Abstract
Community respiratory viral infections (CRVI) are associated with pulmonary function impairment, alloimmune lung syndromes, and inferior survival in HLA-matched allogeneic hematopoietic stem cell transplant (HCT) recipients. Although the incidence of viral infections in HLA-haploidentical HCT recipients who receive post-transplant cyclophosphamide (PTCy)-based GVHD prophylaxis is reportedly increased, there are insufficient data describing the incidence of CRVI and the impact of donor source and PTCy on transplant outcomes. Analyzing patients receiving their first HCT between 2012 and 2017 for AML, ALL, and MDS, we describe comparative outcomes between matched sibling transplants receiving either calcineurin-based GVHD prophylaxis (SibCNI, N=1605) or PTCy (SibCy, N=403), and related haploidentical transplants receiving PTCy (HaploCy, N=757). The incidence of CRVI was higher for patients receiving PTCy, regardless of donor type. Patients in the HaploCy cohort who developed a CRVI by day +180 had both a higher risk of treatment-related mortality (TRM) [HR 2.14 (99% CI: 1.13 – 4.07, p=0.002] and inferior two-year overall survival (OS) [HR 1.65 (99% CI: 1.11 – 2.43, p = 0.001] compared to SibCNI with no CRVI. This finding justifies further research into long-term antiviral immune recovery as well as development of preventive and treatment strategies to improve long term outcomes in such patients.
Introduction
HLA-haploidentical hematopoietic stem cell transplantation (HCT) has wide applicability as an alternative source for stem cells in patients without matched donors with the reported success of PTCy used with T-cell replete (TCR) stem cell infusions from peripheral blood or bone marrow (1–5). Prior to the development of the PTCy strategy for haploidentical HCT, alternative T-cell depletion (TCD) strategies included graft manipulation for CD34 selection as well as in-vivo T cell depletion with anti-thymocyte globulin (ATG) or alemtuzumab. TCD strategies in the context of haploidentical transplant were limited by severe GVHD, graft rejection, and increased infectious complications (6–8). Comparisons of TCR haploidentical strategies predominantly involving PTCy (HaploCy) to T cell depleted (TCD) haploidentical strategies demonstrate superior non-relapse mortality (NRM) accompanied by better immune reconstitution of T cell subsets in the first 6 months post–transplant for the HaploCy approach (9–14). However, reports of high rates of infections following HaploCy continue despite improvements in survival and composite outcome measures (15–19). Viral infections are reported in this setting in the range of 70% at 100 days and 77% at 1 year (19). Despite the recognition of increased risk of viral infection, there is a lack of information regarding the incidence of community respiratory viral infections (CRVI) in haploidentical stem cell transplant recipients and the impact of those infections on transplant outcomes. Furthermore, it is unknown whether the degree of mismatch, the use of PTCy, or both impacts infection and transplant outcomes, and the limited data that are available are conflicting (20–21).
Although the incidence of CRVI is reportedly low in matched allogeneic HCT recipients receiving calcineurin inhibitor (CNI) based GVHD prophylaxis, both retrospective and prospective studies have found associations between early CRVI and pulmonary function, alloimmune lung syndromes (allo-LS), and transplant related mortality (TRM). Furthermore, co-viral infections and in particular CMV viremia has been associated with increased progression of CRVI to lower respiratory tract infections. Given the high rates of viral infections reported with HaploCy HCT, understanding the incidence and impact that CRVI has on transplant outcomes in this setting may impact the choice of donors and post-transplant management strategies relating to infection prophylaxis and treatment of graft versus host disease (GVHD).
This study aims to identify the comparative incidence of CRVI infections occurring by day +180 post-transplant by donor source and the impact of CRVI on outcomes including survival, relapse, chronic GVHD, and transplant related mortality (TRM) using the Center for International Blood and Marrow Transplant Research (CIBMTR) registry. Our target population was selected to evaluate the impact of PTCy and donor and included matched sibling transplants with calcineurin based GVHD prophylaxis (SibCNI ) compared to matched siblings with PTCy based GVHD prophylaxis (SibCy) and haploidentical related transplants receiving PTCy based GVHD (HaploCy).
Materials and Methods
Study Population
A total of 11,964 patients 2 years of age or older receiving first HCT transplant for AML, ALL, and MDS between 2012 and 2017 were identified in the CIBMTR registry. Cohorts examined included recipients of related haploidentical (≥ 2 antigen/allele mismatched) donors with PTCy (HaploCy), HLA identical siblings with PTCy (SibCy), and HLA identical siblings with calcineurin based GVHD prophylaxis (SibCNI) of either tacrolimus/cyclosporine plus MMF ± other, or tacrolimus/cyclosporine + methotrexate ± other. The HaploCy and SibCy cohorts received PTCy with other agents as GVHD prophylaxis. Patients who received ATG or alemtuzumab were excluded. Patients who received PTCy without other immune suppression were also excluded given low numbers (N=10). Other exclusion criteria included umbilical cord blood transplants, matched unrelated donor transplants, CD34 selection or other forms of ex vivo-T cell depletion, and patients who experienced infections prior to day 0. Matched unrelated donors were excluded due to the lack of sufficient MUD PTCy comparator cohort with detailed infection data. To minimize bias, patients transplanted at centers which had no reported haploidentical HCT patients were excluded. The final patient cohort analyzed included 757 HaploCy, 403 SibCy, and 1605 SibCNI recipients (Supplemental Table 1).
Data Source
The CIBMTR is a research consortium consisting of over 500 transplant centers internationally. Through a collaboration between the Medical College of Wisconsin and the National Marrow Donor Program, patient and outcomes data from these centers are collected and analyzed. Central auditing of the data is performed to ensure consistency and quality. The CIBMTR collects the Transplant Essential Data (TED) form and Comprehensive Report Form (CRF) prior to transplantation, at 100 days (D100), 6 months (D180), and 1 year after transplantation and annually thereafter. All patients included in this study gave written consent to participate in the CIBMTR Research Database and to have their data included in observational research. This study was approved by the institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program.
Infection data are reported only on the CRF. Centers report infections in accordance with instructions in the forms manual (22). Data collected include an organism, site of infection, and date of onset. There are no data on prophylaxis, diagnostic methodology, or treatment of infection. Additionally, forms do not collect specifics on viral load or preemptive protocols for surveillance.
Statistical analysis
Comparative analysis was performed between three cohorts— HaploCy, SibCy, and SibCNI— to assess the impact of CRVI occurring by day +180 post-transplant. Univariate and multivariable analyses were used to determine 2-year outcomes of overall survival (OS), transplant related mortality (TRM), relapse, and chronic GVHD. The cumulative incidence function with death as a competing risk was used to estimate the probability of TRM, relapse, and chronic GVHD. OS was estimated using the Kaplan Meier analysis.
