Abstract
Background
The evidence for the associations between early-life adiposity and female cancer risks is mixed. Little is known about the exact shape of the relationships and whether the associations are independent of adult adiposity.
Methods
We conducted dose–response meta-analyses of prospective studies to summarise the relationships of early-life body mass index (BMI) with breast, endometrial, and ovarian cancer risks. Pubmed and Embase were searched through June 2020 to identify relevant studies. Using random-effects models, the summary relative risks (RRs) and 95% confidence intervals (CIs) were estimated per 5-kg/m2 increase in BMI at ages ≤ 25 years. A nonlinear dose–response meta-analysis was conducted using restricted cubic spline analysis.
Results
After screening 33,948 publications, 37 prospective studies were included in this analysis. The summary RRs associated with every 5-kg/m2 increase in early-life BMI were 0.84 (95% CI = 0.81–0.87) for breast, 1.40 (95% CI = 1.25–1.57) for endometrial, and 1.15 (95% CI = 1.07–1.23) for ovarian cancers. For breast cancer, the association remained statistically significant after adjustment for adult BMI (RR = 0.80, 95% CI = 0.73–0.87). For premenopausal breast, endometrial, and ovarian cancers, the dose–response curves suggested evidence of nonlinearity.
Conclusions
With early-life adiposity, our data support an inverse association with breast cancer and positive associations with ovarian and endometrial cancer risks.
Subject terms: Risk factors, Epidemiology
Introduction
Breast, endometrial, and ovarian cancers are commonly diagnosed female cancers that together account for 32% of cancer incidence and 22% of cancer deaths in women worldwide [1]. These three cancer sites share many common risk factors including younger age at menarche [2–5], nulliparity [4–7], and menopausal hormone therapy use [8–11]. Among the known risk factors, adult body fatness is also an important risk factor. Previous meta-analyses estimated that every 5-kg/m2 increase in adult body mass index (BMI) is associated with a 12% increased risk of postmenopausal breast cancer [12], a 54% increased risk of endometrial cancer [13], and a 7% increased risk of ovarian cancer [14].
Some studies also suggest the long-term effects of early-life body fatness on the risks of breast [15–23], endometrial [24–28], and ovarian cancers [29–31]. However, the direction and strength of the associations varied across studies [22–26, 31–34]. It is unknown whether the inconsistency in study results can be explained by the differences in population characteristics (e.g., menopausal status, geographic region) and methodological issues (e.g. prospective vs. retrospective, measured vs. recalled body fatness, with vs. without adjustment for adult body fatness, body fatness measures at different ages) among the studies. Some studies also suggested that the associations may vary by tumour subtypes [17, 21, 23, 35, 36]. Particularly, little is known about whether the associations with early-life body fatness are independent of adult body fatness and whether the associations vary by menopausal status. Further, the exact shape of the relationships (e.g. linear, U-shape) are not clear.
While previous meta-analyses have primarily focused on the associations with adult BMI, in this study we conducted a meta-analysis of prospective studies for the associations between early-life BMI and risks of breast, endometrial, and ovarian cancers, the three major female cancers that share many common biologic mechanisms. By performing linear and non-linear dose–response meta-analyses, we explored the shape of the relationships and quantified the risks associated with specific levels of early-life BMI. We also included a larger number of cases from both pre- and postmenopausal women and additional studies that were not part of the previous pooling studies [37, 38] and recent meta-analyses [13, 14, 39]. Furthermore, to clarify the associations, we performed various subgroup analyses to investigate whether the associations varied by menopausal status, adjustment for adult BMI, age of BMI, assessment method of BMI, region, and tumour subtypes.
Methods
Search strategy
The Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [40] were closely followed for the design, analysis, and reporting of this meta-analysis. D.B., Y.N., S.R., H.J. and Y.C. conducted the literature search, study selection, and data extraction independently and H.O. checked for accuracy. Studies published through June 2020 were identified by searching Pubmed and Embase databases. Detailed search terms are provided in Supplementary Table 1. The reference lists of previous meta-analyses and all the articles included in our analysis were also reviewed for additional studies.
Study selection
This meta-analysis was restricted to prospective studies that provided relative risk (RR) estimates (hazard ratios or risk ratios) and 95% confidence intervals (CIs) for the associations between early-life BMI and three major female cancer (breast, endometrial, and ovarian cancer) risks. We defined early-life period as age ≤25 years including childhood, adolescence, and early adulthood. When body fatness measures (BMI or pictogram) at multiple ages were provided in the study, we selected the age closest to 18 years because BMI at age 18 was the most common variable reported in the studies. When the studies reported RRs and 95% CIs separately for multiple mutually exclusive subgroups (e.g. premenopausal vs. postmenopausal women, women with vs. without family history), we extracted the estimates from each subgroup. For dose–response meta-analysis, we restricted to studies that reported at least three categories of body fatness variable with information on RR, 95% CI, and the category-specific number of cases and person-years. When category-specific number of person-years were not reported, we estimated by multiplying category-specific number of participants by the average duration of follow-up. When quantile-based studies did not provide the distributions of person-years, total person-years were equally divided across the quantiles. Studies that reported RR and 95% CI for the linear trend (e.g. per 5-kg/m2 increase in BMI) but did not report category-specific information (RR, 95% CI, or number of cases and person-years) [32, 41–46] were included in linear dose–response meta-analysis only. Studies were excluded if they used data from cancer survivors or documented cancer mortality only. Articles without full-text or not written in the English language were also excluded. When multiple studies were published from the same cohort or data, we included the study with the longest follow-up, the largest sample size, or was most recently published. There was a total of 37 publications (21 for breast, 10 for endometrial, 6 for ovarian cancer) eligible for this dose–response meta-analysis. Study selection procedure is summarised in Supplementary Fig. 1.
Data extraction
For each study, we extracted the following data: last name of the first author, publication year, country, study name, study design, age distribution and menopausal status of study population, number of cases and person-years of all categories required for calculation, category-specific range of exposure variables, recorded or estimated average follow up time, assessment method of exposure (measured vs. recalled), adjustment variables, and RRs and corresponding 95% CIs by categories or per unit. We extracted RRs and 95% CIs from the fully adjusted model. However, if both models with and without adjustment for adult BMI were reported, we extracted the estimates from the two models separately [23] for subgroup analysis and used the estimates from the model without adjustment in the primary analysis. When the lowest categories of body fatness were not selected as the reference group in the studies, we changed the reference group to the lowest category and converted the corresponding RRs and 95% CIs using variance formula for rate ratio (with normal approximation). In studies [17, 23] that assessed early-life body fatness using pictogram only (Stunkard’s [47] or Sørensen’s [48]), we imputed the BMI value using results from the validation study [49]. Because the validation study provided data for BMI at age 15 years, we used pictogram data for the age closest to 15 years when multiple age data were available. The extracted data are shown in Supplementary Table 2 (breast cancer), Supplementary Table 3 (endometrial cancer), and Supplementary Table 4 (ovarian cancer). The quality of studies was evaluated using the Newcastle-Ottawa Scale [50].
