Abstract
Background
Since December 2019, coronavirus disease 2019 (COVID-19) has become a global pandemic caused by highly transmissible severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although respiratory disease and multisystem inflammatory syndrome in children (MIS-C) are main clinical presentations in children, numerous neurological manifestations are being described increasingly. We aimed to investigate new onset neurological symptoms associated with SARS-CoV-2 in pediatric patients in order to establish a possible relationship as well as to understand the underlying pathophysiological mechanisms between SARS-CoV-2 infection and neurological findings.
Methods
We analyzed retrospectively children who had neurologic manifestations temporally associated with SARS-CoV-2 infection at Hacettepe University İhsan Doğramaci Children's Hospital. We performed a literature search between March 20, 2020 and March 30, 2021. Articles that report children with COVID-19 related neurological manifestations were included.
Results
We have observed 15 consecutive cases with new onset neurological manifestations along with confirmed SARS-CoV-2 infection. Age at hospitalization ranged from three months to 17 years. Ten patients had central nervous system involvement, and most common manifestation was encephalopathy (5/10), which is also one of the most common manifestations of the patients mentioned in the relevant 39 articles we reviewed.
Conclusion
Children with COVID-19 can present with neurologic findings such as encephalopathy, seizures, cerebrovascular events as well as abnormal eye movements. Clinical suspicion and awareness are required to show the association between neurologic manifestations and COVID-19.
Keywords: Neurology, SARS-CoV-2, COVID-19, Children, Seizure, MIS-C
1. Introduction
Since December 2019, the coronavirus disease 2019 (COVID-19), caused by the highly transmissible severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global pandemic [1]. The disease remains a challenge to clinicians due to its different manifestations. Numerous neurological complications have been described in patients with SARS-CoV-2 infection. These include both the central and peripheral nervous system, range from mild to severe, and can occur in patients with severe to asymptomatic COVID-19 due to different pathophysiological mechanisms [[1], [2], [3], [4], [5], [6]].
Since neurological complications have long been identified in association with other respiratory viruses, including influenza and coronaviruses, it is not surprising that SARS-CoV-2 may also lead to neurological problems [[5], [6], [7]]. In pediatric patients although respiratory disease and multisystem inflammatory syndrome in children (MIS-C) are the predominant concerns, increasing data shows the nervous system is a possible target for SARS-CoV-2 [[1], [2], [3], [4]].
In adults, nervous system involvement is more likely in severe cases [8,9]. Although headache, dizziness, and loss of smell and taste are the most commonly reported symptoms in adult patients, severe clinical outcomes such as acute cerebrovascular disease, transverse myelitis, encephalopathy, encephalitis, acute hemorrhagic necrotizing encephalopathy, seizures, and Guillain-Barré syndrome (GBS) have been reported [[5], [6], [7], [8], [9]]. Considering diverse neurological manifestations, multiple mechanisms like direct viral invasion of nervous system or activation of the inflammatory system and inflammatory mediators including cytokines may play a role in the pathogenesis of nervous system involvement in SARS-CoV-2 infection [[7], [8], [9], [10], [11], [12]]. Nervous system manifestations in COVID-19 pediatric patients have been reported. In this study we aimed to investigate acute neurological symptoms and signs associated with SARS-CoV-2 in pediatric patients in order to establish a possible temporal relationship as well as to understand the underlying pathophysiological mechanisms between SARS-CoV-2 infection and neurological findings.
2. Materials and methods
2.1. Patients
We retrospectively analyzed the patients hospitalized from March 11, 2020, to June 1, 2021, at Hacettepe University İhsan Doğramacı Children's Hospital with COVID-19-related illness in terms of neurological findings. Fifteen patients under 18 years old fulfilling the WHO's clinical criteria for case definition of SARS-CoV-2 were included [13].
Clinical and neuroradiological findings, neurophysiological tests, neurological diagnosis, and outcomes of each patient were analyzed. Patients with headache and loss of smell and taste due to COVID-19 were not included in the study. Two patients with prior neurological disorders, one with sensorineural hearing loss and the other with a history of stroke and factor V Leiden mutation were included due to presence of new-onset neurological findings.
Nasopharyngeal swab samples were tested for SARS-CoV-2 (Orf1ab, N and E genes) by real-time polymerase chain reaction (RT-PCR). Immunoglobulin G (IgG) anti-SARS-CoV-2 antibodies were quantified in the serum of patients with a negative SARS-CoV-2 PCR test. Euroimmun (received EUA from FDA) anti-SARS-CoV-2 enzyme-linked-immuno-adsorbent assay (ELISA) IgG Test Kits were used for detecting anti-SARS-CoV-2 IgG. The cerebrospinal fluid (CSF) of two patients was analyzed for SARS-CoV-2 PCR and anti-SARS-CoV-2 IgG.
Patients who had neurological manifestations associated with SARS-CoV-2 infection were divided into para-infectious and postinfectious groups. Those who were SARS-CoV-2 PCR positive and had neurological symptoms alone or in conjunction with other COVID-19 infectious symptoms were included in the para-infectious group. Patients who were SARS-CoV-2 PCR negative but anti-SARS-CoV-2 IgG positive and had neurological manifestations were included in the postinfectious group. The patients in the postinfectious group did not have acute COVID-19 symptoms. They had either a history of suggesting COVID-19 clinical findings at least three to eight weeks ago or an epidemiological link. No other obvious etiological factor existed in either group.
Descriptive statistics were used to assess the incidence and prevalence of neurological findings. The study was approved by the Hacettepe University Ethical Committee (June 15, 2021, 2021/12–02).
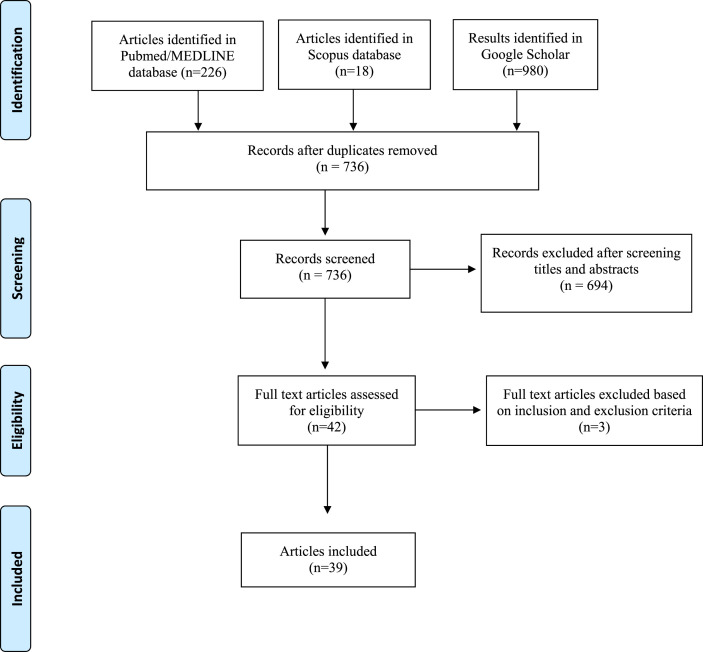
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (www.prisma-statement.org/) statement. The PRISMA checklist and flow diagram were used [14]. Flow diagram is given in Fig. 1 .
Fig. 1.
Literature review flow diagram.
2.2. Literature search
A detailed literature search was performed of the PubMed/MEDLINE, Scopus, and Google Scholar databases using the following keywords: “Coronavirus disease-19,” “COVID-19,” “2019 novel coronavirus diseases,” “severe acute respiratory syndrome coronavirus 2,” “SARS-CoV-2,” “2019-nCoV,” “MIS-C,” “brain,” “neurology,” “neurologic complication,” “central nervous system,” “status,” “seizure”, “convulsion,” “cranial nerves,” “severe neurological disease,” life-threatening neurological manifestations.” The words “child,” “children,” and “pediatric” were also used in every article. We searched the literature, filtered for articles published in English, from the databases’ inception to March 30, 2021. The resulting published articles were mostly limited to case reports and case studies.
2.2.1. Literature eligibility criteria
Articles reporting patients with COVID-19 and those presenting with neurological manifestations (with or without other manifestations), aged under 18 years and any gender, were included. Articles reporting children with primary neurological disorders developed or complicated by COVID-19 were excluded. Animal studies, studies with full-text articles unavailable, conference papers, and ongoing trials were excluded. Minor neurological manifestations such as loss of smell and/or taste, fatigue, and headache were not included.
2.2.2. Data extraction
The following parameters were extracted from the included studies: age, gender, diagnosis, diagnostic tests for COVID-19, neurological diagnosis, radiological findings, neurophysiological studies, and outcomes. The type of study, date of publication, and publishing journal were also noted. All the studies were screened and the data were manually extracted by an author (S.L.G.).
2.2.3. Outcome measures
The results were divided into central and peripheral nervous system manifestations. The literature review aimed to reveal the possible link between SARS-CoV-2 infection and neurological complications.
3. Results
3.1. Patients
During the study period, a total of 312 children with COVID-19-related illness were hospitalized. Their median age was 10.8 years (range: 1 month–17.5 years). Fifteen (4.8%) consecutive cases had acute new-onset neurological manifestations associated with SARS-CoV-2 infection. The age of these patients at admission ranged from 3 months to 17 years, with a median age of 5 years. The female–male ratio was 6 (40%) to 9 (60%). Three patients had underlying disease: one with asthma, one with factor V Leiden mutation, and one with sensorineural hearing loss. The clinical presentations and outcomes of patients are given in Table 1 .
Table 1.
