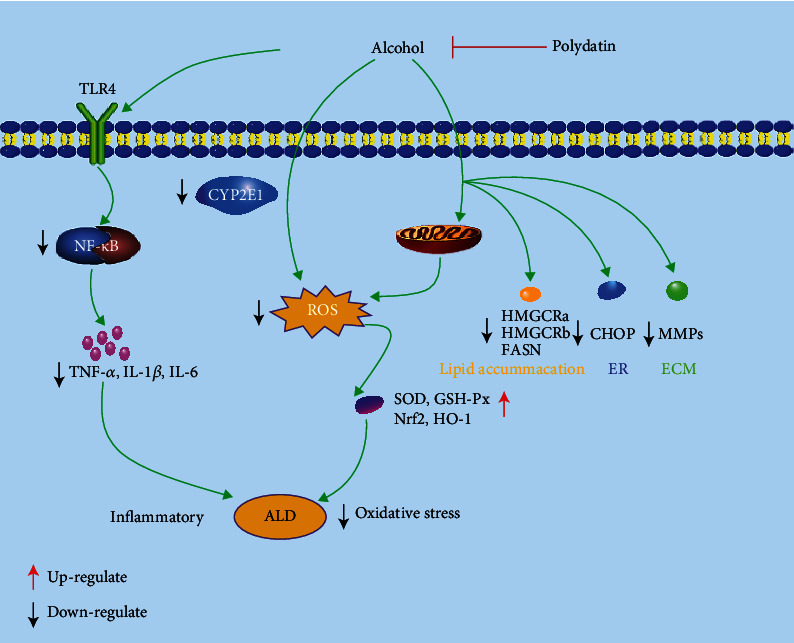
Cellular and molecular mechanisms of PD in the prevention of oxidative-associated alcoholic liver disease.
There is increasing evidence that long-term excessive alcohol intaking can increase the release of inflammatory cytokines [48]. PD treatment decreased the levels of proinflammatory cytokines including interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) through downregulating toll-like receptor 4 (TLR4) and nuclear factor kappa B (NF-κB) p65 [41]. Besides, PD could improve the liver function by decreasing the levels of lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) in the serum [41, 46].
To conclude, previous studies indicated that pretreatment of PD could alleviate liver diseases induced by alcohol. PD exerts its protective activity by resisting the oxidative stress induced by alcohol and restoring the antioxidant balance and the MMP/TIMP ratio of hepatic tissue.
2.2. Polydatin and Nonalcoholic Fatty Liver Diseases
Nowadays, nonalcoholic fatty liver disease (NAFLD) is emerging as one of the most common causes of chronic liver disease due to the increasing incidence of obesity, diabetes, and metabolic syndrome in the general population [49, 50]. NAFLD had affected about 173 million to 338 million people in China, and its prevalence was estimated by 25.2% in the world [36]. NAFLD encompasses a broad spectrum of liver injury ranging from simple triglyceride (TG) accumulation in the liver (steatosis) to nonalcoholic steatohepatitis (NASH), which may lead to fibrosis and cirrhosis [51]. The pathogens of NAFLD involve in lipid accumulation, oxidative stress, and inflammation (Figure 4). It was reported that the first stage of NAFLD was lipid accumulation in the hepatocytes [52]. In normal circumstances, insulin inhibits adipose tissues releasing free fatty acid (FFA). However, with the development of insulin resistance, the increased plasma concentrations of glucose and fatty acids promote hepatic fatty acid synthesis and damage β-oxidation, leading to hepatic steatosis. Hepatic steatosis conversely exacerbates the degree of insulin resistance and accelerates the subsequent transition to steatohepatitis and fibrosis [53, 54]. Supplemented with PD for 12 weeks in methionine- and choline-deficient- (MCD-) induced model rats can alleviate the insulin resistance and improve basal insulin resistance values and glucose tolerance test in homeostasis model assessment. Besides, abnormal adiponectin and leptin levels were also corrected by PD supplementation. Additionally, PD could enhance insulin sensitivity via upregulating expression levels of insulin receptor substrate 2 and Akt phosphorylation in the rat liver induced by a high-fat diet (HFD) [55]. Besides, PD abrogated slight liver steatosis, increased carnitine palmitoyl transferase-1 (CPT-1) and peroxisome proliferator-activated receptor-α (PPAR-α) protein levels, decreased stearoyl-CoA desaturase-1 (SCD-1) and sterol regulatory element binding protein 1 (SREBP-1) protein levels, and reduced total cholesterol (TC) and TG levels in the fructose-fed liver of rats [56]. These results demonstrated that PD could inhibit hepatosteatosis via the reduction of the lipid accumulation.
Figure 4.
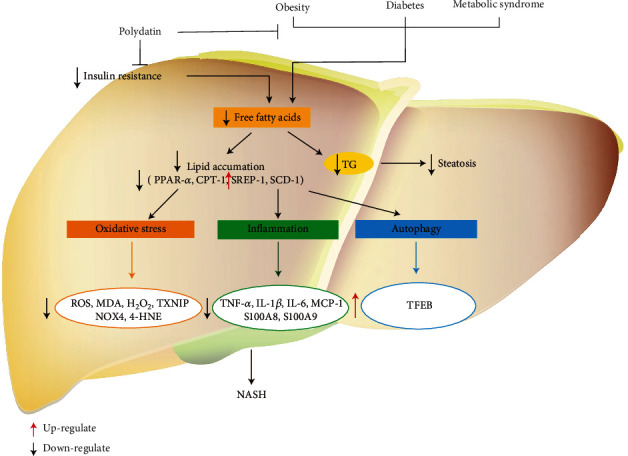
Cellular and molecular mechanisms of PD in the prevention of oxidative-associated nonalcoholic liver diseases.
In NAFLD pathogenesis, oxidative stress is considered as a vital factor [57]. It is reported that PD could alleviate liver oxidative stress in vivo and in vitro. In vivo, PD reduced the levels of malondialdehyde (MDA), ROS, and hydrogen peroxide (H2O2) and decreased thioredoxin-interacting protein (TXNIP) at concentrations of 7.5-30 mg/kg. In vitro, PD could reduce the levels of ROS and TXNIP and enhance miR-200a targeting Keap1/Nrf2 pathway in fructose-induced HepG2 and BRL-3A cells [56]. In pathological conditions, ROS overproduction was induced by nicotinamide adenine dinucleotide phosphate (NADPH) oxidative (NOX). In the NOX family, abnormal expression of NOX4 has been implicated in mice with diet-induced steatohepatitis and patients with NASH related to oxidative stress [58]. Intraperitoneally injected with 5 mg/kg PD reduced oxidative stress by decreasing the levels of NOX4, ROS, and 4-hydroxynonenal (4-HNE) in MCD-induced NASH C57BL/6 mice [59].
Apart from lipid accumulation and oxidative stress, inflammation also plays a crucial role in the development of NAFLD. Excessive fructose and HFD consumption cause NAFLD pathogenesis. PD could downregulate apoptosis-associated speck-like protein (ASC), and the NOD-like receptor family, pyrin domain containing 3 (NLRP3) protein levels and IL-1β were released in the liver of fructose-induced rats [56]. In another study, it was reported that PD treatment for 4 weeks can remarkably reduce Gr-1+ cells and alleviate hepatocyte steatosis and decrease expressions of proinflammatory factors including S100A8, S100A9, and monocyte chemoattractant protein-1 (MCP-1) in the liver tissues of HFD mice [60, 61]. Besides, treatment with PD reduced mRNA levels of proinflammatory cytokines such as IL-6, TNF-α, and CD68 macrophage activation related to the suppression of toll-like receptor (TLR) 4/NF-κB p65 signaling pathway [58, 60].
