Abstract
The complex determinants of health and disease can be determined when approached as a system of interactions of biological agents at different scales. Like the physicochemical properties that govern nucleic acids and proteins, there should be a finite set of rules that dictate the behavior of cells to form tissues. Thus, the occurrence of disease can be seen as flaws in processes that are governed by rules pertaining to multicellular structures. Multiplexed imaging is a technology that connects information that bridges multiple biological scales (i.e., molecules, cells, and tissues) and enables elucidation of rules associated with formation of multicellular structures. Uncovering important multicellular structures associated with disease will propel a wave of development of new categories of diagnostics and therapeutics.
Keywords: Multiplexed tissue imaging, multicellular structures, spatial biology, diagnostics, therapeutics, cancer immunotherapy
Spatial Biology as the Next Frontier of Biological Signatures
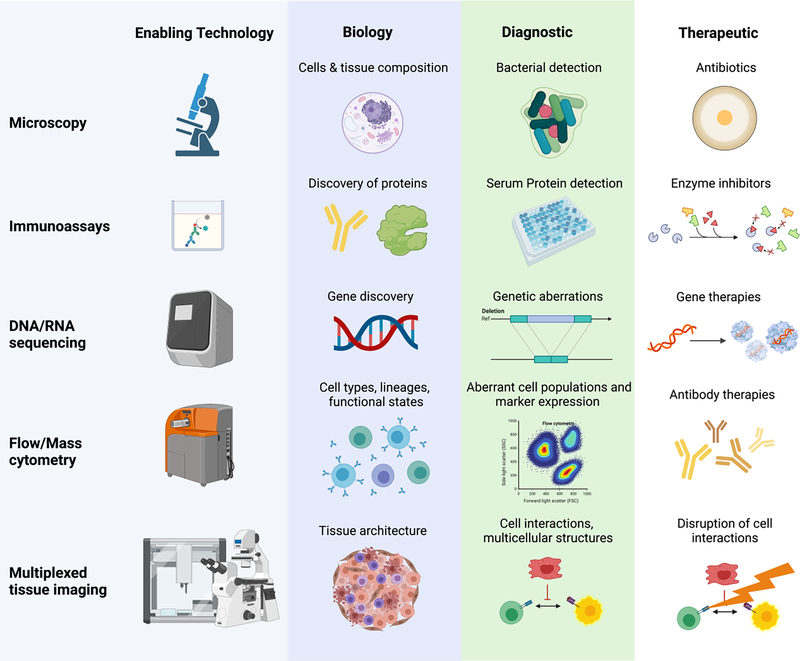
Behind every biological signature there is an enabling technology (Fig. 1). The development of the microscope revealed the world of microbes and led to identification of pathogens responsible for infections and antibiotic development. Similarly, the ability to detect and quantify proteins, nucleic acids, and single cells using recombinant antibodies, DNA sequencing devices, and multiparameter flow and mass cytometers have led to development of serum-based diagnostic assays, prognostic gene expression scores, and therapeutics such as small-molecule drugs that inhibit enzymes, gene therapies, and immunotherapies.
Figure 1: Knowledge of biology has been fueled by technology development, which has resulted in new diagnostics and therapeutics benefiting human health.
Microscopy enabled discovery of cellular structures, revealed bacteria as infection-causing agents, and spurred the development of antibiotics. Immunoassays have been used to discover distinct proteins such as enzymes allowing development of drugs targeting of specific proteins and creation of diagnostics such as viral antigen tests. DNA and RNA sequencing devices have enabled diagnostic differentiation of infectious agents such as viruses and have driven development of targeted gene therapies. Flow and mass cytometry allow characterization and quantification of multiple populations of cells and are used for diagnosis of blood-based cancers and in the development of antibody-based therapies. Finally, multiplexed imaging reveals novel spatial relationships fueling the next wave of diagnostics and therapies.
Recently, multiplexed imaging technologies have been developed that allow simultaneous probing of single cells for more than 50 protein targets or 1000 RNA targets with spatial resolution [1]. Multiplexed imaging workflows incorporate computational analysis pipelines to extract and analyze quantitative single-cell expression profiles. The resultant data reveals diverse cell types and granular cell states. Combined with the additional dimension of spatial information, cell-cell interactions, cell-cell correlations, cellular enrichments and depletions, restricted marker expression, and morphologic characterization are possible.
