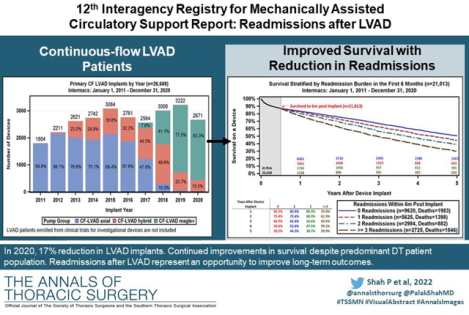
Abstract
The twelfth annual report from the Society of Thoracic Surgeons (STS) Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs) highlights outcomes for 26,688 continuous-flow LVAD patients over the past decade (2011–2020). In 2020, we observed the largest drop in yearly LVAD implant volumes since the registry’s inception, which reflects the effects of the COVID-19 pandemic on cardiac surgical volumes in the United States. The 2018 heart transplant allocation policy change in the U.S. continues to affect LVAD implantation volumes and device strategy, with 78.1% of patients now implanted as destination therapy. Despite an older and sicker patient cohort, survival in the recent era (2016–2020) at one- and two-years continues to improve at 82.8% and 74.1%. Patient adverse event profile has also improved in the recent era, with significant reductions in stroke, gastrointestinal bleeding, infection, and device malfunction/pump thrombosis. Finally, we review the burden of readmissions after LVAD implant and highlight an opportunity to improve patient outcomes by reducing this frequent and vexing problem.
Graphical Abstract
Introduction
The landscape of durable mechanical circulatory support (MCS) and left ventricular assist device (LVAD) therapy has undergone a significant transformation over the past 5-years. This evolution has been shaped by five noteworthy events. First was the landmark decision by the United Network for Organ Sharing (UNOS) in October 2018 to approve a new heart allocation policy for the U.S. to provide increased discernment to the sickest cohort of transplant candidates and to balance geographical inequities in organ distribution. These changes resulted in an immediate and profound disadvantage to implantation of durable MCS systems favoring instead the use of temporary MCS devices to achieve a higher priority transplant status. As a result, the use of the BTT indication has fallen dramatically. Secondly, a series of reports from the largest to-date clinical trial of LVAD therapy demonstrated significant reductions in the rates of device thrombosis, stroke, gastrointestinal bleeding (GIB) and mortality with the newest generation centrifugal-flow device, Heartmate 3 compared to the older generation axial-flow Heartmate II (Abbott Labs, Chicago IL).(1) Third, was the announcement on June 3, 2021, by Medtronic Inc., to withdraw the Heartware™ Ventricular Assist Device (HVAD™) from the global market. This was caused by years of concern around a higher burden of neurologic events(2) and all-cause mortality with the HVAD as well as several Class I U.S. Food and Drug Administration (FDA) recalls attributed to device malfunction related to a failure to restart the pump following pump stoppages that resulted in patient deaths. The net culmination of these events has left the MCS community, and the patients they serve, with only one FDA-approved durable LVAD option, while we await newer generation devices to undergo preclinical and clinical evaluation. Fouth, one cannot overstate the myriad effects that the devasting COVID-19 pandemic has had on the care of patients with heart failure. The restricted access to in-person heart failure clinic visits and reluctance to seek acute care in hospitals resulted in patients experiencing a higher rate of hospitalization and mortality during the pandemic.(3,4) Yearly improvements in cardiovascular mortality preceding the pandemic may have been short-lived given the approximate increase in excess cardiovascular deaths occurring during the pandemic.(5) Finally, the pandemic led to a 52.7% reduction in cardiac surgical volumes and a concomitant increase in mortality after surgery, without a return to baseline.(6)
The Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs) has in parallel undergone a significant transformation. Originally developed as a public-private partnership involving the National Heart, Lung, and Blood Institute (NHLBI), FDA, industry, hospitals, Centers for Medicaid and Medicare Services (CMS), and University of Alabama at Birmingham in 2005. Registry oversight and administration was transferred to The Society of Thoracic Surgeons (STS) National Databases in January of 2018. Undoubtedly, since its inception, Intermacs has provided critical data to understand real-world outcomes of durable MCS and identified opportunities for improvements in device design and clinical management. At STS Intermacs, these objectives continue to be priorities of the Registry, with enhanced focus on providing high-quality data on patient outcomes that can be used by individual centers to benchmark their own performance. In addition, STS Intermacs can identify quality metrics for improving programmatic outcomes as well as identify opportunities to inform the design of the next generation of durable MCS devices. Individual centers now have available to them an online dashboard to benchmark their program against STS Intermacs for several quality measures of potential interest, including hospital readmission, infection, stroke, GIB, and heart transplant rates.
In this year’s annual STS Intermacs report, we continue to enhance the standardized reporting structure which is common to all STS National Databases. The report should provide a reference benchmark that can inform payors, providers, patients, industry, and national as well as international agencies on the current state of durable MCS. In addition, we perform the first comprehensive analysis of hospital readmissions using STS Intermacs after LVAD implant, describe the epidemiology, and highlight the consequences of readmission.
Patients and Methods
The twelfth STS Intermacs Annual Report includes all adult (aged ≥19 years) patients who underwent implantation of an FDA-approved durable MCS device from January 1, 2011, to December 31, 2020, with follow-up through June 30, 2021. Individuals on total artificial heart (TAH) support, pulsatile-flow LVAD, isolated right ventricular assist device (RVAD) support, or those receiving a biventricular assist device (BiVAD) support at the time of index LVAD operation are included in Figure 1, but data analyses were subsequently focused solely on those patients who received an isolated primary continuous-flow (CF) LVAD (n=26,688).
Figure 1:
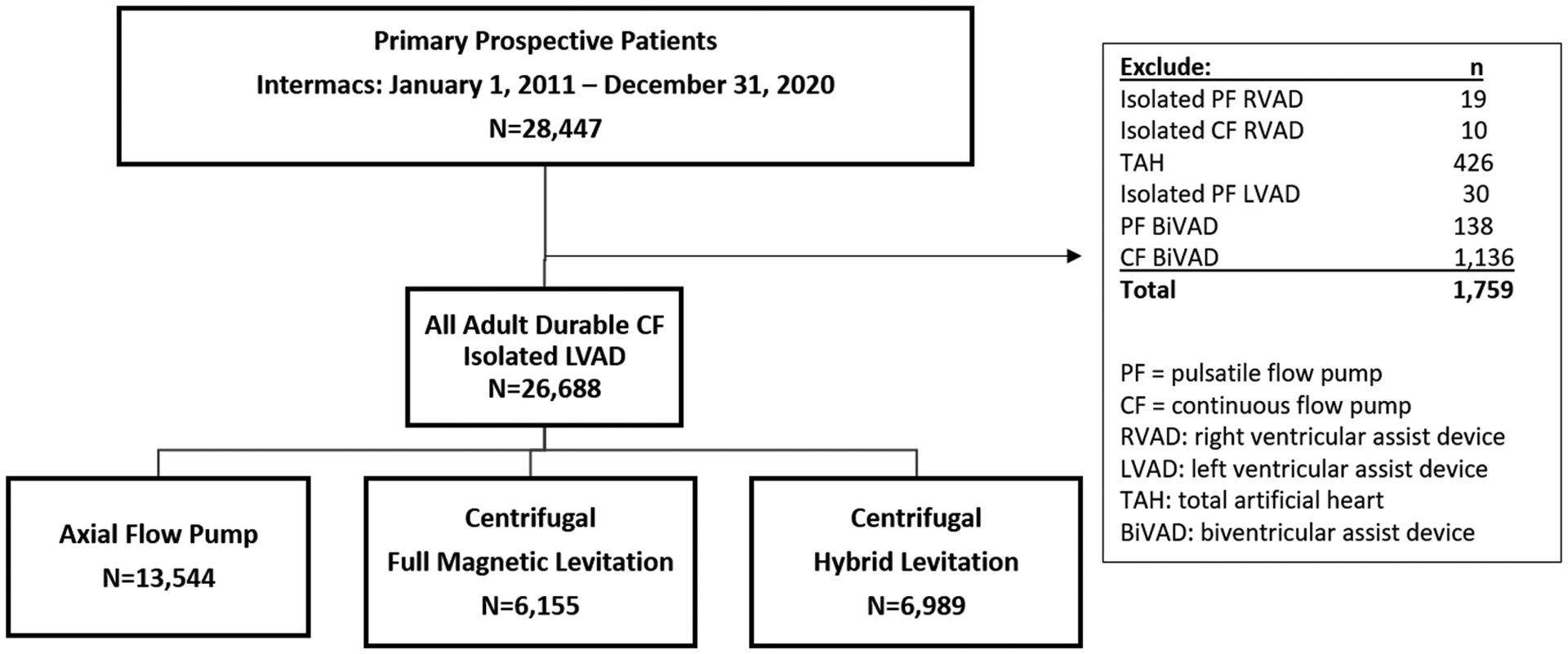
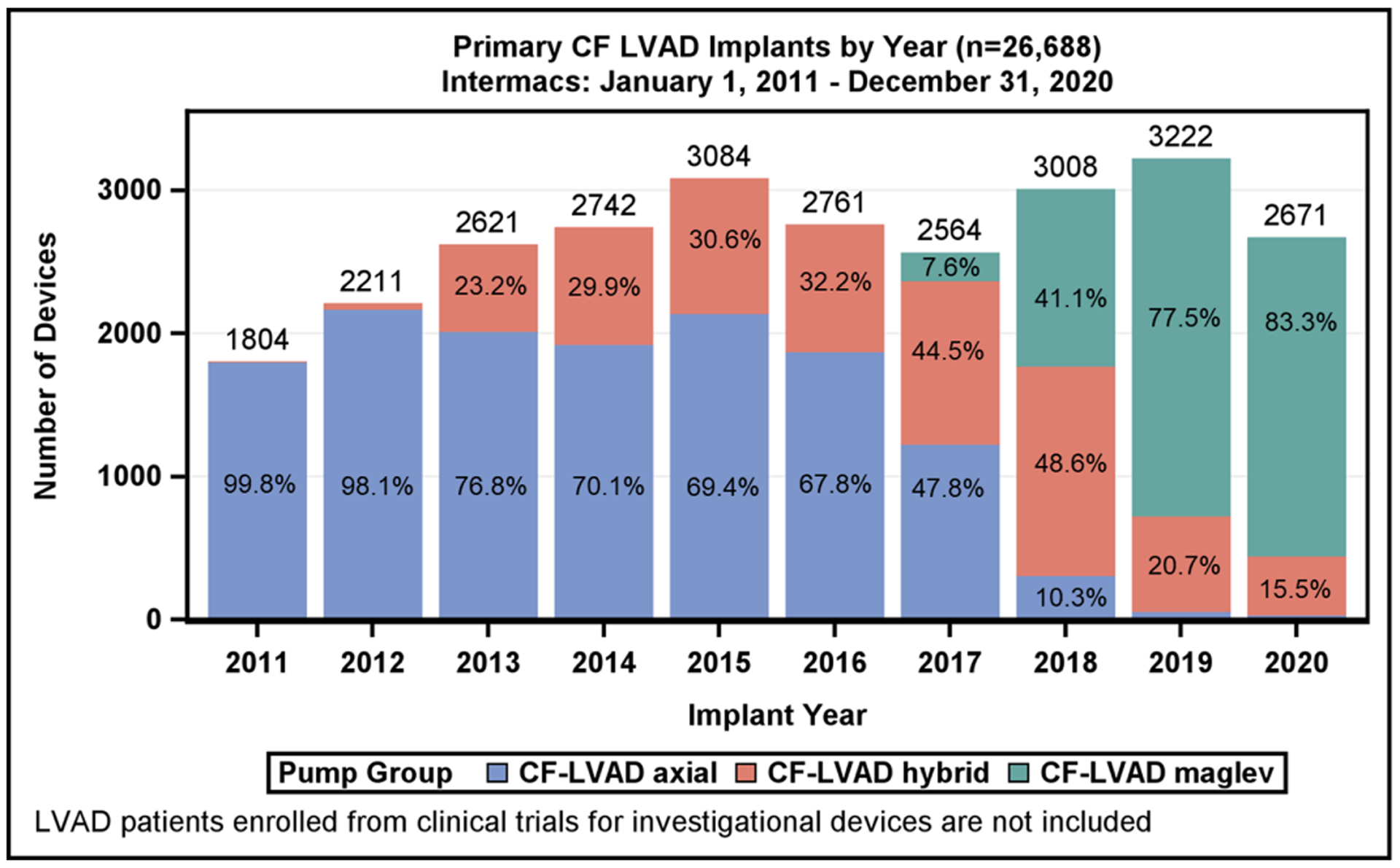
Consort Diagram with All Mechanical Circulatory Support Devices and Left Ventricular Assist Device Implants based on Flow-Type Over Time. A) Diagram depicts all durable mechanical circulatory support (MCS) devices entered into the Society of Thoracic Surgeons Interagency Registry for Mechanically Assisted Circulatory Support (STS Intermacs), January 1, 2011 to December 31, 2020. B) Annual yearly continuous-flow left ventricular assist device (CF-LVAD) implants by flow configuration.