Autophagy is a system which could regulate intracellular degradation. The development of NASH is considered to be related to impaired autophagic degradation of intracellular lipids. Autophagy regulates lipid metabolism and insulin resistance in the liver and protects hepatocytes from injury and cell death [62]. In vivo, oral administration of 100 mg/kg PD decreased hepatic lipid accumulation and alleviated inflammation and hepatocyte injury in MCD-induced db/db mice. In vitro, PD reduced palmitic acid-induced lipid accumulation in cultured hepatocytes. Both in vivo and in vitro, PD could restore lysosomal function and autophagic flux which was damaged by steatosis or NASH. In conclusion, PD inhibited PI3K/Akt/mTOR signaling pathway and increased the expression and activity of transcription factor EB (TFEB), a known master regulator of lysosomal function [63].
2.3. Polydatin and Liver Injury and Fulminant Hepatic Failure
Currently, lots of the commonly used drugs, including analgesic, anticancer drugs, agent antiphlogistic, and antidepressant, might be hepatotoxicity for human being [64]. Acetaminophen (APAP), a commonly used drug in clinical, was applied for ameliorating fever and pain. At standard doses, APAP exerts remarkable healing effects; however, when taken in overdose amounts, it could initiate acute hepatotoxicity and hepatic injury [65]. In addition, PD was found to show protective effect against APAP-induced hepatotoxicity via improving liver functions, alleviating oxidative stress, and suppressing apoptosis. Pretreatment of PD for 7 days at the doses of 25-100 mg/kg could effectively increase the survival rate of APAP-treated mice, significantly relieve histopathologic alterations in liver, and decrease the levels of AST and ALT in serum. Besides, PD treatment markedly and dose-dependently decreased oxidative stress by decreasing the levels of ROS, MDA, nitric oxide (NO), and GSSG and increasing the liver activities of GSH-Px, GSH, and the GSH/GSSG. Meanwhile, iNOS and NOX2 were also inhibited by PD treatment. Additionally, it is reported that PD significantly inhibited apoptosis of hepatocytes via increasing Bcl-2 and decreasing Bax, Apaf-1, Cyt-C, cleaved- (C-) caspase-9, and C-caspase-3 [66]. In another study, treatment with PD at the doses of 25-100 mg/kg/day has significant protective effect against cisplatin-induced oxidative stress and enhances antioxidant defense enzymes in mice [67].
Sulfur mustard (SM), a chemical warfare agent applied in a series of military conflicts, possesses serious threat to civilians and military soldiers [68]. Although the molecular mechanisms of SM induced hepatotoxicity were still unclear, it is recognized that oxidative stress plays predominant roles in the SM-induced liver damage [69]. PD treatment could dramatically increase the survival rate of mice with subcutaneously injection of SM. Additionally, PD treatment decreased the serum aminotransferase and alleviated SM-induced liver damage in mice. What is more, PD can also remarkably upregulate sirtuin-1 (Sirt1), NAD(P)H, quinone oxidoreductase-1 (NQO1), and Nrf2 and HO-1 in L02 cells and liver tissues of mice [70]. CCl4 can cause severe hepatocellular injury due to its highly toxic metabolite trichloromethyl free radical via the action of the cytochrome P450 system [71]. Intraperitoneal injection of CCl4 (50 μL/kg) markedly induced liver injury in mice with increased serum levels of AST and ALT and upregulated IL-1β, TNF-α, iNOS, COX-2, and NF-κB in hepatic tissues. Besides, CCl4 increased the MDA and decreased the GSH, SOD, GST, CAT, and GPx in liver tissues. Interestingly, pretreatment with PD (25-100 mg/kg/day) for 5 days before CCl4 injection can improve the liver injury via upregulation of transforming growth factor-beta1 (TGF-β1) in the liver tissues [72].
Almost all organ systems including humans and animals could be affected by arsenic (As), as could cause several hazardous effects on animals and humans via inducing oxidative stress [73, 74]. Previous researches have shown that PD treatment could ameliorate the As-induced histopathological damage in tissues, lipid peroxidation, and DNA damage in rats [75]. Another heavy metal named Cd also has a high toxic potential for humans and animals, and long-term exposure in Cd would result in serious damage in liver [76]. Treatment with PD (120 mg/kg) significantly increased the liver total oxidant status (TOS) and decreased the MDA in liver tissue of mice exposed in Cd [77].
Cholestasis might be induced by intrahepatic and systemic retention of toxic hydrophobic bile salts. Cholestatic liver diseases can develop into periportal inflammation, liver fibrosis, cirrhosis, and even hepatic failure [78, 79]. The toxicity of hydrophobic bile salt exposure in the liver can induce oxidative stress, subsequently leading to apoptosis and inflammatory necrosis [80]. PD could improve SOD activity, reduce MDA and serum AST, ALT, ALP, total bile acid (TBA), and total bilirubin (TBIL) levels, and inhibit ER stress, p-elf2α, CHOP, and hepatocellular apoptosis in the cholestatic mice induced by alpha-naphthylisothiocyanate (ANIT) and bile duct ligation (BDL). The results suggest that PD may alleviate cholestatic liver damage by inhibition of oxidative stress, ER stress, and apoptosis [81].
Fulminant hepatic failure (FHF) is characterized by overwhelming hepatic injury with failure of hepatocyte function, resulting in a devastating clinical syndrome of hepatic encephalopathy, severe coagulopathy, jaundice, and hydroperitoneum [82, 83]. Pretreatment with PD (10-100 mg/kg) could decrease the mortality of lipopolysaccharide/D-galactosamine- (LPS/D-GaIN-) induced FHF mice by alleviating liver damage and reducing AST and ALT. Furthermore, PD could also inhibit the TNF-α, endothelial cell adhesion molecule-1 (ECAM-l), intercellular cell adhesion molecule-1 (ICAM-l), NF-κB, and myeloperoxidase (MPO) activities induced by LPS [84].
