Abstract
A large amount of disposable plastic face masks (DPFs) is produced and used during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, which results in an inevitable consequence of the dramatic increase of DPFs waste. However, the impact of DPFs exposure to the environment on their toxicity is rarely considered. In this study, a range of 76–276 items/L microplastics (MPs) was detected in the DPFs leachates, and fibrous ( 80.3%) and polypropylene (PP, 89.2%) MPs were dominant. Co, Cu, Ni, Sr, Ti and Zn, were commonly detected in all leachates of the tested DPFs. Organics, such as acetophenone, 2,4-Di-tert-butylphenol, benzothiazole, bisphenol-A and phthalide, were found in the DPFs leachate, which were including organic solvents and plasticizer. Besides, we first found an emerging environmental risk substance, namely environmentally persistent free radicals (EPFRs), was generated in the DPFs leachates. The characteristic g-factors of the EPFRs was in a range of 2.003–2.004, identified as mixture of carbon- and oxygen-centered radicals. By means of in vitro toxicity assay, the DPFs leachate were confirmed to cause cytotoxicity and oxidative stress. Significantly, it is found that the formed EPFRs could contribute more toxic effects. Furthermore, when compared to N95 respirators, the tested surgical masks tend to release more MPs, leach more metals and organics, and generate more EPFRs. Surgical masks were thus showed higher risk than N95 respirators after exposure to water. This work highlights the importance of understanding the chemical complexity and possible toxicity of DPFs for their risk assessment.
Keywords: COVID-19 pandemic, Plastic face masks (DPFs), Leachate, Microplastics (MPs), Heavy metals, Health risk, Environmentally persistent free radicals (EPFRs)
Graphical abstract
1. Introduction
As the spread of a pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the worldwide, in order to avoid the human to human nature of transmission of coronavirus, face mask is recommended to wear for protecting from the viral infection. As the World Health Organization (WHO) projected, a monthly global demand of disposable plastic face masks (DPFs) is of nearly 90 million in the current pandemic (WHO, 2020). A skyrocket global demand of face masks wearing leads to an unprecedented increase of face masks production. It is seen that this global health crisis causes an extra problem that an unusual rise of DPFs waste (Adyel, 2020).
To date, DPF has been observed in various receiving waters around the world. Recent studies have evidenced the occurrence of DPFs on beaches and coasts of South America and in the lakes and rivers of Africa and Europe ( Akhbarizadeh et al., 2021; Aragaw, 2020; Ardusso et al., 2021; De-la-Torre et al., 2021; Kutralam-Muniasamy and Shruti, 2022; Okuku et al., 2021; Prata et al., 2020; Kalina and Tilley, 2020). It is speculated that more than 1 billion face masks have entered the oceans and around 3.5 million metric tons of masks have been landfilled worldwide in the first year of the Covid-19 pandemic (COVID-19 Facemasks & Marine Plastic Pollution, 2020). The rise of DPFs exposure to the aquatic environment may cause severe detrimental effects on the ecological environment. As such, more exploration of the environmental impacts and the potential human health risk caused by DPFs entering the environment is, therefore, necessary given the concern regarding the increase of DPFs waste in the SARS-CoV-2 pandemic.
DPFs are commonly made from polymeric materials, such as polypropylene (PP), polyethylene (PE) or polycarbonate (PC) (Fadare and Okoffo, 2020; Potluri and Needham, 2005). When the DPFs waste is subjected to light irradiation, mechanical abrasion, oxidation or biodegradation after exposing to the environment, it can be a potential source of microplastics (MPs) (Ma et al., 2021). It is estimated that around 2.3 × 1021 particles MPs are released to the environment by leachates of the landfilled DPFs in 2020 (Patrício Silva et al., 2021). With increasing production and consumption of DPFs in the SARS-CoV-2 pandemic, an increased amount of MPs could be released from the DPFs waste to both the terrestrial and aquatic environment (Kutralam-Muniasamy et al., 2022). Furthermore, DPFs are complex mixtures which consist of more than one polymer and various additives, or other presented chemicals (including nonintentional added substances (NIAS, impurities and side or breakdown products)) (Zimmermann et al., 2019). Metals and organic contaminants is possibly leached from DPFs waste and then have an adverse effect on the organisms (Sullivan et al., 2021). In general, the current studies on the impacts of the DPFs waste after exposing to the environment mainly focus on MPs releasing and chemicals leaching (Wang et al., 2021; Saliu et al., 2021; Sullivan et al., 2021).
Besides, a recent study has reported that an emerging environmental risk substance, namely environmentally persistent free radical (EPFR), can be formed during the plastic aging (Zhu et al., 2019), which has also been demonstrated to exhibit toxicity to organisms (Pan et al., 2019; Zhao et al., 2019). For example, the generated EPFRs can induce the formation of reactive oxygen species (ROS) and overloaded ROS may induce oxidative stress, further causing cardiopulmonary dysfunction and chronic respiratory diseases (Balakrishna et al., 2009; Lucas and Maes, 2013). Therefore, for a comprehensive assessment of the ecological impact caused by MPs releasing and chemicals leaching after plastic products exposing to the environment, the generation of EPFRs and its toxic effects should be taken into the consideration. However, limited work has been conducted to assess the generation of EPFRs, and thus critical information is missing for the evaluation of environmental impact of the DPFs waste associated with EPFRs.
As mentioned above, it is hypothesized that EPFRs generation possibly occurs in the process of the DPFs waste exposure to the environment and then, besides organics, metals and MPs, the generated EPFRs also contribute a toxic effect on organisms and human. To testify the hypothesis, we characterized the chemicals leaching and MPs releasing, as well as EFPR generation from different kinds of DPFs (e.g., surgical masks and N95 respirators) exposing to water. The main objectives of this study are to verify the generation of EPFRs after the different kinds of the DPFs exposing to the aquatic environment, and assess the toxicity of the DPFs leachate by in vitro bioassays, including cytotoxicity and intracellular ROS levels. The results of this study would bring to light the contributions of generated EPFRs to the toxicity of DPFs exposure to the aquatic environment and the health risk induced by DPFs waste.
2. Materials and methods
2.1. Tests materials and DPFs leachate preparation
According to the recommendations from WHO, surgical masks and respirators without exhalation valves were suggested to use to suppress Coronavirus disease transmission (WHO, 2020.). Therefore, DPFs, including surgical masks and respirators without exhalation valve (e.g., N95 respirators), were used in this study, rather than other types of masks, such as cloth masks or respirators with exhalation valve. DPFs were purchased from different manufacturers and suppliers (SI, Table S1). DPF leachate was prepared by soaking two pieces of DPFs (from same brand) into 1 L deionized water which was of MilliPore® MilliQ quality for different time (i.e., 5 d, 10 d, and 15 d). The mixtures were slowly agitated in rotating incubators (120 rpm) at room temperature (∼25 °C) during preparing DPF leachate to ensure that DPFs could always be in full contact with water. Rotating incubators were kept covering with aluminum foil and without external light source (under natural indoor light) during preparing leachates. DPFs leachates were prepared for tests in triplicates.
Three sets of DPFs leachates were tested for evaluating their potential impacts after exposing to the aquatic environment. Each of all sets contained 8 separate batches with using 8 different DPFs leachates (6 surgical masks and 2 N95 respirators) which were prepared by soaking for the same days under the same conditions. The prepared leachates were used for identifying the organic and inorganic components, counting and characterizing MPs, and detecting and qualifying EPFRs, respectively. Each test for characterizing DPFs leachates was in triplicates. In addition, the pH of leachate in the incubator was measured and recorded as well (SI, Table S2).
