Abstract
We have carried out epizootiologic surveys at various sites in Japan to investigate wild animals that serve as reservoirs for the agents of human babesiosis in the country. Small mammals comprising six species, Apodemus speciosus, Apodemus argenteus, Clethrionomys rufocanus, Eothenomys smithii, Crocidura dsinezumi, and Sorex unguiculatus, were trapped at various places, including Hokkaido, Chiba, Shiga, Hyogo, Shimane, and Tokushima Prefectures. Animals harboring Babesia microti-like parasites were detected in all six prefectures. Inoculation of their blood samples into hamsters gave rise to a total of 20 parasite isolates; 19 were from A. speciosus, and the other 1 was from C. rufocanus. Sequencing of the parasite small-subunit rRNA gene (rDNA) sequence revealed that 2 of the 20 isolates were classified as Kobe type because their rDNAs were identical to that of the Kobe strain (the strain from the Japanese index case). The other 18 isolates were classified as a new type, designated the Hobetsu type, because they all shared an identical rDNA sequence which differed significantly from both that of Kobe-type isolates and that of northeastern United States B. microti (U.S. type). The parasites with Kobe-, Hobetsu- and U.S.-type rDNAs were phylogenetically closely related to each other but clearly different from each other antigenically. The isolates from rodents were demonstrated to be infective for human erythrocytes by inoculation into SCID mice whose erythrocytes had been replaced with human erythrocytes. The results suggest that a new type of B. microti-like parasite, namely, the Hobetsu type, is the major one which is prevalent among Japanese wild rodents, that A. speciosus serves as a major reservoir for both Kobe- and Hobetsu-type B. microti-like parasites, and that C. rufocanus may also be an additional reservoir on Hokkaido Island.
Babesia microti is an erythroparasitic protozoon frequently seen in small wild rodents. This parasite is the causative agent of human babesiosis (8, 12, 30), an emerging tick-bone zoonosis which has been increasingly recognized in the northeastern and upper midwestern United States, where both Lyme borreliosis (13) and human granulocytic ehrlichiosis (15, 19) concomitantly occur due to sharing of the same tick vector and rodent reservoir. The presence of B. microti in various rodent species has been documented throughout the northern temperate zone of North America (4, 5, 7, 29, 34), Europe (9, 27, 33), and Eurasia (25, 26, 32), but symptomatic human cases have been reported almost exclusively in the United States (12, 30). Although the absence of human cases in Europe is ascribed to the strict preference of the vector ticks for rodents as blood-supplying animals (9, 12, 30), it is not known whether that is also the case in the other regions where B. microti is enzootic but not zoonotic. Further epidemiological studies across countries should therefore be undertaken.
Human babesiosis had not been detected in Japan until very recently, but in 1999 the first symptomatic case of human babesiasis was found at Kobe City in Hyogo Prefecture, Japan (17). The patient proved to be infected by a blood transfusion from an asymptomatic carrier on Awaji Island of Hyogo Prefecture, as virtually identical B. microti-like parasites were isolated from both the patient and the blood donor (23, 35). Another B. microti-like parasite which had the same small-subunit rRNA gene (rDNA) sequence as the Kobe strain (the strain from the index case) was also isolated from a field mouse (Apodemus speciosus) trapped near the donor's residence (35), indicating that babesiosis has already been enzootic and zoonotic around the area. These Japanese strains differed significantly from the B. microti strains in the United States in terms of rDNA sequence and antigenicity.
Nearly two decades ago, Shiota et al. (26) carried out a field survey to examine the prevalence of erythroparasitic protozoa among Japanese wild rodents and found a parasite resembling B. microti in as many as 30.2% of A. speciosus mice captured in Shiga Prefecture, Japan. However, because the Babesia spp. described in their study had not been fully identified and are no longer available, the relationship between those strains and the Kobe strain is not known. Hence, it still remains unanswered as to whether the causative agent for the first Japanese human babesiosis case has been in the country for a long time or may have recently entered the country somehow from another place where babesiosis is endemic.
In the northeastern United States, the roles of Peromyscus leucopus and Ixodes scapularis as the rodent reservoir and the tick vector for human babesiosis, respectively, have been well established (12, 30). In Japan, however, neither the reservoir nor the vector has been investigated thoroughly. The objective of the present study was to conduct epizootiologic surveys at various places in Japan for the detection and isolation of Babesia parasites in small wild rodents. The surveys revealed that a new type of B. microti-like parasites was isolated from many A. speciosus mice trapped at various places in Japan. This new type of parasite was clearly distinguishable from both the Kobe strain and U.S. B. microti, antigenically and genotypically.
MATERIALS AND METHODS
Field collections.
