Abstract
To clarify the predominance of Th1 or Th2 immune responses in malignant and tuberculous pleural effusion (MPE and TPE, respectively), we performed a meta-analysis of previously published results of the levels of Th1/Th2 cytokines associated with these two types of pleural effusion to evaluate the use of Th1/Th2 cytokine profiles in distinguishing TPE from MPE. We searched the PubMed and EMBASE databases for studies indexed from 2000 to March 2021. We included studies that (a) diagnosed TPE and MPE based on culture or pleural tissue biopsy and that (b) compared levels of Th1/Th2 cytokines between TPE and MPE. Pooled data based on a random-effects model or fixed-effects model and standardized mean differences (SMDs) across studies were used to compare TPE and MPE. We also performed Egger’s test to assess publication bias. Of 917 identified studies, a total of 42 studies were selected for the meta-analysis. Compared with MPE subjects, TPE subjects had a significantly higher level of TNF-α [2.22, (1.60–2.84)], an elevated level of IFN-γ [3.30, (2.57–4.40)] in pleural effusion, a situation where the Th1 immune response dominated. Conversely, the levels of interleukin-4 (IL-4) and IL-10 (Th2 cytokines) were higher in the MPE subjects than in the TPE subjects, showing statistically nonsignificant tiny effects [−0.15, (−0.94 to 0.63) and −0.04, (−0.21 to 0.12), respectively]. We confirmed that TPE, a situation in which the Th1 cytokines are predominant. The slight preponderance of Th2 cytokines in MPE, which is not convincing enough to prove.
Subject terms: Lung cancer, Tuberculosis
Introduction
Malignant and tuberculous pleural effusion (MPE and TPE, respectively) are the two most common types of exudative pleural effusions, and both are associated with a typical accumulation of lymphocytes1,2. Naïve CD4 + T cells are activated by the antigen-MHC complex then differentiate into functional T-helper 1(Th1) or T-helper 2 (Th2) subsets3. Th1 cytokines include interleukin (IL)-2, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ, whereas IL-4, IL-5, and IL-10 are considered Th2 cytokines4. IL-6 is an inflammatory cytokine produced by monocytes, macrophages, and dendritic cells5,6. IL-6 has emerged as an important regulator of Th1/Th2 differentiation, promoting the IL-4-dependent induction of Th2 differentiation and inhibiting Th1 differentiation by upregulating suppressor of cytokine signaling (SOCS)-1 expression7.
Immune responses mediated by either the Th1 or Th2 subset dominate depending on different types of pleural effusion. The Th1-dominated immune response is considered an important factor in the containment of mycobacteria, and the main immune effector mechanism involves classically activated macrophages that arise in response to Th1 cytokine signals8. Th1 cytokines have been shown to predominate in immunity to lung tuberculosis (TB)9,10. Notably, TPE is considered a common localized form of extrapulmonary TB. However, whether the mechanism of localized immune response in TPE is dominated by the Th1 or Th2 subset remains to be investigated. MPE, on the other hand, has been associated with Th2 cytokine predominance11,12. Although some reports have shown a bias towards Th2 predominance in MPE, some reports have not13.
Therefore, in this study, we performed a meta-analysis of all available studies to quantitatively evaluate the Th1/Th2 cytokine profiles in TPE and MPE as well as to assess the potentially diagnostic value of these cytokines in discriminating TPE from MPE.
Materials and methods
This meta-analysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) Statement14, and it had been registered with International Platform of Registered Systematic Review and Meta-analysis Protocols (No. INPLASY202210005). An approval from the institutional review board was not necessary, as we extracted only summary information from previously published articles.
Search strategy
A systematic search was conducted (Zeng Y.L. and Qi Y.) of the PubMed and EMBASE databases from 2000 to March 2021. We selected eligible studies documenting the levels of pleural effusion about Th1/Th2 cytokine profiles. The following key words were used in the database search: malignant pleural effusion, tuberculous pleural effusion, tumor necrosis factor-alpha, TNF-α, interferon gamma, IFN-γ, interleukin 2, IL-2, interleukin 4, IL-4, interleukin 5, IL-5, interleukin 6, IL-6, interleukin 10, and IL-10. The details of the strategy are available in Supplementary Methods.
Study selection and data extraction
The inclusion criteria were as follows: (1) original and human studies; (2) studies with a title or abstract including the terms “tuberculous and malignant pleural effusion” and “cytokines”; (3) studies reporting the pleural effusion levels of TNF-α, IFN-γ, IL-2, IL-4, IL-5, IL-6, and IL-10 in patients; (4) MPE diagnosed based on malignant cells in pleural fluid or pleural biopsy; (5) TPE diagnosed based on Ziehl–Neelsen staining or positive mycobacteria culture, or positive pleural biopsy tissue samples; and (6) studies available with full text. No limitation was applied regarding the histological type of MPE, stages of cancer, severities of the disease, or region and race of the study subjects. We extracted demographic details from each included study as follows: the country of origin, surname of the first author, year of publication, sample size, research design, and patient's age. The standard deviation (SD), median, and interquartile range (IQR) of the pleural effusion levels of Th1/Th2 cytokines were extracted for each potentially included study. We eliminated studies that were reviews, case reports, meta-analyses or conference abstracts; nonhuman experiments; non-English language studies; or studies that presented insufficient data for pooling. Data extraction was conducted by two independent investigators (Z.Y.L. and Z.H.) via a form created for this study. Divergences were resolved by consensus or by consulting a senior investigator.
Quality evaluation
We used the Newcastle–Ottawa Scale (NOS) to assess the methodological quality of the studies describing differences in Th1/Th2 cytokine levels in TPE and MPE and considered any study with a score of 6 or more (the highest score was 9) as being of good quality. The results are displayed in Supplementary Methods. Two reviewers independently evaluated the quality of each study.
