Abstract
The genetic diversity of recent clinical isolates of Candida albicans in Japan was studied on the basis of amplified DNA band lengths determined with a specific PCR primer reported to have been designed to span a transposable intron region in the 25S rRNA gene. Our analyses of 301 clinical isolates of C. albicans showed that they could be classified into five genotypes: genotype A (172 isolates), genotype B (66 isolates), genotype C (56 isolates), genotype D (C. dubliniensis; 5 isolates), and a new genotype (designated genotype E; 2 isolates). The new genotype E was characterized to have a group I intron-like sequence, which is longer than hitherto reported ones and which has a nucleotide sequence length of 962 bp. Our analysis of the 962-bp sequence indicated that it is composed of an intron similar to that of C. dubliniensis of 621 bp with a 341-bp insertion. Analysis of the sequence of the internal transcribed spacer (ITS) region of the genotype E strain showed that its sequence is identical to those of strains of other genotypes, with only a few base substitution differences. Throughout the study, the possible horizontal transfer of the group I intron between C. dubliniensis and C. albicans was suggested. A high degree of correlation between the presence of a group I intron in C. albicans genotype E and susceptibility to the antifungal agent flucytosine was observed. The five isolates of C. dubliniensis examined in the present study showed genetic diversity when they were compared by randomly amplified polymorphic DNA fingerprinting pattern analysis, and this diversity was also confirmed by the analysis of ITS region sequences.
The increasing incidence of AIDS and the recent development of a new treatment strategy for patients with hematologic malignancies and organ transplants have led to steady increases in the number of immunocompromised patients with fungal infections (14). Although the number of fungal species responsible for infection in such patients continues to increase, Candida species remain the most frequently encountered fungal pathogens (2, 14). Among the Candida species, Candida albicans is still considered the most important fungal pathogen. However, an increasing number of reports have described atypical C. albicans strains among human clinical isolates, and C. albicans strains have been subdivided into some biological groups, including genetic subtypes (8). Recent advances in molecular biology-based technology enable detailed analysis of the genetic diversity of C. albicans, and some groups of C. albicans strains have been genetically characterized and reported (5, 6).
The usefulness of ribosomal sequences for genetic typing has been demonstrated and widely applied to the identification of several fungal pathogens (7–9). McCullough et al. (8) reported that a PCR primer designed to span the 25S rRNA gene (rDNA) region can classify C. albicans strains into four genotypes on the basis of the amplified PCR product length: genotype A (450-bp product), genotype B (840-bp product), genotype C (450- and 840-bp products), and genotype D (1,080-bp product). In their report, they confirmed that C. albicans genotype D belongs to the same taxon as C. dubliniensis. We have also confirmed that this genotype analysis method is simple and reproducible when reference C. albicans strains are used. Since no systematic study on the genetic subtyping of Japanese C. albicans isolates has been reported thus far, we were interested in this method from a molecular epidemiological point of view. Here, we report on the molecular characterization of a new genotype of C. albicans and new isolates of C. dubliniensis which were found in analyses of 301 isolates phenotypically identified as C. albicans. We also discuss the possible origin of the group I intron in C. albicans and its association with antifungal susceptibility.
MATERIALS AND METHODS
C. albicans and other Candida sp. strains.
The following reference strains of C. albicans and C. dubliniensis were used: C. albicans ATCC 90028, ATCC 90029, and CY1123 and C. dubliniensis CBS 7987 and 70-12539 (16). Three hundred one isolates of C. albicans obtained from clinical specimens (from July 1999 to March 2000) submitted to the Hiroshima Red Cross-Atomic Bomb Survivors Hospital (Hiroshima, Japan), Kurashiki Central Hospital (Okayama, Japan), Kochi Municipal Hospital (Kochi, Japan), Chiba University Hospital (Chiba, Japan), Kitasato University Hospital (Kanagawa, Japan), and Showa University Hospital (Tokyo, Japan) were used in the present study. These clinical isolates were identified as C. albicans in each regional hospital on the basis of their cultural and morphological characteristics such as colony color on CHROMagar Candida and chlamydospore formation on cornmeal agar, respectively. The isolates were confirmed to be C. albicans and C. dubliniensis with the API ID32C system (Biomerieux SA, Marcy l'Etoile, France) following the recommendations of the manufacturer. They were inoculated onto potato dextrose agar (PDA; Difco) slants and were incubated at 37°C for approximately 48 to 72 h before DNA extraction.
Extraction of DNA.
