Abstract
Background
We examined the relationship between the tumour microenvironment and the clinical efficacy of neoadjuvant chemotherapy in patients with cT2-4aN0M0 bladder cancer using multiplex fluorescence immunohistochemistry.
Methods
The study retrospectively evaluated 51 patients who underwent radical cystectomy following neoadjuvant chemotherapy for cT2-4aN0M0 muscle-invasive bladder cancer. Patients were divided into responders (<pT2) and non-responders (≥pT2). We assessed the density of each immune cell type in intratumoural and peritumoural areas in both groups via multiplex fluorescence immunohistochemical analysis.
Results
The median age was 69 years; 39 patients were male. Twelve (23.5%), 17 (33.3%), 10 (19.7%) and 12 (23.5%) patients were pT0, pT1, pT2 and ≥pT3, respectively. Responders had a significantly higher 5-year cancer-specific survival rate (96.6%) than non-responders (48.4%; p = 0.0018). CD8+ T cell (p = 0.0056) and CD204+ cell (p = 0.0394) densities were significantly higher in the intratumoural area in non-responders than in responders. Patients with higher CD204+ cell densities in cancerous areas had worse prognosis.
Conclusions
This comprehensive analysis of the immune microenvironment of a muscle-invasive bladder cancer specimen revealed that preexisting tumour-infiltrating proliferating CD8+ T cells and CD204+ cells are indicators of the response to neoadjuvant chemotherapy and that CD204+ cells can be considered an unfavourable prognostic factor in these patients.
Subject terms: Cancer microenvironment, Tumour biomarkers, Bladder cancer, Immunoediting
Introduction
Muscle-invasive bladder cancer (MIBC) is an aggressive malignancy that is associated with a poor prognosis, and the standard treatment for MIBC is radical cystectomy. However, the 5-year survival rate for total cystectomy alone is approximately 50% [1]. Therefore, cisplatin-based neoadjuvant chemotherapy (NAC) administered before cystectomy is a standard treatment that is recommended by several guidelines [2–4] for MIBC. The use of cisplatin-based NAC for MIBC is underpinned by several randomised clinical trials and meta-analyses, which found a 6% survival benefit at 10 years [5]. Recently, the partial results of a randomised phase III trial comparing the efficacy of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (dd-MVAC) or gemcitabine and cisplatin in the MIBC perioperative setting have been reported, and the full results are expected [6].
Although the efficacy of NAC is supported by these studies, NAC remains underutilized in clinical practice for various reasons, including the proportions of elderly patients with MIBC, poor performance status, multiple comorbidities and impaired renal function [7]. Particularly, 40% of patients are ineligible for cisplatin-based NAC because of nephrotoxicity [8, 9]. Consequently, it is important to identify a clear biomarker of NAC and improve the outcomes in MIBC with novel therapies, such as immune checkpoint inhibitors (ICIs).
The tumour microenvironment (TME) plays an essential role in cancer therapy. The TME consists of immune cells, mesenchymal cells, endothelial cells, inflammatory mediators and extracellular molecules [10, 11]. The context of TME determined at diagnosis reflects the immune response [12, 13] and the effect of chemotherapy [14], and changes in the number of various immune cells infiltrating the TME are associated with clinical outcomes [15, 16]. For instance, the presence of increased tumour-infiltrating lymphocytes (TILs), such as CD8+ T cells or CD4+ T cells, has been established as a prognostic factor in various tumours, including urothelial cancer [17, 18]. In contrast, myeloid inhibitory cells such as tumour-associated macrophages (TAMs) can limit responses to chemotherapy, irradiation and angiogenic inhibitors [19, 20]. Makino and colleagues [21] reported that the marked pretherapy infiltration of M2 macrophages might be a useful biomarker of the response to NAC in oesophageal cancer. Therefore, a deeper understanding of TME should not only help with NAC as it is currently practiced but could also be the basis for immunotherapy including neoadjuvant settings.
Multiplex fluorescence immunohistochemistry (mFIHC) is a powerful tool for the comprehensive analysis of the immune cell type in TME, compared with traditional immunohistochemistry. This technology enables the separate assessment of the immune profiles of intratumoural and peritumoural areas in TME, excluding the subjectivity of the evaluator. In addition, mFIHC enables a more detailed characterisation of each lineage cell [22]. Previous studies reported on intratumoural or stromal lymphocytes in addition to analysing TIL subsets and reported that TIL localisation in the epithelial compartment of tumours is important because the nonspecific infiltration of lymphocytes into the tumour or stroma is often observed after treatment [23, 24].
In this study, we assessed the association of TME and efficacy in patients treated with NAC for MIBC using mFIHC. To the best of our knowledge, this is the first study demonstrating immune profiling by mFIHC before and after NAC treatment in MIBC.