Several funding agencies and scientific communities have realized the importance of the new technology by establishing large consortia efforts to use multiplexed imaging techniques to create spatial atlases of human and animal model tissues. These consortia include the Human BioMolecular Atlas Program (HuBMAP), which seeks to create an atlas of healthy human organs, Human Tumor Atlas Network (HTAN), which has the goal of mapping different types of cancer, and Human Cell Atlas (HCA), which is working to map both healthy and diseased tissue [2–4], amongst others. These efforts have built on the rich history of clinical immunohistochemistry both in diagnostics and therapeutic development. In this perspective, we highlight how multiplexed imaging technologies have already produced unique spatial biological insights and have the potential to uncover multicellular modules as signatures that will fuel the next wave of diagnostic and therapeutic discoveries.
To Grok is to Know: Multiplexed Imaging Approaches for Understanding Local and Global Cellular Mechanisms
Intracellular proteins come together to create multiprotein structures with novel functions, convey signals, and then disassociate to partake in yet other multiprotein arrangements. Such flexible and plastic rearrangements underscore how just a relatively few protein species in a cell can carry out the extraordinary complexity of cellular processes. Similarly, cells such as those within a dynamic system such as the immune system might be expected to arrange themselves alternately as multiform information carriers and effectors in manners according to need and context. So, how does one address understanding how given arrangements of cells represent tissue function at the local and global scale?
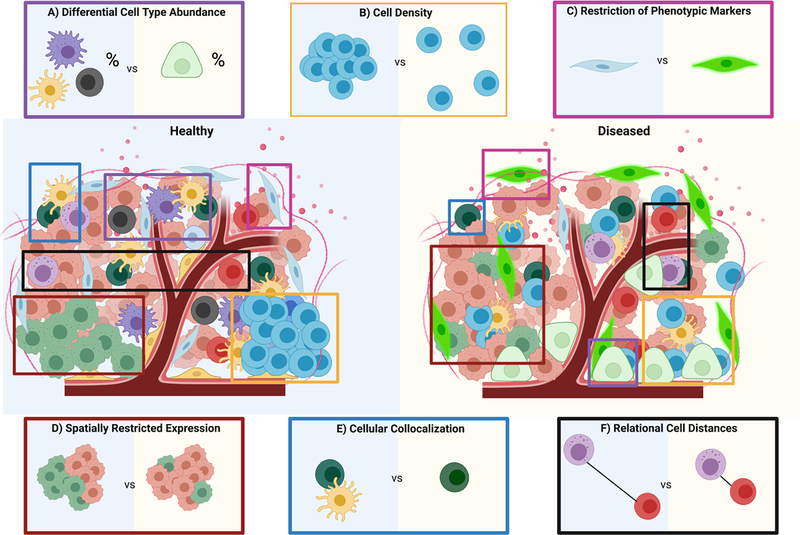
Multiplexed tissue imaging is advancing our understanding of spatial context in areas such as oncology [5–12], immunology [13,14], and microbiology [15,16]. An early focus of the field has been the tumor immune microenvironment and its implications for immunotherapy. Immunotherapies (e.g., immune checkpoint inhibitors and cellular therapies) target interactions between subsets of immune cells and cancer cells. These interactions are difficult to characterize based on single biomarkers and without spatial context [17]. With only two or three markers, multiplexed imaging can better predict response to anti-PD1/PD-L1 therapy than standard immunohistochemistry, tumor mutational burden, or gene expression profiling [18]. At the most basic level, multiplexed imaging can identify cell-type compositional differences between tumor samples (Fig. 2A). Additionally, the expression levels of markers like PD-1 and PD-L1 can be quantified on multiple cellular subsets (e.g., CD8+ T cells, CD163+ macrophages, and tumor cells) in the same tissue section. Such data have revealed that specific tissue areas (e.g. stroma vs. tumor vs. tumor-stroma boundary) have higher densities of cells that express (or do not express) certain markers (CD8+ FoxP3+ PD-1low/mid cells, PDL1− CD163+ macrophages) (Fig. 2B) and that presence of these cell types correlates with response to immunotherapy [7] (Fig. 2C).
