Abstract
Background and aim
The COVID-19 pandemic has severely affected the world’s population in the last two years. Along with non-pharmacological public health interventions, major efforts have also been made to identify effective drugs or active substances for COVID-19 prevention and treatment. These include, among many others, the trace elements zinc and selenium, based on laboratory studies and some observational human studies. However, both of these study designs are not adequate to identify and approve treatments in human medicine, and experimental studies in the form of randomized controlled trials are needed to demonstrate the effectiveness and the safety of any interventions.
Methods
We undertook a systematic review in which we searched for published and unpublished clinical trials using zinc or selenium supplementation to treat or prevent COVID-19 in the Pubmed, Scopus and ClinicalTrials databases up to 10 January 2022.
Results
Amongst the published studies, we did not find any trial with selenium, whereas we retrieved four eligible randomized clinical trials using zinc supplementation, only one of which was double-blind. One of these trials looked at the effect of the intervention on the rate of new SARS-CoV-2 infections, and three at the COVID-19 clinical outcome in already infected individuals. The study populations of the four trials were very heterogeneous, ranging from uninfected individuals to those hospitalized for COVID-19. Only two studies investigated zinc alone in the intervention arm with no differences in the endpoints. The other two studies examined zinc in association with one or more drugs and supplements in the intervention arm, therefore making it impossible to disentangle any specific effects of the element. In addition, we identified 22 unpublished ongoing clinical trials, 19 on zinc, one on selenium and two on both elements.
Conclusion
No trials investigated the effect of selenium supplementation on COVID-19, while the very few studies on the effects of zinc supplementation did not confirm efficacy. Therefore, preventive or therapeutic interventions against COVID-19 based on zinc or selenium supplementation are currently unjustified, although when the results of the on-going studies are published, this may change our conclusion.
Keywords: COVID-19, Clinical trial, Selenium, Systematic review, Supplementation, Zinc
1. Introduction
Coronavirus disease (COVID-19) is a severe and potentially fatal condition caused by infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), an airborne virus that spread globally after its initial outbreak in late 2019 in China [1]. The clinical spectrum of SARS-CoV-2 severity ranges from an asymptomatic condition to mild symptoms such as fever, cough, ageusia, anosmia and asthenia [2], [3], up to most severe conditions, as acute respiratory distress syndrome (ARDS) and multi organ failure [4]. When the outbreak swept across Italy, which was the first country to be seriously and extensively hit by the epidemic [5], the case-fatality rate was as high as 15% [6]. To reduce the risk of transmission of SARS-CoV-2, several public health and preventive measures have been advised, including mobility restrictions through lockdown, and hand and respiratory hygiene [7], [8]. In the last months specific vaccines have been developed, with subsequent implementation of an impressive and most effective vaccination campaign [4], [9]. For COVID-19 patients, supportive care measures, such as mechanical ventilation, and a few pharmacological therapies, such as systemic corticosteroids, remain the standard of care, in the absence of a specific antiviral therapy [3], [10], [11].
In the current situation, there is an enormous interest about the possible preventive or supportive therapies against SARS-CoV-2 infection and the related disease, COVID-19. Among these, supplementation with vitamins and minerals has been suggested to help in counteracting the COVID-19 pandemic, and it has therefore increased in some populations [12], [13], [14], [15], [16], [17], [18]. Focusing on trace elements, zinc and selenium are the two minerals that triggered most of the interest by researchers and the general population, and there is evidence that self-supplementation with these two minerals has considerably increased due to this perception in the areas characterized by a high COVID-19 prevalence [19].
Some support for the possibility that zinc and selenium may be helpful in the prevention of the SARS-CoV-2 infection and the therapy of COVID-19 comes from the antioxidant and immunomodulatory properties of these two elements or related proteins [20], [21], [22], from their antiviral activity [23], [24], as well as from some nonexperimental human studies that suggested an involvement of a ‘low’ zinc and selenium status in favoring COVID-19 incidence and lethality [25], [26]. It has also been noted that zinc deficiency increases IL-6 levels [27], an observation of interest given the cytokine storm characterizing COVID-19 [28]. Concerning selenium, it has been proposed that the selenium-containing antioxidant enzyme glutathione peroxidase 1 could be related to the main protease (Mpro) of SARS-CoV-2, and therefore selenium status might have a role in COVID-19 onset [29], [30]. In addition, the zinc supplement appears to have mild beneficial effects on acute viral respiratory tract infections [31]. Some human non-experimental studies have suggested an involvement of low zinc and selenium status in COVID-19 development, but not all studies have yielded consistent results, and the observational design substantially weaken their capacity to demonstrate causal links [25], [32], [33], [34], [35], [36], [37]. This also explains why a recent meta-analysis including both experimental (clinical trials) and non-experimental human studies on zinc and COVID-19 concluded that zinc was not proven to be effective against COVID-19 [38].
However, in human medicine any claims of efficacy (and safety) of supplementation of substances, such as drugs or nutrients, will only be accepted if the data is derived from well-designed experimental studies, the prime choice being randomized controlled trials (RCTs) [39], [40]. COVID-19 is no exception to this general rule [41], [42]. The need to obtain experimental evidence for safety and efficacy before permitting supplementation with trace elements is important, and particularly relevant for selenium where the safety margin (between adequacy and toxicity) is narrow [43]. In the past it has been claimed that selenium could reduce the risk of cancer, cardiovascular disease and diabetes, mainly on the basis of observational epidemiologic studies and one single small trial [44]. However, experimental human studies in the form of RCTs have later shown that no such effects exist, and have even reported that adverse effects such as advanced prostate cancer and type 2 diabetes may be favored by selenium supplementation [45], [46].
In the light of our improved understanding of the controversies surrounding nutrient supplementation studies, we reviewed published clinical trials to see if there is any evidence for possible effects of zinc or selenium on COVID-19, and therefore if there is any indication that increased intake of these trace elements is warranted and appropriate to prevent the current COVID-19 pandemic. In particular, according to the PICO approach, we searched for studies which investigated subjects with a clinical diagnosis of SARS-CoV-2 infection or with developed COVID-19. The considered interventions were zinc or selenium, with the control group allocated to placebo only. Finally, we considered all possible clinical outcomes showing a potential effect of the intervention on the risk of SARS-CoV-2 infection and COVID-19 onset and progression, including, for instance, symptoms of reduction or clearance from the nasopharyngeal tract.
In addition, we wanted to determine whether the current recommendations not to use zinc supplements that exceed the recommended dietary allowance [47] should be changed. Excess intake of any nutrient, including that of these two trace elements, may lead to toxicity when the upper intake level is exceeded, therefore inappropriate reliance on these or other alleged therapeutic tools may have serious health consequences. In addition, we searched for unpublished RCTs and assessed their quality where possible, in order to predict which high quality evidence would be available for meta-analysis in the future.