Materials and methods
Patients
We retrospectively evaluated 51 patients who received NAC following radical cystectomy at Iwate Medical University Hospital from October 2010 to October 2019. All patients were treated with curative intent. Tissue samples were obtained through transurethral biopsy or resection before the administration of NAC in all patients. The patients received two or three cycles of NAC, following radical cystectomy.
We divided the patients into two groups according to the histopathological stage in surgical tissues after NAC. Patients with ≥pT2 and <pT2 were classified into the non-responder and responder groups, respectively. Three patients who underwent immediate cystectomy without chemotherapy were recruited for the control group.
The study was conducted in accordance with the principles of the Declaration of Helsinki. The human ethics board of each institution approved this study and written informed consent was obtained from all patients prior to enrolment (Iwate Medical University; protocol no. 2019-083, National Cancer Center Hospital East; Protocol No. 2019-194).
Multiplex fluorescence immunohistochemistry
Four-micrometre-thick tissue sections obtained from formalin-fixed, paraffin-embedded (FFPE) blocks were subjected to mFIHC staining using the Opal Kit (AKOYA Biosciences, California, USA). We prepared thin FFPE sections of specimens obtained from all eligible cases, and immunostaining was performed as soon as possible after specimen sectioning. The antibodies, dilutions and activation conditions used are listed in Supplementary Table S1. The slides were scanned using the Vectra slide scanner (AKOYA Biosciences). For each marker, the mean fluorescent intensity per case was determined as a base point from which positive calls could be established. For multispectral analysis, each individually stained section was used to establish the spectral library of the fluorophores. Five to 20 random areas equally presenting the parenchyma and peritumor portions of the tumour in each sample were blindly reviewed and analysed by two pathologists at ×20 magnification. We selected these tumour areas from within the area where viable cancer cells existed to the invasive edge via visually inspecting H&E specimens according to the consensus of TIL assessment in breast cancer [25].
The summary of the analysis is described below, following previously reported methods [23, 26, 27]. An image analysis program (Inform; AKOYA Biosciences) was used to segment tumour tissues into carcinoma and stromal areas and to detect immune cells with specific phenotypes, after which the distribution of immune cells was analysed. Training sessions for tissue segmentation and phenotype recognition were conducted (Supplementary Fig. S1). After phenotyping typical CD4+ and CD8+ cells using Inform software, gated CD3+ populations based on the mean fluorescence intensity of CD3, CD3+ CD4+ and CD3+ CD8+ cells were determined as CD4+ and CD8+ T cells, respectively. A similar gating strategy was used for the analysis of Foxp3, Ki-67, PD-1 and Tim-3 high population in CD4+ or CD8+ T cells using an analytical program (Spotfire version 7.8; TIBCO Software, California, USA). The area of each tissue category, divided into intratumoural and peritumoural areas, was evaluated to assess the density of each immune cell type, as represented below:
T cells in the intratumoural and peritumoural areas were defined as intratumoural and peritumoural T cells, respectively. Intratumoural area was selected from the area where the tumour was viable and peritumoural area from bordering the tumour.
Statistical analysis
Statistical comparisons of the NAC responder and non-responder groups regarding clinicopathological features and mFIHC results were performed using the Mann–Whitney U test, Pearson χ2 test and analysis of variance. The paired t test was used to compare the densities of immune cells between pre-NAC and post-NAC tissue, excluding the analysis of patients with pT0. Receiver-operating characteristic (ROC) analysis of the NAC response was used to determine the cutoff density for each immune cell type, and patients were classified into ‘high’ and ‘low’ groups.
Progression-free survival (PFS) and cancer-specific survival (CSS) rates were calculated using the Kaplan–Meier method. PFS was defined as the time from radical cystectomy to radiographic or clinical progression. CSS was defined as the time from the radical cystectomy to cancer-specific death or loss-to-follow-up censoring. All statistical analyses were performed using JMP 14.0 software (SAS Institute Inc., Cary, NC, USA). For all statistical comparisons, differences with p < 0.05 were considered statistically significant.
Results
Patient characteristics
The clinical and pathological features of the patient cohort in the NAC responder (n = 22) and non-responder (n = 29) groups are described in Table 1. Patient characteristics were not significantly different between the two groups. The median follow-up duration after radical cystectomy was 38.1 (range, 0.1–114.6) months. The 5-year PFS and CSS were 70.8% and 76.3%, respectively (Supplementary Fig. S2a). In addition, the NAC response was significantly related to PFS and CSS (PFS: p = 0.0018; CSS: p = 0.0011; Supplementary Fig. S2b), indicating a strong association between tumour response to NAC treatment and patient prognosis.
Table 1.