2.4. Polydatin and Liver Fibrosis
It is reported that uncontrolled simple steatosis might develop to some serious liver diseases such as hepatitis, fibrosis, and cirrhosis [64, 85]. During liver fibrosis, a mass of cellular and molecular events participates in the complex pathological process (Figure 5). Hepatocyte injury is the initial event in response to continuous wounding stimulation [86]. Besides, hepatic stellate cells (HSCs) have been considered playing a vital role in course of liver fibrosis [87, 88]. After a chronic liver damage, HSCs are activated and proliferated and then developed to a myofibroblastic phenotype with upregulated α-smooth muscle actin (α-SMA) that synthesizes ECM proteins, such as type I collagen [89]. However, PD treatment for 3-6 weeks could remarkably downregulate the α-SMA and suppress the increased collagen I and hydroxyproline, an amino acid contained in collagen in liver of mice [91]. Sphingosine kinase 1 (SphK1) plays critical roles in the activation of HSCs and liver fibrosis [92], and SphK1 was strongly induced in mice exposed to CCl4. It is reported that PD could attenuate the proliferation and activation of HSCs via inhibiting SphK1 signaling pathway in CCl4-induced mice, contributing to the suppression of liver fibrosis [95]. Using human immortalized HSC line of LX-2 induced by the platelet-derived growth factor-BB (PDGF-BB) or adenovirus-SphK1, it is reported that PD attenuated the collagen synthesis and apoptosis of hepatocyte and showed significantly antiproliferative effect against HSCs induced by PDGF-BB. Epithelial-mesenchymal transition (EMT) is a crucial biological process for development of fibrosis, and TGF-β1 signaling is one of the most critical profibrotic pathways [97, 98]. Zhao et al. reported that PD treatment (7.5, 15, and 30 mg/kg) for 11 weeks antagonized the nuclear translocation of the Zinc finger E-box binding homeobox 1 (ZEB1) and inhibit survivin-activated TGF-β1/Smad signaling, which was consistent with its protective effect on fructose-induced EMT and liver fibrosis. Inhibiting the nuclear translocation of ZEB1 by PD may be a new strategy to ameliorate EMT of liver fibrosis associated with high-fructose diet [99].
Figure 5.
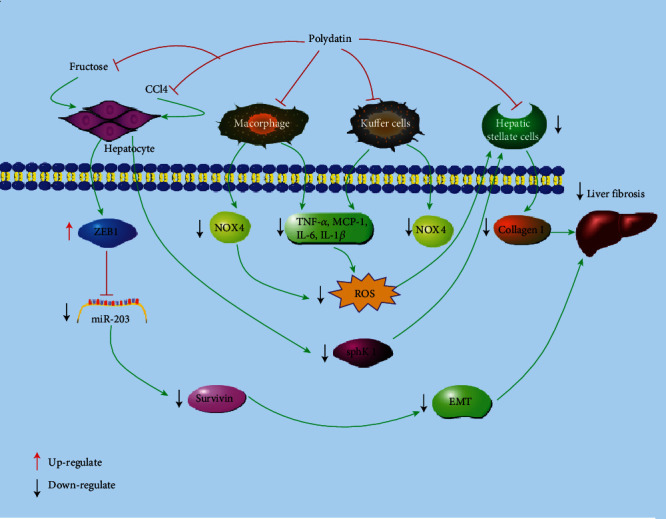
Cellular and molecular mechanisms of PD in the prevention of oxidative-associated liver fibrosis.
However, liver fibrosis was a complex pathophysiological process. If therapeutic methods are only aimed at decreasing the activation of HSCs, it often leads to undesirable outcomes [97]. Liver fibrosis is involving the mutual interaction between parenchymal hepatocytes and nonparenchymal liver cells, including HSCs, Kupffer cells (KCs), and macrophages [94, 95]. The activated KCs constitute a central component of the inflammatory response in liver fibrosis by producing amount proinflammatory cytokines such as MCP-1, TNF-α, and TGF-β1 and mediators related to oxidative stress that induce quiescent HSCs to differentiate into activated myofibroblasts, the principle ECM-synthesizing cells which play as the vital executor in hepatic fibrogenesis. In return, the activated HSCs promote the recruitment of macrophages from the bone marrow to augment the already-large number of KCs, further aggravating the deterioration of inflammation and fibrogenesis [90, 96]. Both in vivo and in vitro results suggested that polydatin-loaded-micelle (PD-MC) could remarkably decrease liver cell apoptosis and avoid HSCs and macrophage activation and inhibit inflammatory response by suppressing the activation of TLR4/NF-κB p65 signaling and proinflammatory cytokines secretion in macrophages and oxidative stress [97].
2.5. Polydatin and Hepatocellular Carcinoma
Cancers are the leading killers for human being in the world, especially for aged people over than 55 years old. Besides surgery, chemotherapy remains the best choice for treating various cancers. In recent years, increasing scientific evidences have suggested that natural agents are precious resources for finding more novel and safe candidate drugs for treating cancers, including hepatocellular carcinoma (HCC), lung cancer, and breast cancer [100, 101]. Patients with chronic liver diseases and cirrhosis might result in HCC, a common primary malignancy in the liver. HCC is the third leading cause of cancer-related deaths in the world [94]. Unfortunately, the currently available chemotherapeutic agents are not practical for the treatment of advanced HCC [101–103]. In this regard, it is necessary to develop more effective compounds, which may provide a novel therapy for HCC treatment, especially in the advanced stage. PD can promote SMMC-7721 and HepG2 cell apoptosis via upregulating Bax/Bcl-2 ratio and suppressing proliferation by decreasing the Wnt/β-catenin signaling in hepatocellular carcinoma. The invasion and migration of cancer cells are believed to promote the metastasis of cancer to a large extent. HCC cell invasion and migration invasion assay and wound healing assay were suppressed by treatment with PD [104]. Therefore, PD might be a promising natural small molecule drug for early liver cancer treatment.
3. Clinical Reports and Toxicity Studies
In clinical practice, there was no research published on PD single used for treating liver diseases. However, lots of Chinese patent medicines, such as Yi-du-Tiao-gan mixture, liver Corelle tablet, and Hu-gan-ning capsule, have been used in clinical practice for the treatment of liver disease in China. In these Chinese patent medicines, P. cuspidatum was one of the primary medicinal materials and PD was one of the main active ingredients [105–107]. Only one team studied the clinical efficacy of PD in treating coronary heart disease in the elderly. The results illustrated that the effective of experimental group treatment rate was 91.67%, dramatically higher than that of the control group, 76.67%. The effectiveness of PD to treat elderly coronary heart disease was definite [108]. Besides, PD injection, applied in treating myocardial ischemia, cerebral ischemia, shock, and other cardiovascular and cerebrovascular diseases, has been approved to enter phase II clinical trials by the US Food and Drug Administration [109].
PD has a favorable safety profile in animals (up to a dose of 200 mg/kg) and was well tolerated in humans (40 mg twice a day for 90 days in phase II clinical trial). The safe evaluation was completed by the New Drugs Safety Evaluation and Research Center in the Chinese Academy of Medical Sciences, which demonstrated that no significant toxic effect existed after intravenous injection of PD for 30 days [110]. Another research reported that the LD50 of PD was (1000 ± 57.3) mg/kg injected intraperitoneally [111]. However, few researches are assessing the adverse reactions of PD in liver diseases. Only in one randomized clinical trial PD has been shown to exert a marked effect on abdominal pain in patients with irritable bowel syndrome through dietary supplementation. Peritonitis, some liver cell necrosis, and bone marrow fat hyperplasia would occur in varying degrees when intraperitoneal injection concentrations of 50, 150, and 700 mg/kg of PD for 42 days [112]. Gavaged with the maximum concentration and the maximum gavage volume of PD to mice, the survival rate of mice was 100%, and the accumulated maximum tolerable dose (MTD) amount per day is 75.5 g/kg. The IC50 of PD for human normal liver cells L02 is 263.05 μg/mL [113].
