Abstract
The kinetics of dengue virus (DEN)-specific serum immunoglobulin classes (immunoglobulin M [IgM] and IgA) and subclasses (IgG1 to IgG4) were studied in patients suffering from dengue fever (DF), dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS). Serum samples from non-DEN febrile patients were included as controls. IgM, IgG1, and IgG3 serum antibodies were the predominant immunoglobulins throughout the course of illness in all three patient groups. In contrast, IgA antibodies were significantly higher in the acute phase in DSS patients compared to those in DF patients (P < 0.05). The levels of IgG1 differed significantly between patients with DF and those with DHF and DSS (P < 0.05). A significant difference was also found in IgG3 levels between DF patients and DHF patients (P < 0.05) but not between DF patients and DSS patients. Finally, levels of IgG4 antibodies differed significantly between DF patients and DSS patients (P < 0.05). Collectively, these data show that increased levels of DEN-specific IgA, IgG1, and IgG4 serum antibodies are risk markers for the development of DHF and DSS and that their measurement may provide valuable guidance for early therapeutic intervention.
Dengue virus (DEN) is a mosquito-borne virus belonging to the family Flaviviridae. The four serotypes (DEN 1 to 4) are transmitted to humans through the bite of infected mosquitoes, which mainly belong to the Aedes aegypti and Aedes albopictus species. An estimated 50 million people annually are infected with DEN, and more than 2 billion people are at risk of acquiring DEN infection in tropical and subtropical regions (27). Infection with DEN may either be asymptomatic or be characterized by a variety of clinical manifestations. The majority of dengue patients develop an illness characterized by fever, chills, frontal headache, myalgia, arthralgia, and a rash, symptoms which together form the clinical syndrome of dengue fever (DF). More severe manifestations of the disease are associated with the development of hemorrhagic phenomena with plasma leakage (dengue hemorrhagic fever [DHF]) and shock (dengue shock syndrome [DSS]) (26). DHF and DSS mainly affect young children, accounting for approximately 250,000 deaths annually (18, 26). The above-mentioned features of DEN infection, as well as the fact that the mosquito vectors have a wide distribution in tropical and subtropical areas, have led to the emergence of DEN as one of the most important public health problems worldwide (11).
Despite decades of research, the pathogenesis of DEN infection remains poorly understood. Several hypotheses have been formulated to explain the development of DHF and DSS, with antibody-dependent enhancement (ADE) of infection (13, 21) being the most widely accepted. It has also been speculated that viremia plays an important role in the pathogenesis of severe DEN infections; however, it was recently demonstrated that the magnitude and duration of viremia were not significantly different among patients with primary versus secondary DEN infections (19). Other studies have demonstrated the indirect implication of circulating adhesion molecules in the pathogenesis of severe DEN infection (1, 17).
Different immunoglobulin G (IgG) subclasses can fix and activate complement (2, 5) and bind to Fcγ receptors (12, 14, 20, 24). These factors may also play an important role in the development of ADE and thus in the pathogenesis of DHF and DSS (6, 25).
The laboratory diagnosis of DEN is based on virus isolation, detection of viral RNA, or detection of DEN-specific IgM and IgG serum antibodies (9, 26). The ratio between acute-phase IgM and IgG antibodies is indicative of primary or secondary infection (26). Recent studies have indicated the diagnostic value of DEN-specific IgA serum antibodies (10, 22) and a relationship between levels of DEN-specific IgG1 serum antibodies and disease severity (23).
Here we have studied the possible correlation between the kinetics of DEN-specific serum Ig classes and subclasses on the one hand and disease severity on the other. Besides having direct diagnostic and prognostic implications, the data contribute to our understanding of the pathogenesis of DEN infections of different severity.
MATERIALS AND METHODS
Serum samples.
During the DEN epidemic in Indonesia in 1995 and 1996, serial serum samples were obtained from 171 patients with confirmed DEN infection and from 21 patients with nondengue (ND) febrile illness to serve as controls. Table 1 summarizes the characteristics of the DEN-infected patients and the controls. Of the DEN-infected patients 72 had DF, 30 had DHF, and 69 had DSS according to the criteria defined by the World Health Organization (26). All patients had been admitted to the hospital on different days after onset of fever (range, 0 to 20 days), and serial samples had been collected after admission. All patients were citizens of Yogyakarta and Semarang in Indonesia. The age varied between 7 months and 14 years (mean, 7.6 years), and 53% of the patients were females. The mean duration of fever for DF, DHF grade I (DHF I), DHF II, DHF III, and DHF IV patients was 7.0, 8.6, 9.1, 9.6, and 11.8 days, respectively. During this period all DEN serotypes were circulating, of which DEN 3 was the most predominant serotype (8). The ND febrile patients were residents of the same areas of Indonesia and belonged to the same age group (mean, 7.7 years; range, 2 to 14 years). Of this group 43% were females, and the mean duration of fever was 9.0 days. ND febrile patients tested negative for malaria, Epstein-Barr virus, measles virus, rubella virus, influenza virus, and rickettsia species, whereas only one of these patients tested IgM positive for chikungunya virus.