Patients characteristics.
| Variable | Level | Responder (n = 29) | Non-responder (n = 22) |
|---|---|---|---|
| Age | Median | 68 (43–78) | 69 (44–74) |
| Sex | Male | 23 (79.3%) | 15 (68.2%) |
| Female | 6 (20.7%) | 7 (31.8%) | |
| Smoking | Yes | 17 (58.6%) | 13 (59.1%) |
| No | 5 (17.2%) | 8 (36.3%) | |
| Unknown | 7 (24.1%) | 1 (4.6%) | |
| Clinical stage | cT2 | 20 (68.9%) | 10 (45.4%) |
| cT3 | 9 (31.1%) | 11 (50%) | |
| cT4 | 0 | 1 (4.6%) | |
| Histology | Pure UC | 28 (96.6%) | 21 (95.4%) |
| UC + variant histology | 1 (3.4%) | 1 (4.6%) | |
| TURBT | Yes | 11 (37.9%) | 6 (27.3%) |
| No | 18 (62.1%) | 16 (72.7%) | |
| NAC regimen | GC/CaG | 25 (86.2%) | 15 (68.2%) |
| MVAC | 4 (13.8%) | 7 (31.8%) | |
| Pathological stage | pT0 | 12 (41.4%) | 0 |
| pTis/1 | 17 (58.6%) | 0 | |
| pT2 | 0 | 10 (45.4%) | |
| ≥pT3 | 0 | 12 (54.6%) |
UC urotherial carcinoma, TURBT trans-urethral resection of bladder cancer, NAC neoadjuvant chemotherapy, GC gemcitabine + cisplatin, CaG carboplatin + gemcitabine, MVAC methotrexate + vinblastine + doxorubicin (adriamycin) + cisplatin.
Analysis of various immune cells between NAC responders and non-responders
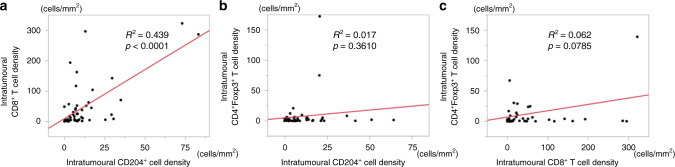
We assessed the association between the NAC response and tumour infiltration of various immune cells on pretreatment specimens via haematoxylin/eosin staining and mFIHC (Fig. 1a–d). The density of CD8+ T cells and CD204+ cells were significantly higher in tumoural areas in NAC non-responders than in NAC responders (CD8+ T cell: p = 0.0056; CD204+ cell: p = 0.0394, respectively), whereas the density of CD4+ T cells, CD4+Foxp3+ T cells, CD20+ and CD38+ cells were not associated with the NAC response (Fig. 1f). To investigate the relationship among those immune cells that showed significant differences in the two groups, we analysed the correlation of intratumoural immune cell density among CD8+ T cells, CD4+Foxp3+ cells and CD204+ cells. The results showed that CD204+ cells were positively correlated with CD8+ T cells, while CD8+ T cells and CD204+ cells were uncorrelated with CD4+Foxp3+ cells based on linear regression models (Fig. 2a–c).
Fig. 1. Assessment of tumor immune microenvironment.
Representative image of haematoxylin–eosin staining in pre-NAC tissue in the a responder group (×100) and b non-responder group (×100). Multiplex fluorescence immunohistochemistry in pre-NAC tissue in the c responder group and d non-responder group for the following markers: CD3, CD4, CD8, CD204, FoxP3, cytokeratin and 4′,6-diamidino-2-phenylindole. Original magnification, ×20. e, f Relationship of the density of each immune cell type in the intratumoural and peritumoural areas between the responder and non-responder groups.
Fig. 2. Correlation of intratumoral immune cells in pre-NAC tissues.
Linear regression modelling of the density of a CD204+ cell values for CD8+ T cell, b CD204+ cell values for CD4+Foxp3+ T cell and c CD8+ T cell values for CD4+Foxp3+ T cell in the intratumoural area in pre-NAC tissue as indicated.