Preimplant patient characteristics and demographics, perioperative details, and adverse events during isolated CF LVAD support are reported for patients enrolled into STS Intermacs during the last 10 years. For all adverse events, STS Intermacs definitions from version 4.0 were used. Generally, analyses were dichotomized into two eras: 2011–2015 and 2016–2020. Reporting of adverse events, rehospitalizations, and causes of death were limited to the latter era (January 2016 to December 2020). Events were categorized as early (occurring 0–90 days after implant) and late (occurring >90 days postoperatively).
The analyses reported here were approved by the STS Intermacs/PediMACS Committee of the STS Access & Publications Task Force under the Workforce on Research Development.
Statistical Analysis
For descriptive purposes, categorical variables are expressed as frequencies and percentages. Continuous variables are expressed as means ± standard deviation. Categorical variables were compared with chi-squared testing and continuous variables with the t-test. Kaplan-Meier survival estimates were calculated, censoring patients at the time of transplantation or cessation of device support. Patients undergoing a device exchange were not censored in the analysis. For all survival analyses, differences for specific subsets of data were compared with log-rank testing. Outcomes associated with specified strategies at the time of implant, including bridge to transplant (BTT), bridge to candidacy (BTC), and destination therapy (DT), were examined using the competing outcomes analysis by Fine and Gray, in which multiple mutually exclusive outcomes are tracked over time. At any point in time, the sum of the proportion (percentage) of patients in each outcome category equals 100%. Adverse events were calculated as event count, event rate (per patient-year), patient count, and patient percentage. Multiphase parametric hazard modeling was used to identify the shape of hazard (instantaneous risk) for post-LVAD death. This method has been used extensively to identify the changing hazard profiles post-surgery and the association of risk factors with different phases of risk.(7) Up to three phases of risk (early declining phase, constant phase, and late phase) are evaluated. For this analysis an early and constant phase best fit the shape of the hazard. A p-value of <0.05 was considered statistically significant. Statistical analysis was quantified with SAS 9.4 software (SAS Institute, Inc, Cary, NC).
Results
Intermacs MCS Implant Volumes Over Time for All Devices
From January 2011 to December 2020, 28,447 adult patients received an FDA-approved durable MCS device with STS Intermacs registration. The distribution of patients by device type is shown in Figure 1. For the reported 10-year period, 29 patients (0.1%) received an isolated right ventricular assist device (RVAD), 426 (1.5%) underwent total artificial heart (TAH) implant, 168 (0.6%) received a pulsatile-flow durable LVAD or pulsatile BiVAD, and 27,824 (97.8%) underwent implantation of a durable CF LVAD or CF BiVAD during the index LVAD operation. Most patients, 26,688 (96%), were supported by an isolated CF LVAD, and 1,136 (4%) had a CF BiVAD (the majority, 91.2%, were temporary CF RVADs). Implant volumes for all MCS devices over the last 10 years are shown in Supplemental Figure 1. A significant drop occurred in the number of TAHs performed from 278 in the first era (2011–2015) to 148 in the most recent era (2016–2020); on the other hand, the number of CF BiVADs increased across eras from 475 to 661. The yearly frequency of isolated LVAD implants according to device flow type is shown in Figure 1. As noted in prior reports, a decline in the total number of CF LVADs implanted occurred in 2016 and 2017, attributed largely to the ~1,200 US LVAD patients being enrolled into the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 (MOMENTUM 3) investigation device exemption (IDE) trial(1) and continued access protocol (CAP)(8) prior to FDA approval of the device. A rebound followed in 2018 and 2019 with the subsequent FDA approval of the HeartMate 3 for both short- and long-term use as well as FDA approval of the HVAD for DT indication and established a record number of primary CF LVAD implants (3222) in 2019.
In 2020, a dramatic 17% reduction in LVAD implant volume was seen in the U.S. This reduction likely resulted from a combination of the COVID-19 pandemic (decreased cardiac operations in general) and the reduced transplant priority for LVAD patients with the revised allocation system (increased use of temporary MCS as BTT therapy).(9)
Isolated CF LVAD Cohort Characteristics
From the first era (2011–2015) to the current era (2016–2020), the populations receiving a CF LVAD exhibited some notable differences (Table 1). In the current era, there was a decrease in representation of White patients (67.8% to 62.0%, p<0.0001), and patients with Hispanic ethnicity had modestly higher representation (6.3% to 7.3%, p=0.0011). In the current era, there was a lower proportion of patients with severe diabetes, prior implantable cardioverter defibrillator (ICD), and history of cardiac surgery, but modestly higher proportion of patients on dialysis compared to the prior era. Overall, patients had higher clinical severity in the most recent era. Patients in the most recent era had higher proportion of inotrope use or bridging with temporary MCS prior to LVAD (p<0.0001). In addition, the growing recognition of heart failure cardiogenic shock(10) plus the greater availability and expertise with temporary MCS across U.S. hospitals will likely lead to a continued increase in the number of patients supported with temporary MCS prior to LVAD.
Table 1:
Baseline Patient Characteristics on Continuous-Flow Left Ventricular Assist Device Support
| Patient Characteristics | All Patients | 2011–2015 Era | 2016–2020 Era | p-value* |
|---|---|---|---|---|
| (n=26,668) | (n=12,462) | (n=14,226) | ||
| Demographics | ||||
| Age at Implant (years) | 57.2 ± 13.0 | 57.2 ± 12.9 | 57.2 ± 13.0 | 0.7 |
| Female | 5766 (21.6) | 2643 (21.2) | 3123 (22.0) | 0.14 |
| Race | <.0001 | |||
| White | 17275 (64.7) | 8452 (67.8) | 8823 (62.0) | |
| Black | 6902 (25.9) | 2926 (23.5) | 3976 (27.9) | |
| Other | 2511 (9.4) | 1084 (8.7) | 1427 (10.0) | |
| Hispanic | 1784 (6.8) | 777 (6.3) | 1007 (7.3) | 0.0011 |
| BMI | 28.7 ± 7.2 | 28.6 ± 7.0 | 28.7 ± 7.5 | 0.27 |
| Medical History | ||||
| Severe Diabetes | 2897 (10.9) | 1492 (12.0) | 1405 (9.9) | <.0001 |
| Dialysis | 735 (2.8) | 301 (2.4) | 434 (3.1) | 0.0016 |
| Current ICD | 20857 (78.7) | 10092 (81.5) | 10765 (76.1) | <.0001 |
| History of Cardiac Surgery | 8436 (31.6) | 4258 (34.2) | 4178 (29.4) | <.0001 |
| Indication | ||||
| Device Strategy | <0.0001 | |||
| Bridge to Transplant - Listed | 5,607 (21.9) | 2,647 (24.2) | 2,960 (20.3) | |
| Bridge to Candidacy | 6,874 (26.9) | 3,559 (32.5) | 3,315 (22.7) | |
| Destination Therapy | 12,865 (50.4) | 4,669 (42.7) | 8,196 (56.1) | |
| Other | 205 (0.8) | 69 (0.6) | 136 (0.9) | |
| Severity of Illness | ||||
| Patient Profile | <.0001 | |||
| 1. Critical Cardiogenic Shock | 4406 (16.5) | 1850 (14.8) | 2556 (18.0) | |
| 2. Progressive Decline | 9193 (34.4) | 4396 (35.3) | 4797 (33.7) | |
| 3. Stable but inotrope dependent | 9160 (34.3) | 4022 (32.3) | 5138 (36.1) | |
| 4. Resting Symptoms | 3851 (14.4) | 2126 (17.1) | 1725 (12.1) | |
| 5–7. Ambulatory Heart Failure | 78 (0.3) | 68 (0.5) | 10 (0.1) | |
| Inotropes | 22034 (82.9) | 10061 (81.0) | 11973 (84.5) | <.0001 |
| Temporary Circulatory Support | 7381 (33.8) | 2734 (27.6) | 4647 (38.9) | <.0001 |
| ECMO | 1284 (4.8) | 398 (3.2) | 886 (6.2) | <.0001 |
| IABP | 6408 (24.0) | 2763 (22.2) | 3645 (25.6) | <.0001 |
| Cardiac primary diagnoses | ||||
| Primary Heart Failure Etiology | <.0001 | |||
| Ischemic Cardiomyopathy | 10316 (38.7) | 5045 (40.5) | 5271 (37.1) | |
| Non-ischemic Cardiomyopathy | 13757 (51.5) | 6226 (50.0) | 7531 (52.9) | |
| Other | 2615 (9.8) | 1191 (9.6) | 1424 (10.0) | |
| Laboratory values | ||||
| Albumin (g/L) | 34.1 ± 6.4 | 34.0 ± 6.7 | 34.2 ± 6.1 | 0.0218 |
| BUN (mg/dL) | 29.1 ± 17.4 | 29.2 ± 17.9 | 29.0 ± 16.9 | 0.3789 |
| Creatinine (mg/dL) | 1.4 ± 0.7 | 1.4 ± 0.7 | 1.4 ± 0.7 | 0.4484 |
| INR | 1.3 ± 0.5 | 1.3 ± 0.5 | 1.3 ± 0.5 | <.0001 |
| Platelets (x10/uL) | 196.1 ± 80.3 | 196.6 ± 79.2 | 195.7 ± 81.4 | 0.3277 |
| ALT (u/L) | 60.2 ± 185.3 | 67.0 ± 228.9 | 54.5 ± 137.7 | <.0001 |
| AST (u/L) | 51.5 ± 178.9 | 55.5 ± 203.5 | 48.1 ± 154.9 | 0.001 |
| Total Bilirubin (mg/dL) | 1.3 ± 1.7 | 1.4 ± 1.9 | 1.3 ± 1.6 | 0.0043 |
| Echocardiography | ||||
| LVEDD (cm) | 6.8 ± 1.1 | 6.8 ± 1.1 | 6.8 ± 1.1 | <.0001 |
| LVEF | <.0001 | |||
| ≥ 40 | 153 (0.6) | 80 (0.6) | 73 (0.5) | |
| 30–39 | 938 (3.5) | 481 (3.9) | 457 (3.2) | |
| 20–29 | 6459 (24.2) | 2994 (24.0) | 3465 (24.4) | |
| <20 | 17532 (65.7) | 7923 (63.6) | 9609 (67.5) | |
| Unknown | 1606 (6.0) | 984 (7.9) | 622 (4.4) | |
| RV function: Severe dysfunction | 2886 (14.4) | 1234 (15.1) | 1652 (14.0) | 0.0311 |
| Aortic Regurgitation: Severe | 142 (0.6) | 60 (0.6) | 82 (0.7) | 0.3688 |
| Mitral Regurgitation: Severe | 5668 (23.2) | 2654 (23.6) | 3014 (22.8) | 0.1711 |
| Tricuspid Regurgitation: Severe | 2796 (11.5) | 1276 (11.4) | 1520 (11.6) | 0.6471 |
| Hemodynamics | ||||
| Systolic Blood Pressure (mmHg) | 106.1 ± 16.3 | 105.1 ± 16.0 | 107.0 ± 16.4 | <.0001 |
| RA Pressure (mmHg) | 12.9 ± 8.4 | 13.4 ± 8.6 | 12.4 ± 8.2 | <.0001 |
| PA Systolic Pressure (mmHg) | 49.7 ± 14.9 | 50.0 ± 14.7 | 49.5 ± 15.1 | 0.0117 |
| PA Wedge Pressure (mmHg) | 24.8 ± 9.3 | 24.6 ± 9.1 | 24.9 ± 9.5 | 0.0231 |
| Cardiac Index (L/min/m2) | 2.2 ± 0.9 | 2.2 ± 1.0 | 2.1 ± 0.8 | <.0001 |
p-value is for comparing between the two eras
Continuous data are reported as the mean ± SD and categorical data as number (percentage) of patients.
AST, aspartate aminotransferase; ALT, alanine transaminase; BMI, body mass index; BUN, blood urea nitrogen; ECMO, extra-corporeal membrane oxygenator; IABP, intra-aortic balloon pump; ICD, implantable cardioverter defibrillator; INR, international normalized ratio; LVEDD, left-ventricular end diastolic dimension; LVEF, left-ventricular ejection fraction; PA, pulmonary artery; RA, right atrial; RV, right ventricular.