Two or three loopfuls of fungal yeast cells from PDA slants were suspended in 200 μl of TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA) in an Eppendorf tube (1.5 ml). DNA extraction was carried out by the procedure described by Imai et al. (3) and Tamura et al. (16). Briefly, 250 μl of GPT reagent (6 M guanidine thiocyanate in 50 mM Tris [pH 8.3]) and 450 μl of Tris (pH 8.0)-buffered phenol were added to a suspension of washed yeast cells in an Eppendorf tube, and the mixture was boiled for 15 min to kill the fungal cells and extract the DNA. Chloroform-isoamyl alcohol (250 μl) was then added; and the aqueous phase was separated by centrifugation at 12,000 × g, mixed with an equal amount of 100% isopropanol and a 1/10 volume of 3 M sodium acetate, and placed at −20°C for 1 h. Samples were centrifuged at 12,000 × g for 20 min; and the nucleic acid pellet obtained was washed with ice-cold 70% ethanol, dried, and resuspended in sterile TE buffer at a concentration of 5 μg/ml.
Genotype determination by PCR.
The primer pairs whose sequences span the site of the transposable intron in the 25S rDNA were those described by McCullough et al. (8). The PCR primer pairs used were CA-INT-L (5′-ATAAGGGAAGTCGGCAAAATAGATCCGTAA-3′) and CA-INT-R (5′-CCTTGGCTGTGGTTTCGCTAGATAGTAGAT-3′). Amplification reactions were performed in 25 μl of distilled water containing 2.5 μl of each primer (20 pm), 2.5 μl of genomic DNA (5 μg/ml), and one PCR bead (Ready-to-Go PCR beads; Amersham Pharmacia Biotech, Piscataway, N.J.). The PCR conditions used were as follows: denaturation by incubation for 3 min at 94°C prior to 30 cycles of 94°C for 1 min, 65°C for 1 min, and 72°C for 2.5 min and a final extension at 72°C for 10 min in a thermoreactor. All reaction products were characterized by electrophoresis on 1.5% agarose gels in 1× TBE (Tris-borate-EDTA) buffer at 70 V for 100 min and were then stained in a solution of 0.5 μg of ethidium bromide per ml.
DNA sequencing of ITS1-5.8S-ITS2 region of rDNA and introns of C. albicans strains.
The PCR primers used for amplification and sequencing of the internal transcribed spacer (ITS) regions were the same as those described by White et al. (20). The PCR primers used for ITS region DNA sequencing were ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). Primers CA-INT-L, CA-INT-R, Car1F (5′- AAACGGCGGGAGTAACTAT-3′), Cae2F (5′-TACTTTATGACGACAAC-3′), and Cae1R (5′-TGGCTACCTTAAGC-3′) were used as the primers for sequencing of the intron region sequences. Amplification reactions were performed in 25 μl of distilled water containing 2.5 μl of each primer (20 pm), 2.5 μl of genomic DNA (5 μg/ml), and one PCR bead. PCR was performed by initial denaturation at 94°C for 4 min, followed by 35 cycles at 94°C for 2 min, 55°C for 2 min, and 72°C for 2 min and a final extension at 72°C for 10 min (14). The PCR products were purified with a PCR product presequencing kit (U.S. Biochemical Corp., Cleveland, Ohio) and were then sequenced directly with a Big Dye terminator reagent kit with Taq polymerase and by the protocol recommended by the manufacturer of the model 310 automated DNA sequencer; Perkin-Elmer/Applied Biosystems, Chiba, Japan). The DNA sequences were aligned with the Clustal W program (version 1.74) (17), and the alignment was visually corrected.
RAPD PCR analysis of new C. dubliniensis isolates.
Two primers were used for randomly amplified polymorphic DNA (RAPD) analysis: primers R-1 (5′-ATGGATCGGC-3′) and R-2 (5′-ATTGCGTCCA-3′). These were prepared on the basis of the reports of Poonwan et al. (13) and Aoki et al. (1). Amplification reactions were performed in 30 μl of distilled water containing 2.5 μl of each primer (20 pm), 2.5 μl of genomic DNA (5 μg/ml), and one PCR bead. The PCR was performed under the same conditions as those described previously (1, 3).
Candida species identity confirmation by slide agglutination tests, with a yeast identification system, and by phenotypic characterization tests.
After two transfers on PDA slants, the identities of the new isolates of Candida species were confirmed by slide agglutination tests (Candida Check; Iatron Co., Tokyo, Japan). The API ID32C yeast identification system (Biomerieux SA) was also used for confirmation of the identities of the Candida species. CHROMagar Candida (Kanto Kagaku Co., Tokyo, Japan) (11) was used for observation of colony color, and Tween 80-Oxgall-caffeic acid agar medium (Remel, Lenexa, Kans.) was used for the chlamydospore formation test.
Drug susceptibility testing.
