Abstract
Sulfonylureas (SUs) are one of the commonly prescribed oral anti-hyperglycemic agents (AHA) in low- and middle-income countries (LMICs), either in combination with metformin therapy or alone. However, concern about cardiovascular safety has limited the use of SUs in the management of type 2 diabetes mellitus (T2DM). Additionally, lack of uniformity in the national and international guidelines regarding the positioning of SUs in the management of diabetes has also been reported. The objective of this review was to assess the various national and international guidelines on diabetes management and understand the recommendations specific to SUs in various scenarios. A total of 33 national and international guidelines on the management of T2DM published in English were evaluated. These guidelines have considered the latest evidence and suggest the use of certain second-generation SUs as second-line therapy or in combination with other AHAs in select population and specific scenarios. Identification of the appropriate population, classification based on underlying risk, thorough assessment of the comorbid conditions, and a step-wise approach for the selection of appropriate SUs is essential for the effective management of T2DM. Additionally, cost-to–benefit ratio should be considered, particularly in LMICs, and SUs could continue to play an important role in such settings.
Keywords: Sulfonylureas, national and international guidelines, type 2 diabetes
Introduction
The incidence of diabetes mellitus (DM) has quadrupled over the past few decades and continues to increase at an alarming rate. 1 As per the estimates provided by the International Diabetes Federation (IDF), the global prevalence of DM in 2019 was 9.3% (463 million people) and is projected to increase to 10.9% (700 million people) by 2045. 2
A wide array of anti-diabetic agents categorized under at least 12 classes are currently available for type 2 diabetes mellitus (T2DM) management. 3 The choice of these medications are generally influenced by the multiple international and national guidelines developed by various organizations with an intention to optimize the management of DM.4,5 These guidelines recommend specific treatment algorithms aimed at targeted management of T2DM. Metformin has been widely recommended as frontline therapy in the management of T2DM, followed by combination therapy with other oral antihyperglycemic agents (AHA).4,5 In specific cases where metformin therapy fails to achieve/maintain glycemic targets or is contraindicated due to the presence of certain risk factors, other AHAs, such as sulfonylureas (SUs), are recommended either in combination with metformin or as monotherapy.4,5
Sulfonylureas are one of the commonly prescribed oral AHAs in low- and middle-income countries (LMICs) of Asia and Africa, either alone or in combination with metformin. This class of drugs acts by stimulating the release of insulin from β-cells, thereby lowering hyperglycemia and glycated hemoglobin (HbA1c) levels in people with T2DM. 6 The SUs are classified into first- and second-generation SUs, based on the hierarchy of development, and also as short, intermediate- and long-acting SUs, based on their duration of action. Fixed-dose combinations of SUs along with metformin or other glucose-lowering medications have shown beneficial clinical outcomes in terms of efficacy and safety. The SUs have been included as one of the essential AHAs in the National List of Essential Medicines (NLEM) published by several countries in Africa, Middle East and North Africa, and South East Asian region. 6
Despite being used regularly for the management of T2DM, there is no uniform recommendation regarding the position of SUs (as first or second-line therapy) in both national and international diabetes management guidelines. Thus, there is a need to obtain greater clarity on the timely and precise use of SUs in current diabetes management protocol. In this review, data from various national and international guidelines regarding the role of SUs in the management of T2DM, especially in LMICs have been consolidated to assess and recommend the role of SUs in the current era.
Methodology
A literature search was performed in MEDLINE-PubMed and Google Scholar to identify national and international guidelines on the management of DM, published between January 2010 and January 2021 and available in English. The search strings used included diabetes AND guidelines, sulfonylureas AND guidelines, type 2 diabetes mellitus AND management, and diabetes guidelines AND updates. The literature search results were screened and 33 pertinent guidelines (7 international and 26 national) that met the inclusion criteria were selected. National guidelines encompassed countries in Asia (India, Bangladesh, Nepal, Sri Lanka, Indonesia, Malaysia, Singapore, Korea, and China), North and South America (the United States of America and Columbia), Europe (Britain), and the Pacific region (Australia). Global guidelines included those published by the American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD), American Association of Clinical Endocrinologists (AACE), International Society for Pediatric and Adolescent Diabetes (ISPAD), World Health Organization (WHO), International Diabetes Federation (IDF), and National Institute for Health and Clinical Excellence (NICE). Other region-specific diabetes guidelines published in regional languages were not considered in this review. Additionally, global guidelines are followed in few of the regional guidelines and were hence not included.