In the recent era, a wider gap favoring non-ischemic (52.9%) etiology versus an ischemic cardiomyopathy (37.1%) is evident. Improved national protocols and implementation of guidelines for treatment of hypertension, cholesterol lowering, tobacco cessation, and management of coronary artery disease have contributed to a decline in prevalence of patients with ischemic cardiomyopathy.(11) These observations align with national trends that reveal an age-adjusted decline in deaths due to ischemic heart disease.(12)
Trends in Intermacs, LVAD Indications and Device Flow Type
The Intermacs Profile at the time of CF LVAD implantation, stratified according to era, is provided in Table 1. During the reported 10-year period, half of the implants were performed in critically ill Profile 1 or 2 patients with a shift towards higher patient acuity in the most recent era. In the most recent era, there was an overall increase in Profile 1 patients (18.0% vs 14.8%) among critically ill patients and an increase in Profile 3 implants (36.1% vs 32.3%) among more stable patients (Profiles 3–7). The patient profile by implant year for primary CF LVAD is shown in Figure 2A.
Figure 2:
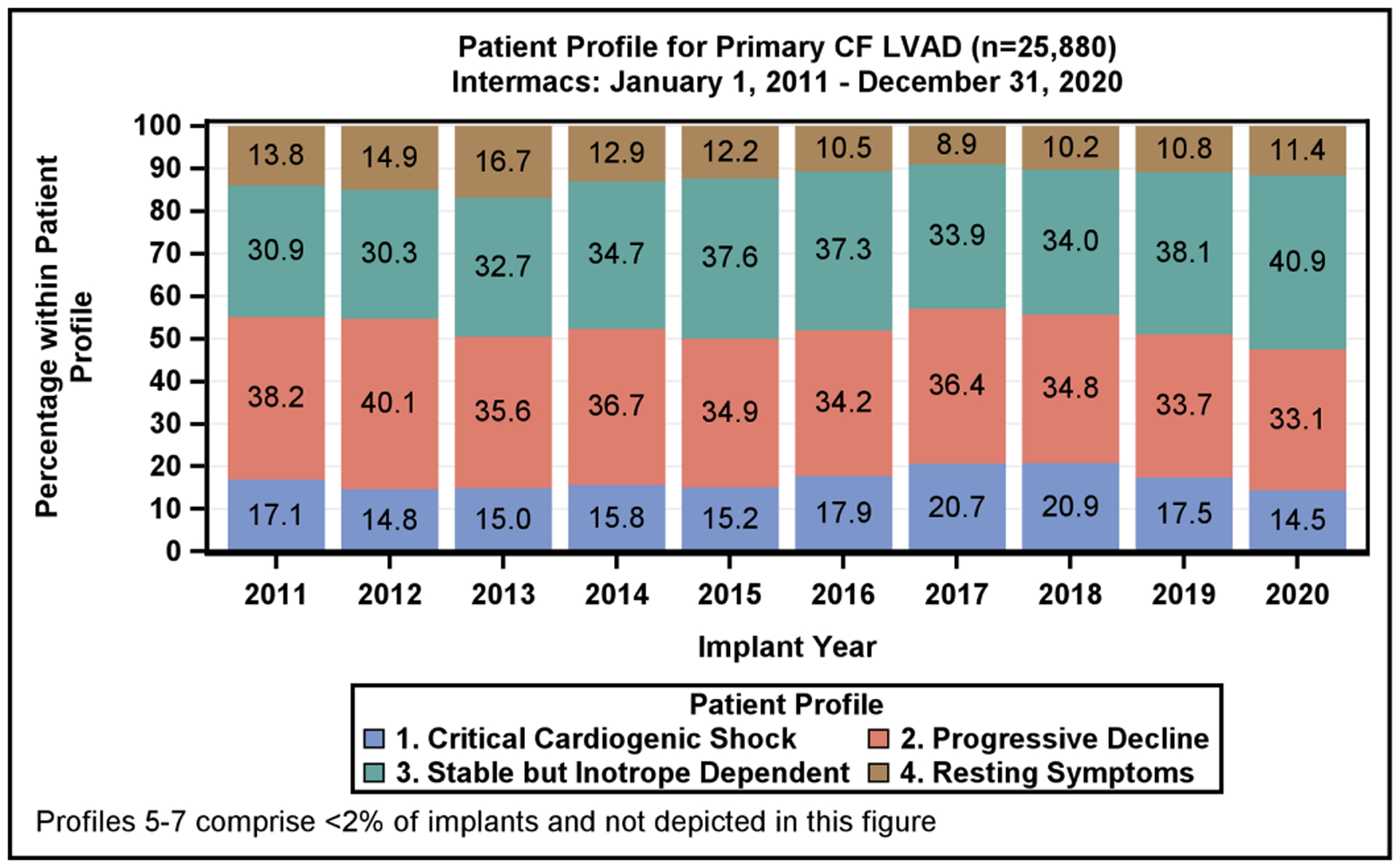
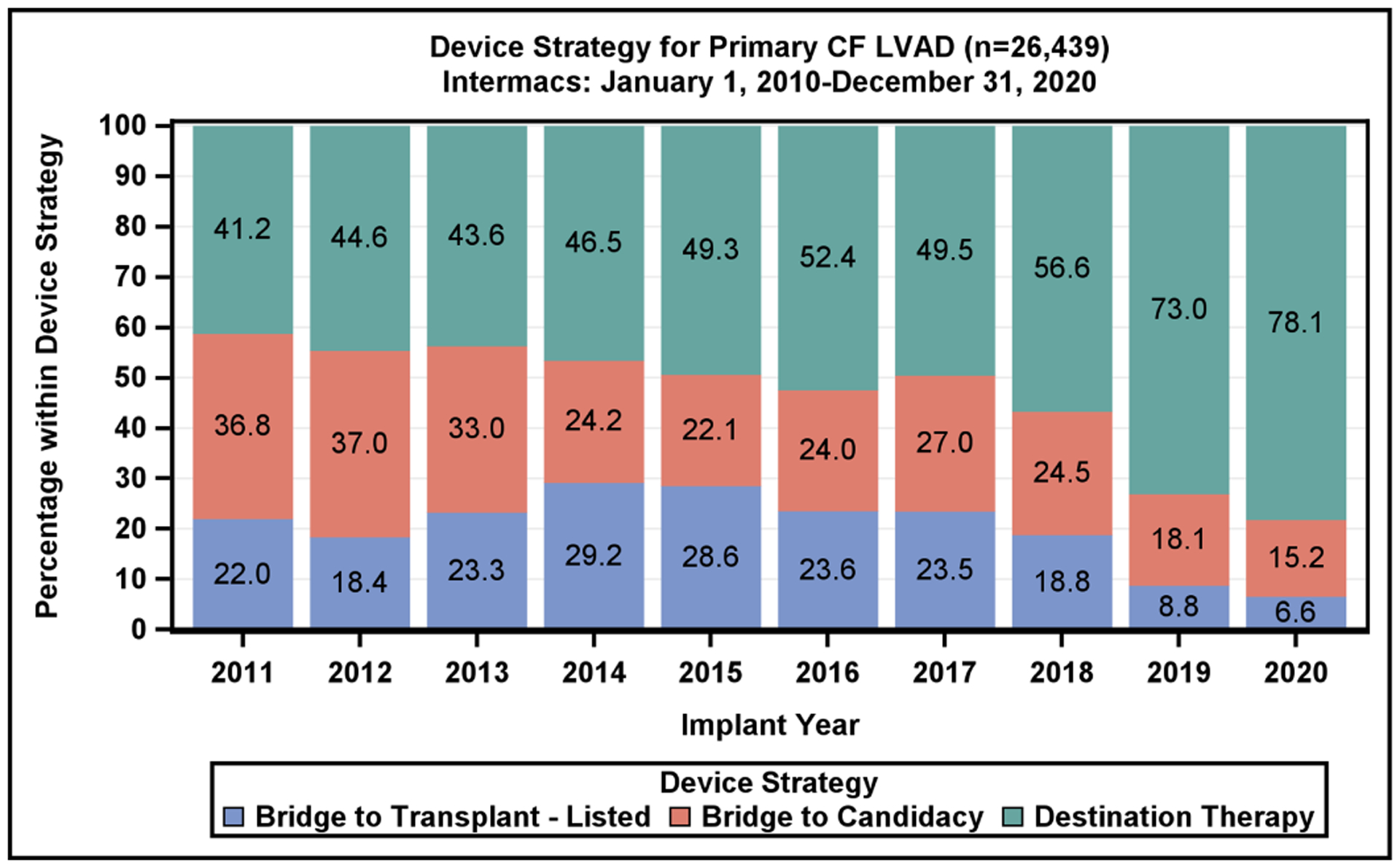
Patient Profile and Device Strategy by Implant Year. A) Distribution of Intermacs Profiles by year of continuous-flow left ventricular assist device implantation. Profiles 5–7 account for <2% of implants and are excluded from the figure. B) The device strategy at time of continuous-flow left ventricular assist device implantation by year
The new six-tiered heart allocation system assigns stable LVADs to Status 4.(13,14) Status 4 transplants account for 18.3% of all transplants currently, while in the previous allocation system, stable LVAD patients were afforded a Status 1B, which accounted for 28.3% all heart transplants.(15) A growing proportion of patients are being listed for transplant at Status 1–3, which further reduces access to transplantation for LVAD patients. These changes in the allocation priority are reflected by a reduced proportion of BTT and BTC LVAD implants being performed. The BTT or BTC indications, which had previously accounted for 51% of all device implants in 2017, now account for only 22% of all device implants (Figure 2B). This raises the importance of shared decision-making prior to implant as to whether the intent is BTT, BTC or DT.(16,17) Given the decreased transplant availability to stable LVAD patients with the current allocation algorithm, further studies are needed to examine the selection process for durable devices vs transplantation.
In the current era, DT is the predominant indication, with 78% of CF LVADs implanted as DT in 2020 (Figure 2B), a dramatic increase from 49.5% in 2017. The marked increase in DT implants since 2017 is coincident with the change in heart transplant allocation, growth of standalone DT programs, improvement in LVAD patient survival, and approval of the fully magnetically levitated centrifugal-flow device in 2018, with its improved adverse event profile.(1)
During 2011–2015, axial-flow devices were the predominant implanted device type but have now declined to 1% of total implants in 2020 (Figure 1). The use of the hybrid-levitation centrifugal CF LVAD increased rapidly following FDA approval for BTT in 2012, achieving 49% of the total implants in 2018%, followed by a decline in favor of the fully magnetically levitated centrifugal-flow pump. In 2020, this pump now accounts for 83% of all CF LVAD implants.
Survival Outcomes for Patients on Isolated LVAD Support
During the study period, 26,688 index CF LVADs (13,544 axial flow and 13,144 centrifugal flow) were implanted. The overall survival with current generation CF LVADs continues to be favorable, with 1-year survival for the entire cohort of 81.9 % and 44.2% at 5 years (Figure 3A). Importantly, the survival by Kaplan-Meier (with censoring at transplant or cessation of support) is only 16.8% at 10 years (Supplemental Figure 2). When comparing the two eras studied, 1- and 5-year survival have improved modestly (82.8% vs 80.8% at 1 year, p<0.0001; 48.2 vs 42.0% at 5 years, p<0.0001), increased from 4.0 years to 4.6 years in the current era. (Figure 3B). Across Intermacs profiles, patients have exhibited higher survival in the more recent era (Supplemental Figure 3). The starkest difference in survival by Intermacs profile occurs at 1-year, where profile 1 patients have lower survival at 75.2% compared to 84% in profile 3 patients. By five years, this survival difference is diminished, and profile 1 patients have 42% survival compared to 45.9% in profile 3 patients (Figure 4A).