CRVI is a time-dependent variable and events may occur early after HCT. To account for impacts of CRVI prior to day 180, univariate analyses for outcomes were examined applying dynamic landmark analyses using multiple landmark time points based on the median and interquartile range for CRVI (23). This approach allows appropriate categorization of the patient as infection/no infection for patients still alive at the landmarks examined. Multivariable analyses employed the Cox proportional hazard models for outcomes by 2 years post-transplant. The main effect of the presence/absence of respiratory viral infection was kept in all models as a time-dependent variable. Variables examined in the Cox model are shown in Table 1. The proportional hazards assumptions for each factor in the Cox model were tested. If covariates violated the proportional hazards assumptions, time-dependent covariates were added. A stepwise selection procedure was used to identify significant risk factors with the significance level of 0.01. Interactions between main effect and significant covariates were tested. Center effects were tested using the score test and all models were adjusted for center effect (24).
Table 1:
Variables included in the MVA
|
Analyses: 2y OS, 2y DFS, 2y Rel, 2y NRM, 2y cGVHD |
| Main Effect Variable: Groups by CRV infection • HaploPTCy with CRV infection vs Haplo PTC no CRV infection vs MRDPTCy with CRV infection vs MRD PTCy no CRV infection vs MRD no PTCy with CRV infection vs MRD no PTCy no CRV infection (ref) |
| Other variables to be examined • Graft type: Marrow (ref) vs PB • D/R Gender: M-M (ref) vs M-F v F-F v F-M • HCT-CI: 0 (ref) vs 1 −2 vs 3–4 vs 5+ • Disease risk: AL favorable cyto, early/intermediate stage (ref) vs AL intermediate/nl cyto, early stage, vs AL poor cyto, early stage; vs AL int/nl cyto, intermediate stage vs AL poor cyto, intermediate stage vs AL advanced (all cyto categories) vs MDS very low/low vs MDS intermediate vs MDS high/very high • Recipient Age: ≤ 20 (ref) vs 21 – 40 vs 41 – 60 v >60 • KPS: ≥ 90 (ref) vs 80 – 89 vs <80 • Conditioning intensity: Myeloablative (ref) vs RIC/NMA • TBI: No (ref) vs Yes • Time from dx to HCT: <6 m (ref) vs 6 – 12 m vs >12 m • Year of HCT: 2012 – 2014 (ref) vs 2015 – 2017 • Neutrophil engraftment prior to infection (time dependent) • # of Viral infections: None vs 1 vs 2 vs 3+ • Co-Infection: No infection (ref) vs Viral + Co-infection vs Viral + Co-infection vs other infection by day 180 • Acute GVHD grade 2 – 4 (time dependent) |
Results
Table 2 shows the patient characteristics. The identified CRVI included rhinovirus, parainfluenza virus, respiratory syncytial virus, influenza, adenovirus, enterovirus, human metapneumovirus, and coronavirus. Rhinovirus, parainfluenza, and respiratory syncytial virus accounted for approximately 70% of all CRVI reported (Table 3). Some patients had multiple viruses; however, more patients receiving PTCy developed a CRVI [HaploCy 14%; SibCy 16%; SibCNI 9%; p <0.001). Notably, the frequency of individual viruses was not statistically different among groups.
Table 2:
Characteristics of the three cohorts as defined by presence/absence of CRVI by day 180.
| CRV Infection by day 180 | No CRV infection | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | HaploCy N(%) N = 117 |
SibCy N(%) N = 65 |
SibCNI N(%) N = 151 |
HaploCy N(%) N = 640 |
SibCy N(%) N = 338 |
SibCNI N(%) N = 1454 |
| Number of centers | 46 | 34 | 56 | 97 | 72 | 99 |
|
| ||||||
|
Patient Related
| ||||||
| Gender, Male | 77 (66) | 39 (60) | 79 (52) | 382 (60) | 204 (60) | 854 (59) |
|
| ||||||
| Age at transplant, years | ||||||
| ≤10 | 8 ( 7) | 1 ( 2) | 11 ( 7) | 27 ( 4) | 3 (<1) | 30 ( 2) |
| 11–20 | 12 (10) | 4 ( 6) | 4 ( 3) | 39 ( 6) | 19 ( 6) | 81 ( 6) |
| 21–30 | 12 (10) | 16 (25) | 13 ( 9) | 56 ( 9) | 53 (16) | 102 ( 7) |
| 31–40 | 5 ( 4) | 10 (15) | 8 ( 5) | 39 ( 6) | 52 (15) | 129 ( 9) |
| 41–50 | 17 (15) | 9 (14) | 22 (15) | 54 ( 8) | 58 (17) | 183 (13) |
| 51–60 | 25 (21) | 11 (17) | 35 (23) | 127 (20) | 71 (21) | 357 (25) |
| 61–70 | 29 (25) | 12 (18) | 51 (34) | 226 (35) | 73 (22) | 498 (34) |
| >70 | 9 ( 8) | 2 ( 3) | 7 ( 5) | 72 (11) | 9 ( 3) | 74 ( 5) |
|
| ||||||
| Karnofsky/Lansky performance status | ||||||
| ≥90 | 64 (55) | 37 (57) | 91 (60) | 326 (51) | 196 (58) | 855 (59) |
| 80–89 | 34 (29) | 18 (28) | 45 (30) | 195 (30) | 84 (25) | 404 (28) |
| <80 | 16 (14) | 10 (15) | 15 (10) | 103 (16) | 55 (16) | 185 (13) |
| Missing | 3 ( 3) | 0 | 0 | 16 ( 3) | 3 (<1) | 10 (<1) |
|
| ||||||
| HCT-CI | ||||||
| 0 | 32 (27) | 10 (15) | 37 (25) | 167 (26) | 93 (28) | 355 (24) |
| 1–2 | 29 (25) | 17 (26) | 40 (26) | 180 (28) | 107 (32) | 407 (28) |
| 3–4 | 38 (32) | 18 (28) | 48 (32) | 173 (27) | 86 (25) | 428 (29) |
| 5+ | 18 (15) | 20 (31) | 26 (17) | 119 (19) | 51 (15) | 259 (18) |
| Missing | 0 | 0 | 0 | 1 (<1) | 1 (<1) | 5 (<1) |
|
| ||||||
|
Donor Related
| ||||||
| Donor/recipient gender match | ||||||