Statistical analysis
Linear and nonlinear dose–response meta-analyses were conducted for breast, endometrial, and ovarian cancer risks. In primary analysis, we did not stratify by menopausal status because some studies did not specify the menopausal status of the participants or did not stratify the analyses by menopausal status. For linear dose–response meta-analyses, we used the methods described by Greenland and Longnecker [51] to compute study-specific slopes of linear trends and corresponding 95% CIs from the natural logs of the RRs and CIs extracted across categories of early-life BMI. In estimating study-specific linear trends, several approximations were made: the midpoint of each exposure category was assigned to the corresponding RR; the width of the open-ended extreme categories was assumed to be same as that of the adjacent interval. Then, the estimated study-specific RRs and variances were pooled using the DerSimonian-Laird random-effects models [52] to estimate the summary RR and 95% CI. Forest plots of the linear dose–response meta-analysis were presented for RRs and 95% CIs associated with each 5-kg/m2 increment in early-life BMI.
Nonlinear dose–response meta-analysis was conducted using restricted cubic spline analysis [53, 54]. For each study, cubic splines were modelled with three knots fixed at the 10th, 50th, and 90th percentiles of the distribution of early-life BMI, accounting for correlation across category-specific RRs and 95% CIs within each study [53]. The reference was set to the early-life BMI of 18.5 kg/m2, the lowest cutoff for normal weight in men and women aged 18 years or older [55]. Then, the derived curves were combined using multivariate random effects meta-analysis [56]. The p-value for nonlinearity was obtained from the test of the null hypothesis that the regression coefficient of the second spline transformation was equal to zero.
Heterogeneity in the relationship across studies was assessed by Cochran’s Q test [57] and I2, the percentage of total variation across studies that is attributable to true heterogeneity rather than to chance [58]. I2 values around 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively [59]. To identify sources of heterogeneity and assess study quality, subgroup analyses and meta-regression were conducted based on linear dose–response meta-analysis by a priori selected variables that are related to potential effect modifiers (menopausal status, region, age of BMI) and methodological characteristics (adjustment for current adult BMI, assessment method of early-life BMI). In subgroup analysis by adjustment for current adult BMI, we restricted analyses to publications that reported both models with and without adjustment (6 studies for breast cancer, 4 studies for endometrial cancer) to increase the comparability between the results (e.g. number of studies included, participant characteristics). Among the studies that reported tumour subtype-specific RRs and 95% CIs [21, 23, 36, 60, 61], we performed subgroup analysis by tumour subtypes (e.g. oestrogen receptor [ER]-positive vs. ER-negative breast cancers). We conducted subgroup analyses only when there were at least three studies in each stratum. Potential for publication bias was tested using Egger’s test. Diverse sensitivity analyses including the influence analysis were performed to check robustness of the results. For statistical significance, two-sided significance level was set at alpha level 0.05. All statistical analyses were conducted using STATA 12 (StataCorp, College Station, TX).
Results
After screening 33,948 publications, 37 prospective studies (21 for breast cancer [15–23, 32, 36, 41–45, 60–64], 10 for endometrial cancer [24–28, 46, 65–68], and 6 for ovarian cancer [30, 31, 34, 69–71]) were identified and included in this dose–response meta-analysis. Of these, 19 studies were from the United States [19, 21, 23–25, 27, 28, 42, 43, 46, 60, 61, 63, 65, 67–71], 14 studies were from Europe [15, 17, 20, 22, 26, 30–32, 34, 41, 45, 62, 64, 66], and 4 studies [16, 18, 36, 44] were from other countries. All studies received the Newcastle-Ottawa score ≥7, indicating high quality studies (Supplementary Table 5).
Breast cancer
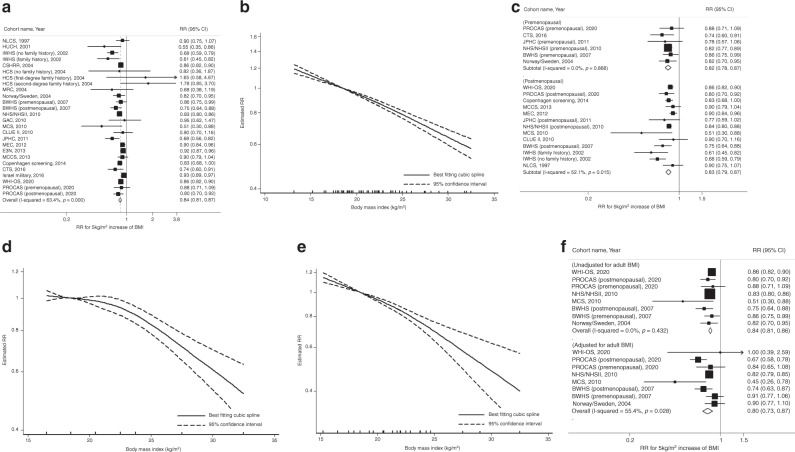
A total of 39,733 breast cancer cases from 1,849,875 participants were included in the meta-analyses of early-life BMI and breast cancer risk (range of BMI: 13.2–32.5 kg/m2). Each 5-kg/m2 increase in early-life BMI was statistically significantly associated with a 16% reduced breast cancer risk (summary RR = 0.84, 95% CI = 0.81–0.87) with evidence of moderate to high heterogeneity (I2 = 63.4%, p < 0.001; Fig. 1a). Although there was some heterogeneity in the magnitude of RRs among the studies, all studies except one consistently showed an inverse association. Small study effects, such as publication bias, were not indicated by Egger’s test (p = 0.08). In the nonlinear dose–response meta-analysis, we found no evidence of nonlinearity (p-nonlinearity = 0.19; Fig. 1b).
Fig. 1. Linear and nonlinear dose–response relationships for early-life BMI and breast cancer risk.
This figure shows the results from a linear dose–response relationship with total breast cancer; b nonlinear dose–response relationship with total breast cancer (p-nonlinearity = 0.19); c subgroup analysis by menopausal status (p-heterogeneity = 0.78); d nonlinear dose–response relationship with premenopausal breast cancer (p-nonlinearity = 0.004); e nonlinear dose–response relationship with postmenopausal breast cancer (p-nonlinearity = 0.16); f subgroup analysis by adjustment for adult BMI (p-heterogeneity = 0.36). The black squares and horizontal lines represent study-specific relative risks and their 95% confidence intervals. The area of each black square reflects the weight each study contributes to the meta-analysis. The middle and horizontal tips of diamonds represent summary RRs and their 95% confidence intervals, respectively. The P values were calculated from Cochran’s Q test; all statistical tests were two-sided.