Amphotericin B, fluconazole, flucytosine, and itraconazole were gifts from Bristol-Myers Squibb Japan (Tokyo, Japan), Pfizer Pharmaceuticals (Nagoya, Japan), Nihon Roche (Tokyo, Japan), and Janssen Pharmaceutica (Beerse, Belgium), respectively. Susceptibilities to the antifungal agents were determined by a modified NCCLS M27-A broth microdilution method by use of yeast nitrogen base with 1% glucose (Difco, Detroit, Mich.) as the assay medium (10). C. albicans ATCC 90028, C. albicans ATCC 90029 (which is resistant to flucytosine), and C. albicans CY1123 (which is resistant to flucytosine and the azoles) were used as reference strains.
Nucleotide sequence accession number.
The whole sequence of the group I intron of the genotype E strains was deposited in the DDBJ database under accession number AB049125. The sequences of the ITS1-5.8S-ITS2 regions of C. albicans ATCC 90028 (genotype A), ATCC 90029 (genotype B), CY1123 (genotype C), and IFM 49826 (genotype E) and C. dubliniensis CBS 7987 and IFM 49833 are deposited in the DDBJ database with accession numbers AB049119 to AB049124, respectively.
RESULTS
Genotypes of newly isolated C. albicans strains from clinical specimens in Japan.
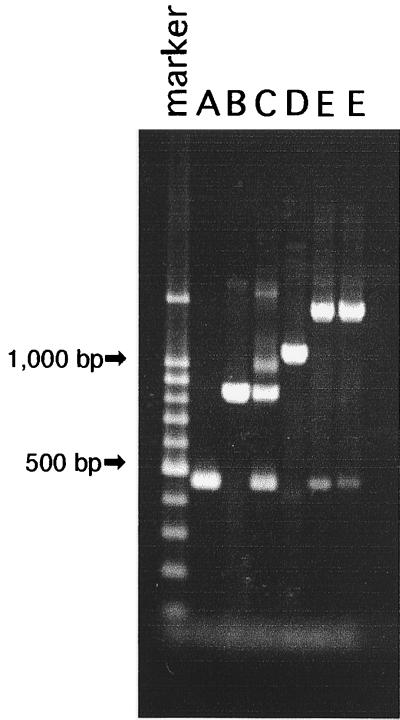
All the C. albicans strains were inoculated into CHROMagar Candida, and the resultant colony color was used to exclude possible contamination with non-C. albicans isolates. The genotypes of all 301 isolates of C. albicans were analyzed by the PCR method, and the results are shown in Table 1. The lengths of the PCR products of reference strains C. albicans ATCC 90028 (serotype A) and ATCC 90029 (serotype B) were about 450 and 840 bp, respectively. C. albicans CY1123, which produced two PCR products of about 450 and 840 bp, respectively, was used as the genotype C reference strain. C. dubliniensis CBS 7987 exhibited a band of approximately 1,080 bp and was used as the genotype D reference strain (Fig. 1).
TABLE 1.
Genotyping of 301 recent clinical C. albicans and C. dubliniensis isolates from Japana
| Source | No. (%) of strains of the following genotype:
|
|||||
|---|---|---|---|---|---|---|
| Total | A | B | C | D (C. dubliniensis) | E | |
| Hiroshima Red Cross-Atomic Bomb Survivors Hospital (western Japan) | 46 | 28 | 9 | 9 | 0 | 0 |
| Kurashiki Central Hospital (western Japan) | 28 | 11 | 6 | 9 | 2 | 0 |
| Kouchi Municipal Hospital (Shikoku Island, western Japan) | 15 | 7 | 5 | 2 | 1 | 0 |
| Chiba University Hospital (central Japan) | 152 | 93 | 29 | 26 | 2 | 2 |
| Kitasato University Hospital (central Japan) | 30 | 17 | 10 | 3 | 0 | 0 |
| Showa University Hospital (central Japan) | 30 | 16 | 7 | 7 | 0 | 0 |
| Total | 301 | 172 (57.1) | 66 (21.9) | 56 (18.6) | 5 (1.7) | 2 (0.7) |
All strains were isolated in Japan from July 1999 to March 2000.
FIG. 1.
Genotyping DNA band pattern profiles of reference strains of C. albicans genotypes A, B, and C; C. dubliniensis genotype D; and two C. albicans genotype E strains (lanes A to E, respectively).
Results showed that of the 301 isolates tested, 172 were classified as genotype A, 66 were classified as genotype B, 56 were classified as genotype C, and 5 were classified as genotype D (C. dubliniensis). In addition, we observed two new strains that produced a different PCR product of about 1,400 bp (Table 2; Fig. 1), which is longer than that of C. dubliniensis (1,080 bp). Therefore, we considered these two strains to belong to a new genotype, designated genotype E. C. albicans genotype E strains were isolates from non-AIDS patients in Chiba, which is in the central part of Japan, while C. dubliniensis strains were distributed among all geographical regions of Japan. The C. dubliniensis strains were also found not to be associated with AIDS patients, although the human immunodeficiency virus (HIV) infection status of one patient was not clear (Table 2).