2.2. DPF leachate characterization and quantification
2.2.1. Counting and characterization of microplastics in DPF leachate
After removing bulk soaked DPFs, the leachates for characterizing MPs release were stored in rotating incubators with covering aluminum foil. Before the next step MPs counting and characterization, the leachates were processed MPs extraction (SI, section S1) (Wang et al., 2020a). After extractions, MPs were collected from solution to the surface of the filters and then, examined with stereomicroscopes (augmentations between ×14 and x70, SZM and SDZ-PL, Kyowa, Japan) and scanning electronic microscopy (SEM, Hitachi, Japan) to determined MPs’ size, color, morphology and types (SI, section S2). In this study, only MPs with a diameter of less than 5 mm were measured by visible observation. The identification of polymers was analysis with using Fourier Transform Infrared Spectroscopy (FTIR) with the Attenuated Total Reflectance (ATR) accessory (Thermo Nicolet FTIR, Nexus) (Wang et al., 2020b). Throughout the study, several laboratory quality control measures were implemented to circumvent the contamination of samples from plastic material and the environment and more information has been provided in the Section S3 in the supplement.
2.2.2. Organics and mental analysis in DPFs leachates
Organics in the leachates was analyzed by gas chromatography-mass spectrometry (GC-MS, an Agilent 6890 GC Series coupled to a Hewlett Packard 5973 mass selective detector) using non-target and target screening method (SI, section S4 and Tables S3–S4) after leachates extraction. More details on leachate extraction for GC-MS analysis has been shown in the Supporting Information (SI, section S5). NIST library was used for organics qualitative analysis by extracting best hits.
For the analysis of trace metals in the DPFs leachate, a Thermo Scientific ICAP 7200 Inductive Coupled Plasma-Optical Emission Spectrometry (ICP-OES) analyzer was used. External calibration was performed for the quantification of trace metals. More details on methods and quality control for ICP-OES analysis can be found in the appendix (SI, section S6 and Table S5).
The control samples were set without adding DPFs for comparing with the leachate samples, which were also prepared with deionized water. The control samples were subjected to a non-target screening by ICP-OES and GC-MS for identifying the chemical components. All compounds detected in the control samples were removed from data set when results discussing.
2.2.3. EPR analysis of EPFRs on DPFs
The leachates were detected by EPR analysis for quantifying the possible generated EPFRs. Specifically, the obtained leachate was first transferred and placed in an I.D. quartz tube. Afterwards, the quartz tube with the leachate was inserted into the cavity of the EPR instrument for EPFRs analysis. EPR measurements were conducted with using a Bruker EMXmicro-6/1/P/L spectrometer (Karlsruhe, Germany) at room temperature (∼25 °C). In addition, ROS in the leachates was also measured by EPR coupling with spin-trapping agent, i.e., DMPO. Briefly, a volume of 2 mL of each leachate sample was taken and mixed with 500 μL DMPO solution (100 mM), 500 μL DMPO/DMSO solution (100 mM) and 500 μL TEMP solution (of 100 mM) for detecting •OH, O2 •− and 1O2, respectively. Then, the obtained mixture was used for the measurement of EPR. The parameters of EPR measurement were based on our previous study (Zhu et al., 2019), and also provided in the supplement (SI, Table S6).
2.3. Toxicity of DPF leachate
2.3.1. Cytotoxicity assessment
Mc3t3e1cell were used as the model cell for bioassays in this study. The cytotoxicity assay was performed by the viability of mc3t3e1cell in a 96-well plate. The viable cell number of mc3t3e1cell was quantitated by the method of Cell Counting Kit-8 (CCK-8, purchased from Sigma-Aldrich). The number of mc3t3e1 cell was adjusted to 50000 cells/ml in each well. For cell culture, 100 μL inoculate and 200 μL complete medium were added to each well of a 96-well plate and the edge of the well, respectively. Cells were seeded in a cell incubator (37 °C and 5% CO2). DPFs leachates (25 μL) which were taken from the samples and not concentrated was added to each well. For control groups, a volume of 25 μL sterile phosphate buffer saline (PBS, pH = 7.4) was added in the wells. The culture medium was removed and washed with PBS after 24 h incubation. A 100 μL of culture medium containing a 10 μL of CCK solution was added into each well, and then measured by a microplate reader (iMark™ Microplate Absorbance Reader, BIO-RAD) at 450 nm after 1h-incubation in the incubator. Each test was repeated 6 times.
To identify the cytotoxicity induced by EPFRs, N-acetyl-l-cysteine (NAC, 5 mM, Sigma-Aldrich, St. Louis, MO) as a free-radical scavenger was used (Aldini et al., 2018), which has non-impact on the cell viability (Jia et al., 2020). Cells which were incubated with DPFs leachate with or without NAC was used to evaluate the cytotoxicity.
2.3.2. Intracellular ROS levels
The intracellular ROS levels was evaluated by cell-permeable non-fluorescent probe 2′,7′-dichlorofluorescin diacetate (DCFH-DA, Sigma) dying assay. Cell culture was carried on a 12-well plate with adjusting mc3t3e1 cell number to 100,000 cells/mL in each well and inoculate 500 μL in each well. After incubation for 24 h in the inoculator (37 °C and 5% CO2), 125 μL DPFs leachate was added to each well. The controls were without adding DPFs leachate but rather the same amount of deionized water. The culture medium was removed and washed with PBS after 24 h-incubation, and then adding 1 mL of serum-free culture medium containing 10 μM/mL DCFH-DA to each well. With incubating in the dark for 15 min, samples were processed by removing the culture medium, washing with PBS twice, and adding 1 mL PBS. And then samples were used to observe and analyzing the DCFH-DA dying results by an inverted fluorescence microscope (Nikon ECLIPSE Ti) with ImageJ (National Institutes of Health, USA).
2.4. Statistical analysis
t-test analysis (R software) was used to test the data on chemicals leaching and MPs releasing for evaluating the statistically significant differences of the organics and metal’ concentrations and the MPs abundance in the processes of DPFs leaching. Data collected from toxicity tests were tested for nonlinear regressions (GraphPad Prism 7). In order to analysis the significant differences between experimental groups and control groups, data collected from toxicity tests were tested for normality and equal various variance (SPSS statistics 22.0 and Sigmaplot 13) to analysis the significant differences between experimental groups and control groups. p 0.05 was regarded as a statistically significant difference.