2. Methods
To assess all the evidence on the efficiency of zinc or selenium against SARS-CoV-2 transmission and COVID-19 onset and progression, we searched for clinical trials in the Pubmed, Scopus and ClinicalTrials databases from inception until 10 January 2022, using “(zinc OR selenium) AND (COVID-19 OR SARS-CoV-2)” as MeSH terms for Pubmed and as keywords for Scopus. We filtered the research including “Clinical Trial” option on Pubmed and adding INDEXTERMS (“clinical trials”) AND (LIMIT-TO (DOCTYPE, “ar”)) on Scopus. On the ClinicalTrials.gov database we set COVID-19 in the “condition or disease” field and alternatively zinc or selenium in the “Intervention/treatment” field. Included clinical trials had to report results on the effects of zinc or selenium compared to no intervention or placebo, with the use of the trace element, combined or not with other treatments, being the only difference between the treatment and control groups.
We assessed the risk of bias in both published and unpublished clinical trials with RoB 2 guidelines [48]. Two investigators (EB and FZ) assessed the risk of bias and any discrepancy was resolved with the help of a third author (TF). Outcomes of primary interest were mortality and Intensive Care Unit admissions. Secondary outcomes were all outcomes related to SARS-CoV-2 infection and COVID-19 onset and progression, e.g. hospitalization, time after symptoms reduction or time for nasopharyngeal tract clearance.
3. Results
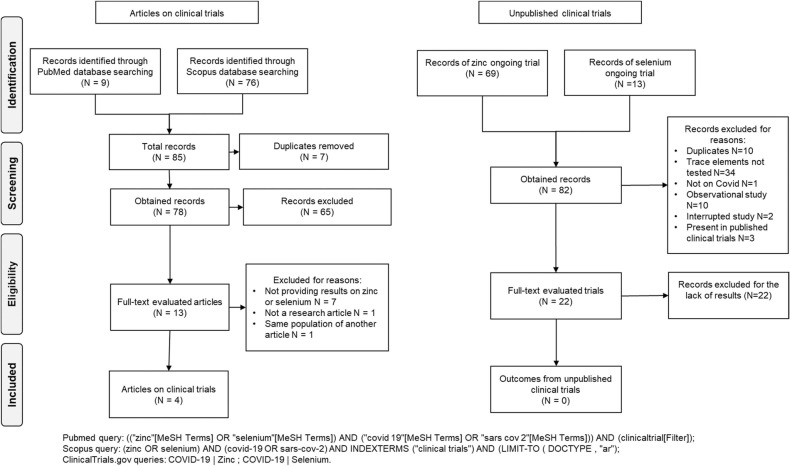
We retrieved 78 records from Pubmed and Scopus databases after removing duplicates ( Fig. 1). After title and abstract screening, we assessed the full-text of the remaining 13 papers. After removing duplicate studies and investigations not reporting any endpoints, we retrieved 4 trials, all of them evaluating the effect of zinc on COVID-19. Two of these four trials were registered on ClinicalTrials.gov database. No published clinical trial on selenium was found.
Fig. 1.
Flow chart for clinical trials on zinc or selenium for COVID-19 prevention and treatment.
The characteristics of the four eligible studies are shown in Table 1 [49], [50], [51], [52]. The first study was a multi-center randomized clinical trial, considering a generic population of patients with a confirmed diagnosis of COVID-19 [49]. It included a total of 191 participants from Egypt with a mean age of 43 years and with possible comorbidities of hypertension, diabetes or hepatic diseases. The observation period was 28 days and the intervention consisted of 220 mg of zinc sulfate, which contained 50 mg of elemental zinc, twice a day for 15 days. Both the intervention and the placebo arms also received hydroxicloroquine, a drug which could act as a zinc ionophore. They did not evaluated baseline zinc concentration or time between zinc initiation and symptoms onset [49]. Results did not show a significant effect of zinc treatment: recovery after 28 days and death rate were 79.2% and 5.2% in the intervention group respectively, and 77.9% and 5.3% in the control group respectively.
Table 1.
Description of eligible studies on clinical trials on the effects of zinc and selenium against COVID-19.
| Study | Trial type and duration |
Population | Population characteristics | Location | Interventions | Conclusions of the study |
|---|---|---|---|---|---|---|
| Abd-Elsalam 2021 [49] | RCT 23 June 2020–23 August 2020 28 days |
Confirmed diagnosis of COVID-19 infection N = 191 |
Female: 44 IG group-31 CG; Male: 52 IG-64 CG Excluded: patients with hypokalemia or hypomagnesemia, porphyria, neutrophilia, myasthenia gravis, maculopathy or changes in the visual field, heart failure, prolonged QT interval in ECG, liver cirrhosis, psoriasis, epilepsy, anemia from pyruvate kinase and G6PD deficiencies, chronic kidney disease, and pregnant or lactating females |
Egypt | Combination of: -CQ/HCQ -220 mg of zinc sulfate twice daily (50 mg of elemental zinc twice daily) |
Zinc supplements did not modify the clinical efficacy of HCQ: recovery after 28 days and death fate were 79.2% and 5.2% in intervention group respectively, and 77.9% and 5.3% in control group respectively. |
| Margolin 2021 [50] | Non-randomized clinical trial March 2020–July 2020 20 weeks |
Healthy exposed population N = 113 |
53 IG-60 CG Female/male: approximately 60/40% Excluded: oxygen saturation <94%, afebrile temperature |
United States (Ohio) | Combination of: -Zinc 25 mg once a day -Vitamin C -Vitamine D3 -Vitamine E -l-lysine -Quercetin -Quina™ |
15% were diagnosed with SARS-CoV-2 infection in the control group after 20 weeks, 0% in intervention group. |
| Patel 2021 [51] | Phase IIa pilot double‐blind RCT From May 2020 28 days |
COVID‐19 confirmed hospitalized adults with oxygen saturation (SpO2) of ≤94% N = 33 |
Female: 4 IG-8 CG Male: 11 IG-10 CG Excluded: age<18, pregnant, lactating female, allergy to zinc, severe hepatic impairment, history of organ transplant which requires immunosuppressive treatment which can interfere with kidney function, HIV infection, patient required cardiopulmonary resuscitation, imminent or inevitable death, eGFR<60 mL/min/1.73 m2 or patient requiring dialysis, haemochromatosis |
Australia | -0.5 mg/kg/day of zinc chloride (0.24 mg/kg/day elemental zinc) by intravenous infusion | The primary outcome in non-ventilated patients was oxygen flow required to maintain blood oxygen levels above 94%. Did not reach its target enrollment. Same clinical results in the two groups. HDIVZn increased serum zinc levels above the deficiency cutoff of 10.7 μmol/L in the intervention arm. |
| Thomas 2021 [52] | RCT 27 April 2020–14 October 2020 10 days |
Diagnosis of SARS-CoV-2 infection confirmed with a polymerase chain reaction assay N = 214 |
Female: 37 only zinc IG-31 CG Male: 21 only zinc IG-19 CG Excluded: hospitalized, resided outside of Ohio or Florida, pregnant, actively lactating, had advanced chronic kidney disease, liver disease awaiting transplantation, or history of calcium oxalate kidney stones |
United States (Ohio and Florida) | Separately: -50 mg of zinc gluconate once a day (7 mg of elemental zinc once a day [79]) -Ascorbic acid -Combination of both treatments |
Study was stopped. Treatment had no effect on SARS-CoV-2 symptoms. 50% reduction in symptoms occurred in a mean of 6.7 days in standard care group and 5.9 days in zinc only group. |
Abbreviations: CG, control group; CQ, chloroquine; eGFR, estimated glomerular filtration rate; HCQ, hydroxychloroquine; HDIVZn, high‐dose intravenous zinc; IG, intervention group; RCT, randomized controlled trial.