Figure 3:
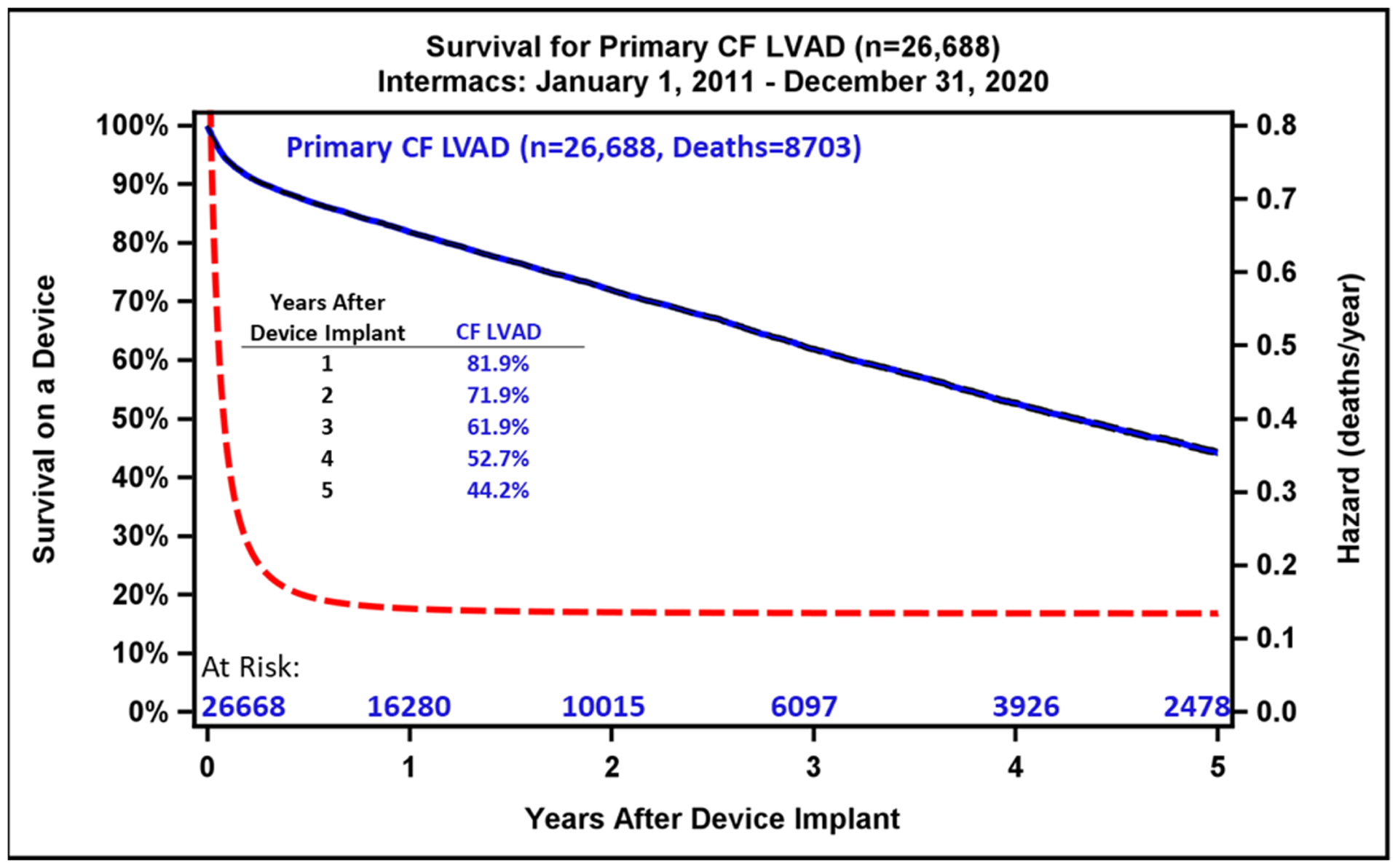
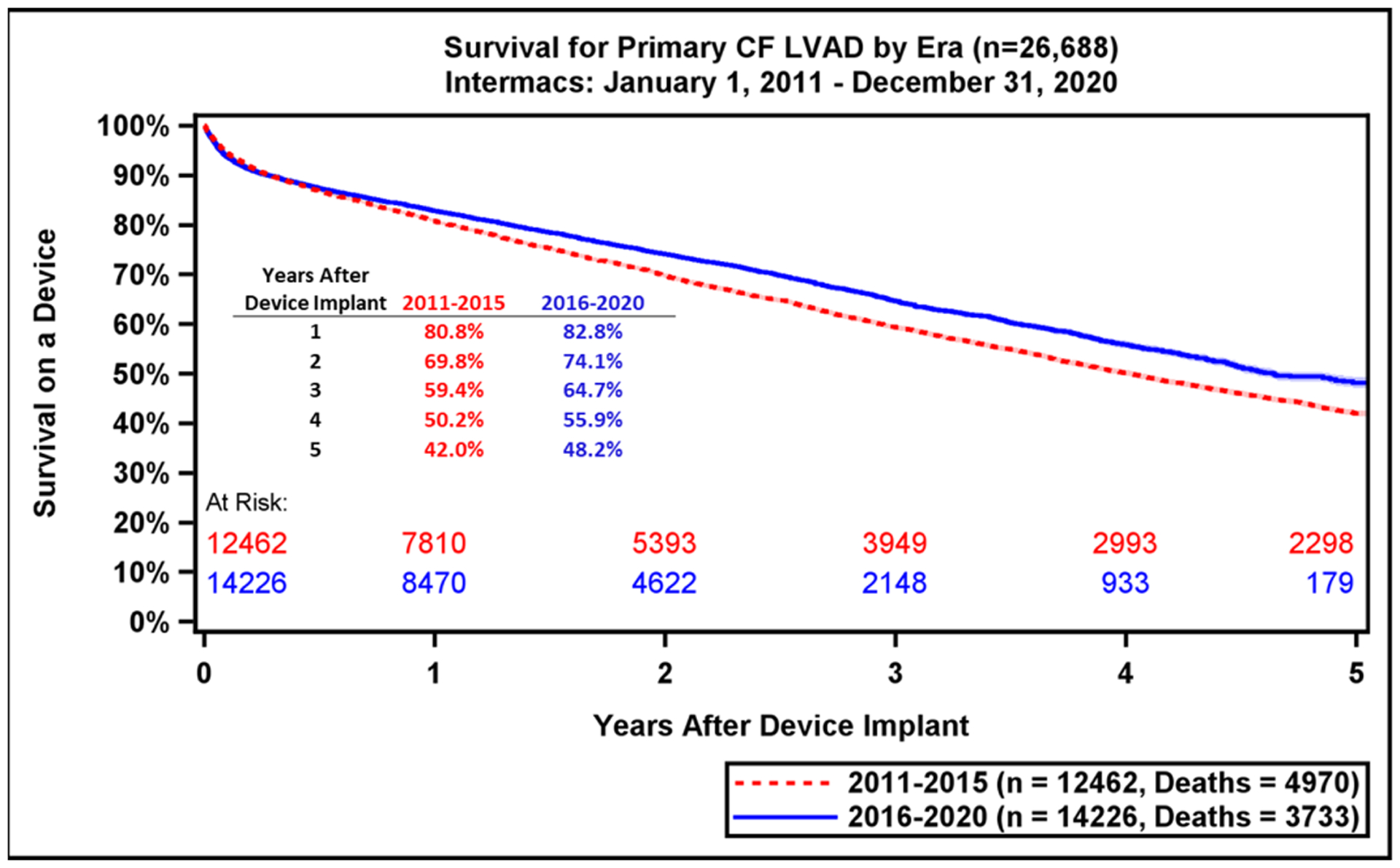
Kaplan-Meier Survival Analysis for All Patients and by LVAD Era. A) Kaplan-Meier estimated survival after continuous-flow left ventricular assist device implantation for the past decade. Hazard rates are depicted by dashed red line. B) The estimated survival is compared between the previous era (2011–2015) and the current era (2016–2020). Shaded areas indicate 70% confidence limits, p (log-rank) = <0.0001, Event: Death (censored at transplant or cessation of support).
Figure 4:
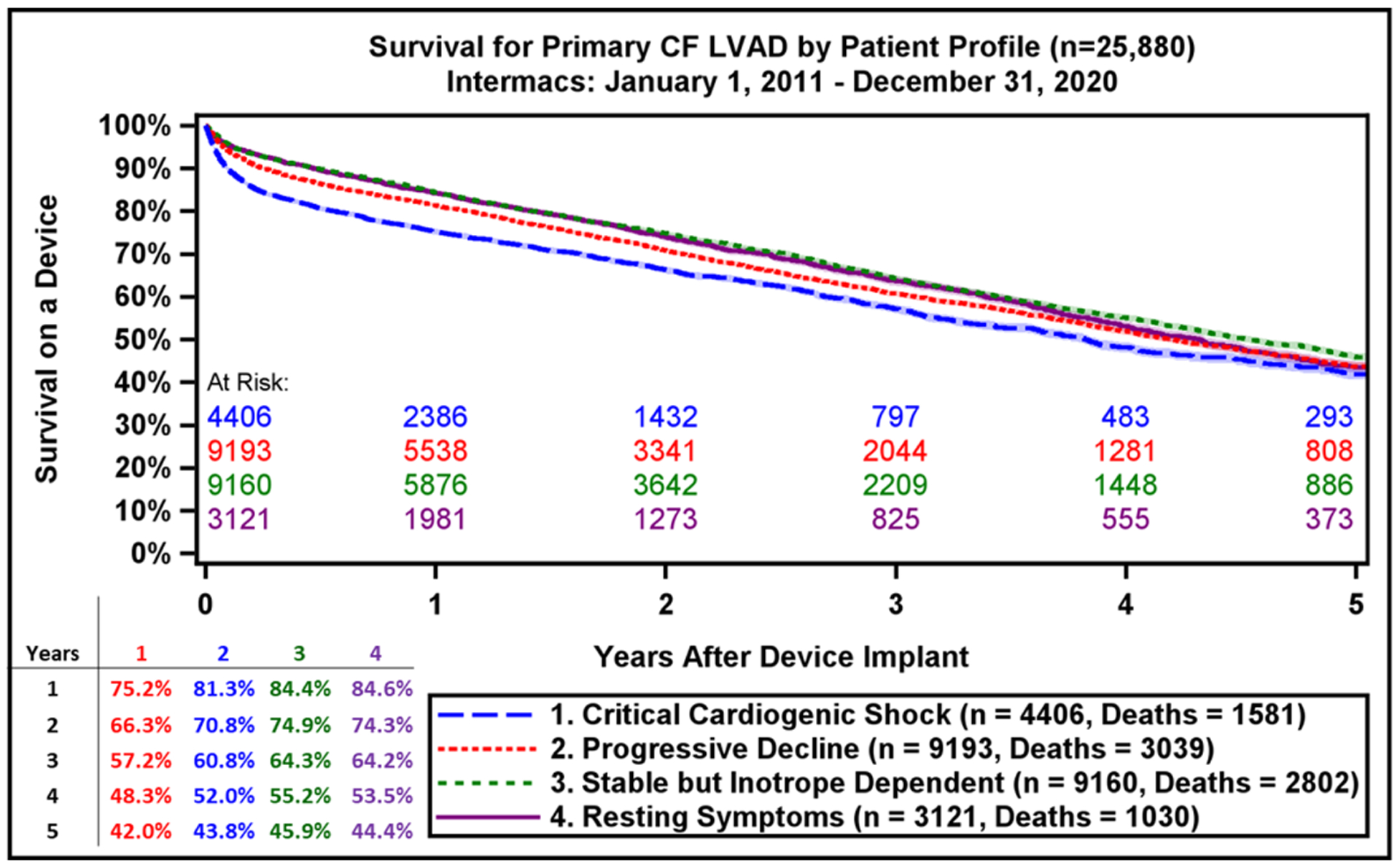
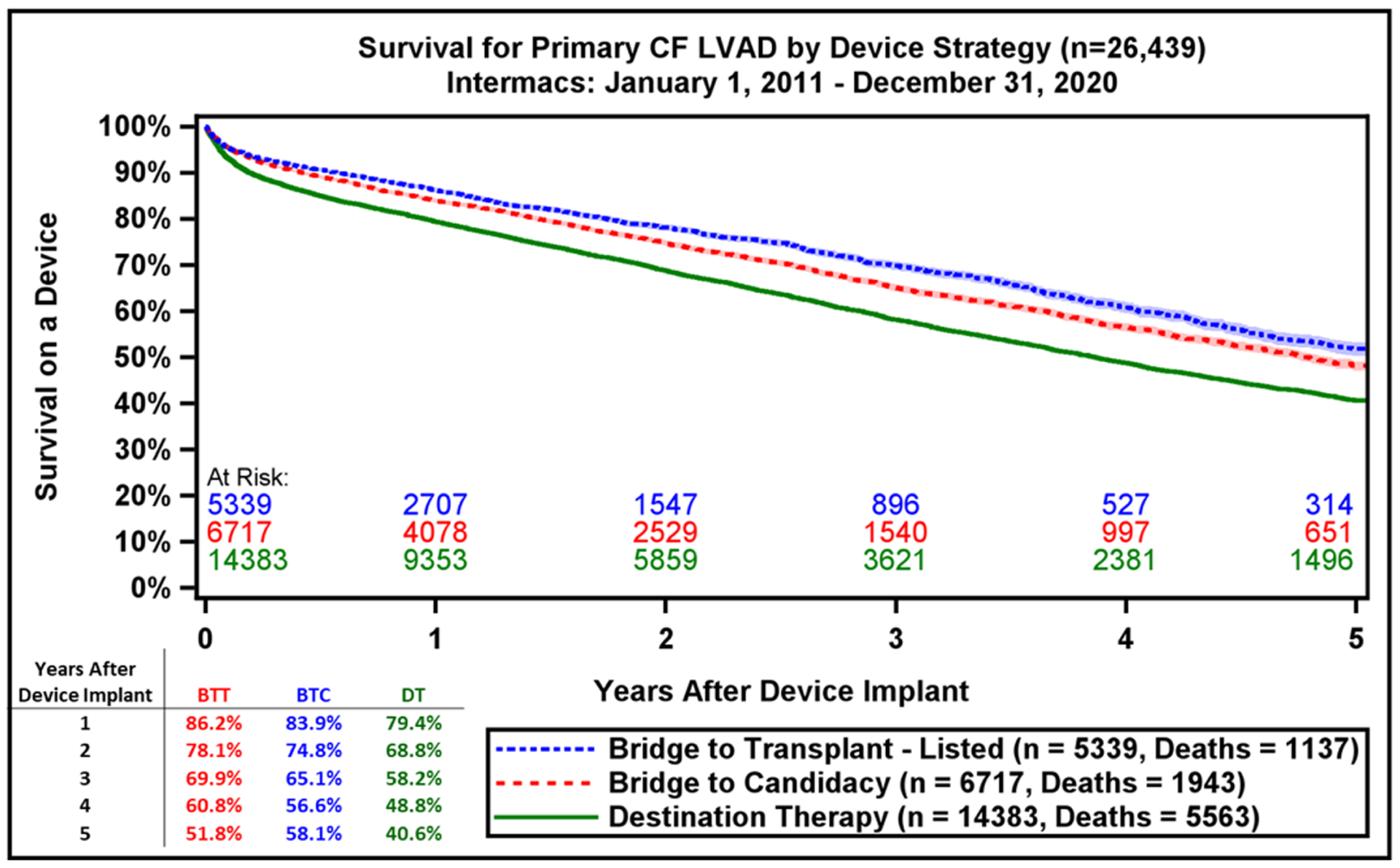
Survival by Intermacs Profile and Device Implant Strategy. A) Kaplan-Meier estimated survival after continuous-flow left ventricular assist device implantation by Intermacs Profile and B) Device intent or strategy at time of implant. BTC, bridge to candidacy; BTT, bridge to transplant; DT, destination therapy. Shaded areas indicate 70% confidence limits, p (log-rank) = <0.0001, Event: Death (censored at transplant or cessation of support)
Implant intent is associated with survival, with those implanted as BTT having superior survival compared with BTC and DT patients (Figure 4B). In the competing risk analysis, for the entire cohort of all primary CF LVADs by 5 years after implant, 20.9% were alive on support, 34% were transplanted, and a small minority (4.3%) had cessation of support for myocardial recovery (Supplemental Figure 4). The various competing outcomes differ across implant indications with a progressive decline in transplant rates for BTT vs. BTC vs. DT patients (Supplemental Figure 5A–C). All patients who were implanted in the era 2016–2020 had a lower likelihood of getting transplanted at all years of follow up irrespective of the treatment intent when compared to the earlier era. Survival data from the current report also supports against arbitrary categorization of patients as BTT vs. BTC vs. DT, as most patients are on support for longer periods of time (everyone is functionally DT) and the absolute increase in survival was observed in the most recent era was irrespective of original implant strategy (Supplemental Figure 6). Finally, when comparing the recent era with prior era, we see that all patients are alive on device support for longer periods of time (Supplemental Figure 7).