4. Conclusion and Perspectives
Collectively, according to the abovementioned effects and mechanisms of polydatin (PD) on liver diseases, it is highly suggested PD is an effective natural product for treating oxidative stress-associated liver diseases, including alcoholic liver diseases, nonalcoholic liver diseases, liver injury, liver fibrosis, and hepatocellular carcinoma. Experimental evidence indicated that PD exhibits curative effect against liver diseases through various signaling pathways, such as PI3K/Akt/Nrf2/HO-1 and Sirt1/Nrf2, PI3K/Akt/mTOR. Overall, this review highlights the potential application of PD as a potential agent against liver diseases.
In past decades, lots of researches have focused on the preclinical therapeutical effects of PD against various liver diseases. However, the detail molecular mechanisms were not thoroughly explored. Modern research methods such as genomics, metabolomics, proteomics, and metagenomics can be used to conduct more in-depth investigations on the corresponding mechanisms of PD on liver diseases, which provide strong support and theoretical basis for the subsequent clinical research and ultimately developed this natural compound to a new drug for liver disorders [114–116]. Secondly, although researchers have concentrated on LD50 of PD, the study of reproductive, carcinogenic, and teratogenic toxicity was not involved. PD has a higher bioavailability than resveratrol, but its concentration in liver tissue was relatively low; intravenous injection of 20 mg/kg of PD, the maximum concentration in liver is 5.22 g ± 0.46 μg/kg; for oral administration of 50 mg/kg of PD, the maximum concentration in liver is 4.47 ± 2.51 μg/kg [117, 118]. Recently, it is suggested that the targeted drug delivery systems based on microenvironment sensitive polymeric nanocarriers had great potentials to increase the drugs' bioavailability, improving the therapeutic efficacy and minimizing the drug side effects [119]. It is necessary to find or synthesize related biopharmaceutical materials corresponding the characteristics of liver diseases to prepare new dosage forms of PD to ameliorate the therapeutic efficacy [120]. Therefore, potential safety hazard and restricted efficacy of PD remain to conquer for further clinical practices [96]. Finally, PD and curcumin have similar pharmacological effects on oxidative stress associated with liver disorders [4]. Will combination of PD and curcumin shows a more substantial therapeutic effect on liver diseases? It is worth to be further studied.
Acknowledgments
This research was supported by the Project of Administration of Traditional Chinese Medicine of Sichuan Province of China (No. 2021MS460 and No. 2020HJZX001), Sichuan Science and Technology Program (No. 2020YFS0523), and Xinglin Scholar Discipline Promotion Talent Program of Chengdu University of Traditional Chinese Medicine (No. BSH2018006).
Abbreviations
- α-SMA:
α-Smooth muscle actin
- AGEs:
Advanced glycation end products
- ADH:
Alcohol dehydrogenase
- ALB:
Albumin
- ALDs:
Alcohol liver diseases
- ALDH:
Aldehyde dehydrogenase
- ALP:
Alkaline phosphatase
- ALT:
Alanine aminotransferase
- ANIT:
Alpha-naphthylisothiocyanate
- APAP:
Acetaminophen
- ASC:
Apoptosis-associated speck-like protein
- As:
Arsenic
- AST:
Aspartate aminotransferase
- Bax:
BCL-2-associated
- BDL:
Bile duct ligation
- Bcl-2:
B-cell lymphoma-2
- CAT:
Catalase
- CD:
Cadmium chlorine
- CHOP:
C/EBP homologous protein
- CYP2E1:
Cytochrome P4502E1
- CPT-1:
Carnitine palmitoyl transferase-1
- Cyt-C:
Cytochrome c
- ECAM-l:
Endothelial cell adhesion molecule-1
- ECM:
Extracellular matrix
- EMT:
Epithelial-mesenchymal transition
- ER:
Endoplasmic reticulum stress
- FASN:
Fatty acid synthase
- FFA:
Free fatty acid
- FHF:
Fulminant hepatic failure
- GADD45αa:
Growth arrest and DNA damage-inducible gene, 45αa
- GPx:
Glutathione peroxidase
- GR:
Glutathione reductase
- GSH:
Glutathione
- H2O2:
Hydrogen peroxide
- HCC:
Hepatocellular carcinoma
- HSCs:
Hepatic stellate cells
- HO-1:
Heme oxygenase-1
- IL-6:
Interleukin-6
- IL-1β:
Interleukin-1β
- ICAM-1:
Intercellular cell adhesion molecule-1
- KCs:
Kupffer cells
- LDH:
Lactate dehydrogenase
- LX-2:
HSC line
- MCD:
Methionine-and choline-deficient
- MMPs:
Matrix metalloproteinases
- LPS:
Lipopolysaccharide
- MCP-1:
Monocyte chemoattractant protein-1
- MDA:
Malondialdehyde
- mTOR:
Phosphoinosmde-3-kinase/the mammalian target of rapamycin
- MTD:
Maximum tolerable dose
- MPO:
Myeloperoxidase
- NADPH:
Nicotinamide adenine dinucleotide phosphate oxidative (NOX)
- NAFLDs:
Nonalcoholic liver diseases
- NASH:
Nonalcoholic steatohepatitis
- NF-κB:
Nuclear factor kappa B
- NLRP3:
The NOD-like receptor family, pyrin domain containing 3
- NQO1:
NAD(P)H, quinone oxidoreductase-1
- NO:
Nitric oxide
- NOX:
Nicotinamide adenine dinucleotide phosphate oxidative
- Nrf2:
Nuclear factor erythroid 2-related factor 2
- PD:
Polydatin
- PDGF-BB:
Platelet-derived growth factor-BB
- PD-MC:
Polydatin-loaded-micelle
- PI3K:
Phosphatidylinositol 3-kinase
- PPAR-α:
Peroxisome proliferator activated receptor-α
- RNS:
Reactive nitrogen species
- ROS:
Reactive oxygen species
- SCD-1:
Stearoyl-CoA desaturase-1
- Sirt1:
Sirtuin-1
- SM:
Sulfur mustard
- SOD:
Superoxide dismutase
- SphK1:
Sphingosine kinase 1
- SREBP-1:
Sterol regulatory element binging protein 1
- TBA:
Total bile acid
- TBIL:
Total bilirubin
- TC:
Total cholesterol
- TFEB:
Transcription factor EB
- TG:
Triglyceride
- TGF-β1:
Transforming growth factor-beta1
- TLR4:
Toll-like receptor 4
- TNF-α:
Tumor necrosis factor-α
- TOS:
Total oxidant status
- TXNIP:
Thioredoxin-interacting protein
- ZEB1:
Zinc finger E-box binding homeobox 1.
Contributor Information
Wei Peng, Email: pengwei@cdutcm.edu.cn.
Chunjie Wu, Email: wucjcdtcm@163.com.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Dandan Tang and Qing Zhang contributed equally to this work.