The second study was a single-center non-randomized and apparently unblinded clinical trial [50]. It included 113 healthy exposed participants from United States, with a duration of intervention of 20 weeks encompassing the adminitration of 25 mg/day of zinc together with zinc ionophores (quina plant bark extract and quercetin), vitamins C, D3 and E, and l-lysine. Population age mode was 59 years and considered comorbidities were hypertension, coronary artery disease and type 2 diabetes mellitus. Also in this case, zinc baseline concentration was not considered. This non-randomized trial suggested possible differences between the intervention arm and the control arm (apparently not given placebo), as it reported a lower infection rate in the intervention arm (15% were diagnosed with SARS-CoV-2 infection in the control group after 20 weeks, 0% in intervention group).
The third study was a single-center double-blind randomized clinical trial on 33 COVID-19 confirmed hospitalized adults with oxigen saturation of 94% or less, having a mean age of 60 years [51]. The population was from Australia and it included participants with hypertension, diabetes mellitus, chronic cardiovascular disease, chronic respiratory disease, cirrhosis and hepathic failure. The period of intervention (0.24 mg elemental zinc/kg body weight/day) was 7 days and the period of observation of both intervention and placebo arms was 28 days. In contrast to other studies, serum baseline zinc concentration was assessed. The study did not reach the enrollment target because public health measures reduced the number of people eligible for enrollment [51]. As the only conclusion of the study, the authors reported the increased serum zinc levels induced by intravenous zinc supplementation, and the capaciity of zinc supplementation to restore adequate zinc status in the supplemented participants. Additional results reported a worse outcome concerning hospitalization in the first 7 days in the intervention arm (85% of patients hospitalized versus 67% in the placebo arm), while no differences between the two arms was observed at 28 days.
The last study was a multi-center and apparently unblinded randomized clinical trial carried out in the US, with 214 participants with SARS-CoV-2 infection who were receiving outpatient care in Ohio and Florida [52]. The mean age of the population was 45 years, and a substantial part of it reported comorbidities such as diabetes, hypertension, dyslipidemia, asthma, anxiety and depression. The intervention arm received a daily supplementation of 50 mg gluconate zinc for 10 days, while the control group received a standard ‘usual care’ for COVID-19 without further specification. The time between symptom onset and zinc intervention was assessed. The trial was stopped early for futility. A 50% reduction in symptoms occurred in a mean period of 6.7 days after the start of the trial in the standard care group, while the corresponding period in the zinc-only group was 5.9 days. Additionally, hospitalization occurred in 8.6% of the intervention group and 6.0% of the control group, while death rate was 0% for both.
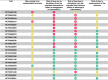
Risk of bias evaluation of clinical trials with published results is shown in Table 2. All the studies had at least one source of high risk of bias: all studies analyzed, except one [51], have high risk of bias in the deviation from the intendeed interventions. It should be noted that only two out of four trials were registered on ClinicalTrial.gov [49], [52], one [51] had published its protocol in a previous study [53], and one not neither [50].
Table 2.
Risk of bias of the eligible clinical trials evaluated with RoB 2 tool. Red, yellow and green marks correspond to high, medium and low risk of bias, respectively.
 |
3.1. Ongoing Trials
For currently unpublished clinical trials searched on ClinicalTrials.gov database, we retrieved 82 records, 22 of which were included even if they did not upload results ( Table 3): five clinical trials are completed but the results are not yet published; ten clinical trials are in the recruitment phase. The main reason for excluding a study was the fact that the effects of trace elements were not tested, as they were administrated to both the control and case group. Nineteen ongoing trials evaluated the administration of zinc, fourteen ongoing trials specified the dose in various zinc coumpounds. The minimum daily dose of zinc was 10 mg and the maximum was 220 mg. One study analyzed selenium through administering a dose of 1000 μg daily intravenously. In other two ongoing trials, zinc and selenium together were administered at daily doses of 7.5 mg and 15 μg and of 10 mg and 110 mg, respectively. The risk of bias assessment was performed without considering the two domains referred to result reporting ( Table 4). Most of unpublished clinical trials performed a randomized allocation for the interventions but did not provide any information about allocation concealment. Twenty ongoing trials performed a randomization, one was not randomized and another had no information available. Some studies did not blind all the participants, trial personnel and outcome assessors e.g. analysts: five studies were open label, two had a single masking: in one of the two the evaluators were blinded, in the other the participants were blinded. The remaining studies were at least double-blind.
Table 3.
Description of unpublished clinical trials on the effects of zinc and selenium against COVID-19.