Adverse Events & Cause of Death for LVAD Patients
Major adverse event rates occur most frequently during the early period after LVAD implant (Table 2). Notably, adverse event rates in all major categories were lower in patients implanted during the most recent era (Supplemental Table 1). During the recent era, patients experienced greater freedom from GIB (79.9% vs. 75.3%, p<0.0001) and device malfunction/pump thrombosis (90.4% vs. 80.4%, p<0.0001) compared to prior era (Figure 5A–B). There were also notable, but smaller differences in freedom from first stroke at 1-year (88.1% vs. 86.7%, p<0.0001) and freedom from MCS Infection (86.0% vs. 84.7%, Figure 5C–D). This likely reflects the lower stroke incidence with the fully magnetically levitated centrifugal-flow pump in the recent era.(1) Freedom from first bleeding, neurological dysfunction, infection, and first non-MCS infection are shown in Supplemental Figure 8A–D.
Table 2:
Adverse events in 14,226 Continuous-flow Left Ventricular Assist Device Patients (January 1, 2016-December 31, 2020) With Follow-up Through June 30, 2021
| CF LVAD | |||||
|---|---|---|---|---|---|
| Event | Perioda | Event Count | AE Rateb | Patient Count | Patient Percent |
| Rehospitalization (all cause) | Early | 7,438 | 2.25 | 5,042 | 35.4% |
| Late | 34,893 | 1.78 | 9,327 | 65.6% | |
| Major Bleeding | Early | 4,304 | 1.30 | 2,939 | 20.7% |
| Late | 6,199 | 0.32 | 3,099 | 21.8% | |
| GI Bleeding | Early | 2,114 | 0.64 | 1,557 | 10.9% |
| Late | 4,423 | 0.23 | 2,249 | 15.8% | |
| Non-GI Reoperation for Bleeding | Early | 698 | 0.21 | 626 | 4.4% |
| Late | 169 | 0.009 | 152 | 1.1% | |
| Cardiac Arrhythmia | Early | 3,058 | 0.93 | 2,375 | 16.7% |
| Late | 2,288 | 0.12 | 1,523 | 10.7% | |
| Device Malfunction/Pump Thrombus | Early | 677 | 0.21 | 594 | 4.2% |
| Late | 2,361 | 0.12 | 1,728 | 12.1% | |
| Device Malfunction | Early | 250 | 0.076 | 234 | 1.6% |
| Late | 1,111 | 0.057 | 889 | 6.2% | |
| Pump Thrombus | Early | 442 | 0.13 | 385 | 2.7% |
| Late | 1,311 | 0.067 | 1001 | 7.0% | |
| Major Infection | Early | 4,323 | 1.31 | 3,169 | 22.3% |
| Late | 8,905 | 0.45 | 4,699 | 33.0% | |
| MCS Infection | Early | 499 | 0.15 | 469 | 3.3% |
| Late | 3,377 | 0.17 | 2,157 | 15.2% | |
| Non-MCS Infection | Early | 3956 | 1.20 | 2898 | 20.4% |
| Late | 6,192 | 0.32 | 3,609 | 25.4% | |
| Stroke | Early | 946 | 0.29 | 864 | 6.1% |
| Late | 1,705 | 0.087 | 1,393 | 9.8% | |
| Renal Dysfunction | Early | 1,412 | 0.43 | 1,301 | 9.1% |
| Late | 862 | 0.044 | 702 | 4.9% | |