TABLE 2.
History and useful differential features between C. albicans genotype E and C. dubliniensis strains isolated in the present study
| Strain number | Source | Patient characteristic (age [yr]) | Chlamydospore pattern | API ID32C profile identification | Growth at 45°C | Approximate PCR product length (bp) | RAPD group
|
|
|---|---|---|---|---|---|---|---|---|
| R-1 primer | R-2 primer | |||||||
| C. albicans genotype A ATCC 90028 | Singlet | C. albicans | + | 450 | ||||
| C. albicans genotype B ATCC 90029 | Singlet | C. albicans | + | 840 | ||||
| C. albicans genotype C CY1123 | Singlet | C. albicans | + | 450, 840 | ||||
| C. dubliniensis CBS 7987 | Doublet | C. dubliniensis | − | 1,080 | 1 | A | ||
| C. dubliniensis | ||||||||
| IFM 49829 | Feces | Female (70), roentgen ulcer | Chain | C. dubliniensis | − | 1,080 | 1 | A |
| IFM 49830 | Feces | Male (75), leukemia | Doublet | No IDa | − | 1,080 | 2 | A |
| IFM 49831 | Sputum | Female (85), anemia | Chain | C. albicans | − | 1,080 | 1 | C |
| IFM 49832 | Sputum | Female (79), diabetes | Doublet | No ID | − | 1,080 | 1 | A |
| IFM 49833 | Unknown | Unknown | Singlet and triplet | C. dubliniensis | − | 1,080 | 1 | B |
| C. albicans genotype E | ||||||||
| IFM 49826 | Sputum | Female (56), subarachnoid hemorrhage | Singlets | C. albicans | + | 1,400 | ||
| IFM 49827 | Urine | Male (80), emphysema of lungs, intensive care unit | Singlet | C. albicans | + | 1,400 | ||
No ID, not identified; the profile does not resemble any of those stored in the database.
Predicted structure of C. albicans genotype E group I intron.
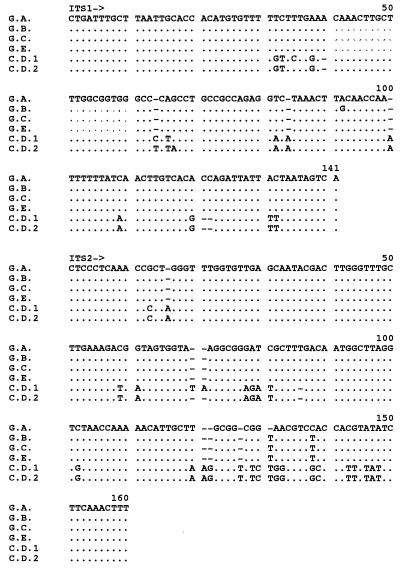
The 1,400-bp intron-like sequence of genotype E strains suggested the existence of an intron within the 25S rDNA, which could be removed during maturation of rRNA transcripts. The sequence of the intron-like structure was determined, and the sequence was compared with those of the reported sequences of C. dubliniensis CBS 7987 (EMBL database accession number Z70663) and C. albicans genotype B (EMBL database accession number X74272) (Fig. 2). The length of C. albicans genotype E intron-like sequence was 962 bp, and subsequent base analysis indicated that it belonged to group I introns, as it contained conserved elements of group I introns, such as P, Q, R, and S regions, identical to those that commonly exist in the group I introns of C. albicans genotype B and C. dubliniensis. The sequence was most similar to that of the C. dubliniensis group I intron, with an insertion of a 341-bp fragment at what appeared to be position 255 bp of the internal guide sequence (IGS) site (Fig. 2). Our search with the BLAST program did not reveal a DNA sequence similar to the 341-bp insertion sequence, and the origin of the insertion sequence could not determined.
FIG. 2.
Alignment of group I introns of C. albicans genotype E (G.E.), C. dubliniensis (C.D.), and C. albicans genotype B (C.A.). The underlining indicates the group 1 intron conserved sequence elements.