Statistical analysis
The outcomes were all continuous variables: Th1/Th2 cytokine levels in TPE and MPE. Data provided as the mean and SD were extracted. Data provided as the median and IQR were transformed to the mean and SD according to the method of S.P. Hozo (2005)15. We used standardized mean differences (SMDs) and 95% confidence intervals (CIs) to assess the levels of Th1/Th2 cytokines in TPE compared to MPE. The Cochran’s Q test, I2 statistic and P value were used to assess the heterogeneity. A P ≥ 0.1 or I2 ≤ 50% was considered as no significant heterogeneity, and the fixed-effects model (FEM) was applied; otherwise, the random-effects model (REM) was used. We used the sensitivity analysis to assess the robustness. Subgroup analyses were used to stratify the studies by covariables including country, study design, etiology, publishing year and quality scores based on meta-regression, in order to explore potential sources of heterogeneity. The software RevMan 5.2 and STATA version 14.0 were used for the statistical analyses and images. Begg's test was used to assess the asymmetry of the funnel plot. p < 0.05 was considered publication bias, and if present, the trim-and-fill method was adopted to determine the influence of publication bias on the results.
Results
Characteristics of the included studies
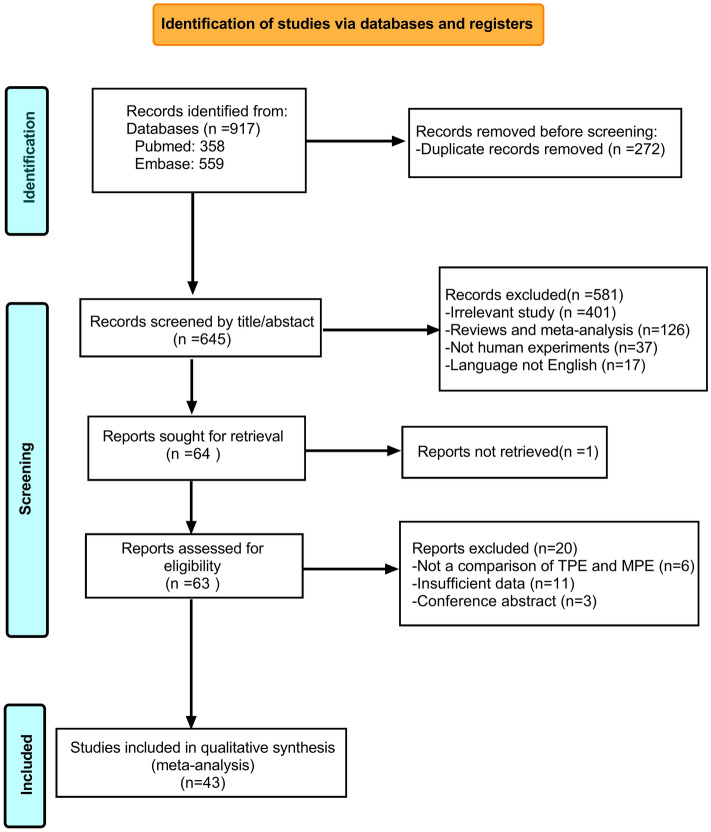
A total of 917 potentially relevant studies identified from the database were retrieved, of which 43 studies were included in the final meta-analytical processes. The study selection process is detailed in Fig. 1. In addition, we provide the details of the selected studies in Table 1. Considering the large number of included studies with information on TNF-α and IFN-γ, we only included the ELISA results, but other measurement methods [including cytometric bead array (CBA), chemiluminescent enzyme immunoassay (CLEIA), radioimmunoassay (RIA)] were included for assessments of the remaining cytokines. One study was excluded due to CBA measurements of IFN-γ levels56. The quality ratings for each study and characteristics of the included studies are presented in Supplementary Methods.
Figure 1.
PRISMA flow chart of the selection of studies.
Table 1.
Characteristics of the included original studies regarding the Th1/Th2 cytokine profiles for tuberculous and malignant pleural effusion.
| Cytokines | No. of studies | No. of patients | Assay methodology | SMD (95%) | Model | Heterogeneity (%) | p value | |
|---|---|---|---|---|---|---|---|---|
| I2 | P | |||||||
| TNF-α | 1916–34 |
TPE: 571 MPE: 661 |
ELISA (N = 19) | 2.22 | Random | 95 | < 0.001 | < 0.001 |
| IFN-γ | 2011,13,17,18,20,29,33,35–47 |
TPE: 728 MPE: 992 |
ELISA (N = 20) | 3.82 | Random | 97 | < 0.001 | < 0.001 |
| IL-2 | 335,48,49 |
TPE: 179 MPE: 210 |
ELISA (N = 1) Others (N = 2) |
-0.07 | Fixed | 46 | 0.16 | 0.47 |
| IL-4 | 613,20,35,40,49,50 |
TPE:201 MPE:288 |
ELISA (N = 4) Others (N = 2) |
-0.15 | Random | 92 | < 0.001 | 0.70 |
| IL-6 | 1222,27,32,39,48–55 |
TPE: 361 MPE: 731 |
ELISA (N = 7) Others (N = 5) |
3.53 | Random | 98 | < 0.001 | < 0.001 |
| IL-10 | 811,13,20,21,29,48–50 |
TPE: 280 MPE: 549 |
ELISA (N = 5) Others (N = 3) |
0.17 | Random | 66 | 0.005 | 0.25 |
Th1/Th2 cytokine levels in TPE and MPE
Analysis of TNF-α
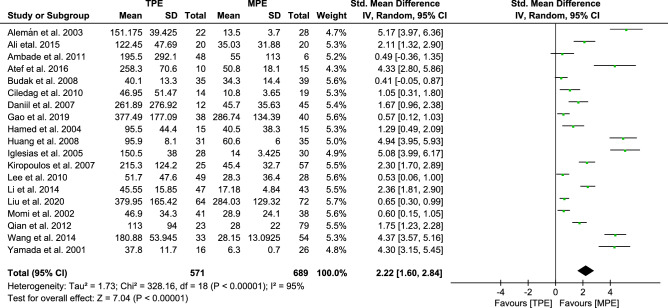
In a pooled analysis of all 19 trials, the results revealed that there was a significant increase in the pleural effusion levels of TNF-α in TPE subjects compared to MPE subjects [2.22, (1.60–2.84, p < 0.00001)] (Fig. 2). However, there was statistically significant heterogeneity among the studies (I2 95%). Subsequently, sensitivity analysis (Fig. S1) was conducted by excluding studies one by one at a time, but neither the magnitude nor the direction of the effect size was substantially altered. We showed that the pooled SMD was stable and reliable, implying higher TNF-α levels in TPE. To explore the sources of the heterogeneity, we performed meta-regression analysis based on subgroup stratification by variables, including country, design, etiology, publishing year and quality scores. In the stratified analyses (Table S1), there was no reliable evidence that the above variables represented the main source of heterogeneity. Therefore, we considered that the heterogeneity might be due to differences in ELISA manufacturers and the severity of the diseases selected for study. In this analysis, there was publication bias based on Begg's test (p < 0.05). We therefore used the trim-and-fill method to adjust for publication bias and examined its effect on the pooled SMD (Supplementary Methods), yielding an adjusted effect (SMD = 1.038, 95% CI 0.328–1.748, p < 0.01).