Guideline Recommendations on the Diagnosis and Treatment of Type 2 Diabetes Mellitus
Majority of the guidelines are harmonious in terms of diagnostic criteria and initial therapy with oral AHA for people with T2DM. The glycemic targets recommended by guidelines include fasting plasma glucose (FPG) levels of 80-130 mg/dL (4.4-7.2 mmol/L), HbA1c <7.0% (8.6 mmol/L), and post- prandial glucose (PPG) levels <180 mg/dL (10.0 mmol/L). 7
Currently, several different classes of oral AHAs and insulins are available for the management of T2DM. Metformin initiation along with lifestyle intervention has been recommended by many guidelines following initial diagnosis of T2DM. However, metformin is contraindicated in people with severe kidney impairment, hypersensitivity reactions, cardiac failure, impaired hepatic function, and respiratory insufficiency. 8 Hence, many of the current guidelines recommend annual renal function monitoring in people with T2DM who are on metformin therapy. 9 According to most guidelines, SUs are the commonly used second-line AHAs along with metformin. 6
International Guideline Recommendations for Sulfonylureas in Management of Type 2 Diabetes Mellitus
International diabetes management guidelines instruct on the use and contraindications for SUs in different clinical circumstances for the treatment of T2DM. International recommendations for the use of SUs are summarized in Table 1.
Table 1.
Recommendations from international organizations/associations/societies related to the use of sulfonylureas.5,10-15
| Sl. No | International guidelines | Guideline recommendations for use of SUs |
|---|---|---|
| 1 | ADA 5 | • Combination therapy with any of the six preferred medications has been recommended if target HbA1C levels are not attained with metformin monotherapy. • The preferred medications include: SUs, DPP-4 inhibitors, thiazolidinedione, GLP-1 RA, SGLT2 inhibitors, or basal insulin. Patient factors and drug-specific effects are to be considered while deciding the therapy. • SUs are recommended: – as third-line therapy in people with T2DM and ASCVD or CKD, following the failure of SGLT2i or GLP-1RA – in individuals without underlying cardiac or renal problems. – as the second-line therapy option when the cost of treatment is a major factor; if there is no risk or established CKD, ASCVD, or HF. |
| 2 | IDF 10 | • When metformin is not tolerated, SUs (except glibenclamide/glyburide) can be prescribed. • Metformin can be combined with SUs (except glyburide/glibenclamide), SGLT2 inhibitors or DPP4 inhibitors. • When starting an SU, the patient should be educated about how to prevent, recognize, and treat hypoglycemia. |
| 3 | ISPAD 11 | • SUs are not approved for use in those <18 years of age. • People on SUs should be encouraged to do self-monitoring of blood glucose to detect asymptomatic hypoglycemia. |
| 4 | NICE 12 | • SUs are recommended as an initial treatment regimen in individuals who are intolerant to metformin. • The use of insulin or SUs is recommended in people with T2DM who are symptomatically hyperglycemic. • Recommend dual therapy with metformin and SUs in adults with T2DM, whose HbA1c levels remain uncontrolled with initial metformin monotherapy. • SUs are also recommended as third-line therapy as part of a triple-drug regimen along with metformin and DPP-4 inhibitors or with metformin and pioglitazone. |
| 5 | WHO 13 | • SUs are recommended when glycemic control is not achieved with metformin monotherapy or in those metformin intolerance. • Recommend the usage of modern SUs, such as gliclazide, for better safety. |
| 6 | EASD 14 | • Addition of SU effectively reduces CV risk compared to lifestyle interventions alone; hence, it is recommended in people with T2DM. • Newer SUs, such as glimepiride, are associated with comparatively lesser adverse events, such as hypoglycemia and cardiovascular toxicity. |
| 7 | AACE 15 | • SU is recommended as first-line therapy, as an alternative to metformin in select patients. • In addition to metformin, dual or triple therapy using SU is recommended with caution. |
AACE, American Association of Clinical Endocrinologists; ADA, American Diabetes Association; ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DPP4i, dipeptidyl peptidase 4 inhibitor; EASD, European Association for the Study of Diabetes; GLP1RA, glucagon-like peptide-1 receptor agonist; HF, heart failure; IDF, International Diabetes Federation; ISPAD, International Society for Pediatric and Adolescent Diabetes; NICE, National Institute for Health and Care Excellence; SGLT2i, sodium–glucose co-transporter-2 inhibitor; SU, sulfonylurea; WHO: World Health Organization.