Figure 2: Multiplexed imaging has uncovered novel spatial markers and cell-cell interactions underlying diseased states.
In this schematic, two representative tissue samples are compared. Healthy tissue is represented by a blue background and diseased tissue by a yellow background. Multiplexed imaging studies have revealed i) differential cell-type abundance in healthy and diseased tissue with distinct cell types present in diseased tissue that are not present or that are present at different levels in healthy tissue; ii) differences in local cell density iii) restriction of certain phenotypic markers to healthy or diseased cells; iv) spatially restricted expression; v) co-localization of certain cell types only in healthy or diseased tissue; and vi) unique distance relationships between cell types.
In addition to broad cellular landscape characterization, highly multiplexed imaging can be leveraged for granular spatial analysis of a limited number of cell types to provide information on cell-intrinsic molecular programs. This has been applied to study how CD8+ T cell metabolic pathways are associated with immunoregulatory marker expression, demonstrating that metabolic activity is spatially restricted at the tumor-immune boundary [14] (Fig. 2D). Thus, multiplexed imaging can identify metabolic pathways that could be targeted for therapeutic benefit.
Furthermore, multiplexed imaging enables quantification of co-localization of different stromal, immune, and tumor cell subsets (Fig. 2E). In a study that detected 36 protein markers in breast cancer samples, the interdependence of immune cell types (e.g., natural killer cell infiltration only in tumors with B cells and CD4+ and CD8+ T cells) supported coordinated cellular recruitment in the anti-tumor response [5]. Diffuse infiltration of immune cells into tumors, as opposed to high immune cell density, was identified as a clinical biomarker of outcome [5]. Strategies to interrupt or promote such occurrences have potential as cancer immunotherapies.
Co-localization of cell types often implies direct interactions between these cell types. Multiplexed imaging data can be used to create interaction signature scores based on recurrent co-localization of cells expressing cognate functional markers (e.g., immunoregulatory proteins and receptors) (Fig. 2C & 2E). This technique applied to triple negative breast cancer samples revealed that co-localization of memory T cells with tumor cells and with antigen presenting cells was important for controlling tumor biology and had prognostic value [9].
However, cellular interactions may not always become evident by direct cellular contact in the tissue sample, which represents a snapshot in time of mobile cellular elements. Consequently, a signature derived from the physical distances between PD1+ CD4+ T cells, regulatory T cells, and tumor cells was identified as a strong predictor of response to anti-PD1 immunotherapy in cutaneous T cell lymphoma (Fig. 2F) [8]. This signature captures the balance between T cell effector activity and suppression. This study also demonstrated the central role of PD1+ CD4+ T cells in the response to anti-PD1 treatment [8]. While these examples represent only a fraction of the discoveries being made, multiplexed imaging is uncovering novel mechanistic and clinical insights related to restricted cell marker expression, differential cell type abundance, and unique spatial cell-cell interactions. Basically, to understand the logic for why given local processes exist in the first place, one must understand the gestalt of the system.
Multiplex Imaging Uncovers Multicellular Modules Associated with Disease
Tissues consist of many cell types and cells in multiple functional states that interact at the molecular scale to form conserved units that coordinate tissue function. An understanding of principles of spatial organization under physiological and pathological conditions will be reached by considering expression patterns in individual cells and their direct interactions. Merging such information will enable the detection of multicellular modules within tissues as a higher level of organization. Multiplexed imaging is being used to characterize cellular interactions required for function of a given tissue, the driving forces behind recurrent tissue features, and how multicellular structures are altered in disease [19]. Such structures may be obvious (e.g., lymph follicles) or only detectable through in-depth computational analysis of multiplexed tissue imaging datasets.