References
- 1.Muriel P. Liver Pathophysiology . Amsterdam, The Netherlands: Elsevier; 2017. The Liver: General Aspects and Epidemiology. [Google Scholar]
- 2.Buzzetti E., Pinzani M., Tsochatzis E. A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism . 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Cichoż-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World Journal of Gastroenterology . 2014;25(25):8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammad F., Mahdi Z., Fatemeh P. Curcumin in liver diseases: a systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients . 2018;10(7):p. 855. doi: 10.3390/nu10070855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palma H. E., Wolkmer P., Gallio M., et al. Oxidative stress parameters in blood, liver, and kidney of diabetic rats treated with curcumin and/or insulin. Molecular and Cellular Biochemistry . 2014;386(1-2):199–210. doi: 10.1007/s11010-013-1858-5. [DOI] [PubMed] [Google Scholar]
- 6.Xu L. Q., Xie Y. L., Gui S. H., et al. Polydatin attenuates D-galactose-induced liver and brain damage through its anti-oxidative, anti-inflammatory and anti-apoptotic effects in mice. Food & Function . 2016;7(11):4545–4555. doi: 10.1039/C6FO01057A. [DOI] [PubMed] [Google Scholar]
- 7.Valko M., Rhodes C. J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions . 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Sarma A. D., Mallick A. R., Ghosh A. K. Free radicals and their role in different clinical conditions: an overview. International Journal of Pharma Sciences and Research . 2010;1(3):185–192. [Google Scholar]
- 9.Li S., Tan H. Y., Wang N., et al. The role of oxidative stress and antioxidants in liver diseases. International Journal of Molecular Sciences . 2015;16(11):26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Videla L. A. Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. World Journal of Hepatology . 2009;1(1):72–78. doi: 10.4254/wjh.v1.i1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Świderska M., Maciejczyk M., Zalewska A., Pogorzelska J., Flisiak R., Chabowski A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radical Research . 2019;53(8):841–850. doi: 10.1080/10715762.2019.1635691. [DOI] [PubMed] [Google Scholar]
- 12.Chen L., Zhao X., Wei S., et al. Mechanism of paeoniflorin on ANIT-induced cholestatic liver injury using integrated metabolomics and network pharmacology. Frontiers in Pharmacology . 2021;12, article 737630 doi: 10.3389/fphar.2021.737630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sang R., Yu Y., Ge B., Xu L., Wang Z., Zhang X. Taraxasterol fromTaraxacumprevents concanavalin A-induced acute hepatic injury in mice via modulating TLRs/NF-κB and Bax/Bc1-2 signalling pathways. Artifical Cells Nanomedicine and Biotechnology . 2019;47(1):3929–3937. doi: 10.1080/21691401.2019.1671433. [DOI] [PubMed] [Google Scholar]
- 14.Xu H., Chen G. F., Ma Y. S., et al. Hepatic proteomic changes and Sirt 1/AMPK signaling activation by oxymatrine treatment in rats with non-alcoholic steatosis. Frontiers in Pharmacology . 2020;11:p. 216. doi: 10.3389/fphar.2020.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang A. H., Sun H., Wang X. J. Recent advances in natural products from plants for treatment of liver diseases. European Journal of Medicinal Chemistry . 2013;63(33):570–577. doi: 10.1016/j.ejmech.2012.12.062. [DOI] [PubMed] [Google Scholar]
- 16.Peng W., Qin R. X., Li X. L., Zhou H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: A review. Journal of Ethnopharmacology . 2013;148(3):729–745. doi: 10.1016/j.jep.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C. S., Xiang Y. H., Xiao J. B., Lei Q. F. Quantitative determination of resveratrol and piceid in Polygonum Cuspidatum Sieb.et Zucc.by HPLC. Chinese Journal of Pharmaceutical Analysis . 2005;25(5):534–536. [Google Scholar]
- 18.Wang H. Y. Effect of GA3 Treatment on Quality of Two Table Grape Varieties . Shuo Shi Lun Wen: Northwest A & F University; 2017. [Google Scholar]
- 19.Chen X. X. The Content of Resveratrol, Piceid in Polygonum Cuspidatum and Resveratrol in Arachis Hypogaes . Shuo Shi Lun Wen: Fujian Normal University; 2004. [Google Scholar]
- 20.Wang Y. S., Zhu G. H., Wang B., et al. Influences of ancient processing method steaming with black bean and drying and pharmacopoeia processing method continuous steaming with black bean decoction on 12 components of Polygoni Multiflori Radix. Chinese Traditional and Herbal Drugs . 2020;51(19):4972–4982. [Google Scholar]
- 21.Zhao K., Su Z. R., Yang B. W., Tan F., Deng J. Determination of resveratrol and polydatin in mulberry. Food Science . 2010;31(14):241–244. [Google Scholar]
- 22.Chen L., Lan Z. Polydatin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation by inhibiting NF-κB/NLRP3 inflammasome activation via the AMPK/SIRT1 pathway. Food & Function . 2017;8(5):1785–1792. doi: 10.1039/C6FO01561A. [DOI] [PubMed] [Google Scholar]
- 23.Guan S. Y., Zhang K., Wang X. S., et al. Anxiolytic effects of polydatin through the blockade of neuroinflammation in a chronic pain mouse model. Molecular Pain . 2020;16 doi: 10.1177/1744806919900717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordaro M., Impellizzeri D., Siracusa R., et al. Effects of a co-micronized composite containing palmitoylethanolamide and polydatin in an experimental model of benign prostatic hyperplasia. Toxicology and Applied Pharmacology . 2017;329:231–240. doi: 10.1016/j.taap.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Hu T. Z., Fei Z. H., Su H. F., Xie R. Y., Chen L. T. Polydatin inhibits proliferation and promotes apoptosis of doxorubicin- resistant osteosarcoma through LncRNA TUG1 mediated suppression of Akt signaling. Toxicology and Applied Pharmacology . 2019;371:55–62. doi: 10.1016/j.taap.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Xu G., Kuang G., Jiang W. G., Jiang R., Jiang D. Polydatin promotes apoptosis through upregulation the ratio of Bax/Bcl-2 and inhibits proliferation by attenuating the β-catenin signaling in human osteosarcoma cells. American Journal of Translational Research . 2016;8(2):922–931. [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Ye J. T., Li J., et al. Polydatin ameliorates lipid and glucose metabolism in type 2 diabetes mellitus by downregulating proprotein convertase subtilisin/kexin type 9 (PCSK9) Cardiovascular Diabetology . 2016;15(1):p. 19. doi: 10.1186/s12933-015-0325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M. M., Zhao Z. J., Shen M., et al. Polydatin protects cardiomyocytes against myocardial infarction injury by activating Sirt3. Biochimica et Biophysica Acta-Molecular Basis Disease . 2017;1863(8):1962–1972. doi: 10.1016/j.bbadis.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y., Xue L., Du W., et al. Polydatin restores endothelium-dependent relaxation in rat aorta rings impaired by high glucose: a novel insight into the PPARβ-NO signaling pathway. PLoS One . 2015;10(5, article e0126249) doi: 10.1371/journal.pone.0126249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q., Tan Y., Zhang N., Yao F. Polydatin prevents angiotensin II-induced cardiac hypertrophy and myocardial superoxide generation. Experimental Biology and Medicine . 