Majority of the international guidelines recommend SUs as second-line therapy (either with metformin or insulin-based therapies; Table 1).
Regional Guideline Recommendations for Sulfonylureas in the Management of Type 2 Diabetes Mellitus
Different national guidelines provide guidance on usage of pharmacological agents for managing hyperglycemia based on resource availability at regional levels. The guideline recommendations that have been proposed regarding the use of SUs in diabetes care are highlighted in Table 2.
Table 2.
| Sl. no. | Region | Guidelines on the use of SUs |
|---|---|---|
| 1 | Canada, 201916 | • Use SUs with caution among the elderly due to the increased risk of hypoglycemia. • Start with half the dose in elderly and up-titrate gradually. • Prefer gliclazide, gliclazide MR, or glimepiride as the risk of hypoglycemia is lesser. |
| 2 | Columbia, 201617 | • People with recently diagnosed T2DM and HbA1c >9% who are intolerant to the combination of metformin and DPP4 or SGLT2 inhibitors are recommended a combination of metformin with a newer SU (glimepiride or gliclazide). • People receiving SUs are recommended to implement glucose self-monitoring to detect and treat any episodes of hypoglycemia appropriately. • All SUs (except glipizide) are contraindicated if the glomerular filtration rate is >30 mL/min. |
| 3 | Australia, 201818 | • Recommends SUs as second-line therapy following metformin or as an add-on along with metformin or insulin. • Individuals on SUs should be encouraged to self-monitor their glucose levels. |
| 4 | Nigeria, 202019 | • Recommends modern SUs especially in individuals aged >40 years old, who have had DM for <10 years. |
| 5 | Uganda, 201620 | • Add-on therapy with glibenclamide or glimepiride is recommended if the glycemic targets are not achieved with both lifestyle therapy and metformin. |
| 6 | The Middle East and North Africa, 201921 | • Recommends SUs as fixed-dose combinations along with two or three OADs. |
| 7 | China 22 | • Xiao Ke Wan, a fixed-dose combination of SU (glyburide) and certain traditional Chinese medications are recommended, as risk of hypoglycemia is lower compared to glyburide alone. |
| 8 | Korea, 201923 | • Recommends SUs in individuals who are intolerant to metformin. |
| 9 | Singapore, 201424 | • Recommends SUs as a reasonable alternative to metformin in first-line pharmacotherapy. • Recommends self-monitoring of blood glucose in individuals using SUs. |
| 10 | India, 202025 | • SUs as second-line agents to be used in persons with T2DM who are not obese. • Risk of hypoglycemia is lower with SUs (especially gliclazide MR) in South Asians and are hence preferred in this population. • The dose of SUs should be reduced when prandial insulin is introduced. • Glimepiride and gliclazide MR, are recommended in persons at increased risk of or with CVD. • Second-generation SUs are recommended in the treatment of diabetic kidney disease. • Self-monitoring of blood glucose (4 times/day) is recommended in individuals with new-onset/uncontrolled/acute illness receiving SUs and should include prandial and bedtime values. |
| 11 | Austria, 20203 | • SUs are preferred after SGLT-2 inhibitors, GLP-1RAs, pioglitazone, and DPP-4 inhibitors. |
| 12 | Belgium, 20203 | • SUs are used as a second-line therapy. |
| 13 | Canada, 20203 | • SUs are considered second-line therapy. |
| 14 | Germany, 20203 | • SUs are a second-line therapy option. |
| 15 | Greece, 20203 | • Second-line therapy with SUs or pioglitazone if cost is an issue. |
| 16 | Hungary, 2020†3 | • SUs to be considered as first-line therapy if metformin is intolerable or contraindicated. • GPs widely use SUs (mainly gliclazide) as they can prescribe only metformin and SUs as initial therapy; although SUs are not the preferred as an add-on therapy. • Age, body weight, co-morbidities, hypoglycemia risk, cost and preference should be considered before initiating an add-on medication. |