When stratified by menopausal status, the summary RRs for the association between early-life BMI and breast cancer risk were similar between premenopausal (RR = 0.82, 95% CI = 0.78–0.87) and postmenopausal women (RR = 0.83, 95% CI = 0.79–0.87, p-heterogeneity = 0.78; Fig. 1c). However, in the nonlinear dose–response meta-analysis, there was evidence of nonlinearity, suggesting a steeper risk reduction at BMI > 23 kg/m2 (e.g., threshold effect), in premenopausal women (p-nonlinearity = 0.004; Fig. 1d) but not in postmenopausal women (p-nonlinearity = 0.16; Fig. 1e). In most studies, except for two [36, 64], menopausal status at baseline was consistent with menopausal status at cancer diagnosis. Results were similar after excluding these two studies that have missing information on menopausal status at cancer diagnosis (data not shown).
To evaluate the associations independent of adult BMI, we compared results by adjustment for adult BMI among 6 publications (8 distinct populations) that reported both models with and without the adjustment. The inverse association between early-life BMI and breast cancer risk did not materially change and remained statistically significant in the adult BMI-adjusted models (summary RR = 0.80, 95% CI = 0.73–0.87 adjusted vs. RR = 0.84, 95% CI = 0.81–0.86 unadjusted; p-heterogeneity = 0.36; Fig. 1f).
Table 1 (for overall results) and Supplementary Fig. 2 (for study-specific results) present results from other subgroup analyses. In subgroup analysis by tumour subtype, significantly inverse associations were observed with both ER-positive (RR = 0.75, 95% CI = 0.65–0.87) and ER-negative breast cancers (RR = 0.60, 95% CI = 0.38–0.98). Although the summary RR was slightly stronger for ER-negative tumours, the difference was not statistically significant (p-heterogeneity = 0.29). In subgroup analysis by age of BMI, we compared the results from studies that included BMI at age <18 years (RR = 0.87, 95% CI = 0.82–0.91) vs. at 18 years (RR = 0.79, 95% CI = 0.72–0.87) vs. >18 years (RR = 0.85, 95% CI = 0.79–0.91) and found similar results (p-heterogeneity = 0.63). The summary RRs were also similar in studies with self-reported BMI (RR = 0.83, 95% CI = 0.79–0.87) vs. measured BMI (RR = 0.86, 95% CI = 0.80–0.93; p-heterogeneity = 0.49) and in studies from the USA (RR = 0.82, 95% CI = 0.78–0.87) vs. Europe (RR = 0.86, 95% CI = 0.82–0.90) vs. other regions (RR = 0.81, 95% CI = 0.69–0.96; p-heterogeneity = 0.45).
Table 1.
Summary of subgroup analyses for early-life BMI and breast and ovarian cancer risks.
| Cancer site | Subgroups | Number of studies included | Summary RR (95% CI) | I2 | P-heterogeneity | ||
|---|---|---|---|---|---|---|---|
| Within subgroup | Between subgroups | ||||||
| Breast cancer | Tumour subtype | Oestrogen receptor-positive | 5 | 0.75 (0.65, 0.87) | 74.9% | 0.003 | 0.29 |
| Oestrogen receptor-negative | 3 | 0.60 (0.38, 0.98) | 77.5% | 0.01 | |||
| Age of BMI | BMI at age <18 | 7 | 0.87 (0.82, 0.91) | 77.8% | <0.001 | 0.63 | |
| BMI at age 18 | 10 | 0.79 (0.72, 0.87) | 58.4% | 0.01 | |||
| BMI at age >18 | 9 | 0.85 (0.79, 0.91) | 38.9% | 0.11 | |||
| BMI assessment method | Measured | 6 | 0.86 (0.80, 0.93) | 68.0% | 0.008 | 0.49 | |
| Self-reported | 20 | 0.83 (0.79, 0.87) | 60.4% | <0.001 | |||
| Region | USA | 12 | 0.82 (0.78, 0.87) | 59.1% | 0.005 | 0.45 | |
| Europe | 10 | 0.86 (0.82, 0.90) | 33.8% | 0.14 | |||
| Other | 4 | 0.81 (0.69, 0.96) | 79.2% | 0.002 | |||
| Ovarian cancer | Region | USA | 4 | 1.18 (1.05, 1.31) | 0.0% | 0.66 | 0.57 |
| Europe | 3 | 1.11 (0.97, 1.27) | 33.8% | 0.22 | |||
This table shows the results from subgroup analyses by tumour subtype, age of BMI, BMI assessment method, and region.
BMI body mass index, CI confidence interval, RR relative risk.
Endometrial cancer
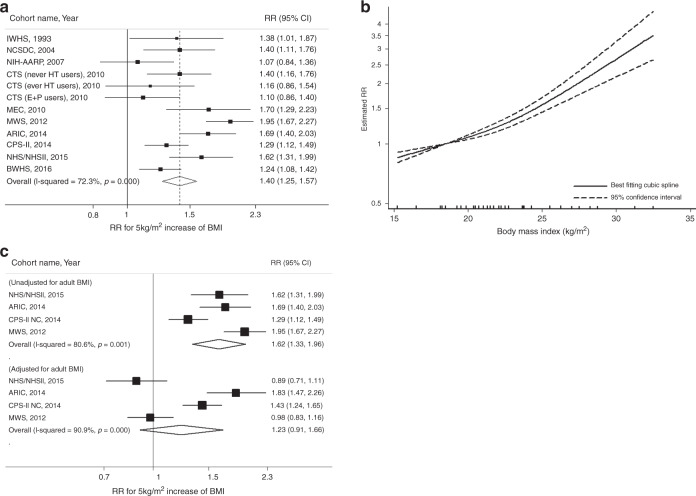
A total of 4,539 endometrial cancer cases from 662,779 participants were included in the meta-analyses of early-life BMI and endometrial cancer risk (range of BMI: 15.3–32.5 kg/m2). Each 5-kg/m2 increase in early-life BMI was statistically significantly associated with a 1.40-fold increased risk of endometrial cancer (summary RR = 1.40, 95% CI = 1.25–1.57) with evidence of high heterogeneity (I2 = 72.3%, p < 0.001; Fig. 2a). Although we observed heterogeneity in the magnitude of associations among studies, with RRs ranging from 1.07 to 1.95, there was no variation in the direction of associations. Small study effects, such as publication bias, were not evident with Egger’s test (p = 0.63). In the nonlinear dose–response meta-analysis, there was evidence of nonlinearity, suggesting a steeper elevation of risk at BMI > 23 kg/m2 (p-nonlinearity = 0.004; Fig. 2b). In subgroup analysis by adjustment for adult BMI, the positive association with early-life BMI was attenuated in the adult BMI-adjusted models (summary RR = 1.23, 95% CI = 0.91–1.66 adjusted vs. RR = 1.62, 95% CI = 1.33–1.96 unadjusted) but the difference was not statistically significant (p-heterogeneity = 0.19; Fig. 2c).