Figure 2.
Forest plot of 19 studies comparing TNF-α levels in TPE and MPE subjects. The standardized mean difference (the pleural effusion levels of TNF-α in TPE subjects minus that in MPE subjects) was estimated by meta-analysis.
Analysis of IFN-γ
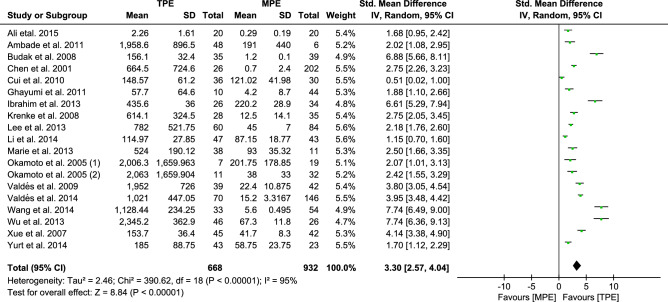
Pooled analysis of all 20 trials revealed that there was a significant increase in the pleural effusion levels of IFN-γ in TPE subjects compared to MPE subjects [3.82, (2.97–4.66, p < 0.00001)] (Fig. S2), with statistically significant between-study heterogeneity (I2 97%). Subsequently, we performed sensitivity analysis, which indicated that there was considerable variation in one trial47. After removing that trial, the remaining results were stable through sensitivity analysis, the REM of pooled SMDs [3.30, (2.57–4.40, p < 0.00001)] (Fig. 3). Our pooled data suggested higher IFN-γ levels in TPE. However, despite a decrease in heterogeneity (I2 from 97 to 95%) with the removal of that trial, substantial heterogeneity still existed. To explore possible sources of heterogeneity, we further performed meta-regression analysis based on subgroup stratification by country, design, etiology, publishing year and quality scores. In the stratified analyses (Table S2), there was no reliable evidence that the above variables were the main source of heterogeneity; however, Egger's test indicated evidence of publication bias (p < 0.05). The trim and fill method was used to impute 3 missing studies to the left of the funnel plot to ensure symmetry, and the REM of pooled SMDs was adjusted [2.567, 95% CI 1.750–3.384, p < 0.001] (Supplementary Methods).
Figure 3.
Forest plot of 19 studies comparing IFN-γ levels in TPE and MPE subjects. The standardized mean difference (the pleural fluid levels of IFN-γ in TPE subjects minus that in MPE subjects) was estimated by meta-analysis.
Analysis of IL-2
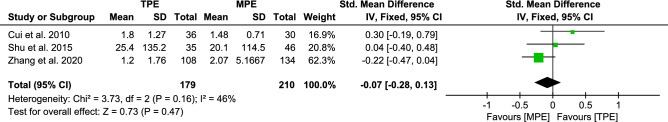
As can be seen in Fig. 4, although pooled analysis of all 3 included trials revealed that the FEM of pooled SMDs [−0.07, (−0.28 to 0.13, p < 0.00001)] suggested that IL-2 levels were higher in MPE subjects than TPE subjects, these differences were not statistically significant [p = 0.47 > 0.05]. The reason for this result is probably due to the small number of included studies. An assessment of publication bias was not accomplished because fewer than ten trials were included.
Figure 4.
Forest plot of 3 studies comparing IL-2 levels in TPE and MPE subjects. The standardized mean difference (the pleural effusion levels of IL-2 in TPE subjects minus that in MPE subjects) was estimated by meta-analysis.
Analysis of IL-4
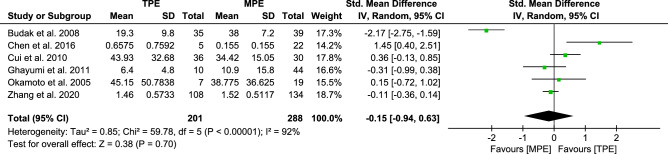
Pooling the data from the six trials revealed that the REM of pooled SMDs [−0.15, (−0.94 to 0.63)] had statistically significant between-trial heterogeneity (I2 92%); the results are presented in Fig. 5. Although MPE subjects had higher levels of IL-4 than TPE subjects, this difference was not statistically significant (p = 0.70 > 0.05).
Figure 5.
Forest plot of 6 studies comparing IL-4 levels in TPE and MPE subjects. The standardized mean difference (the pleural effusion levels of IL-4 in TPE subjects minus that in MPE subjects) was estimated by meta-analysis.
Analysis of IL-10
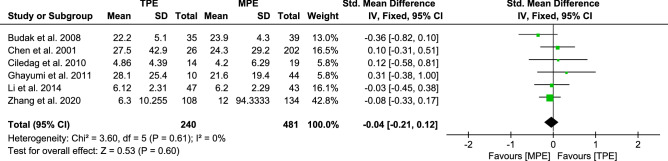
A meta-analysis of the data from eight trials on IL-10 was performed as shown in Figure S3a, the REM of pooled SMDs [0.17, (−0.12 to 0.46)]. However, there was statistically significant heterogeneity among the above results (I2 66%, p < 0.00001). We excluded two studies48,50 according to the results of the Galbraith radial plot (Fig. S3b), and the heterogeneity was decreased appreciably (I2 from 66 to 0%) as shown in Fig. 6. On the basis of the 6 remaining trials, the FEM of pooled SMDs [−0.04, (−0.21 to 0.12)] suggested higher IL-10 levels in MPE subjects.
Figure 6.
Forest plot of 6 studies comparing IL-10 levels in TPE and MPE subjects. The standardized mean difference (the pleural effusion levels of IL-10 in TPE subjects minus that in MPE subjects) was estimated by meta-analysis.