| 17 | Israel, 2020‡3 | • SUs preferred as fourth-line therapy if cost is a concern. |
| 18 | Italy, 20203 | • Gliclazide can be preferred, if an SU is necessary; glibenclamide is contraindicated. |
| 19 | The Netherlands, 20203 | • SUs (gliclazide) is preferred only as a second-line add-on therapy. |
| 20 | Poland, 20203 | • Individualize the choice of further drugs considering the side-effects, effectiveness, risk of hypoglycemia and weight gain, cost, and patient preferences. |
| 21 | Portugal, 20203 | • The choice is individualized according to HbA1c levels (efficacy), side effects, weight, hypoglycemia risk, age, health status, life expectancy, patient preferences, and cost. |
| 22 | Romania, 2020‡3 | • First-line monotherapy with metformin or SUs (if metformin is not tolerated) or insulin (if required). • Metformin or SU along with all other classes of drugs (SGLT2 inhibitors, DPP4 inhibitors, GLP-1RAs, pioglitazone, insulin, acarbose, or repaglinide), or basal insulin (initial diagnosis) along with all other classes are preferred triple/quadruple drug combinations for second-line therapy. |
CVD, cardiovascular disease; DPP4, dipeptidyl peptidase 4; HbA1C, glycated hemoglobin; MR, modified release; SGLT2, sodium–glucose co-transporter-2; SU, sulfonylureas; T2DM, type 2 diabetes mellitus. †The guideline was published in January 2017 and will expire on 31 December 2019; current work is underway on the updated version, which will be available from 1 January 2020. CV events and risks will be considered as important factors; SGLT2-is and GLP1-RAs will be preferred in patients with CV events and/or risks; ‡Guidelines currently being revised.
An international task force comprising members of Asian and African countries (India, Bangladesh, Nepal, Sri Lanka, Indonesia, Malaysia, Singapore, and Egypt) has put forward the following recommendations regarding the use of second-generation SUs in diabetes care 6 :
Modern SUs (including glimepiride and gliclazide MR) can be preferred as second-line agents when glycemic targets are not achieved/reached with metformin monotherapy.
Sulfonylureas can be used along with all classes of oral AHAs (except glinides).
Second-generation SUs can be used as third-line agents with dual combination therapy for the management of uncontrolled diabetes as they are safer than older SUs.
Sulfonylureas are also suggested owing to better safety, glucose lowering efficacy and tolerability instead of up-titrating metformin beyond half-maximal dose.
Combinations containing second-generation SUs are recommended in elderly patients.
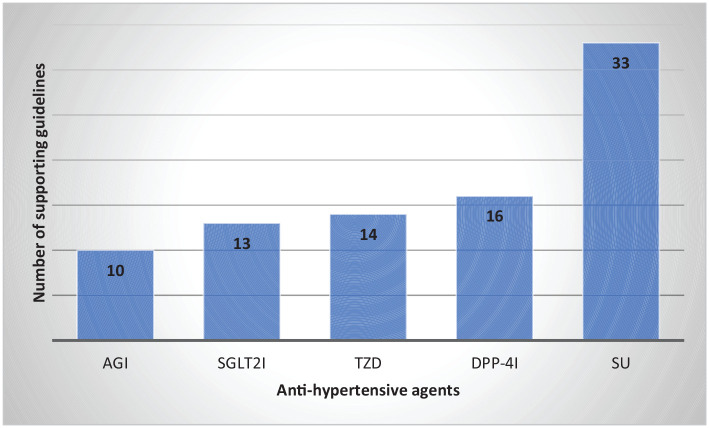
Sulfonylureas were recommended as an add-on to metformin therapy in all national and international guidelines evaluated in the current review (Figure 1).3,5,11-25
Figure 1.
Guidelines recommending different oral AHAs as second-line therapy in T2DM.3,5,11-25
AGI, alpha-glucosidase inhibitors; DPP-4i, dipeptidyl peptidase 4 inhibitor; SGLT-2i, sodium–glucose transport protein 2 inhibitor; SU, sulfonylurea; TZD, thiazolidinediones.
Guidelines: International: American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD), International (IDF), World Health Organization (WHO), American Association of Clinical Endocrinologists (AACE), National Institute for Clinical Excellence (NICE), International Society for Pediatric and Adolescent Diabetes (ISPAD); National: India, Nepal, Bangladesh, Pakistan, Sri Lanka, Canada, Colombia, Australia, Egypt, Nigeria, Uganda, Middle East and North Africa, China, Korea, Singapore, Austria, Belgium, Germany, Greece, Hungary, Israel, Italy, Netherlands, Poland, Portugal, and Romania.