TABLE 1.
Characteristics of DEN and ND febrile patients
| Disease severity | Grade | No. (%) gender
|
Mean (range) age (yr) | Mean duration of fever (days) | |
|---|---|---|---|---|---|
| Male | Female | ||||
| Febrile ND | 12 (57) | 9 (43) | 7.7 (2–14) | 9.0 | |
| DF | 36 (50) | 36 (50) | 7.4 (0.6–14) | 7.0 | |
| DHF | DHF I | 8 (53) | 7 (47) | 8.7 (4–13) | 8.6 |
| DHF II | 7 (47) | 8 (53) | 8.1 (3–13) | 9.1 | |
| DSS | DHF III | 25 (44) | 32 (56) | 7.2 (3–14) | 9.6 |
| DHF IV | 4 (33) | 8 (67) | 5.4 (3–10) | 11.8 | |
| Total | 92 (48) | 100 (52) | 7.4 (0.6–14) | 8.4 | |
DEN virus antigens.
DEN 1 (strain CDC), DEN 2 (strain N. Guinea C), DEN 3 (strain H 87), and DEN 4 (strain Hawaii 241) were used to infect C6/36 insect cells. The infected cells were cultured in Leibovitz medium supplemented with antibiotics and 1% fetal bovine serum for 5 to 6 days. When more than 90% of the cells were infected by the virus (as determined by immunofluorescence assay), the culture supernatants were discarded. Subsequently the cells were harvested and acetone extracted as follows. One part DEN-infected cell suspension was mixed with 20 parts ice-cold acetone (−20°C). The mixture was centrifuged for 10 min at 187 × g, and the supernatant acetone was discarded. The procedure was repeated one more time, and the pellet was left to dry in a 37°C incubator for 2 h. The pellet was resuspended in phosphate-buffered saline (PBS) and stored in aliquots at −70°C until use in the DEN IgA and IgG subclass capture enzyme immunoassay (capture EIA). The protein concentration of the DEN antigens was determined with the Bradford assay as 1 mg/ml for all DEN serotypes.
Serology. (i) IgM EIA.
A commercial kit (Focus Technologies, Cypress, Calif.) was used for the measurement of DEN-specific IgM antibodies in the serum samples of the patients. The test was performed according to the procedures described by the manufacturer (9).
(ii) IgA EIA.
The assay used for the detection of DEN-specific IgA antibodies was a modification of a method described earlier (22). Briefly, commercially available plates coated with monoclonal anti-human IgA antibody (Meddens Diagnostics, Vorden, The Netherlands) were used for the assay. After blocking the plates with 10% (wt/vol) skim milk (ELK Campina, Eindhoven, The Netherlands), in PBS for 1 h at 37°C, 50 μl of serum samples was added on the plates. Serum samples were diluted 1:500 in PBS containing 2% (wt/vol) milk, 5% (vol/vol) normal goat serum (ICN Biochemicals Inc.), and 5% (vol/vol) each normal rabbit and normal mouse serum (Dako, Glostrup, Denmark) (EIA buffer). After 1 h of incubation at 37°C, unbound antibodies were washed away and 50 μl of DEN 1 to 4 antigens diluted 1:50 in EIA buffer with 5% (vol/vol) normal human serum (NHS) was added to the plates. The plates were incubated for 2 h at 37°C and washed again to remove unbound antigen and an anti-flavivirus monoclonal antibody conjugated with horseradish peroxidase (Focus Technologies), diluted 1:2,000 in EIA-NHS buffer was added to the wells for 1 h. Plates were washed again and developed with 2,2,4,4-tetramethylbenzidine as the substrate. The reaction was stopped with the addition of 1 N H2SO4 (100 μl/well), and the extinction was read at 450 nm (with a 620-nm reference filter).
(iii) IgG subclass EIA.