Analysis of ITS regions of C. albicans and C. dubliniensis
For the establishment of the phylogenetic position of C. albicans genotype E, the sequences of the ITS1-5.8S-ITS2 regions of C. albicans ATCC 90028 (genotype A), ATCC 90029 (genotype B), CY1123 (genotype C), and IFM 49826 and IFM 49827 (genotype E) and C. dubliniensis CBS 7987 and IFM 49833 were determined. The results showed that the sequences of the ITS1-5.8S-ITS2 regions of C. albicans genotypes C and E were identical and that only a 1-bp difference in the sequences between genotypes B and C was observed. The sequence difference between genotypes A and E was minor, with a 1-bp difference and a 1-bp deletion observed in C. albicans genotype A compared with the sequence of genotype E (Fig. 3).
FIG. 3.
Alignment of ITS1 and ITS2 region sequences of C. albicans genotype E strains and C. dubliniensis CBS 7987 and IFM 49833 in comparison with those of C. albicans genotype A, B, and C isolates. G.A., C. albicans genotype A, ATCC 90028; G.B., C. albicans genotype B, ATCC 90029; G.C., C. albicans genotype C, CY1123; G.E., C. albicans genotype E, IFM 49826; C.D.1, C. dubliniensis CBS 7987; C.D.2, C. dubliniensis IFM 49833.
Phenotypic characterization of C. albicans genotype E.
All C. albicans genotypes, including the five C. dubliniensis strains, grew well at 45°C on culture media such as PDA and Sabouraud dextrose agar. However, reference isolate C. dubliniensis CBS 7987 did not grow at the restricted temperature. Genotype E isolates produced chlamydospores on TOC agar medium, and most of them were terminal singlets, similar to those of C. albicans. The number of chlamydospores produced by C. albicans genotype E was lower than the number produced by C. dubliniensis CBS 7987. On the basis of this information, some useful differential techniques enabled us to separate genotype E strains from strains of the other genotypes of C. albicans and C. dubliniensis (Table 2). Additionally, C. albicans genotype E also produced a green color on CHROMagar, similar to genotype A to D strains, appeared to be serotype A, and was biochemically confirmed to be C. albicans with the API ID32C system.
RAPD analysis and comparison of ITS region sequences of Japanese C. dubliniensis isolates.
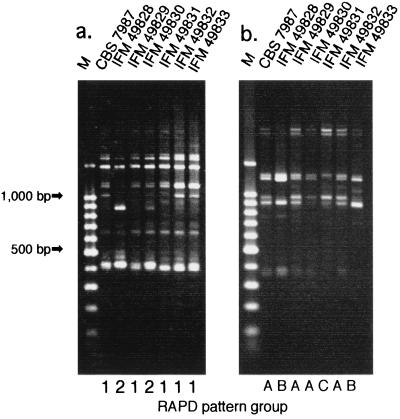
Five strains of C. dubliniensis were compared to the type strain (strain CBS 7987) and the other reference strain (strain IFM 49828 [which is the same as strain 70–12539]) (16) by their fingerprinting patterns obtained by RAPD analysis with two 10-mer primers. Genetic typing of the five C. dubliniensis strains revealed heterogeneous RAPD fingerprinting patterns. Typing with primer R-2 showed a more characteristic RAPD fingerprinting pattern for each strain than typing with primer R-1, with primer R-2 being able to classify the six strains of C. dubliniensis into three groups, while primer R-1 classified them into two groups (Fig. 4). These results suggested the existence of at least three different genotypic groups of C. dubliniensis in Japan. The sequences of the ITS regions of the remaining four strains were identical to that of the types strain of C. dubliniensis (strain CBS 7987).
FIG. 4.
RAPD band patterns of five C. dubliniensis isolates in comparison with those of the C. dubliniensis type strain and strain IFM 49828 from our culture collection. (a) The R-1 primer was used. (b) The R-2 primer was used. Lanes M, molecular size markers.
The results of the analyses of the sequences of the ITS regions of five epidemiologically unrelated C. dubliniensis strains from different hospitals in Japan are shown in Fig. 3 and are compared with those for C. dubliniensis strains isolated from different geographic regions. The sequence data show the heterogeneity of the sequences of the ITS regions of C. dubliniensis strains. Among the five strains of C. dubliniensis tested, the sequence of the ITS region of C. dubliniensis IFM 49833, from Kochi Hospital, was clearly different from those of the type strain of C. dubliniensis (strain CBS 7987) and the four other Japanese clinical isolates of C. dubliniensis.
The API ID32C identification kit gave phenotypic profiles compatible with that expected for two strains of C. dubliniensis, while the remaining three strains had unidentifiable profiles.