| Male-Male | 49 (42) | 24 (37) | 47 (31) | 240 (38) | 132 (39) | 460 (32) |
| Male-Female | 21 (18) | 19 (29) | 32 (21) | 159 (25) | 80 (24) | 315 (22) |
| Female-Male | 28 (24) | 15 (23) | 32 (21) | 142 (22) | 72 (21) | 394 (27) |
| Female-Female | 19 (16) | 7 (11) | 40 (26) | 99 (15) | 54 (16) | 284 (20) |
| Missing | 0 | 0 | 0 | 0 | 0 | 1 (<1) |
|
| ||||||
|
Disease Related
| ||||||
| Disease | ||||||
| AML | 87 (74) | 53 (82) | 105 (70) | 441 (69) | 257 (76) | 920 (63) |
| ALL | 5 ( 4) | 2 ( 3) | 7 ( 5) | 21 ( 3) | 17 ( 5) | 53 ( 4) |
| MDS | 25 (21) | 10 (15) | 39 (26) | 178 (28) | 64 (19) | 481 (33) |
|
| ||||||
| Disease status | ||||||
| AML/ALL, early | 49 (42) | 31 (48) | 59 (39) | 259 (40) | 158 (47) | 660 (45) |
| AML/ALL, intermediate | 27 (23) | 11 (17) | 27 (18) | 116 (18) | 66 (20) | 183 (13) |
| AML/ALL, advanced | 15 (13) | 13 (20) | 22 (15) | 82 (13) | 48 (14) | 122 ( 8) |
| AML/ALL, unknown | 1 (<1) | 0 | 5 ( 3) | 5 (<1) | 2 (<1) | 10 (<1) |
| MDS, early | 12 (10) | 3 ( 5) | 10 ( 7) | 64 (10) | 21 ( 6) | 169 (12) |
| MDS, advanced | 13 (11) | 7 (11) | 28 (19) | 114 (18) | 43 (13) | 310 (21) |
|
| ||||||
| Cytogenetics for AML/ALL | ||||||
| Normal | 10 ( 9) | 3 ( 5) | 7 ( 5) | 32 ( 5) | 25 ( 7) | 76 ( 5) |
| Favorable | 3 ( 3) | 2 ( 3) | 2 ( 1) | 20 ( 3) | 16 ( 5) | 37 ( 3) |
| Intermediate | 36 (31) | 20 (31) | 54 (36) | 220 (34) | 120 (36) | 464 (32) |
| Poor | 42 (36) | 28 (43) | 40 (26) | 161 (25) | 104 (31) | 334 (23) |
| Other | 1 (<1) | 1 ( 2) | 7 ( 5) | 19 ( 3) | 5 ( 1) | 42 ( 3) |
| Not tested/Missing | 0 | 1 ( 2) | 2 ( 1) | 10 ( 2) | 4 ( 1) | 20 ( 1) |
| MDS N/A | 25 (21) | 10 (15) | 39 (26) | 178 (28) | 64 (19) | 481 (33) |
|
| ||||||
| IPSS-R prior to transplant (MDS only) | ||||||
| Very low | 1 (<1) | 3 ( 5) | 1 (<1) | 18 ( 3) | 11 ( 3) | 59 ( 4) |
| Low | 9 ( 8) | 4 ( 6) | 11 ( 7) | 58 ( 9) | 18 ( 5) | 122 ( 8) |
| Intermediate | 8 ( 7) | 3 ( 5) | 13 ( 9) | 45 ( 7) | 19 ( 6) | 147 (10) |
| High | 6 ( 5) | 0 | 7 ( 5) | 24 ( 4) | 10 ( 3) | 66 ( 5) |
| Very high | 0 | 0 | 4 ( 3) | 13 ( 2) | 3 (<1) | 34 ( 2) |
| Missing | 1 (<1) | 0 | 3 ( 2) | 20 ( 3) | 3 (<1) | 53 ( 4) |
| AML/ALL N/A | 92 (79) | 55 (85) | 112 (74) | 462 (72) | 274 (81) | 973 (67) |
|
| ||||||
|
Transplant Related
| ||||||
| Graft type | ||||||
| Bone Marrow | 51 (44) | 24 (37) | 22 (15) | 257 (40) | 107 (32) | 178 (12) |
| Peripheral blood | 66 (56) | 41 (63) | 129 (85) | 383 (60) | 231 (68) | 1276 (88) |
|
| ||||||
| Conditioning regimen intensity | ||||||
| Myeloablative | 53 (45) | 38 (58) | 89 (59) | 261 (41) | 184 (54) | 846 (58) |
| RIC/NMA | 64 (55) | 27 (42) | 62 (41) | 379 (59) | 154 (46) | 608 (42) |
|
| ||||||
| TBI, yes | 78 (67) | 40 (62) | 48 (32) | 453 (71) | 194 (57) | 388 (27) |
|
| ||||||
| Time from diagnosis to transplant | ||||||
| <6 month | 49 (42) | 33 (51) | 81 (54) | 266 (42) | 147 (43) | 809 (56) |
| 6 month-12 months | 30 (26) | 17 (26) | 30 (20) | 165 (26) | 100 (30) | 318 (22) |
| >12 months | 38 (32) | 15 (23) | 39 (26) | 208 (32) | 90 (27) | 324 (22) |
| Missing | 0 | 0 | 1 (<1) | 1 (<1) | 1 (<1) | 3 (<1) |
|
| ||||||
| Year of transplant | ||||||
| 2012 – 2014 | 24 (21) | 13 (20) | 101 (68) | 146 (23) | 74 (22) | 718 (49) |
| 2015 – 2017 | 93 (79) | 52 (80) | 63 (42) | 494 (67) | 264 (68) | 736 (51) |
Table 3.
CRV type and frequency based on donor type. The values (n, %) for the organisms in the table are for the patients with the individual infections by a specific organism. Some patients had more than one CRVI reported and a patient may be included in more than one organism group. The percentages noted represent the percent for individual organisms of the total number of CRVI. The p-value for each organism is from the Chi-squared analysis of the organism based upon the number of total patients with infection for that specific organism.
| Organism | HaploCy n=757 (%) |
SibCy n=403 (%) |
SibCNI n=1605 (%) |
P-value |
|---|---|---|---|---|
| Number of patients with CRVI | 107 (14%) | 63 (16%) | 147 (9%) | <0.001 |
| Organisms Reported (not mutually exclusive) | ||||
| Rhinovirus | 27 (25%) | 30 (48%) | 53 (36%) | 0.011 |
| Parainfluenza | 24 (22%) | 15 (24%) | 41 (28%) | 0.588 |
| Respiratory syncytial virus | 32 (30%) | 10 (16%) | 35 (24%) | 0.118 |
| Influenza | 16 (15%) | 8 (13%) | 24 (16%) | 0.796 |
| Adenovirus | 20 (19%) | 12 (19%) | 15 (10%) | 0.098 |
| Enterovirus | 6 (6%) | 5 ( 8%) | 7 ( 5%) | 0.660 |
| Human Metapneumovirus | 2 (2%) | 0 | 4 ( 3%) | 0.415 |
| Coronavirus | 3 (3%) | 0 | 3 ( 2%) | 0.425 |
The cumulative incidences of CRVI in the HaploCy, SibCy and SibCNI at day 30 were: 3% (99% CI, 1.6–4.8), 3% (1.3–5.5) and 2.4 % (1.5–3.5) respectively (P =0.649). However, the incidence of CRVI at day 180 was notably higher at 15.5% (12.3–19) for HaploCy, 16.2% (11.7–21.2) for SibCy, and 9.4 %(7.6–11.4) for SibCNI at 6 months (P<.001) post-transplant, with incidence of CRVI in SibCy and HaploCy significantly higher than seen with SibCNI. (Figure 1).