Fig. 2. Linear and nonlinear dose–response relationships for early-life BMI and endometrial cancer risk.
This figure shows the results from a linear dose–response relationship with endometrial cancer; b nonlinear dose–response relationship with endometrial cancer (p-nonlinearity = 0.004); c subgroup analysis by adjustment for adult BMI (p-heterogeneity = 0.19). The black squares and horizontal lines represent study-specific relative risks and their 95% confidence intervals. The area of each black square reflects the weight each study contributes to the meta-analysis. The middle and horizontal tips of diamonds represent summary RRs and their 95% confidence intervals, respectively. The P values were calculated from Cochran’s Q test; all statistical tests were two-sided.
Ovarian cancer
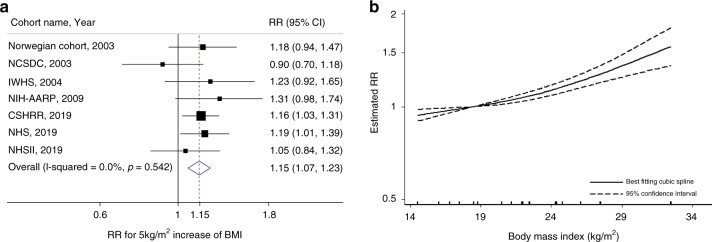
A total of 2,692 ovarian cancer cases from 496,391 participants were included in the meta-analyses of early-life BMI and ovarian cancer risk (range of BMI: 14.6–32.5 kg/m2). The summary RR for each 5-kg/m2 increase in early-life BMI in relation to ovarian cancer risk was 1.15 (95% CI = 1.07–1.23) with no evidence of heterogeneity (I2 = 0%, p = 0.54; Fig. 3a). Small study effects, such as publication bias, were not evident with Egger’s test (p = 0.72). In the nonlinear dose–response meta-analysis, there was suggestive evidence of nonlinearity with a steeper risk elevation at BMI > 23 kg/m2 (p-nonlinearity = 0.05) (Fig. 3b). In the subgroup analysis by region, summary RRs were similar between studies from USA (RR = 1.18, 95% CI = 1.05–1.31) and Europe (RR = 1.11, 95% CI = 0.97–1.27; p-heterogeneity = 0.57; Table 1 and Supplementary Fig. 3).
Fig. 3. Linear and nonlinear dose–response relationships for early-life BMI and ovarian cancer risk.
This figure shows the results from a linear dose–response relationship with ovarian cancer; b nonlinear dose–response relationship with ovarian cancer (p-nonlinearity = 0.05). The black squares and horizontal lines represent study-specific relative risks and their 95% confidence intervals. The area of each black square reflects the weight each study contributes to the meta-analysis. The middle and horizontal tips of diamonds represent summary RRs and their 95% confidence intervals, respectively. The P values were calculated from Cochran’s Q test; all statistical tests were two-sided.
Sensitivity analyses
Similar results were observed in sensitivity analyses that excluded studies with pictogram assessment and those with the converted reference group (data not shown). The results were also robust in the influence analysis that excluded one study at a time (data not shown).
Discussion
Our dose–response meta-analysis of 37 prospective studies estimated a 16% reduced breast cancer risk, a 40% increased endometrial cancer risk, and a 15% increased ovarian cancer risk associated with every 5-kg/m2 increase in early-life BMI. The inverse association with breast cancer risk was similar between pre- and postmenopausal women and remained statistically significant after additional adjustment for current adult BMI, suggesting that the association is independent of adult body fatness and not modified by menopausal status. Our findings also suggest possible nonlinear relationships (e.g. threshold effects) of early-life BMI with premenopausal breast cancer, endometrial cancer, and ovarian cancer risks showing a steeper risk change occurring at BMI >23 kg/m2.
Our findings provide the evidence supporting the long-term effects of early-life BMI on three major female cancer risks. In our analyses, the positive associations with endometrial and ovarian cancer risks and the inverse association with breast cancer risk were consistently observed in most studies that adjusted for various adult risk factors including parity, age at first birth, and physical activity, suggesting that the associations are independent of these adult risk factors. Our findings are also consistent with those from previous meta-analyses [13, 14, 39]. In the current meta-analysis, we additionally performed various subgroup analyses and observed that the associations were also largely consistent across different populations and assessment methods. Our subgroup analyses demonstrated that there was no systematic difference in risk estimates between the studies with self-reported vs. measured data and thus the associations are unlikely to be fully explained by the related potential biases and methodological issues. The associations are also unlikely to be fully explained by confounding because in many studies the associations were similar between age-adjusted and multivariable-adjusted models that included potential confounders such as birthweight and lifestyle risk factors [18–20, 23, 25, 27, 32, 34, 61, 62, 64, 69, 70]. Further, although we observed moderate to high heterogeneity across the studies, the heterogeneity was primarily due to the variation in the magnitude of associations, not the direction of the associations. Compared with large studies, small studies tended to show variable estimates, possibly contributing to the heterogeneity in the estimates among the studies.
For breast and endometrial cancers, we also conducted a subgroup analysis by the adjustment for adult BMI, a potential mediator, among the studies that reported both models with and without adjustment for current adult BMI. In previous meta-analyses [13, 14, 39], different numbers of studies were included for adjusted vs. unadjusted estimates and thus it is difficult to clarify whether any variation in RRs and 95% CIs are due to statistical power or study population difference. By restricting the analysis to the studies that reported both models, we increased the comparability between the results and reduced the possibility of differences in statistical power (e.g. number of studies included) creating spurious heterogeneity. For breast cancer, we observed that the association did not materially change and remained statistically significant after additional adjustment for adult BMI, further supporting the independent association of early-life BMI and the pathways not mediated by adult BMI. On the other hand, the association for endometrial cancer was substantially attenuated in the adult BMI-adjusted models, suggesting that a large proportion of the association may be explained by the adult BMI-mediated pathways and that adult adiposity may be more aetiologically relevant to endometrial carcinogenesis. Similarly, a previous meta-analysis of case–control studies reported that the association between early-life BMI and ovarian cancer disappeared after the adjustment for adult BMI [35]. Overweight and obese girls are more likely to become obese adults [72], and adult obesity is an important risk factor for various cancer sites [12, 14] including endometrial [13] and ovarian cancers [14, 35]. Adipose tissue secretes adipokines, such as leptin, that promotes inflammation and cellular proliferation [73]. Leptin can also regulate sex hormone binding globulin expression [74] and increase bioavailability of oestrogens. Postmenopausal obesity also increases circulating levels of oestrogens via aromatase activity in adipose tissues [75], promoting cellular proliferation and tumorigenesis. However, for breast cancer, we observed inverse associations of early-life BMI with both pre- and postmenopausal breast cancer risks. These findings suggest that the adult BMI-independent pathways may also exist to counteract the adverse effect of the adult BMI-mediated pathways. Further, given that the direction of associations for early-life BMI is consistent with that of adult BMI and premenopausal, but not postmenopausal, breast cancer [12], the related mechanisms for early-life BMI are more likely to be similar with those of adult BMI and premenopausal breast cancer (e.g. anovulation) as opposed to those of adult BMI and postmenopausal breast cancer (e.g. aromatisation). Moreover, the inverse association specifically found with breast cancer but not with other cancer sites (e.g. ovarian and endometrial cancers) suggest that the differential mechanisms, such as breast tissue-specific effects, are likely to be involved in breast carcinogenesis.