3. Results and discussion
3.1. Characterization of DPFs leachates
3.1.1. MPs release from DPFs
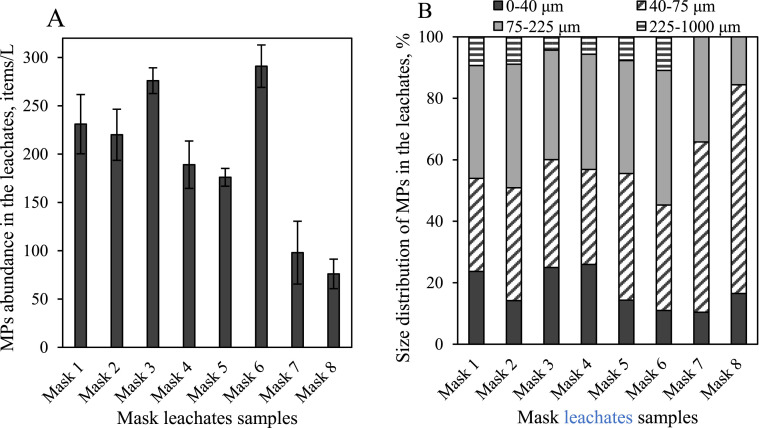
All samples taken from the leachates prepared by soaking the DPFs from 8 different brands were subjected to microscopy (including SEM and stereomicroscopes) and FTIR analysis. MPs were observed in all leachates’ samples and the abundance of plastic-like particles for all DPFs leachates were in a range of 76–276 items/L (Fig. 1 and SI-Fig. S1). This result is significantly lower than some other studies. Such as, Liang et al. reported as much as more than 800 items/L was identified by the face mask leasing (2021), and Morgana et al. found around 2.6 × 103 items/mask MPs presents in the leachates after soaking (2021). However, it is difficult to directly compare the results of MPs from different studies since the different test methods were used. Liang et al. agitated masks twice as fast as we did (2021). Morgana et al. cut the masks into pieces with scissors before soaking (2021). Both faster agitating and cutting mask into pieces could lead to more MPs release to the water. Noteworthy, the pollution of MPs caused by the exposure of DPFs to the environment may be even worse since much more MPs could be released from the DPFs waste due to the weathering by e.g. wind and light irradiation. For example, Wang et al. reported that a single surgical mask after 18 h UV weathering can release more than 1.5 million microplastics to the aqueous environment (2021); Saliu et al. found that a single surgical mask can release up to 173,000 fibers/day with processing of 180 h UV weathering and vigorous stirring in artificial seawater (2021). According to the obtained results, it seemed that surgical masks released more MPs than N95 respirators. The results of FTIR analysis show all particles are identified as MPs, mainly including PP (89.2–97.2%) and PA (2.8–10.8%) (SI-Figs. S2 and S3). The results of microscopy analysis showed that only fibrous MPs (80.3–97.4%) and particle MPs (<10%) were found in the DPF leachates and their main colors were blue and transparent (SI-Fig. S2). It appeared that no obvious differences characteristics of MPs were found in the leachates for the DPFs which were same type but from different brand.
Fig. 1.
Abundance (A) and size distribution (B) of MPs in 15 d-samples of different DPFs leachates. The abundance of MPs in the control group has been deducted from test group. Error bars represent standard deviation of three replicates.
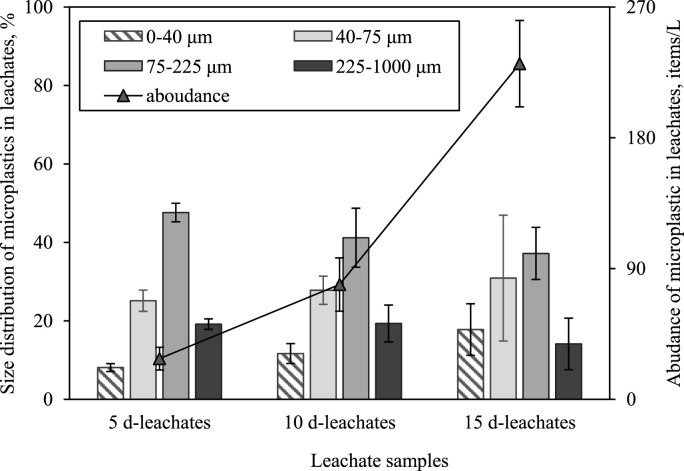
In addition to investigate the characteristics of the released MPs in DPFs leachates with time, samples of the DPFs leachates were taken and analyzed after various soaking time. Taking samples from Mask 1 leachates as an example, an average of 231 items/L particles were observed in 15 d-leachates using stereomicroscopes, which were mainly in blue (65.3%) and transparent (34.7%) (Fig. 2 and SI-Fig. S2). The abundance of MPs in their 5 d-samples and 10 d-samples was with an average of 28 items/L and 79 items/L, respectively, suggesting that the number of MPs increased with the increasing the exposure time. It may be mainly due to that fractures of fibers occurred during soaking and agitating. It can be seen from SEM images (SI-Figure S4 A-B) that DPFs had a smooth surface before agitated in deionized water. After 15 days of soaking and agitating, through the majority of fibers were intact, deformation and damage were observed on the surface and some small particles and fiber fragments formed where the damage occurred (SI-Figure S4 C-D). The results in Fig. 2 showed that the abundance of MPs of 40–75 μm and 57–225 μm in the DPFs leachates was 37.1–47.6% and 25.1–30.9%, respectively, which were the dominant presented in the leachates. The abundance of MPs of <40 μm in leachates tended to be increased with time, but the increase was still not significant (p = 0.14). According to the microscope observation, only particles and fibrous MPs were found in the leachates and more than 90% MPs were fibrous in the leachates, which was also consistent with the SEM analysis. Interestingly, fibrous and particles MPs were all reported to be observed in the gastrointestinal tract of the organisms (Mazurais et al., 2015), and were considered to be frequently ingested by terrestrial and marine organisms (Avio et al., 2015; Choi et al., 2018).
Fig. 2.
Abundance and size distribution of MPs in DPFs leachates (Mask 1). The abundance of MPs in the control group has been deducted from test group. Error bars represent standard deviation of three replicates.
3.1.2. Metals leaching
Various heavy metals were identified in the DPFs leachates. As shown in Table 1 , Co, Cu, Ni, Sr, Ti and Zn were detected in all samples of the DPFs leachates and the values of control samples processing concentrations were below the LOD of ICP-OEC analysis. Besides, it is observed that other heavy metals, including Cd, Cr, Mn and Pb, presented in the surgical masks leachates. For examples, Mn and Pb were detected in all surgical masks leachates and Cd and Cr were detected in the DPF leachates for Mask 1, 2, 3, 6 and 7 and Mask 1, 2, 3, 4 and 5, respectively. Comparatively, for N95 respirators, only less than 1 μg/L Cr was found in the leachates of Mask 7. Meanwhile, the concentrations of all detected heavy metals in the surgical masks leachates were commonly higher than those in N95 respirators leachates. It indicates that the amount of DPFs used and discarded could lead to a potential environmental risk that more heavy metals possibly enter the environment via leaching from DPFs waste. It is reasonable to speculate that surgical masks waste more easily leach heavy metals into the aquatic environmental than N95 respirators waste. Furthermore, some of these heavy metals are potentially bio-accumulated by organisms and could cause acute and chronic adverse effects on human health by ingestion, dermal contact and inhalation exposure. As such, contact allergy to Cr, Ni and Co are the most common metal allergy and approximately 1–3% of the adult general population have encountered this (Thyssen and Menné, 2010). Ingesting Cd, Co, Cr and Pb was reported to have potential carcinogenic risk to both children and adults (Hu et al., 2012). In addition, multiple metal–metal interactions of, e.g. Cd, Cu, Ni, and Zn, may contribute to an higher toxicity in a mixture (Traudt et al., 2017). Therefore, the huge amount of DPFs waste generated during the COVID-19 pandemic may not only cause that more heavy metals are potentially released into the environment and accumulated by organisms, but also give rise to a risk of exposure of heavy metals to human by regularly wearing DPFs.
Table 1.