2015;240(10):1352–1361. doi: 10.1177/1535370214561958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park B., Jo K., Lee T. G., Hyun S. W., Kim J. S., Kim C. S. Polydatin inhibits NLRP3 inflammasome in dry eye disease by attenuating oxidative stress and inhibiting the NF-κB pathway. Nutrients . 2019;11(11):p. 2792. doi: 10.3390/nu11112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peritore A. F., D’Amico R., Cordaro M., et al. PEA/polydatin: anti-inflammatory and antioxidant approach to counteract DNBS-induced colitis. Antioxidants . 2021;10(3):p. 464. doi: 10.3390/antiox10030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gugliandolo E., Fusco R., Biundo F., et al. Palmitoylethanolamide and polydatin combination reduces inflammation and oxidative stress in vascular injury. Pharmacology Research . 2017;123:83–92. doi: 10.1016/j.phrs.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Chen G., Yang Z., Wen D., et al. Polydatin has anti-inflammatory and antioxidant effects in LPS-induced macrophages and improves DSS-induced mice colitis. Immunity, Inflammation and Disease . 2021;9(3):959–970. doi: 10.1002/iid3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loi E. S., Pontis A., Cofelice V., et al. Effect of ultramicronized- palmitoylethanolamide and co-micronized palmitoylethanolamide/polydatin on chronic pelvic pain and quality of life in endometriosis patients: an open-label pilot study. International Journal of Women’s Health . 2019;11:443–449. doi: 10.2147/IJWH.S204275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao J., Wang F., Wong N. K., et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. Journal of Hepatology . 2019;71(1):212–221. doi: 10.1016/j.jhep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Wang M., Zhang X. J., Liu F., et al. Saponins isolated from the leaves of Panax notoginseng protect against alcoholic liver injury via inhibiting ethanol-induced oxidative stress and gut-derived endotoxin-mediated inflammation. Journal of Functional Foods . 2015;19:214–224. doi: 10.1016/j.jff.2015.09.029. [DOI] [Google Scholar]
- 38.Albano E. Oxidative stress in alcoholic liver disease. In: Albano E., Parola M., editors. Studies on Hepatic Disorders. Oxidative Stress in Applied Basic Research and Clinical Practice . Cham: Humana Press; 2015. [DOI] [Google Scholar]
- 39.Liangpunsakul S., Haber P., McCaughan G. W. Alcoholic liver disease in Asia, Europe, and North America. Gastroenterology . 2016;150(8):1786–1797. doi: 10.1053/j.gastro.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashita H., Goto M., Matsui-Yuasa I., Kojima Yuasa A. Ecklonia cava polyphenol has a protective effect against ethanol-induced liver injury in a cyclic AMP-dependent manner. Marine Drugs . 2015;13(6):3877–3891. doi: 10.3390/md13063877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Q. H., Xu L. Q., Liu Y. H., et al. Polydatin Protects Rat Liver against Ethanol-Induced Injury: Involvement of CYP2E1/ROS/Nrf2 and TLR4/NF-B p65 Pathway. Evidence-Based Complementary and Alternative Medicine . 2017;2017:15. doi: 10.1155/2017/7953850.7953850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai Y. L., Zhou C. Y., Huang P., et al. Polydatin alleviated alcoholic liver injury in zebrafish larvae through ameliorating lipid metabolism and oxidative stress. Journal of Pharmacological Sciences . 2018;138(1):46–53. doi: 10.1016/j.jphs.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Rutkowski D. T., Wu J., Back S. H., et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Developmental Cell . 2008;15(6):829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaeschke H., Gores G. J., Cederbaum A. I., Hinson J. A., Pessayre D., Lemasters J. J. Mechanisms of hepatotoxicity. Toxicological Sciences . 2002;65(2):166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 45.Banerjee P., Jana S., Chakraborthy S., Swarnakar S. Inflammation and MMPs in alcohol-induced liver diseases and protective action of antioxidants. Indian Journal of Biochemistry & Biophysics . 2013;50(5):377–386. [PubMed] [Google Scholar]
- 46.Koneru M., Sahu B. D., Gudem S., et al. Polydatin alleviates alcohol-induced acute liver injury in mice: relevance of matrix metalloproteinases (MMPs) and hepatic antioxidants. Phytomedicine . 2017;27:23–32. doi: 10.1016/j.phymed.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Nassir F., Ibdah J. A. Role of mitochondria in alcoholic liver disease. World Journal of Gastroenterology . 2014;20(9):2136–2142. doi: 10.3748/wjg.v20.i9.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J. W., Chen X. Y., Hu P. Y., et al. Effects of linderae radix extracts on a rat model of alcoholic liver injury. Experimental & Therapeutic Medicine . 2016;11(6):2185–2192. doi: 10.3892/etm.2016.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marra F., Gastaldelli A., Svegliati Baroni G., Tell G., Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends in Molecular Medecine . 2008;14(2):72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Younossi Z. M., Koenig A. B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology . 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 51.Brunt E. M. Pathology of Nonalcoholic Fatty Liver Disease. American Journal of Clinical Pathology . 2007;128(5):837–847. doi: 10.1309/RTPM1PY6YGBL2G2R. [DOI] [PubMed] [Google Scholar]
- 52.Ko J., Kim K. Effects of exercise and diet composition on expression of MCP-1 and oxidative stress-related mRNA of adipose tissue in diet-induced obese mice. Journal of Exercise Nutrition & Biochemistry . 2013;17(4):181–188. doi: 10.5717/jenb.2013.17.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jou J., Choi S., Diehl A. Mechanisms of disease progression in nonalcoholic fatty liver disease. Seminars Liver Diseases . 2008;28(4):370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 54.Tilg H., Moschen A. R. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends in Endocrinology & Metabolism . 2008;19(10):371–379. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Q., Ying Y., Zhang N., Yao F. R. Polydatin supplementation ameliorates diet-induced development of insulin resistance and hepatic steatosis in rats. Molecular Medicine Reports . 2015;11(1):603–610. doi: 10.3892/mmr.2014.2708. [DOI] [PubMed] [Google Scholar]
- 56.Zhao X. J., Yu H. W., Yang Y. Z., et al. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biology . 2018;18:124–137. doi: 10.1016/j.redox.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spahis S., Delvin E., Borys J. M., Levy E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxidants & Redox Signaling . 2017;26(10):519–541. doi: 10.1089/ars.2016.6776. [DOI] [PubMed] [Google Scholar]
- 58.Bettaieb A., Jiang J. X., Sasaki Y., et al. Hepatocyte nicotinamide adenine dinucleotide phosphate reduced oxidase 4 regulates stress signaling, fibrosis, and insulin sensitivity during development of steatohepatitis in mice. Gastroenterology . 2015;149(2):468–480.e10. doi: 10.1053/j.gastro.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li R., Li J. Z., Huang Y. J., et al. Polydatin attenuates diet-induced nonalcoholic steatohepatitis and fibrosis in mice. International Journal of Biological Sciences . 2018;14(11):1411–1425. doi: 10.7150/ijbs.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J. M., Tan Y. Y., Yao F. R., Zhang Q. Polydatin alleviates non-alcoholic fatty liver disease in rats by inhibiting the expression of TNF-α and SREBP-1c. Molecular Medicine Reports . 2012;6(4):815–820. doi: 10.3892/mmr.2012.1015. [DOI] [PubMed] [Google Scholar]