Clinical characteristics of patients.
| Central Nervous System Diseases | |||||||
|---|---|---|---|---|---|---|---|
| Case no, Sex, Age, Underlying disease | SARS-CoV-2 exposure | COVID-19 Symptoms | Neurological manifestations | SARS-Co-V-2 PCR/Serology | CSF Examination | Diagnosis | Treatment & Outcome |
|
Case 1 F/3 m, None |
Her father was SARS-CoV-2 PCR positive a month ago | None | Afebrile generalized convulsive status epilepticus (left focal clonic to bilateral) | PCR negative (Nasopharyngeal swab) SARS-CoV-2 IgG positive (serum) |
Glucose:73 mg/dL Protein:85.7 mg/dL Direct microscopic examination: RBC (Traumatic LP) CSF culture: negative Viral encephalitis PCR panel: negative |
First seizure as SE Postinfectious |
During hospitalization: Midazolam infusion, Levetiracetam and phenobarbital. Patient had a smooth clinical course with no focal deficits Recovered Seizure free during follow up for six months with Levetiracetam mRS: 0 |
|
Case 2 F/7 m, None |
Her parents were SARS-CoV-2 PCR positive at admission | Fever | New onset abnormal ocular movements (tonic eye deviation in upward and lateral gaze followed by faster compensatory downward eye movement) Neurologic examination was otherwise normal. |
PCR negative (Nasopharyngeal swab) SARS-CoV-2 IgG positive (serum) |
Glucose:72 mg/dL Protein:24.4 mg/dL Direct microscopic examination: no cell CSF culture: negative Viral encephalitis PCR panel: negative |
Temporary abnormal ocular movements Para-infectious |
No treatment. Abnormal ocular movements resolved in two days. No further similar attack on up to 6 months follow up mRS:0 |
|
Case 3 M/7 m, None |
His mother was SARS-CoV-2 PCR positive at admission | Fever | Febrile generalized motor (atonic) seizure at admission. | PCR negative (Nasopharyngeal swab) SARS-CoV-2 IgG positive (serum) |
Glucose:55 mg/dL Protein: 21.9 mg/dL Direct microscopic examination: no cell CSF culture: negative Viral encephalitis PCR panel: negative |
Febrile seizure Para-infectious |
No treatment Complete recovery Seizure free on up to five months follow up No ASM mRS:0 |
|
Case 4 M/2 y, None |
Household contact | Fever, cough | Febrile generalized convulsive status epilepticus | PCR positive (Nasopharyngeal swab) CSF SARS-CoV-2 PCR: negative CSF SARS-CoV-2 IgG: negative |
Glucose:130 mg/dL Protein:13.2 mg/dL Direct microscopic examination: no cell CSF culture: negative Viral encephalitis PCR panel: negative |
Febrile SE Para-infectious |
Iv midazolam, Iv levetiracetam Ceftriaxone Recovered, Seizure free on up to six months follow up Levetiracetam mRS:0 |
|
Case 5 M/3 y, Factor V Leiden Homozygous mutation, history of stroke one year ago, using ASA |
Unknown | None | Numbness on the left side of the body NE: Slight pyramidal findings on the left (preexisting?) |
PCR negative (Nasopharyngeal swab) SARS-CoV-2 IgG positive (serum) |
Glucose:59 mg/dL Protein:11.5 mg/dL Direct microscopic examination: no cell CSF cytocentrifuge and cytopathologic examination: Activated lymphoid cells, monocytes, ependymal cells Activated macrophages Bacterial culture: negative Culture for Mycobacterium Tuberculosis: negative Viral encephalitis PCR panel: negative |
COVID-19 related CNS manifestations, Acute hydrocephalus Postinfectious |
Endoscopic third ventriculostomy Back to his baseline on up to three months follow up mRS:0 |
|
Case 6 M/3 y, None |
None (His father is a dentist) He had complaints of mild fever 20 days ago. |
MIS-C Fever, rashes, conjunctival injection, hypotension, hepatosplenomegaly |
Headache NE: leptomeningeal irritation findings on admission. Nuchal rigidity Kernig's positive Brudzinski positive Irritability, lethargy and visual hallucinations (4t day) |
PCR negative (Nasopharyngeal swab) SARS-CoV-2 IgG positive (serum) |
Glucose: 58 mg/dL Protein: 40 mg/dL Direct microscopic examination: no cell CSF culture: negative Viral encephalitis PCR panel: negative |
MIS-C related Encephalopathy Meningeal irritation findings Postinfectious |
IVIg, Steroids, Immunomodulatory treatment, Plasma exchange Prophylactic anticoagulation Antiviral Recovered with continued treatment of his inflammatory syndrome. mRS:0 |
|
Case 7 M/5 y, None |
Household contact | Otitis externa, fever | Left focal tonic-clonic seizure | PCR positive (Nasopharyngeal swab) | Glucose:55 mg/dL Protein:18.4 mg/dL Direct microscopic examination: no cell CSF culture: negative Viral encephalitis PCR panel: negative |
First febrile seizure Para-infectious |
Levetiracetam Recovered Seizure free at three month follow up. mRS:0 |
|
Case 8 F/11.5 y, None |
Household contact a month ago | None | Headache, diplopia, nausea and vomiting Grade 4 papilledema 6th nerve palsy Neurologic examination was otherwise normal. |
PCR negative (Nasopharyngeal swab) SARS-CoV-2 IgG positive (serum) CSF SARS-CoV-2 PCR: negative CSF SARS-CoV-2 IgG: negative |
CSF pressure: 33 cm H2O Glucose:59 mg/dL Protein:84.3 mg/dL Direct microscopic examination: RBCs CSF cytocentrifuge and cytopathologic examination: Polymorphic lymphomononuclear cells consisting of small mature lymphocytes& histiocytes Activated macrophages |
COVID-19 related CNS manifestation Secondary intracranial hypertension Postinfectious |
Topiramate, Acetazolamide Complete recovery Papilledema and abducens palsy recovered in three months mRS:0 |
|
Case 9 F/14 y, None |
Her father was SARS-CoV-2 PCR positive a month ago | MIS-C Fever, Rash, conjunctival injection, diarrhea and abdominal pain She had severe hemodynamic impairment |
Visual hallucinations. Irritability, confusion. Fluctuating attention and cognition. Delirium state for two days On 22nd day had blurred vision |
PCR negative (Nasopharyngeal swab) SARS-CoV-2 IgG positive (serum) |
N/p | MIS-C related encephalopathy, vasculitis and PRESS Postinfectious |
IVIg, Steroids, Immunomodulatory treatment, Prophylactic anticoagulation Antiviral Plasma exchange, ECMO Discharged with moderate muscle strength loss and need assistance for walking. Difficulty in climbing stairs and tremor on up to six months follow up. mRS: 2 |
|
Case 10 F/15 y, None |
None History of fever for a few days was present a month ago, but no COVID-19 PCR confirmation |
MIS-C Fever, abdominal pain, diarrhea and maculopapular rashes on the trunk. Hypotension |
Visual, tactile hallucinations. She developed ongoing auditory hallucinations. Fluctuating cognition. She was very agitated and had delirium state for two days. |
PCR negative (Nasopharyngeal swab) SARS-CoV-2 IgG positive (serum) |
N/p |
MIS-C related delirium and encephalopathy Postinfectious |
IVIg, Steroids, Immunomodulatory treatment, Prophylactic anticoagulation Antiviral Recovered with continued treatment of her inflammatory syndrome mRS:0 |
|
Central Nervous System and Peripheral Nervous System Diseases | |||||||
|
Case 11 F/6 y, Asthma |
Household contact, SARS-CoV-2 PCR was positive 3 weeks ago |
MIS-C Fever, rashes, conjunctival injection, abdominal pain |
Irritability. Fluctuations in cognition. Visual hallucinations on admission Weakness (upper and lower limbs: 2/5) on 7th day of hospitalization |
PCR negative (Nasopharyngeal swab) SARS-CoV-2 IgG positive (serum) |
N/p | MIS-C related encephalopathy and critical illness neuromyopathy Postinfectious |
IVIg, Steroids, Immunomodulatory treatment, Plasma exchange Prophylactic anticoagulation Antiviral Discharged with mild muscle strength loss and need assistance for walking. mRS:1 |
|
Case 12 M/17 y, Sensorineural hearing loss with cochlear implant |
History of contact with a relative who had COVID-19 3 weeks ago |
MIS-C Fever Vomiting Hypotension Tachycardia Hepatomegaly Splenomegaly |
Severe headache, vomiting. Irritability, lethargy. Fluctuating cognition. Nuchal rigidity, Kernig's positive, Brudzinski positive (at referral hospital) On extubation at 26thday; Lethargy Weakness lower > upper limbs (3/5,4/5) Muscle athropy Difficulty in swallowing liquids |
PCR negative (Nasopharyngeal swab and deep tracheal aspirate) SARS-CoV-2 IgG positive (serum) |
Glucose: 58 mg/dL Protein: 23 mg/dL Direct CSF microscopic examination: no cell CSF culture: negative |
MIS-C related encephalopathy and critical illness neuromyopathy Postinfectious |
IVIg, Steroids, Immunomodulatory treatment, Plasma exchange, Prophylactic anticoagulation, Antiviral treatment Partial recovery with continued treatment of his inflammatory syndrome. Discharged with moderate muscle strength loss and need assistance for walking. Steppage gait, impaired vibration sensation in the lower limbs on up to 5 months follow up. mRS:2 |
| Peripheral Nervous System Diseases | |||||||
|
Case no, Sex, Age, Underlying disease |
SARS-CoV-2 exposure |
COVID-19 Symptoms |
Neurological manifestations |
SARS-Co-V-2 PCR/Serology |
CSF Examination |
Diagnosis |
Treatment & Outcome |
|
Case 13 M/13 y, Asthma |
Household contact | MIS-C Fever, Rash, conjunctival injection, diarrhea and abdominal pain |
15th day of admission tingling and pain in the hands and feet with no motor deficit but hyperesthesia | PCR negative (Nasopharyngeal swab) SARS-CoV-2 IgG positive (serum) |
N/p | MIS-C, neuropathic pain Postinfectious |
IVIg, Steroids, Immunomodulatory treatment, Prophylactic anticoagulation Antiviral Recovered with gabapentin mRS:0 |
|
Case 14 M/16y, None |
Household contact, | Fever | Severe myalgia, CK::1112 U/L (at admission) CK maximum level was 4511 U/L on third day of hospitalization. |
PCR positive (Nasopharyngeal swab) | N/p | COVID-19 related myositis Para-infectious |
CK level improved in seven days with IV alkaline hydration therapy. CK:119 U/L mRS:0 |
|
Case 15 M/4.5 m, None |
Household contact | Fever, diarrhea, vomiting | No weakness Serum CK level was 39078 U/L at admission |
PCR positive (Nasopharyngeal swab) | N/p | COVID-19 related myositis, Rhabdomyolysis Para-infectious |
His CK level and clinical findings improved in 38 days with IV alkaline hydration mRS:0 |
*Cerebrospinal fluid (CSF); Creatin Kinase (CK); Female (F); Intravenouse (IV); Intravenouse Immunoglobulin (IVIg); Lumpar puncture (LP); Male (M); Modified Rankin Scale (mRS); Multisystem inflammatory syndrome in children (MIS-C); N/p: Not performed; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); Status Epilepticus (SE).