Susceptibilities of C. albicans genotype E and C. dubliniensis isolates to four antifungal drugs in comparison with those of other reference strains of C. albicans
The two C. albicans genotype E strains had susceptibilities to amphotericin B, fluconazole, and itraconazole almost identical those of the genotype A, B, and C reference strains. On the other hand, the MICs of flucytosine for the two strains of C. albicans genotype E were the lowest of those for all groups. All strains of C. dubliniensis had similar susceptibilities to amphotericin B, itraconazole, fluconazole, and flucytosine. Although the susceptibilities of five strains of C. dubliniensis to amphotericin B, itraconazole, and fluconazole were almost identical to those of the reference C. albicans strains, the MICs were lower than the MIC for C. albicans (data not shown).
DISCUSSION
C. albicans is a ubiquitous commensal organism and has been considered a major pathogen for immunocompetent patients as well as immunocompromised patients. Different methods have been developed to differentiate isolates of C. albicans (11, 14) and to determine the relationships of these subtypes to human disease. Moreover, advances in molecular biology have enabled the use of various new molecular biology-based genetic methods to answer a variety of epidemiological questions regarding infection with this organism (11, 14).
Mercure et al. (9) reported that C. albicans produces a well-characterized EcoRI restriction fragment length polymorphism pattern whose bands are intensively stained by ethidium bromide. Of these bands, the dimorphic (3.7- and 4.2-kbp) fragment, which was shown to have originated from the rRNA-encoding regions (rDNA), has been used to classify C. albicans into two types (genotypes A and B). Their further studies confirmed that the presence or absence of group I introns among C. albicans strains accounts for the difference in the DNA band lengths of the rDNA fragment (3.7 or 4.2 kbp) observed (12). Those studies led to the preparation by McCullough et al. of a PCR primer pair that can demonstrate the presence of group I introns in the 25S rDNA (8). Our present studies confirmed the usefulness of the PCR primer pair for the genotyping of C. albicans. An additional advantage of using this primer pair is that it can detect C. dubliniensis as well as determine the genotypes of C. albicans.
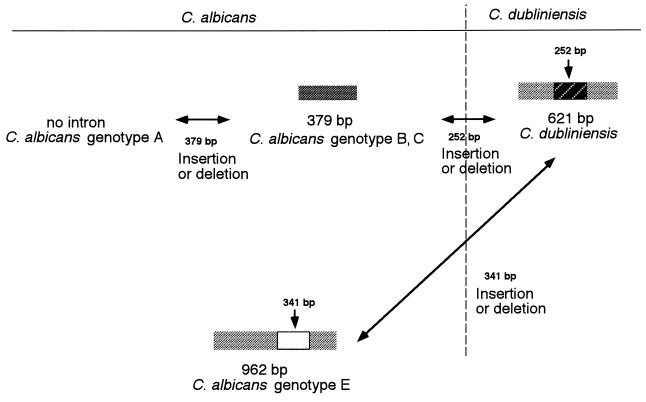
Analysis of 301 C. albicans isolates obtained from 1999 to 2000 in Japan revealed that they could be classified into the four previously recognized genotypes: genotypes A (172 isolates), B (66 isolates), C (56 isolates), and D (C. dubliniensis; 5 isolates). Interestingly, two isolates that had unclassifiable PCR band patterns were also found. Since the API ID32C identification system and slide agglutination tests indicated that both strains were phenotypically C. albicans, we designated them as a new genotype of C. albicans, genotype E. The sequences of the ITS regions of the new genotype E strains also confirmed their identities as C. albicans. However, the genotype E strain was different, in that a 341-bp insertion occurred within the group I intron. Thus, the most significant observation in the present study concerns analysis of this insertion fragment within the group I intron. However, this genotype E strain showed a high degree of similarity to C. dubliniensis compared to the degree of similarity of strains of other C. albicans genotypes when the similarity was determined on the basis of the group I intron sequence. These data suggest a possible horizontal transfer of the group I intron from C. dubliniensis to C. albicans genotype E, or vice versa (Fig. 5). In the present experiment, no group I intron was observed in other Candida species tested such as C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis (data not shown). Therefore, this intron transfer might be possible only between microorganisms of highly related taxons, such as between C. albicans and C. dubliniensis. This observation that the C. albicans group I intron is confined to C. albicans genotypes B, C, and E, including C. dubliniensis, is consistent with the fact that sexual reproduction has yet to be reported for C. albicans (18). In addition, the lack of a group I intron in C. albicans genotype A suggests an active mechanism for the prevention of intron acquisition by this taxon. Therefore, the present results seem to support the usefulness of this intron-based PCR genotyping method for epidemiological and taxonomic studies.
FIG. 5.
Proposed horizontal transfer of group I intron between C. albicans and C. dubliniensis.