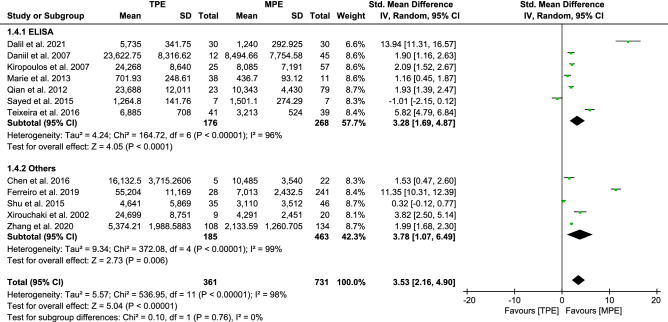
Analysis of IL-6 between TPE and MPE
IL-6 was the most commonly measured inflammatory cytokine. On the basis of 12 trials, the REM of pooled SMDs [3.53, (2.16–4.90)] indicated substantial heterogeneity between studies (I2 = 98%, p < 0.00001) as shown in Fig. 7. The pooled results confirmed that the levels of IL-6 in TPE patients were higher than those in MPE patients. To assess the stability of the pooled results of the meta-analysis of IL-6, sensitivity analysis was conducted by excluding studies one by one at a time. Neither the magnitude nor the direction of the effect size was substantially altered, indicating that our results were robust. Furthermore, we excluded two trials22,53 based on the sensitivity analysis (Fig. S4a). After the deletion of these two trials, the remaining results were stable through sensitivity analysis (Fig. S4b) and caused no appreciable change in the pooled SMDs [1.92, (1.13–2.71)], but the heterogeneity was decreased (I2 from 98 to 94%) (Fig. S4c). To test the robustness of our results, we perform stratified analyses to detect the sources of the heterogeneity in this study as shown in Table S3. Among the 12 trials, 7 presented ELISA measurements, and the remaining five reported other measurements (2 with CBA results, 2 with RIA results, and 1 with CLEIA results). Consequently, we considered that the main heterogeneity arises from differences in measurement methods. However, nonsignificant differences in between-group heterogeneity were found in some stratified analyses, and measurement methods did not indicate additional sources of heterogeneity. In this analysis, there was no publication bias based on Egger’s test (p = 0.150, Supplementary Methods).
Figure 7.
Forest plot of 12 studies comparing IL-6 levels in TPE and MPE subjects. The standardized mean difference (the pleural fluid levels of IL-6 in TPE subjects minus that in MPE subjects) was estimated by meta-analysis.
Discussion
To the best of our knowledge, this is the first large-scale meta-analysis to systematically investigate the levels of Th1/Th2 cytokines in MPE and TPE. Our results showed that the Th1 cytokine profile was predominant in TPE compared to MPE. We confirmed that the immune response in TPE is dominated by Th1 polarization. Although significant heterogeneity was detected, trials with extreme SMDs were removed based on sensitivity analysis and Galbraith radial plot assessment, which diminished the heterogeneity and provided more stable results. Despite the slight bias toward Th2 cytokines in MPE subjects, the pooled SMD was not statistically significant.
Exudative pleural effusion results from a vicious loop of interactions between the immune system and a pleural or parenchymal disease like TB infection, malignancy or inflammation. It results in abnormal accumulation of fluid in the pleural space via altering the permeability of the pleural membranes to enhance plasma extravasation. TB and malignant disease are among the most frequent causes of exudative pleural effusions. Th cells are known to play important roles in the pathogenesis of exudative pleural effusion and host defense.
Th1 cells have been observed to have an anti-infectious role in TB57. Th1-mediated host immunity inhibits further multiplication of Mycobacterium tuberculosis (Mtb) via the secretion of IFN-γ and other Th1 cytokines, which activate macrophages, promote phagosome maturation to stimulate phagocytosis, and stimulate the production of reactive nitrogen intermediates58. TNF-α is essential for stimulating the chemotaxis of inflammatory cells to sites of infection and leads to granulomatous response to containment disease progression59. Normally, IL-6 is a proinflammatory cytokine, inducing the production of acute phase proteins to promote inflammation. Nevertheless, macrophages infected with Mtb induce the production of IL-6, which inhibits the response of macrophages to IFN-γ, resulting in the inability to eradicate Mtb infection60. The increased IL-6 levels also contribute to increased expression of suppressor of cytokine signaling (SOCS) in TB61. SOCS1 is regarded as an important mediator that inhibits IFN-γ secretion by macrophages, which in turn hampers the early clearance of Mtb by macrophages62. Consistent with the results of other studies, our study found higher levels of IL-6 in TPE subjects, which suggested that IL-6, as an important cytokine, may be involved in the formation of TPE and play an important role in the occurrence and development of pulmonary tuberculosis (PTB).
Different from Th1 cytokines, IL-10 is considered an inhibitory and anti-inflammatory Th2 cytokine that negatively regulates IFN-γ-mediated host immunity, limits pathogen clearance in the early immune response to Mtb and mediates long-term chronic infection63. The IFN-γ/IL-10 ratio is a useful objective marker of the clinical severity of TB64. Additionally, IL-4 is a classic Th2 cytokine that may subvert mycobacterial containment in macrophages by perturbating Th1-related pathways and regulatory T cells (Tregs)65.
Early studies have suggested that Th1- and Th2-cell-producing cytokines play important roles in the immune microenvironment of tumors; Th1 cytokines relevant to antitumor immunity have been reported, whereas Th2 cytokines are related to tumor invasiveness and metastasis66. Notably, in advanced cancer, a shift from Th1 to Th2 cells in the tumor environment is often observed, and impaired Th1 cell-mediated immunity has been reported to be associated with cancer progression67,68. Anthony et al. reported that patients with advanced squamous cell carcinoma had a diminished Th1 antitumor immune response but stronger underlying Th2 immune response69. This shift was further demonstrated in malignant effusions70. Accordingly, these studies collectively indicate that a shift from a Th1 to a Th2 cell response in the microenvironment of tumors may play a crucial role in the development and progression of tumors. Moreover, evidence indicates that tumor-derived TGF-β-induced overexpression of IL-10 may drive the shift in the Th1/Th2 balance toward a Th2 response and inhibit the Th1 response71. The balance of Th1/Th2 cytokines in MPE subjects remains controversial. We speculate that the shift from Th1 to Th2 along with tumor progression may explain the discrepancy regarding whether Th2 cytokines predominate in MPE subjects, but this needs to be studied further.