List of concentrations of main heavy metals detected in the 15d-DPFs leachates (μg/L). Standard deviation analysis is based on triplicate tests. N.D represents “non-detected”.
| Sample |
Cd |
Co |
Cr |
Cu |
Mn |
Ni |
Pb |
Sr |
Ti |
Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.3 | N.D | N.D | 0.2 | 0.1 | N.D | 0.1 | N.D | N.D | 0.1 |
| Mask 1 | 1.2 ± 0.1 | 7.3 ± 0.1 | 0.8 ± 0.1 | 6.6 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.1 | 1.3 ± 0.1 | 13.1 ± 1.5 | 7.7 ± 1.0 | 13.9 ± 3.6 |
| Mask 2 | 0.6 ± 0.1 | 5.6 ± 0.5 | 0.8 ± 0.3 | 4.7 ± 0.8 | 0.9 ± 0.1 | 1.1 ± 0.3 | 0.8 ± 0.2 | 10.2 ± 1.6 | 5.2 ± 1.3 | 10.3 ± 1.6 |
| Mask 3 | 1.0 ± 0.3 | 5.8 ± 0.2 | 0.7 ± 0.1 | 8.3 ± 1.1 | 2.1 ± 0.9 | 1.3 ± 0.6 | 1.1 ± 0.3 | 14.4 ± 1.1 | 7.8 ± 2.3 | 15.6 ± 1.5 |
| Mask 4 | N.D | 7.2 ± 0.9 | 0.8 ± 0.3 | 7.7 ± 0.5 | 1.6 ± 0.6 | 1.9 ± 0.5 | 0.7 ± 0.2 | 10.3 ± 2.7 | 9.2 ± 2.7 | 12.7 ± 2.6 |
| Mask 5 | N.D | 7.6 ± 0.6 | 0.4 ± 0.1 | 7.3 ± 0.7 | 1.4 ± 0.6 | 0.7 ± 0.3 | 0.7 ± 0.2 | 11.6 ± 2.3 | 6.6 ± 2.5 | 10.8 ± 3.4 |
| Mask 6 | 1.3 ± 0.3 | 8.0 ± 0.7 | N.D | 6.5 ± 0.3 | 2.0 ± 0.3 | 0.9 ± 0.4 | 0.9 ± 0.2 | 12.5 ± 1.6 | 6.9 ± 1.3 | 17.7 ± 2.6 |
| Mask 7 | 0.8 ± 0.1 | 3.1 ± 1.0 | N.D | 1.2 ± 0.4 | N.D | 0.8 ± 0.2 | N.D | 0.5 ± 0.1 | 0.9 ± 0.2 | 10.2 ± 1.6 |
| Mask 8 | N.D | 6.4 ± 2.1 | N.D | 0.9 ± 0.4 | N.D | 0.5 ± 0.1 | N.D | 0.8 ± 0.2 | 0.7 ± 0.3 | 9.3 ± 2.2 |
3.1.3. Organic components leaching
By means of GC-MS SCAN analysis with the NIST mass spectral library, organic additive chemicals, including plasticizers and antioxidants, were tentatively identified which all more than 90% mass spectral match to reference compounds in the library and that were not found in the controls. The tentatively identified organic compounds and their potential toxicity are summarized and listed in SI-Table S7. Acetophenone (AP), 2,4-Di-tert-butylphenol (DTBP) and bis(2-ethylhexyl) phthalate were found in all tested samples, however, some compounds, like tributyl acetylcitrate and benzaldehyde, 2,4-dimethyl- were identified in only one or two samples of DPFs leachates. This may be due to the more complex nature of the DPFs leachates, resulting in the component peaks being unresolvable in the chromatograms from the samples DPFs leachates. Meanwhile, as reported by G.L.Sullivan et al. various compounds, like polyamide-66, oligomers of polyamide, polyethylene glycol-like and N-Undecyl-1-undecanamine were identified in the DPFs 18 h-leachates (2021). Even though a broad range of organic compounds are potentially leached from DPF, only a few were in detectable amounts in the full scan analyses for the leachate samples (SI-Fig. S5). Thus, from the set of organic compounds identified in the leachates and the reported organic components which are identified in the leachates of PP products (Capolupo et al., 2020), five organic compounds, including AP, benzothiazole, DTBP, bisphenol-A (BPA) and phthalide, were selected as target compounds for target analysis and their standards were purchased as references to do target screening with GC-MS. The results of these five organic compounds’ concentrations in DPFs leachates analyzed by GC-MS SIM method have been shown in Table 2 .
Table 2.
Concentrations of selected organic compounds in the DPFs leachates quantified by GC-MS in SIM model (μg/L). “±” represents standard deviation of three replicates.
| Samples |
AP |
benzothiazole |
DTBP |
BPA |
phthalide |
|---|---|---|---|---|---|
| Controls | 0.6 ± 0.1 | N.D | N.D | N.D | N.D |
| Mask 1 | 6.2 ± 1.2 | 1.7 ± 0.6 | 1.6 ± 0.3 | 1.1 ± 0.3 | 1.7 ± 0.5 |
| Mask 2 | 1.2 ± 0.7 | 2.5 ± 0.5 | 0.9 ± 0.3 | 3.2 ± 1.2 | 1.5 ± 0.3 |
| Mask 3 | 6.8 ± 1.7 | 2.9 ± 0.8 | 3.8 ± 0.9 | 1.1 ± 0.6 | 0.9 ± 0.1 |
| Mask 4 | 4.6 ± 2.1 | 6.7 ± 2.3 | 1.5 ± 0.8 | 1.5 ± 0.7 | 2.8 ± 1.0 |
| Mask 5 | 1.8 ± 0.9 | 9.2 ± 2.7 | 0.9 ± 0.4 | 0.8 ± 0.2 | 4.1 ± 1.2 |
| Mask 6 | 2.4 ± 0.9 | 1.6 ± 0.4 | 3.1 ± 1.1 | 0.8 ± 0.2 | 3.3 ± 1.7 |
| Mask 7 | 0.9 ± 0.4 | 1.2 ± 0.3 | N.D | N.D | N.D |
| Mask 8 | N.D | 1.5 ± 0.7 | 0.8 ± 0.1 | N.D | N.D |
All five selected organic compounds were detected with GC-MS SIM method in the surgical masks leachates while BPA and phthalide were not found in all N95 respirators leachates. Especially, although BPA was only identified in surgical masks leachates with a low concentration of 0.8–3.2 μg/L, it was not found with non-target GC-MS screening. It indicated that the sensitivity of the GC-MS SIM method was higher than that of the SCAN methods and allowed to quantify organic additives in DPFs leachates. By means of comparing preliminary investigations of the organic compounds detection in DPFs leachates by target and non-target GC-MS analysis, the results vary greatly and presumably, a great deal of organic additives are possible to be leached into the environment with the increasing DPFs leaching. These organic additives with complex natures which toxicity have already been demonstrated (Table 2) may have an adverse impact on the environment and human health when leaching from DPFs.
By comparing the released MPs and leached components in both surgical masks and N95 respirators leachates from different brands, as a consequence, surgical masks leachates tend to contain more MPs, heavy metals and organics than N95 respirators leachate. Moreover, for DPFs of the same type but from different brands, no significant differences on the characteristics of the released MPs and leached components were observed in their leachate.