Cardiovascular Safety of Sulfonylureas: Review of Guidelines
The efficacy of SUs in reducing hyperglycemia is well established. However, limited evidence on long-term cardiovascular (CV) safety may account for the differences in guidelines on the use of SUs.
The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial assessed the effects of intensive glucose control by gliclazide with other drugs, on major vascular outcomes in people with T2DM. The combined outcome of major macro- and microvascular events was relatively reduced by 10% following intensive glucose control. 26
Numerous clinical studies have assessed the role of antidiabetic therapy in CV outcomes. The CV safety of second-generation SUs was established by 3 cardiovascular outcome trials (CVOTs): Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (CARMELINA), Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients with Type 2 Diabetes (CAROLINA), and Thiazolidinediones or SUs Cardiovascular Accidents Intervention Trial (TOSCA.IT).27-29
CAROLINA was the first and only active-comparator CVOT that demonstrated noninferior CV safety of glimepiride compared with linagliptin as a second-line glucose-lowering treatment option. Since the participants in the CAROLINA trial mirrored real-world population in clinical practice, the results unequivocally established the robust safety of a second-generation SU. 28
In an indirect comparison between glimepiride and placebo in in the CARMELINA and CAROLINA trials, glimepiride was considered non-inferior to placebo in terms of 3-point MACE (HR 1.04, 95% CI 0.850, 1.274), all-cause mortality (HR 1.08, 95% CI 0.880, 1.317), CV death (HR 0.96, 95% CI 0.732, 1.259), and non-CV death (HR 1.24, 95% CI 0.893, 1.733). 30
The incidence of total CV events with SUs (mostly gliclazide and glimepiride) in the TOSCA.IT trial following its addition in people with T2DM inadequately controlled with metformin was similar to that with pioglitazone. 29
Although the CV safety and tolerability of SUs has been reported in a few clinical studies, differences still exist in the national and international guidelines regarding the positioning of SUs in the management of T2DM. According to the EASD/ADA consensus report, SUs are the last choice of drug in people with T2DM with or without established ASCVD or CKD and/or heart failure (HF). However, the second-generation SUs (glimepiride, gliclazide, and glipizide) have been reported to have a lower hypoglycemia risk, compared to other SUs. Thus, according to EASD/ADA, second-generation SUs remain a reasonable choice among the oral AHAs, owing to the favorable efficacy and safety profiles, as well as cost. 3 The Canadian guidelines do not recommend SUs for elderly patients or those with CKD. Nevertheless, if necessary, gliclazide is recommended owing to the lower risk of hypoglycemia, cardiovascular (CV) events, and mortality. 31
The RSSDI also recommends newer SUs, such as gliclazide- MR and glimepiride in people with T2DM due to less cardiovascular adverse events. 25 The South Asian Federation of Endocrine Societies (SAFES) recommend glimepiride and gliclazide modified-release (MR) to be preferred SUs over conventional SUs owing to better CV outcomes, reduced mortality and renal protection. 32 Further, the Royal Australian College of General Practitioners (RACGP)/Diabetes Australia, the Society for Endocrinology, Metabolism and Diabetes of South Africa (SEMDSA), the Dutch and Italian guidelines recommend gliclazide as it is associated with lower CV risk, lesser hypoglycemic episodes, weight neutrality, along with established microvascular benefits and lower costs.18,33
Patient-Centric Approach to Sulfonylurea Therapy
According to the most recent guideline updates, a patient-centric approach is recommended while choosing the appropriate pharmacologic therapies.3,4 While selecting the appropriate therapeutic regimen, clinicians need to consider the hypoglycemia risk, comorbidities, weight gain, adverse effects, cost, and patient preferences. Besides, the cost and benefit–risk ratio should be evaluated while choosing AHAs, 4 and a nonauthoritative management plan that involves patients as a part of “shared decision-making” should be implemented. 34
The key factors playing major roles in adopting a patient-centric approach are: (i) presence of comorbidities such as ASCVD, risk factors of high ASCVD, HF, and CKD; (ii) body weight; (iii) hypoglycemia risk; (iv) cost, (v) side effects, and (vi) patient preferences. 4
Treatment of T2DM in South Asia needs to be individualized, considering the phenotype, diverse and heterogeneous lifestyle, environmental, cultural, social, and economic factors. 35 The ADA recommends second-generation SUs for use in ASCVD or patients with HF or diabetic kidney disease (DKD) owing to non-aggravating effects on such comorbidities as well as high glucose-lowering efficacy. Moreover, cost-effective AHAs should be preferred wherever cost is a rate-limiting factor toward optimal pharmacotherapy. However, SUs should be used with caution among patients at risk of weight gain and hypoglycemia. Furthermore, SUs such as glimepiride and glipizide should be initiated conservatively in patients with renal impairment to avoid risk of hypoglycemia. 4