An in-house capture EIA system was developed for the determination of DEN-specific IgG subclasses in the DEN-infected individuals and the control patients. Commercially available EIA microwells coated with monoclonal anti-human IgG1, -2, -3, and -4 (Central Laboratory Bloodbank, Amsterdam, The Netherlands) were blocked with 10% (wt/vol) skim milk (ELK, Campina) in PBS for 1 h at 37°C. The microwells were then washed three times with washing buffer (0.5% [vol/vol] Tween 20 in PBS). Serum samples were diluted 1:100 (or 1:1,000 when tested for IgG1) in PBS supplemented with 2% (wt/vol) skim milk (PBS-M). Fifty microliters of diluted serum samples was then added into the microwells and incubated for 1 h at 37°C. The wells were washed again three times, and a mixture of DEN 1 to 4 antigens, diluted 1:20 (50 ng/ml) in PBS-M supplemented with 5% (vol/vol) NHS, was added into the wells (50 μl/well). The wells were incubated for 2 h at 37°C and washed again as described above. An antiflavivirus monoclonal antibody conjugated with horseradish peroxidase (Focus Technologies) diluted 1:2,000 in PBS-M supplemented with 5% NHS was added to the wells for 1 h. The wells were washed six times in the final step before they were developed with 2,2,4,4-tetramethylbenzidine as the substrate. The reaction was stopped with the addition of 1 N H2SO4 (100 μl/well), and the extinction was read at 450 nm (with a 620-nm reference filter).
Calculations.
The ratios of the DEN-specific IgA and IgG subclass antibodies were calculated with the following formula: ratio = (OD of sample − OD of blank)/3 × (mean of the negative controls), where OD is optical density (extinction) of each sample. For the calculation of IgM ratios the cutoff serum of the manufacturer was used. For the calculation of statistical parameters an SPSS program was used. Since the distribution of the data was normal as determined by computer analysis, the significance of differences was calculated using the one-way analysis of variance test. A two-sided P value of <0.05 was considered to represent a significant difference.
RESULTS
Kinetics of DEN-specific Ig classes and subclasses in DF patients.
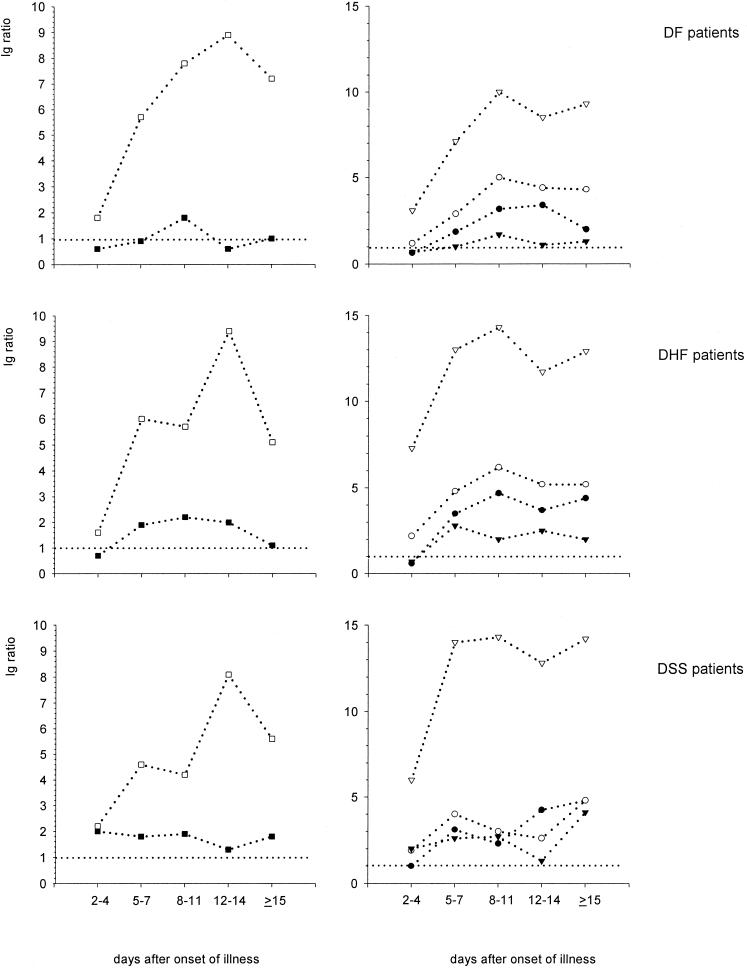
The kinetics of DEN-specific class and subclass serum antibodies in different patient groups are depicted in Fig. 1. In DF patients DEN-specific IgM antibodies increased from a mean ratio of 2.2 ± 2.4 (mean ± standard deviation) in the acute phase (days 2 to 4), to a mean ratio 7.6 ± 5.0 in the early convalescent phase (days 8 to 11), reached the highest values on days 12 to 14 (mean ratio, 8.9 ± 6.3), and declined (mean ratio, 6.9 ± 6.0) in the convalescent phase (≥15 days). DEN-specific IgA