In the current study, we were able to conduct various subgroup analyses that was not performed in previous meta-analyses of early-life BMI [13, 14, 39]. For breast cancer, we observed that the associations were similar between studies using BMI at different ages. Although some studies have suggested that carcinogenic exposure before vs. after menarche and before vs. after the first childbirth may differentially influence mammographic density [76] and breast cancer risk [77], our subgroup analysis found no evidence of heterogeneity in breast cancer risk associations by age of BMI (10–17 years vs. 18 years vs. 19–25 years). Our findings suggest that the inverse association between early-life BMI and breast cancer risk may originate as early as age 10 years, possibly prior to menarche. In our analysis, we were not able to estimate summary RRs for BMI at very young ages (<10 years) because only few studies reported body fatness at age <10 years. In the current study, we were also able to perform subgroup analysis by tumour subtype for breast cancer. We observed that early-life BMI was significantly inversely associated with both ER-positive and ER-negative breast cancers, suggesting that both oestrogen-dependent and oestrogen-independent pathways may be involved. For ovarian and endometrial cancers, we were not able to examine the differential associations by tumour subtype because only few recent studies have examined the associations by tumour subtypes. However, some studies reported that, for ovarian cancer, the association with early-life BMI may be restricted to non-serous ovarian cancer [31, 35, 71]. Further studies are needed to clarify the aetiology of early-life BMI on different tumour subtypes in women.
Although the exact mechanisms for the inverse association between early-life BMI and breast cancer risk are unknown, there are potential pathways that may explain the associations. Previous studies have shown lower circulating levels of adult insulin-like growth factor (IGF)-I in women who were overweight or obese (vs. normal weight) during childhood [78]. Circulating levels of adult IGF-I are associated with an increased breast cancer risk [79] and lower levels of age-related lobular involution (the age-related atrophy of epithelial structures) of breast tissue [80]. Studies also suggest the lifelong impact of early-life body fatness on breast density (lower overall density [81, 82] and image intensity variation of dense tissue on a mammogram [82]), breast tissue composition (higher proportion of adipose tissue and lower proportion of stromal tissue) [83], breast tissue expression of cell proliferation marker Ki67 [84], and breast transcriptome [85]. Early-life body fatness may influence breast cancer risk by contributing to lifelong setpoints of these intermediate markers. In the Nurses’ Health Study (NHS) and NHSII cohorts, a mediation analysis was conducted and found that a large proportion of the association between early-life body fatness and breast cancer was mediated by percent mammographic density in both pre- (71%–82%) and postmenopausal (26%–98%) women [86]. It is possible that a greater proportion of the association may be explained when multiple breast tissue-specific pathways are considered in the future studies.
Our dose–response meta-analysis also suggested nonlinear relationships of early-life BMI with premenopausal breast, endometrial, and ovarian cancer risks. Our finding is largely consistent with a previous pooled analysis of premenopausal breast cancer, which observed leveling of risk for underweight women [87]. These findings suggest that the effects of early-life BMI may require certain BMI thresholds. However, while the previous pooled analysis focused only on premenopausal women [38], our analysis additionally included a large number of cases from postmenopausal breast cancers and observed no evidence of nonlinearity with postmenopausal breast cancer risk, suggesting that the potential threshold effect may not universally apply to all cancer sites. Further studies are needed to clarify the possible threshold effects of early-life BMI on female cancer risks.
Our study has several limitations. Although we included fully adjusted models in the analysis, we cannot exclude the possibility of residual confounding if the individual studies included in our meta-analysis did not adequately adjust for potential confounders. Studies rarely had adequate data on genetic factors and early-life environmental exposures that are likely to influence the associations. Our meta-analysis is also not free form publication bias as we included published studies only. However, we observed no indication of publication bias based on Egger’s test. As the recall of early-life exposures is often very difficult, measurement error is likely to occur in the assessment of early-life BMI. However, in our subgroup analyses, we observed no significant differences between results from studies with measured vs. self-reported early-life BMI.
Despite the limitations, our study has important strengths. We examined both linear and non-linear dose–response relationships of early-life BMI with breast, endometrial, and ovarian cancer risks. Previous meta-analyses focused on linear dose–response relationships and the comparison of the highest vs. lowest categories of early-life BMI. In our nonlinear dose–response meta-analysis, we were able to explore the shape of the relationships and quantify the risks associated with specific levels of early-life BMI. In our meta-analysis, we included prospective studies only and thus recall bias is likely to be reduced and any measurement error in early-life BMI is likely to be non-differential. When examining the associations independent of adult BMI, we compared studies that reported both models with and without additional adjustment for adult BMI. This method increased the comparability between results from these two models. Further, our meta-analysis included 15 additional studies for breast cancer and 4 additional studies for ovarian cancer that were not part of the previous pooling studies [37, 38], allowing us to comprehensively perform various subgroup analyses to investigate potential effect modification and variation in associations due to methodologic differences. Compared with the most recent meta-analyses [13, 14, 39], we additionally included 9 studies for breast cancer, 2 studies for endometrial cancer, and 1 study for ovarian cancer analyses. Lastly, we observed similar results in sensitivity analyses and thereby confirmed the robustness of our results.
Our findings enhanced our understanding of the associations between early-life body fatness and female cancer risks. In summary, our dose–response meta-analysis of 37 prospective studies supports the positive associations of early-life body fatness with ovarian and endometrial cancers and an inverse association with breast cancer risk. Despite the reduced breast cancer risk associated with early-life body fatness, childhood obesity should not be promoted as a preventive strategy for breast cancer as it is also associated with increased risks of various other cancer sites including endometrial and ovarian cancers.
Supplementary information
Acknowledgements
Not applicable.
Author contributions
DB conducted literature search, performed data extraction, and wrote the paper. SH performed statistical analyses and wrote the paper. SR, YN, HJ and YC conducted literature search and performed data extraction. HO and NK designed the research and made substantial contributions to interpretation of data, and critical revision and editing of the manuscript. All authors revised the manuscript for important intellectual content and gave final approval of the version to be published.