The first strains identified as C. dubliniensis were recovered from the oral cavities of Irish HIV-infected individuals (15). However, since then there have been a number of reports on the isolation of C. dubliniensis in laboratories throughout the world, including Europe, North America, and Australia (15, 16). Kamei et al. reported the first case of C. dubliniensis infection in Asia (4). We have reported 1 C. dubliniensis isolate among 100 isolates of C. albicans being maintained in our laboratory (16). We became interested in determining the number of C. dubliniensis isolates among recent clinical isolates of C. albicans in Japan because no previous systematic study had been done to determine the frequency of this yeast over a large geographical area. Herein we have reported on the isolation of five additional strains of C. dubliniensis among the 301 clinical yeast isolates surveyed in the present study, confirming the existence of the taxon in Japan. Interestingly, these C. dubliniensis strains were found to be genetically heterogeneous. When their genetic patterns were analyzed by the RAPD method, these strains of C. dubliniensis could be classified into at least three groups. In addition, we found sequence differences at three positions in the ITS1 region and a 2-bp deletions in the ITS2 region between IFM 49833 (an isolate from Kochi Hospital) and the remaining four C. dubliniensis strains (Fig. 3). To our knowledge, this is the first report of heterogeneity among sequences of the ITS region of C. dubliniensis. The present study also suggests that C. dubliniensis is more widespread in the western part of Japan than in the eastern part because of 30 C. albicans isolates obtained from Kurashiki Hospital and 15 isolates obtained from Kochi Hospital, 2 and 1 isolates, respectively, were identified as C. dubliniensis.
The prevalence of C. dubliniensis in the oral cavities of HIV-infected individuals and AIDS patients has been reported by many researchers (5, 15). However, those investigations have been hampered by the lack of a simple and reliable method capable of unequivocally differentiating C. dubliniensis from C. albicans in a clinical laboratory. Although we have reported on a new PCR primer pair that is useful for differentiating C. dubliniensis from C. albicans (16), the present PCR system is designed to detect genotypes A, B, C, and E and C. dubliniensis (genotype D) and was considered to be more useful for these studies because all C. albicans genotypes, including C. dubliniensis, could be determined by only a single PCR run.
Doonelly et al. reported that 1.8% of their isolates recovered from asymptomatic healthy individuals and 16.5% of their isolates recovered from HIV-infected individuals are C. dubliniensis (2). In a similar study, Odds and Bernaerts reported that approximately 2% of the 2,500 yeast isolates stored in an archival culture collection and originally identified as C. albicans were reclassified as C. dubliniensis (11). The present experimental data that indicate a 1.6% rate of isolation of C. dubliniensis isolates among newly isolated C. albicans isolates in Japan is similar to the rates obtained by other investigators (2, 15) and suggests that C. dubliniensis is distributed all over the world. Moreover, of the five isolates of C. dubliniensis detected in the present study, four were found not to be associated with AIDS, indicating that this fungal infection may not necessarily be associated with AIDS in Japan, although further epidemiological studies are necessary.
We also wanted to confirm whether common phenotypic tests could be used to confirm the species identities of the five strains as C. dubliniensis, i.e., growth at 45°C and colony color on CHROMagar Candida. None of the five isolates grew at 45°C, but reference C. albicans strains including the C. albicans genotype E strains grew well at this temperature. These results confirm that the temperature susceptibility pattern test is useful for the differentiation of C. dubliniensis from C. albicans. In contrast, the dark blue color of colonies on CHROMagar Candida, which has been considered one of the more useful features for the identification of C. dubliniensis, was not confirmed for four of the five isolates. Therefore, differentiation of the two taxa on the basis of colony color on CHROMagar Candida may not be as reliable as was previously thought. We did confirm that the ITS region sequence information is highly useful and reliable for the identification of C. dubliniensis species, particularly for the differentiation of C. dubliniensis from C. albicans.
Mercure et al. (9) reported that there may be some correlation between the presence of a self-splicing intron in the 25S rDNA of C. albicans strains and susceptibility to flucytosine. They also indicated that C. albicans strains that have the group I intron are more susceptible to flucytosine (9). We found that the two C. albicans genotype E strains with the group I intron are more susceptible to flucytosine than other reference C. albicans strains, supporting the hypothesis described above. However, recently, we found that some C. albicans genotype A strains without the group I intron as well as genotype B and C strains with the group I intron are susceptible to flucytosine. Therefore, the contribution of the group I intron to the susceptibilities of Candida spp. to flucytosine may be only one factor, and other factors (18, 19) may play more important roles in the flucytosine susceptibilities of Candida species. Detailed studies are in progress in our laboratory in order to more clearly define the role of the group I intron in antifungal susceptibility or resistance among the various C. albicans genotypes and C. dubliniensis.
ACKNOWLEDGMENT
This work was performed through Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government (to Y.M.).