| NCT Number | Interventions | Enrollment | Study Design | Location | Status |
|---|---|---|---|---|---|
| NCT04323228 | Dietary Supplement: Oral supplement enriched in antioxidants (15 μg selenium and 7.5 mg zinc once a day) Dietary Supplement: Cellulose-containing placebo capsules |
40 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Double (Participant, Care Provider) Primary Purpose: Supportive Care |
Saudi Arabia | Recruiting |
| NCT04334512 | Drug: Hydroxychloroquine Drug: Azithromycin Dietary Supplement: Vitamin C Dietary Supplement: Vitamin D Dietary Supplement: Zinc (not specified) |
600 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Double (Participant, Investigator) Primary Purpose: Treatment |
California, United States | Recruiting |
| NCT04335084 | Drug: Hydroxychloroquine Dietary Supplement: Vitamin C Dietary Supplement: Vitamin D Dietary Supplement: Zinc (not specified) |
600 | Allocation: Randomized Intervention Model: Single Group Assignment Masking: Double (Participant, Investigator) Primary Purpose: Prevention |
California, United States | Recruiting |
| NCT04435587 | Drug: Ivermectin pills/zinc sulfate (100 mg two times a day for three days) Drug: Combined ART/hydroxychloroquine/zinc sulfate (23 mg two times a day for five days) |
80 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Single (Outcomes Assessor) Primary Purpose: Treatment |
Thailand | Recruiting |
| NCT04446104 | Drug: Hydroxychloroquine sulfate tablets Drug: Ivermectin 3 mg tablets Drug: Zinc (80 mg once a day) Drug: Povidone-iodine Dietary Supplement: Vitamin C |
4257 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: None (Open Label) Primary Purpose: Prevention |
Singapore | Completed |
| NCT04468139 | Drug: Quercetin Dietary Supplement: Bromelain Drug: Zinc (50 mg once a day) Drug: Vitamin C |
60 | Allocation: N/A Intervention Model: Single Group Assignment Masking: None (Open Label) Primary Purpose: Treatment |
Saudi Arabia | Recruiting |
| NCT04472585 | Drug: Ivermectin injectable solution Other: Injectable placebo Drug: Zinc (20 mg three times a day) Drug: Placebo empty capsule Drug: Oral ivermectin |
180 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) Primary Purpose: Treatment |
Pakistan | Recruiting |
| NCT04542993 | Dietary Supplement: Zinc picolinate (50 mg three times a day) Dietary Supplement: Resveratrol Dietary Supplement: Zinc picolinate placebo Dietary Supplement: Resveratrol placebo |
60 | Allocation: Randomized Intervention Model: Single Group Assignment Masking: Single (Participant) Primary Purpose: Supportive Care |
Washington, United States | Active, not recruiting |
| NCT04558424 | Dietary Supplement: Zinc gluconate (220 mg once a day) and ascorbic acid | 50 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) Primary Purpose: Supportive Care |
Bangladesh | Not yet recruiting |
| NCT04621149 | Other: Chlorine dioxide Dietary Supplement: Zinc acetate (not specified) Drug: Famotidine Other: Placebo Dietary Supplement: Lactoferrin, green tea extract |
120 | Allocation: Randomized Intervention Model: Factorial Assignment Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) Primary Purpose: Treatment |
Arizona, United States | Recruiting |
| NCT04621461 | Dietary Supplement: Zinc sulfate (220 mg once a day) Drug: Placebo |
3 | Allocation: Randomized Intervention Model: Single Group Assignment Masking: Double (Participant, Investigator) Primary Purpose: Treatment |
New York, United States | Completed |
| NCT04641195 | Dietary Supplement: Vitamin D3 (cholecalciferol) Dietary Supplement: Zinc gluconate (40 mg once a day) Dietary Supplement: Zinc gluconate (40 mg once a day) and vitamin D (cholecalciferol) Other: Placebo |
700 | Allocation: Randomized Intervention Model: Factorial Assignment Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) Primary Purpose: Treatment |
India | Recruiting |
| NCT04751669 | Dietary Supplement: Vitamin and trace elements (zinc 10 mg once a day and selenium 110 mg once a day) Dietary Supplement: Placebo |
300 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) Primary Purpose: Treatment |
Spain | Not yet recruiting |
| NCT04828538 | Dietary Supplement: Vitamin D Dietary Supplement: Omega DHA/EPA Dietary Supplement: Vitamin C, vitamin B complex and zinc acetate (100 mg once a day) |
3600 | Allocation: Randomized Intervention Model: Factorial Assignment Masking: Triple (Participant, Care Provider, Investigator) Primary Purpose: Other |
Mexico | Active, not recruiting |
| NCT04869579 | Drug: Selenious acid (first day 2000 μg, following days 1000 μg) Other: Placebo |
100 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Double (Participant, Investigator) Primary Purpose: Treatment |
Texas, United States | Not yet recruiting |
| NCT04935515 | Drug: Oral antibiotic, antihistamine, anti-inflammatory, multivitamins and zinc (not specified) Drug: Oral low dose steroid Drug: Intravenous antibiotics with low dose steroid Drug: Oral anti-coagulant |
25 | Allocation: Non-Randomized Intervention Model: Parallel Assignment Masking: None (Open Label) Primary Purpose: Supportive Care |
India | Completed |
| NCT04937556 | Dietary Supplement: Probiotic: Lactobacillus salivarius + vitamin D + zinc (not specified) Dietary Supplement: Placebo |
60 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Triple (Participant, Care Provider, Investigator) Primary Purpose: Treatment |
Spain | Recruiting |
| NCT05003492 | Combination Product: Combination therapy with zinc (15 mg twice daily) plus standard therapy Radiation: Photodynamic therapy Drug: Standard therapy |
2 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: None (Open Label) Primary Purpose: Treatment |
Saudi Arabia | Not yet recruiting |
| NCT04377646 | Drug: Hydroxychloroquine Drug: Hydroxychloroquine (placebo) Drug: Zinc (15 mg once a day) Drug: Zinc (placebo) |
660 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Triple (Participant, Care Provider, Investigator) Primary Purpose: Prevention |
Tunisia | Not yet recruiting |
| NCT04551339 | Dietary Supplement: PreserVision AREDS formulation soft gels or tablets (69.6 mg/day of zinc) Dietary Supplement: Multivitamin with 11 mg of zinc |
2700 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: None (Open Label) Primary Purpose: Other |
Minnesota, United States | Completed |
| NCT04584567 | Drug: Doxycyclin and placebo Drug: Doxycyclin and zinc (15 mg once a day) Drug: Placebo |
194 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Double (Participant, Investigator) Primary Purpose: Prevention |
Tunisia | Completed |
| NCT04780061 | Drug: Vitamin D3 50,000 IU Dietary Supplement: Vitamin C/zinc (8.3 mg three times a day) Dietary Supplement: Vitamin K2/D Other: Microcrystalline cellulose capsules Other: Medium chain triglyceride oil |
200 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) Primary Purpose: Treatment |
Canada | Recruiting |
Table 4.
Risk of bias evaluation of the ongoing clinical trials having used RoB 2 tool. Red, yellow and green marks correspond to high, medium and low risk of bias, respectively. The last two domains of the tool have not been evaluated as no result was presented.
 |
4. Discussion
The relation between zinc, selenium and SARS-CoV-2 infection and COVID-19 has been discussed in the biomedical literature [49], [50], [51], [52], and the possibility of beneficial effects of supplementation with these two trace elements has been proposed based on the purported effects of zinc and selenium on the immune system, the capacity of these elements to counteract viral infections and diseases [24], [54], [55], and, finally, on the results of a few nonexperimental human studies [56], [57], [58]. The hypothesis arises from mechanistic evidence generated from biochemical and toxicological studies in vitro or in laboratory animals, showing that zinc and selenium may support the immune system [13], [20] and may have effects against viral pathologies and viral replication [24], [54]. Indeed, zinc and selenium are involved in both the innate and cell-mediated immune response [20], [59], [60], [61], [62], [63], [64], [65], and zinc supplementation has been proposed to enhance the immune response [13], [66], [67].