As future studies clarify the roles of Th1/Th2 cytokine profiles and their effect on the local immune response, new therapeutic approaches to immunotherapy may be developed. Currently, a useful cytokine/anti-cytokine therapy involves supplementation of anti-inflammatory recombinant cytokines as well as neutralizing cytokines or antagonizing receptors by monoclonal antibodies (mAbs). Recombinant IL-2 has been used successfully for cancer therapy and was approved by the FDA for the treatment of metastatic renal-cell carcinoma in 1992 and for metastatic melanoma in 1998. Infliximab, a chimeric anti-TNFα monoclonal antibody, has been demonstrated to be an effective therapy for rheumatoid arthritis, Crohn's disease, ankylosing spondylitis and other autoimmune diseases. Despite these beneficial effects of infliximab treatment in autoimmune diseases, it carries the risk of TB occurrence and latent TB reactivation72. Based on the immunosuppressive ability of IL-10 in tumor immunity, therapeutic strategies targeting the IL-10 signaling pathway have evolved considerably in cancer immunotherapy73. In addition, overexpressed IL-4 and IL-13 receptors on cancer cells are specific targets for receptor-directed cancer immunotherapy74. Our study confirms changes in the Th1/Th2 cytokine profiles of patients with TPE and MPE, which may be helpful for future investigations of cytokine therapies.
Moreover, combining multiple biomarkers has previously been shown to enhance diagnostic accuracy. The combination of TNF-α and adenosine deaminase 2 (ADA2) has been shown to improve the specificity and accuracy of diagnosis for the differentiation of TPE from MPE29. Future studies are needed to determine if Th1/Th2 cytokine profiles confer supplementary diagnostic value for pleural effusions.
There are some unavoidable limitations of our study that are deserving of attention. First, significant heterogeneity was observed in some of our results; although stratified analyses were done, we did not identify the source of heterogeneity. Second, some of the trials provided only the median and IQR levels of the cytokines, which we converted to the mean and SD by Hozo et al.’s method, which might lead to slightly skewed data. Third, the sample sizes included in studies on Th2 cytokines were relatively small, and only six trials were included in the pooled results. Fourth, we did not examine the effect of the stages of cancer on cytokine levels in the included studies, which might lead to heterogeneity and deviation in this analysis. Fifth, few studies have reported the primary tumor causing MPE and the histological type of the primary tumor; therefore, future studies should investigate its effect on cytokine levels.
Conclusions
In summary, this systematic review and meta-analysis confirms that pleural effusions caused by TB, a situation in which the Th1 cytokines are predominant. Although MPE appears to be slightly bias toward Th2 cytokines, this finding was not sufficiently convincing to prove. Our findings suggest that these cytokines are likely to serve supplementary diagnostic value for the differentiation of TPE from MPE. Moreover, it is important to know the role of cytokines derived from Th cells on the pathogenesis of diseases and host defense, which may provide new clues for cytokine immunotherapies.
Supplementary Information
Acknowledgements
This work was funded by the Science and Technology Planning Project of Xuzhou, China (No. KC21251).
Author contributions
Z.Y.L. conceived and designed the study; Z.Y.L. and Q.Y. performed the systematic literature search and quality assessment; Z.Y.L. and Z.H. extracted and analyzed the data; Z.Y.L drafted the initial manuscript; and W.L.W reviewed the manuscript and critically edited and revised the manuscript. All authors approved the final version of the manuscript as submitted.
Data availability
The data sets supporting the results of this article are included within the article and its supplementary material.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hai Zhou, Email: zhouhai339339@163.com.
Yu Qi, Email: qiyu1987xiaobao@163.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06685-8.
References
- 1.Aguiar LMZD, Antonangelo L, Vargas FS, Zerbini MCN, Sales MM, Uip DE, Saldiva PHN. Malignant and tuberculous pleural effusions: Immunophenotypic cellular characterization. Clinics (Sao Paulo, Brazil) 2008;63(5):637–644. doi: 10.1590/S1807-59322008000500012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oshikawa K, Yanagisawa K, Ohno S, Tominaga S-I, Sugiyama Y. Expression of ST2 in helper T lymphocytes of malignant pleural effusions. Am. J. Respir. Crit. Care Med. 2002;165:1005–1009. doi: 10.1164/ajrccm.165.7.2105109. [DOI] [PubMed] [Google Scholar]
- 3.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 2003;4(9):835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 4.Lee HL, Jang JW, Lee SW, Yoo SH, Kwon JH, Nam SW, Bae SH, Choi JY, Han NI, Yoon SK. Inflammatory cytokines and change of Th1/Th2 balance as prognostic indicators for hepatocellular carcinoma in patients treated with transarterial chemoembolization. Sci. Rep. 2019;9(1):3260. doi: 10.1038/s41598-019-40078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choy, E. & Rose-John, S. Interleukin-6 as a multifunctional regulator: Inflammation, immune response, and fibrosis. J. Scleroderma Relat. Disord. 2(2), 1–5 (2017).