Figure 1:

The Incidence of CRV infections based on donor type
The cumulative incidences of CRVI in the HaploCy, SibCy and SibCNI at day 30 were: 3% (99% CI, 1.6–4.8), 3% (1.3–5.5) and 2.4 %(1.5–3.5) respectively (P =0.649). However, the incidence of CRVI at day 180 was notably higher at 15.5% (12.3–19) for HaploCy, 16.2% (11.7–21.2) for SibCy, and 9.4 %(7.6–11.4) for SibCNI at 6 months (P<.001) post-transplant, with incidence of CRVI in SibCy and HaploCy significantly higher than seen with SibCNI
HaploCy + CRVI was associated with a decreased overall survival and increased treatment related mortality (TRM) by day 180. On multivariable analysis, the HaploCy cohort who develop a CRVI (n = 114) by day +180 have an inferior OS [HR 1.65 (99% CI: 1.11 – 2.43), p< .002] and increased TRM [HR 2.14 (995 CI: 1.13 – 4.07) p=0.0022] compared to reference group, SibCNI with no CRVI (n = 1421) (Figures 2A–B, Table 4, supplemental 2). The SibCy cohort (n = 65) also demonstrated an inferior overall survival [HR 1.48 (99% CI: 0.96 – 2.27), p = 0.02] and increased TRM [HR 1.84 (99% CI: 0.80 – 4.21), p = 0.057] but did not meet statistical significance. Additional factors associated with inferior overall survival include transplant for high/very high risk MDS; higher HCT-CI; and older age. Additional factors associated with an increase TRM include female donor to male recipient; older age; and development of grade II-IV aGVHD. Relapse of primary disease was the primary cause of death across the 3 cohorts. Infection as a primary or contributory cause of death occurred in 131 (35%) patients following HaploCy, 45 (25%) patients following SibCy, and 193 (26%) in the SibCNI cohort (Supplemental table 3).
Figure 2:
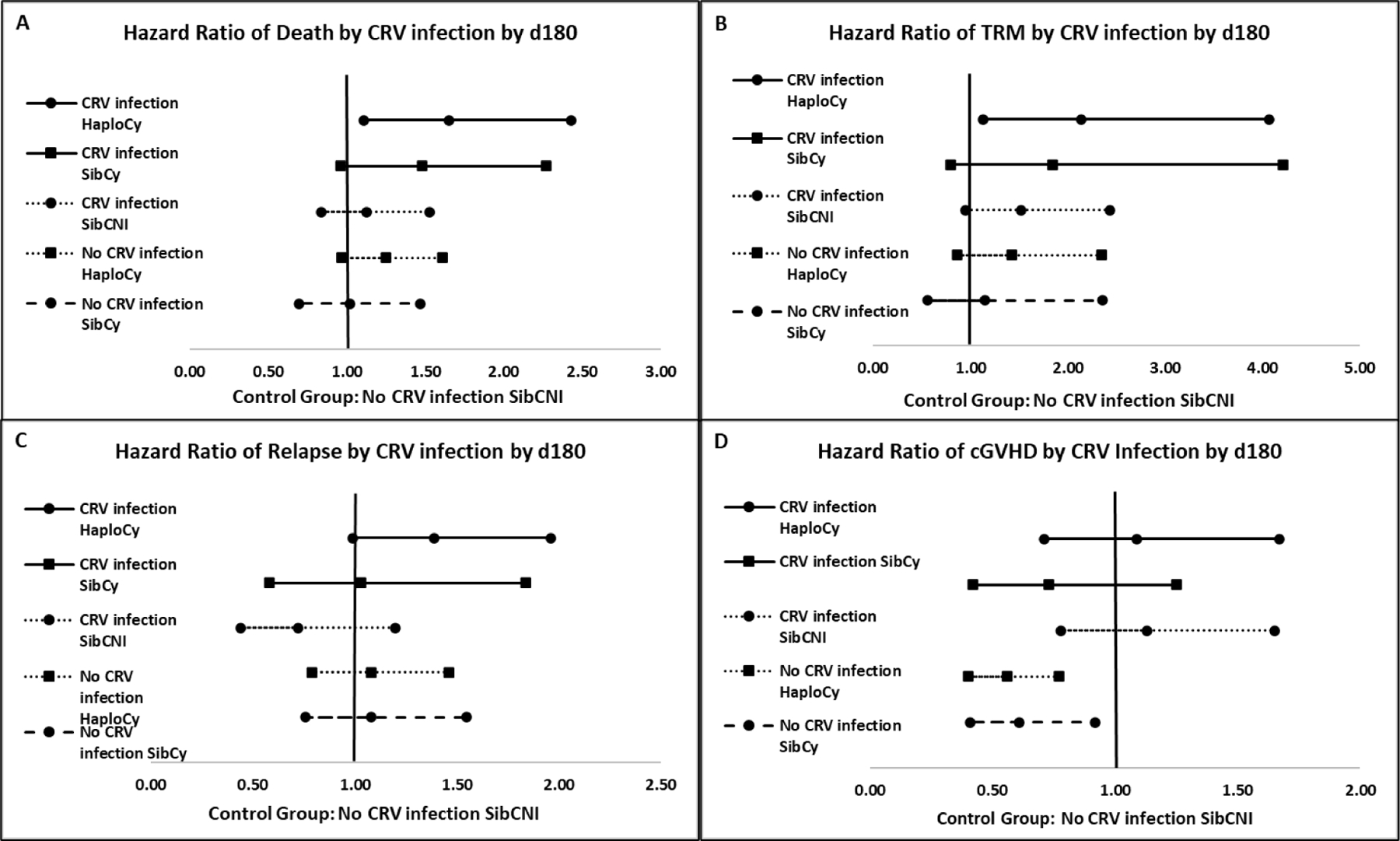
Forest plots of the main effect variable of the presence/absence of infection by HaploCy, SibCy, and SibCNI cohorts. The reference group for each panel is the SibCy cohort without infection. Hazard ratio for death (A), transplant related mortality (B), relapse (C), and chronic GVHD (D) are shown.