Special Considerations
Ramadan
Fasting during Ramadan can lead to excessive gluconeogenesis in people with diabetes, thereby increasing the risk of dehydration, diabetic ketoacidosis, both hypoglycemia and hyperglycemia, and thrombosis. 36
During Ramadan fasting, switching to newer SUs (gliclazide and glimepiride), is recommended as the risk of hypoglycemia is lower compared to glibenclamide.6,36 This has also been supported in the recent IDF-DAR practical guidelines for safe fasting during Ramadan. 36
Elderly population
Risk of hypoglycemia is higher among the elderly with sulfonylurea therapy. Nevertheless, second-generation SUs, such as gliclazide and glimepiride, may be used in elderly people due to relatively lower risk of hypoglycemia than the conventional SUs. Longer-acting second-generation SUs like glyburide should, however, be avoided in the elderly population. 37
Other special populations
During pregnancy, SUs have been reported to cross placenta in sufficient levels to induce neonatal hypoglycemia. 38 Although the second-generation SUs do not significantly cross the placenta, there is paucity and inconsistency of data regarding the use of SUs during pregnancy and lactation. 6
Sulfonylureas are not recommended in children and adolescent populations, as there is scarcity of data on the safety and efficacy of SUs6,39
In patients with comorbidities, such as CVD, second-generation SUs are preferred over first-generation SUs. Short-acting SUs like glipizide should be preferred in people with moderate-to–severe renal impairment, while second-generation SUs can be used at low doses in this group of people. 6
In people with mild-to–moderate hepatic impairment, lowering the dose of SU and/or increasing the intervals between dosing is recommended. 6
Positioning of Sulfonylurea: Authors’ Views
A patient-centered approach is advised while considering SUs therapy. Proper patient selection and patient education will help in appropriate use of this important class of drugs. Sulfonylureas are widely recommended as second-line therapy among persons with T2DM in developing countries. A careful choice among various SUs should be considered since the clinical concerns are majorly associated with use of different SU generations. Second generation SUs including gliclazide MR and glimepiride can be preferred as they are associated with better safety, glucose lowering efficacy, tolerability and are cost effective. Gliclazide MR and glimepiride have also been reported to have a relatively lower risk of hypoglycemia than the conventional SUs, patient education on hypoglycemia and the use of self-monitoring of blood glucose should be considered to minimize hypoglycemia risk especially in the elderly. Additionally, the CV safety of second-generation SUs has been established in, ADVANCE, CAROLINA and TOSCA.IT trials. However, uniformity in the recommendations still seems lacking among national and international guidelines.
A summary of recommendations and positioning of SUs based on the overall assessment of the national and international guidelines has been depicted in Table 3.
Table 3.
Appropriate and optimal use of SUs as recommended by guidelines.
| Therapy type | Usage |
|---|---|
| SU monotherapy | Monotherapy as first line if metformin is not tolerated or contraindicated.3,10,12 |
| SU as second line | When there are no indicators of high-risk or established CKD, ASCVD, or HF, and cost of treatment is a major factor. 5 |
| SU as triple-combination therapy | As third-line therapy with metformin and other agents (DPP4i, insulin, pioglitazone, GLP1-RA). 12 |
| SU as quadruple-combination therapy | In combination with metformin + GLP1RA + DPP4i or metformin + DPP4i + SGLT2i. 3 |
| SUs in combination with insulins | Gliclazide and glimepiride may be used as second line with insulin-based therapies and metformin.3,18 |
| SUs in elderly population | Gliclazide and glimepiride may be used in elderly patients, but with caution.6,37 |
| SUs in Ramadan fasting | Second-generation SUs, such as gliclazide and glimepiride, are recommended, and avoid glibenclamide. Dose reduction may be required.6,36 |
| SUs in CVD or renal impairment | Can be used in patients with CVD.
6
Patients with renal impairment are at risk of developing hypoglycemia following SU therapy; SUs to be used with caution in this group of patients. 6 |
ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DPP4i, dipeptidyl peptidase 4 inhibitor; GLP1RA, glucagon-like peptide-1 receptor agonist; HF, heart failure; SGLT2i, sodium–glucose co-transporter-2 inhibitor, SU: sulfonylurea.