Six patients were classified as para-infectious: four were PCR positive, and two tested negative but had more than one SARS-CoV-2 PCR positive close household contact. Both PCR negative para-infectious patients tested seropositive for SARS-CoV-2 IgG on the 18th day after their initial symptoms. Nine postinfectious patients tested PCR negative for SARS-CoV-2, but all were SARS-CoV-2 IgG positive at admission. All had a history of contact with at least one SARS-CoV-2 PCR positive close household contact three to six weeks before admission. One patient was PCR positive three weeks before admission while two had symptoms of upper respiratory infections at the time of contact. Six of the nine patients classified as postinfectious diagnosed with MIS-C.
3.1.1. Isolated central nervous system involvement
Of the 15 patients with neurological manifestations, 10 (66.6%) had CNS involvement, most commonly encephalopathy. Five of 10 patients (50%) with CNS involvement presented with encephalopathy, and two adolescents had hallucinations and delirium; all had diagnoses of MIS-C, and one developed posterior reversible encephalopathy syndrome (PRES) during follow-up. The second most common CNS manifestation was seizure (4/10; 40%): two patients were admitted with febrile seizure, one with febrile status epilepticus, and the other with afebrile status epilepticus. Three of the patients with seizures were in the para-infectious group. One patient with abnormal eye movements had tonic eye deviation in upward and lateral gaze followed by faster compensatory downward eye movement, with no associated nystagmus or abnormal head or extremity movements. On her magnetic resonance imaging (MRI), punctate diffusion restriction in the dorsal medulla oblongata was detected suggestive of brainstem involvement.
Another patient presented with diplopia, papilledema, and sixth cranial nerve palsy due to increased intracranial pressure (ICP). A 3-year-old boy with a 1-year history of previous stroke associated with factor V Leiden mutation, maintained on acetylsalicylic acid (ASA) prophylaxis, presented with left localized numbness. He was eventually diagnosed with acute tri-ventricular hydrocephalus due to cerebral aqueduct obstruction.
3.1.2. Isolated peripheral nervous system involvement
Two patients (13.3%) had myositis and elevated creatine kinase (CK) levels during the acute phase of COVID-19. Both were treated with intravenous alkaline hydration, and one (Case 2) experienced rhabdomyolysis.
One patient (6.6%) with MIS-C had tingling and pain in both hands and feet on the 15th day of admission. On neurological examination, he had no motor deficits. Electromyography (EMG) was performed on the day of emerged symptoms. The pain was suspected to be neuropathic and he was started on gabapentin treatment. Although his EMG was reported as normal, his symptoms resolved completely under gabapentin treatment.
3.1.3. Both central and peripheral nervous system involvement
Two (13.3%) MIS-C patients had both central and peripheral nervous system involvement, presenting with encephalopathy and developing critical illness neuromyopathy on the 7th and 26th days of admission, respectively.
3.1.4. Cerebrospinal fluid examination
Nine (60%) patients had lumbar punctures; only three cerebrospinal fluids (CSF) were analyzed for SARS-CoV-2 PCR and SARS-CoV-2 IgG, with negative results (Table 1). Two patients had high CSF protein levels attributed to lumbar puncture procedures being traumatic hence cross-contamination of CSF. Red blood cells were observed on direct microscopic examination. The remaining patients’ CSF samples direct microscopic examination did not reveal any cells.
A viral panel including herpes simplex virus (HSV) types 1 and 2, varicella zoster virus (VZV), Epstein Barr virus (EBV), cytomegalovirus (CMV), human herpesvirus (HHV) type 6, HHV7, adenovirus, parvovirus B19, and enterovirus as well as bacterial cultures were performed for all nine patients. Both viral panels and cultures were negative for all patients.
Cerebrospinal fluid cytocentrifuge and cytopathologic examination were performed for two patients diagnosed with ICP to exclude neoplastic causes. The examination results revealed activated macrophages.
3.1.5. Neurophysiological studies
The neurophysiological findings of the cases are summarized in Table 2 . A total of six patients had electroencephalogram (EEG) study, four patients, diagnosed with seizure, had EEGs recorded at admission, all but one had normal EEGs. Electrographic seizure activity on right parieto-occipital region and low voltage background detected in one patient, diagnosed as febrile status, at admission and on the 3rd day of hospitalization.
Table 2.
Neuroimaging and neurophysiological study findings.
| Case no | Neuroimaging | EEG/EMG |
|---|---|---|
| Case 1 | CT: normal Cranial MRI: Punctate diffusion restriction in the posterior limb of internal capsule and increased leptomeningeal contrast enhancement. (MRA was not available therefore focal vasculopathy could not be documented.) |
EEG (at admission): electrographic seizures on right parieto-occipital region EEG (third day): no epileptiform discharges, low voltage background EEG (three months later): normal |
| Case 2 | CT: normal Cranial MRI: Punctate diffusion restriction in the dorsal medulla oblongata |
EEG (at admission and two months later): normal |
| Case 3 | Non-contrast-enhanced cranial MRI: Normal findings | EEG (two weeks later): normal |
| Case 4 | Non-contrast-enhanced cranial CT: Normal | EEG (two months later): normal |
| Case 5 | CT: Triventricular hydrocephalus Cranial MRI: Triventricular hydrocephalus associated with aquaductal stenosis and increased leptomeningeal contrast enhancement |
N/p |
| Case 6 | Non-contrast-enhanced cranial CT: Normal findings | N/p |
| Case 7 | CT: normal Non-contrast-enhanced cranial MRI: normal | EEG (at admission): normal |
| Case 8 | Initial cranial MRI: Bifrontal nonspecific white matter lesions and increased leptomeningeal contrast enhancement (after LP). Follow-up cranial MRI: Distension of the peri-optic nerve subarachnoid CSF space and vertical tortuosity of the optic nerve consistent with increased intracranial pressure |
N/p |
| Case 9 | Cranial MRI (26th day of admission): Cranial MRI: MIS-C related findings: Infra/supratentorial multiple microhemorrhages suggesting small-vessel vasculitis and bilateral MCA stenosis consistent with medium-vessel vasculitis, meningo-ependymal contrast enhancement MRI (36th day): PRES MRI (54th day): PRES (regression) |
N/p |
| Case 10 | N/p | EEG (one month later): normal |
| Case 11 | Cranial CT: normal Contrast-enhanced cranial MRI: Normal findings | EMG (during inflammatory syndrome): myopathic changes |
| Case 12 | Contrast-enhanced cranial CT: Normal findings Cranial CT angiography: stenosis in the upper truncus of the right MCA and paucity of distal branches of the MCA |
EMG (two months later): motor axonal polyneuropathy in lower extremities |
| Case 13 | N/p | EMG (at admission): normal |
a Cerebrospinal fluid (CSF); Computed Tomography (CT); Electroencephalography (EEG); Electromyography (EMG); Middle cerebral arteria (MCA); Magnetic resonance imaging (MRI); Magnetic resonance angiography (MRA); Not performed (N/p); Posterior reversible encephalopathy syndrome (PRES).
EMG was performed in three patients; all were hospitalized with the diagnosis of MIS-C. One patient experienced extremity pain without motor deficit on the 15th day, and EMG obtained the same day was normal.
Case 11 had diffuse weakness on the 7th day of admission. She could move her limbs only when gravity was eliminated, and her EMG revealed myopathic changes.
Case 12 experienced more pronounced weakness in the lower extremities, noted just after extubation on the 26th day. He could move his legs against gravity and was capable of minimal resistance in his arms. He was diagnosed with motor axonal polyneuropathy on EMG performed 2 months after discharge.
3.1.6. Neuroimaging Studies
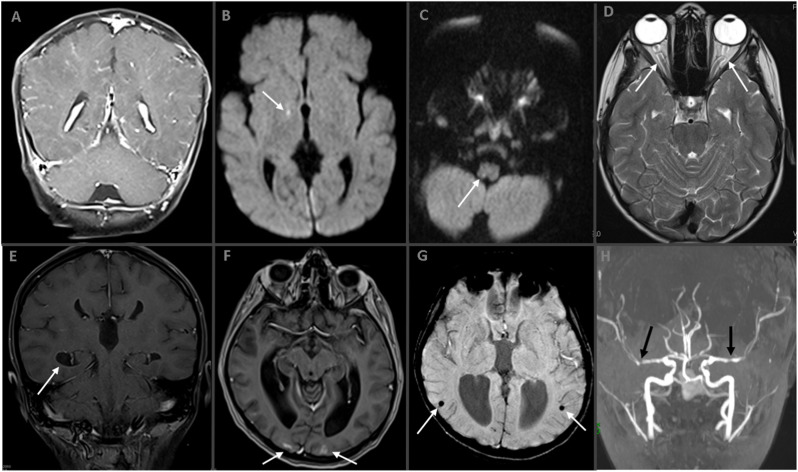
The neuroimaging findings in all cases are summarized in Table 2. Eleven patients underwent neuroimaging. Three had only cranial computed tomography (CT) while two underwent non-contrast-enhanced cranial magnetic resonance imaging (MRI), and six underwent contrast-enhanced MRI. Some of the selected patients’ neuroimaging findings are shown in Fig. 2 (A–H). The most common neuroimaging finding was leptomeningeal contrast enhancement (4/11 cases with neuroimaging, 36.3%; 4/6 cases with contrast-enhanced cranial MRI, 66.7%). Two cases had micro-ischemic lesions. Two had increased intracranial hypertension (one case with tri-ventricular hydrocephalus and aqueduct stenosis). Of the six patients with MIS-C, four underwent neuroimaging, and one had positive MRI findings suggesting vasculitis and meningioependymitis as shown in Fig. 2 (E–H: Patient 13). Furthermore, PRES occurred on follow-up MRI of this patient.
Fig. 2.
(A–H). Neuroimaging Studies of Cases.
A. Postcontrast coronal T1W MR image shows diffuse leptomeningeal contrast enhancement in Case 1. B. Axial diffusion-weighted imaging (DWI) demonstrates a punctate diffusion restriction (arrow) in Case 1. C. In another patient (Case 3), axial DWI showing dorsal medullar punctate diffusion restriction (arrow). D. Axial T2W MR image reveals distension of the peri-optic nerve subarachnoid space and tortuosity in Case 11 (arrows). Bottom line is MR images of Case 13 with ‘multisystem inflammatory syndrome in children’ (E–H). Postcontrast coronal T1W MR image shows ependymal contrast enhancement (E, arrow) and axial postcontrast image shows cortical contrast enhancement (F, arrows) in the bilateral occipital lobes. Susceptibility-weighted image revealing parenchymal microhemorrhages (G, arrows) and bilateral middle cerebral artery stenosis in time-of-flight MR angiography (H, arrows).