antibodies were only detectable in DF patients between days 8 and 11 after onset of fever (mean ratio, 1.8 ± 1.7). The levels of DEN-specific IgG1 antibodies in DF patients were low in the acute phase (mean ratio, 3.0 ± 4.3) and increased in the early convalescent (mean ratio, 10.0 ± 6.7) and convalescent phases (mean ratio, 9.4 ± 6.4). At days 2 to 4 after onset of fever, the levels of DEN-specific IgG2, IgG3, and IgG4 were low, with mean ratios of 0.6 ± 1.2, 1.2 ± 2.0, and 0.6 ± 1.5 respectively, whereas these subclasses reached their highest levels on day 8 to 14 after onset of fever (mean ratios, 3.2 ± 2.1, 4.4 ± 2.2, and 1.7 ± 2.5, respectively) and declined after 15 days (2.4 ± 2.0, 4.2 ± 2.6, and 1.3 ± 2.0 respectively).
FIG. 1.
Kinetics of DEN-specific IgA, IgM, and IgG subclass responses in DEN patients with various disease severity, over a period of 2 weeks after onset of fever. Results are expressed as mean ratios. □, IgM response; ■, IgA response; ▿, IgG1 response; ●, IgG2 response; ○, IgG3 response; ▾, IgG4 response.
Kinetics of DEN-specific Ig classes and subclasses in DHF patients.
DEN-specific IgM antibodies in the DHF patients had lower mean values (mean ratio, 3.4 ± 4.0) on days 2 to 4 than on days 5 to 14 (mean ratio, 9.4 ± 9.1) or from day 15 onwards (mean ratio, 6.0 ± 5.5). The levels of DEN-specific IgA antibodies were undetectable in the DHF patients in the acute phase of illness (days 2 to 4) (mean ratio, 0.7 ± 0.7) but increased in the following early convalescent phase (days 5 to 14) to a mean ratio of 2.2 ± 1.2 and decreased to a mean ratio of 1.1 ± 0.8 from day 15 onwards. DEN-specific IgG1 was the predominant subclass in this patient group, with ratios as high as 7.3 ± 5.2, on days 2 to 4, increasing to the highest levels (mean ratio, 14.3) on days 8 to 11 and slightly declining from day 15 onwards (mean ratio, 12.0 ± 5.2). DEN-specific IgG2 was undetectable in acute DHF patients (mean ratio, 0.6 ± 0.6) but increased to the highest levels on days 8 to 11 (mean ratio, 4.7 ± 1.5) and slightly decreased 15 days after onset of fever (mean ratio, 4.0 ± 2.1). In contrast, DEN-specific IgG3 antibody levels were detectable in acute-phase DHF patients (mean ratio, 2.6 ± 2.3), increased to the highest values in early convalescent phase to a mean ratio of 6.2 ± 0.6, and decreased in the convalescent phase to a mean ratio of 5.4 ± 2.0. DEN-specific IgG4 antibodies were not detectable in the acute DHF but increased rapidly to their highest values (mean ratio, 2.6 ± 2.4) by days 5 to 7 after onset of fever and declined again to a mean ratio of 2.0 ± 2.2 in the convalescent phase (Fig. 1).
Kinetics of DEN-specific Ig classes and subclasses in DSS patients.