Computational and statistical techniques have been developed to analyze multiplexed imaging data to define multicellular structures. Multicellular modules can be defined from raw imaging data or from segmented single-cell data as recurrent combinations of spatially restricted proteins or cell types (Fig. 3a) [6,8,10,15,16]. One approach registers neighboring cells in a local group of cells by a sliding window across the tissue, indexing each cell and then recording to varying levels the context of neighboring cells (cell type, geographic relative position, shape, etc.). Clustering of these windows revealed conserved cellular neighborhoods in the colorectal cancer invasive front [6]. These cellular neighborhoods are functionally important tissue units as the frequency of PD1+ CD4+ T cells in the granulocyte-enriched neighborhood, but not their overall frequency in the tissue, was prognostic for patient survival [6]. The important role of the functional state of a neighborhood structure extends the concept of functional cellular states. Analyzing neighborhoods rather than only individual cell-cell interactions has also proven useful in the contexts of microbial infection [15,16] and tumor subclone patterns [10].
Figure 3: Top-down and bottom-up approaches to discover multicellular structures from multiplexed imaging.
(a) Section of tonsil tissue stained with hematoxylin and eosin (H&E). Area 1 is a blood vessel-associated area, area 2 is a lymphoid structure, and area 3 is not defined by readily identifiable structural elements. (b) Example of graph from top-down analysis: e.g. fractions of cells determined by multiplexed imaging in regions pre-selected as areas of interest marked 1, 2, and 3 in panel a. (c) Bottom-up analysis enables identification of multicellular structures that are not obvious in H&E-stained images (area 3 from panel a) while also capturing known structures (areas 1 and 2 from panel a). Tissue representations colored by cell type identity (left panel) and cell neighborhood association (middle panel) as identified using bottom-up clustering of single-cell marker expression and cell co-localization profiles are shown. The right panel shows the network of neighborhood interaction rules (i.e. a hierarchical representation of recurrent co-localization of neighborhoods) that defines the structural composition of the tissue.
Inasmuch as the CNs appear to bound homogeneous processes in each region, the inter-CN regions are places where CNs might interact. Statistical methods have been developed to define important cellular neighborhood interactions. One technique investigates the border where adjacent cellular neighborhoods come into contact as an interface for cellular communication and potential signal exchange [19]. The enrichment and conservation of borders between neighborhoods defines spatial contexts that are required for maintenance of multi-cellular structures. Thus, the specific functions of a tissue are reflected in the assembly of its cellular neighborhoods with respect to the spatial contexts formed between them.
With the ability to capture tissue organization at different scales of cell types, cell-cell interactions, and cellular neighborhoods comes the need to integrate this information. This has been achieved by decomposition into tissue modules using tensor techniques. Decomposition of the colorectal cancer immune microenvironment revealed coupling of T cell- and macrophage-enriched neighborhoods that were anti-correlated in terms of their cytotoxic and regulatory T cell content [6]. This analysis suggests that inter-neighborhood communication is a constituting principle of the tumor microenvironment and of the anti-tumor immune response.
A second computational method to map tissue composition from simpler parts is based on the identification of assembly rules that govern conserved tissue architecture. For example, there are repeating patterns of connections among cellular neighborhoods within immune follicles in different tissues with similar functions (e.g., in lymphatic organs and the tumor immune microenvironment). Moreover, tissue-specific assembly rules highlight unique tissue functions linked to disease processes. Illustrating these principles, corruption of actively assembled higher order tissue motifs in the immune microenvironment by an infiltrating tumor is prognostic for patient survival in colorectal cancer [19].
The strategies described so far rely on “bottom-up” analysis of multiplex imaging data using spatial statistics to infer higher order structure from marker expression profiles and spatial location of single cells (Fig. 3a). Conversely, “top-down” analysis begins from established histopathologic features with known relevance and dissects their molecular underpinnings using computational methods (Fig. 3b). The top-down strategy builds upon the decades of pathology knowledge about larger structures associated with disease. One recent example leveraged previously established pathologic features of colorectal cancer (e.g., tumor differentiation, grade, subtype, lymphatic/vascular/immune density, budding) and found pronounced variability in immunosuppressive interactions at different morphologically defined tumor areas [11].