Funding information
HO was supported by the National Research Foundation of Korea (NRF) grants (2019R1G1A1004227, 2019S1A3A2099973) and Korea University grant L1906811. NK was supported by the National Research Foundation of Korea (NRF) grants (2018R1C1B6008822, 2018R1A4A1022589). The funders were not involved in the study design or data analysis and the views expressed in this publication are those of the authors.
Data availability
Available upon request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dohyun Byun, SungEun Hong, NaNa Keum, Hannah Oh.
Contributor Information
NaNa Keum, Email: nak212@mail.harvard.edu.
Hannah Oh, Email: hananhoh@korea.ac.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01625-1.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Peeters PHM, Verbeek ALM, Krol A, Matthyssen MMM, Dewaard F. Age at menarche and breast-cancer risk in nulliparous women. Breast Cancer Res Treat. 1995;33:55–61. doi: 10.1007/BF00666071. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh CC, Trichopoulos D, Katsouyanni K, Yuasa S. Age at menarche, age at menopause, height and obesity as risk-factors for breast-cancer - associations and interactions in an international case-control study. Int J Cancer. 1990;46:796–800. doi: 10.1002/ijc.2910460508. [DOI] [PubMed] [Google Scholar]
- 4.McPherson CP, Sellers TA, Potter JD, Bostick RM, Folsom AR. Reproductive factors and risk of endometrial cancer—the Iowa women’s health study. Am J Epidemiol. 1996;143:1195–202. doi: 10.1093/oxfordjournals.aje.a008707. [DOI] [PubMed] [Google Scholar]
- 5.Fujita M, Tase T, Kakugawa Y, Hoshi S, Nishino Y, Nagase S, et al. Smoking, earlier menarche and low parity as independent risk factors for gynecologic cancers in japanese: a case-control study. Tohoku J Exp Med. 2008;216:297–307. doi: 10.1620/tjem.216.297. [DOI] [PubMed] [Google Scholar]
- 6.Layde PM, Webster LA, Baughman AL, Wingo PA, Rubin GL, Ory HW. The independent associations of parity, age at 1st full term pregnancy, and duration of breastfeeding with the risk of breast-cancer. J Clin Epidemiol. 1989;42:963–73. doi: 10.1016/0895-4356(89)90161-3. [DOI] [PubMed] [Google Scholar]
- 7.Titus-Ernstoff L, Perez K, Cramer DW, Harlow BL, Baron JA, Greenberg ER. Menstrual and reproductive factors in relation to ovarian cancer risk. Br. J Cancer. 2001;84:714–21. doi: 10.1054/bjoc.2000.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salagame U, Banks E, O’Connell DL, Egger S, Canfell K. Menopausal hormone therapy use and breast cancer risk by receptor subtypes: results from the new south wales cancer lifestyle and evaluation of risk (CLEAR) study. PLoS ONE. 2018;13:e0205034. doi: 10.1371/journal.pone.0205034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakken K, Fournier A, Lund E, Waaseth M, Dumeaux V, Clavel-Chapelon F, et al. Menopausal hormone therapy and breast cancer risk: impact of different treatments. The European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2011;128:144–56. doi: 10.1002/ijc.25314. [DOI] [PubMed] [Google Scholar]
- 10.Doherty, JA, Cushing-Haugen, KL, Saltzman, BS, Voigt, LF, Hill, DA, Beresford, SA, et al. Long-term use of postmenopausal estrogen and progestin hormone therapies and the risk of endometrial cancer. Am J Obstet Gynecol. 2007;197: 139-e1. [DOI] [PubMed]
- 11.Lacey JV, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–41. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 12.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 13.Aune D, Rosenblatt DAN, Chan DSM, Vingeliene S, Abar L, Vieira AR, et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Ann Oncol. 2015;26:1635–48. doi: 10.1093/annonc/mdv142. [DOI] [PubMed] [Google Scholar]
- 14.Aune D, Rosenblatt DAN, Chan DSM, Abar L, Vingeliene S, Vieira AR, et al. Anthropometric factors and ovarian cancer risk: A systematic review and nonlinear dose-response meta-analysis of prospective studies. Int J Cancer. 2015;136:1888–98. doi: 10.1002/ijc.29207. [DOI] [PubMed] [Google Scholar]
- 15.Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI. Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351:1619–26. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 16.Keinan-Boker L, Levine H, Derazne E, Molina-Hazan V, Kark JD. Measured adolescent body mass index and adult breast cancer in a cohort of 951,480 women. Breast Cancer Res Treat. 2016;158:157–67. doi: 10.1007/s10549-016-3860-6. [DOI] [PubMed] [Google Scholar]
- 17.Fagherazzi G, Guillas G, Boutron-Ruault MC, Clavel-Chapelon F, Mesrine S. Body shape throughout life and the risk for breast cancer at adulthood in the French E3N cohort. Eur J Cancer Prev. 2013;22:29–37. doi: 10.1097/CEJ.0b013e328355ec04. [DOI] [PubMed] [Google Scholar]
- 18.Kawai M, Minami Y, Kuriyama S, Kakizaki M, Kakugawa Y, Nishino Y, et al. Adiposity, adult weight change and breast cancer risk in postmenopausal Japanese women: the Miyagi Cohort Study. Brit J Cancer. 2010;103:1443–7. doi: 10.1038/sj.bjc.6605885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer JR, Adams-Campbell LL, Boggs DA, Wise LA, Rosenberg L. A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomark Prev. 2007;16:1795–802. doi: 10.1158/1055-9965.EPI-07-0336. [DOI] [PubMed] [Google Scholar]
- 20.Weiderpass E, Braaten T, Magnusson C, Kumle M, Vainio H, Lund E, et al. A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomark Prev. 2004;13:1121–7. [PubMed] [Google Scholar]
- 21.Sellers TA, Davis J, Cerhan JR, Vierkant RA, Olson JE, Pankratz VS, et al. Interaction of waist/hip ratio and family history on the risk of hormone receptor-defined breast cancer in a prospective study of postmenopausal women. Am J Epidemiol. 2002;155:225–33. doi: 10.1093/aje/155.3.225. [DOI] [PubMed] [Google Scholar]
- 22.Hilakivi-Clarke L, Forsen T, Eriksson JG, Luoto R, Tuomilehto J, Osmond C, et al. Tallness and overweight during childhood have opposing effects on breast cancer risk. Br J Cancer. 2001;85:1680–4. doi: 10.1054/bjoc.2001.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171:1183–94. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens VL, Jacobs EJ, Patel AV, Sun JZ, Gapstur SM, McCullough ML. Body weight in early adulthood, adult weight gain, and risk of endometrial cancer in women not using postmenopausal hormones. Cancer Cause Control. 2014;25:321–8. doi: 10.1007/s10552-013-0333-7. [DOI] [PubMed] [Google Scholar]