DSS patients showed low levels of DEN-specific IgM antibodies on days 2 to 4 (mean ratio, 2.2 ± 2.1), which had increased on days 12 to 14 to a mean ratio of 8.1 ± 5.0, and decreased to a mean ratio of 5.6 ± 4.0 on day 15. DEN-specific IgA antibodies in DSS patients in the acute phase had a mean ratio of 2.0 ± 2.5, increased to the highest levels (mean ratio, 1.9 ± 1.4) on days 8 to 11, and slightly decreased 15 days after onset of fever (mean ratio, 1.8 ± 1.6). Low DEN-specific IgG1 antibody levels in the acute phase (mean ratio, 6.0 ± 5.0) reached the highest values in the early convalescent phase (ratio, 14.3) and remained at high levels in the convalescent phase (mean ratio, 14.1 ± 0.5). DEN-specific IgG2 antibodies were hardly detectable on days 2 to 4 in DSS patients (mean ratio, 1.0 ± 0.8), but the levels of DEN-specific IgG2 increased slowly to reach their highest levels on day 15 (mean ratio, 4.8 ± 1.5). DEN-specific IgG3 antibodies were low in acute DSS patients (mean ratio, 1.9 ± 1.6) and reached their highest levels ≥15 days after onset of fever (mean ratio, 4.7 ± 1.7). The same pattern was seen in the levels of DEN-specific IgG4 antibodies (acute-phase mean ratio, 2.0 ± 2.1; highest convalescent-phase mean ratio, 3.7 ± 2.4) (Fig. 1).
Statistical analysis of kinetics of DEN-specific Ig classes and subclasses.
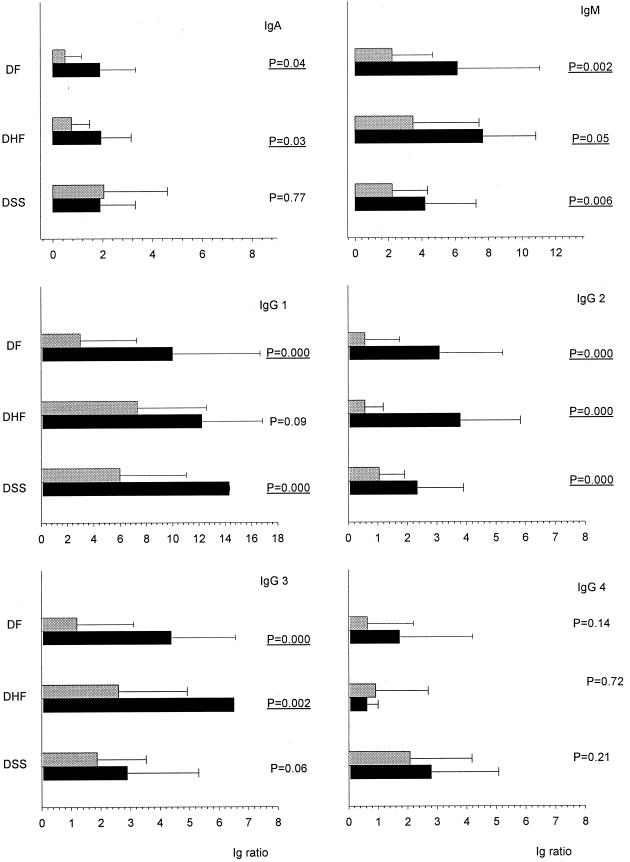
Statistical analysis revealed a significant increase between the acute and convalescent phase of DEN infection in several Ig class and subclass antibodies in the respective DEN patient groups (Fig. 2). In DF patients the levels of DEN-specific IgM, IgA, IgG1, IgG2, and IgG3 serum antibodies increased significantly in the convalescent phase (P = 0.002, P = 0.04, P = 0.000, P = 0.000, and P = 0.000, respectively). In contrast, in DHF patients only DEN-specific IgM, IgA, IgG2, and IgG3 increased significantly from the acute to the convalescent phase of illness (P = 0.05, P = 0.03, P = 0.000, and P = 0.002 respectively). Finally, in DSS patients DEN-specific IgM, IgG1, and IgG2 revealed a significant increase in the convalescent phase of illness (P = 0.006, P = 0.000, and P = 0.000, respectively).
FIG. 2.
Comparison of DEN-specific Ig class and subclass responses in DEN patients with various severity, in the acute phase of disease (light grey bars) and the convalescent phase (black bars), of DEN disease. Error bars, standard deviation.
Comparison of Ig class and subclass antibodies in patients with DEN infection and ND febrile patients.