Table 4:
Multivariable analyses for events by 2 years
| Variable | N | Hazard Ratio [99% CI] |
p-value |
|---|---|---|---|
|
Overall Mortality (adjusted for center effects)
| |||
| Main Effect Variable, Infection | 0.0017 | ||
| SibCNI, no infection | 1421 | 1.00 | |
| HaploCy with infection | 114 | 1.65 [1.11 – 2.43] | 0.0010 |
| SibCy with infection | 65 | 1.48 [0.96 – 2.27] | 0.0203 |
| SibCNI with infection | 148 | 1.13 [0.84 – 1.53] | 0.2787 |
| HaploCy no infection | 615 | 1.25 [0.97 – 1.61] | 0.0246 |
| SibCy no infection | 330 | 1.20 [ 0.70 – 1.47] | 0.8979 |
|
| |||
| Disease/Stage/Cytogenetics (IPSS) | < 0.0001 | ||
| AL, early/intermediate, favorable cyto | 68 | 1.00 | |
| AL early, normal/intermediate cyto | 667 | 1.00 [0.56–1.78] | 0.9892 |
| AL early, poor cyto | 434 | 0.96 [0.55–1.69] | 0.8655 |
| AL intermediate, normal/intermediate cyto | 213 | 1.07 {0.59 – 1.92] | 0.7695 |
| AL intermediate, poor cyto | 124 | 1.27 [0.69 – 2.33] | 0.3208 |
| AL advanced, any cyto | 291 | 1.97 [1.08 – 3.60] | 0.0036 |
| MDS very low/low | 307 | 0.90 [0.51 – 1.59] | 0.6248 |
| MDS intermediate | 229 | 1.54 [ 0.87 – 2.72] | 0.0539 |
| MDS high/very high | 162 | 2.05 [ 1.09 – 3.86] | 0.0034 |
| Missing | 198 | 1.29 [ 0.66 – 2.50] | 0.3255 |
|
| |||
| HCT-CI | 0.0004 | ||
| 0 | 679 | 1.00 | |
| 1 – 2 | 762 | 0.95 [0.67 – 1.34] | 0.6945 |
| 3 – 4 | 768 | 1.12 [ 0.80 – 1.56] | 0.3815 |
| 5+ | 484 | 1.32 [ 0.94 – 1.85] | 0.0380 |
|
| |||
| Age at HCT, years | <0.0001 | ||
| 0 – 20 | 234 | 1.00 | |
| 21 – 40 | 479 | 0.88 [0.60 – 1.30] | 0.4042 |
| 41 – 60 | 945 | 1.27 [0.89 – 1.82] | 0.0790 |
| 60 | 1035 | 1.62 [1.08 – 2.42] | 0.0023 |
|
| |||
|
Treatment Related Mortality (adjusted for center effects)
| |||
| Main Effect Variable, Infection | 0.0164 | ||
| SibCNI, no infection | 1415 | 1.00 | |
| HaploCy with infection | 116 | 2.14 [1.13 – 4.07] | 0.0022 |
| SibCy with infection | 65 | 1.84 [ 0.80 – 4.21] | 0.0574 |
| SibCNI with infection | 146 | 1.52 [0.95 – 2.43] | 0.0223 |
| HaploCy no infection | 621 | 1.42 [0.86 – 2.35] | 0.0736 |
| SibCy no infection | 329 | 1.15 [ 0.56 – 2.36] | 0.6290 |
|
| |||
| Donor/Recipient Gender Match | <0.0001 | ||
| Male/Male | 927 | 1.00 | |
| Male/Female | 616 | 1.05 [0.77 – 1.43] | 0.6610 |
| Female/Male | 658 | 1.45 [1.13 – 1.87] | 0.0002 |
| Female/Female | 491 | 0.82 [0.56 – 1.19] | 0.1631 |
|
| |||
| Age at HCT, years | 0.0025 | ||
| 0 – 20 | 234 | 1.00 | |
| 21 – 40 | 477 | 0.80 [0.47 – 1.37] | 0.2857 |
| 41 – 60 | 946 | 1.23 [ 0.68 – 2.25] | 0.3673 |
| > 60 | 1035 | 1.77 [ 0.88 – 3.55] | 0.0341 |
|
| |||
| Acute GVHD, grade II-IV | <0.0001 | ||
| No | 1837 | 1.00 | |
| Yes | 855 | 2.66 [ 1.79 – 3.96] | |
|
| |||
|
Relapse (adjusted for center effects)
| |||
| Main Effect Variable, Infection | 0.1185 | ||
| SibCNI, no infection | 1407 | 1.00 | |
| HaploCy with infection | 113 | 1.39 [0.99 – 1.96] | 0.0118 |
| SibCy with infection | 65 | 1.03 [0.58 – 1.84] | 0.8883 |
| SibCNI with infection | 146 | 0.72 [0.44 – 1.20] | 0.0992 |
| HaploCy no infection | 605 | 1.08 [0.79 – 1.46] | 0.5416 |
| SibCy no infection | 326 | 1.08 [0.76 – 1.55] | 0.5700 |
|
| |||
| Disease/Stage/Cytogenetics (IPSS) | <0.0001 | ||
| AL, early/intermediate, favorable cyto | 67 | 1.00 | |
| AL early, normal/intermediate cyto | 659 | 0.69 [0.40 – 1.21] | 0.0890 |
| AL early, poor cyto | 430 | 0.96 [0.54 – 1.70] | 0.8366 |
| AL intermediate, normal/intermediate cyto | 208 | 0.75 [0.43 – 1.33] | 0.1945 |
| AL intermediate, poor cyto | 124 | 1.03 [0.55 – 1.94] | 0.8889 |
| AL advanced, any cyto | 289 | 2.00 [1.18 – 3.40] | 0.0007 |
| MDS very low/low | 305 | 1.03 [0.61 – 1.76] | 0.8827 |
| MDS intermediate | 227 | 1.20 [0.67 – 2.16] | 0.4246 |
| MDS high/very high | 159 | 2.27 [1.25 – 4.10] | 0.0004 |
| Missing | 194 | 1.12 [0.62 – 2.01] | 0.6141 |
|
| |||
| Conditioning Intensity | <0.0001 | ||
| Myeloablative | 1419 | 1.00 | |
| RIC/NMA | 1243 | 1.46 [1.23 – 1.73] | |
|
| |||
| Acute GVHD, grade II-IV | <0.0001 | ||
| No | 1816 | 1.00 | |
| Yes | 846 | 0.78 [0.67 – 0.91] | |
|
| |||
|
Chronic GVHD (adjusted for center effects)
| |||
| Main Effect Variable, Infection | <0.0001 | ||
| SibCNI, no infection | 1433 | 1.00 | |
| HaploCy with infection | 117 | 1.09 [0.71 – 1.67] | 0.6031 |
| SibCy with infection | 65 | 0.73 [0.42 – 1.25] | 0.1283 |
| SibCNI with infection | 148 | 1.13 [0.78 – 1.65] | 0.3971 |
| HaploCy no infection | 632 | 0.56 [0.40 – 0.77] | <0.0001 |
| SibCy no infection | 332 | 0.61 [0.41 – 0.92] | 0.0017 |
|
| |||
| Graft Type | <0.0001 | ||
| Bone Marrow | 636 | 1.00 | |
| Peripheral Blood | 2091 | 2.23 [1.66 – 3.00] | |
|
| |||
| Donor/Recipient Gender Match | 0.0005 | ||
| /Male | 937 | 1.00 | |
| Male/Female | 623 | 1.01 [0.82 – 1.25] | 0.8804 |
| Female/Male | 670 | 1.26 [1.04 – 1.54] | 0.0021 |
| Female/Female | 496 | 1.24 [1.01 – 1.53] | 0.0074 |
|
| |||
| Acute GVHD, grade II-IV | 0.0004 | ||
| No | 1820 | 1.00 | |
| Yes | 907 | 1.30 [1.07 – 1.58] | |
There was no association of relapse risk based upon the main effect variable of CRVI and donor type with or without PTCy (p=0.119) (Figure 2C, Supplemental table 2). Patients without CRVI and receiving PTCy have a lower risk of cGVHD, regardless of donor (p<.001) (Figures 2D, Supplemental table 2). A higher risk of relapse was associated with transplant for high/very high risk MDS or advanced acute leukemia, as well as NMA/RIC conditioning. Development of grade II-IV aGVHD was protective against relapse (Table 4). Factors associated with increased cGVHD included receipt of peripheral blood stem cells, female donor for a recipient of either gender, and the development of grade II-IV aGVHD (Table 4).