- 25.Dougan MM, Hankinson SE, De Vivo I, Tworoger SS, Glynn RJ, Michels KB. Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. Int J Cancer. 2015;137:625–37. doi: 10.1002/ijc.29427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang TYO, Cairns BJ, Allen N, Sweetland S, Reeves GK, Beral V, et al. Postmenopausal endometrial cancer risk and body size in early life and middle age: prospective cohort study. Brit J Cancer. 2012;107:169–75. doi: 10.1038/bjc.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SL, Goodman MT, Zhang ZF, Kolonel LN, Henderson BE, Setiawan VW. Body size, adult BMI gain and endometrial cancer risk: the multiethnic cohort. Int J cancer. 2010;126:490–9. doi: 10.1002/ijc.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sponholtz TR, Palmer JR, Rosenberg L, Hatch EE, Adams-Campbell LL, Wise LA. Body size, metabolic factors, and risk of endometrial cancer in black women. Am J Epidemiol. 2016;183:259–68. doi: 10.1093/aje/kwv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubin F, Chetrit A, Freedman LS, Alfandary E, Fishler Y, Nitzan H, et al. Body mass index at age 18 years and during adult life and ovarian cancer risk. Am J Epidemiol. 2003;157:113–20. doi: 10.1093/aje/kwf184. [DOI] [PubMed] [Google Scholar]
- 30.Engeland A, Tretli S, Bjørge T. Height, body mass index, and ovarian cancer: a follow-up of 1.1 million Norwegian women. J Natl Cancer Inst. 2003;95:1244–8. doi: 10.1093/jnci/djg010. [DOI] [PubMed] [Google Scholar]
- 31.Aarestrup J, Trabert B, Ulrich LG, Wentzensen N, Sorensen TIA, Baker JL. Childhood overweight, tallness, and growth increase risks of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2019;28:183–8. doi: 10.1158/1055-9965.EPI-18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burton A, Martin R, Galobardes B, Smith GD, Jeffreys M. Young adulthood body mass index and risk of cancer in later adulthood: historical cohort study. Cancer Cause Control. 2010;21:2069–77. doi: 10.1007/s10552-010-9625-3. [DOI] [PubMed] [Google Scholar]
- 33.Baer HJ, Hankinson SE, Tworoger SS. Body size in early life and risk of epithelial ovarian cancer: results from the Nurses’ Health Studies. Brit J Cancer. 2008;99:1916–22. doi: 10.1038/sj.bjc.6604742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schouten LJ, Goldbohm RA, van den Brandt PA. Height, weight, weight change, and ovarian cancer risk in the Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol. 2003;157:424–33. doi: 10.1093/aje/kwf224. [DOI] [PubMed] [Google Scholar]
- 35.Olsen CM, Nagle CM, Whiteman DC, Ness R, Pearce CL, Pike MC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;20:251–62. doi: 10.1530/ERC-12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki R, Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, et al. Body weight at age 20 years, subsequent weight change and breast cancer risk defined by estrogen and progesterone receptor status-the Japan public health center-based prospective study. Int J Cancer. 2011;129:1214–24. doi: 10.1002/ijc.25744. [DOI] [PubMed] [Google Scholar]
- 37.Schouten LJ, Rivera C, Hunter DJ, Spiegelman D, Adami HO, Arslan A, et al. Height, body mass index, and ovarian cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomark Prev. 2008;17:902–12. doi: 10.1158/1055-9965.EPI-07-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Premenopausal Breast Cancer Collaborative G, Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, et al. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018;4:e181771. doi: 10.1001/jamaoncol.2018.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan DSM, Abar L, Cariolou M, Nanu N, Greenwood DC, Bandera EV, et al. World Cancer Research Fund International: continuous update project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30:1183–200. doi: 10.1007/s10552-019-01223-w. [DOI] [PubMed] [Google Scholar]
- 40.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 41.De Stavola B, dos Santos Silva I, McCormack V, Hardy R, Kuh D, Wadsworth M. Childhood growth and breast cancer. Am J Epidemiol. 2004;159:671–82. doi: 10.1093/aje/kwh097. [DOI] [PubMed] [Google Scholar]
- 42.Torio CM, Klassen AC, Curriero FC, Caballero B, Helzlsouer K. The modifying effect of social class on the relationship between body mass index and breast cancer incidence. Am J Public Health. 2010;100:146–51. doi: 10.2105/AJPH.2007.126979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White KK, Park SY, Kolonel LN, Henderson BE, Wilkens LR. Body size and breast cancer risk: the MULTIETHNIC Cohort. Int J Cancer. 2012;131:E705–16. doi: 10.1002/ijc.27373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishnan K, Bassett JK, MacInnis RJ, English DR, Hopper JL, McLean C, et al. Associations between weight in early adulthood, change in weight, and breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22:1409–16. doi: 10.1158/1055-9965.EPI-13-0136. [DOI] [PubMed] [Google Scholar]
- 45.Andersen ZJ, Baker JL, Bihrmann K, Vejborg I, Sørensen TI, Lynge E. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast Cancer Res. 2014;16:R4. doi: 10.1186/bcr3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canchola AJ, Chang ET, Bernstein L, Largent JA, Reynolds P, Deapen D, et al. Body size and the risk of endometrial cancer by hormone therapy use in postmenopausal women in the California Teachers Study cohort. Cancer Cause Control. 2010;21:1407–16. doi: 10.1007/s10552-010-9568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stunkard AJ. Use of the Danish adoption register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis. 1983;60:115–20. [PubMed] [Google Scholar]
- 48.Sørensen T, Stunkard A, Teasdale T, Higgins M. The accuracy of reports of weight: children’s recall of their parents’ weights 15 years earlier. Int J Obes. 1983;7:115–22. [PubMed] [Google Scholar]
- 49.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138:56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 50.Wells, GA, Shea, B, O’Connell, D, Peterson, J, Welch, V, Losos, M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed 15, July, 2021.