3.2. Generation and evolution of EPFRs in the leachate of DPFs
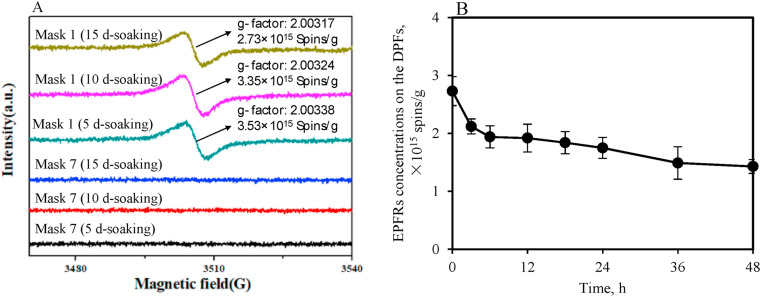
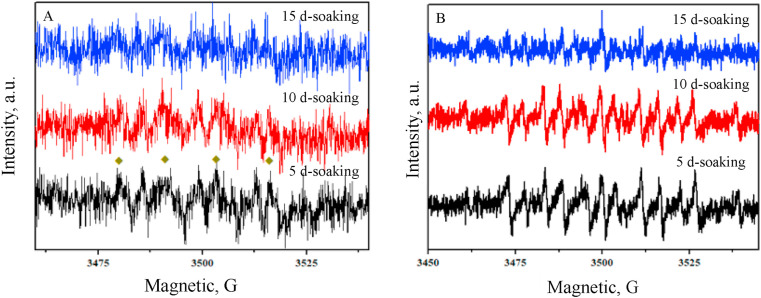
As hypothesized, EPFRs is suspect to be generated during DPFs exposing in water. To test this hypothesis, as there were no significant differences on the characteristics of the leachates for the DPFs which were the same type but from different brands based on the above results of the DPFs leachates, one kind of surgical masks (Mask 1) and one kind of N95 respirators (Mask 7) was used to analyze the possible generation of EPFRs. The DPF leachates were detected by EPR after 5 d-, 10 d- and 15 d-soaking (at room temperature ∼25 °C) and the obtained EPR spectra are shown in Fig. 3 . Distinct EPR signals were detected in the leachates samples of Mask 1 which confirmed that EPFRs could form during DPFs exposure to water. The g-values and ΔH p-p of the EPR signals were in a range of 2.003∼2.004 and 4.6∼5.4 G, respectively, which suggested that the generated EPFRs were likely carbon-centered radicals with a nearby heteroatom or the mixture of carbon- and oxygen-centered radicals (Valavanidis et al., 2008). Conversely, no obvious EPR single was found in all leachates samples of Mask 7. It seems that besides N95 respirator could release less MPs and leachate less chemicals to water compared to surgical mask, it is likely more difficult to form EPFRs in the process of N95 respirator exposure to water.
Fig. 3.
(A) EPR spectra of the DPFs samples (Mask 1 and Mask 7) in the leachates. The values in the figure are the g-factors and EPFRs concentrations for EPR spectra for Mask 1 leachates samples. No EPR single was found in the samples which were without soaking (0 d-soaking) for both Mask 1 and Mask 7 leachates and the results were not shown in the figure. (B) The mean concentrations of the EPFRs of 15 days of Mask 1 samples under dark condition. Error bars represent standard deviation of three replicates.
It has been demonstrated that the formation of EPFRs can be attributed by electron-transformation between aromatic compounds and transition metals or chemical bonds cleavage in polymers (Han and Chen, 2017). As reported above, organic additives with aromatic structure and various metals were identified in the DPFs leachates. It was thus considered that the electrons transfer from organic additives (e.g. AP and BPA) to metal ions (e.g. Cu, Ni and Zn), which promoted that EPFRs was generated and shielded by the polymeric network of DPFs (Feld-Cook et al., 2017; Jia et al., 2019; Kuzina et al., 2004). As previous study reported, EPFRs generated by chemical bond cleavage tends to be occurred on aromatic polymers (e.g. polystyrene) (Jia et al., 2018; Zhu et al., 2019). However, even although DPFs may contain a small part of PS, the main materials of DPFs are PP and PA, which are without benzene rings. Therefore, it could be inferred that chemical bond cleavage is not the dominant process for EPFRs formation on the DPFs.
It was observed that g-factor and spin density in DPFs were slightly decreased as a function of soaking time (from 2.00317 to 2.00338, Fig. 3A). This could be due to a higher contribution of carbon-centered radicals which was suspected to lead to a decreased g-factor due to the electrons transfer of organic additives. The slightly decreased spin density seems to indicate that the generated EPFRs decayed slowly. However, it was also suspected that EPFRs were not only generated during soaking, but also consumed rapidly in the solution or in the air. To further study carefully the stability of EPFRs on the DPFs, bulk masks were removed from leachates after 15 days of soaking and agitating and then, the samples were with monitoring the EPR singles in the dark. The spin density was observed to decay 47.6% in 48 h (Fig. 3B). It indicated that the generated EPFRs were reactive, which could react with small molecules, e.g. O2 and H2O, presented in the air or in the solution and result in the decay of EPFRs (Liu et al., 2021). Furthermore, according to the results shown in Fig. 4 , superoxide radical (O2 •−, Fig. 4 A) and methyl radical (•CH3, Fig. 4 B) were found in the leachates samples of Mask 1. O2 •− has been demonstrated to be generally generated by carbon-centered radicals reacting with O2 (Zhu et al., 2020a). Therefore, it can be concluded that EPFRs are constantly produced during the surgical mask soaking process and retained in the leachates. Meanwhile, the reaction of EPFRs occurred during surgical mask exposing to water is able to generate reactive radicals (e.g. O2 •−), which may cause more potential environmental risks. Subsequently, surgical masks were further used to study the toxicity evaluation of EPFRs.
Fig. 4.
EPR spectra obtained from leachates samples (Mask 1) (A) with DMPO/DMSO (100 mM, 500 μL), and (B) with DMPO (100 mM, 500 μL). Black lines represent 5 d-samples, red lines represent 10 d-samples and blue lines represent 15 d-samples. No EPR single was found in the samples which were without soaking (0 d-soaking) and the results were not shown in the figure. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Preliminary assessment of the toxicity of DPF leachate
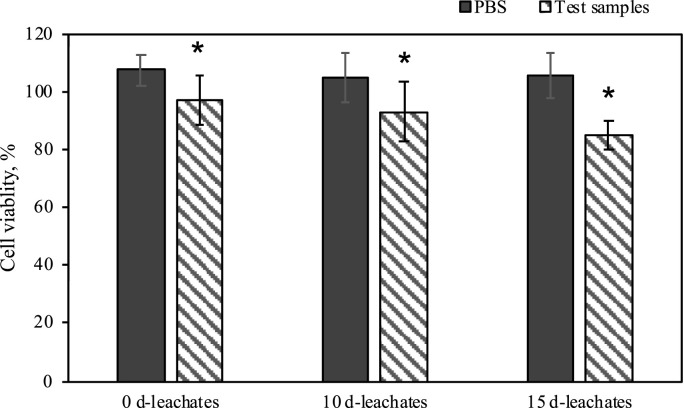
Preliminary trials showed that all of the analyzed leachate samples exhibited varying degrees of toxicity. The viability of mc3t3e1cell was significantly decreased after exposing to leachate (Mask 1) which were soaked for 0–15 d in comparation with the controls (Fig. 5 ). Exposing to the leachate, the cells were damaged and possibly, the cell membrane integrity was impaired (Zhu et al., 2020b). Around 76.2% of cell viability was observed for the samples with adding 15 d-leachates, which was lower than those exposing to 5 d-leachates and 10 d-leachates. Preliminary trials suggested that the DPFs leachates showed toxicity to the cells and inhibited the growth of the cells. The toxic effect of the leachates on cell was likely more obvious over time. This is also consistent with the above results of time accumulation of MPs releasing and metal and organic compounds leaching, and constantly generation of EFPRs.
Fig. 5.
Average viability of mc3t3e1cell (hatched bars) in the cell culture supernatants following exposure to the DPFs leachates (Mask 1) and controls which were added PBS with the same amount as the DPFs leachates (black bars). The exposure concentration of DPFs leachates was 25 μL/100 μL cell culture. Asterisk represents the significant difference from controls (*p < 0.05). Error bars represent standard deviation of six replicates.