However, in order to establish causal connections between these two trace elements and both SARS-CoV-2 infection and COVID-19 disease, randomized controlled trials are needed. In fact, this is the only reliable study design, and the gold standard for clinical research, human medicine and pharmacology to assess and confirm the efficacy and safety of any intervention [68], [69], [70]. For zinc and selenium, safety is also an issue and an endpoint of major relevance, due to the potential toxicity of high doses of these two elements, particularly of selenium, an element known to have a narrow safe range of intake [71], [72]. In this review, we were unable to retrieve any trials on selenium supplementation and COVID-19-related endpoints, and therefore the current evidence does not justify selenium supplementation to prevent and treat COVID-19, especially given potential safety issues. The RCTs on zinc were characterized by substantially null and non-conclusive findings, and, in addition, several methodological issues that hampered the interpretation of results. In particular, one study did not implement any randomization, since allocation to the treatment was on a voluntary basis [50]. The results were interpreted independently from the comments and conclusions of the authors of the corresponding studies, and from the implementation of any statistical analysis.
Two of the eligible studies evaluated the effect of zinc in monotherapy supplementation, both showing lack of effect against COVID-19 severity [51], [52]. Conversely, one trial suggested possible differences between the control and intervention groups, but it was not randomized, and, in addition, zinc was part of a multi-pharmacological intervention, so the effect of zinc itself could not be disentangled. The included studies showed high heterogeneity also in terms of employed elemental zinc doses, ranging from 7 mg/day to 100 mg/day, as well as mode of administration, i.e. intravenous or oral. In addition, the study populations in these trials were heterogeneous, since they included both participants uninfected and infected by SARS-CoV-2, and without COVID-19 or with very different severity of the disease (mild, moderate, severe and critical COVID-19, or unknown severity). Three studies were unblinded, thus being exposed to very serious risk of bias for possible difference in behavior among participants based on the allocation arm, and no RCTs with low risk of bias appear to have been carried out so far. An aspect that can mislead is the lack of baseline zinc measurement at the time of enrollment, especially if no randomization has been done. Only one study performed this measurement at baseline [51].
Zinc status is notoriously difficult to assess, except for severe deficiency, and plasma zinc concentration changes in the presence of infection, so it would be difficult to determine zinc status in patients infected with COVID-19 [73], [74]. For all these reasons, any utility of zinc supplementation in COVID-19 prevention and treatment has not been proven so far, thus supporting the recommendation not to rely on zinc supplementation in COVID-19 therapy [47], [75].
For the prevention and therapy of COVID-19, as more generally with any human disease, it is mandatory to use only treatments and drugs with scientifically established efficacy, and these may only be identified through randomized controlled trials adequately testing the efficacy and safety of an intervention in the target populations [69], [70]. Claims based on observational studies or laboratory studies are insufficient and may be misleading and therefore have serious consequences, such as false expectations of protection, choice of ineffective treatment, and decrease in the use of the appropriate measures to prevent SARS-CoV-2 infection and COVID-19 severity (starting from the specific vaccination). Finally, in some cases, mineral supplementation may lead to intakes that exceed the upper safe level (UL), a serious instance particularly for selenium given its uncertain but narrow safe range of intake [46], [72], [76]. The intake of zinc exceeded the UL derived by various agencies [77], [78] in one analyzed trial [49]. As things stand, in the absence of data from clinical trials to support zinc and selenium supplementation in COVID-19 prevention and therapy, supplementation of these trace elements for these goals should be avoided. It should also be noted that the use of zinc and selenium has not been supported or authorized by Drug Regulatory Agencies and by bodies as the World Health Organization and the Center for Disease Control [47], [75]. Nevertheless, the presence of so many ongoing trials compared to those published until now could in future modify the indications that emerge from our review.
Funding
Drs. Balboni, Filippini, and Vinceti were supported by grant ‘Dipartimenti di Eccellenza 2018–2022” to the Department of Biomedical, Metabolic and Neural Sciences of the University of Modena and Reggio Emilia from the Italian Ministry of Education, University and Research. Dr. Filippini was supported by grant ‘UNIMORE FAR 2020 Interdisciplinare Linea FOMO - Fondazione di Modena’ and by grant ‘UNIMORE FAR IMPULSO 2020’ (no. 494/2020) from the University of Modena and Reggio Emilia.
CRediT authorship contribution statement
TF and MV conceived the study. EB and FZ extracted and analyzed data with TF. All authors interpreted the data. EB and FZ prepared the first of the manuscript with contribution of TF, SJF-T and MV. All authors read and approved the final manuscript.
Declarations of interest
None.