Changes in intratumoural immune cells pre- and post-NAC treatment
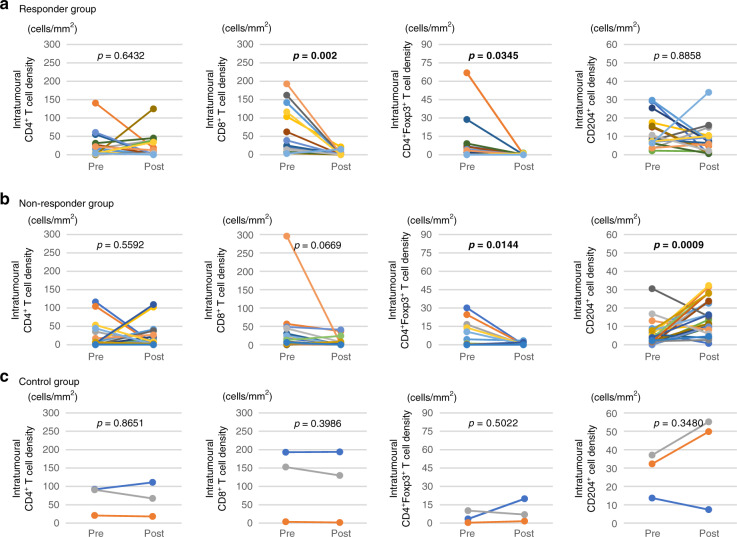
Next, we examined differences in the immune TMEs in pre- and post-NAC treatment. We assessed the densities of each immune cell type in the intratumoural area based on the pre-NAC tissue compared with the matched post-NAC tissue among the responder, non-responder, and control groups, excluding patients with pT0. In responder and non-responder groups, we observed decreased levels of CD8+ T cells and CD4+Foxp3+ T cells in the post-NAC tissue compared with pre-NAC tissue, which were significant or tended toward significance. Notably, we observed increased levels of CD204+ cells in the post-NAC tissue compared with pre-NAC tissue in the non-responder group (p = 0.0009), while we observed no significant difference in the responder group (Fig. 3).
Fig. 3. Changes in intratumoral immune cells pre- and post-NAC treatment.
Relationship between the density of each immune cell type in the intratumoural area in pre-NAC tissue and post-NAC tissue in a responder, b non-responder, and c control groups. In responder and nonresponder groups, CD8+ T cells, and CD4+Foxp3+ T cells were significantly decreased in the post-NAC tissue compared with pre-NAC tissue. CD204+ cells were significantly increased in the post-NAC tissue compared with pre-NAC tissue in the non-responder group (p = 0.0009). In control group, each immune cells were not changed between pre-NAC tissue and post-NAC tissue.
Analysis of intratumoural CD8+ T cell function
To investigate the function of intratumoural CD8+ T cells, we examined the frequencies of Ki-67 expression as a proliferative marker, PD-1 and TIM3 expression as a suppressor, or exhausted immune checkpoint molecules in CD8+ T cells between the responder and non-responder groups. The proportion of Ki-67+CD8+ T cells within the CD8+ T cells in pre-NAC tissue was significantly higher in the responder group than in non-responder group (p = 0.0413), while there were no significant differences between the two groups regarding the proportion of PD-1+ or PD-1+TIM3+CD8+ T cells within CD8+ T cells in pre-NAC tissue (Fig. 4).
Fig. 4. Assessment of intratumoral CD8+ T cell function.
Representative image of a haematoxylin–eosin staining and b multiplex fluorescence immunohistochemistry in pre-NAC tissue showing the distribution of CD8+ T cell subsets in the intratumoural and peritumoural areas: pink arrows (CD8+ Ki-67+), cyan arrows (CD8+ PD-1+), and magenta arrows (CD8+PD-1+TIM3+). c Comparisons of the proportions of Ki-67+, PD-1+ and PD-1+TIM3+ among CD8+ T cells in the intratumoural and peritumoural areas between the responder and non-responder groups. The proportion of Ki-67+CD8+ T cells within the CD8+ T cells in pre-NAC tissue was significantly higher in the responder group than in non-responder group (p = 0.0413).
Impact of M2 macrophage infiltration on MIBC prognosis
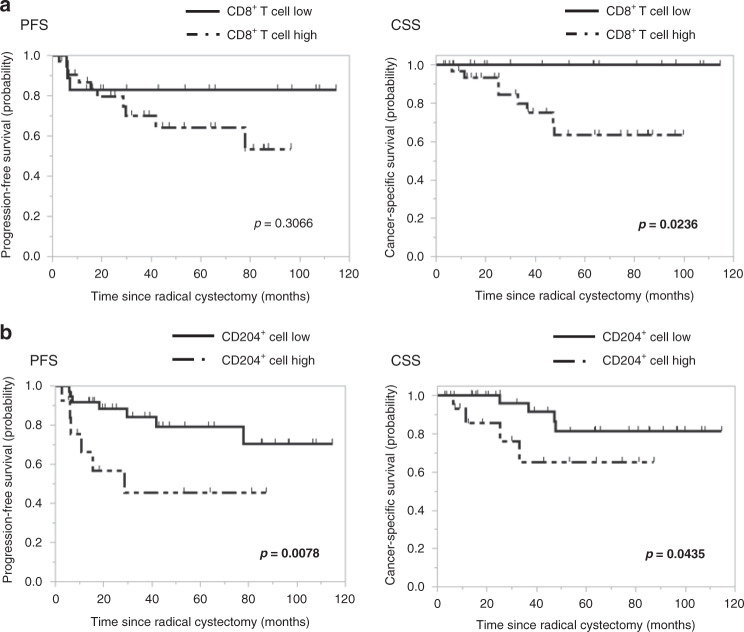
Next, we assessed the association between intratumoural CD8+ T cells and CD204+ cells in pre-NAC tissue, which we divided into high and low groups using ROC analysis, and the prognosis of NAC patients with MIBC (Supplementary Fig. S3). Patients with high tumour infiltration of CD8+ T cells had a significantly shorter CSS than those with low infiltration, but this finding was unnoted for PFS. Notably, patients with a high tumour infiltration of CD204+ cells had a significantly poorer prognosis than those with low infiltration (Fig. 5, 5-year PFS: 50.5% vs 80.2%, p = 0.0078; 5-year CSS: 65.2% vs 81.3%, p = 0.0435).