3.1.7. Outcomes
All the patients were re-evaluated on the 15th day and at the first, second, third, and sixth months after discharge. Outcomes were assessed at the final visit (median: 3 months, range: 1–6 months). Overall, 12 (80%) patients had no disability, assessed by the modified Rankin Scale (mRS = 0), and three (20%) had mild disability.
Four patients who had seizures experienced no seizures after discharge. Three were on antiseizure medication with levetiracetam. The abnormal eye movements detected in one patient resolved spontaneously. The papilledema and sixth nerve paralysis of the patient with secondary intracranial hypertension resolved in 3 months.
One patient with acute tri-ventricular hydrocephalus returned his baseline neurological status following surgery. Two patients with myositis and elevated CK had no neurological or renal impairment at hospital discharge or follow-up visits.
Of the six patients with MIS-C, three recovered completely. However, the remaining three were discharged with mild to moderate muscle strength loss and required assistance for walking.
3.2. Literature review
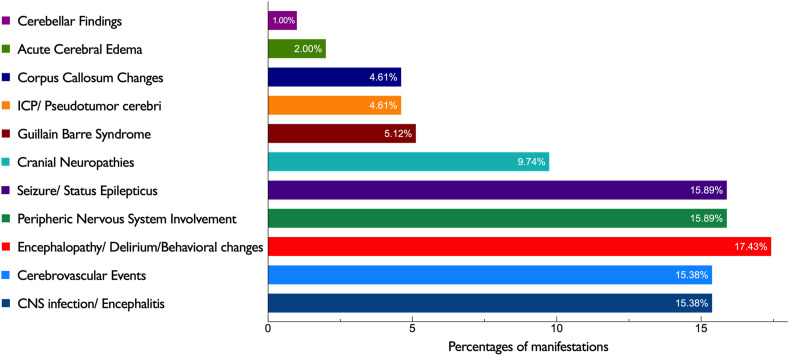
An increasing number of neurological complications in patients with COVID-19 continue to be reported worldwide. Common features of the 211 children with neurological manifestations in the literature we reviewed are summarized in Table 3 . Yea et al. [15] reported 31 patients with only headache, so we excluded these and studied 180 children with specific neurological manifestations temporally associated with SARS-CoV-2 infection. We prepared a chart of the most common presenting neurological features in children with COVID-19, using a combination of our data and the literature review (Fig. 3 ). The ages ranged from 2 months to 17 years. Ninety-five (53.4%) patients were female. Children with MIS-C comprised half of the patients with neurologic manifestations (n = 90, 50.6%).
Table 3.
Literature review of neurological manifestations associated with SARS-CoV-2 in pediatric patients [[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53]].
| First author [reference number] | Type of study, Country | Journal | Patients with Severe Neurological Manifestations, Sex, Ages | Severe Neurological Manifestations | EEG/EMG | Neuroimaging CT and/or MRI | Outcome |
|---|---|---|---|---|---|---|---|
| Aghdam et al., 2020 [30] | Case report, Iran | Journal of Pediatric Neurology | n = 1 2-month-old female |
COVID-19: Seizures | EEG: low voltage slow wave activity | Brain CT: a hyperdense center with diameter of 5 mm in left temporal lobe cortex, hemorrhages | Discharged with neurologic baseline |
| Enner et al., 2020 [19] | Clinical letter, United States of America | Pediatric Neurology | n = 1 14-year-old female |
COVID-19: Seizures and apneic episodes | EEG: epileptiform activity | Brain and Spine MRI: normal | Discharged well with no apneic episodes after antiepileptics |
| McAbee et al., 2020 [31] | Correspondence, United States of America |
Pediatric Neurology | n = 1 11-year-old male |
COVID-19: Encephalitis, status epilepticus | EEG: Frontal intermittent delta activity | Head CT: normal | Recovered |
| Frank et al., 2020 [28] | Case report, Brazil | Journal of Tropical Pediatrics | n = 1 15-year-old male |
COVID-19: Guillain-Barré syndrome | EMG: Compatible with GBS, acute motor axonal neuropathy (AMAN type GBS) | Brain MRI: normal | Currently undergoing motor physiotherapy |
| Khalifa et al., 2020 [29] | Case report, Egypt |
Journal of Pediatric Infectious Diseases Society | n = 1 11-year-old male |
COVID-19: Guillain Barre syndrome |
EMG: Compatible with GBS (Acute inflammatory demyelinating polyneuropathy variant) | Brain and Spine MRI: Contrast enhancement of cauda equina nerve roots | Discharged with improved motor activity, but still deep tendon reflexes were hypoactive. |
| Bektaş et al., 2020 [32] | Original article, Turkey | Brain and Development | n = 2 10-year-old male 11-year-old female |
MIS-C: Reversible corpus callosum splenial lesions |
EEG: 1 diffused slowed background activity | Brain MRI: hyperintensity on T2-weighted images in the splenium of the corpus callosum with restricted diffusion | Recovered |
| Abdel-Mannan et al., 2020 [24] | Case series, United Kingdom | JAMA Neurology | n = 4 2 male, 2 female 11.75 years (min-max, 8-15) |
4 MIS-C: 4 encephalopathy, 2 global proximal weakness 1 global flaccid paralysis 1 proximal leg weakness |
EEG: 2 diffuse slow activity, 1 slow activity over the anterior regions EMG: 2 myopathic changes 1 myopathic and neuropathic changes |
Brain MRI: 3 patients have signal changes in the splenium of the corpus callosum on MRI, | No death Encephalopathy resolved. 2 fully ambulant 2 wheelchairs bound |
| de Miranda Henriques-Souza et al., 2020 [33] | Case report, Brazil |
Neuroradiology | n = 1 12-year-old female |
COVID-19: ADEM Acute bilateral progressive weakness |
N/A | Brain MRI: extensive bilateral and symmetric restricted diffusion involving the subcortical and deep white matter. Cervical Spine MRI: longitudinally extensive cervical myelopathy involving both white and gray matter |
Recovered |
| Kaur et al., 2020 [34] | Clinical letter, New Mexico | Pediatric Neurology | n = 1 3-year-old female |
COVID-19: Transverse Myelitis | N/A | Brain & Orbital MRI: normal Spine MRI: swelling of the cervical spinal cord with T2-hyperintense edema involving most of the transverse aspect of the spinal cord, extending from the lower medulla to the midthoracic level. |
Still hospitalized |
| Basirjafari et al., 2020 [35] | Case report, Iran | Journal of Medical Virology | n = 1 9-year-old male |
COVID-19: Subarachnoid hemorrhage Low level of consciousness |
N/A | Brain CT: hyperdensity at basal cisterns, interhemispheric, and bilateral Sylvian fissures in favor of subarachnoid hemorrhage, without intraventricular hemorrhage and hydrocephalus (green arrow); decreased density of white matter in favor of brain edema | Died |
| García-Howard et al., 2020 [20] | Case report, Spain | Frontiers in Pediatrics | n = 1 3-month-old female |
COVID-19: Repeated afebrile seizures | EEG: normal | Brain MRI: normal | Recovered |
| Appavu et al., 2021 [36] | Case report, United States of America | Pediatrics | n = 2 8-year-old female 16-year-old male |
COVID-19: Arteritis and large vessel occlusive stroke 2 acute hemiplegia |
N/A | Case 1, Brain MRI: small completed infarctions in the middle cerebral artery (MCA) territories bilaterally. MRA: proximal left M1 occlusion. Case 2, Brain MRI and MRA: complete left MCA territory infarction, irregularity of left M1 (suggestive of arteritis), and occlusion of left MCA bifurcation. |
No death. Both discharged with need for rehabilitation |
| Bhatta et al., 2020 [21] | Case report, United States of America | Cureus | n = 1 11-year-old male |
COVID-19: seizure | N/A | Head CT: normal | Recovered |
| Bhavsar et al., 2020 [25] | Case report, United States of America | Neurology Clinical Practice | n = 1 16-year-old male |
COVID-19: Encephalitis Low level of consciousness |
EEG: encephalopathy with no seizure findings | Head CT: normal | Recovered |
| Ippolito Bastidas et al., 2020 [18] | Case report, Spain | Neurology Clinical Practice | n = 1 13-year-old female |
COVID-19: Cerebral sinus vein thrombosis Headache, impaired consciousness |
N/A | Head CT: Right occipital intracerebral hemorrhage Brain MRA: bilateral transverse sinus thrombosis with extension to the right sigmoid sinus and internal jugular vein |
Recovered |
| Kihira et al., 2020 [37] | Case report, United States of America | Pediatric Radiology | n = 1 5-year-old male |
COVID-19: Cerebral infarct | N/A | Head CT: large acute right anterior and middle cerebral artery territory infarction and subarachnoid hemorrhage in the left hemisphere. | Death |
| Dugue et al., 2020 [38] | Case report, United States of America | Neurology | n = 1 6-week-old male |
COVID-19: Seizure | EEG: an excess of temporal sharp transients for age and intermittent vertex delta slowing with normal sleep-wake cycling | Brain MRI: normal | Recovered |
| Gaur et al., 2020 [39] | Brief report, United Kingdom | American Journal of Neuroradiology | n = 2 9-year-old male 12-year-old male |
MIS-C & COVID-19: Cytotoxic lesions of corpus callosum Lethargy and headache |
N/A | Case 1, Brain MRI: hyperintense lesion in the splenium of the corpus callosum that exhibited restricted diffusion Case 2, Brain MRI: extensive abnormal T2-weighted hyperintense signal and restricted diffusion in the entire corpus callosum and frontoparietal cerebral white matter |
|
| Abel et al., 2020 [40] | Case report, United States of America | Neurology | n = 1 33-month-old male |
MIS-C: Encephalopathy and bilateral thalamic lesions | İnitial Video EEG: moderate background slowing Control EEG on day 12: mild diffuse slowing |
First Brain MRI: restricted diffusion in the bilateral lateral thalamic nuclei without T2/fluid-attenuated inversion recovery changes. Control MRI on day 15: resolution of thalamic lesions. |
Recovered Discharged with mild residual weakness |
| Verkuil et al., 2020 [41] | Case report, United States of America | The Lancet | n = 1 14-year-old female |
MIS-C: Pseudotumor cerebri Papilledema and abducens nerve paralysis |
N/A | MRI and MRV: revealed abnormalities consistent with increased intracranial pressure | Recovered |