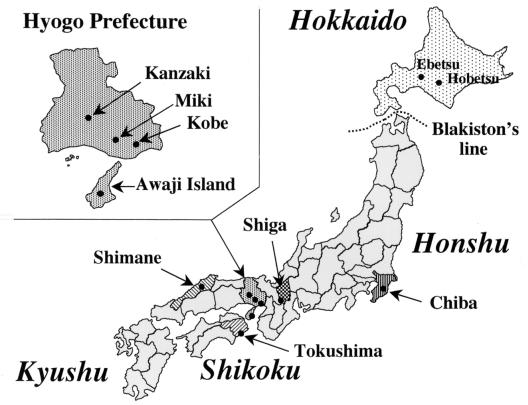
During 1999 and 2000, small wild mammals were trapped at various places in Japan (Fig. 1) using Sherman live traps (H. B. Sherman Traps, Inc., Tallahassee, Fla.). The trapped animals were euthanatized by chilling on ice followed by exposure to carbon dioxide gas. Their blood was immediately collected by heart puncture with heparinized syringes and kept at 4°C. To aid identification, animals were weighed and their total body, tail, and rear foot lengths were measured. Identification of species was done according to the key characteristics described by Abe et al. (1). Blood samples were centrifuged at 1,200 × g for 10 min, and the supernatant fractions were stored as plasma samples. The red blood cells (RBCs) were washed three times in phosphate-buffered saline (PBS) (pH 7.2) and processed for preparation of thin-smear blood films, extraction of genomic DNA, and inoculation into hamsters.
FIG. 1.
Map of Japan showing the locations of field survey points (●).
Experimental animals.
Golden Syrian hamsters and BALB/c mice were purchased from SLC Inc. (Hamamatsu, Japan). NOD/shi-scid mice (11) were maintained in the laboratory animal facility at Rakuno-Gakuen University. The method used to prepare SCID mice with circulating RBCs replaced by human RBCs (designated hu-RBC-SCID mice) has been described elsewhere (20, 23). All hamsters and mice, except those used to prepare immune sera, were splenectomized and used for experiments after the surgical wounds had healed completely. All animals were housed in isolators at temperatures of between 22 and 25°C and were provided with a gamma-irradiated pellet diet and autoclaved tap water. Animal experimentation was carried out according to the Laboratory Animal Control Guidelines at Rakuno-Gakuen University.
Isolation of Babesia parasites.
RBC samples for the field collections were inoculated into splenectomized hamsters for isolation of parasites. Blood samples were collected periodically from the tail veins of the inoculated animals, and Giemsa-stained thin-smear blood films were prepared for microscopic detection of parasitemia. When the level of parasitemia reached 20 to 40%, blood was harvested by cardiocentesis from anesthetized animals, washed in PBS, resuspended in a cell freezing solution (Cell Banker; Nippon Zenyaku Co. Ltd., Kohriyama, Japan), and cryopreserved in liquid nitrogen. The isolates were further propagated by subpassage into new splenectomized hamsters, and their RBCs (parasitemia level, 30 to 50%) were washed in PBS and stored at −80°C without cryopreservatives for subsequent use to prepare parasite DNA and antigens for Western blot analysis. For production of antibodies, hamsters with intact spleens were infected with parasites, and serum samples were collected from the animals when they showed high antibody titers.
Analyses of rDNA sequences.
Genomic DNAs were prepared from the frozen parasitized RBC stocks described above using a whole-blood DNA extraction kit (GenTLE; TaKaRa Biochemicals, Otsu, Japan). Sequences of eukaryotic nuclear small-subunit rDNA were amplified from the DNA samples by PCR with the primer set described by Medlin et al. (18). The specific PCR products, approximately 1.8 kb in size, were cloned and sequenced as described elsewhere (23). Analyses of DNA sequences and phylogenetic relationships were done by using the MacVector software package, version 7.0 (Genetics Computer Group Inc., Madison, Wis.). The rDNA sequences (GenBank accession numbers are given in parentheses) used for phylogenetic analysis were from the Kobe strain (AB032434), Ho226 (AB050732), a Babesia sp. from a Spanish dog (AF188001), B. microti (U09833), Babesia rodhaini (AB049999), Babesia felis (AF244912), Babesia bigemina (X59604), Babesia canis (L19079), Babesia caballi (Z15104), Theileria equi (Babesia equi; Z15105), Theileria parva (L02366), Theileria annulata (M64243), Theileria sergenti (AB016074), and Cryptosporidium parvum (L16996). The sequences were aligned with the program Clustal W Alignment (31), and a phylogenetic tree was constructed by the neighbor-joining method (24) from the aligned sequences with the program Phylogenetic Analysis in the MacVector software. Support for tree nodes was calculated with 1,000 bootstrap replicates by use of the bootstrap tree algorithm. Nested PCR was used to detect Babesia parasites in the blood specimens from the field collections according to previously published protocols (22, 35). DNA samples were prepared with a DNA Extractor WB kit (Wako Pure Chemical Industries, Osaka, Japan); approximately 1/10 of a sample was used for the first round of PCR with the primer set Bab1A-Bab4A, followed by the second round of PCR with 1 μl of the first-round PCR product and the primer set Bab2A-Bab3A (35).
Antigenic analyses.
The indirect immunofluorescent-antibody test (IFAT) was carried out by a method described previously (23). A mixture of fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (IgG) plus IgM and anti-rat IgG plus IgM (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) was used for the detection of antibodies in the animals collected from the fields. Western blot analysis was performed as described previously (2, 35). Frozen stocks of Babesia-infected RBCs were thawed and washed five times at 4°C in 10 mM Tris-HCl–10 mM EDTA (pH 7.5) by centrifugation at 10,000 × g for 10 min. The resulting pellets were dissolved in 125 mM Tris-HCl (pH 6.5) containing 5% β-mercaptoethanol, 2% sodium dodecyl sulfate, 10% glycerol, and 0.1% bromophenol blue, heated at 98°C for 5 min, and vigorously vortexed. The samples were diluted such that each contained material from equivalent numbers of parasitized RBCs and were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by blotting onto Fluorotrans membranes (Pall BioSupport, Port Washington, N.Y.). After blocking was done with PBS containing 0.5% casein, the membranes were reacted with appropriately diluted immune sera and subsequently with secondary antibodies (alkaline phosphatase-conjugated affinity-purified goat anti-mouse IgG heavy and light chains or anti-Syrian hamster IgG heavy and light chains; Jackson ImmunoResearch Laboratories). Immunoreactive antigens were detected with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium–alkaline phosphatase substrate kit IV (Vector Laboratories, Inc., Burlingame, Calif.).