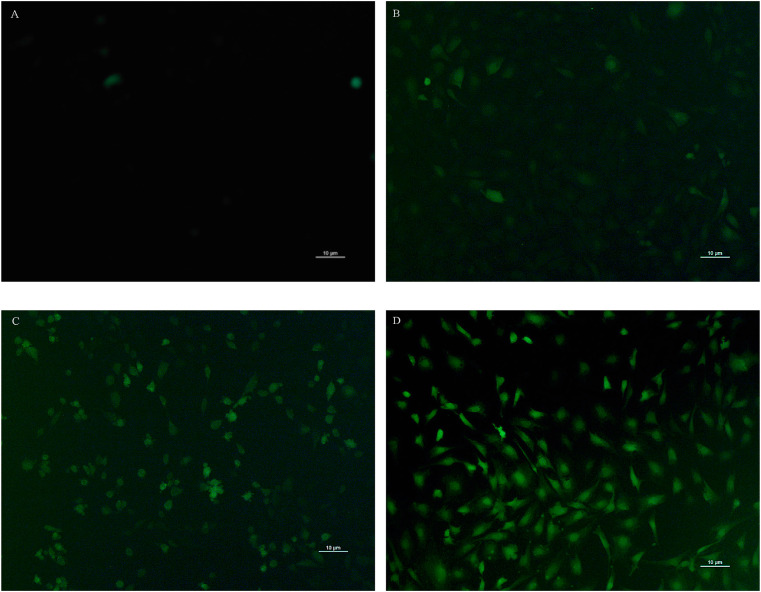
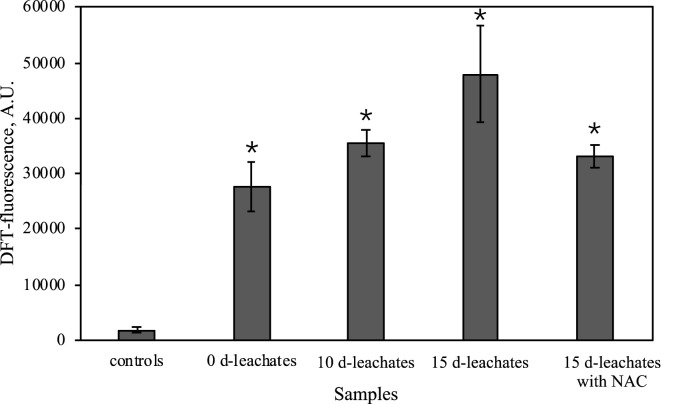
An investigation of in vitro detection of mc3t3e1cell for evaluating ROS generation after exposing the DPFs leachates was done by fluorescence microscopic image analysis using the fluorescent probe DCFH-DA. The microscopic images of DCF fluorescence showed DCHF-DA exhibited widespread background green fluorescence after exposing to the DPFs leachates (Fig. 6 B–D), while almost no fluorescence was shown in the controls (Fig. 6A). Compared to controls, a noticeable increase of fluorescence in the cells was observed over time after the DPFs were added into the cell culture supernatants (Fig. 7 ). This indicates that the exposure of the DPFs leachates exerts excessive oxidative stress to the test cells, which may result in a decrease of cell viability. Hence, the exposure of DPFs in water may have a threat to cause diseases by causing oxidative stress.
Fig. 6.
Fluorescence images of mc3t3e1cell in the cell culture supernatants following exposure to (B) 0-d leachates, (C) 10-d leachates and (D) 15-d leachates with a concentration of 100 μL leachates/1000 μL cell culture. Cell culture supernatants with adding deionized water (100 μL deionized water/1000 μL cell culture) were used as a negative control (A). Mc3t3e1cells show intracellular green 2′,7′-dichlorofluorescein (DCF) fluorescence as a result of ROS production. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 7.
DCF fluorescence intensities of mc3t3e1cell in the cell culture supernatants following exposure to 0 d-, 10 d- and 15 d-DPFs leachates without adding NAC and 15 d-DPFs leachates with adding NAC (5 mM). Control group was cell culture supernatants without exposing to the DPFs leachates. Fluorescence intensities was obtained by the analysis of fluorescence images with ImageJ. Asterisk represents the significant difference from controls (*p < 0.05). Error bars represent standard deviation of six replicates.
To further evaluate the contribution of EPFRs in the samples to the overall cytotoxicity, cell viability with or without adding a free radical scavenger (NAC) was determined. According to the results shown in Fig. S6, cytotoxicity was suppressed by using NAC (85.3% for 15 d-leachates with adding NAC). As discussed above, the generated EPFRs induced the generation of reactive radicals (e.g. O2 •− and •CH3 in Fig. 4), leading to cell death or cellular excessive oxidative stress (Figs. S6–S7). The results of NAC quenching, which show the effect of radicals, confirmed this finding. Cytotoxicity for samples without quenching free radicals is likely induced by other pollutants, including the detected metals and organics. Currently, the concentration of plastic-associated EPFRs to in vitro toxicity of DPFs is not well-known. These results indicate the generated EPFRs induce cytotoxicity and enhance cell oxidative stress, demonstrating that beside leached metals and organics, the generated EFPRs were also a non-negligible cause of in vitro toxicity.
4. Limitations and future directions
In view of the ecotoxicity of the DPF waste exposure to the aquatic environment, our investigation and assessment with taking surgical masks and N95 respirators exposing in water for 15 days is certainly not representative. Nevertheless, as far as we know, it represents the most comprehensive study of evaluating on the potential risk of DPFs waste exposing to the environment. In previous studies, DPFs leachate is mainly restricted to the analysis chemical composition leaching and MP releasing. In this study, in addition to tentatively identify MPs, metals and organics, we also focus on the generation of EPFRs, with providing data on the DPFs leachate triggering an oxidative stress response. The results of this study indicate that the leachates containing chemicals and EPFRs inducing unspecific toxicity are prevalent in DPFs waste, especially during the COVID-19 pandemic in which the widely use of DPFs is unprecedented. It is worth to highlight that the aim is not to draw a conclusion regarding that the wide use of DPFs has a potential impact on health, but to assess the concentrations of the leaching of the DPFs to the overall pollution and to evaluate the risks of the excessive exposure of DPFs waste to the aquatic environment during and after the COVID-19 pandemic from a new perspective: the basis of reducing DPFs’ environmental impact is to acknowledge their chemical complexity and possible toxicity. However, more accurate methods of MPs counting and identification of plastic-associated chemicals may be limited and the current detection methods may have a high rate of false identifications. Furthermore, the separately identify origin and assessing toxicity of EPFRs was challenging and hampered by the lack of related valid research data. Correspondingly, the first step to address the limitations is developing a standard of MPs sampling and detecting, as well as scientific and comprehensive approaches for compound identification. More research on the generation, transformation and occurrence of EPFRs and more bioassays assessment for various of EPFRs to explore the toxic effect is helpful to address the limitations. To a large extent, we need to perform effect-directed analysis to identify the compounds causing the toxicity which are not only leached but also generated during the DPF waste exposure to water.