References
- 1.Yesudhas D., Srivastava A., Gromiha M.M. COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics. Infection. 2021;49(2):199–213. doi: 10.1007/s15010-020-01516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao Z., Xu Y., Sun C., Wang X., Guo Y., Qiu S., Ma K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021;54(1):12–16. doi: 10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan S.S., Yan B., Saw S., Lee C.K., Chong A.T., Jureen R., Sethi S. Practical laboratory considerations amidst the COVID-19 outbreak: early experience from Singapore. J. Clin. Pathol. 2021;74(4):257–260. doi: 10.1136/jclinpath-2020-206563. [DOI] [PubMed] [Google Scholar]
- 4.Docea A.O., Tsatsakis A., Albulescu D., Cristea O., Zlatian O., Vinceti M., Moschos S.A., Tsoukalas D., Goumenou M., Drakoulis N., Dumanov J.M., Tutelyan V.A., Onischenko G.G., Aschner M., Spandidos D.A., Calina D. A new threat from an old enemy: reemergence of coronavirus (Review) Int. J. Mol. Med. 2020;45(6):1631–1643. doi: 10.3892/ijmm.2020.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinceti M., Filippini T., Rothman K.J., Di Federico S., Orsini N. SARS-CoV-2 infection incidence during the first and second COVID-19 waves in Italy. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filippini T., Zagnoli F., Bosi M., Giannone M.E., Marchesi C., Vinceti M. An assessment of case-fatality and infection-fatality rates of first and second COVID-19 waves in Italy. Acta Biomed. 2021;92(S6) doi: 10.23750/abm.v92iS6.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemmer C.J., Hufert F., Siewert S., Reisinger E. Protection from COVID-19: the efficacy of face masks. Dtsch Arztebl Int. 2021;118(5):59–65. doi: 10.3238/arztebl.m2021.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinceti M., Filippini T., Rothman K.J., Ferrari F., Goffi A., Maffeis G., Orsini N. Lockdown timing and efficacy in controlling COVID-19 using mobile phone tracking. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J. New strategy for COVID-19 vaccination: targeting the receptor-binding domain of the SARS-CoV-2 spike protein. Cell Mol. Immunol. 2021;18(2):243–244. doi: 10.1038/s41423-020-00584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Castelnuovo A., Costanzo S., Antinori A., Berselli N., Blandi L., Bonaccio M., Bruno R., Cauda R., Gialluisi A., Guaraldi G., Menicanti L., Mennuni M., My I., Parruti A., Patti G., Perlini S., Santilli F., Signorelli C., Stefanini G.G., Vergori A., Ageno W., Aiello L., Agostoni P., Al Moghazi S., Arboretti R., Aucella F., Barbieri G., Barchitta M., Bartoloni A., Bologna C., Bonfanti P., Caiano L., Carrozzi L., Cascio A., Castiglione G., Chiarito M., Ciccullo A., Cingolani A., Cipollone F., Colomba C., Colombo C., Crosta F., Dalena G., Dal Pra C., Danzi G.B., D’Ardes D., de Gaetano Donati K., Di Gennaro F., Di Tano G., D’Offizi G., Filippini T., Maria Fusco F., Gaudiosi C., Gentile I., Gini G., Grandone E., Guarnieri G., Lamanna G.L.F., Larizza G., Leone A., Lio V., Losito A.R., Maccagni G., Maitan S., Mancarella S., Manuele R., Mapelli M., Maragna R., Marra L., Maresca G., Marotta C., Mastroianni F., Mazzitelli M., Mengozzi A., Menichetti F., Milic J., Minutolo F., Molena B., Mussinelli R., Mussini C., Musso M., Odone A., Olivieri M., Pasi E., Perroni A., Petri F., Pinchera B., Pivato C.A., Poletti V., Ravaglia C., Rossato M., Rossi M., Sabena A., Salinaro F., Sangiovanni V., Sanrocco C., Scorzolini L., Sgariglia R., Simeone P.G., Spinicci M., Trecarichi E.M., Veronesi G., Vettor R., Vianello A., Vinceti M., Visconti E., Vocciante L., De Caterina R., Iacoviello L., Covid R., Treatments C. Lopinavir/ritonavir and darunavir/cobicistat in hospitalized COVID-19 patients: findings from the multicenter Italian CORIST Study. Front. Med. 2021;8 doi: 10.3389/fmed.2021.639970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavriatopoulou M., Ntanasis-Stathopoulos I., Korompoki E., Fotiou D., Migkou M., Tzanninis I.G., Psaltopoulou T., Kastritis E., Terpos E., Dimopoulos M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021;21(2):167–179. doi: 10.1007/s10238-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrao S., Mallaci Bocchio R., Lo Monaco M., Natoli G., Cavezzi A., Troiani E., Argano C. Does evidence exist to blunt inflammatory response by nutraceutical supplementation during COVID-19 pandemic? An overview of systematic reviews of vitamin D, vitamin C, melatonin, and zinc. Nutrients. 2021;13(4):1261. doi: 10.3390/nu13041261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S., Yang Y., Lin X., Li Z., Ma G., Su Z., Zhang S. A novel particulate delivery system based on antigen-Zn(2+) coordination interactions enhances stability and cellular immune response of inactivated foot and mouth disease virus. Mol. Pharm. 2020;17(8):2952–2963. doi: 10.1021/acs.molpharmaceut.0c00365. [DOI] [PubMed] [Google Scholar]
- 14.Jovic T.H., Ali S.R., Ibrahim N., Jessop Z.M., Tarassoli S.P., Dobbs T.D., Holford P., Thornton C.A., Whitaker I.S. Could vitamins help in the fight against COVID-19? Nutrients. 2020;12(9):2550. doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrelli F., Luciani A., Perego G., Dognini G., Colombelli P.L., Ghidini A. Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies. J. Steroid Biochem. Mol. Biol. 2021;211 doi: 10.1016/j.jsbmb.2021.105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakoor H., Feehan J., Al Dhaheri A.S., Ali H.I., Platat C., Ismail L.C., Apostolopoulos V., Stojanovska L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schomburg L. Selenium deficiency due to diet, pregnancy, severe illness, or COVID-19-A preventable trigger for autoimmune disease. Int. J. Mol. Sci. 2021;22(16):8532. doi: 10.3390/ijms22168532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taheri S., Asadi S., Nilashi M., Ali Abumalloh R., Ghabban N.M.A., Mohd Yusuf S.Y., Supriyanto E., Samad S. A literature review on beneficial role of vitamins and trace elements: evidence from published clinical studies. J. Trace Elem. Med. Biol. 2021;67 doi: 10.1016/j.jtemb.2021.126789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aysin E., Urhan M. Dramatic increase in dietary supplement use during COVID-19. Curr. Dev. Nutr. 2021;5:207. doi: 10.1093/cdn/nzab029_008. [DOI] [Google Scholar]
- 20.Avery J.C., Hoffmann P.R. Selenium, selenoproteins, and immunity. Nutrients. 2018;10(9):1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haryanto B., Suksmasari T., Wintergerst E.S., Maggini S. Multivitamin supplementation supports immune function and ameliorates conditions triggered by reduced air quality. Vitam. Min. 2015;4(2) doi: 10.4172/2376-1318.1000128. [DOI] [Google Scholar]