| Swarz et al., 2020 [22] | Correspondence, United States of America | Pediatric Neurology | n = 1 9-year-old female |
COVID-19: Focal status epilepticus | Video EEG: continuous delta slowing throughout the right hemisphere without epileptiform features. | Head CT: normal Brain MRI: normal |
Recovered |
| Mirzaee et al., 2020 [42] | Case report, Iran | Radiology | n = 1 9-year-old male |
COVID-19: Focal cerebral arteriopathy Generalized seizures |
N/A | Brain MRI: acute infarction without microhemorrhages, along with focal irregular narrowing and banding of the proximal M1 segment of the left middle cerebral artery with a slightly reduced distal flow | Discharged with hemiparesis |
| Ahsan et al., 2021 [26] | Case report, United States of America | Clinical Experimental Pediatrics | n = 1 7-year-old female |
COVID-19: Myelin oligodendrocyte glycoprotein antibody encephalitis Status epilepticus |
EEG: cerebral slowing with left focal slowing | Brain MRI: Left perirolandic cortex and posterior parietal lobe cerebral edema | Discharged |
| Sandoval et al., 2021 [23] | Original article, Chile | Journal of Child Neurology | n = 13 8 female, 5 male, 6.5 years (min-max, 1-17) |
8 MIS-C: 6 acute flaccid tetra paresis, 1 seizure, 1 ageusia 5 COVID-19: 1 anosmia and ageusia, 1 febrile seizure, 1 status epilepticus, 1 Guillain Barre Syndrome |
EEG: 2 normal, 1 severely abnormal with slow continuous background activity EMG: 1 moderate acute motor axonal neuropathy (AMAN) |
3 normal CT findings 1 Brain MRI: unenhanced right frontal nodular white matter hypo intensity. 1 Brain and spine MRI: multifocal demyelinating lesions |
No death 2 dysgeusia, 3 no new seizure, 7 complete strength recovery |
| Becker et al., 2021 [43] | Brief report, United States of America | The Journal of Pediatrics | n = 4 3 female, 1 male, 11,25 years (min-max, 6-14) |
4 MIS-C: increased intracranial pressure | 3 EEG: no seizures | 3 Brain CT: normal 1 Brain CT: cerebral edema 1 MRI: normal 1 MRI: papilledema, flattened sinuses |
No death, Discharged with neurologic baseline |
| Saeed et al., 2020 [44] | Case report, Iran | IDCases | n = 1 3-year-old male |
MIS-C: Status epilepticus | N/A | Brain CT on day 1: diffuse cerebral edema Brain MRI: intracerebral hemorrhage in the right occipital lobe. |
Discharged with neurologic baseline |
| Shupper et al., 2020 [45] | Letter to editor, United States of America | Child's Nervous System | n = 1 5-year-old male |
MIS-C: MCA Infarction and Subarachnoid hemorrhage during ECMO | EEG: nonconvulsive status epilepticus | Head CT: a right middle cerebral artery (MCA) infarction, cerebral edema, and diffuse contralateral subarachnoid hemorrhage | Still in ICU |
| Tiwari et al., 2021 [46] | Case report, India |
Lancet Child Adolescent Health | n = 1 9-year-old female |
MIS-C: Acute ischemic stroke | N/A | Head CT: infarct in the genu and adjacent body of the corpus callosum, left basal ganglia and bilateral thalami. CT angiography: multifocal smooth stenosis of intracranial ICAs, right MCA and both A2 segments of ACA. Diffuse narrowing of M2 and M3 segment branches of both MCA. |
No death. Followed up for rehabilitation |
| Regev et al., 2020 [47] | Case report, Israel | The Pediatric Infectious Journal | n = 1 16-year-old male |
MIS-C: central nervous system involvement | N/A | Brain MRI and MRA: diffuse small low signal foci of hemosiderosis in subcortical White matter of both hemispheres and the corpus callosum. | Discharged with improved muscle strength and consciousness |
| De Paulis et al., 2020 [48] | Brief report, Brazil | The Pediatric Infectious Disease Journal | n = 1 4-year-old female |
MIS-C: Confusion and mental somnolence | N/A | Head CT: normal | Recovered |
| Hutchison et al., 2020 [49] | Case report, United States of America |
Psychosomatics | n = 1 14-year-old male |
MIS-C: Delirium | N/A | N/A | Recovered |
| Yousefi et al., 2021 [50] | Case report, Iran |
The Pediatric Infectious Disease Journal | n = 1 9-year-old female |
COVID-19: viral meningitis | N/A | N/A | Recovered |
| Sa et al., 2021 [51] | Case series, United Kingdom |
Neurology, Neuroimmunology and Neuroinflammation | n = 9 5 female, 4 male, 10 years (min-max, 2-15) |
9 MIS-C: 3 altered consciousness 3 acute behavioral changes 2 headache 1 Encephalopathy |
4 patients had EEG and 3 of them were abnormal (2 EEG were compatible with encephalopathy and 1 with seizure) | 4 abnormal neuroimaging 1 acute infarction 1 subtle cortical change 1 splenial and hippocampal changes 1 intraparenchymal hemorrhage and infarction |
1 patient with extensive infarction died, 4 completely recovered, 3 had problems with memory and behavior |
| Akhondian et al., 2021 [52] | Case series, Iran |
International Clinical Neuroscience Journal | n = 5 3 female, 2 male, 7 years (min-max, 5m-12) |
COVID-19: 3 febrile seizures 1 ataxia and drowsiness 1 low level of consciousness and ischemia |
N/A | Brain MRI: 1; consistent with acute infarctions in bifrontal and biparietal lobes 1; hyperintense signals in the brain stem and deep gray matter 1; a small ischemic area in the left centrum semi-oval 1; bilateral scattered subcortical white matter involvement 1; Brain CT scan revealed intraventricular hemorrhage. And had multiple ischemic regions on MRI |
1 died 3 recovered |
| Sánchez-Morales et al., 2021 [27] | Case series, Mexico | Child's Nervous System | n = 10 5 female, 5 male, 13 years (min-max, 2-16) |
3 Guillain Barre Syndrome 2 Optic Neuritis 1 Anti NMDA encephalitis 1 Myositis, 2 Ischemic stroke, 1 infection related ataxia |
3 NCS: AIDP | 2 MRI: optic nerve hyperintensities 2 MRI: Acute Ischemic Stroke 1 CT: normal |
8 recovered 1 Dysphasia, hemicorporeal weakness 1 died |
| Yea et al., 2021 [15] | Preprint, International | Preprint with The Lancet | n = 51 21 female, 30 male 6.6 y (IQR; 3.7–9.7) |
31 patients had only headache 12, Seizure 1, Encephalopathy 3, altered mental status 1 psychosis 1, cranial neuropathy 1, speech impairment |
N/A | 1, Brain and Spine MRI: T2/FLAIR signal hyperintensity in the deep cerebellar hemispheres. Abnormal enhancement of the cauda equina associated with mild thickening of the nerve roots. 1, Brain MRI: diffusion changes in the subcortical white matter |
2 died Others' outcome was not mentioned. |
| LaRovere et al., 2021 [16] | Case series, United States of America | JAMA Neurology | n = 43 16 female, 27 male 12 years (min-maz, 7-15) |
15, Severe encephalopathy (8 MIS-C) 12, Ischemic or hemorrhagic stroke (3 MIS-C) 8, Acute CNS infection or ADEM (6 MIS-C) 4, Acute fulminant cerebral edema (2 MIS-C) 4, Gullian Barré Syndrome (1 MIS-C) |
N/A | Brain CT: 23 Brain MRI:26 5 patients with encephalopathy had brain MRI with abnormal signal intensity and restricted diffusion in corpus callosum and periventricular white matter. 3 fulminant cerebral edema with uncal/tonsillar herniation on imaging (MRI/CT) |
n = 15; Discharged home n = 17; new CNS deficits required rehabilitation n = 11; died |
| Lindan et al., 2020 [17] | Case series, International | The Lancet Child & Adolescent Health | n = 38 18 female, 20 male |
16, ADEM-like disease pattern 12, neuritis 8, myelitis 7, Thrombotic/vasculitis |
N/A | Category 1 (acute COVID-19): 4 patients had patchy T2 hyperintensity involving gray and white matter which was referred to ADEM. 2 patients with myelitis 2 patients with enhancement of CN VII & XII; CN V &VIII 4 patients with co-infections and 3 of them had vasculitic/thrombotic findings. Category 2 (acute/subacute COVID-19): 3 patients had patchy T2 hyperintensity referred to ADEM and 2 had myelitis. 3 patients had enhancement of CN and cauda equina 1 patient with superior sagittal sinus thrombosis Category 3 (MIS-C): 7 had CC Splenial lesion T2 hyperintensity. 4 of them with ADEM like lesions; 2 had CN enhancement and 1 had enhancement of cauda equina Category 4 (indeterminate): 4 patients had enhancement of CN and/or cauda equina. 1 patient with infarction 2 patients with ADEM-like lesions | n = 27; normal at discharge/improvement at follow-up n = 4; died n = 5; residual neurological deficits |
| Baccarella et al., 2021 [53] | Clinical Letter, United States of America | Pediatric neurology | n = 2 9-year-old male 6-year-old male |
MIS-C: increased intracranial pressure Case 2 was with abducens palsy |
N/A | Brain MRI and MR venography of case1: normal Brain MRI of case 2: Magnetic resonance imaging of the brain and orbits was notable for kinking and distention of both optic nerve sheaths with protrusion of the optic discs into the globes, consistent with increased ICP. |
Resolved |
aAnterior cerebral artery (ACA); Acute disseminated encephalomyelitis (ADEM); acute inflammatory demyelinating polyneuropathy (AIDP); acute motor axonal neuropathy (AMAN); Corpus Callosum (CC); Cranial nerve (CN); Central nervous system (CNS); Computed tomography (CT); Electroencephalography (EEG); Electromyography (EMG); Extracorporeal membrane oxygenation (ECMO); Guillain-Barré syndrome (GBS); Magnetic resonance imaging (MRI); Magnetic resonance angiography (MRA); Middle cerebral arteria (MCA); Multisystem inflammatory syndrome in children (MIS-C); Month/year (m/y); Not available (N/A); Number (n); Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); Status Epilepticus (SE).
Fig. 3.
Most common presenting neurological features in children with COVID -19 based on the review of 39 case reports and series from around the World.