- 6.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006;8(2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002;39(9):531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 8.Flynn JL, Chan J, Lin PL. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 2011;4(3):271–278. doi: 10.1038/mi.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai X, Wilson SE, Chmura K, Feldman NE, Chan ED. Morphometric analysis of Th1 and Th2 cytokine expression in human pulmonary tuberculosis. Tuberculosis. 2004;84(6):375–385. doi: 10.1016/j.tube.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 1996;9(4):532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y-M, Yang W-K, Whang-Peng J, Tsai C-M, Perng R-P. An analysis of cytokine status in the serum and effusions of patients with tuberculous and lung cancer. Lung Cancer. 2001;31(1):25–30. doi: 10.1016/s0169-5002(00)00165-3. [DOI] [PubMed] [Google Scholar]
- 12.Lieser EA, Croghan GA, Nevala WK, Bradshaw MJ, Markovic SN, Mansfield AS. Up-regulation of pro-angiogenic factors and establishment of tolerance in malignant pleural effusions. Lung Cancer. 2013;82(1):63–68. doi: 10.1016/j.lungcan.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Ghayumi MA, Mojtahedi Z, Fattahi MJ. Th1 and Th2 cytokine profiles in malignant pleural effusion. Iran. J. Immunol. 2011;8(4):195–200. [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alemán C, Alegre J, Monasterio J, Segura RM, Armadans L, Anglés A, Varela E, Ruiz E, Fernández De Sevilla T. Association between inflammatory mediators and the fibrinolysis system in infectious pleural effusions. Clin. Sci. 2003;105(5):601–607. doi: 10.1042/CS20030115. [DOI] [PubMed] [Google Scholar]
- 17.Ali, A. & Mohmoud, T. Differential diagnostic efficiency of T cells subsets versus interferon-gamma, tumor necrosis factor-alpha, and adenosine deaminase in distinguishing tuberculous from malignant pleural effusions. Chest148(4), 68 (2015).
- 18.Ambade V, Arora MM, Rai SP, Nikumb SK, Basannar DR. Markers for differentiation of tubercular pleural effusion from non-tubercular effusion. Med. J. Armed Forces India. 2011;67(4):338–342. doi: 10.1016/S0377-1237(11)60080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atef, H.M., Okab, A.A., Al Mehy, G.F. & El Beheisy, M.M. The role of tumor necrosis factor alpha in differentiation between malignant and non malignant pleural effusion. Egypt. J. Chest Dis. Tuberculosis65(3), 605–612 (2016).
- 20.Budak F, Uzaslan EK, Cangür S, Göral G, Oral HB. Increased pleural soluble fas ligand (sFasL) levels in tuberculosis pleurisy and its relation with T-helper type 1 cytokines. Lung. 2008;186(5):337–343. doi: 10.1007/s00408-008-9107-5. [DOI] [PubMed] [Google Scholar]
- 21.Ciledag A, Kaya A, Erol S, Sen E, Celik G, Cesur S, Fidan Y, Kinikli S. The comparison of pleural fluid TNF-alpha and IL-10 levels with ADA in tuberculous pleural effusion. Curr. Med. Chem. 2010;17(19):2096–2100. doi: 10.2174/092986710791233652. [DOI] [PubMed] [Google Scholar]
- 22.Daniil ZD, Zintzaras E, Kiropoulos T, Papaioannou AI, Koutsokera A, Kastanis A, Gourgoulianis KI. Discrimination of exudative pleural effusions based on multiple biological parameters. Eur. Respir. J. 2007;30(5):957–964. doi: 10.1183/09031936.00126306. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Song L, Li D, Peng L, Ding H. Clinical value of haptoglobin and soluble CD163 testing for the differential diagnosis of tuberculous and malignant pleural effusions. Medicine. 2019;98(42):e17416. doi: 10.1097/MD.0000000000017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamed EA, El-Noweihi AM, Mohamed AZ, Mahmoud A. Vasoactive mediators (VEGF and TNF-alpha) in patients with malignant and tuberculous pleural effusions. Respirology. 2004;9(1):81–86. doi: 10.1111/j.1440-1843.2003.00529.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang LY, Shi HZ, Liang QL, Wu YB, Qin XJ, Chen YQ. Expression of soluble triggering receptor expression on myeloid cells-1 in pleural effusion. Chin. Med. J. 2008;121(17):1656–1661. [PubMed] [Google Scholar]
- 26.Iglesias Sáenz, D. et al. Metalloproteinases and tissue inhibitors of metalloproteinases in exudative pleural effusions. Eur. Respir. J.25(1), 104–109 (2005). [DOI] [PubMed]
- 27.Kiropoulos TS, Kostikas K, Oikonomidi S, Tsilioni I, Nikoulis D, Germenis A, Gourgoulianis KI. Acute phase markers for the differentiation of infectious and malignant pleural effusions. Respir. Med. 2007;101(5):910–918. doi: 10.1016/j.rmed.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Lee MH, Nahm CH, Choi JW. Thrombin-antithrombin III complex, proinflammatory cytokines, and fibrinolytic indices for assessing the severity of inflammation in pleural effusions. Ann. Clin. Lab. Sci. 2010;40(4):342–347. [PubMed] [Google Scholar]
- 29.Li M, Wang H, Wang X, Huang J, Wang J, Xi X. Diagnostic accuracy of tumor necrosis factor-alpha, interferon-gamma, interleukin-10 and adenosine deaminase 2 in differential diagnosis between tuberculous pleural effusion and malignant pleural effusion. J. Cardiothorac. Surg. 2014;9:118. doi: 10.1186/1749-8090-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Wang S, Zhang Z, Jie J, Song L, Hua S. Diagnostic value of soluble form of mer tyrosine kinase (sMerTK) in tuberculous pleural effusion and malignant pleural effusion. BioMed. Res. Int. 2002;2020:1496935. doi: 10.1155/2020/1496935. [DOI] [Google Scholar]
- 31.Momi H, Matsuyama W, Inoue K, Kawabata M, Arimura K, Fukunaga H, Osame M. Vascular endothelial growth factor and proinflammatory cytokines in pleural effusions. Respir. Med. 2002;96(10):817–822. doi: 10.1053/rmed.2002.1364. [DOI] [PubMed] [Google Scholar]