Reference strains of B. microti.
The Gray strain (6) was propagated in hamsters and used as the type strain of B. microti isolated in the northeastern United States. The Gray-Mo strain (16), a mouse-adapted substrain of the Gray strain, was used to produce antibodies in BALB/c mice. The Kobe strain (24) was propagated in hamsters and used as the type strain of the Kobe-type B. microti-like parasite. The Australia strain of B. rodhaini, kindly provided by the National Institute of Animal Health, Tsukuba, Japan, was propagated in NOD/shi-scid mice, and antibodies were generated in BALB/c mice by repeated injections of killed parasites prepared by freezing and thawing parasitized RBCs from infected NOD/shi-scid mice.
Nucleotide sequence accession number.
The rDNA sequences of strain Ho226 (an isolate from the town of Hobetsu in Hakkaido Prefecture) and B. rodhaini have been submitted to DDBJ and have been given accession numbers AB050732 and AB049999, respectively.
RESULTS
Epizootiologic surveys.
Trapping of small wild mammals was attempted at various places in six prefectures in Japan, which included Hokkaido Island, the Chiba, Shiga, Hyogo, and Shimane Prefectures on Honshu Island (the mainland), and Tokushima Prefecture on Shikoku Island (Fig. 1). The results of the field surveys are summarized in Table 1. A total of 112 mammals comprising six species, which included 77 A. speciosus, 20 Apodemus argenteus, 10 Clethrionomys rufocanus, 3 Eothenomys smithii, 1 Crocidura dsinezumi, and 1 Sorex unguiculatus, were obtained. The blood specimens from these animals were processed to prepare thin-smear blood films and DNA samples, which were examined for B. microti-like parasites by microscopy and by rDNA-based PCR, respectively. The results indicate that A. speciosus is the major reservoir and that the parasites are distributed in all six prefectures. Inoculation of the parasite-positive blood samples into splenectomized hamsters resulted in a total of 20 parasite isolates, of which 19 were from A. speciosus and the other 1 was from C. rufocanus. Splenomegaly was often observed in the animals whose blood was parasitemic.
TABLE 1.
Summary of field surveys of B. microti-like parasites among small wild mammals in Japan from 1999 to 2000
| Prefecture | Site | Species | No. of animals trapped | No. positive/no. tested by:
|
No. of animals with the following rDNA genotypee
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Microscopya | PCRb | IFATc | Isolationd | Kobe | Hobetsu | ||||
| Hokkaido | Hobetsu | Apodemus speciosus | 10 | 7/10 | 7/10 | 7/10 | 7/9 | 0 | 7 |
| Apodemus argenteus | 2 | 0/2 | 0/2 | 0/2 | 0/2 | ||||
| Clethrionomys rufocanus | 3 | 0/3 | 1/3 | 2/3 | 1/3 | 0 | 1 | ||
| Ebetsu | Apodemus speciosus | 22 | 0/22 | 0/22 | 0/22 | 0/11 | |||
| Apodemus argenteus | 15 | 0/15 | 0/15 | 0/15 | 0/8 | ||||
| Clethrionomys rufocanus | 7 | 0/7 | 0/7 | 0/7 | 0/4 | ||||
| Sorex unguiculatus | 1 | 0/1 | 0/1 | 0/1 | ND | ||||
| Chiba | Ohtaki | Apodemus speciosus | 2 | 2/2 | 2/2 | 2/2 | 2/2 | 0 | 2 |
| Shiga | Yamanaka | Apodemus speciosus | 1 | 1/1 | 1/1 | 1/1 | 1/1 | 0 | 1 |
| Hyogo | Kobe | Apodemus speciosus | 1 | 0/1 | 0/1 | 1/1 | 0/1 | ||
| Miki | Apodemus speciosus | 1 | 0/1 | 0/1 | 0/1 | ND | |||
| Kanzaki | Apodemus speciosus | 8 | 0/8 | 0/8 | 0/8 | ND | |||
| Apodemus argenteus | 1 | 0/1 | 0/1 | 0/1 | ND | ||||
| Awaji | Apodemus speciosus | 7f | 4/7 | 4/7 | 4/7 | 4/7 | 2 | 2 | |
| Crocidura dsinezumi | 1 | 0/1 | 0/1 | 0/1 | 0/1 | ||||
| Shimane | Daito | Apodemus speciosus | 14 | 4/14 | 7/14 | 4/8 | 3/4 | 0 | 3 |
| Apodemus argenteus | 2 | 0/2 | 0/2 | 0/2 | ND | ||||
| Eothenomys smithii | 3 | 0/3 | 0/3 | 0/3 | ND | ||||
| Tokushima | Anan | Apodemus speciosus | 11 | 1/11 | 2/11 | 3/11 | 2/3 | 0 | 2 |
| Total | 112 | 19/112 | 24/112 | 24/106 | 20/56 | 2 | 18 | ||
Detection of parasitized RBCs by microscopy of thin-smear blood films.