Fig. 5. Progression-free survival and cancer-specific survival in patients with high and low infiltration of CD8+ T cell and CD204+ cell.
Kaplan–Meier curves for PFS, and CSS after radical cystectomy according to the infiltration of a CD8+ T cell and b CD204+ cell in pre-NAC tissue divided into high and low groups. Patients with a high tumor infiltration of CD8+ T cells had a significantly shorter CSS than those with low infiltration (p = 0.0238). Patients with a high tumor infiltration of CD204+ cells had a significantly shorter PFS and CSS than those with low infiltration (p = 0.0078, p = 0.0435).
Discussion
In the present study, the density of intratumoural CD8+ T cells and CD204+ cells in pre-NAC tissue was higher in the NAC non-response group than that in the response group. Furthermore, NAC response was significantly associated with prognosis, and patients with high intratumoural CD8+ T cells and CD204+ cells (M2 macrophages) in pre-NAC tissue had a poorer prognosis than those with low infiltration. A previous meta-analysis reported a relationship between the high infiltration of TAMs and a poor prognosis in most solid tumours [28]. In bladder cancer, several studies have reported that TAMs were associated with poorer survival with various treatment modalities, such as Bacillus Calmette–Guerin therapy, chemotherapy and immunotherapy [29–32]. However, Aljabery et al. reported that TAMs were associated with a lower rate of lymph node metastases and higher CSS in patients with bladder cancer patients [33]. However, almost all the studies assessed TAMs using traditional immunohistochemistry for CD68 as pan-macrophages, and only a few reports focused on M2 macrophages. Therefore, the role of TAMs has remained contentious in bladder cancer [34]. This is the first report to demonstrate that intratumoural proliferating CD8+ T cells and CD204+ cells in pre-NAC tissue are predictors in the NAC setting for MIBC using mFIHC to localise each immune cell in the TME.
TAMs are known to directly or indirectly inhibit T cell responses in the TME. The direct inhibitory effects of TAMs on T cell responses include the production of inhibitory cytokines such as interleukin-10 and tumour growth factor-β, involvement in immune checkpoints via the expression of molecules such as PD-L1, and metabolic activities such as the depletion of metabolites and production of reactive oxygen species. Moreover, TAMs indirectly inhibit T cell responses by controlling the immune microenvironment, including the recruitment of immunosuppressive cells (e.g. Tregs) or by inhibiting stimulatory cells (e.g. dendritic cells) [35]. Our data showed that intratumoural CD8+ T cells as effecter cells exhibited a strong positive correlation with intratumoural CD204+ cells as immune-suppressive cells in pre-NAC tissue. The data suggest that M2 macrophages directly inhibit T cell function. In a recent study, Yang et al. reported that urinary bladder cancer (UBC) can be classified based on immune gene profiling using the following four subtypes: cold tumour, immune ignorant, immune inactive and hot tumour. Among these subtypes, only cold tumours were correlated with a favourable prognosis and early-stage UBC, including luminal type with chemosensitivity [36, 37]. To clearly evaluate the four immune states and immune-excluded subtypes in the tumour specimens analysed in this study, we analysed the correlation between intratumours and peritumours of CD8+ T and CD204+ cells and found a positive correlation between both intra- and peritumoural CD8+ T cells and CD204+ cells (Supplementary Fig. S4). The results suggest that immune-excluded subtypes are suitable, with some exceptions. Thus, based on these results, the non-responder group with high infiltration of CD8+ T and CD204+ cells could be classified as a hot tumour subtype, whereas the responder group with low infiltration of CD8+ T and CD204+ cells could be classified as a cold subtype. However, in this study, intratumoural CD204+ and CD8+ T cells were not correlated with intratumoural CD4+Foxp3+ T cells as Tregs in pre-NAC tissue. Because Tregs are composed of functionally distinct subpopulations, including naive Tregs, effector Tregs and non-suppressive Tregs, it is difficult to distinguish the suppressive function of tumour-infiltrating Tregs based on Foxp3 expression alone [38]. Furthermore, Foxp3 is not only expressed in Tregs but also weakly in activated T cells, and it would be difficult to distinguish these subpopulations by immunohistochemistry, in which quantitative evaluation of antigen expression levels is difficult [39]. However, Ki-67+CD8+ T cells in pre-NAC tissue showed a significantly higher expression in the responder group than in the non-responder group. In tumour immunology, T cells primed by antigen-presenting cells are believed to be activated to initiate cell proliferation. Therefore, we believe that higher infiltration of Ki-67-positive proliferating T cells into the tumour reflects the occurrence of the adaptive immune response. Imaizumi et al. reported that the evaluation of activated T cells expressing Ki-67 with their localisation in tumours is a reliable immunological marker for determining the prognosis of locally advanced rectal cancer patients [24].