- 22.Prentice S. They are what you eat: can nutritional factors during gestation and early infancy modulate the neonatal immune response? Front. Immunol. 2017;8:1641. doi: 10.3389/fimmu.2017.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cermelli C., Vinceti M., Scaltriti E., Bazzani E., Beretti F., Vivoli G., Portolani M. Selenite inhibition of Coxsackie virus B5 replication: implications on the etiology of Keshan disease. J. Trace Elem. Med. Biol. 2002;16(1):41–46. doi: 10.1016/S0946-672X(02)80007-4. [DOI] [PubMed] [Google Scholar]
- 24.te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Younesian O., Khodabakhshi B., Abdolahi N., Norouzi A., Behnampour N., Hosseinzadeh S., Alarzi S.S.H., Joshaghani H. Decreased serum selenium levels of COVID-19 patients in comparison with healthy individuals. Biol. Trace Elem. Res. 2022;200:1562–1567. doi: 10.1007/s12011-021-02797-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubourg G., Lagier J.C., Brouqui P., Casalta J.P., Jacomo V., La Scola B., Rolain J.M., Raoult D. Low blood zinc concentrations in patients with poor clinical outcome during SARS-CoV-2 infection: is there a need to supplement with zinc COVID-19 patients? J. Microbiol. Immunol. Infect. 2021;54(5):997–1000. doi: 10.1016/j.jmii.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gammoh N.Z., Rink L. Zinc in infection and inflammation. Nutrients. 2017;9(6):324. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neagu M., Calina D., Docea A.O., Constantin C., Filippini T., Vinceti M., Drakoulis N., Poulas K., Nikolouzakis T.K., Spandidos D.A., Tsatsakis A. Back to basics in COVID-19: antigens and antibodies-completing the puzzle. J. Cell Mol. Med. 2021;25(10):4523–4533. doi: 10.1111/jcmm.16462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seale L.A., Torres D.J., Berry M.J., Pitts M.W. A role for selenium-dependent GPX1 in SARS-CoV-2 virulence. Am. J. Clin. Nutr. 2020;112(2):447–448. doi: 10.1093/ajcn/nqaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Huttenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.P., Liu Y., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O'Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu-Ozturk D., Wang H.Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d'Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., Garcia-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunter J., Arentz S., Goldenberg J., Yang G., Beardsley J., Myers S.P., Mertz D., Leeder S. Zinc for the prevention or treatment of acute viral respiratory tract infections in adults: a rapid systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2021;11(11) doi: 10.1136/bmjopen-2020-047474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moghaddam A., Heller R.A., Sun Q., Seelig J., Cherkezov A., Seibert L., Hackler J., Seemann P., Diegmann J., Pilz M., Bachmann M., Minich W.B., Schomburg L. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12(7):2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fromonot J., Gette M., Ben Lassoued A., Gueant J.L., Gueant-Rodriguez R.M., Guieu R. Hypozincemia in the early stage of COVID-19 is associated with an increased risk of severe COVID-19. Clin. Nutr. 2021 doi: 10.1016/j.clnu.2021.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duncan A., Talwar D., McMillan D.C., Stefanowicz F., O’Reilly D.S. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am. J. Clin. Nutr. 2012;95(1):64–71. doi: 10.3945/ajcn.111.023812. [DOI] [PubMed] [Google Scholar]
- 35.Yao J.S., Paguio J.A., Dee E.C., Tan H.C., Moulick A., Milazzo C., Jurado J., Della Penna N., Celi L.A. The minimal effect of zinc on the survival of hospitalized patients with COVID-19: an observational study. Chest. 2021;159(1):108–111. doi: 10.1016/j.chest.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlucci P.M., Ahuja T., Petrilli C., Rajagopalan H., Jones S., Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J. Med. Microbiol. 2020;69(10):1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derwand R., Scholz M., Zelenko V. COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study. Int. J. Antimicrob. Agents. 2020;56(6) doi: 10.1016/j.ijantimicag.2020.106214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szarpak L., Pruc M., Gasecka A., Jaguszewski M.J., Michalski T., Peacock F.W., Smereka J., Pytkowska K., Filipiak K.J. Should we supplement zinc in COVID-19 patients? Evidence from a meta-analysis. Pol. Arch. Intern Med. 2021;131(9):802–807. doi: 10.20452/pamw.16048. [DOI] [PubMed] [Google Scholar]
- 39.European Medicine Agency, Clinical trials in human medicines. 〈https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials-human-medicines〉, 2021 (Accessed 18 January, 2022).
- 40.Food and Drug Administration, Conducting clinical trials. 〈https://www.fda.gov/drugs/development-approval-process-drugs/conducting-clinical-trials〉, 2021 (Accessed 18 January, 2022).
- 41.Ben-Zuk N., Dechtman I.D., Henn I., Weiss L., Afriat A., Krasner E., Gal Y. Potential prophylactic treatments for COVID-19. Viruses. 2021;13(7):1292. doi: 10.3390/v13071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouffak S., Shubbar Q., Saleh E., El-Awady R. Recent advances in management of COVID-19: a review. Biomed. Pharm. 2021;143 doi: 10.1016/j.biopha.2021.112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jablonska E., Vinceti M. Selenium and human health: witnessing a Copernican revolution? J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2015;33(3):328–368. doi: 10.1080/10590501.2015.1055163. [DOI] [PubMed] [Google Scholar]
- 44.Clark L.C., Combs G.F., Jr., Turnbull B.W., Slate E.H., Chalker D.K., Chow J., Davis L.S., Glover R.A., Graham G.F., Gross E.G., Krongrad A., Lesher J.L., Jr., Park H.K., Sanders B.B., Smith Jr, C.L., Taylor J.R. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional prevention of cancer study group. JAMA. 1996;276(24):1957–1963. doi: 10.1001/jama.1996.03540240035027. [DOI] [PubMed] [Google Scholar]
- 45.Vinceti M., Filippini T., Del Giovane C., Dennert G., Zwahlen M., Brinkman M., Zeegers M.P., Horneber M., D’Amico R., Crespi C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018;1 doi: 10.1002/14651858.CD005195.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinceti M., Filippini T., Wise L.A., Rothman K.J. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111210. [DOI] [PubMed] [Google Scholar]
- 47.National Institute of Health, COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 〈https://www.covid19treatmentguidelines.nih.gov/〉, 2021 (Accessed 18 January, 2022).