The “top-down” approach is especially powerful in tissues with heterogeneous distribution of distinct architectural features, especially tissues captured with whole slide imaging. This can inform selection of areas to image and guide analysis towards phenotypic patterns of interest. In contrast, tissues in microarray (TMA) formats require pre-selection of pathologic or structural features. Although the microarray format enables high throughput, this type of targeted imaging may limit the ability to make statements about tissue heterogeneity, the contributions of other non-included tissue structures, and larger gradations [6,16]. Ideally, synergy of both bottom-up and top-down approaches could be leveraged. Such an approach has been used to understand the immune response to breast cancer with multiplexed imaging. It was found that immune infiltrate sizes and the numbers of blood vessels are correlated with the presence of specific immune subsets [5].
Uncovering disease-associated multicellular structures within tissues from archived samples will spark targeted, mechanistic multiplexed imaging studies to identify the factors that underlie coordinated behavior of these networks and the molecular markers associated with these structures—as both are necessary for therapeutic development. Studying the tumor microenvironment will identify factors that could be targeted to disrupt cancer-promoting tissue networks. Such therapeutics could actively interfere with the composition and organization of tumor-promoting tissue structures or could facilitate formation of structures associated with tumor-suppression [20]. This has already been realized clinically with the advent of the checkpoint inhibitors. Through understanding of more complex cell interactions, and coupled with predictive signatures developed from multiplexed imaging, we envision additional therapies that can be complementary with current cancer cell- and T cell-directed treatments in a tumor subtype- or patient-specific way.
Concluding Remarks and Future Directions
We expect several technical and computational advances to occur in the next few years that will further enable translational research using multiplexed imaging technologies. To ensure clinically and statistically relevant data, methods will be developed to increase throughput and standardization. Currently, multiplexed techniques leverage lower-plex (1–7 markers) but higher throughput assays as validation [8]; however, more powerful sample processing automation and computational frameworks are already beginning to be developed [7]. Further progress in throughput will be critical as diagnostic signatures from multiplexed imaging become more complex. In large part through consortia efforts, there has been standardization in both the technical and computational spaces that will enable data cross-comparisons [21–23]. This will be essential for widespread adoption of multiplexed imaging techniques and the ability to leverage pre-existing datasets.
Beyond higher throughput, we expect an increase in the depth of information as well. Currently, most techniques rely on either protein or RNA readouts, though many groups have analyzed for both on different sections of the same sample [8,16]. Current methods for simultaneous protein and RNA detection from the same tissue are still limited by the number of markers and/or the lack of single cell resolution [15,24]. We expect techniques to be developed to enable protein and RNA analysis simultaneously at the single cell level. Another avenue that will increase the depth of information is computational association of multimodal datasets, like what has been developed for integrating individual single-cell RNA-seq and single-cell ATAC-seq datasets. Govek et al. have already developed a technique for aligning CITE-seq data with CODEX multiplexed imaging data [25]. The ability to perform both protein and RNA measurements on the same sample will be critical for associating diagnostic multicellular modules with therapeutically targetable signaling networks.
We expect that computational advances will also facilitate identification and dissection of complex multicellular modules that can serve as the basis for new diagnostics and therapeutics. Machine learning is used to segment out individual cells in images for single-cell quantification [26] and to quantify lower-plex immunohistochemistry data [27]. Machine learning approaches could be extended to enable segmentation of complex, multicellular structures such as the glomeruli of the kidney and to automate cell type annotation. We also expect that bottom-up approaches will be developed that provide a statistical framework to define rules governing formation of multicellular structures and to quantitatively describe complex multicellular structures within tissues. Both top-down and bottom-up approaches will be necessary for accurate validation of mechanisms of structure formation and to compare compositions within distinct tissue structures to establish diagnostics and evaluate therapeutics.
As our ability to identify multicellular modules advances, it will be necessary to increase our ability to create and perturb such structures in vitro and in silico. The development of more complex in vitro models can be accomplished using organs-on-a-chip, organoids, and biomaterials used for tissue engineering [28–30]. We expect that such models will complement multiplexed imaging and will allow testing of novel therapeutics designed to destroy targeted multicellular structures or to facilitate their formation. Similarly, we expect that in silico models such as multiscale agent-based models will be used as companions for testing complex networked hypotheses related to the formation of multicellular structures [31].