3.2.1. Central nervous system involvement
3.2.1.1. Cerebrovascular disease
Cerebrovascular diseases including ischemic and hemorrhagic strokes, venous thrombosis, cerebral arteriopathies, and vasculitis were the most common neurological manifestations related to SARS-CoV-2 infection. We reviewed 36 (20.22%) cases with cerebrovascular complications. Some were due to underlying diseases, and some had strokes during ECMO and life support. For example, LaRovere et al. [16] reported 12 cases with acute ischemic or hemorrhagic stroke, five of whom experienced cerebrovascular event during ECMO. Two cases had underlying vascular malformations (one with arteriovenous malformation and the other with Moya syndrome). In a study of neuroimaging manifestations in children, Lindan et al. [17] reported seven cases with thromboembolic or vasculitic stroke. In addition to ECMO treatment complications, acute cerebral artery infarctions and subarachnoid hemorrhages were reported [16]. Bastidas et al. [18] reported a 13-year-old girl with sinus vein thrombosis. She presented to the hospital with severe headache and impaired consciousness. On the fifth day of admission, a body angio-CT scan was performed; she had multiple vein thrombosis including superior sagittal sinus and pulmonary thromboembolism and bilateral deep femoral and iliac vein thrombosis.
3.2.1.2. Seizures, status epilepticus
Seizures were reported in 28 (15.5%) patients. Four (2.2%) were febrile seizures and were the only presenting complaint of SARS-CoV-2 infection. All the seizure episodes were acutely symptomatic, and most appeared during the febrile period. A 14-year-old girl presented with fever, cough, and hypoxia. During hospitalization, she had episodes of seizures and apnea. Neurologic examination was normal. Cerebrospinal fluid examination was in normal ranges, and CSF culture was negative. She had epileptiform activity on EEG. Craniospinal MRI was normal. She was discharged with no apneic or seizure episodes after levetiracetam [19]. Afebrile seizures were also reported as the only presenting complaint. Garcia Howard et al. [20] and Bhatta et al. [21] reported two patients with afebrile seizures. Garcia Howard et al. [20] reported that a 3-month-old girl with respiratory symptoms had afebrile seizure episodes. She had a normal EEG pattern and brain MRI. Bhatta et al. reported an 11-year-old boy with two episodes of generalized tonic-clonic seizures. He had no history of fever or respiratory symptoms. Both were asymptomatic during follow-up.
Swarz et al. [22] reported that a 9-year-old boy with no medical history presented with focal SE. He was afebrile and had no meningeal irritation findings. Head CT was normal, and EEG showed continuous delta slowing throughout the right hemisphere without epileptiform activity. In a case series reported from Chile, one patient among 13 cases presented with SE [23].
3.2.1.3. Encephalopathy, encephalitis and encephalomyelitis
Of the 180 patients, 26 (14.4%) were reported to have encephalopathy or a low level of consciousness. Encephalopathy was reported in patients with MIS-C and acute COVID-19. Abdel-Mannan et al. [24] reported four cases of encephalopathy with MIS-C in the United Kingdom. Three had diffuse slow activity on EEG and signal changes in the splenium of the corpus callosum on MRI. LaRovere et al. [16] reported 15 cases with severe encephalopathy, and eight were diagnosed with MIS-C. Most patients recovered while their inflammatory response resolved.
Encephalitis associated with SARS-CoV-2 infection has been reported. Bhavshar et al. [25] reported a 16-year-old boy with somnolence for two days and generalized weakness. He could not ambulate without assistance. During the emergency department evaluation, he had seizures. CT was normal, and he had a lumbar puncture. CSF findings were compatible with encephalitis. Ahsan et al. [26] reported myelin oligodendrocyte glycoprotein antibody encephalitis (MOG) in a 7-year-old girl from the United States of America. Sánchez-Morales et al. [27] reported a 14-year-old boy with anti-NMDA-R encephalitis. He had altered behavior and mental status, seizures, insomnia, and orolingual dyskinesias. Anti-NMDA-R antibody was positive in the CFS sample. His seizures recovered, but psychiatric symptoms continued. Acute disseminated encephalomyelitis (ADEM) was reported in 9 (5%) cases. Lindan et al. [17], also reported 16 cases of neuroimaging compatible with an ADEM-like pattern, but this did not mean that patients met the clinical definition for ADEM. A 12-year-old girl presented with neurologic complaints such as acute, progressive, and bilateral motor deficit. She developed respiratory symptoms two days after the neurological complaints.
3.2.2. Peripheral nervous system involvement
3.2.2.1. Guillain-barre syndrome
Ten (5.5%) patients were reported with the diagnosis of GBS and eight (80%) were male. The patients were aged between 8 and 15 years. All had positive SARS-CoV-2 antibody results. Sandoval et al. [23] reported that an 8-year-old boy presented with a progressive ascendant acute flaccid tetraparesis, areflexia, and multiple cranial nerve palsies, with an EMG and nerve conduction study (NCS) compatible with the acute motor axonal neuropathy (AMAN) GBS variant. Frank et al. [28] also reported AMAN type GBS. Sánchez-Morales et al. [27] and Khalifa et al. [29] reported four cases of GBS with acute inflammatory demyelinating polyneuropathy (AIDP). All patients were discharged, and some needed physical rehabilitation for a period.
3.2.2.2. Myositis
A previously healthy 10-year-old girl who presented with fever and myalgia was diagnosed with myositis and rhabdomyolysis [27]. She had a positive SARS-CoV-2 PCR result. She had acute myositis with respiratory symptoms.
3.2.3. Geographic location of cases with nervous system involvement due to SARS-CoV-2
The most common published cases with nervous system involvement were in the United States of America, followed by the United Kingdom, Chile, and Mexico (Supplementary Table 1).
4. Discussion
In the present study conducted in a tertiary center involving case series of hospitalized children and adolescents with COVID-19 and/or MIS-C, neurologic involvement incidence was 4.8%. Most of the patients (80%, n = 12) with neurologic findings were previously healthy and most of the neurological manifestations (n = 15) were transient and resolved at the time of hospital discharge. Three patients had mild to moderate new neurologic disabilities persisting at discharge.
In both adults and children, a wide range of neurological symptoms have been reported, ranging from relatively mild symptoms like fatigue, headache, and anosmia to more severe clinical presentations like encephalopathy, seizures, and stroke [[2], [3], [4], [5]]. In these reports neurologic symptoms varied by age; while seizures and status epilepticus observed in young patients, headache, loss of taste and smell and fatigue were observed in older patients. Consistent with these findings, four patients with seizures (three with para-infectious febrile seizure and one with postinfectious afebrile SE) included in the study were young children. A 3-month-old girl had her first seizure during the postinfectious period and had punctate diffusion restriction in the posterior limb of the internal capsule on MRI. No other etiological factor than COVID-19 was diagnosed. The clinical outcome was good, and all these patients were seizure-free during three-to-six-month follow-up with monotherapy. They had no neurological sequelae (mRS:0).
SARS-CoV-2 virus was initially thought unable to pass through the blood–brain barrier (BBB) or enter the nervous system. However, postmortem studies on the cerebral pathology of SARS-CoV-2 have identified that it could pass the BBB, and neuro-invasion could use olfactory nerve channels and trans-synaptic viral transfer [10,54]. Also possible is a “Trojan horse” mechanism of the virus using macrophages to enter the central nervous system [55]. Neural cells, astrocytes, microglia, and brain microvascular endothelial cells express ACE2, a SARS-CoV-2 spike (S) protein binding receptor [10,11]. Once inside the nervous system, the virus can infect those cells and cause neural damage or inflammation. This might help to explain the para-infectious events. Possible pathophysiological mechanisms of neurological involvement in our pediatric cases might be the direct viral invasion of neuronal structures through infecting olfactory system, or through types 2 and 3 innate immune cells. The role and distribution of ACE-2 receptors of over different areas of brain, especially brainstem should be considered in pathophysiology. Postinfectious inflammatory processes, dysregulated cytokine cascade, autoimmunity, and vascular events including thrombosis, infarction, or hemorrhages might be responsible for pathophysiology of neurological involvement [[56], [57], [58]].
Few studies have suggested the role of cytokines in febrile seizures [59,60]. Some have reported that proinflammatory cytokines such as interleukin-6 (IL-6), interleukin-8 (IL-8), and interferon-γ (IFN-γ) are increased in febrile seizures. We did not study the cytokine/chemokine profiles of our cases because of the retrospective nature of the study. Cytokines were reported to play a role in the pathophysiology of SARS-CoV-2 infection [61]. They may also be involved in the pathophysiology of COVID-19-related febrile or afebrile seizures. Important proinflammatory cytokines such as IL-6, TNF-α, IFN-γ, and IL-17 have been linked to the pathogenesis of SARS-CoV-2 infection [62,63]. They may play a key role in epileptic pathogenesis. They also lead to seizures by increasing glutamate and decreasing GABA in the cerebral cortex and hippocampus [62,64]. Therefore, we hypothesize that proinflammatory cytokines might have a crucial role in the pathogenesis of seizures due to SARS-CoV-2 both during the para-infectious and postinfectious periods.
We observed global cerebral involvement in five MIS-C patients with severe encephalopathy. All had changes in cognition: three had visual, auditory, or tactile hallucinations, and two were in a delirium state for two days. Two MIS-C patients had meningeal irritation at admission. Two MIS-C patients with severe and progressive disease course had vascular findings on MRI. MRI of one patient revealed infra- and supratentorial multiple microhemorrhages, suggestive of small-vessel vasculitis, bilateral middle cerebral artery (MCA) stenosis consistent with medium-vessel vasculitis and meningioependymal contrast enhancement. Computed tomography angiography imaging of the other patient showed stenosis in the upper truncus of the right MCA and paucity of the distal branches of the MCA. These neuroimaging findings suggested vascular endothelial cell damage, consistent with the cases in the literature review [[16], [17], [18],27,36,37,45,46]. Postinfectious neurological manifestations of MIS-C cases, might be the result of immune-mediated responses. The exaggerated cytokine release leading to endothelial damage, the hallmark of MIS-C pathogenesis, might be responsible for the disruption of BBB [54,65].
Previous case reports in children stated the association between COVID-19 and secondary ICP [53]. Consistently, in our series, one patient with increased ICP was diagnosed with secondary ICP related with COVID-19. Another patient diagnosed with factor V Leiden homozygous mutation, maintained on ASA and had a stroke history had increased ICP. He was diagnosed with acute hydrocephalus due to a web formation in Aquaeductus Sylvius and underwent an endoscopic third ventriculostomy. During the course of COVID-19 he did not have a new-onset stroke and his MRI only revealed contrast enchantment. Therefore, we hypothesized that the web formed in the Aquaeductus Sylvius might have resulted from inflammation associated with COVID-19.