| Respiratory Failure | Early | 2,316 | 0.70 | 1865 | 13.1% |
| Late | 883 | 0.045 | 736 | 5.2% | |
| Wound Dehiscence | Early | 123 | 0.037 | 116 | 0.8% |
| Late | 54 | 0.003 | 50 | ||
Early = ≤ 90 days post implant; Late = > 90 days post implant,
Rates are reported per patient-year of LVAD support.
AE, adverse event; GI: gastrointestinal; MCS: mechanical circulatory support.
Figure 5:
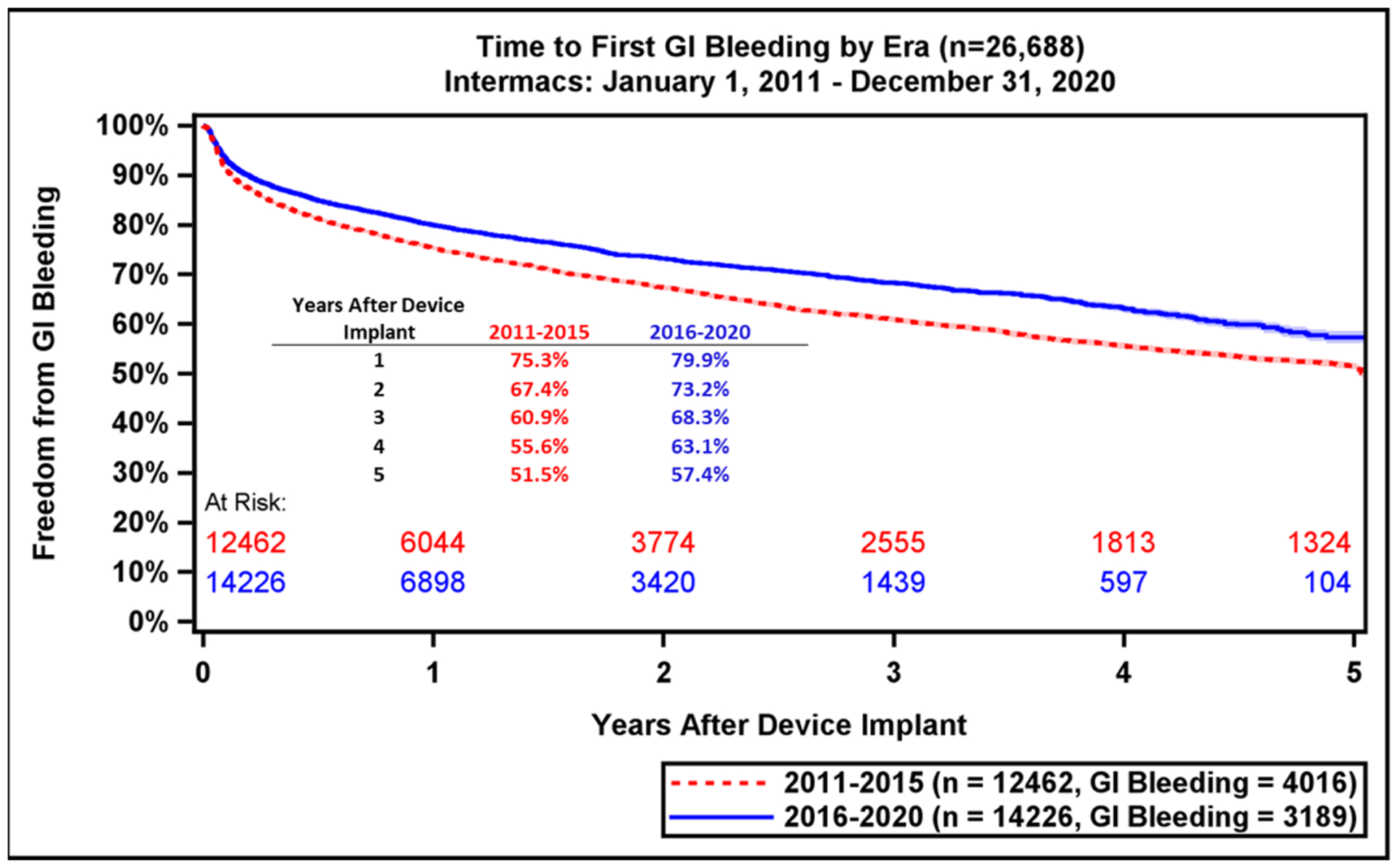
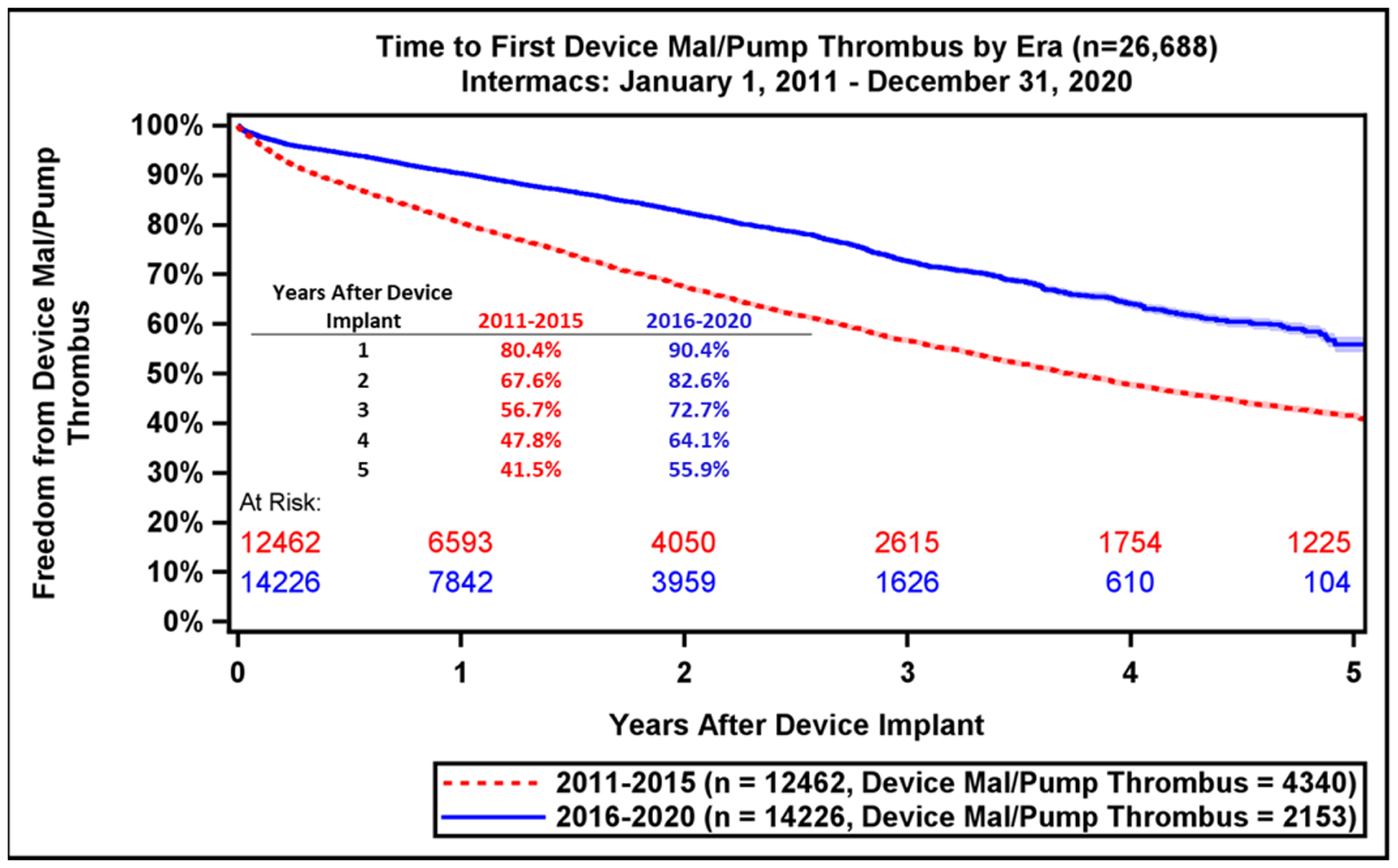
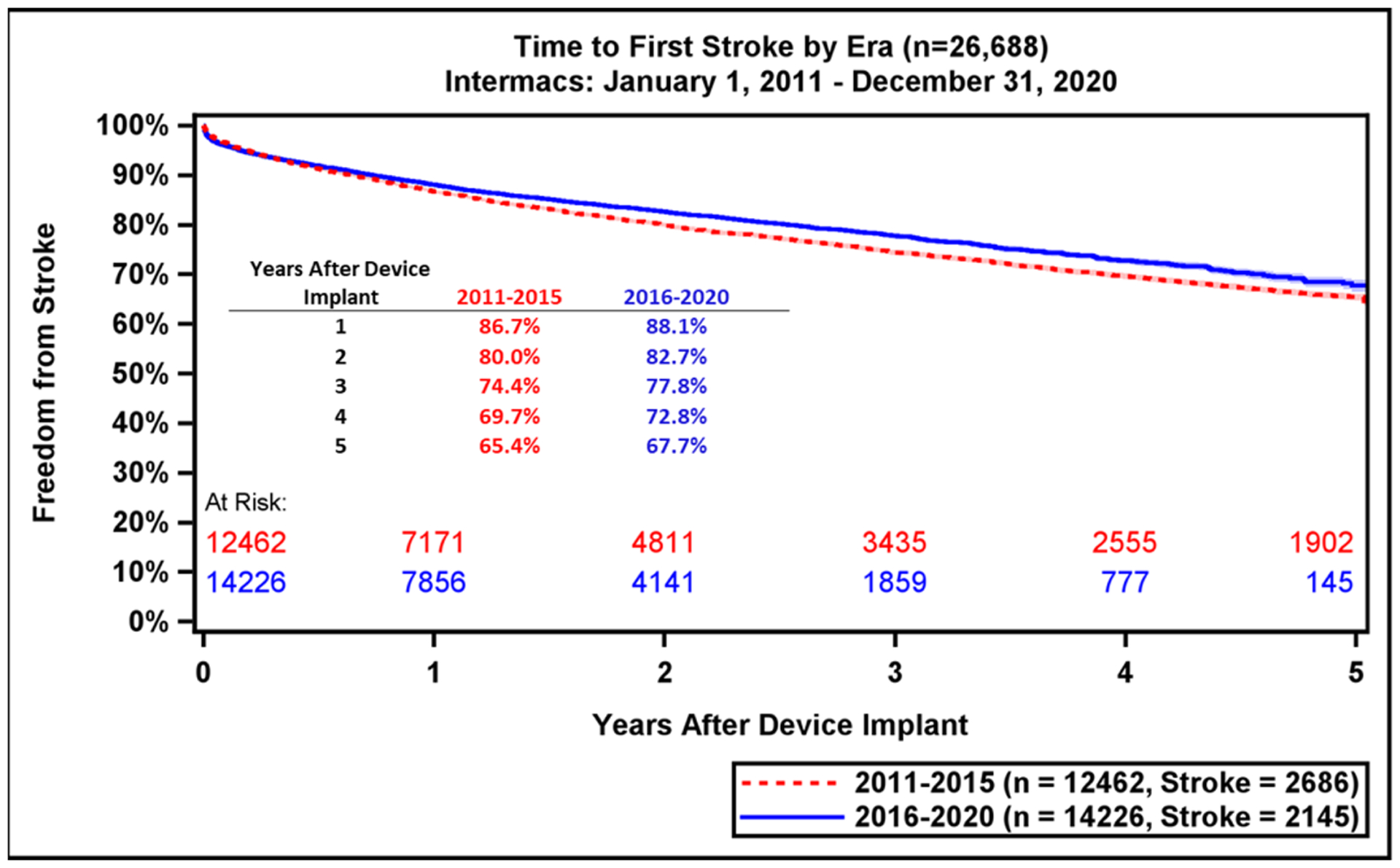
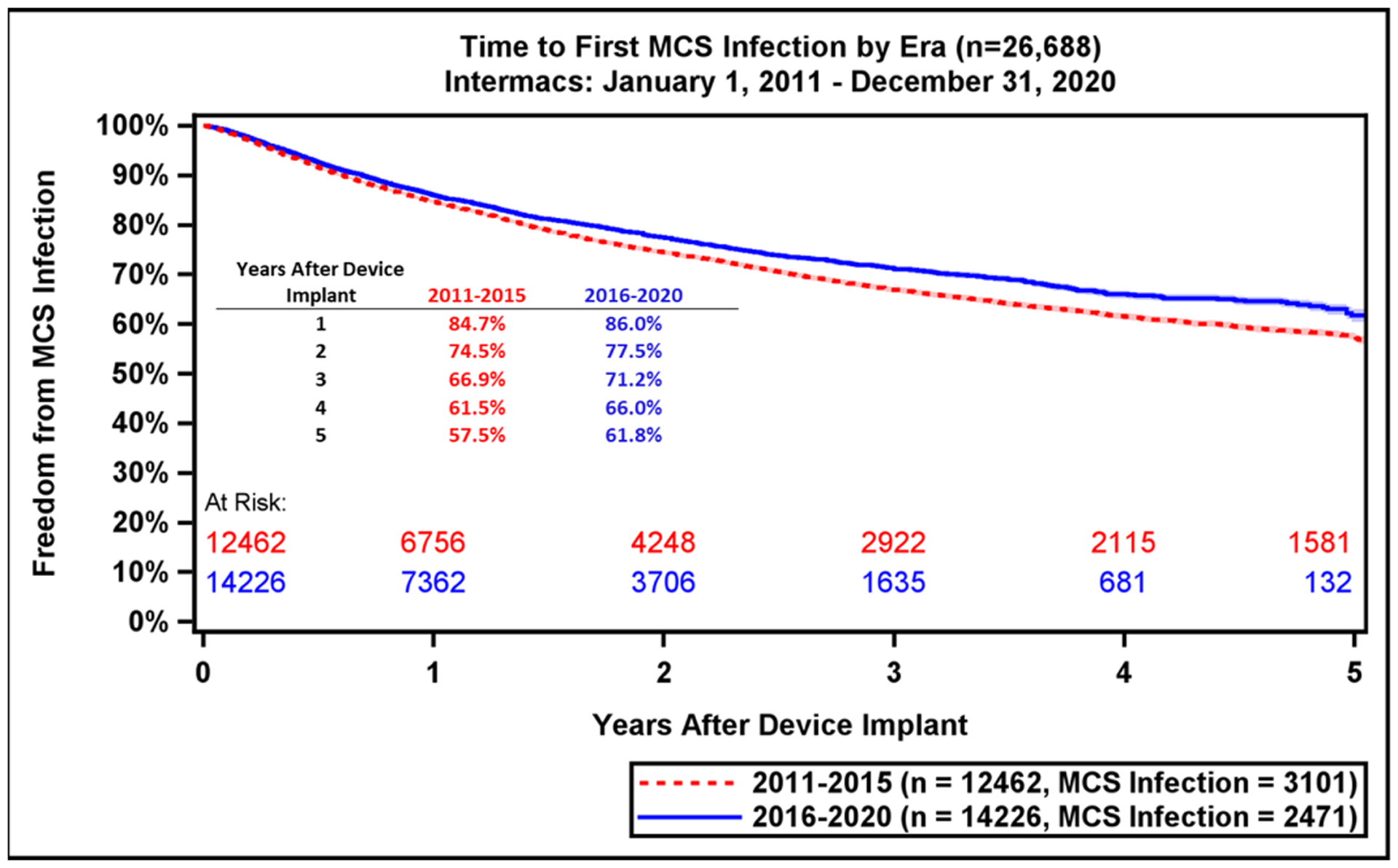
Freedom from GI Bleeding, Device Malfunction, Stroke, and MCS Infection by Era. Freedom from adverse events as compared across eras (2011–2015 vs. 2016–2020): (A) GI bleeding, (B) Device Malfunction / Pump Thrombosis, (C) Stroke, (D) MCS Infection. GI, gastrointestinal; MCS, mechanical circulatory support. Shaded areas indicate 70% confidence limits, p (log-rank) = <0.0001, Event: Death (censored at transplant or cessation of support).
Cause of Death for Patients on Isolated LVAD Support
As LVAD patients are supported for longer durations, a shift in the cause of death has been observed for patients in the recent era, with withdrawal of support (n=672, 18%), replacing neurologic dysfunction (n=898, 18.1%) as the most common cause. It is important to note that withdrawal of support may be reflective of earlier adverse events, co-morbidities or other unexplained factors and warrants further research. Neurologic dysfunction as a cause of death is expected to continue to improve as the newest and only available LVAD technology, is associated with lower rates of stroke. Both eras shared similar rates of major bleeding, respiratory failure and other (not specified) causes of death (Table 3).
Table 3:
Cause of Death Across Device Eras for Left Ventricular Assist Device Patients
| Primary Cause of Death | Era 2011–2015 | Era 2016–2020 | *p-value |
|---|---|---|---|
| (n=4,970) | (n=3,733) | ||
| <.0001 | |||
| Circulatory Other | 326 (6.6) | 241 (6.5) | |
| Device Malfunction | 150 (3.0) | 49 (1.3) | |
| Heart Failure | 602 (12.1) | 482 (12.9) | |
| Major Bleeding | 90 (1.8) | 68 (1.8) | |
| Major Infection | 388 (7.8) | 216 (5.8) | |
| Multisystem Organ Failure (MSOF) | 745 (15.0) | 593 (15.9) | |
| Neurological Dysfunction | 898 (18.1) | 532 (14.3) | |
| Other | 679 (13.7) | 584 (15.6) | |
| Respiratory | 291 (5.9) | 195 (5.2) | |
| Sudden Death | 208 (4.2) | 101 (2.7) | |
| Withdrawal of Support | 593 (11.9) | 672 (18.0) |
P < .0001 for era comparisons.