Detection of babesial rDNA by nested PCR.
IFAT titers that were higher than 1:200 against either strain Kobe or strain Ho234 were taken as positive.
Isolation of B. microti-like parasites by inoculation of blood specimens into splenectomized hamsters. ND, not done.
Determined by sequencing of the rDNA amplified from the isolated parasites.
Including two A. speciosus mice included in a previous study (35).
Genotypic and phylogenetic analyses.
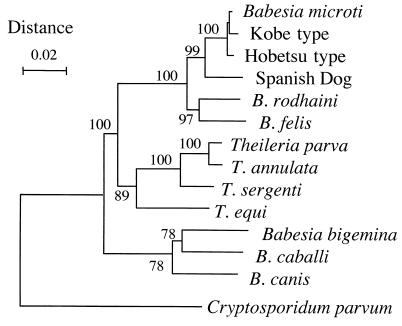
Sequencing of the rDNA amplified from each parasite isolate revealed that 2 out of the 20 isolates had rDNA sequences which were identical to that of the Kobe strain (GenBank accession no. AB032434). The two isolates, designated strains Aw1 and Aw7, were both obtained from A. speciosus mice trapped on Awaji Island of Hyogo Prefecture and were classified as Kobe type. The other 18 isolates had identical rDNA sequences (GenBank accession no. AB050732 for strain Ho226 as a representative) and were classified as Hobetsu type. This type of parasite was closely related to both the Kobe type and the U.S. type (B. microti isolated in the United States), as indicated by the phylogenetic tree shown in Fig. 2, but there were substantial differences in the rDNA sequences (Table 2). A parasite with the U.S.-type rDNA sequence was not found in the present survey.
FIG. 2.
Phylogenetic tree constructed by the neighbor-joining method with rDNA sequences of various apicomplexan parasites. A portion corresponding to bases 22 to 1715 of the rDNA sequence of strain Ho226 (GenBank accession no. AB050732) was included for analysis. The number on each branch shows the percent occurrence in 1,000 bootstrap replicates.
TABLE 2.
Differences and identities among rDNA sequences of four closely related Babesia parasites
| Parasite (type) | Size (bp)a | No. of differences or % identityb for:
|
|||
|---|---|---|---|---|---|
| Aw1 | Ho234 | Gray | B. rodhaini | ||
| Aw1 (Kobe) | 1,768 | 99.4 | 99.2 | 96.0 | |
| Ho234 (Hobetsu) | 1,764 | 22 | 99.3 | 96.1 | |
| Gray (U.S.) | 1,763 | 15 | 15 | 96.1 | |
| B. rodhaini | 1,751 | 84 | 81 | 78 | |
Size of the rDNA sequence amplified by PCR.
The numbers of differences, including both nucleotide differences and gaps inserted for alignment, are shown at the lower left; the percent identity values are shown at the upper right.
Serological analysis.
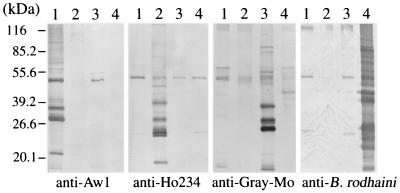
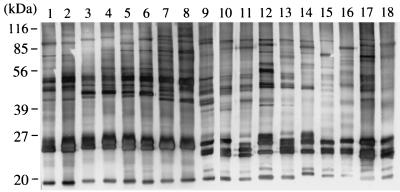
Plasma samples from the field mice were examined by IFAT for the detection of specific antibodies against the Kobe- and Hobetsu-type parasites. Antibody-positive samples were generally in good agreement with those found positive by PCR and parasite isolation (Table 1). To examine antigenic cross-reactivities among Kobe-, Hobetsu-, and U.S.-type parasites and B. rodhaini (another rodent Babesia species closely related to B. microti), we performed pairwise comparisons with IFATs (Table 3) and Western blot analyses (Fig. 3). High IFAT titers and strongly reacting bands in Western blots were observed against only the homologous antisera, although a weakly cross-reactive band with an apparent molecular mass of 50 kDa was seen in all four Babesia parasites examined, indicative of their minimal antigenic relatedness. To examine intragenotypic variations, 18 Hobetsu-type parasites that were isolated from various places in Japan were also compared in Western blot analyses (Fig. 4). All 18 parasites reacted against an immune serum raised against a Hobetsu-type isolate (strain Ho234) with approximately equal intensities and displayed similar banding patterns; however, minor variations were seen in the numbers, sizes, and intensities of the bands with all the strains, demonstrating microheterogeneity within the parasites belonging to the same rDNA genotype. The degree of heterogeneity between isolates, however, appeared in parallel with their geographic distance, as nearly identical banding patterns were seen with isolates obtained from the same survey site. In IFATs, on the other hand, nearly comparable antibody titers were obtained with a given immune serum, regardless of any Hobetsu-type isolate being used as an antigen; for example, the antibody titer determined with strain Ho234 and that determined with strain Ya501 usually varied within a fourfold dilution. Therefore, the influence of intragenotypic heterogeneities on serodiagnosis by IFATs appeared to be only minimal.
TABLE 3.