Discussion
In this retrospective registry study our goal was to 1) determine the comparative incidence of community respiratory virus infections (CRVI) occurring by day +180 post-transplant in 3 cohorts defined by donor source and GHVD prophylaxis; and 2) determine the impact of CRVI on outcomes including survival, TRM, relapse, and chronic GVHD within the cohorts. We found a statistically significant increase in the 6-month cumulative incidence of CRVI in the cohorts who received PTCy regardless of donor type. The occurrance of CRVI in the HaploCy prior to day 180 was associated with an increase in TRM and decrease in OS. These findings did not extend to the SibCy group, although the small number of patients with CRVI in this cohort may have prevented findings of significance at 99% confidence. This suggests, that for CRVI, the increased risk of TRM and decrease in OS is driven predominantly by the platform of haploidentical donor and PTCy, rather than just PTCy alone. The findings from our analysis evaluated in the context of previously reported CRVI related phenomena following allogeneic HCT highlight the importance of understanding the impact of viral infection and immune recovery in haploidentical HCT with PTCy.
Recipients of allogeneic HCT are particularly susceptible to severe respiratory viral infections. Several previously identified factors contribute to the development and severity of respiratory viral infection following allogeneic HCT (25–28). Cytomegalovirus (CMV) seropositivity is a risk factor for CRVI following HCT and CMV viremia is associated with increased mortality in this setting. Progression of CRVI from upper tract disease to lower tract disease appears to impact mortality and long term pulmonary complications. Conversion from upper tract infections (URI) to lower tract infections (LRTI) is associated with CMV viremia, CRVI within the first 100 days of HCT (29), high-dose steroids at the time of CRVI, GVHD, cord blood, or antigen mismatch allo-HCT. Prospective studies reported by EBMT in 1997 showed high mortality rates from fatal pneumonias following RSV and Adenovirus (28). Subsequent studies suggest that these infections contribute to morbidity and mortality in the short and long term with increases in non-infectious pulmonary syndromes including obstructive airway disease and bronchiolitis obliterans (25, 27, 30).
Pulmonary complications represent a major cause of morbidity and mortality after allogeneic HCT, with alloimmune lung syndromes (allo-LS) including Idiopathic pneumonia syndrome (IPS), bronchiolitis obliterans syndrome (BOS), and bronchiolitis obliterans and organizing pneumonia (BOOP). Both retrospective and prospective studies have found associations between early CRVI associated airflow decline (30) and short- and long- term pulmonary function as well as the development of allo-LS, and data show that this complication contributes to transplant related outcomes (31). In allogeneic HCT as a whole, improvements in care associated with prospective interventional clinical surveillance programs for CRVI have suggested that all-cause mortality is substantially reduced with intervention when compared to retrospective controls (32). Addressing the higher rates of viral infections, as seen in HaploCy HCT, and understanding the characteristics of immune reconstitution provide opportunities for improving transplant outcomes by focusing on infection control measures and early identification of patients at risk for increased morbidity and mortality from the sequelae of these infections.
Our study supports the importance of respiratory viral infections in transplant outcome and particularly in the setting of HaploCy. While the cumulative incidences of community respiratory viral infections following allogeneic HCT in our population is relatively low particularly within the first 30 days after HCT, CRVI increases in frequency over subsequent months. This timing correlates with patients leaving the protection of the transplant center and early isolation measures and resultant increased exposure to community based pathogens. By day 180 post HCT there is a statistically significant increase in community respiratory viral infections in the patients who received post-transplant cyclophosphamide as compared to standard calcineurin inhibitor based GVHD prophylaxis, regardless of donor type. We saw decreased survival and increased treatment related mortality in HaploCy cohort as compared to SibCNI with no CRVI. Our findings suggest that the degree of mismatch as well as the use of PTCy may impact the incidence of CRVI and, subsequently, transplant outcome. The small number of patients in the SibCy cohort who developed CRVI likely contributed to the inability to find statistical significance at the 99% confidence level making it difficult to evaluate the contributions of mismatch versus PTCy with respect to outcomes. Ongoing efforts to better define immune reconstitution in the setting of PTCy and different donor sources are needed.
There are clear limitations to this analysis. These data are collected retrospectively across multiple institutions and could be impacted by substantial variation related to practice patterns. Testing for CRVI is not done pre-emptively and there are no data captured on indications nor the methodology utilized for CRVI testing. For patients who have left the transplant center, even if tested, the results may not be reported to the transplant center for subsequent CIBMTR reporting. These limitations are likely to underestimate the absolute incidence of CRVI as testing is more likely to be completed in patients with more persistent or severe symptoms or those seen at the transplant center instead of in the community. In addition, the data available through the CIBMTR do not define the severity of infection including a lack of information on the progression from upper tract infection to lower tract infection, duration of infection, or requirement for hospitalization or more intensive support. Furthermore, we were unable to comment on correlations to immune reconstitution due to limited reported data for quatitative immunoglobulins and lymphocyte subsets.
Additional limitations of this study include our inability to determine outcome of specific viral infections and whether infections associated with adenovirus were primary infections or reactivation. While the outcome for individual respiratory viral infections are expected to differ by organism, even a CRVI with a less virulent organism has potential to lead to LRTI and additional morbidity. Therefore we included all common CRVI regardless of expected virulence. Rhinovirus progression to LRTI in allogeneic HCT recipients is significant ranging from 17–29% in recent publications (37, 38). Similarly, human coronavirus infections are common causes of CRVI in allogeneic HCT recipients and have been reported to progress to LRTI in 5–30% of cases (39, 40). While we are unable to determine whether adenoviral infections were related to primary infections or reactivation, the incidence of adeniviral infections in allogeneic HCT recipients is significant with high mortality rates in the setting of LRTI and disseminated infections (29, 41). Consequently, inclusion of all reported CRVI is supported.
The higher overall mortality for HaploCy patients developing CRVI warrants consideration for pre-emptive, therapeutic, and long term follow up studies of such patients in an effort to identify strategies for improving outcomes. Antiviral options for treatment of respiratory viral infections, with the exception of influenza, remain limited. However, evolving and expanding treatment for respiratory viral infections are in clinical trials, including the use of antiviral cytotoxic T cells, virus-specific monoclonal antibodies or nanobodies. Other novel antiviral therapies such as the new fusion or entry inhibitors may offer potential for improvement in high risk settings. The development of bronchiolitis obliterans, which has been associated with early respiratory viral infections after allogeneic HCT is frequently not diagnosed until substantial airway obstruction has occurred and the patient has experienced irreversible lung damage causing progressive respiratory impairment, increased infections and an increase in non-relapse mortality (20, 25, 29). Strategies for early detection of developing allo-LS such as bronchiolitis obliterans are critical to prevent irreversible consequences of advanced bronchiolitis obliterans. Application of prevention and treatment strategies may be more effective once the important deficits in immune recovery are better defined. Pro-active surveillance, aggressive efforts to limit the incidence of CRVI, including wearing masks, and detection of early disease may offer opportunities to intervene and prevent adverse outcomes related to the immunologic sequela of CRVI in these patients. (32–36)
Conclusion
In conclusion, the findings from this retrospective registry study show that the use of PTCy increases the incidence of CRVI and composite viral infections regardless of donor type. The occurrence of CRVI in the setting of PTCy impacts OS and TRM and is statistically significant in the HaploCy cohort. While we are unable to comment on the specific pathophysiology, previously reported studies related to CRVI in allogeneic HCT are intriguing and suggest that there are opportunities to develop pre-emptive and therapeutic strategies that may have substantial impact on outcome in this setting.