There are a few limitations in this article. While an attempt was made to cover all published guidelines, the search was limited to guidelines published (language limited to English) between January 2010 and January 2021. Guidelines published post January 2021 were not considered as the article was developed in March 2021.
Conclusion
Sulfonylureas continue to play a vital role in the management of T2DM. Majority of the international and national guidelines reviewed in this article suggest newer SUs as second-line therapy for treatment of people with T2DM. The newer SUs, such as glimepiride and gliclazide MR, are efficacious, comparatively less expensive and are associated with low rates of hypoglycemia, weight gain, and cardiovascular toxicity compared to the conventional SUs. Hence, despite the continuous advent of newer glucose-lowering therapies, SUs may still be an ideal pharmacological treatment choice in developing countries.
Acknowledgments
Medical writing and editorial support has been provided by Rajshri Mallabadi of BioQuest India Private Limited and paid for by Sanofi, India. Editorial support has also been provided by Anahita Gouri and Rohan Mitra of Sanofi, India.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Sanofi, India. This initiative was supported by Sanofi India. Medical writing related charges were paid for by Sanofi India. The authors received no honoraria from Sanofi directly or indirectly related to the development of this publication.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S Amarnath is an employee of Sanofi, India. All other authors declare no conflict of interest.
Author Contributions: All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published. All authors had full access to the articles reviewed in this manuscript, take responsibility for the writing of this manuscript, including critical review and editing of each draft, and take complete responsibility for the integrity and accuracy of this manuscript.
Compliance with Ethics Guidelines: This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
ORCID iDs: Banshi Saboo  https://orcid.org/0000-0001-7293-8864
https://orcid.org/0000-0001-7293-8864
Viswanathan Mohan  https://orcid.org/0000-0001-5038-6210
https://orcid.org/0000-0001-5038-6210
References
- 1. Bashier A, Bin Hussain A, Abdelgadir E, Alawadi F, Sabbour H, Chilton R. Consensus recommendations for management of patients with type 2 diabetes mellitus and cardiovascular diseases. Diabetol Metab Syndr. 2019;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 3. Consoli A, Czupryniak L, Duarte R, et al. Positioning sulphonylureas in a modern treatment algorithm for patients with type 2 diabetes: expert opinion from a European consensus panel. Diabetes Obes Metab. 2020;22:1705-1713. [DOI] [PubMed] [Google Scholar]
- 4. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S111-S124. [DOI] [PubMed] [Google Scholar]
- 6. Kalra S, Bahendeka S, Sahay R, et al. Consensus recommendations on sulfonylurea and sulfonylurea combinations in the management of type 2 diabetes mellitus - International Task Force. Indian J Endocrinol Metab. 2018;22:132-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S73-S84. [DOI] [PubMed] [Google Scholar]
- 8. Stoica RA, Ștefan DS, Rizzo M, et al. Metformin Indications, Dosage, Adverse Reactions, and Contraindications. IntechOpen; 2020. [Google Scholar]
- 9. Stasinopoulos J, Bell JS, Manski-Nankervis J-A, Hogan M, Jenkin P, Sluggett JK. Medication management of type 2 diabetes in residential aged care. Aust J Gen Pract. 2018;47:675-681. [DOI] [PubMed] [Google Scholar]
- 10. IDF. IDF clinical practice recommendations for managing type 2 diabetes in primary care. 2018. Accessed January 12, 2021. https://www.idf.org/e-library/guidelines/128-idf-clinical-practice-recommendations-for-managing-type-2-diabetes-in-primary-care.html [DOI] [PubMed]
- 11. Zeitler P, Arslanian S, Fu J, et al. ISPAD clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19:28-46. [DOI] [PubMed] [Google Scholar]
- 12. NICE. Type 2 diabetes in adults: management. The NICE guidelines. 2015. Accessed June 20, 2020. https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#drug-treatment-2
- 13. WHO. Guidelines on Second-and Third-Line Medicines and Type of Insulin for the Control of Blood Glucose Levels in Non-Pregnant Adults with Diabetes Mellitus. World Health Organization; 2018. Accessed June 24, 2020. http://www.ncbi.nlm.nih.gov/books/NBK532229/ [PubMed] [Google Scholar]
- 14. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [DOI] [PubMed] [Google Scholar]