- 32.Qian Q, Sun WK, Zhan P, Zhang Y, Song Y, Yu LK. Role of monocyte chemoattractant protein-1, tumor necrosis factor-alpha and interleukin-6 in the control of malignant pleural effusion and survival in patients with primary lung adenocarcinoma. Int. J. Biol. Markers. 2012;27(2):e118–e124. doi: 10.5301/JBM.2012.9197. [DOI] [PubMed] [Google Scholar]
- 33.Wang M, Zhang Z, Wang X. Superoxide dismutase 2 as a marker to differentiate tuberculous pleural effusions from malignant pleural effusions. Clinics (Sao Paulo) 2014;69(12):799–803. doi: 10.6061/clinics/2014(12)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada Y, Nakamura A, Hosoda M, Kato T, Asano T, Tonegawa K, Itoh M. Cytokines in pleural liquid for diagnosis of tuberculous pleurisy. Respir. Med. 2001;95(7):577–581. doi: 10.1053/rmed.2001.1103. [DOI] [PubMed] [Google Scholar]
- 35.Cui HY, Zhang Q, Su B, Li W, Tang SJ. Differential levels of cytokines and soluble fas ligand between tuberculous and malignant effusions. J. Int. Med. Res. 2010;38(6):2063–2069. doi: 10.1177/147323001003800621. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim L, Salah M, Rahman AAE, Zeidan A, Ragb M. Crucial role of CD4+CD 25+ FOXP3+ T regulatory cell, interferon-γ and interleukin-16 in malignant and tuberculous pleural effusions. Immunol. Invest. 2013;42(2):122–136. doi: 10.3109/08820139.2012.736116. [DOI] [PubMed] [Google Scholar]
- 37.Krenke R, Safianowska A, Paplinska M, Nasilowski J, Dmowska-Sobstyl B, Bogacka-Zatorska E, Jaworski A, Chazan R. Pleural fluid adenosine deaminase and interferon-gammas diagnostic tools in tuberculous pleurisy. J. Physiol. Pharmacol. 2008;59(SUPPL. 6):349–360. [PubMed] [Google Scholar]
- 38.Lee KS, Kim HR, Kwak S, Choi KH, Cho JH, Lee YJ, Lee MK, Lee JH, Park SD, Park DS. Association between elevated pleural interleukin-33 levels and tuberculous pleurisy. Ann. Lab. Med. 2013;33(1):45–51. doi: 10.3343/alm.2013.33.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marie MAM, John J, Krishnappa LG, Gopalkrishnan S, Bindurani SR. Role of interleukin-6, gamma interferon and adenosine deaminase markers in management of pleural effusion patients. West Indian Med. J. 2013;62(9):803–807. doi: 10.7727/wimj.2012.253. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto M, Hasegawa Y, Hara T, Hashimoto N, Imaizumi K, Shimokata K, Kawabe T. T-helper type 1/T-helper type 2 balance in malignant pleural effusions compared to tuberculous pleural effusions. Chest. 2005;128(6):4030–4035. doi: 10.1378/chest.128.6.4030. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto M, Kawabe T, Iwasaki Y, Hara T, Hashimoto N, Imaizumi K, Hasegawa Y, Shimokata K. Evaluation of interferon-gamma, interferon-gamma-inducing cytokines, and interferon-gamma-inducible chemokines in tuberculous pleural effusions. J. Lab Clin. Med. 2005;145(2):88–93. doi: 10.1016/j.lab.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Valdés L, José ES, Dobaño JMA, Golpe A, Valle JM, Penela P, Barcala FJG. Diagnostic value of interleukin-12 p40 in tuberculous pleural effusions. Eur. Respir. J. 2009;33(4):816–820. doi: 10.1183/09031936.00085008. [DOI] [PubMed] [Google Scholar]
- 43.Valdés L, José ES, Ferreiro L, Golpe A, Gude F, Álvarez-Dobaño JM, Pereyra MF, Toubes ME, González-Barcala FJ. Interleukin 27 could be useful in the diagnosis of tuberculous pleural effusions. Respir. Care. 2014;59(3):399–405. doi: 10.4187/respcare.02749. [DOI] [PubMed] [Google Scholar]
- 44.Wu YB, Ye ZJ, Qin SM, Wu C, Chen YQ, Shi HZ. Combined detections of interleukin 27, interferon-γ, and adenosine deaminase in pleural effusion for diagnosis of tuberculous pleurisy. Chin. Med. J. (Engl.) 2013;126(17):3215–3221. [PubMed] [Google Scholar]
- 45.Xue K, Xiong S, Xiong W. Clinical value of vascular endothelial growth factor combined with interferon-gamma in diagnosing malignant pleural effusion and tuberculous pleural effusion. J. Huazhong Univ. Sci. Technol. Med. Sci. 2007;27(5):495–497. doi: 10.1007/s11596-007-0504-4. [DOI] [PubMed] [Google Scholar]
- 46.Yurt, S. et al. Diagnostic utility of serum and pleural levels of adenosine deaminase 1–2, and interferon-γ in the diagnosis of pleural tuberculosis. Multidiscip. Respir. Med.9(1), 113 (2014). [DOI] [PMC free article] [PubMed]
- 47.Zhang M, Xiong D, Li H, Wang Z, Li R. Diagnostic value of T-spot TB combined with INF-γ and IL-27 in tuberculous pleurisy. Exp. Ther. Med. 2018;15(2):1871–1874. doi: 10.3892/etm.2017.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shu CC, Wang JY, Hsu CL, Keng LT, Tsui K, Lin JF, Lai HC, Yu CJ, Lee LN, Luh KT. Diagnostic role of inflammatory and anti-inflammatory cytokines and effector molecules of cytotoxic T lymphocytes in tuberculous pleural effusion. Respirology. 2015;20(1):147–154. doi: 10.1111/resp.12414. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Chen Y, He G, Jiang X, Chen P, Ouyang J. Differential diagnosis of tuberculous and malignant pleural effusions: Comparison of the Th1/Th2 cytokine panel, tumor marker panel and chemistry panel. Scand. J. Clin. Lab. Invest. 2020;80(4):265–270. doi: 10.1080/00365513.2020.1728784. [DOI] [PubMed] [Google Scholar]
- 50.Chen KY, Feng PH, Chang CC, Chen TT, Chuang HC, Lee CN, Su CL, Lin LY, Lee KY. Novel biomarker analysis of pleural effusion enhances differentiation of tuberculous from malignant pleural effusion. Int. J. Gen. Med. 2016;9:183–189. doi: 10.2147/IJGM.S100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalil RN, Marjani M, Dezfuli NK, Tabarsi P, Moniri A, Varahram M, Adcock IM, Mortaz E. Potential diagnostic value of pleural fluid cytokines levels for tuberculous pleural effusion. Sci. Rep. 2021;11(1):660. doi: 10.1038/s41598-020-79685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El Sayed R, Okab A, El-Mahdy M, Kasb I, Ismail Y. Role of interleukin-6 (IL-6) in diagnosis of malignant pleural mesothelioma. Egypt. J. Chest Dis. Tuberculosis. 2015;64(2):419–424. [Google Scholar]