Results of IFATs with four closely related Babesia parasites
| Seraa | Reciprocal titer against the following parasite (type):
|
|||
|---|---|---|---|---|
| Aw1 (Kobe) | Ho234 (Hobetsu) | Gray (U.S.) | B. rodhaini | |
| Anti-Aw1 | 6,400 | <25 | <25 | <25 |
| Anti-Ho234 | <25 | 12,800 | <25 | <25 |
| Anti-Gray-Mo | 100 | 200 | 6,400 | 200 |
| Anti-B. rodhaini | 50 | 50 | 200 | 51,200 |
Convalescent-phase sera from infected hamsters (anti-Aw1 and anti-Ho234) or mice (anti-Gray-Mo and anti-B. rodhaini).
FIG. 3.
Western blot analyses of four closely related Babesia parasites. Lanes 1 through 4 contained strains Aw1 (Kobe type), Ho234 (Hobetsu type), and Gray (U.S. type) and B. rodhaini, respectively. Parasite antigens were probed with convalescent-phase sera from hamsters (anti-Aw1 and anti-Ho234) or mice (anti-Gray-Mo and anti-B. rodhaini) that were infected with each parasite.
FIG. 4.
Western blot analyses of Hobetsu-type B. microti-like parasites isolated from various places in Japan. The respective parasite strains analyzed were Ho226, Ho232, Ho233, Ho234, Ho235, Ho236, Ho237, and Ho240 from Hobetsu in Hokkaido Prefecture (lanes 1 to 8); Ya501 from Yamanaka in Shiga Prefecture (lane 9); An2 and An3 from Anan in Tokushima Prefecture (lanes 10 and 11); Da111, Da112, and Da116 from Daito in Shimane Prefecture (lanes 12 to 14); Ot1 and Ot2 from Ohtaki in Chiba Prefecture (lanes 15 and 16); and Aw3 and Aw6 from Awaji in Hyogo Prefecture (lanes 17 and 18). Parasite antigens were probed with a convalescent-phase serum from a hamster that was infected with strain Ho234.
Morphology of piroplasms.
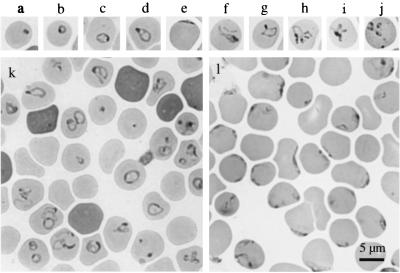
Intraerythrocytic parasites in the infected hamsters displayed various morphologies (Fig. 5 a through j), including dot form, ring form, ovoid form, pyriform, pleiomorphic ameboid forms near the center of the erythrocytes, or crescent arch forms on the margin of the erythrocytes. Parasites mostly occurred singly within an erythrocyte, but paired forms and Maltese cross (tetrad) forms were also seen occasionally. Multiply infected erythrocytes were often observed when parasitemia levels increased. In comparisons between Kobe- and Hobetsu-type isolates, the Kobe type seemed slightly larger and displayed the ring form, ovoid form, and pyriform more frequently than the Hobetsu type (Fig. 5k), whereas the Hobetsu type displayed the marginal crescent arch form more frequently than the Kobe type (Fig. 5l). However, these dissimilarities were not clear enough to be used as morphological criteria to distinguish between the two types of Japanese B. microti-like parasites or between the Japanese and the U.S. parasites, because the morphology varied significantly depending on the level of parasitemia, parasite strains, and conditions of the hosts (species of animal, splenectomized or not, acute phase or convalescent phase, and so forth).
FIG. 5.
Photomicrograph of Giemsa-stained thin-smear blood films showing various forms of piroplasms in hamster erythrocytes. (Upper Panels) a, dot form; b, ring form; c, ovoid form; d, pyriform; e, crescent arch form; f to i, ameboid forms; j, Maltese cross form. (Lower Panels) Selected microscopic views of strains Aw7 (k) and Ho234 (l), emphasizing the difference between Kobe- and Hobetsu-type parasites, respectively.
Infectivity for human RBCs.
To test whether or not parasites isolated from rodent reservoirs are capable of infecting human RBCs, parasite isolates were inoculated into hu-RBC-SCID mice. It has already been shown (35) that strain Aw1, a Kobe-type isolate, was propagated readily in human RBCs in hu-RBC-SCID mice. When two Hobetsu-type isolates, strains Ya501 and Ho234, were tested, the former strain proliferated readily in hu-RBC-SCID mice, whereas the latter strain grew only very poorly. Strain Ho234, however, showed increased infectivity for human RBCs during the process of adaptation, which was achieved by successive passages in hu-RBC-SCID mice for 3 months (data not shown).
DISCUSSION
The present survey demonstrated that there are two types of B. microti-like parasites in Japan, designated the Kobe and Hobetsu types, and that the Hobetsu type is the major parasite which is widely distributed throughout the country, including Hokkaido, Honshu, and Shikoku Islands. An interesting finding was that a Hobetsu-type parasite was isolated from a field mouse, A. speciosus, which was captured in the town of Yamanaka in Shiga Prefecture. The captured site was within the area where Shiota et al. (26) had first detected B. microti-like parasites in A. speciosus nearly two decades ago, indicating that the original parasite described in their study, which is no longer available, probably was of this type as well. In order to make this determination, we attempted to amplify the babesial rDNA sequence from the blood smear slides which Shiota et al. had prepared from infected animals two decades ago (26), but no successful result was obtained.