- 15. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 executive summary. Endocr Pract. 2020;26:107-139. [DOI] [PubMed] [Google Scholar]
- 16. Ivers NM, Jiang M, Alloo J, et al. Diabetes Canada 2018 clinical practice guidelines: key messages for family physicians caring for patients living with type 2 diabetes. Can Fam Physician. 2019;65:14-24. [PMC free article] [PubMed] [Google Scholar]
- 17. Aschner PM, Muñoz OM, Girón D, et al. Clinical practice guideline for the prevention, early detection, diagnosis, management and follow up of type 2 diabetes mellitus in adults. Colomb Med. 2016;47:109-131. [PMC free article] [PubMed] [Google Scholar]
- 18. RACGP. General practice management of type 2 diabetes. 2016. Accessed November 8, 2020. https://static.diabetesaustralia.com.au/s/fileassets/diabetes-australia/5d3298b2-abf3-487e-9d5e-0558566fc242.pdf
- 19. Chinenye S, Ofoegbu EN, Onyemelukwe GC, et al. Clinical Practice Guidelines for Diabetes Management in Nigeria. 2nd ed. 2013. Accessed October 8, 2020. http://gracelanddiabetesfoundation.org/wp-content/uploads/2018/03/Guideline-For-Diabetes-Management-In-Nigeria-2nd-Edition.pdf
- 20. Ministry of Health-Uganda. Uganda Clinical Guidelines 2016:National Guidelines forManagement of Common Conditions. 2016. Accessed October 8, 2020. https://health.go.ug/sites/default/files/Uganda%20Clinical%20Guidelines%202016_FINAL.pdf
- 21. Shera AS, Basit A, Fawwad A. Middle East and North Africa region guidelines for the management of type 2 diabetes. J Diabetol. 2019;10:134. [Google Scholar]
- 22. Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35:e3158. [DOI] [PubMed] [Google Scholar]
- 23. Kim MK, Ko SH, Kim BY, et al. 2019 clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J. 2019;43:398-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goh SY, Ang SB, Bee YM, et al. Ministry of Health Clinical Practice Guidelines: diabetes mellitus. Singapore Med J. 2014;55:334-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chawla R, Madhu S, Makkar B, et al. RSSDI-ESI clinical practice recommendations for the management of type 2 diabetes mellitus 2020. Indian J Endocrinol Metab. 2020;24:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New Engl J Med. 2008;358:2560-2572. [DOI] [PubMed] [Google Scholar]
- 27. Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. J Am Med Assoc. 2019;321:69-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 Diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalra S, Ghosh S, Das AK, et al. Unravelling the utility of modern sulfonylureas from cardiovascular outcome trials and landmark trials: expert opinion from an international panel. Indian Heart J. 2020;72:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghosh S, Mukhopadhyay P, Pandey P, Chatterjee P, Pandit K. Cardiovascular safety of glimepiride: an indirect comparison from CAROLINA and CARMELINA. Diabetes Vasc Dis Res. 2020;17:1479164120973653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lipscombe L, Booth G, Butalia S, et al. Pharmacologic glycemic management of type 2 diabetes in adults. Can J Diabetes. 2018;42:S88-S103. [DOI] [PubMed] [Google Scholar]
- 32. Kalra S, Aamir A, Das A, et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: a consensus statement. Indian J Endocrinol Metab. 2015;19:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amod A. The place of sulfonylureas in guidelines: why are there differences? Diabetes Ther. 2020;11:5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Basu S, Sharma N. Diabetes self-care in primary health facilities in India - challenges and the way forward. World J Diabetes. 2019;10:341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Misra A, Sattar N, Tandon N, et al. Clinical management of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018;6:979-991. [DOI] [PubMed] [Google Scholar]
- 36. Hassanein M, Al-Arouj M, Hamdy O, et al. Diabetes and Ramadan: practical guidelines. Diabetes Res Clin Pract. 2017;126:303-316. [DOI] [PubMed] [Google Scholar]
- 37. American Diabetes Association. 12. Older adults: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S168-S179. [DOI] [PubMed] [Google Scholar]
- 38. American Diabetes Association. 14. Management of diabetes in pregnancy: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S200-S210. [DOI] [PubMed] [Google Scholar]
- 39. American Diabetes Association. 13. Children and adolescents: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S180-S199. [DOI] [PubMed] [Google Scholar]