5. Conclusion
This study provides new insight into the potential health risk of the disposable face mask waste with the COVID-19 pandemic by not only leaching metals and organics and releasing MPs but also the formed EPFRs. More MPs were released to the leachates from the DPFs with the increasing of the exposure time, for example, the abundance of MPs of surgical mask was enhanced from 28 items/L in 5 d-leachate to 231 items/L in 15 d-leachates. It is found that MPs were fragmented with soaking time as the abundance of smaller MPs (e.g. < 40 μm) in leachates was increased. Metals (including Co, Cu, Ni, Sr, Ti and Zn) and organics (including acetophenone, DTBP and bis(2-ethylhexyl) phthalate) were commonly presented in the leachates. Furthermore, more various metals (like Cd, Cr, Mn and Pb) and organics (like tributyl acetylcitrate and benzaldehyde) were found in surgical masks leachates than in N95 respirators leachates. From the perspective of plastic-related chemicals, the DPFs was confirmed as a source of MPs, metals and organics, and hence could cause an ecosystem risk after exposing to the aquatic environment. Significantly, the carbon-centered EPFRs were detected to be generated on the DPFs, which could be attributed to the electron-transfer reaction between metals and organics contained in the DPFs. In order to estimate the potential adverse effects of the DPF waste, we further conducted in vitro toxicity assessment and demonstrated that the DPFs leachate could cause cytotoxicity and oxidative stress. Noteworthy, the generated EPFRs enhanced the toxic effects of DPFs leachates. Overall, this work provides detailed data and systemic estimation to the generated EPFRs after DPFs exposure to water. These findings contributed to bridging the knowledge gap in better understanding the environmental effects and toxic risk of DPFs waste exposure to the aquatic environment.
Credit author statement
Ze Liu: Conceptualization, Methodology, Software, Data curation, Writing – original draft, Writing – review & editing. Jianqun Wang: Methodology, Software, Data curation, Writing – review & editing. Xuetong Yang: Validation, Investigation, Writing – review & editing. Qian'en Huang: Validation, Investigation, Writing – review & editing. Kecheng Zhu: Validation, Investigation, Writing – review & editing. Yajiao Sun: Validation, Investigation. Stijn Van Hulle: Validation, Writing – review & editing. Hanzhong Jia: Validation, Writing – review & editing, Supervision
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Ze Liu, Jianqun Wang, and Xuetong Yang contributed to the work equally and were regarded as co-first author. This study was funded by the National Natural Science Foundation of China (Grants Nos. 42077351), the National Key R&D Program of China (Grant No. 2019YFC1804203), Shaanxi Key R&D Program of China (Grant No. 2019ZDLNY01-02-01), the “One Hundred Talents” program of Shaanxi Province (SXBR9171), and the Shaanxi Science Fund for Distinguished Young Scholars (Grant No. 2019JC-18). We would also like to thank to Mr. Xiaolong Hu for the design of the graphic.
Footnotes
This paper has been recommended for acceptance by Da Chen.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envpol.2022.119019.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adyel T.M. Accumulation of plastic waste during COVID-19. Science. 2020;369:1314–1315. doi: 10.1126/science.abd9925. [DOI] [PubMed] [Google Scholar]
- Akhbarizadeh R., Dobaradaran S., Nabipour I., Tangestani M., Abedi D., Javanfekr F., Jeddi F., Zendehboodi A. Abandoned Covid-19 personal protective equipment along the Bushehr shores, the Persian Gulf: an emerging source of secondary microplastics in coastlines. Mar. Pollut. Bull. 2021;168:112386. doi: 10.1016/j.marpolbul.2021.112386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldini G., Altomare A., Baron G., Vistoli G., Carini M., Borsani L., Sergio F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic. Res. 2018;52:751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- Aragaw T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull. 2020;159:111517. doi: 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardusso M., Forero-López A.D., Buzzi N.S., Spetter C.V., Fernández-Severini M.D. COVID-19 pandemic repercussions on plastic and antiviral polymeric textile causing pollution on beaches and coasts of South America. Sci. Total Environ. 2021;763:144365. doi: 10.1016/j.scitotenv.2020.144365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avio C.G., Gorbi S., Regoli F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: first observations in commercial species from Adriatic Sea. Mar. Environ. Res., Particles in the Oceans: Implication for a safe marine environment. 2015;111:18–26. doi: 10.1016/j.marenvres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Balakrishna S., Lomnicki S., McAvey K.M., Cole R.B., Dellinger B., Cormier S.A. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Part. Fibre Toxicol. 2009;6:11. doi: 10.1186/1743-8977-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capolupo M., Sørensen L., Jayasena K.D.R., Booth A.M., Fabbri E. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 2020;169:115270. doi: 10.1016/j.watres.2019.115270. [DOI] [PubMed] [Google Scholar]
- Choi J.S., Jung Y.-J., Hong N.-H., Hong S.H., Park J.-W. Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus) Mar. Pollut. Bull. 2018;129:231–240. doi: 10.1016/j.marpolbul.2018.02.039. [DOI] [PubMed] [Google Scholar]
- COVID-19 Facemasks & Marine Plastic Pollution, (n.d).
- De-la-Torre G.E., Rakib MdR.J., Pizarro-Ortega C.I., Dioses-Salinas D.C. Occurrence of personal protective equipment (PPE) associated with the COVID-19 pandemic along the coast of Lima, Peru. Sci. Total Environ. 2021;774:145774. doi: 10.1016/j.scitotenv.2021.145774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadare O.O., Okoffo E.D. Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci. Total Environ. 2020;737:140279. doi: 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld-Cook E.E., Bovenkamp-Langlois L., Lomnicki S.M. Effect of particulate matter mineral composition on environmentally persistent free radical (EPFR) formation. Environ. Sci. Technol. 2017;51:10396–10402. doi: 10.1021/acs.est.7b01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Chen B. Generation mechanism and fate behaviors of environmental persistent free radicals. Prog. Chem. 2017;29:1008–1020. doi: 10.7536/PC170566. [DOI] [Google Scholar]
- Hu X., Zhang Y., Ding Z., Wang T., Lian H., Sun Y., Wu J. Bioaccessibility and health risk of arsenic and heavy metals (Cd, Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing, China. Atmos. Environ. Times. 2012;57:146–152. doi: 10.1016/j.atmosenv.2012.04.056. [DOI] [Google Scholar]
- Jia H., Li Shuaishuai, Wu L., Li Shiqing, Sharma V.K., Yan B. Cytotoxic free radicals on air-borne soot particles generated by burning wood or low-maturity coals. Environ. Sci. Technol. 2020;54:5608–5618. doi: 10.1021/acs.est.9b06395. [DOI] [PubMed] [Google Scholar]
- Jia H., Zhao S., Shi Y., Zhu K., Gao P., Zhu L. Mechanisms for light-driven evolution of environmentally persistent free radicals and photolytic degradation of PAHs on Fe(III)-montmorillonite surface. J. Hazard Mater. 2019;362:92–98. doi: 10.1016/j.jhazmat.2018.09.019. [DOI] [PubMed] [Google Scholar]
- Jia H., Zhao S., Shi Y., Zhu L., Wang C., Sharma V.K. Transformation of polycyclic aromatic hydrocarbons and formation of environmentally persistent free radicals on modified montmorillonite: the role of surface metal ions and polycyclic aromatic hydrocarbon molecular properties. Environ. Sci. Technol. 2018;52:5725–5733. doi: 10.1021/acs.est.8b00425. [DOI] [PubMed] [Google Scholar]