Hospital Readmissions for LVAD Patients
Frequent hospital readmissions remain a significant burden for LVAD patients and care providers and are a major contributor to lifetime LVAD healthcare expenditures.(18) Approximately 20–30% of LVAD patients are readmitted within 30 days of discharge from their implant admission.(19,20) The most reported reasons for readmission include major infection (13.5%), major bleeding (12.9%), fluid overload (5.1%), arrhythmias (5.1%), and neurologic dysfunction inclusive of stroke (4.8%, Figure 6A). Notably, many readmissions are elective hospitalizations for heart transplantation (4.7%), planned procedures (4.4%), and anticoagulation adjustment (3.7%). Finally, a large proportion of readmissions are uncategorized (18.7%), providing an opportunity for a detailed evaluation. Despite these statistics, the overall incidence of readmission has improved over time, dropping from 78.0% at 1 year in the prior era to 70.9% in the current era (Supplemental Figure 9).
Figure 6:
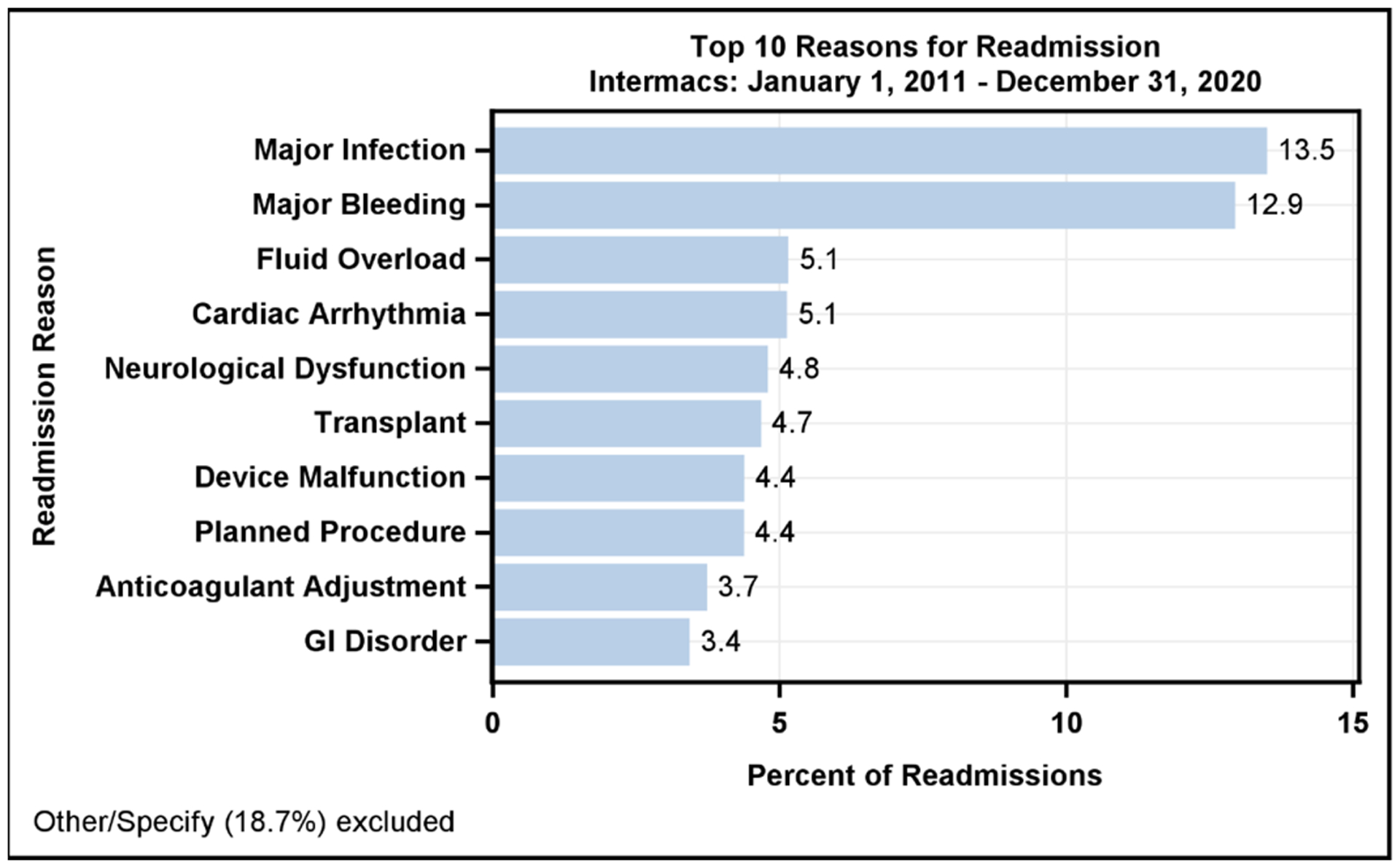
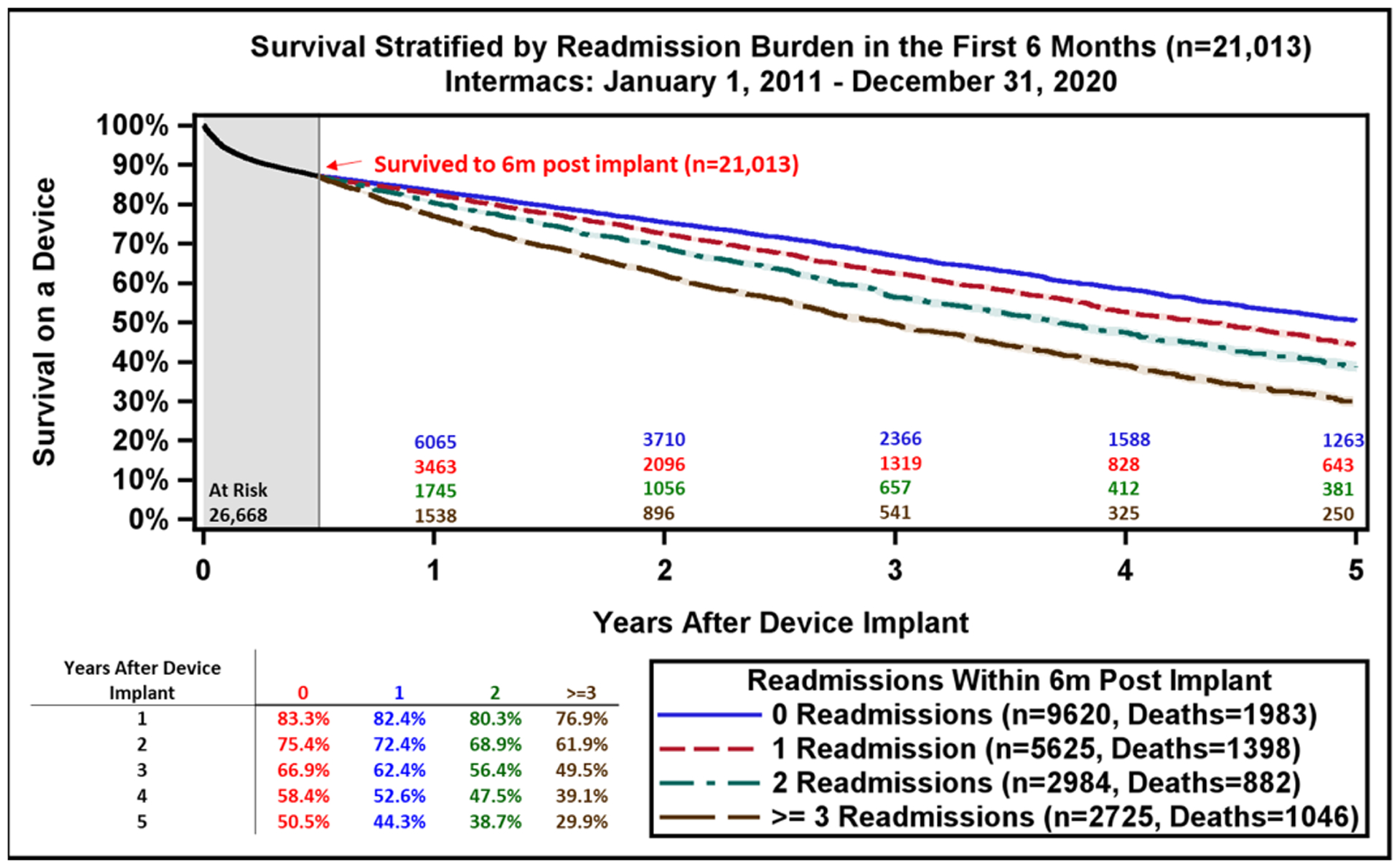
Reasons for Readmission after LVAD and Patient Survival after Readmissions Occurring in the first 6-months. A) The major reasons for readmission after continuous-flow left ventricular assist device implantation. B) All continuous-flow left ventricular assist device patients who survived to 6-months (n=21,013) are stratified by the number of hospital readmissions occurring during the first 6-months after implant. Kaplan-Meier estimated survival is then provided based on number of readmissions during the initial 6-month period. Shaded areas indicate 70% confidence limits, p (log-rank) = <0.0001, Event: Death (censored at transplant or cessation of support).
To determine the impact of hospital readmissions on survival, patients were stratified by the number of readmissions occurring in the first 6 months. Overall survival for all LVAD patients at 6 months post-implant was 87%. Patients without a readmission in the first 6 months had the largest survival advantage in each successive year. Estimated 5-year survival on LVAD therapy without 6-month readmission was 51% compared to 30% in those readmitted three or more times during the first 6-months following LVAD implant (Figure 6B). Aside from patient survival, each LVAD readmission costs approximately $35,000,(20) and reduces the overall cost-effectiveness of this therapy.(18) Future strategies to reduce readmissions may include multidisciplinary care with standardized protocols for infection prevention, anticoagulation management, heart failure medication optimization, routine biomarker, and hemodynamic assessment to further improve quality of life and long-term patient survival.
2020 STS-Sponsored Intermacs Research and Studies from the Participant User File Program
For centers and investigators interested in utilizing the STS Intermacs database for research, there are two methods to access national de-identified data (https://www.sts.org/research-center/programs-and-data-access). The first path involves the STS Participant User File (PUF), which permits users to perform their own analyses of the dataset without oversight by STS Intermacs Task Force. A cost to the investigators is associated with this option. The second pathway involves the Access and Publications (A&P) research program which entails a competitive process by which investigators propose well thought out research proposals that are peer reviewed at STS, and generally 1–2 projects are supported each cycle, with 2 cycles per year. Key publications from STS Intermacs in 2020 are provided in Table 4.