- 61.Mo J. F., Wu J. Y., Li Z. Therapeutic efficacy of polydatin for nonalcoholic fatty liver diseaseviaregulating inflammatory response in obese mice. RSC Advances . 2018;8(54):31194–31200. doi: 10.1039/C8RA05915B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neuschwander-Tetri B. A. Nontriglyceride hepatic lipotoxicity: the new paradigm for the pathogenesis of NASH. Current Gastroenterology Reports . 2010;12(1):49–56. doi: 10.1007/s11894-009-0083-6. [DOI] [PubMed] [Google Scholar]
- 63.Chen X. T., Chan H., Zhang L., et al. The phytochemical polydatin ameliorates non-alcoholic steatohepatitis by restoring lysosomal function and autophagic flux. Journal of Cellular and Molecular Medicine . 2019;23(6):4290–4300. doi: 10.1111/jcmm.14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mortezaee K., Khanlarkhani N. Melatonin application in targeting oxidative- induced liver injuries: a review. Journal of Cellular Physiology . 2017;233(5):4015–4032. doi: 10.1002/jcp.26209. [DOI] [PubMed] [Google Scholar]
- 65.Jaeschke H. Acetaminophen: dose-dependent drug hepatotoxicity and acute liver failure in patients. Digestive Diseases . 2015;33(4):464–471. doi: 10.1159/000374090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y. H., Huang Q. H., Wu X., et al. Polydatin protects against acetaminophen-induced hepatotoxicity in miceviaanti-oxidative and anti-apoptotic activities. Food & Function . 2018;9(11):5891–5902. doi: 10.1039/C8FO01078A. [DOI] [PubMed] [Google Scholar]
- 67.Ince S., Acaroz D. A., Neuwirth O. Protective effect of polydatin, a natural precursor of resveratrol, against cisplatin-induced toxicity in rats. Food and Chemical Toxicology . 2014;72:147–153. doi: 10.1016/j.fct.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 68.Sun J. H., Min X. G., Huang Y., Gang L. I., Pang Q. X. Experience and enlightenment of medical support in ‘8.4’ intoxication accident. Hospital Administration Journal of Chinese People’s Liberation Army . 2004;11(6):p. 365. [Google Scholar]
- 69.Wei M. Q., Pei Z. P., Feng Y. W. Neglected role of hydrogen sulfide in sulfur mustard poisoning: Keap1 S-sulfhydration and subsequent Nrf2 pathway activation. Scientific Reports . 2017;7(1):p. 9433. doi: 10.1038/s41598-017-09648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang H., Chen Y. C., Pei Z. P., et al. Protective effects of polydatin against sulfur mustard-induced hepatic injury. Toxicology and Applied Pharmacology . 2019;367:1–11. doi: 10.1016/j.taap.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 71.Weerachayaphorn J., Chuncharunee A., Jariyawat S., et al. Protection of centrilobular necrosis by _Curcuma comosa_ Roxb. in carbon tetrachloride-induced mice liver injury. Journal of Ethnopharmacology . 2010;129(2):254–260. doi: 10.1016/j.jep.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 72.Zhang H., Yu C. H., Jiang Y. P., et al. Protective effects of polydatin from Polygonum cuspidatum against carbon tetrachloride-induced liver Injury in mice. Plos One . 2012;7(9) doi: 10.1371/journal.pone.0046574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin Y. P., Sun G. F., Li X., Li G. X., Lu C. W., Qu L. Study on the toxic effects induced by different arsenicals in primary cultured rat astroglia. Toxicology and Applied Pharmacology . 2004;196(3):396–403. doi: 10.1016/j.taap.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 74.Sinha M., Manna P., Sil P. C. Protective effect of arjunolic acid against arsenic-induced oxidative stress in mouse brain. Journal of Biochemical and Molecular Toxicology . 2008;22(1):15–26. doi: 10.1002/jbt.20209. [DOI] [PubMed] [Google Scholar]
- 75.Acaroz D. A., Zemheri F., Demirel H. H., Kucukkurt I., Ince S., Eryavuz A. In vivo assessment of polydatin, a natural polyphenol compound, on arsenic-induced free radical overproduction, gene expression, and genotoxicity. Environmental Science and Pollution Research International . 2018;25(3):2614–2622. doi: 10.1007/s11356-017-0391-6. [DOI] [PubMed] [Google Scholar]
- 76.Khan M. A., Khan S., Khan A., Alam M. Soil contamination with cadmium, consequences and remediation using organic amendments. Science of the Total Environment . 2017;601-602:1591–1605. doi: 10.1016/j.scitotenv.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 77.Evcimen M., Aslan R., Gulay M. S. Protective effects of polydatin and grape seed extract in rats exposed to cadmium. Drug and Chemical Toxicology . 2020;43(3):1–9. doi: 10.1080/01480545.2018.1480629. [DOI] [PubMed] [Google Scholar]
- 78.Beuers U., Boyer J. L., Paumgartner G. Ursodeoxycholic acid in cholestasis: potential mechanisms of action and therapeutic applications. Hepatology . 1998;28(6):1449–1453. doi: 10.1002/hep.510280601. [DOI] [PubMed] [Google Scholar]
- 79.de Vries E., Beuers U. Management of cholestatic disease in 2017. Liver International . 2017;37(S1):123–129. doi: 10.1111/liv.13306. [DOI] [PubMed] [Google Scholar]
- 80.Burban A., Sharanek A., Guguen-Guillouzo C., Guillouzo A. Endoplasmic reticulum stress precedes oxidative stress in antibiotic-induced cholestasis and cytotoxicity in human hepatocytes. Free Radical Biology Medecine . 2018;115:166–178. doi: 10.1016/j.freeradbiomed.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 81.Fang J. N., Luo L. L., Ke Z. L., et al. Polydatin protects against acute cholestatic liver injury in mice via the inhibition of oxidative stress and endoplasmic reticulum stress. Journal of Functional Foods . 2019;55:175–183. doi: 10.1016/j.jff.2019.02.029. [DOI] [Google Scholar]
- 82.Antoniades C. G., Berry P. A., Wendon J. A., Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. Journal of Hepatology . 2008;49(5):845–861. doi: 10.1016/j.jhep.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 83.Possamai L. A., Antoniades C. G., Anstee Q. M. Role of monocytes and macrophages in experimental and human acute liver failure. World Journal of Gastroenterology . 2010;16(15):1811–1819. doi: 10.3748/wjg.v16.i15.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu M. J., Gong X., Jiang R., Zhang L., Li X. H., Wan J. Y. Polydatin protects against lipopolysaccharide-induced fulminant hepatic failure in D-galactosamine-sensitized mice. International Journal of Immunopathology and Pharmacology . 2012;25(4):923–934. doi: 10.1177/039463201202500410. [DOI] [PubMed] [Google Scholar]