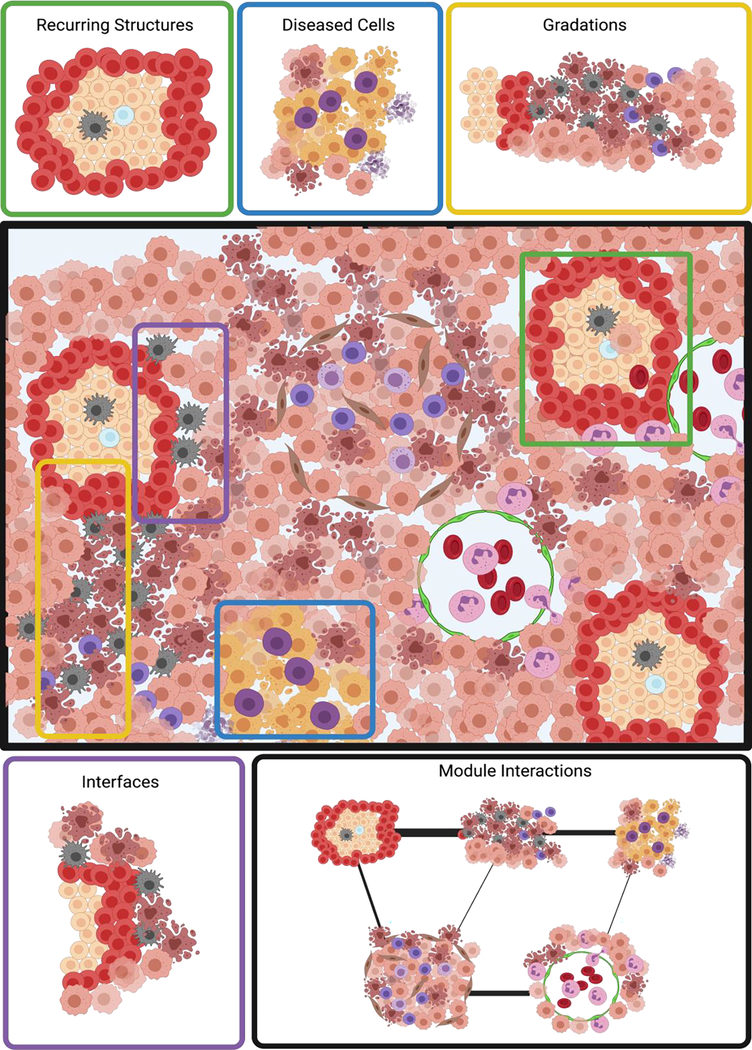
Traditionally multicellular structures have been studied by top-down histological techniques. Multiplexed imaging technologies now allow both detailed top-down study of structures and bottom-up reconstruction of multicellular structures. Although this type of imaging has only been made possible recently, multiplexed imaging studies have already identified cellular (cell density, localization or presence of phenotypic markers, co-localization or distance between cell types) and multicellular (cell neighborhoods, multicellular structures) determinants of diseased tissue. Analyses of multiplexed images have led to discovery of recurring cellular neighborhoods [6], critical tissue interfaces [5], functional gradients [11], diseased cell microenvironments [15], and conserved networks of multicellular structure interactions (Fig. 4). Technological and computational innovations are expected to fuel further breakthroughs that will provide critical missing biological insights into disease and that will lead to targeted approaches to cancer therapy development and patient stratification (Outstanding questions box).
Figure 4: Multicellular structures of particular interest that could be identified by multiplex tissue imaging.
In-depth computational analysis of multiplexed imaging datasets enables identification of recurring, multicellular structures within tissues based on the spatially restricted presence of individual cell types and combinations thereof. Centering cellular neighborhoods around diseased cells (e.g., cancer cells) allows for analysis of their immediate local cellular context. Gradations of cell density may imply underlying spatial differences in cytokine and chemokine concentrations (top row). Regions where different structures interface are likely important for inter-region communication and thus for tissue assembly, which can be abstracted as a network of individual neighborhoods (bottom row).
Outstanding questions box.