- 48.Sterne J.A.C., Savovic J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., Emberson J.R., Hernan M.A., Hopewell S., Hrobjartsson A., Junqueira D.R., Juni P., Kirkham J.J., Lasserson T., Li T., McAleenan A., Reeves B.C., Shepperd S., Shrier I., Stewart L.A., Tilling K., White I.R., Whiting P.F., Higgins J.P.T. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 49.Abd-Elsalam S., Soliman S., Esmail E.S., Khalaf M., Mostafa E.F., Medhat M.A., Ahmed O.A., El Ghafar M.S.A., Alboraie M., Hassany S.M. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine? A randomized, multicenter trial. Biol. Trace Elem. Res. 2021;199(10):3642–3646. doi: 10.1007/s12011-020-02512-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Margolin L., Luchins J., Margolin D., Margolin M., Lefkowitz S. 20-week study of clinical outcomes of over-the-counter COVID-19 prophylaxis and treatment. J. Evid. Based Integr. Med. 2021;26:1–13. doi: 10.1177/2515690X211026193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel O., Chinni V., El-Khoury J., Perera M., Neto A.S., McDonald C., See E., Jones D., Bolton D., Bellomo R., Trubiano J., Ischia J. A pilot double-blind safety and feasibility randomized controlled trial of high-dose intravenous zinc in hospitalized COVID-19 patients. J. Med. Virol. 2021;93(5):3261–3267. doi: 10.1002/jmv.26895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., Il’Giovine Z.J., Mehra R., McWilliams C., Nissen S.E., Desai M.Y. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perera M., El Khoury J., Chinni V., Bolton D., Qu L., Johnson P., Trubiano J., McDonald C.F., Jones D., Bellomo R., Patel O., Ischia J. Randomised controlled trial for high-dose intravenous zinc as adjunctive therapy in SARS-CoV- 2 (COVID-19) positive critically ill patients: trial protocol. BMJ Open. 2020;10(12):e040580. doi: 10.1136/bmjopen-2020-040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beck M.A., Shi Q., Morris V.C., Levander O.A. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat. Med. 1995;1(5):433–436. doi: 10.1038/nm0595-433. [DOI] [PubMed] [Google Scholar]
- 55.Kaushik N., Subramani C., Anang S., Muthumohan R., Shalimar B., Nayak C.T., Ranjith-Kumar M., Surjit Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J. Virol. 2017;91(21):e00754–17. doi: 10.1128/JVI.00754-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Razeghi Jahromi S., Moradi Tabriz H., Togha M., Ariyanfar S., Ghorbani Z., Naeeni S., Haghighi S., Jazayeri A., Montazeri M., Talebpour M., Ashraf H., Ebrahimi M., Hekmatdoost A., Jafari E. The correlation between serum selenium, zinc, and COVID-19 severity: an observational study. BMC Infect. Dis. 2021;21(1):899. doi: 10.1186/s12879-021-06617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagher Pour O., Yahyavi Y., Karimi A., Khamaneh A.M., Milani M., Khalili M., Sharifi A. Serum trace elements levels and clinical outcomes among Iranian COVID-19 patients. Int. J. Infect. Dis. 2021;111:164–168. doi: 10.1016/j.ijid.2021.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh S., Diwaker A., Singh B.P., Singh R.K. Nutritional immunity, zinc sufficiency, and COVID-19 mortality in socially similar European populations. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.699389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma C., Hoffmann P.R. Selenoproteins as regulators of T cell proliferation, differentiation, and metabolism. Semin. Cell Dev. Biol. 2021;115:54–61. doi: 10.1016/j.semcdb.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maywald M., Wessels I., Rink L. Zinc signals and immunity. Int. J. Mol. Sci. 2017;18(10):2222. doi: 10.3390/ijms18102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wessels I., Fischer H.J., Rink L. Update on the multi-layered levels of zinc-mediated immune regulation. Semin. Cell Dev. Biol. 2021;115:62–69. doi: 10.1016/j.semcdb.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Wintergerst E.S., Maggini S., Hornig D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006;50(2):85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 63.Kim B., Lee W.W. Regulatory role of zinc in immune cell signaling. Mol. Cells. 2021;44(5):335–341. doi: 10.14348/molcells.2021.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin R.S., Rodriguez C., Veillette A., Lodish H.F. Zinc is essential for binding of p56(lck) to CD4 and CD8alpha. J. Biol. Chem. 1998;273(49):32878–32882. doi: 10.1074/jbc.273.49.32878. [DOI] [PubMed] [Google Scholar]
- 65.Romir J., Lilie H., Egerer-Sieber C., Bauer F., Sticht H., Muller Y.A. Crystal structure analysis and solution studies of human Lck-SH3; zinc-induced homodimerization competes with the binding of proline-rich motifs. J. Mol. Biol. 2007;365(5):1417–1428. doi: 10.1016/j.jmb.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 66.Singh K.P., Zaidi S.I., Raisuddin S., Saxena A.K., Murthy R.C., Ray P.K. Effect of zinc on immune functions and host resistance against infection and tumor challenge. Immunopharmacol. Immunotoxicol. 1992;14(4):813–840. doi: 10.3109/08923979209009237. [DOI] [PubMed] [Google Scholar]
- 67.Wong C.P., Rinaldi N.A., Ho E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol. Nutr. Food Res. 2015;59(5):991–999. doi: 10.1002/mnfr.201400761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim C.Y., In J. Randomization in clinical studies. Korean J. Anesth. 2019;72(3):221–232. doi: 10.4097/kja.19049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hariton E., Locascio J.J. Randomised controlled trials - the gold standard for effectiveness research: study design: randomised controlled trials. BJOG. 2018;125(13):1716. doi: 10.1111/1471-0528.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lash T., VanderWeele T., Haneuse S., Rothman K. Fourth ed., Lippincott Williams and Wilkins; 2021. Modern Epidemiology. [Google Scholar]
- 71.Vinceti M., Filippini T., Wise L.A. Environmental selenium and human health: an update. Curr. Environ. Health Rep. 2018;5(4):464–485. doi: 10.1007/s40572-018-0213-0. [DOI] [PubMed] [Google Scholar]
- 72.Fairweather-Tait S.J., Bao Y., Broadley M.R., Collings R., Ford D., Hesketh J.E., Hurst R. Selenium in human health and disease. Antioxid. Redox Signal. 2011;14(7):1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 73.Arnaud J., Patriarca M., Fofou-Caillierez B.M., Gonzalez-Estecha M., Gomez M.G., De Graaf I., Patriarca V., Ropert-Bouchet M., Schroer-Janssen L., Siebelder C., Te Winkel M., Ventura Alemany M., Weykamp C. External quality assessment schemes for inorganic elements in the clinical laboratory: lessons from the OELM scheme. J. Trace Elem. Med. Biol. 2020;59 doi: 10.1016/j.jtemb.2019.126414. [DOI] [PubMed] [Google Scholar]
- 74.Chovelon B., Arnaud J. Influence of delayed separation of plasma from whole blood on Cu, I, Mn, Se, and Zn plasma concentrations. Clin. Chem. Lab. Med. 2018;56(3):e69–e71. doi: 10.1515/cclm-2017-0358. [DOI] [PubMed] [Google Scholar]
- 75.World Health Organization, Coronavirus disease (COVID-19): food safety and nutrition. 〈https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-food-safety-and-nutrition〉, 2020 (Accessed 18 January, 2022).
- 76.Vinceti M., Mandrioli J., Borella P., Michalke B., Tsatsakis A., Finkelstein Y. Selenium neurotoxicity in humans: bridging laboratory and epidemiologic studies. Toxicol. Lett. 2014;230(2):295–303. doi: 10.1016/j.toxlet.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 77.Institute of Medicine . Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press; 2001. Panel on micronutrients. [PubMed] [Google Scholar]
- 78.European Food Safety Authority, Tolerable upper intake levels for vitamins and minerals, 2006.
- 79.Saper R.B., Rash R. Zinc: an essential micronutrient. Am. Fam. Physician. 2009;79(9):768–772. [PMC free article] [PubMed] [Google Scholar]