A phylogenetic tree based on rDNA sequences indicates that both the Hobetsu and the Kobe types are closely related to B. microti (sensu stricto) from the northeastern United States (designated U.S. type in the present study). Differences were seen at 15 to 22 positions in the aligned rDNA sequences of Kobe-, Hobetsu-, and U.S.-type parasites. Even though these numbers are only 0.85 to 1.25% of the total length sequenced, the differences seem to be significant, when taking into account high sequence conservation within a certain rDNA genotype. Virtually no sequence variation was seen in the rDNAs of U.S.-type B. microti isolated in the northeastern and upper midwestern United States, regardless of whether the isolates were from rodent reservoirs or human patients (S. R. Telford, personal communication). Only two nucleotide differences have been reported between B. microti in the United States and that in Europe (37). Likewise, there was no sequence variation in the rDNAs of the 18 Hobetsu-type isolates from various places in Japan.
In the literature, many erythroparasitic protozoa found in various rodent species have been referred to as B. microti (sensu lato), primarily on the basis of their morphology and the host species (5, 9, 14, 27, 33, 34). However, recent advancement in phylogenetic analyses (3, 8, 23, 37) is making it increasingly clear that this “species” may be a complex including closely related, yet significantly heterogeneous, parasites. Whether this heterogeneity should be taken as evidence for dividing it into different species (or subspecies) or simply regarded as diversity within a single species remains to be seen.
In contrast to Hobetsu-type parasites, Kobe-type parasites were found only on Awaji Island of Hyogo Prefecture. It was recently reported (24) that the first symptomatic case of transfusion-acquired human babesiosis due to the Kobe-type parasite occurred at Kobe City, which is near Awaji Island but is located on the mainland of Hyogo Prefecture (Fig. 1); the transfusion donor was proven to be an asymptomatic carrier (35) who was a resident of Awaji Island. In the present survey, all the rodents captured at several places on the mainland of Hyogo Prefecture, including Kobe City, were negative for Babesia infection. On Awaji Island, in contrast, four of the seven trapped animals were found to be parasitemic, and both Kobe- and Hobetsu-type parasites were isolated from them. Based on two lines of circumstantial evidence, that the Kobe-type parasite was probably not the one which had been detected by Shiota et al.(26) two decades ago and that this type appeared only on Awaji Island, we speculate that the Kobe-type parasite may have somehow emerged in the place very recently and may currently be expanding its geographic distribution. If so, this expansion may have resulted from a recent change in the landscape: an express highway with two bridges that connect Honshu, Awaji, and Shikoku Islands was recently built across Awaji Island. Therefore, careful monitoring is needed to determine whether or not the Japanese index case of 1999 represents the beginning of a newly emerging disease due to the range expansion of an insular strain.
The vast majority of B. microti-like parasites were isolated from A. speciosus, which is a Muridae species unique to Japan and one of the most abundant field mice distributed throughout the country, as it was trapped the most at all the places in the present survey. This finding was consistent with that previously reported by Shiota et al. (26), collectively indicating that A. speciosus serves as the major reservoir for human babesiosis in Japan. This field mouse is also known as a host for both Borrelia afzelii (21), the agent of Lyme disease in Japan, and Ehrlichia muris (10), which is analogous to P. leucopus in the United States, serving as a reservoir for three emerging zoonoses of public heath concern: Lyme disease, human babesiosis, and human granulocytic ehrlichiosis (28). In addition to A. speciosus, C. rufocanus was found to carry a Hobetsu-type parasite. Although we were able to obtain only a single isolate from C. rufocanus, this vole may also serve as a reservoir on Hokkaido Island, as supportive evidence is being obtained in an ongoing field survey on Hokkaido Island (M. Tsuji and C. Morita, unpublished data). This rodent species is known to exist on Hokkaido Island, but not in the other parts of Japan, owing to the presence of a zoogeographical border called Blakiston's line (Fig. 1). Besides being present on Hokkaido Island, C. rufocanus is widely distributed in northern Far East Asia, including Siberia and Sakhalin in Russia and northeastern China. It will be interesting to investigate Babesia parasites carried by the rodents in those regions, because such study may provide a new insight into the origin of the Japanese B. microti-like parasites.
Using the hu-RBC-SCID mouse model (23), we were able to demonstrate that the isolates from wild rodents, both Kobe and Hobetsu types, are potentially capable of infecting humans. Our results, however, also demonstrated that the degree of this capability may vary substantially. Strains Ya501 and Aw1, Hobetsu type and Kobe type, respectively, were both readily infective for human RBCs, whereas strain Ho234, another Hobetsu-type strain, was only very poorly infective; however, infectivity for human RBCs could be enhanced by adaptation. An analogous finding was also obtained with the Gray strain (6), which is the B. microti strain isolated from the U.S. index case patient by hamster inoculation. It was recently reported that this strain was propagated in hu-RBC-SCID mice only very poorly (23); more recently, however, this strain was demonstrated to regain a higher degree of infectivity for human RBCs after 6 weeks of maintenance in hu-RBC-SCID mice (M. Tsuji, unpublished data).
