Abstract
Functional autoantibodies directed to the M2 muscarinic acetylcholine receptor (M2R) could affect the heart rate directly by altering cardiac M2R activity and/or indirectly by changing vagal-mediated cardiac M2R activity. We measured M2R autoantibody activity in sera from 10 subjects with postural tachycardia syndrome (POTS) and 5 healthy control subjects using a cell-based bioassay. Half of the POTS subjects demonstrated presence of elevated M2R autoantibody activity, while no significant M2R autoantibody activity was found in the healthy subjects. Serum-derived immunoglobulin G (IgG) from antibody-positive POTS patients induced a dose-dependent activation of M2R, which was blocked by the muscarinic antagonist atropine. Moreover, antibody-positive POTS IgG decreased the responsiveness to oxotremorine, an orthosteric muscarinic agonist, indicating an indirect inhibitory effect. These data suggest that M2R autoantibodies may contribute to the pathophysiology of POTS by increasing the normal vagal withdrawal during upright posture through its negative allosteric modulation of M2R activity.
Keywords: M2 muscarinic acetylcholine receptor, Postural tachycardia syndrome, Activating autoantibody
Graphical Abstract
M2 muscarinic receptor-activating autoantibodies are present in a subgroup of patients with POTS and act as a negative allosteric modulator of the orthosteric ligand response
Postural tachycardia syndrome (POTS) is a common disorder characterized by an unexplained and exaggerated increase in the cardiac rate (≥ 30 bpm) during upright posture in the absence of orthostatic hypotension. POTS is a heterogeneous condition associated with multiple pathophysiological mechanisms. The strong female to male ratio, relatively young age and frequent association with a previous infection or stressful event led some investigators to suspect an autoimmune basis [1]. Our group has proposed that activating autoantibodies (AAb) to certain G protein-coupled receptors (GPCRs) including the adrenergic receptors are operative [2]. The increased sympathetic activity associated with these allosteric autoantibodies may help explain the cardiovascular pathophysiology of POTS. There is evidence that POTS patients also have decreased parasympathetic activity. This leads to consideration for the presence of autoantibodies to the M2 muscarinic acetylcholine receptor (M2R) in POTS. Antimuscarinic autoantibodies have recently been reported in POTS subjects using an ELISA-based assay [3]. The activity or function of these autoantibodies, however, is still unknown. The present study was designed to examine how M2R autoantibodies, if present and functional, might contribute to the postural tachycardia in POTS subjects.
We evaluated 10 female subjects with POTS (33 ± 5 years) and 5 female healthy control subjects of a similar age range as part of a joint study between Vanderbilt University Medical Center and University of Oklahoma Health Sciences Center [2]. POTS diagnosis was made based on consensus diagnostic criteria [4]. No autoimmune comorbidities were present in the POTS or control subjects. Serum M2R autoantibody activity was measured using a functional cell-based bioassay, the PathHunter eXpress CHRM2 CHO-K1 β-arrestin GPCR assay (DiscoveRx). These luminescence-based assays provide a highly specific platform suitable for identification of functional antibodies in complex biological samples.
Among the 10 POTS subjects, five had elevated functional M2-AAb activity, while none of the controls had significant functional M2R-AAb activity (Fig. 1a). Antibody-positive POTS IgG induced a dose-dependent increase in M2R activation, and the maximal effect appeared to occur at approximately 0.2 mg/mL of IgG (Fig. 1b). The muscarinic blocker atropine markedly suppressed the elevated M2R-AAb activity in POTS IgG (Fig. 1c). Moreover, antibody-positive POTS IgG significantly attenuated the M2R response to the muscarinic agonist oxotremorine, and shifted the dosage response curve to the right (Fig. 1d), indicating a distinct inhibitory allosteric effect of M2R-AAb on the cholinesterase-resistant M2R orthosteric agonist. These autoantibodies, therefore, appear to act as a partial agonist in vitro.
Fig. 1.