REFERENCES
- 1.Aoki F H, Imai T, Tanaka R, Mikami Y, Taguchi H, Nishimura N F, Nishimura K, Miyaji M, Schreiber A Z, Branchini M L M. New PCR primer pairs specific to Cryptococcus neoformans serotype A or B prepared on the basis of random amplified polymorphic DNA fingerprint pattern analysis. J Clin Microbiol. 1999;37:315–320. doi: 10.1128/jcm.37.2.315-320.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doonelly S M, Sullivan D J, Shanley D B, Coleman D C. Phylogenetic analysis and rapid identification of Candida dubliniensis based on analysis of ACT1 intron and exon sequences. Microbiology. 1999;145:1871–1882. doi: 10.1099/13500872-145-8-1871. [DOI] [PubMed] [Google Scholar]
- 3.Imai T, Watanabe K, Tamura M, Mikami Y, Tanaka R, Nishimura K, Miyaji M, Poonwan N, Branchini M L. Geographic grouping of Cryptococcus neoformans var. gattii by random amplified polymorphic DNA fingerprint patterns and ITS sequences. Clin Lab. 2000;46:345–354. [PubMed] [Google Scholar]
- 4.Kamei K, McCullough M J, Stevens D A. Initial case of Candida dubliniensis infection from Asia: non-mucosal infection. Med Mycol. 2000;38:81–83. doi: 10.1080/mmy.38.1.81.83. [DOI] [PubMed] [Google Scholar]
- 5.Kirkpatrick W R, Revankar S G, McAtee R K, Lopez-Ribot J L, Fothergill A W, McCarthy D I, Sanche S E, Cantu R A, Rinaldi M G, Patterson T F. Detection of Candida dubliniensis in oropharyngeal samples from human immunodeficiency virus-infected patients in North America by primary CHROMagar Candida screening and susceptibility testing of isolates. J Clin Microbiol. 1998;36:3007–3012. doi: 10.1128/jcm.36.10.3007-3012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtzman C P, Robnett C J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek. 1998;73:331–371. doi: 10.1023/a:1001761008817. [DOI] [PubMed] [Google Scholar]
- 7.Maleszka R, Clark-Walker G D. Yeasts have a four-fold variation in ribosomal DNA copy number. Yeast. 1993;9:53–58. doi: 10.1002/yea.320090107. [DOI] [PubMed] [Google Scholar]
- 8.McCullough M J, Clemons K V, Stevens D A. Molecular and phenotypic characterization of genotypic Candida albicans subgroups and comparison with Candida dubliniensis and Candida stellatoidea. J Clin Microbiol. 1997;37:417–421. doi: 10.1128/jcm.37.2.417-421.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercure S, Montplaisir S, Lemay G. Correlation between the presence of a self-splicing intron in the 25S rDNA of C. albicans and isolate susceptibility to 5-fluorocytosine. Nucleic Acids Res. 1993;21:6020–6027. doi: 10.1093/nar/21.25.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 11.Odds F C, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32:1923–1929. doi: 10.1128/jcm.32.8.1923-1929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Person W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1998;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poonwan N, Imai T, Mekha N, Yazawa K, Mikami Y, Ando A, Nagata Y. Genetic analysis of Histoplasma capsulatum isolates isolated from clinical specimens in Thailand by a PCR-based random amplified polymorphic DNA method. J Clin Microbiol. 1998;36:3073–3076. doi: 10.1128/jcm.36.10.3073-3076.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandhu G S, Cline B C, Stockman L, Roberts G D. Molecular probes for diagnosis of fungal infections. J Clin Microbiol. 1995;33:2913–2919. doi: 10.1128/jcm.33.11.2913-2919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–334. doi: 10.1128/jcm.36.2.329-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura M, Watanabe K, Imai T, Mikami Y, Nishimura K. New PCR primer pairs specific for Candida dubliniensis and detection of the fungi from the Candida albicans clinical isolates. Clin Lab. 2000;46:33–40. [PubMed] [Google Scholar]
- 17.Thompson J D, Higgens D G, Gibbson T D. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties, and weight matrix choice. Nucleic Acids Res. 1994;22:2673–2680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Ahsen U, Schroeder R. Streptomycin inhibits splicing of group I intron by competition with guanosine substrate. Nucleic Acids Res. 1991;19:2261–2265. doi: 10.1093/nar/19.9.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Ahsen U, Davies J, Schroeder R. Antibiotic inhibition of group I intron on ribozyme function. Nature. 1991;353:368–370. doi: 10.1038/353368a0. [DOI] [PubMed] [Google Scholar]
- 20.White T J, Bruns T, Lee S, Taylor J W. Amplication and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M A, Gelfard D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications. San Diego, Calif: Academic Press, Inc.; 1990. pp. 315–322. [Google Scholar]