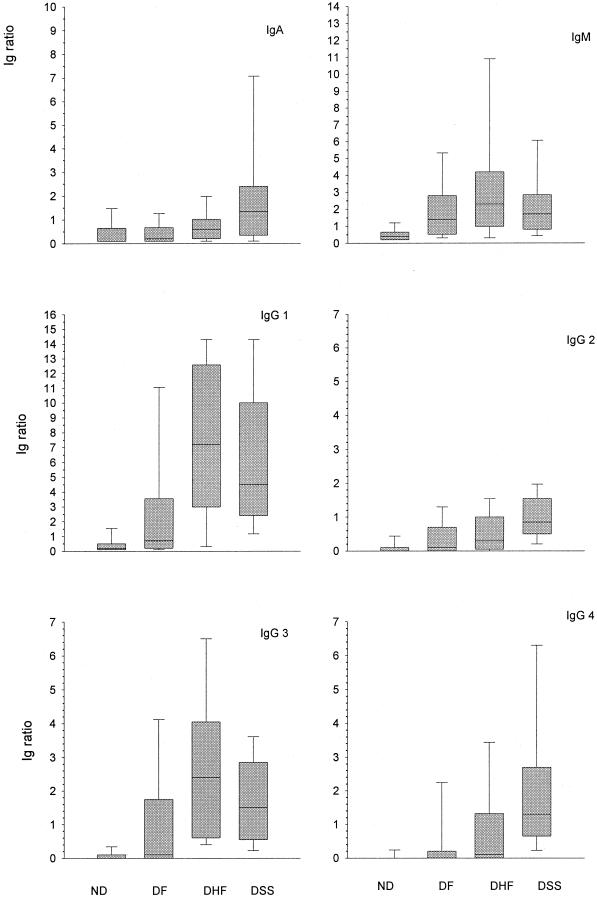
Figure 3 depicts the comparison between patients with DEN infection and ND febrile patients (controls) in the acute phase of infection. ND patients had significantly lower levels of DEN-specific IgM antibodies compared with DF, DHF, and DSS patients (P = 0.006, P = 0.004, and P = 0.003 respectively). The levels of DEN-specific IgA antibodies were significantly higher in DSS patients compared with the ND patients (P = 0.007) but were not significantly different between ND patients and DF or DHF patients (P = 0.814 and P = 0.199, respectively). DEN-specific IgG1 and IgG3 antibodies were significantly lower in controls than in all DEN patients (P = 0.011 for DF, P = 0.000 for DHF, and P = 0.000 for DSS, for both subclasses), whereas DEN-specific IgG2 antibodies were significantly lower in the controls than in DHF and DSS patients (P = 0.017 and P = 0.000, respectively) but were not significantly different between ND and DF patients (P = 0.199). Finally, in the ND febrile patients the levels of DEN-specific IgG4 antibodies were significantly lower than the levels of this subclass in the DSS patients (P = 0.000) but were not significantly different between controls and DF or DHF patients (P = 0.133 and P = 0.057, respectively).
FIG. 3.
Comparison of DEN-specific Ig class and subclass responses in the acute phase of illness in patients with DEN disease of various severity ND febrile patients. Boxes indicate the interquartile range for each patient group. The median is indicated by the line within each box. The whiskers represent the extremes of Ig ratios.
Acute-phase DEN-specific IgM and IgG2 antibodies were the only antibodies that did not differ significantly among the respective DEN patients (Fig. 3). DEN-specific IgA and IgG4 antibodies were significantly higher in the DSS group than in the DF group (Fig. 3). DEN-specific IgG1 antibodies differed significantly when DF patients were compared with DHF or DSS patients, whereas DEN-specific IgG3 antibodies differed only between DF and DHF patients (Fig. 3).
DISCUSSION
In the present study we measured the kinetics of specific IgM, IgA, and IgG subclass antibodies against DEN in patients with varying disease severity upon DEN infection. We demonstrated that DEN-specific IgM, IgG1, and IgG3 antibodies were the predominant Igs throughout the course of illness in DF, DHF, and DSS patients, whereas DEN-specific IgA, IgG1, and IgG4 serum antibodies were parameters associated with the development of DHF and DSS.
All DEN patients had significantly higher DEN-specific IgM antibodies than ND febrile patients in the acute phase of illness (days 2 to 4), and these levels increased significantly in the convalescent phase (days ≥15). These findings indicate that the presence of DEN-specific IgM serum antibodies is rather associated with primary or secondary DEN infection than disease severity. Low levels of DEN-specific IgM serum antibodies in some ND patients may re-present up to 8 months past infection and may not be associated with present DEN infection (4).
Previous studies have underlined the importance of detection of IgA serum antibodies in patients with suspected DEN infections (10, 22). Acute DF patients display low or undetectable DEN-specific IgA serum antibody levels. In the acute phase of the illness, patients who developed shock had significantly higher levels of DEN-specific IgA when compared with DF or DHF patients.