Table 4:
The Society of Thoracic Surgery - Interagency Registry for Mechanically Assisted Circulatory Support Research Publications from 2020
| Study Title | Aim | Key Findings |
|---|---|---|
| Differences in health-related quality of life by implant strategy: Analyses from the Interagency Registry for Mechanically Assisted Circulatory Support (White-Williams et al, J Heart Lung Transplant)(22) | To examine differences in Health-Related Quality of Life (HRQOL) based on EQ-5D-3L and KCCQ-12 questionnaires between preop and 2 years postimplant time periods. The analysis was stratified by pre-operative left ventricular assist device (LVAD) implant strategy. | This study provides overall and domain-specific evidence of differences in HRQOL both cross-sectionally and over time by implant strategy. Overall HRQOL was poor before MCS implantation and significantly improved through 2 years after surgery, regardless of implant strategy. Notably, some cross-sectional differences by group in overall HRQOL before and midterm after implant were not always clinically meaningful. Significant differences were detected in HRQOL domain scores by implant strategy, using both generic and heart failure–specific instruments. Understanding HRQOL may assist patients and caregivers with shared decision-making when considering MCS as a treatment option and provide guidance for healthcare clinicians to develop targeted interventions to improve post-implant HRQOL. |
| Extracorporeal Membrane Oxygenation as a Bridge to Durable Mechanical Circulatory Support: An Analysis of the STS Intermacs Database (Loyaga-Rendon et al, Circ Heart Fail)(23) | To compare the clinical characteristics, outcomes, and risk factors for poor outcomes among Intermacs Profile 1 patients who are supported by extracorporeal membrane oxygenation (ECMO) as a bridge to durable MCS. | This propensity matched analysis showed that Intermacs Profile 1 patients bridged on ECMO to LVAD had lower survival than those without ECMO. The proportion of patients successfully bridged to transplantation after LVAD over 2 year follow up was similar between groups. The highest hazard of death occurred in the first 6 months post-implantation and ECMO use was an independent risk factor for poor outcome. In a selected group of ECMO supported patients, the survival was similar to equally ill matched patients. Multiple identified risk factors can help in patient risk stratification. These data support the implantation of LVADs in carefully selected ECMO patients. |
| Identifying Temporal Relationships Between in-Hospital Adverse Events After Implantation of Durable Left Ventricular Assist Devices (Kilic et al, J Amer Heart Assoc)(24) | To examine the impact of specific perioperative adverse events (AEs) on subsequent AEs after LVAD implantation. | This study, which relied on risk-adjusted Cox proportional hazard models of temporal relationships between AEs, demonstrated that index hospitalization AEs following LVAD implant are significantly associated with the development of subsequent AEs. Specific sequences and patterns of AE were identified. The most profound impact was found to be with a primary respiratory or renal failure AE. Targeted efforts to reduce the incidence of these two highly morbid AEs may be useful in reducing the overall AE burden and subsequent mortality in the LVAD patient population. |
| Postimplant Phosphodiesterase Type 5 Inhibitors Use Is Associated With Lower Rates of Thrombotic Events After Left Ventricular Assist Device Implantation (Xanthopoulos et al, J Amer Heart Assoc)(25) | To examine whether the postimplant use of Phosphodiesterase type 5 (PDE-5) inhibitors is associated with a lower incidence of thrombotic events (composite of pump thrombosis and ischemic stroke) in a Intermacs LVAD population. | The postimplant use of a PDE-5 inhibitor was associated with a significant reduction in thrombotic events, and in both of the components (pump thrombus, ischemic stroke) separately. In addition, PDE-5 inhibitor use was associated with a reduction in all-cause mortality at 48-months postoperatively. A randomized clinical trial to confirm these salutary effects is warranted. |
| Quantifying the impact from stroke during support with continuous flow ventricular assist devices: An STS Intermacs analysis (Kirklin et al, J Heart Lung Transplant)(26) | To analyze the incidence, recurrence, risk factors for, and outcomes after stroke on CF-LVAD devices in STS Intermacs. | This study confirms the 20% incidence of stroke over the first 2 years with the axial flow and non-magnetically levitated centrifugal flow pumps. Patients are somewhat more susceptible to a subsequent ischemic stroke after the first one. The 6-month mortality is >30% after an ischemic stroke and >50% after a hemorrhagic stroke. Based on a limited sub-group of patients with Modified Rankin Score (MRS) data available, only about one-third of patients with major disability showed improvement between 1 and 3 months, and this study suggests a major increase in 1- and 2-year mortality among those with an initial disabling stroke. Better reporting of longitudinal MRS and HRQOL data after a stroke are warranted. |
| Right Atrial Pressure Predicts Mortality Among LVAD Recipients: Analysis of the Intermacs Database (Guglin et al, Heart Lung Circ)(27) | To identify preoperative hemodynamic predictors of all-cause mortality. Standard right heart catheterization hemodynamic variables were compared to find which variable is superior in predicting mortality. Significant variables were examined longitudinally to see their ability to predict short, intermediate, and long-term mortality. | In general, hemodynamic variables, as well as other criteria including Intermacs profiles, were found to be weak predictors of mortality after LVAD. Right atrial pressure (RAP) is the only consistent and significant hemodynamic predictor of mortality in LVAD recipients, independent of Intermacs profile. A RAP ≥ 13mmHg was found to be optimal cutoff for prediction of mortality after implant. RAP remains a significant predictor of mortality during the 1st year of LVAD support. |
AE, adverse event; ECMO, extracorporeal membranous oxygenation; EQ-5D-3L, 3-level version of EQ-5D and consists of the EQ-5D descriptive system and the EQ visual analogue scale; KCCQ-12, Kansas City Cardiomyopathy Questionnaire short version; HRQOL, Health-Related Quality of Life; LVAD, left ventricular assist device; MCS, mechanical circulatory support; MRS, modified rankin score; PDE-5, phosphodiesterase type 5; RAP, right atrial pressure.
Summary
The twelfth annual report highlights the impact of the COVID-19 pandemic on LVAD implantation volumes across the U.S. and the ongoing effect of the 2018 change in the UNOS heart allocation policy. Despite the vast majority of LVADs being implanted in older, sicker and transplant ineligible patients, we see continued improvements in patient outcomes across categories, with median time on pump support approximating 5-years. With the recent withdrawal of the HVAD from the market and minimal implantation of the older generation axial-flow devices, we expect to see a further reduction in hemocompatibility related events and device malfunction, leading to improve patient outcomes. The recent decision in the U.S. by the Centers for Medicare and Medicaid Services (CMS) to remove the requirement for formal assessment of transplant eligibility as a requirement for LVAD coverage reflects the fact that most patients are implanted as long-term therapy, perhaps reducing the need for specific designated strategies (BTT, BTC, and DT).(21) Finally, the first Intermacs analysis of readmissions after LVAD implant and has found stark differences in patient survival for those with and without readmissions. This highlights an opportunity to reinforce appropriate and aggressive medical management, utilization of multidisciplinary care to reduce readmissions, and further improve LVAD patient outcomes.
Supplementary Material
Acknowledgement
The authors wish to acknowledge all the centers who participate in the STS Intermacs Database and the coordinators who spend countless hours collecting and entering data. They would also like to acknowledge the Data Coordinating Center at the Kirklin Institute for Research in Surgical Outcomes at the University of Alabama at Birmingham for its assistance in preparing the data, performing the statistical analysis, and creating the resulting tables and figures.
Funding:
P.S. has an NIH Career Development Award 1K23HL143179; VKT, MY no funding; O.W.P. has research funding from the NIH 5K23HL150322; K.B. has research funding from the NIH R56HL159216, K01HL142848, and L30HL148881; and Women as One Escalator Award. FDP receives research funding from R01HL146619 and R01 HS026003.
Disclosures:
PS – reports related grant support from Roche and Abbott and consulting fees from Procyrion.
VKT – reports unrelated research grant and consulting fee from Medtronic.
JKK- receives partial salary support paid to his institution from Society of Thoracic Surgeons in his role as Director of the Data Coordinating Center for STS INTERMACS.
FDP – reports non-compensated scientific advisor for FineHeart, CH Biomedical, Medtronic, and Abbott. Data Safety Monitoring Board member for Carmat and National Institutes of Health, PumpKIN Study.
DG – consultant and speaker for Abbott Inc., Abiomed Inc. National Co-Principal Investigator, MOMENTUM 3 trial.
The other authors report no relevant disclosures.
Abbreviations and Acronyms
- BiVAD
biventricular assist device
- BTC
bridge to candidacy
- BTT
bridge to transplant
- CF
continuous-flow
- DT
destination therapy
- FDA
Food and Drug Administration
- GIB
gastrointestinal bleeding
- HVAD
HeartWare Ventricular Assist Device
- Intermacs
Interagency Registry for Mechanically Assisted Circulatory Support
- LVAD
left ventricular assist device
- MCS
mechanical circulatory support
- RVAD
right ventricular assist device
- STS
The Society of Thoracic Surgeons
- TAH
total artificial heart
- UNOS
United Network for Organ Sharing
- US
United State
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mehra MR, Uriel N, Naka Y et al. A Fully Magnetically Levitated Left Ventricular Assist Device - Final Report. N Engl J Med 2019;380:1618–1627. [DOI] [PubMed] [Google Scholar]
- 2.Cho SM, Mehaffey JH, Meyers SL et al. Cerebrovascular Events in Patients With Centrifugal-Flow Left Ventricular Assist Devices: Propensity Score-Matched Analysis From the Intermacs Registry. Circulation 2021;144:763–772. [DOI] [PubMed] [Google Scholar]
- 3.Yonas E, Alwi I, Pranata R et al. Effect of heart failure on the outcome of COVID-19 - A meta analysis and systematic review. Am J Emerg Med 2021;46:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, Mamas MA, Mohamed MO et al. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart 2021;107:113–119. [DOI] [PubMed] [Google Scholar]
- 5.Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess Deaths From COVID-19 and Other Causes, March-April 2020. Jama 2020;324:510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen TC, Thourani VH, Nissen AP et al. The Effect of COVID-19 on Adult Cardiac Surgery in the United States in 717 103 Patients. The Annals of thoracic surgery 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackstone EH, Naftel DC, Turner ME. The Decomposition of Time-Varying Hazard Into Phases, Each Incorporating a Separate Stream of Concomitant Information. Journal of the American Statistical Association 1986;81:615–624. [Google Scholar]
- 8.Mehra MR, Cleveland JC Jr., Uriel N et al. Primary results of long-term outcomes in the MOMENTUM 3 pivotal trial and continued access protocol study phase: a study of 2200 HeartMate 3 left ventricular assist device implants. Eur J Heart Fail 2021;23:1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golbus JR, Gupta K, Colvin M et al. Changes in Type of Temporary Mechanical Support Device Use Under the New Heart Allocation Policy. Circulation 2020;142:1602–1604. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Montfort J, Sinha SS, Thayer KL et al. Clinical Outcomes Associated With Acute Mechanical Circulatory Support Utilization in Heart Failure Related Cardiogenic Shock. Circulation Heart failure 2021;14:e007924. [DOI] [PubMed] [Google Scholar]
- 11.Mensah GA, Wei GS, Sorlie PD et al. Decline in Cardiovascular Mortality: Possible Causes and Implications. Circulation research 2017;120:366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virani SS, Alonso A, Aparicio HJ et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 13.Goff RR, Uccellini K, Lindblad K et al. A change of heart: Preliminary results of the US 2018 adult heart allocation revision. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2020;20:2781–2790. [DOI] [PubMed] [Google Scholar]
- 14.Cogswell R, John R, Estep JD et al. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation 2020;39:1–4. [DOI] [PubMed] [Google Scholar]
- 15.Estep JD, Soltesz E, Cogswell R. The new heart transplant allocation system: Early observations and mechanical circulatory support considerations. The Journal of thoracic and cardiovascular surgery 2020. [DOI] [PubMed] [Google Scholar]
- 16.Allen LA, McIlvennan CK, Thompson JS et al. Effectiveness of an Intervention Supporting Shared Decision Making for Destination Therapy Left Ventricular Assist Device: The DECIDE-LVAD Randomized Clinical Trial. JAMA Intern Med 2018;178:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris AA, Khazanie P, Drazner MH et al. Guidance for Timely and Appropriate Referral of Patients With Advanced Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2021;144:e238–e250. [DOI] [PubMed] [Google Scholar]
- 18.Baras Shreibati J, Goldhaber-Fiebert JD, Banerjee D, Owens DK, Hlatky MA. Cost-Effectiveness of Left Ventricular Assist Devices in Ambulatory Patients With Advanced Heart Failure. JACC Heart failure 2017;5:110–119. [DOI] [PubMed] [Google Scholar]
- 19.Patel S, Poojary P, Pawar S et al. National Landscape of Unplanned 30-Day Readmissions in Patients With Left Ventricular Assist Device Implantation. Am J Cardiol 2018;122:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agrawal S, Garg L, Shah M et al. Thirty-Day Readmissions After Left Ventricular Assist Device Implantation in the United States: Insights From the Nationwide Readmissions Database. Circulation Heart failure 2018;11:e004628. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Medicare & Medicaid Services Coverage Decision: Ventricular Assist Devices. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=360.
- 22.White-Williams C, Fazeli PL, Kirklin JK, Pamboukian SV, Grady KL. Differences in health-related quality of life by implant strategy: Analyses from the Interagency Registry for Mechanically Assisted Circulatory Support. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation 2020;39:62–73. [DOI] [PubMed] [Google Scholar]
- 23.Loyaga-Rendon RY, Boeve T, Tallaj J et al. Extracorporeal Membrane Oxygenation as a Bridge to Durable Mechanical Circulatory Support: An Analysis of the STS-INTERMACS Database. Circulation Heart failure 2020;13:e006387. [DOI] [PubMed] [Google Scholar]
- 24.Kilic A, Seese L, Pagani F, Kormos R. Identifying Temporal Relationships Between In-Hospital Adverse Events After Implantation of Durable Left Ventricular Assist Devices. Journal of the American Heart Association 2020;9:e015449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xanthopoulos A, Tryposkiadis K, Triposkiadis F et al. Postimplant Phosphodiesterase Type 5 Inhibitors Use Is Associated With Lower Rates of Thrombotic Events After Left Ventricular Assist Device Implantation. Journal of the American Heart Association 2020;9:e015897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirklin JK, Naftel DC, Myers SL, Pagani FD, Colombo PC. Quantifying the impact from stroke during support with continuous flow ventricular assist devices: An STS INTERMACS analysis. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation 2020;39:782–794. [DOI] [PubMed] [Google Scholar]
- 27.Guglin M, Omar HR. Right Atrial Pressure Predicts Mortality Among LVAD Recipients: Analysis of the INTERMACS Database. Heart Lung Circ 2021;30:592–599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