- 85.Cydylo M. A., Davis A. T., Kavanagh K. Fatty liver promotes fibrosis in monkeys consuming high fructose. Obesity . 2017;25(2):290–293. doi: 10.1002/oby.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Canbay A., Friedman S., Gores G. J. Apoptosis: the nexus of liver injury and fibrosis. Hepatology . 2004;39(2):273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 87.Lee U. E., Friedman S. L. Mechanisms of hepatic fibrogenesis. Best Practice & Research Clinical Gastroenterology . 2011;25(2):195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Friedman S. L., Roll F. J., Boyles J., Bissell D. M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proceedings of the National Academy of Sciences . 1985;82(24):8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Novo E., Cannito S., Paternostro C., Bocca C., Miglietta A., Parola M. Cellular and molecular mechanisms in liver fibrogenesis. Archives Biochemistry and Biophysics . 2014;548(1):20–37. doi: 10.1016/j.abb.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 90.Friedman S. L. Mechanisms of hepatic fibrogenesis. Gastroenterology . 2004;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao X. Y., Li R., Liu Y., et al. Polydatin protects against carbon tetrachloride-induced liver fibrosis in mice. Archives Biochemistry and Biophysics . 2017;629(2):1–7. doi: 10.1016/j.abb.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 92.Lan T., Li C., Yang G., et al. Sphingosine kinase 1 promotes liver fibrosis by preventing miR-19b-3p-mediated inhibition of CCR2. Hepatology . 2018;68(3):1070–1086. doi: 10.1002/hep.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lan T., Zhuang L., Li S., Yang G., Guo J. Polydatin attenuates hepatic stellate cell proliferation and liver fibrosis by suppressing sphingosine kinase 1. Biomedicine & Pharmacotherapy . 2020;130:p. 110586. doi: 10.1016/j.biopha.2020.110586. [DOI] [PubMed] [Google Scholar]
- 94.Trautwein C., Friedman S. L., Schuppan D., Pinzani M. Hepatic fibrosis: concept to treatment. Journal of Hepatology . 2015;62(1):S15–S24. doi: 10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 95.Seki E., Schwabe R. F. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology . 2015;61(3):1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Friedman S. L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiological Reviews . 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin L. T., Gong H. Y., Li R., et al. Nanodrug with ROS and pH dual-sensitivity ameliorates liver fibrosis via multicellular regulation. Advanced Science . 2020;7(7):p. 1903138. doi: 10.1002/advs.201903138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park J. H., Park B., Park K. K. Suppression of hepatic Epithelial-to-Mesenchymal transition by melittin via blocking of TGFβ/Smad and MAPK-JNK signaling pathways. Toxins . 2017;9(4):p. 138. doi: 10.3390/toxins9040138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao X. J., Yang Y. Z., Yu H. W., et al. Polydatin inhibits ZEB1-invoked epithelial- mesenchymal transition in fructose- induced liver fibrosis. Journal of Cellular and Molecular Medicine . 2020;24(22):13208–13222. doi: 10.1111/jcmm.15933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu L. S., Jia M., Chen L. Cytotoxic and antifungal constituents isolated from the metabolites of endophytic fungus DO14 from Dendrobium officinale. Molecules . 2016;21(1):p. 14. doi: 10.3390/molecules21010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Newman D. J., Cragg G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. Journal of Natural Product . 2020;83(3):770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 102.Kudo M., Trevisani F., Abou-Alfa G. K., Rimassa L. Hepatocellular carcinoma: therapeutic guidelines and medical treatment. Liver Cancer . 2017;6(1):16–26. doi: 10.1159/000449343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakamoto Y. Promising new strategies for hepatocellular carcinoma. Hepatology Research . 2017;47(4):251–265. doi: 10.1111/hepr.12795. [DOI] [PubMed] [Google Scholar]
- 104.Yang J., Wu Y., Du D. Polydatin inhibits cell proliferation, invasion and migration, and induces cell apoptosis in hepatocellular carcinoma. Brazilian Journal of Medical and Biological Research . 2018;51(4) doi: 10.1590/1414-431x20176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luo H. L., Xiao S. L., Chen K. Content determination of polydatin in Yidu Tiaogan mixture by HPLC. China Pharmaceuticals . 2016;25(20):45–47. [Google Scholar]
- 106.Zhang G. Y., Liu C. H. Content of polydatin in liver corelle table determined by RP-HPLC. China Medicine and Pharmacy . 2017;7(9):39–41. [Google Scholar]
- 107.Wang C., Zhang P., Zhang X., Yu M., Lv Z. 1H NMR quantification of polydatin and Emodin in Huganning, a Chinese patent herbal medicine. Revista Brasileira de Farmacognosia . 2020;30(1):28–33. doi: 10.1007/s43450-020-00031-7. [DOI] [Google Scholar]
- 108.Zhang X. H., Li J. The clinical efficacy of polydatin in the treatment of elderly coronary heart disease and its influence on patients NF-κB and inflammatory factors. Chinese Journal of Integrative Medicine on Cardio-Cerebrovasc Diseases . 2018;16(16):2352–2354. [Google Scholar]
- 109.Zeng Z. H., Chen Z. Q., Li T., et al. Polydatin: a new therapeutic agent against multiorgan dysfunction. Journal of Surgical Research . 2015;198(1):192–199. doi: 10.1016/j.jss.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 110.Li P., Wang X., Zhao M., Song R., Zhao K. S. Polydatin protects hepatocytes against mitochondrial injury in acute severe hemorrhagic shock via SIRT1-SOD2pathway. Expert Opinion on Therapeutic Targets . 2015;19(7):997–1010. doi: 10.1517/14728222.2015.1054806. [DOI] [PubMed] [Google Scholar]
- 111.Zhu L. X. Study on the Resveratrol (glycoside) and Anthraquinone of Polygonum Cuspidatum Sieb.et Zucc . Bo Shi Lun Wen: Jiangnan University; 2005. [Google Scholar]
- 112.Zong N., Hu Y. Q., Liu D. L. Study on the biological activity of resveratrol glycosides. Acta Academiae Medicinae . 2002;11(4):298–300. [Google Scholar]
- 113.Xi P. Study on the Hepatotoxicity of Polygonum Cuspidatum and Reyanning Heji . Shuo Shi Lun Wen: Henan University of Chinese Medicine; 2017. [Google Scholar]
- 114.Oh S., Jo Y., Jung S., Yoon S., Yoo K. H. From genome sequencing to the discovery of potential biomarkers in liver disease. BMB Reports . 2020;53(6):299–310. doi: 10.5483/BMBRep.2020.53.6.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Procopet B., Fischer P., Farcau O., Stefanescu H. Metabolomics: from liver chiromancy to personalized precision medicine in advanced chronic liver disease. World Journal of Hepatology . 2018;10(3):371–378. doi: 10.4254/wjh.v10.i3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Albhaisi S. A. M., Bajaj J. S., Sanyal A. J. Role of gut microbiota in liver disease. American Journal of Physiology Gastrointestinal and Liver Physiology . 2020;318(1):G84–G98. doi: 10.1152/ajpgi.00118.2019. [DOI] [PubMed] [Google Scholar]
- 117.Mathew S., Hedstroem M., Adlercreutz P. Enzymatic synthesis of piceid glycosides by cyclodextrin glucanotransferase. Process Biochemistry . 2012;47(3):528–532. doi: 10.1016/j.procbio.2011.11.012. [DOI] [Google Scholar]
- 118.Du Q. H., Peng C., Zhang H. Polydatin: a review of pharmacology and pharmacokinetics. Pharmaceutical Biology . 2013;51(11):1347–1354. doi: 10.3109/13880209.2013.792849. [DOI] [PubMed] [Google Scholar]
- 119.Nance E. Careers in nanomedicine and drug delivery. Advance Drug Delivery Reviews . 2019;144:180–189. doi: 10.1016/j.addr.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Su D., Cheng Y., Liu M., et al. Comparision of piceid and resveratrol in antioxidation and antiproliferation activities in vitro. Plos One . 2013;8(1) doi: 10.1371/journal.pone.0054505. [DOI] [PMC free article] [PubMed] [Google Scholar]