What level of structural detail (cell-cell interaction, cellular neighborhood or multi-neighborhood interactions) will be most valuable in terms of diagnostic and therapeutic targetability?
How can significant, multicellular structures be targeted without unintended consequences on tissue function?
Can defined multicellular structures be identified by high-throughput techniques that can be used clinically or can imaging and data analysis workflows be simplified and standardized to enable clinical adoption?
How does one assign meaning to groups of cells that collectively enable abstract, emergent functions that transcend individual cell definitions?
Highlights.
Multiplex tissue imaging enables in situ detection of more than 50 proteins or 1000 RNAs in the same tissue section with single-cell resolution
Analyses of spatially resolved cellular phenotypes, interactions, and neighborhoods have revealed mechanistic insights into physiological and pathological processes
Multicellular, spatially informed signatures can serve as disease biomarkers and provide new targets for therapeutic development
Acknowledgments
Figures were created using BioRender.com.
Funding
This work was supported by the U.S. National Institutes of Health (5U19AI057229-18, 5P01HL10879708, 5R01GM10983604, 5R33CA18365403, 5U01AI101984-09, 5UH2AR06767604, 5R01CA19665703, 5U54CA20997105, 5F99CA212231-02, 1F32CA233203-01, 5U01AI140498-04, 1U54HG010426-03, 5U19AI100627-09, 1R01HL120724-01A1, R33CA183692, R01HL128173-04, 5P01AI131374-04, 5UG3DK114937-02, 5U19AI135976-04, IDIQ17X149, 1U2CCA233238-01, 1U2CCA233195-01, 5U2CCA233195-02, UH3DK114937); the U.S. Department of Defense (W81XWH-14-1-0180, W81XWH-12-1-0591); the U.S. Food and Drug Administration (HHSF223201610018C, DSTL/AGR/00980/01); Cancer Research UK (C27165/A29073); the Bill and Melinda Gates Foundation (OPP1113682); the Cancer Research Institute; the Parker Institute for Cancer Immunotherapy; the Kenneth Rainin Foundation (2020-1463); the Silicon Valley Community Foundation (2017-175329 and 2017-177799-5022); the Beckman Center for Molecular and Genetic Medicine; Juno Therapeutics, Inc. (122401); Pfizer, Inc. (123214); Celgene, Inc. (133826, 134073); Vaxart, Inc. (137364); and the Rachford & Carlotta A. Harris Endowed Chair to G.P.N. J.W.H. was supported by an NIH T32 Fellowship (T32CA196585) and an American Cancer Society - Roaring Fork Valley Postdoctoral Fellowship (PF-20-032-01-CSM). M.A.B. was funded by a Career Development Award of the International Myeloma Society.
Conflicts of Interest
G.P.N. received research support from Pfizer, Vaxart, Celgene, and Juno Therapeutics during the course of this work. G.P.N. is an inventor on US patent 9909167, granted to Stanford University, that covers some aspects of CODEX multiplexed imaging technology. G.P.N. has equity in and is on the scientific advisory board of Akoya Biosciences, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tan WCC et al. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 2020. Apr 1;40(4):135–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rozenblatt-Rosen O et al. The Human Tumor Atlas Network: Charting Tumor Transitions across Space and Time at Single-Cell Resolution. Cell. 2020. Apr 16;181(2):236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The human body at cellular resolution: the NIH Human Biomolecular Atlas Program. Nature. 2019. Oct 10;574(7777):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regev A et al. The human cell atlas. Elife. 2017. Dec 5;6:e27041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keren L et al. A Structured Tumor-Immune Microenvironment in Triple Negative Breast Cancer Revealed by Multiplexed Ion Beam Imaging. Cell. 2018. Sep 6;174(6):1373–1387.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schürch CM et al. Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front. Cell. 2020. Sep 3;182(5):1341–1359.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry S et al. Analysis of multispectral imaging with the AstroPath platform informs efficacy of PD-1 blockade. Science (80−). 2021. Jun 11;372(6547):eaba2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips D et al. Immune cell topography predicts response to PD-1 blockade in cutaneous T cell lymphoma. medRxiv. 2020. Dec 8; 10.1101/2020.12.06.20244913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patwa A et al. Multiplexed imaging analysis of the tumor-immune microenvironment reveals predictors of outcome in triple-negative breast cancer. Commun Biol. 2021. Dec 9;4(1):852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rovira-Clave X et al. Spatial Epitope Barcoding Reveals Subclonal Tumor Patch Behaviors. SSRN Electron J. 2021. Jun 17; 10.2139/ssrn.3865280 [DOI] [Google Scholar]