The present study has several limitations because of the relatively small number of patients and the retrospective design. However, our clinical outcomes were comparable to those reported by other authors. In addition, we did not evaluate the association between TMEs and ICI response in the neoadjuvant setting because neoadjuvant ICI is not approved for urothelial carcinoma in Japan. We believe that the information presented herein can contribute toward further understanding of intratumoural immune cell behaviour and expanding the potential therapeutic spectrum of immunotherapy, including the neoadjuvant setting. We are planning a prospective study with a larger sample size to validate the results of this exploratory study. The limitations of mFIHC analysis include the limited number of antibodies that can be simultaneously stained and the inability to guarantee the specificity of antibodies depending on the target molecule. However, when carefully validated, they can contribute to a more comprehensive and objective quantitative assessment of the various intra- and peritumoural immune cells separately in the TME [40].
In this study, the evaluation of CD204+ cells and CD8+ T cells based on pre-NAC tissue obtained by transurethral biopsy or resection was useful for predicting NAC response and unfavourable prognosis.
Once CD204+ and CD8+ T cells are identified in the pretreatment specimen and a poor response to NAC is predicted, conversion to neoadjuvant ICI or combination with radiotherapy may be additional treatment options. Assessing the changes in each immune cell type in pre- and post-NAC treatment tissues revealed that the density of CD8+ T cells was higher in the non-responder group in the pre-NAC tissue, and the densities of CD8+ T cells and Tregs were significantly decreased in both the responder and non-responder groups in the post-NAC tissue. These results may indicate that neoadjuvant ICI will be effective in cases where a poor response to NAC is predicted and may be one of the reasons for the limited response to immune ICI after second-line treatment. In fact, a clinical trial on neoadjuvant pembrolizumab for localised MIBC has been reported with good clinical response [41]. In a recent review, Topalian et al. [42] reported the presence of tumour-specific CD8+ T cells and tertiary lymphoid structures in pretreatment specimens as a factor associated with the efficacy of neoadjuvant ICI therapy. Particularly, they reported that the B cell component had a potential role in mediating anti-PD-1 responses. Although our results did not include neoadjuvant ICI cases and therefore cannot be directly compared with the NAC response, the presence of B and plasma cells in pretreatment specimens has been confirmed, and we believe that understanding TMEs will lead to the development of neoadjuvant ICI therapy. Moreover, in this study, M2 macrophages were significantly increased in post-NAC tissue compared to pre-NAC tissue, while no significant change was observed in the responder group. Considering that M2 macrophages are a poor prognostic factor, M2 macrophage-targeted therapy might be an optional treatment in the future. In fact, a previous study reported that targeting TAMs could significantly improve the efficacy of conventional and immunotherapeutics [35]. In addition, Wang et al. [43] found that the balance between adaptive immune response and protumorigenic inflammation in the TME is associated with PD-1/PD-L1 resistance in urothelial cancer, with the latter being locally and systemically linked to the proinflammatory cellular state of myeloid phagocytic cells. They also suggested that overcoming myeloid-related ICI resistance improves the benefits of ICIs in patients with UC. Hence, macrophage-targeting therapy is expected to improve the therapeutic efficacy of existing conventional chemotherapy and radiotherapy through the activation of anticancer immunity [44].
Conclusion
This is the first report to demonstrate that a preexisting tumour immune microenvironment predicts the clinical response and prognosis of MIBC in the NAC setting. The importance of the quality of intratumoural effector CD8+ T cells in pretreatment specimens was established, suggesting the significance of quantitative as well as a qualitative evaluation of various immune cells in the TME. In addition, intratumoural CD204+ cells as M2 macrophages were found to be associated with NAC response and prognosis, indicating the possibility of clinical development of combination immunotherapy against M2 macrophages as a therapeutic target.
Disclaimer
The work presented in this article is original research. This article has not been previously published and has not been submitted for publication elsewhere while under consideration.
Supplementary information
Acknowledgements
The authors would like to thank all patients and their families. They also would like to thank Enago (www.enago.jp) for the English language review.