Five patients had peripheral nervous system impairment: one had neuropathic pain, two experienced primarily encephalopathy and developed critical illness neuromyopathy during the hospital stay, and two had myositis and elevated CK levels. Severe inflammation, several secondary effects of critical illness, medications and possibly direct effects of SARS-CoV-2 may be responsible for peripheric nervous system involvement [[66], [67], [68]]. A significant number of patients with severe COVID-19 hospitalizations may be prolonged and may require mechanical ventilation, anesthetic medications, hence they are at risk of developing acquired weaknesses such as critical illness polyneuropathy, myopathy, and polyneuromyopathy. All conditions have been reported in adults with COVID-19, and severe inflammation is suggested to increase the risk [67,68].
So far, few data are available to describe SARS-CoV-2 specific CSF findings. In the present study, nine patients had CSF analysis, four had seizures, two had encephalopathy during MIS-C, one had secondary ICP, one had abnormal eye movements, and one underwent ventriculostomy for hydrocephalus. Pleocytosis was not detected in any samples. SARS-CoV-2 PCR and antibody testing could be studied in three out of the nine CSF samples, and all three were negative. Overall, the results of CSF studies of our patients were consistent with non-infectious inflammation processes during the course of disease. Bellon et al. [69] evaluated CSF samples of 31 adults with positive RT-PCR for SARS-CoV-2 from respiratory specimens and with neurological findings. None were positive for SARS-CoV-2 RNA by RT-PCR. All patients tested positive for anti-SARS-CoV-2 IgG antibodies in the serum, and 78% had antibodies in the CSF. According to the findings of this report, SARS-CoV-2 was not detected in most CSF samples. However, the detection of antibodies could suggest the possibility of an immune mechanism rather than viral invasion, requiring further clarification. Cytopathological evaluation of CSF samples has shown increased numbers of lymphocytes and macrophages. High numbers of macrophages were reported in 60% of CSF specimens’ cytological examination [70]. Two of our patients had cytopathological examination of CSF samples, and activated macrophages were prominent, in accordance with the literature. Increased macrophages could suggest microglial activation, known to induce expression of inflammatory responses such as cytokines, chemokines, and matrix metalloproteases [70,71]. These findings could provide a preference for anti-inflammatory or immunomodulatory treatment for neurological complications if supported by further studies.
We reviewed a broad range of severe neurological findings of pediatric patients, including central nervous system diseases (such as ADEM, severe encephalopathy with corpus callosum splenial lesions, seizures, ischemic strokes, cerebral venous sinus thrombosis, acute cerebral edema, and central nervous system infections) and peripheral nervous system diseases (such as GBS, myelitis, cranial neuropathies, and severe myositis) associated with SARS-CoV-2 infection. Neurologic involvement associated with SARS-CoV-2 possibly have multiple underlying mechanisms leading to para- or post-infectious events. The activation of cytokines and chemokines might be one of the key processes of postinfectious neurological involvement in MIS-C patients. In our case series severe encephalopathy observed in MIS-C patients resolved completely with anti-inflammatory treatment.
Some recent studies have reported neuropilin-1 (NRP1) as a receptor for the SARS-CoV-2 spike protein [72]. Moutal et al. [73] reported that the SARS-CoV-2 spike protein interacts with NRP1 to induce signaling in nociceptors. This could be a mechanism for pain during or after SARS-CoV-2 infection; another hypothesis is high cytokine levels during severe COVID-19 or MIS-C [74]. These theories could support the peripheral nervous system involvement that we report in a patient with neuropathic pain during MIS-C and patients with critical illness neuromyopathies.
Our study has several limitations, this single-center retrospective observational study spans a short period. The full spectrum of the neurologic symptoms might have been underestimated in children, especially in young children. Neuroimaging could not be obtained for all patients. We could not have a continuous EEG monitoring, so we might have missed electrographic seizures. The follow-up period of patients is short. Nevertheless, we were able to demonstrate the outcomes of patients with a formal scale.
5. Conclusion
We presented pediatric patients with new onset neurologic manifestations associated with SARS-CoV-2 infection. Our data showed that children, especially infants may present with neurologic findings such as seizures or abnormal eye movements as only symptoms of COVID-19. With growing evidence of the role of immune response playing a key role on many neurologic para-infectious and postinfectious manifestations of COVID-19, it is plausible that children with unusual signs and symptoms should be tested for COVID-19. Pediatricians should be aware of such atypical presentations of COVID-19 infection for early diagnosis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors have indicated they have no potential conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejpn.2022.02.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ellul M.A., Benjamin L., Singh B., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koralnik I.J., Tyler K.L. COVID-19: a global threat to the nervous system. Ann. Neurol. 2020;88(1):1–11. doi: 10.1002/ana.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Favas T.T., Dev P., Chaurasia R.N., et al. Neurologicalmanifestations of COVID-19: a systematic review and metaanalysis of proportions. Neurol. Sci. 2020;41(12):3437–3470. doi: 10.1007/s10072-020-04801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin. Neurol. Neurosurg. 2020;194:105921. doi: 10.1016/j.clineuro.2020.105921. Epub 2020 May 15. PMID: 32422545; PMCID: PMC7227498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng Kee Kwong K.C., Mehta P.R., Shukla G., Mehta A.R. COVID-19, SARS and MERS: a neurological perspective. J. Clin. Neurosci. 2020;77:13–16. doi: 10.1016/j.jocn.2020.04.124. Epub 2020 May 5. PMID: 32417124; PMCID: PMC7198407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi: 10.3390/v12010014. PMID: 31861926; PMCID: PMC7020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. PMID: 32275288; PMCID: PMC7149362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J. Clin. Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. Epub 2020 May 6. PMID: 32409215; PMCID: PMC7200361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183(1):16–27. doi: 10.1016/j.cell.2020.08.028. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ElBini Dhouib I. Does coronaviruses induce neurodegenerative diseases? A systematic review on the neurotropism and neuroinvasion of SARS-CoV-2. Drug Discov Ther. 2021;14(6):262–272. doi: 10.5582/ddt.2020.03106. [DOI] [PubMed] [Google Scholar]
- 12.Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16(11):636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO/2019-nCoV/Surveillance_Case_Definition/2020.2.
- 14.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Yea C, Borton M, Bitnun A et al. Neurological Manifestations of SARS-CoV-2 in Hospitalized Children: A Multi-National Cohort Study. Available at: SSRN: https://ssrn.com/abstract=3831858 or https://doi.org/10.2139/ssrn.3831858.
- 16.LaRovere K.L., Riggs B.J., Poussaint T.Y., et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78(5):536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindan C.E., Mankad K., Ram D., et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2021;5(3):167–177. doi: 10.1016/S2352-4642(20)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ippolito Bastidas H., Márquez-Pérez T., García-Salido A., et al. Cerebral venous sinus thrombosis in a pediatric patient with COVID-19. Neurol Clin Pract. 2021;11(2):e208–e210. doi: 10.1212/CPJ.0000000000000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enner S., Hormozdyaran S., Varughese R., Milillo J., Pavkovic I., Laureta E., Schneider J., Kothare S. Central apnea in an adolescent with COVID-19. Pediatr. Neurol. 2020 Sep;110:87–88. doi: 10.1016/j.pediatrneurol.2020.05.012. Epub 2020 Jun 5. PMID: 32674908; PMCID: PMC7273148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Howard M., Herranz-Aguirre M., Moreno-Galarraga L., et al. Case report: benign infantile seizures temporally associated with COVID-19. Front Pediatr. 2020;8:507. doi: 10.3389/fped.2020.00507. Published 2020 Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatta S., Sayed A., Ranabhat B., Bhatta R.K., Acharya Y. New-onset seizure as the only presentation in a child with COVID-19. Cureus. 2020;12(6) doi: 10.7759/cureus.8820. Published 2020 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swarz J.A., Daily S., Niemi E., Hilbert S.G., Ibrahim H.A., Gaitanis J.N. COVID-19 infection presenting as acute-onset focal status epilepticus. Pediatr. Neurol. 2020;112:7. doi: 10.1016/j.pediatrneurol.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandoval F., Julio K., Méndez G., et al. Neurologic features associated with SARS-CoV-2 infection in children: a case series report [published online ahead of print, 2021 mar 1] J. Child Neurol. 2021 doi: 10.1177/0883073821989164. 883073821989164. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Mannan O., Eyre M., Löbel U., Bamford A., Eltze C., Hameed B., Hemingway C., Hacohen Y. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77(11):1440–1445. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhavsar S.M., Agarwal S., Lewis R., et al. COVID-19 infection associated with encephalitis in an adolescent. Neurol Clin Pract. 2021;11(2):e189–e192. doi: 10.1212/CPJ.0000000000000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahsan N., Jafarpour S., Santoro J.D. Myelin oligodendrocyte glycoprotein antibody encephalitis following severe acute respiratory syndrome coronavirus 2 in a pediatric patient [published online ahead of print, 2021 Feb 1] Clin Exp Pediatr. 2021 doi: 10.3345/cep.2020.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sánchez-Morales A.E., Urrutia-Osorio M., Camacho-Mendoza E., et al. Neurological manifestations temporally associated with SARS-CoV-2 infection in pediatric patients in Mexico [published online ahead of print, 2021 Mar 10] Childs Nerv Syst. 2021:1–8. doi: 10.1007/s00381-021-05104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank C.H.M., Almeida T.V.R., Marques E.A., et al. Guillain-barré syndrome associated with SARS-CoV-2 infection in a pediatric patient. J. Trop. Pediatr. 2021;67(3):fmaa044. doi: 10.1093/tropej/fmaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalifa M., Zakaria F., Ragab Y., et al. Guillain-barré syndrome associated with severe acute respiratory syndrome coronavirus 2 detection and coronavirus disease 2019 in a child. J Pediatric Infect Dis Soc. 2020;9(4):510–513. doi: 10.1093/jpids/piaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aghdam M.K., Bakhtiari H., Diazi D.N., Eftekhari K. Neurological manifestations of novel coronavirus disease in a 2-month-old infant: a case report. J. Pediatr. Neurol. 2020 Jul doi: 10.1055/s-0040-1716393. ISSN 1304-2580. [DOI] [Google Scholar]