- Kalina M., Tilley E. This is our next problem”: cleaning up from the COVID-19 response. Waste Manag. 2020;108:202–205. doi: 10.1016/j.wasman.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutralam-Muniasamy G., Pérez-Guevara F., Shruti V.C. A critical synthesis of current peer-reviewed literature on the environmental and human health impacts of COVID-19 PPE litter: new findings and next steps. J. Hazard Mater. 2022;422:126945. doi: 10.1016/j.jhazmat.2021.126945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutralam-Muniasamy G., Shruti V.C. The case of “public congregation vs. COVID-19 PPE pollution”: evidence, lessons, and recommendations from the annual pilgrimage to the Catholic Holy Site in Mexico City, Mexico. Sci. Total Environ. 2022;821:153424. doi: 10.1016/j.scitotenv.2022.153424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzina S.I., Brezgunov A.Yu, Dubinskii A.A., Mikhailov A.I. Free radicals in the photolysis and radiolysis of polymers: IV. Radicals in γ- and UV-irradiated wood and lignin. High Energy Chem. 2004;38:298–305. doi: 10.1023/B:HIEC.0000041340.45217.bd. [DOI] [Google Scholar]
- Liang H., Ji Y., Ge W., Wu J., Song N., Yin Z., Chai C. Release kinetics of microplastics from disposable face masks into the aqueous environment. Sci. Total Environ. 2021;151650 doi: 10.1016/j.scitotenv.2021.151650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Sun Y., Wang J., Li J., Jia H. In vitro assessment reveals the effects of environmentally persistent free radicals on the toxicity of photoaged tire wear particles. Environ. Sci. Technol. 2021 doi: 10.1021/acs.est.1c05092. [DOI] [PubMed] [Google Scholar]
- Lucas K., Maes M. Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013;48:190–204. doi: 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurais D., Ernande B., Quazuguel P., Severe A., Huelvan C., Madec L., Mouchel O., Soudant P., Robbens J., Huvet A., Zambonino-Infante J. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Mar. Environ. Res. 2015;112:78–85. doi: 10.1016/j.marenvres.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Morgana S., Casentini B., Amalfitano S. Uncovering the release of micro/nanoplastics from disposable face masks at times of COVID-19. J. Hazard Mater. 2021;419:126507. doi: 10.1016/j.jhazmat.2021.126507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuku E., Kiteresi L., Owato G., Otieno K., Mwalugha C., Mbuche M., Gwada B., Nelson A., Chepkemboi P., Achieng Q., Wanjeri V., Ndwiga J., Mulupi L., Omire J. The impacts of COVID-19 pandemic on marine litter pollution along the Kenyan Coast: a synthesis after 100 days following the first reported case in Kenya. Mar. Pollut. Bull. 2021;162:111840. doi: 10.1016/j.marpolbul.2020.111840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B., Li H., Lang D., Xing B. Environmentally persistent free radicals: occurrence, formation mechanisms and implications. Environ. Pollut. 2019;248:320–331. doi: 10.1016/j.envpol.2019.02.032. [DOI] [PubMed] [Google Scholar]
- Patrício Silva A.L., Prata J.C., Duarte A.C., Barcelò D., Rocha-Santos T. An urgent call to think globally and act locally on landfill disposable plastics under and after covid-19 pandemic: pollution prevention and technological (Bio) remediation solutions. Chem. Eng. J. 2021;426:131201. doi: 10.1016/j.cej.2021.131201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potluri P., Needham P. In: Textiles for Protection, Woodhead Publishing Series in Textiles. Scott R.A., editor. Woodhead Publishing; 2005. 6 - technical textiles for protection; pp. 151–175. [DOI] [Google Scholar]
- Prata J.C., Silva A.L.P., Walker T.R., Duarte A.C., Rocha-Santos T. COVID-19 pandemic repercussions on the use and management of plastics. Environ. Sci. Technol. 2020;54:7760–7765. doi: 10.1021/acs.est.0c02178. [DOI] [PubMed] [Google Scholar]
- Saliu F., Veronelli M., Raguso C., Barana D., Galli P., Lasagni M. The release process of microfibers: from surgical face masks into the marine environment. Environ. Adv. 2021;4:100042. doi: 10.1016/j.envadv.2021.100042. [DOI] [Google Scholar]
- Sullivan G.L., Delgado-Gallardo J., Watson T.M., Sarp S. An investigation into the leaching of micro and nano particles and chemical pollutants from disposable face masks - linked to the COVID-19 pandemic. Water Res. 2021;196:117033. doi: 10.1016/j.watres.2021.117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyssen J.P., Menné T. Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem. Res. Toxicol. 2010;23:309–318. doi: 10.1021/tx9002726. [DOI] [PubMed] [Google Scholar]
- Traudt E.M., Ranville J.F., Meyer J.S. Acute toxicity of ternary Cd–Cu–Ni and Cd–Ni–Zn mixtures to Daphnia magna: dominant metal pairs change along a concentration gradient. Environ. Sci. Technol. 2017;51:4471–4481. doi: 10.1021/acs.est.6b06169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A., Iliopoulos N., Gotsis G., Fiotakis K. Persistent free radicals, heavy metals and PAHs generated in particulate soot emissions and residue ash from controlled combustion of common types of plastic. J. Hazard Mater. 2008;156:277–284. doi: 10.1016/j.jhazmat.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Wang Q., Hernández-Crespo C., Santoni M., Van Hulle S., Rousseau D.P.L. Horizontal subsurface flow constructed wetlands as tertiary treatment: can they be an efficient barrier for microplastics pollution? Sci. Total Environ. 2020;721:137785. doi: 10.1016/j.scitotenv.2020.137785. [DOI] [PubMed] [Google Scholar]
- Wang Q., Hernández-Crespo C., Santoni M., Van Hulle S., Rousseau D.P.L. Horizontal subsurface flow constructed wetlands as tertiary treatment: can they be an efficient barrier for microplastics pollution? Sci. Total Environ. 2020;721:137785. doi: 10.1016/j.scitotenv.2020.137785. [DOI] [PubMed] [Google Scholar]
- Wang Z., An C., Chen X., Lee K., Zhang B., Feng Q. Disposable masks release microplastics to the aqueous environment with exacerbation by natural weathering. J. Hazard Mater. 2021;126036 doi: 10.1016/j.jhazmat.2021.126036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, n.d. Shortage of Personal Protective Equipment Endangering Health Workers Worldwide. The World Health Organization (WHO).
- WHO, n.d. Coronavirus Disease (COVID-19): Masks [WWW Document]. URL https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-masks (accessed 8.6.21b).
- Zhao S., Miao D., Zhu K., Tao K., Wang C., Sharma V.K., Jia H. Interaction of benzo[a]pyrene with Cu(II)-montmorillonite: generation and toxicity of environmentally persistent free radicals and reactive oxygen species. Environ. Int. 2019;129:154–163. doi: 10.1016/j.envint.2019.05.037. [DOI] [PubMed] [Google Scholar]
- Zhu K., Jia H., Sun Y., Dai Y., Zhang C., Guo X., Wang T., Zhu L. Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: roles of reactive oxygen species. Water Res. 2020;173:115564. doi: 10.1016/j.watres.2020.115564. [DOI] [PubMed] [Google Scholar]
- Zhu K., Jia H., Sun Y., Dai Y., Zhang C., Guo X., Wang T., Zhu L. Enhanced cytotoxicity of photoaged phenol-formaldehyde resins microplastics: combined effects of environmentally persistent free radicals, reactive oxygen species, and conjugated carbonyls. Environ. Int. 2020;145:106137. doi: 10.1016/j.envint.2020.106137. [DOI] [PubMed] [Google Scholar]
- Zhu K., Jia H., Zhao S., Xia T., Guo X., Wang T., Zhu L. formation of environmentally persistent free radicals on microplastics under light irradiation. Environ. Sci. Technol. 2019 doi: 10.1021/acs.est.9b01474. [DOI] [PubMed] [Google Scholar]
- Zimmermann L., Dierkes G., Ternes T.A., Völker C., Wagner M. Benchmarking the in vitro toxicity and chemical composition of plastic consumer products. Environ. Sci. Technol. 2019;53:11467–11477. doi: 10.1021/acs.est.9b02293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.