Author contributions
Study conception and design: DI, SK and WO; data acquisition and analysis: DI, TT, KT, MY, HM, SM, RK and YK; drafting the manuscript and figures: DI, HO and AS; reviewing the manuscript: SK, TN, TS, TT and WO. All authors have read and approved the final draft for submission.
Data availability
All data supporting the results are presented with results and in the figures.
Competing interests
SK reports personal fees from Astra Zeneca, grants and personal fees from Chugai, personal fees from Pfizer, grants and personal fees from Boehringer Ingelheim, personal fees from Taiho, personal fees from Novartis, grants and personal fees from Daiichi-Sankyo, personal fees from MSD, personal fees from Sumitomo Dainippon Pharma, grants and personal fees from Eisai, grants from Astellas, grants from Gilead Sciences, grants and personal fees from Ono Pharmaceutical Co., Ltd, personal fees from Bristol-Myers Squibb, grants and personal fees from REGENERON, personal fees from AYUMI Pharmaceutical Corporation, personal fees from Rakuten Medical, grants from PACT Pharma, grants from Takara Bio Inc., personal fees from GSK, personal fees from ImmuniT Research Inc., grants and personal fees from Ono Pharmaceutical Co., Ltd, personal fees from PMDA (Pharmaceuticals and Medical Devices Agency), grants from AMED (Japan Agency for Medical Research and Development) and grants from JSPS (Japan Society for the Promotion of Science), outside the submitted work. The remaining authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the principles of the Declaration of Helsinki. The human ethics board of each institution approved this study and written informed consent was obtained from all patients prior to enrolment (Iwate Medical University; Protocol No. 2019-083, National Cancer Center Hospital East; Protocol No. 2019-194).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01628-y.
References
- 1.Gore JL, Litwin MS, Lai J, Yano EM, Madison R, Setodji C, et al. Urologic Diseases in America Project. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010;102:802–11. doi: 10.1093/jnci/djq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: summary of the 2020 guidelines. Eur Urol. 2021;79:82–104. doi: 10.1016/j.eururo.2020.03.055. [DOI] [PubMed] [Google Scholar]
- 3.Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol. 2017;198:552–9. doi: 10.1016/j.juro.2017.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. NCCN Guidelines Insights: bladder cancer, version 5.2018. J Natl Compr Cancer Netw. 2018;16:1041–53. doi: 10.6004/jnccn.2018.0072. [DOI] [PubMed] [Google Scholar]
- 5.Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. 2018;4:1535–42. doi: 10.1001/jamaoncol.2018.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfister C, Gravis G, Fléchon A, Soulié M, Guy L, Laguerre B, et al. VESPER Trial Investigators. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER Trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur Urol. 2021;79:214–21. doi: 10.1016/j.eururo.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Anan G, Hatakeyama S, Fujita N, Iwamura H, Tanaka T, Yamamoto H, et al. Trends in neoadjuvant chemotherapy use and oncological outcomes for muscle-invasive bladder cancer in Japan: a multicenter study. Oncotarget. 2017;8:86130–42. doi: 10.18632/oncotarget.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canter D, Viterbo R, Kutikov A, Wong YN, Plimack E, Zhu F, et al. Baseline renal function status limits patient eligibility to receive perioperative chemotherapy for invasive bladder cancer and is minimally affected by radical cystectomy. Urology. 2011;77:160–5. doi: 10.1016/j.urology.2010.03.091. [DOI] [PubMed] [Google Scholar]
- 9.Koie T, Ohyama C, Hashimoto Y, Hatakeyama S, Yamamoto H, Yoneyama T, et al. Efficacies and safety of neoadjuvant gemcitabine plus carboplatin followed by immediate cystectomy in patients with muscle-invasive bladder cancer, including those unfit for cisplatin: a prospective single-arm study. Int J Clin Oncol. 2013;18:724–30. doi: 10.1007/s10147-012-0447-z. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi M, et al. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res. 2019;7:737–50. doi: 10.1158/2326-6066.CIR-18-0436. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, et al. ImmunoScore Signature: a prognostic and predictive tool in gastric cancer. Ann Surg. 2018;267:504–13. doi: 10.1097/SLA.0000000000002116. [DOI] [PubMed] [Google Scholar]
- 15.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immunecheckpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017;14:655–68. doi: 10.1038/nrclinonc.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, et al. ImmunoScore Signature: a prognostic and predictive tool in gastric cancer. Ann Surg. 2018;267:504–13. doi: 10.1097/SLA.0000000000002116. [DOI] [PubMed] [Google Scholar]