- 31.McAbee G.N., Brosgol Y., Pavlakis S., Agha R., Gaffoor M. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr. Neurol. 2020 Aug;109:94. doi: 10.1016/j.pediatrneurol.2020.04.013. Epub 2020 Apr 24. PMID: 32586676; PMCID: PMC7180343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bektaş G., Akçay N., Boydağ K., Şevketoğlu E. Reversible splenial lesion syndrome associated with SARS-CoV-2 infection in two children. Brain Dev. 2021 Feb;43(2):230–233. doi: 10.1016/j.braindev.2020.10.002. Epub 2020 Oct 13. PMID: 33082059; PMCID: PMC7553133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Miranda Henriques-Souza A.M., de Melo A.C.M.G., de Aguiar Coelho Silva Madeiro B., Freitas L.F., Sampaio Rocha-Filho P.A., Gonçalves F.G. Acute disseminated encephalomyelitis in a COVID-19 pediatric patient. Neuroradiology. 2021;63(1):141–145. doi: 10.1007/s00234-020-02571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur H., Mason J.A., Bajracharya M., et al. Transverse myelitis in a child with COVID-19. Pediatr. Neurol. 2020;112:5–6. doi: 10.1016/j.pediatrneurol.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basirjafari S., Rafiee M., Shahhosseini B., Mohammadi M., Aghayari Sheikh Neshin S., Zarei M. Association of pediatric COVID-19 and subarachnoid hemorrhage. J. Med. Virol. 2021;93(2):658–660. doi: 10.1002/jmv.26434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appavu B., Deng D., Dowling M.M., et al. Arteritis and large vessel occlusive strokes in children after COVID-19 infection. Pediatrics. 2021;147(3) doi: 10.1542/peds.2020-023440. [DOI] [PubMed] [Google Scholar]
- 37.Kihira S., Morgenstern P.F., Raynes H., Naidich T.P., Belani P. Fatal cerebral infarct in a child with COVID-19. Pediatr. Radiol. 2020;50(10):1479–1480. doi: 10.1007/s00247-020-04779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dugue R., Cay-Martínez K.C., Thakur K.T., et al. Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94(24):1100–1102. doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaur P., Dixon L., Jones B., Lyall H., Jan W. COVID-19-Associated cytotoxic lesions of the corpus callosum. AJNR Am J Neuroradiol. 2020;41(10):1905–1907. doi: 10.3174/ajnr.A6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abel D., Shen M.Y., Abid Z., et al. Encephalopathy and bilateral thalamic lesions in a child with MIS-C associated with COVID-19. Neurology. 2020;95(16):745–748. doi: 10.1212/WNL.0000000000010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verkuil L.D., Liu G.T., Brahma V.L., Avery R.A. Pseudotumor cerebri syndrome associated with MIS-C: a case report. Lancet. 2020;396(10250):532. doi: 10.1016/S0140-6736(20)31725-6. [DOI] [PubMed] [Google Scholar]
- 42.Mirzaee S.M.M., Gonçalves F.G., Mohammadifard M., Tavakoli S.M., Vossough A. Focal cerebral arteriopathy in a pediatric patient with COVID-19. Radiology. 2020;297(2):E274–E275. doi: 10.1148/radiol.2020202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker A.E., Chiotos K., McGuire J.L., Bruins B.B., Alcamo A.M. Intracranial hypertension in multisystem inflammatory syndrome in children. J. Pediatr. 2021;233:263–267. doi: 10.1016/j.jpeds.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saeed A., Shorafa E. Status epilepticus as a first presentation of COVID-19 infection in a 3 years old boy; Case report and review the literature. IDCases. 2020;22 doi: 10.1016/j.idcr.2020.e00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schupper A.J., Yaeger K.A., Morgenstern P.F. Neurological manifestations of pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Childs Nerv Syst. 2020;36(8):1579–1580. doi: 10.1007/s00381-020-04755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiwari L., Shekhar S., Bansal A., Kumar S. COVID-19 associated arterial ischaemic stroke and multisystem inflammatory syndrome in children: a case report. Lancet Child Adolesc Health. 2021;5(1):88–90. doi: 10.1016/S2352-4642(20)30314-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regev T., Antebi M., Eytan D., et al. Pediatric inflammatory multisystem syndrome with central nervous system involvement and hypocomplementemia following SARS-COV-2 infection. Pediatr. Infect. Dis. J. 2020;39(8):e206–e207. doi: 10.1097/INF.0000000000002804. [DOI] [PubMed] [Google Scholar]
- 48.De Paulis M., Oliveira D.B.L., Vieira R.P., et al. Multisystem inflammatory syndrome associated with COVID-19 with neurologic manifestations in a child: a brief report. Pediatr. Infect. Dis. J. 2020;39(10):e321–e324. doi: 10.1097/INF.0000000000002834. [DOI] [PubMed] [Google Scholar]
- 49.Hutchison L., Plichta A.M., Lerea Y., Madora M., Ushay H.M. Neuropsychiatric symptoms in an adolescent boy with multisystem inflammatory syndrome in children. Psychosomatics. 2020;61(6):739–744. doi: 10.1016/j.psym.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yousefi K., Poorbarat S., Abasi Z., Rahimi S., Khakshour A. Viral meningitis associated with COVID-19 in a 9-year-old child: a case report. Pediatr. Infect. Dis. J. 2021;40(2):e87–e98. doi: 10.1097/INF.0000000000002979. [DOI] [PubMed] [Google Scholar]
- 51.Sa M., Mirza L., Carter M., et al. Systemic inflammation is associated with neurologic involvement in pediatric inflammatory multisystem syndrome associated with SARS-CoV-2. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e999. doi: 10.1212/NXI.0000000000000999. Published 2021 Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akhondian J., Toosi F.S., Ashrafzadeh F., Hashemi N., Zand N.S. COVID-19; neurological findings in five pediatric patients. Int Clin Neurosci J. 2021 Spring;8(2):96–98. [Google Scholar]
- 53.Baccarella A., Linder A., Spencer R., et al. Increased intracranial pressure in the setting of multisystem inflammatory syndrome in children, associated with COVID-19. Pediatr. Neurol. 2021;115:48–49. doi: 10.1016/j.pediatrneurol.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.928996. Published 2020 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ElBini Dhouib I. Does coronaviruses induce neurodegenerative diseases? A systematic review on the neurotropism and neuroinvasion of SARS-CoV-2. Drug Discov Ther. 2021;14(6):262–272. doi: 10.5582/ddt.2020.03106. [DOI] [PubMed] [Google Scholar]
- 56.Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: a systematic review. J. Neurol. Sci. 2020;413 doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 58.Niazkar H.R., Zibaee B., Nasimi A., Bahri N. The neurological manifestations of COVID-19: a review article. Neurol. Sci. 2020;41(7):1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwon A., Kwak B.O., Kim K., et al. Cytokine levels in febrile seizure patients: a systematic review and meta-analysis. Seizure. 2018;59:5–10. doi: 10.1016/j.seizure.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 60.Kim K., Kwak B.O., Kwon A., et al. Analysis of plasma multiplex cytokines and increased level of IL-10 and IL-1Ra cytokines in febrile seizures [published correction appears in J Neuroinflammation. 2017 Nov 17;14 (1):226] J. Neuroinflammation. 2017;14(1):200. doi: 10.1186/s12974-017-0974-7. Published 2017 Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh S.P., Pritam M., Pandey B., Yadav T.P. Microstructure, pathophysiology, and potential therapeutics of COVID-19: a comprehensive review. J. Med. Virol. 2021;93(1):275–299. doi: 10.1002/jmv.26254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nikbakht F., Mohammadkhanizadeh A., Mohammadi E. How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms. Mult Scler Relat Disord. 2020;46 doi: 10.1016/j.msard.2020.102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galic M.A., Riazi K., Pittman Q.J. Cytokines and brain excitability. Front. Neuroendocrinol. 2012;33(1):116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galanopoulou A.S. GABA(A) receptors in normal development and seizures: friends or foes? Curr. Neuropharmacol. 2008;6(1):1–20. doi: 10.2174/157015908783769653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coperchini F., Chiovato L., Ricci G., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: further advances in our understanding the role of specific chemokines involved. Cytokine Growth Factor Rev. 2021;58:82–91. doi: 10.1016/j.cytogfr.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Attal N., Martinez V., Bouhassira D. Potential for increased prevalence of neuropathic pain after the COVID-19 pandemic. Pain Rep. 2021;6 doi: 10.1097/PR9.0000000000000884. e884-e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kress J.P., Hall J.B. ICU-acquired weakness and recovery from critical illness. N. Engl. J. Med. 2014;371:287–288. doi: 10.1056/NEJMc1406274. [DOI] [PubMed] [Google Scholar]
- 68.Bax F., Lettieri C., Marini A., et al. Clinical and neurophysiological characterization of muscular weakness in severe COVID-19. Neurol. Sci. 2021;42:2173–2178. doi: 10.1007/s10072-021-05110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellon M., Schweblin C., Lambeng N., Cherpillod P., Vazquez J., Lalive P.H., Schibler M., Deffert C. Cerebrospinal fluid features in SARS-CoV-2 RT-PCR positive patients. Clin. Infect. Dis. 2020 Aug 8:ciaa1165. doi: 10.1093/cid/ciaa1165. Epub ahead of print. PMID: 32770235; PMCID: PMC7454353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bindoli S., Felicetti M., Sfriso P., Doria A. The amount of cytokine-release defines different shades of Sars-Cov 2 infection. Exp. Biol. Med. 2020 Jun;245(11):970–976. doi: 10.1177/1535370220928964.Epub.2020.May.28. PMID: 32460624; PMCID: PMC7427176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Obaidi M.M.J., Bahadoran A., Wang S.M., Manikam R., Raju C.S., Sekaran S.D. Disruption of the blood brain barrier is vital property of neurotropic viral infection of the central nervous system. Acta Virol. 2018;62(1):16–27. doi: 10.4149/av.2018.102.PMID:29521099. [DOI] [PubMed] [Google Scholar]
- 72.McFarland A.J., Yousuf M.S., Shiers S., Price T.J. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: implications for COVID-19 and pain. Pain Rep. 2021;6(1):e885. doi: 10.1097/PR9.0000000000000885. Published 2021 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moutal A., Martin L.F., Boinon L., et al. SARS-CoV-2 Spike protein co-opts VEGF-A/Neuropilin-1 receptor signaling to induce analgesia. Preprint. bioRxiv. 2020 doi: 10.1101/2020.07.17.209288. 2020.07.17.209288. Published 2020 Sep. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16(11):636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.