Supplementary Material
Acknowledgements
The authors would like to thank the CIBMTR Infection and Immune Reconstitution Working Committee for their support and collaboration. Additional thanks are extended to Rebecca Schleimer, who assisted with manuscript preparation.
Footnotes
Conflicts of interests
SRG works with Wugen Inc., Consultancy.
RTM works with Celgene/Juno Consulting Advisory Board, Consultancy and Research Funding, Novartis Consulting Consultancy and Research Funding, Kite Consulting Advisory Board, Juno Therapeutics Consultant Consultancy and Honoraria, Incyte Corporation Consultant Consultancy and Honoraria, Athersys, Inc. Patent-holder Financial Benefit and/or patents and Royalty, Incyte Consulting Consultancy and Honoraria, Kite Therapeutics Consultant Honoraria, Novartis Pharmaceuticals Corporation Consultant Consultancy, Honoraria and scientific steering committee.
RR works with Kleo Pharma Collaborator Research Funding, Glycostem Advisory board Advisory Board, Kiadis Pharma Consultant Consultancy.
RFC receives compensation and funding from Merck, Chimerix, Shire/Takeda, Gilead, Ansun pharmaceuticals, Viracor, Karius, Pulmotec, and Janssen. Paid consultant for Merck, Chimerix, Ansun Pharmaceuticals, Kyorin, ReViral, Clinigen, Oxford Immunotec, Janssen, Shire/Takeda, Genentech, Paratek, and Shinogei.
KVK works with Kiadis SAB Member Advisory Board and Consultancy, Atara Advisor Advisory Board and Consultancy, Novartis As hoc Advisor Advisory Board and Consultancy, Novartis Consultant Advisory Board and Consultancy, Celgene/Juno Advisor Advisory Board and Honoraria, Incyte As hoc Advisor Advisory Board and Consultancy, Helocyte Consultant Consultancy, Kiadis Consultant Advisory Board and Consultancy, Kite/Gilead Consultant Advisory Board, Consultancy and Research Funding, Kadmon Advisor Advisory Board and Honoraria, Incyte Consultant Advisory Board and Consultancy, Takeda Consultant Consultancy, Celgene Consultant Consultancy, Kite/Gilead Advisor Advisory Board, Consultancy and Research Funding, Kadmon Consultant Advisory Board and Consultancy.
MAP reports honoraria from Abbvie, Bellicum, Celgene, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, Omeros, and Takeda. He serves on DSMBs for Cidara Therapeutics, Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. He has received research support for clinical trials from Incyte, Kite/Gilead and Miltenyi Biotech. He serves in a volunteer capacity as a member of the Board of Directors of American Society for Transplantation and Cellular Therapy (ASTCT) and Be The Match (National Marrow Donor Program, NMDP). Payments: Consulting > $5,000: Merck, Novartis, Incyte. Research support for clinical trials to institution > $5,000: Kite, Incyte, Miltenyi. Relationships: Board of Directors, BeTheMatch, NMDP.
MLR receives compensation from BioIntellect for Advisory Board.
Other authors have no competing interests.
References
- 1.Baker M, Wang H, Rowley SD, et al. Comparative Outcomes after Haploidentical or Unrelated Donor Bone Marrow or Blood Stem Cell Transplantation in Adult Patients with Hematological Malignancies. Biol Blood Marrow Transplant Published online 2016. doi: 10.1016/j.bbmt.2016.08.003 [DOI] [PubMed]
- 2.Luznik L, O’Donnell PV., Symons HJ, et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biol Blood Marrow Transplant Published online 2008. doi: 10.1016/j.bbmt.2008.03.005 [DOI] [PMC free article] [PubMed]
- 3.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood Published online 2015. doi: 10.1182/blood-2015-01-623991 [DOI] [PMC free article] [PubMed]
- 4.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood Published online 2015. doi: 10.1182/blood-2015-04-639831 [DOI] [PMC free article] [PubMed]
- 5.Passweg JR, Baldomero H, Bader P, et al. Use of haploidentical stem cell transplantation continues to increase: The 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant Published online 2017. doi: 10.1038/bmt.2017.34 [DOI] [PMC free article] [PubMed]
- 6.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: A phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol Published online 2005. doi: 10.1200/JCO.2005.09.117 [DOI] [PubMed]
- 7.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched hla haplotype. N Engl J Med Published online 1998. doi: 10.1056/NEJM199810223391702 [DOI] [PubMed]
- 8.Kato S, Yabe H, Yasui M, et al. Allogeneic hematopoietic transplantation of CD34+ selected cells from an HLA haplo-identical related donor. A long-term follow-up of 135 patients and a comparison of stem cell source between the bone marrow and the peripheral blood. Bone Marrow Transplant Published online 2000. doi: 10.1038/sj.bmt.1702707 [DOI] [PubMed]
- 9.Koh LP, Rizzieri DA, Chao NJ. Allogeneic Hematopoietic Stem Cell Transplant Using Mismatched/Haploidentical Donors. Biol Blood Marrow Transplant 2007;13(11):1249–1267. doi: 10.1016/j.bbmt.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 10.Ciurea SO, Mulanovich V, Saliba RM, et al. Improved Early Outcomes Using a T Cell Replete Graft Compared with T Cell Depleted Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant Published online 2012. doi: 10.1016/j.bbmt.2012.07.003 [DOI] [PMC free article] [PubMed]
- 11.Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated Haploidentical Bone Marrow Transplantation and Posttransplantation Cyclophosphamide for Hematologic Malignancies after Myeloablative Conditioning. Biol Blood Marrow Transplant Published online 2013. doi: 10.1016/j.bbmt.2012.08.014 [DOI] [PubMed]
- 12.Nakamae H, Fujii K, Nanno S, et al. A prospective observational study of immune reconstitution following transplantation with post-transplant reduced-dose cyclophosphamide from HLA-haploidentical donors. Transpl Int Published online 2019. doi: 10.1111/tri.13494 [DOI] [PubMed]
- 13.Chang YJ, Zhao XY, Huang XJ. Effects of the NK Cell Recovery on Outcomes of Unmanipulated Haploidentical Blood and Marrow Transplantation for Patients with Hematologic Malignancies. Biol Blood Marrow Transplant Published online 2008. doi: 10.1016/j.bbmt.2007.12.497 [DOI] [PubMed]
- 14.Zhao XY, Chang YJ, Xu LP, Liu DH, Liu KY, Huang XJ. Association of natural killer cells in allografts with transplant outcomes in patients receiving G-CSF-mobilized PBSC grafts and G-CSF-primed BM grafts from HLA-haploidentical donors. Bone Marrow Transplant Published online 2009. doi: 10.1038/bmt.2009.73 [DOI] [PubMed]
- 15.Ringdén O, Labopin M, Ciceri F, et al. Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors compared with HLA-identical siblings? Leukemia Published online 2016. doi: 10.1038/leu.2015.232 [DOI] [PubMed]
- 16.McCurdy SR, Kasamon YL, Kanakry CG, et al. Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with post-transplantation cyclophosphamide. Haematologica Published online 2017. doi: 10.3324/haematol.2016.144139 [DOI] [PMC free article] [PubMed]
- 17.Or-Geva N, Reisner Y. The evolution of T-cell depletion in haploidentical stem-cell transplantation. Br J Haematol Published online 2016. doi: 10.1111/bjh.13868 [DOI] [PubMed]
- 18.Marek A, Stern M, Chalandon Y, et al. The impact of T-cell depletion techniques on the outcome after haploidentical hematopoietic SCT. Bone Marrow Transplant Published online 2014. doi: 10.1038/bmt.2013.132 [DOI] [PubMed]
- 19.Crocchiolo R, Bramanti S, Vai A, et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis Published online 2015. doi: 10.1111/tid.12365 [DOI] [PMC free article] [PubMed]
- 20.Copelan OR, Sanikommu SR, Trivedi JS, et al. Higher Incidence of Hemorrhagic Cystitis Following Haploidentical Related Donor Transplantation Compared with Matched Related Donor Transplantation. Biol Blood Marrow Transplant Published online 2019. doi: 10.1016/j.bbmt.2018.12.142 [DOI] [PubMed]
- 21.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-Haploidentical Bone Marrow Transplantation with High-Dose Posttransplantation Cyclophosphamide: Effect of HLA Disparity on Outcome. Biol Blood Marrow Transplant Published online 2010. doi: 10.1016/j.bbmt.2009.11.011 [DOI] [PMC free article] [PubMed]
- 22.Forms Instruction Manual. CIBMTR Forms Instr Man 2020;Q428–440:I. https://www.cibmtr.org/manuals/fim/1/en/topic/f2100-q428-440.