In the present survey, we have not been able to find a parasite with the U.S.-type rDNA sequence. This apparent absence of U.S.-type B. microti in Japan may be relevant to the seemingly rare occurrence of symptomatic human babesiosis cases in Japan, in contrast to the relatively frequent clinical case reports from areas of endemicity in the United States (36). It would be an interesting hypothesis that B. microti in the United States may be more pathogenic and virulent for humans than the Japanese B. microti-like parasites. Antigenically, the Kobe-, Hobetsu-, and U.S.-type parasites were poorly cross-reactive with each other. Since IFATs with all three types of parasite antigens are now available, we are currently conducting seroepidemiological surveys of humans to estimate the prevalence of each type of Babesia infection among Japanese.
ACKNOWLEDGMENTS
We thank S. R. Telford III, Harvard School of Public Health, for critical review and helpful discussion of the manuscript. We also thank F. Mahara for kindly providing accommodations for the field survey in Anan City, Tokushima, Japan. We thank H. Murayama, Y. Iwabu, and Y. Saito, Rakuno-Gakuen University, for excellent technical assistance, and I. Kaiho, Public Health Laboratory of Chiba Prefecture, for collection of wild rodents.
This work was supported in part by a grant-in-aid from the Ministry of Education, Science and Culture of Japan (no. 12450139) and by Gakujutsu-Frontier Cooperative Research at Rakuno-Gakuen University.
REFERENCES
- 1.Abe H, Ishii N, Kaneko Y, Maeda K, Miura S, Yoneda M. A pictorial guide to the mammals of Japan. Tokyo, Japan: Tokai University Press; 1994. . (In Japanese.) [Google Scholar]
- 2.Arai S, Tsuji M, Kim S-J, Nakada K, Kirisawa R, Ohta M, Ishihara C. Antigenic and genetic diversities of Babesia ovata in persistently infected cattle. J Vet Med Sci. 1998;60:1321–1327. doi: 10.1292/jvms.60.1321. [DOI] [PubMed] [Google Scholar]
- 3.Bronsdon M A, Homer M J, Magera J M, Harrison C, Andrews R G, Bielitzki J T, Emerson C L, Persing D H, Fritsche T R. Detection of enzootic babesiosis in baboons (Papio cynocephalus) and phylogenetic evidence supporting synonymy of the genera Entopolypoides and Babesia. J Clin Microbiol. 1999;37:1548–1553. doi: 10.1128/jcm.37.5.1548-1553.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkot T R, Schneider B S, Pieniazek N J, Happ C M, Rutherford J S, Slemenda S B, Hoffmeister E, Maupin G O, Zeidner N S. Babesia microti and Borrelia bissetti transmission by Ixodes spinipalpis ticks among prairie voles, Microtus ochrogaster, in Colorado. Parasitology. 2000;121:595–599. [PubMed] [Google Scholar]
- 5.Fay F H, Rausch R L. Parasitic organisms in the blood of arvicoline rodents in Alaska. J Parasitol. 1969;55:1258–1265. [Google Scholar]
- 6.Gleason N N, Healy G R, Western K A, Benson G D, Schultz M G. The “Gray” strain of Babesia microti from a human case established in laboratory animals. J Parasitol. 1970;56:1256–1257. [PubMed] [Google Scholar]
- 7.Healy G R, Spielman A, Gleason N. Human babesiosis: reservoir of infection on Nantucket Island. Science. 1976;192:479–480. doi: 10.1126/science.769166. [DOI] [PubMed] [Google Scholar]
- 8.Homer M J, Aguilar-Delfin I, Telford III S R, Krause P J, Persing D H. Babesiosis. Clin Microbiol Rev. 2000;13:451–469. doi: 10.1128/cmr.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussein H S. Ixodes trianguliceps: seasonal abundance and role in the epidemiology of Babesia microti infection in northwestern England. Ann Trop Med Parasitol. 1980;74:531–539. doi: 10.1080/00034983.1980.11687381. [DOI] [PubMed] [Google Scholar]
- 10.Kawahara M, Ito T, Suto C, Shibata S, Rikihisa Y, Hata K, Hirai K. Comparison of Ehrlichia muris strains isolated from wild mice and ticks and serologic survey of humans and animals with E. muris as antigen. J Clin Microbiol. 1999;37:1123–1129. doi: 10.1128/jcm.37.4.1123-1129.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyanagi Y, Tanaka Y, Kira J I, Ito M, Hioki K, Misawa N, Kawano Y, Yamasaki K, Tanaka R, Suzuki Y, Ueyama Y, Terada E, Tanaka T, Miyasaka M, Kobayashi T, Kumazawa Y, Yamanoto N. Primary human immunodeficiency virus type 1 viremia and central nervous system invasion in a novel hu-PBL-immunodeficient mouse strain. J Virol. 1997;71:2417–2424. doi: 10.1128/jvi.71.3.2417-2424.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krause P J, Telford S R., III . Babesiosis. In: Gilles H M, editor. Protozoal diseases. London, England: Arnold; 1999. pp. 236–248. [Google Scholar]
- 13.Krause P J, Telford S R, III, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing D H. Concurrent Lyme disease and babesiosis: evidence for increased severity and duration of illness. JAMA. 1996;275:1657–1660. [PubMed] [Google Scholar]
- 14.Levine N D. The protozoan phylum Apicomplexa. Vol. 2. Boca Raton, Fla: CRC Press, Inc.; 1988. [Google Scholar]