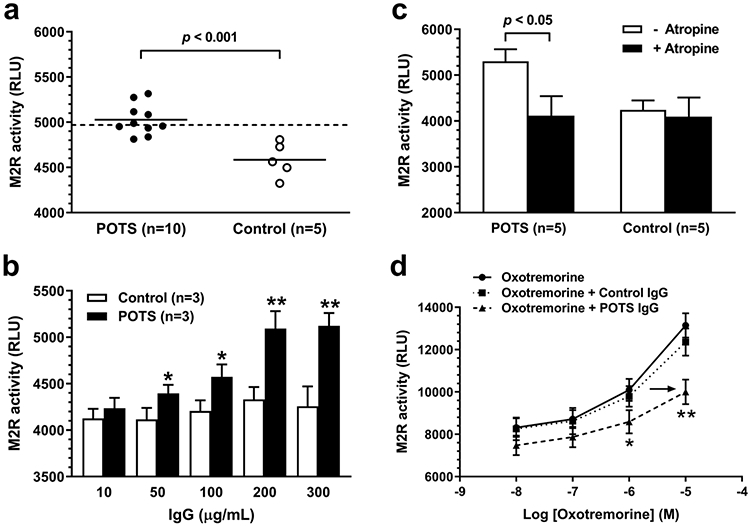
Functional activity of M2R autoantibodies in sera from POTS and control subjects assessed by the cell-based bioassay. a Serum (1:20)-induced M2R activation. The dashed line represents the threshold for “elevated” autoantibody activity defined as 2SD above the mean control value. b Dose effect of serum IgG on M2R activation. *p < 0.05, **p < 0.01 vs. baseline and controls. c Effect of the muscarinic blocker atropine on serum IgG (0.2 mg/mL)-induced M2R activation. d Effects of serum IgG (0.2 mg/mL) from 3 antibody-positive POTS patients and 3 control subjects on oxotremorine dose responsiveness. *p < 0.05, **p < 0.01 vs. oxotremorine alone and control IgG. M2R activation was quantified and expressed as relative luminescence units (RLUs)
The weaker direct effect of M2R-AAb on the atria would be to slow atrial rate during recumbency. On standing, however, the demonstrated negative allosteric effect of M2R-AAb could affect the cardiac rate either directly at the heart or indirectly via the vagus by reducing the efficacy of acetylcholine to activate the same receptor. At the heart, this effect would diminish the inherent negative chronotropic effect of the M2R. Centrally, vagal withdrawal during upright posture is associated with a modestly increased heart rate. If the degree of vagal withdrawal is accentuated by the M2R-AAb-mediated diminished reactivity of the M2R to its natural ligand acetylcholine, then the heart rate would be accentuated over its normal responsiveness. This is in line with the finding of an inhibitory effect of M2R autoantibodies on cardiovagal function observed in patients with Chagas disease [5].
While there are limitations due to the small sample size, this work represents a focused study on M2R-AAb and how they appear to act in so-afflicted subjects with POTS. Our discovery of functional, receptor-specific M2R autoantibodies in a subgroup of POTS patients and how they interact with the receptor in vitro has potential pathogenic implications since POTS is also associated with decreased parasympathetic activity. Thus, the cardiovascular or autonomic effects of previously reported antiadrenergic autoantibodies were not evaluated in these patients at this time. There is a significant likelihood that the presence of M2R-AAb in a subgroup of POTS has an additive effect on the postural tachycardia. This would fit with the hypothesis that POTS cardiovascular pathophysiology represents a concretion of two or more autonomic-oriented autoantibodies directed to their respective GPCR. A combination of the inhibitory allosteric effect from α 1-adrenergic autoantibodies with the positive allosteric effects associated with β 1/2-adrenergic autoantibodies would carry the probability for postural tachycardia. The superimposition of the negative allosteric impact of M2R-AAb either directly on the heart or indirectly via the vagus nerve would enhance this tachycardia so characteristic of subjects with POTS.
In conclusion, we present a novel finding of functional M2R autoantibodies in a subgroup of POTS patients. Our in vitro studies showed a negative allosteric modulation of M2R activity by these autoantibodies, suggesting that they may contribute to the development of parasympathetic dysfunction in a subgroup of POTS patients. Larger studies are needed to determine whether the presence of M2R-AAb makes a clinical difference as well as to fully understand their potential role in the pathophysiology of POTS.
Acknowledgements
This manuscript is dedicated to the memory of our colleague Dr. David Kem, who recently passed away. He proposed the concept of autoantibody-mediated cardiovagal dysfunction in postural tachycardia syndrome.
Funding
This work was supported by funding from the National Heart, Lung, and Blood Institute (R01HL128393), an Exploratory Grant award from Harold Hamm Diabetes Center at the University of Oklahoma, and individual grants from Christy and Aaron Jagdfeld and Francie Fitzgerald family through the University of Oklahoma Foundation Webster Fund.
Footnotes
Human subjects
This study was approved by the Vanderbilt University Medical Center and University of Oklahoma Health Sciences Center Institutional Review Boards as conforming to the overlying ethics principles operative in the United States. All subjects provided written informed consent.
Conflict of interest
The authors declare no competing interests.
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature's terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
References
- 1.Vernino S, Stiles LE (2018). Autoimmunity in postural orthostatic tachycardia syndrome: Current understanding. Auton Neurosci 215:78–82. 10.1016/j.autneu.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 2.Li H, Yu X, Liles C, Khan M, Vanderlinde-Wood M, Galloway A, Zillner C, Benbrook A, Reim S, Collier D, Hill MA, Raj SR, Okamoto LE, Cunningham MW, Aston CE, Kem DC (2014). Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 3:e000755. 10.1161/JAHA.113.000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunning WT 3rd, Kvale H, Kramer PM, Karabin BL, Grubb BP (2019). Postural orthostatic tachycardia syndrome is associated with elevated G-protein coupled receptor autoantibodies. J Am Heart Assoc 8:e013602. 10.1161/JAHA.119.013602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheldon RS, Grubb BP 2nd, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK, Kanjwal K (2015). 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 12:e41–63. 10.1016/j.hrthm.2015.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltrame SP, Carrera Paez LC, Auger SR, Sabra AH, Bilder CR, Waldner CI, Goin JC (2020). Impairment of agonist-induced M2 muscarinic receptor activation by autoantibodies from chagasic patients with cardiovascular dysautonomia. Clin Immunol 212:108346. 10.1016/j.clim.2020.108346 [DOI] [PubMed] [Google Scholar]