- 17.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24:311–35. doi: 10.1097/PAP.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, Pan W, Yang M, Yang W, He W, Chen X, et al. Programmed death ligand-1 is associated with tumor infiltrating lymphocytes and poorer survival in urothelial cell carcinoma of the bladder. Cancer Sci. 2019;110:489–98. doi: 10.1111/cas.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 20.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;2019:6. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto K, Makino T, Sato E, Noma T, Urakawa S, Takeoka T, et al. Tumor-infiltrating M2 macrophage in pretreatment biopsy sample predicts response to chemotherapy and survival in esophageal cancer. Cancer Sci. 2020;111:1103–12. doi: 10.1111/cas.14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70:46–58. doi: 10.1016/j.ymeth.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Igari F, Sato E, Horimoto Y, Takahashi Y, Isomura T, Arakawa A, et al. Diagnostic significance of intratumoral CD8+ tumor-infiltrating lymphocytes in medullary carcinoma. Hum Pathol. 2017;70:129–38. doi: 10.1016/j.humpath.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Imaizumi K, Suzuki T, Kojima M, Shimomura M, Sakuyama N, Tsukada Y, et al. Ki67 expression and localization of T cells after neoadjuvant therapies as reliable predictive markers in rectal cancer. Cancer Sci. 2020;111:23–35. doi: 10.1111/cas.14223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi T, Kohsaka S, Takamochi K, Kishikawa S, Ikarashi D, Sano K, et al. Histological characteristics of lung adenocarcinoma with uncommon actionable alterations: special emphasis on MET exon 14 skipping alterations. Histopathology. 2021;78:987–99. doi: 10.1111/his.14311. [DOI] [PubMed] [Google Scholar]
- 27.Ikarashi D, Kitano S, Ishida K, Nakatsura T, Shimodate H, Tsuyukubo T, et al. Complete pathological response to neoadjuvant pembrolizumab in a patient with chemoresistant upper urinary tract urothelial carcinoma: a case report. Front Oncol. 2020;10:564714. doi: 10.3389/fonc.2020.564714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takayama H, Nishimura K, Tsujimura A, Nakai Y, Nakayama M, Aozasa K, et al. Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette-Guerin instillation. J Urol. 2009;181:1894–900. doi: 10.1016/j.juro.2008.11.090. [DOI] [PubMed] [Google Scholar]
- 30.Tervahartiala M, Taimen P, Mirtti T, Koskinen I, Ecke T, Jalkanen S, et al. Immunological tumor status may predict response to neoadjuvant chemotherapy and outcome after radical cystectomy in bladder cancer. Sci Rep. 2017;7:12682. doi: 10.1038/s41598-017-12892-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Ni S, Chen Q, Ma L, Jiao Z, Wang C, et al. Bladder cancer cells induce immunosuppression of T cells by supporting PD-L1 expression in tumour macrophages partially through interleukin 10. Cell Biol Int. 2017;41:177–86. doi: 10.1002/cbin.10716. [DOI] [PubMed] [Google Scholar]
- 32.Sharifi L, Nowroozi MR, Amini E, Arami MK, Ayati M, Mohsenzadegan M. A review on the role of M2 macrophages in bladder cancer; pathophysiology and targeting. Int Immunopharmacol. 2019;76:105880. doi: 10.1016/j.intimp.2019.105880. [DOI] [PubMed] [Google Scholar]
- 33.Aljabery F, Olsson H, Gimm O, Jahnson S, Shabo I. M2-macrophage infiltration and macrophage traits of tumor cells in urinary bladder cancer. Urol Oncol. 2018;36:159.e19–159.e26. doi: 10.1016/j.urolonc.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Sharifi L, Nowroozi MR, Amini E, Arami MK, Ayati M, Mohsenzadegan M. A review on the role of M2 macrophages in bladder cancer; pathophysiology and targeting. Int Immunopharmacol. 2019;76:105880. doi: 10.1016/j.intimp.2019.105880. [DOI] [PubMed] [Google Scholar]
- 35.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–82. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Li A, Liu F, Zhao Q, Ji S, Zhu W, et al. Immune profiling reveals molecular classification and characteristic in urothelial bladder cancer. Front Cell Dev Biol. 2021;9:596484. doi: 10.3389/fcell.2021.596484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–65. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–84. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28:401–9. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan WCC, Nerurkar SN, Cai HY, Ng HHM, Wu D, Wee YTF, et al. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 2020;40:135–53. doi: 10.1002/cac2.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Lucianò R, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol. 2018;36:3353–60. doi: 10.1200/JCO.18.01148. [DOI] [PubMed] [Google Scholar]
- 42.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367:eaax0182. doi: 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Sfakianos JP, Beaumont KG, Akturk G, Horowitz A, Sebra RP, et al. Myeloid cell-associated resistance to PD-1/PD-L1 blockade in urothelial cancer revealed through bulk and single-cell RNA sequencing. Clin Cancer Res. 2021;27:4287–300. doi: 10.1158/1078-0432.CCR-20-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17:651–62. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the results are presented with results and in the figures.