- 53.L. Ferreiro et al. Diagnosis of infectious pleural effusion using predictive models based on pleural fluid biomarkers. Ann. Thoracic Med.14(4), 254–263 (2019). [DOI] [PMC free article] [PubMed]
- 54.Teixeira LR, Dias MB, Sales RKB, Antonangelo L, Alvarenga VA, Puka J, Marchi E, Acencio MMP. Profile of metalloproteinases and their association with inflammatory markers in pleural effusions. Lung. 2016;194(6):1021–1027. doi: 10.1007/s00408-016-9945-5. [DOI] [PubMed] [Google Scholar]
- 55.Xirouchaki N, Tzanakis N, Bouros D, Kyriakou D, Karkavitsas N, Alexandrakis M, Siafakas NM. Diagnostic value of interleukin-1alpha, interleukin-6, and tumor necrosis factor in pleural effusions. Chest. 2002;121(3):815–820. doi: 10.1378/chest.121.3.815. [DOI] [PubMed] [Google Scholar]
- 56.Liu, Y.C. et al. Differential diagnosis of tuberculous and malignant pleurisy using pleural fluid adenosine deaminase and interferon gamma in Taiwan. J. Microbiol. Immunol. Infect. 44(2), 88–94 (2011). [DOI] [PubMed]
- 57.Lyadova IV, Panteleev AV. Th1 and Th17 cells in tuberculosis: Protection. Pathol. Biomarkers Mediators Inflamm. 2015;2015:854507–854507. doi: 10.1155/2015/854507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavalcanti, Y.V.N., Brelaz, M.C.A., Neves, J.K.D.A.L., Ferraz, J.C. & Pereira, V.R.A. Role of TNF-alpha, IFN-gamma, and IL-10 in the development of pulmonary tuberculosis. Pulm. Med. 2012, 745483–745483 (2012) [DOI] [PMC free article] [PubMed]
- 59.Lin PL, Plessner HL, Voitenok NN, Flynn JL. Tumor necrosis factor and tuberculosis. J. Invest. Dermatol. Sympos. Proceed. 2007;12(1):22–25. doi: 10.1038/sj.jidsymp.5650027. [DOI] [PubMed] [Google Scholar]
- 60.Nagabhushanam V, Solache A, Ting LM, Escaron CJ, Zhang JY, Ernst JD. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-gamma. J. Immunol. (Baltimore, Md. 1950) 2003;171(9):4750–4757. doi: 10.4049/jimmunol.171.9.4750. [DOI] [PubMed] [Google Scholar]
- 61.Diehl, S. et al. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity13(6), 805–815 (2000). [DOI] [PubMed]
- 62.Carow, B. et al. Silencing suppressor of cytokine signaling-1 (SOCS1) in macrophages improves Mycobacterium tuberculosis control in an interferon-gamma (IFN-gamma)-dependent manner. J. Biol. Chem. 286(30), 26873–26887 (2011) [DOI] [PMC free article] [PubMed]
- 63.Redford, P.S., Murray, P.J. & O’Garra, A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol.4(3), 261–270 (2011). [DOI] [PubMed]
- 64.Ali AHK, Mahmoud TM, Ahmed H. Differential diagnostic efficiency of T cells subsets versus interferon-gamma, tumor necrosis factor-alpha and adenosine deaminase in distinguishing tuberculous from malignant pleural effusions. Egypt. J. Chest Dis. Tuberculosis. 2015;64(3):645–651. [Google Scholar]
- 65.Pooran A, Davids M, Nel A, Shoko A, Blackburn J, Dheda K. IL-4 subverts mycobacterial containment in Mycobacterium tuberculosis-infected human macrophages. Eur. Respir. J. 2019;54(2):1802242. doi: 10.1183/13993003.02242-2018. [DOI] [PubMed] [Google Scholar]
- 66.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin W, Zhang H-L, Niu Z-Y, Wang Z, Kong Y, Yang X-S, Yuan F. The disease stage-associated imbalance of Th1/Th2 and Th17/Treg in uterine cervical cancer patients and their recovery with the reduction of tumor burden. BMC Womens Health. 2020;20(1):126. doi: 10.1186/s12905-020-00972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin. Immunopathol. 1999;21(3):339–359. doi: 10.1007/BF00812261. [DOI] [PubMed] [Google Scholar]
- 69.Sparano A, Lathers DMR, Achille N, Petruzzelli GJ, Young MRI. Modulation of Th1 and Th2 cytokine profiles and their association with advanced head and neck squamous cell carcinoma. Otolaryngol.-Head Neck Surg. 2004;131(5):573–576. doi: 10.1016/j.otohns.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 70.Atanackovic D, Block A, de Weerth A, Faltz C, Hossfeld DK, Hegewisch-Becker S. Characterization of effusion-infiltrating T cells. Clin. Cancer Res. 2004;10(8):2600. doi: 10.1158/1078-0432.ccr-03-0239. [DOI] [PubMed] [Google Scholar]
- 71.Maeda H, Shiraishi A. TGF-beta contributes to the shift toward Th2-type responses through direct and IL-10-mediated pathways in tumor-bearing mice. J. Immunol. (Baltimore, Md. 1950) 1996;156(1):73–78. [PubMed] [Google Scholar]
- 72.Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Bréban M, Pallot-Prades B, Pouplin S, Sacchi A, Chichemanian RM, Bretagne S, Emilie D, Lemann M, Lortholary O, Mariette X. G. Research axed on tolerance of biotherapies, risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: The three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009;60(7):1884–1894. doi: 10.1002/art.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qiao J, Liu Z, Dong C, Luan Y, Zhang A, Moore C, Fu K, Peng J, Wang Y, Ren Z, Han C, Xu T, Fu Y-X. Targeting tumors with IL-10 prevents dendritic cell-mediated CD8+ T cell apoptosis. Cancer Cell. 2019;35(6):901–915.e4. doi: 10.1016/j.ccell.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki A, Leland P, Joshi BH, Puri RK. Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine. 2015;75(1):79–88. doi: 10.1016/j.cyto.2015.05.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets supporting the results of this article are included within the article and its supplementary material.