It is known that IgG1 and IgG3 can fix complement most effectively, whereas IgG2 can fix complement to a lower extent (2, 5). The complement system has been associated with shock syndromes (15). In addition, activation of complement may induce clotting and hence may be implicated in intravascular coagulation, both complications seen in DHF and DSS. In our study we demonstrated that in the acute phase of DEN disease (days 2 to 4 after onset of fever), levels of DEN-specific IgG1 differ significantly between ND patients and DF, DHF, and DSS patients; between DF and DHF patients; and between DF and DSS patients. These data suggest an important role for DEN-specific IgG1 in the development of severe forms of DEN disease and as a prognostic marker for the development of severe disease. DEN-specific IgG1 was the predominant subclass throughout the course of DEN illness and remained at high levels during the convalescent phase of all DEN patient groups. DEN-specific IgG2 was present from day 5 after onset of fever onwards in all patient groups, with no differences in the acute phase between any of the patients groups. However, the difference between controls and DHF or DSS patients was significant, differentiating febrile patients from those with hemmorhagic complications. DEN-specific IgG3 followed a pattern similar to that of IgG1 in the acute phase as well as during the convalescent phase. Despite the lack of significant difference between acute DF and DSS and the rather low levels of DEN-specific IgG3 in DSS patients, the results support the idea that high levels of DEN-specific IgG1 in combination with DEN-specific IgG3 levels may be used as a prognostic marker for severe DEN disease in the acute phase of illness. Production of IgG4 serum antibodies is stimulated by production of interleukin 4, a Th2 cytokine (7). Taking into consideration that Th2 responses are associated with severe pathology and with an exacerbation of many other viral infections (such as herpes simplex virus, influenza virus, and human immunodeficiency virus infection [16]) and that a shift towards Th2 responses is seen in DSS (3), it is reasonable to speculate that elevated levels of DEN-specific IgG4 in acute DEN infection may be prognostic for development of DSS. We could confirm that DEN-specific IgG4 was present during acute and convalescent DEN infection, and more importantly that the levels of this subclass were significantly higher in DSS patients than in control or DF patients. Our findings are in agreement with findings of a previous study, where it was also demonstrated that IgG1 and IgG3 are the predominant subclasses in DEN infections (23).
Persistence of the complement-fixing subclasses (IgG1 and -3) and the cytokine-induced IgG4 in DHF and/or DSS patients supports the hypothesis that immunological factors such as ADE and complement activation products may contribute to DEN disease severity.
Taken together, the results of our study showed that measurement of DEN-specific IgA serum antibodies in the acute phase is a valuable tool in the diagnosis of DEN infection along with the traditional methods of measuring IgM or increase in IgG titers in patients suspected of having DEN infection. Furthermore, we showed that in addition to levels of DEN-specific IgA, also those of DEN-specific IgG1, IgG3, and IgG4 correlate with severity of disease outcome. Therefore, measurement of the levels of these specific Igs may provide valuable guidance for decisions about intensity of treatment in the early stages of the infection, thus lowering disease fatality rates.
ACKNOWLEDGMENTS
We thank the following investigators for their contribution in this study: J. W. M. van der Meer and W. M. V. Dolmans, Department of Internal Medicine, University Medical Centre St. Radboud, Nijmegen, The Netherlands; L. G. Thijs and A. J. P. Veerman, University Hospital Free University, Amsterdam, The Netherlands; and C. P. Burghoorn and C. Copra, Institute of Virology, Erasmus Medical Centre Rotterdam, Rotterdam The Netherlands, for excellent technical assistance.
This work was partly supported by a grant from the Royal Dutch Academy of Art and Science (Koninklijke Nederlandse Academie van Kunst en Wetenschappen).