- 23.Kim S, Logan B, Riches M, Chen M, Ahn KW. Statistical methods for time-dependent variables in hematopoietic cell transplantation studies. Biol Blood Marrow Transplant Published online 2020. doi: 10.1016/j.bbmt.2020.09.034. [DOI] [PMC free article] [PubMed]
- 24.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal Published online 1995. doi: 10.1007/BF00985764 [DOI] [PubMed]
- 25.Marinelli T, Wee LYA, Rowe E, et al. Respiratory Viruses Cause Late Morbidity in Recipients of Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant Published online 2020. doi: 10.1016/j.bbmt.2019.12.724 [DOI] [PubMed]
- 26.Chemaly RF, Hanmod SS, Rathod DB, et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood Published online 2012. doi: 10.1182/blood-2011-08-371112 [DOI] [PubMed]
- 27.Garrett Nichols W, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: Risk factors, response to antiviral therapy, and effect on transplant outcome. Blood Published online 2001. doi: 10.1182/blood.V98.3.573 [DOI] [PubMed]
- 28.Ljungman P Respiratory virus infections in bone marrow transplant recipients: The European perspective. Am J Med Published online 1997. doi: 10.1016/s0002-9343(97)00010-7 [DOI] [PubMed]
- 29.Chakrabarti S, Avivi I, Mackinnon S, Ward K, Kottaridis PD, Osman H, Waldmann H, Hale G, Fegan CD, Yong K, Goldstone AH, Linch DC, Milligan DW. Respiratory virus infections in transplant recipients after reduced-intensity conditioning with Campath-1H: high incidence but low mortality. Br J Haematol 2002. Dec;119(4):1125–32. doi: 10.1046/j.1365-2141.2002.03992.x. [DOI] [PubMed] [Google Scholar]
- 30.Erard V, Chien JW, Kim HW, et al. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: The role of community respiratory viruses. J Infect Dis Published online 2006. doi: 10.1086/504268 [DOI] [PMC free article] [PubMed]
- 31.Versluys AB, Rossen JWA, van Ewijk B, Schuurman R, Bierings MB, Boelens JJ. Strong Association between Respiratory Viral Infection Early after Hematopoietic Stem Cell Transplantation and the Development of Life-Threatening Acute and Chronic Alloimmune Lung Syndromes. Biol Blood Marrow Transplant Published online 2010. [DOI] [PMC free article] [PubMed]
- 32.Piñana JL, Montoro J, Aznar C, et al. The clinical benefit of instituting a prospective clinical community-acquired respiratory virus surveillance program in allogeneic hematopoietic stem cell transplantation. J Infect Published online 2020. doi: 10.1016/j.jinf.2019.12.022 [DOI] [PMC free article] [PubMed]
- 33.Hemming VG, Prince GA, Groothuis JR, Siber GR. Hyperimmune globulins in prevention and treatment of respiratory syncytial virus infections. Clin Microbiol Rev Published online 1995. doi: 10.1128/cmr.8.1.22 [DOI] [PMC free article] [PubMed]
- 34.Groothuis JR. Role of antibody and use of respiratory syncytial virus (RSV) immune globulin to prevent severe RSV disease in high-risk children. J Pediatr Published online 1994. doi: 10.1016/S0022-3476(94)70188-1 [DOI] [PubMed]
- 35.Jamani K, He Q, Liu Y, et al. Early Post-Transplantation Spirometry Is Associated with the Development of Bronchiolitis Obliterans Syndrome after Allogeneic Hematopoietic Cell Transplantation: Early Post-Transplantation Spirometry and Risk of BOS. Biol Blood Marrow Transplant Published online 2020. doi: 10.1016/j.bbmt.2019.12.002 [DOI] [PMC free article] [PubMed]
- 36.Cortez K, Murphy BR, Almeida KN, et al. Immune-globulin prophylaxis of respiratory syncytial virus infection in patients undergoing stem-cell transplantation. J Infect Dis Published online 2002. doi: 10.1086/342412 [DOI] [PubMed]
- 37.Waghmare A, Xie H, Kupers J, Sorror ML, Jerome KR, Englund JA, Boeckh M, Leisenring WM. Human Rhinovirus Infections in Hematopoietic Cell Transplant Recipients:Risk Score for Progression to Lower Respiratory Infection. Biol Blood Marrow Transplant 2019. May;25(5):1011–1021. doi 10.1016/j.bbmt.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sim SA,Leung VKY,Ritchie D,Slavin MA, Sullivan SG, Teh BW. Viral Respiratory Tract Infections in Allogeneic Hematopoietic Stem Cell Transplant Recipients in the Era of Molecular Testing. Biol Blood Marrow Transplant 2018. Jul;24(7):1490–1496. doi: 10.1016/j.bbmt.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eichenberger EM, Soave R, Zappetti D, Small CB, Shore T, van Besien K, Douglass C, Westblade LF, Saitlin MJ. Incidence, significance, and persistence of human coronavirus infection in hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2019. Jul;54(7):1058–1066. doi: 10.1038/41409-018-0386-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fontana L, Strasfeld L. Respiratory Virus Infections of the Stem Cell Transplant Recipient and Hematologic Malignancy Patient. Infect Dis Clin North Am 2019. Jun;33(2):523–544. doi: 10.1016/j.idc.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakabarti S, Milligan DW, Moss PA, Mautner V. Adenovirus infections in stem cell transplant recipients: recent developmenta in understanding pathogenesis, diagnosis and management. Leuk Lymphoma 2004. May;45(5)873–85. doi: 10.1080/10428190310001628176 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