- 15.Magnarelli L A, Dumler J S, Anderson J F, Johnson R C, Fikrig E. Coexistence of antibodies to tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in human sera. J Clin Microbiol. 1995;33:3054–3057. doi: 10.1128/jcm.33.11.3054-3057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsubara J, Koura M, Kamiyama T. Infection of immunodeficient mice with a mouse-adapted substrain of the Gray strain of Babesia microti. J Parasitol. 1993;79:783–786. [PubMed] [Google Scholar]
- 17.Matsui T, Inoue R, Kajimoto K, Tamekane A, Katayama Y, Shimoyama M, Chihara K, Saito-Ito A, Tsuji M. First documentation of human babesiosis in Japan. Jpn J Clin Hematol. 2000;41:628–634. . (In Japanese with an English summary.) [PubMed] [Google Scholar]
- 18.Medlin L, Elwood H J, Stickel S, Sogin M L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell P D, Reed K D, Hofkes J M. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J Clin Microbiol. 1996;34:724–727. doi: 10.1128/jcm.34.3.724-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura Y, Tsuji M, Arai S, Ishihara C. A method for rapid and complete substitution of the circulating erythrocytes in SCID mice with bovine erythrocytes and use of the substituted mice for bovine hemoprotozoa infections. J Immunol Methods. 1995;188:247–254. doi: 10.1016/0022-1759(95)00222-7. [DOI] [PubMed] [Google Scholar]
- 21.Nakao M, Miyamoto K. Mixed infection of different Borrelia species among Apodemus speciosus mice in Hokkaido, Japan. J Clin Microbiol. 1995;33:490–492. doi: 10.1128/jcm.33.2.490-492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persing D H, Mathiesen D, Marshall W F, Telford S R, Spielman A, Thomford J W, Conrad P A. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito-Ito A, Tsuji M, Wei Q, He S, Matsui T, Kohsaki M, Arai S, Kamiyama T, Hioki K, Ishihara C. Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J Clin Microbiol. 2000;38:4511–4516. doi: 10.1128/jcm.38.12.4511-4516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 25.Shih C-M, Liu L-P, Chung W-C, Ong S J, Wan C-C. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol. 1997;35:450–454. doi: 10.1128/jcm.35.2.450-454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiota T, Kurimoto H, Haguma N, Yoshida Y. Studies on Babesia first found in murine in Japan: epidemiology, morphology and experimental infection. Zentbl Bakteriol Mikrobiol Hyg Reihe A. 1984;256:347–355. [PubMed] [Google Scholar]
- 27.Shortt H E, Blachie E S. An account of the genus Babesia as found in certain small mammals in Britain. J Trop Med Hyg. 1965;68:37–42. [PubMed] [Google Scholar]
- 28.Stafford K C, III, Massung R F, Magnarelli L A, Ijdo J W, Anderson J F. Infection with agents of human granulocytic ehrlichiosis, Lyme disease, and babesiosis in wild white-footed mice (Peromyscus leucopus) in Connecticut. J Clin Microbiol. 1999;37:2887–2892. doi: 10.1128/jcm.37.9.2887-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steketee R W, Eckman M R, Burgess E C, Kuritsky J N, Dickerson J, Schell W L, Godsey M S, Jr, Davis J P. Babesiosis in Wisconsin: a new focus of disease transmission. JAMA. 1985;53:2675–2678. doi: 10.1001/jama.253.18.2675. [DOI] [PubMed] [Google Scholar]
- 30.Telford S R, III, Gorenflot A, Brasseur P, Spielman A. Babesial infections in humans and wildlife. In: Kreier J P, editor. Parasitic protozoa. 2nd ed. Vol. 5. New York, N.Y: Academic Press, Inc.; 1993. pp. 1–47. [Google Scholar]
- 31.Thompson J D, Higgins D G, Gibson T J. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Peen P F D, Chang S J, Banknieder A R, Santana F J. Piroplasms from Taiwanese rodents. J Protozool. 1977;24:310–312. doi: 10.1111/j.1550-7408.1977.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 33.Walter G. Babesia microti (strain “Hannover I”): transmission by Ixodes ricinus and course of parasitemia in the bank vole (Clethrionomys glareolus) and the field vole (Microtus agrestis) Acta Trop. 1984;41:259–264. [PubMed] [Google Scholar]
- 34.Watkins R A, Moshier S E, O'Dell W D, Pinter A J. Splenomegaly and reticulocytosis caused by Babesia microti infections in natural populations of the montane vole, Microtus montanus. J Protozool. 1991;38:573–576. doi: 10.1111/j.1550-7408.1991.tb06082.x. [DOI] [PubMed] [Google Scholar]
- 35.Wei Q, Tsuji M, Zamoto A, Kohsaki M, Matsui T, Shiota T, Teleford III S R, Ishihara C. Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J Clin Microbiol. 2001;39:2178–2183. doi: 10.1128/JCM.39.6.2178-2183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White D J, Talarico J, Chang H G, Birkhead G S, Heimberger T, Morse D L. Human babesiosis in New York State: review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med. 1998;158:2149–2154. doi: 10.1001/archinte.158.19.2149. [DOI] [PubMed] [Google Scholar]
- 37.Zahler M, Rinder H, Gothe R. Genotypic status of Babesia microti within the piroplasms. Parasitol Res. 2000;86:642–646. doi: 10.1007/pl00008545. [DOI] [PubMed] [Google Scholar]