REFERENCES
- 1.Anderson R, Wang S, Osiowy C, Issekutz A C. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J Virol. 1997;71:4226–4232. doi: 10.1128/jvi.71.6.4226-4232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruggemann M, Williams G T, Bindon C I, Clark M R, Walker M R, Jefferis R, Waldmann H, Neuberger M S. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987;166:1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi U C, Agarwal R, Elbishbishi E A, Mustafa A S. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol. 2000;28:183–188. doi: 10.1111/j.1574-695X.2000.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen W J, Hwang K P, Fang A H. Detection of IgM antibodies from cerebrospinal fluid and sera of dengue fever patients. Southeast Asian J Trop Med Public Health. 1991;22:659–663. [PubMed] [Google Scholar]
- 5.Dangl J L, Wensel T G, Morrison S L, Stryer L, Herzenberg L A, Oi V T. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988;7:1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falconar A K. The potential role of antigenic themes in dengue viral pathogenesis. Recent Res Dev Virol. 1999;1:437–447. [Google Scholar]
- 7.Gascan H, Gauchat J F, Aversa G, Van Vlasselaer P, de Vries J E. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J Immunol. 1991;147:8–13. [PubMed] [Google Scholar]
- 8.Graham R R, Juffrie M, Tan R, Hayes C G, Laksono I, Ma'roef C, Erlin, Sutaryo, Porter K R, Halstead S B. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia. I. Studies in 1995–1996. Am J Trop Med Hyg. 1999;61:412–419. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 9.Groen J, Koraka P, Velzing J, Copra C, Osterhaus A D. Evaluation of six immunoassays for detection of dengue virus-specific immunoglobulin M and G antibodies. Clin Diagn Lab Immunol. 2000;7:867–871. doi: 10.1128/cdli.7.6.867-871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groen J, Velzing J, Copra C, Balentien E, Deubel V, Vorndam V, Osterhaus A D. Diagnostic value of dengue virus-specific IgA and IgM serum antibody detection. Microbes Infect. 1999;1:1085–1090. doi: 10.1016/s1286-4579(99)00208-7. [DOI] [PubMed] [Google Scholar]
- 11.Gubler D J. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyre P M, Morganelli P M, Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Investig. 1983;72:393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurane I, Ennis F A. Immunopathogenesis of dengue virus infections. In: Gubler D J, Kuno G, editors. Dengue and dengue haemorrhagic fever. London, United Kingdom: CAB International; 1997. pp. 273–290. [Google Scholar]
- 14.Looney R J, Abraham G N, Anderson C L. Human monocytes and U937 cells bear two distinct Fc receptors for IgG. J Immunol. 1986;136:1641–1647. [PubMed] [Google Scholar]
- 15.Morgan B P. Clinical complementology: recent progress and future trends. Eur J Clin Investig. 1994;24:219–228. doi: 10.1111/j.1365-2362.1994.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 17.Murgue B, Cassar O, Deparis X. Plasma concentrations of sVCAM-1 and severity of dengue infections. J. 2001. Med. Virol. [PubMed] [Google Scholar]
- 18.Murgue B, Deparis X, Chungue E, Cassar O, Roche C. Dengue: an evaluation of dengue severity in French Polynesia based on an analysis of 403 laboratory-confirmed cases. Trop Med Int Health. 1999;4:765–773. doi: 10.1046/j.1365-3156.1999.00478.x. [DOI] [PubMed] [Google Scholar]
- 19.Murgue B, Roche C, Chungue E, Deparis X. Prospective study of the duration and magnitude of viraemia in children hospitalised during the 1996–1997 dengue-2 outbreak in French Polynesia. J Med Virol. 2000;60:432–438. doi: 10.1002/(sici)1096-9071(200004)60:4<432::aid-jmv11>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Ravetch J V, Kinet J P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 21.Rothman A L. Viral pathogenesis of dengue infections. In: Gubler D J, Kuno G, editors. Dengue and dengue haemorrhagic fever. London, United Kingdom: CAB International; 1997. pp. 245–272. [Google Scholar]
- 22.Talarmin A, Labeau B, Lelarge J, Sarthou J L. Immunoglobulin A-specific capture enzyme-linked immunosorbent assay for diagnosis of dengue fever. J Clin Microbiol. 1998;36:1189–1192. doi: 10.1128/jcm.36.5.1189-1192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thein S, Aaskov J, Myint T T, Shwe T N, Saw T T, Zaw A. Changes in levels of anti-dengue virus IgG subclasses in patients with disease of varying severity. J Med Virol. 1993;40:102–106. doi: 10.1002/jmv.1890400205. [DOI] [PubMed] [Google Scholar]
- 24.Unkeless J C, Scigliano E, Freedman V H. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- 25.van Gorp E C, Suharti C, ten Cate H, Dolmans W M, van der Meer J W, ten Cate J W, Brandjes D P. Review: infectious diseases and coagulation disorders. J Infect Dis. 1999;180:176–186. doi: 10.1086/314829. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. Geneva, Switzerland: Headquarters, World Health Organization; 1997. [Google Scholar]
- 27.World Health Organization. Strengthening implementation of the global strategy for dengue/dengue haemorrhagic fever prevention and control. Report of the informal consulation, October 1999. Geneva, Switzerland: Headquarters, World Health Organization; 1999. [Google Scholar]