- 11.Lin J-R et al. Multiplexed 3D atlas of state transitions and immune interactions in colorectal cancer. bioRxiv. 2021. Apr 2; 10.1101/2021.03.31.437984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rovira-Clavé X et al. Subcellular localization of biomolecules and drug distribution by high-definition ion beam imaging. Nat Commun. 2021. Dec 30;12(1):4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goltsev Y et al. Deep Profiling of Mouse Splenic Architecture with CODEX Multiplexed Imaging. Cell. 2018. Aug 9;174(4):968–981.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann FJ et al. Single-cell metabolic profiling of human cytotoxic T cells. Nat Biotechnol. 2021. Feb;39(2):186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang S et al. Virus-Dependent Immune Conditioning of Tissue Microenvironments. bioRxiv. 2021. May 25; 10.1101/2021.05.21.444548 [DOI] [Google Scholar]
- 16.McCaffrey EF et al. Multiplexed imaging of human tuberculosis granulomas uncovers immunoregulatory features conserved across tissue and blood. bioRxiv. 2020. Jun 9; 10.1101/2020.06.08.140426 [DOI] [Google Scholar]
- 17.Bruni D et al. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020. Aug 4;20(11):662–80. [DOI] [PubMed] [Google Scholar]
- 18.Lu S et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade. JAMA Oncol. 2019. Aug 1;5(8):1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhate SS et al. Tissue schematics map the specialization of immune tissue motifs and their appropriation by tumors. Cell Syst. 2021. Oct 14; 10.1016/J.CELS.2021.09.012 [DOI] [PubMed] [Google Scholar]
- 20.Egen JG et al. Human Anti-tumor Immunity: Insights from Immunotherapy Clinical Trials. Immunity. 2020;52(1):36–54. [DOI] [PubMed] [Google Scholar]
- 21.Hickey JW et al. Spatial mapping of protein composition and tissue organization: a primer for multiplexed antibody-based imaging. arXiv. 2021. Jul 16; https://arxiv.org/abs/2107.07953v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black S et al. CODEX multiplexed tissue imaging with DNA-conjugated antibodies. Nat Protoc. 2021. Jul 2;16(8):3802–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taube JM et al. The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation. J Immunother Cancer. 2020. May 1;8(1):e000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y et al. High-Spatial-Resolution Multi-Omics Sequencing via Deterministic Barcoding in Tissue. Cell. 2020. Dec 10;183(6):1665–1681.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govek KW et al. Single-cell transcriptomic analysis of mIHC images via antigen mapping. Sci Adv. 2021. Mar 1;7(10):eabc5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenwald NF et al. Whole-cell segmentation of tissue images with human-level performance using large-scale data annotation and deep learning. bioRxiv. 2021. Mar 2; 10.1101/2021.03.01.431313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghahremani P et al. DeepLIIF: Deep Learning-Inferred Multiplex ImmunoFluorescence for IHC Image Quantification. bioRxiv. 2021. Jul 27; 10.1101/2021.05.01.442219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B et al. Advances in organ-on-a-chip engineering. Nat Rev Mater 2018 38. 2018. Aug 1;3(8):257–78. [Google Scholar]
- 29.Gaharwar AK et al. Engineered biomaterials for in situ tissue regeneration. Nat Rev Mater 2020 59. 2020. Jul 6;5(9):686–705. [Google Scholar]
- 30.Kim J et al. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 2020 2110. 2020. Jul 7;21(10):571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skalnik CJ et al. Whole-Colony Modeling of Escherichia coli. bioRxiv. 2021. Apr 27; 10.1101/2021.04.27.441666 [DOI] [Google Scholar]