- 51.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 52.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 53.Harre FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 54.Orsini N, Greenland S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. tata J. 2011;11:1–29. [Google Scholar]
- 55.Organization, WH. Obesity: preventing and managing the global epidemic. (World Health Organization, 2000). [PubMed]
- 56.White IR. Multivariate random-effects meta-analysis. Stata J. 2009;9:40–56. [Google Scholar]
- 57.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 58.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 60.Horn-Ross, PL, Canchola, AJ, Bernstein, L, Neuhausen, SL, Nelson, DO & Reynolds, P. Lifetime body size and estrogen-receptor-positive breast cancer risk in the California Teachers Study cohort. Breast Cancer Res. 2016;18:132. [DOI] [PMC free article] [PubMed]
- 61.Luo J, Chen X, Manson JE, Shadyab AH, Wactawski‐Wende J, Vitolins M, et al. Birth weight, weight over the adult life course and risk of breast cancer. Int J Cancer. 2020;147:65–75. doi: 10.1002/ijc.32710. [DOI] [PubMed] [Google Scholar]
- 62.van den Brandt PA, Dirx MJ, Ronckers CM, van den Hoogen P, Goldbohm RA. Height, weight, weight change, and postmenopausal breast cancer risk: the Netherlands Cohort Study. Cancer Cause Control. 1997;8:39–47. doi: 10.1023/a:1018479020716. [DOI] [PubMed] [Google Scholar]
- 63.Cerhan JR, Grabrick DM, Vierkant RA, Janney CA, Vachon CM, Olson JE, et al. Interaction of adolescent anthropometric characteristics and family history on breast cancer risk in a Historical Cohort Study of 426 families (USA) Cancer Cause Control. 2004;15:1–9. doi: 10.1023/B:CACO.0000016566.30377.4e. [DOI] [PubMed] [Google Scholar]
- 64.Renehan AG, Pegington M, Harvie MN, Sperrin M, Astley SM, Brentnall AR, et al. Young adulthood body mass index, adult weight gain and breast cancer risk: the PROCAS Study (United Kingdom) Br J Cancer. 2020;122:1552–61. doi: 10.1038/s41416-020-0807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gapstur SM, Potter JD, Sellers TA, Kushi LH, Folsom AR. Alcohol consumption and postmenopausal endometrial cancer: results from the Iowa Women’s Health Study. Cancer Cause Control. 1993;4:323–9. doi: 10.1007/BF00051334. [DOI] [PubMed] [Google Scholar]
- 66.Schouten LJ, Goldbohm RA. & Van Den Brandt, P. A. Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. J Natl Cancer Inst. 2004;96:1635–1638. [DOI] [PubMed]
- 67.Chang S-C, Lacey JV, Brinton LA, Hartge P, Adams K, Mouw T, et al. Lifetime weight history and endometrial cancer risk by type of menopausal hormone use in the NIH-AARP diet and health study. Cancer Epidemiol, Biomark Prev. 2007;16:723–30. doi: 10.1158/1055-9965.EPI-06-0675. [DOI] [PubMed] [Google Scholar]
- 68.Han X, Stevens J, Truesdale KP, Bradshaw PT, Kucharska‐Newton A, Prizment AE, et al. Body mass index at early adulthood, subsequent weight change and cancer incidence and mortality. Int J Cancer. 2014;135:2900–9. doi: 10.1002/ijc.28930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson JP, Ross JA, Folsom AR. Anthropometric variables, physical activity, and incidence of ovarian cancer: The Iowa Women’s Health Study. Cancer. 2004;100:1515–21. doi: 10.1002/cncr.20146. [DOI] [PubMed] [Google Scholar]
- 70.Leitzmann MF, Koebnick C, Danforth KN, Brinton LA, Moore SC, Hollenbeck AR, et al. Body mass index and risk of ovarian cancer. Cancer: Interdisciplinary. Int J Am Cancer Soc. 2009;115:812–22. doi: 10.1002/cncr.24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang T, Tworoger S, Willett W, Stampfer M, Rosner B. Associations of early life and adulthood adiposity with risk of epithelial ovarian cancer. Ann Oncol. 2019;30:303–9. doi: 10.1093/annonc/mdy546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Preventive Med. 1993;22:167–77. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 73.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 74.Haffner SM, Miettinen H. Karhapää, P., Mykkänen, L. & Laakso, M. Leptin concentrations, sex hormones, and cortisol in nondiabetic men. J Clin Endocrinol Metab. 1997;82:1807–9. doi: 10.1210/jcem.82.6.3978. [DOI] [PubMed] [Google Scholar]
- 75.Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab. 2012;23:83–9. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ginsburg OM, Martin LJ, Boyd NF. Mammographic density, lobular involution, and risk of breast cancer. Br J Cancer. 2008;99:1369–74. doi: 10.1038/sj.bjc.6604635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature. 1983;303:767–70. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 78.Poole EM, Tworoger SS, Hankinson SE, Schernhammer ES, Pollak MN, Baer HJ. Body size in early life and adult levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3. Am J Epidemiol. 2011;174:642–51. doi: 10.1093/aje/kwr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hormones TE, Group BCC. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–42. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oh H, Pfeiffer RM, Falk RT, Horne HN, Xiang J, Pollak M, et al. Serum insulin‐like growth factor (IGF)‐I and IGF binding protein‐3 in relation to terminal duct lobular unit involution of the normal breast in Caucasian and African American women: The Susan G. Komen Tissue Bank. Int J Cancer. 2018;143:496–507. doi: 10.1002/ijc.31333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bertrand KA, Baer HJ, Orav EJ, Klifa C, Shepherd JA, Van Horn L, et al. Body fatness during childhood and adolescence and breast density in young women: a prospective analysis. Breast Cancer Res. 2015;17:95. doi: 10.1186/s13058-015-0601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oh H, Rice MS, Warner ET, Bertrand KA, Fowler EE, Eliassen AH, et al. Early-life and adult anthropometrics in relation to mammographic image intensity variation in the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2020;29:343–51. doi: 10.1158/1055-9965.EPI-19-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oh, H, Yaghjyan, L, Austin-Datta, RJ, Heng, YJ, Baker, GM, Sirinukunwattana, K, et al. Early-life and adult adiposity, adult height, and benign breast tissue composition. Cancer Epidemiol Biomarkers Prev. 2021;30:608–615. [DOI] [PMC free article] [PubMed]
- 84.Oh H, Eliassen AH, Beck AH, Rosner B, Schnitt SJ, Collins LC, et al. Breast cancer risk factors in relation to estrogen receptor, progesterone receptor, insulin-like growth factor-1 receptor, and Ki67 expression in normal breast tissue. NPJ Breast Cancer. 2017;3:1–8. doi: 10.1038/s41523-017-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang, J, Peng, C, Askew, C, Heng, YJ, Baker, GM, Rubadue, CA, et al. Early-life body adiposity and the breast tumor transcriptome. J Natl Cancer Inst. 2020; 10.1093/jnci/djaa169. [DOI] [PMC free article] [PubMed]
- 86.Rice MS, Bertrand KA, VanderWeele TJ, Rosner BA, Liao X, Adami H-O, et al. Mammographic density and breast cancer risk: a mediation analysis. Breast Cancer Res. 2016;18:1–13. doi: 10.1186/s13058-016-0750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, O’Brien KM, et al. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018;4:e181771–e181771. doi: 10.1001/jamaoncol.2018.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available upon request.