Abstract
Human polyomaviruses JC and BK may cause several clinical manifestations in immunocompromised hosts, including progressive multifocal leukoencephalopathy and hemorrhagic cystitis. Molecular detection by PCR is recognized as a sensitive and specific method for detecting human polyomaviruses in clinical samples. In this study, a real-time PCR assay using the LightCycler platform was evaluated and compared to an “in-house” PCR assay using a conventional detection method. A total of 122 urine specimens were tested, and human polyomavirus was detected in 49 specimens (40%) by both conventional PCR and LightCycler PCR. The remaining 73 specimens (60%) were found negative by both assays. For 46 of the 49 positive specimens, LightCycler PCR and conventional PCR identified the same polyomavirus type. These samples included 30 samples with JC virus (JCV), 14 samples with BK virus (BKV), and 2 samples in which both viruses were detected. In the remaining three samples, both JCV and BKV were detected by the conventional assay, but only JCV was detected by the LightCycler assay. The results of this study show that the LightCycler PCR assay displays sensitivity and specificity similar to those of a conventional PCR assay. These data, combined with its rapid turnaround time for results and decreased hands-on time, make the LightCycler PCR assay highly suitable for the rapid detection and differentiation of JCV and BKV in the clinical laboratory.
Human polyomaviruses JC and BK are ubiquitous in the population (8). Primary infections with these viruses are usually asymptomatic and may result in transient viruria. Following primary infection, JC virus (JCV) and BK virus (BKV) both establish latency in renal tissues and in B lymphocytes (20; G. Lecatsas, B. D. Schoub, A. R. Rabson, and M. Joffe, Letter, Lancet 2:907–908, 1976). Polyomavirus-related disease is largely associated with immunological impairment, and rapid detection and differentiation of the etiological agent in immunocompromised patients are important to assist with clinical management. JCV is the causative agent of the neurological disease progressive multifocal leukoencephalopathy, which occurs primarily in AIDS patients (16, 19), whereas BKV-associated disease includes hemorrhagic cystitis, ureteral stenosis, and other urinary tract disease, which are most commonly found in transplant patients undergoing immunosuppressive therapy (5, 9).
Traditional methods for detecting and identifying polyomaviruses include serologic methods, virus isolation by cell culturing, and electron microscopy (15, 17, 21). Recently, several studies have shown PCR to be an effective tool for detecting polyomaviruses in a range of clinical samples (4, 7, 11); as a result, PCR is now emerging as the “gold standard” for polyomavirus detection. However, the routine implementation of nucleic acid amplification techniques in the clinical laboratory has limitations, because of concerns of amplicon carryover contamination and technically cumbersome PCR product detection methods (14, 24). Real-time PCR instrumentation is the latest advance in nucleic acid amplification and detection technology that overcomes many of these limitations. We developed a real-time PCR assay for the detection and characterization of polyomaviruses using the LightCycler instrument (Roche Molecular Biochemicals). This technology applies fluorescence resonance energy transfer (FRET) in the continuous monitoring of amplicon development by use of two fluorophore-labeled hybridization probes (25). Using the LightCycler PCR (LC-PCR) assay, we examined 122 urine specimens for the presence of polyomaviruses and characterized the viruses when present by melting curve analysis. The results were compared with the results obtained by a conventional PCR assay developed in our laboratory, which used solid-phase hybridization and a colorimetric detection system.
MATERIALS AND METHODS
Specimens and nucleic acid extraction.
To ensure that a diverse sample population was tested in this study, we included urine specimens (n = 122) that were obtained from a general hospital population and that were submitted to the diagnostic laboratory of the Queensland Health Pathology Service for the routine investigation of microorganisms. Patient groups represented included (i) sexual health clinic (n = 22), (ii) pediatric clinic (n = 26), (iii) antenatal clinic (n = 20), (iv) indigenous health clinic (n = 10), (v) general adult medical wards (n = 20), and (vi) immunocompromised (n = 24). Nucleic acid was extracted from 0.2 ml of each specimen with a QIAamp DNA blood mini kit (Qiagen) according to the manufacturer's protocol. This procedure has been shown to be suitable for the extraction of viral DNA from urine specimens because of the high DNA yield and the removal of PCR inhibitors (12). DNA was eluted from the column with 50 μl of PCR-grade water (Baxter water for irrigation).
LC-PCR.
The LightCycler instrument provides a platform for the amplification of target nucleic acid and monitors the development of amplification reaction products by fluorescence after the annealing step in each cycle. Primers PoL1s and PoL2as used in the LC-PCR target a sequence of the VP2 gene that is conserved between JCV and BKV, producing a 131-bp product during the reaction (Table 1). The continuous monitoring of amplicon development was based on the FRET principle and was performed using two adjacent hybridization probes. One probe, PoLP1 (probe 1), was labeled with a donor fluorescein fluorophore at the 3′ end, and a second probe, PoLP2 (probe 2), was labeled with an acceptor fluorophore, LC-Red 640, at the 5′ end. Probe 2 was also labeled with a 3′ phosphorylated residue to prevent extension of the probe by Taq DNA polymerase during the reaction (Table 1).
TABLE 1.
Primer and probe sequences used in the detection and characterization of JCV and BKV
| Assay | Target | Primer or probe | Amplicon size (bp) | Sequence (5′-3′) |
|---|---|---|---|---|
| LC-PCR | VP2 gene | Primer: PoL1s | 131 | CACTTTTGGGGGACCTAGT |
| Primer: PoL2as | CTCTACAGTAGCAAGGGATGC | |||
| Probe 1: PoLP1 | TCTGAGGCTGCTGCTGCCACAGGATTTT-fluorescein | |||
| Probe 2: PoLP2 | LC-Red 640-AGTAGCTGAAATTGCTGCTGGAGAGGCTGCT-phosphate | |||
| PCR-ELAHA | VP1 gene | Primer: PoE1s | 434 | GGAGGAGTAGAAGTTCTAGAA |
| Primer: PoE2as | TCTGGGTACTTTGTYCTGTA | |||
| Probe 1: PoEBP1 | Biotinyl-GCTTAACCTTCATGCAGGGTCACA | |||
| Probe 2: PoEJP2 | Biotinyl-GATGAATGTGCACTCTAATGGTCA |
A LightCycler FastStart DNA master hybridization probes kit (Roche Molecular Biochemicals) was used as the basis for the reaction mixture in the LC-PCR assay; a 20-μl volume was used in each reaction capillary. Briefly, capillaries were loaded with 2 μl of LightCycler FastStart DNA master hybridization probes reagent (10×; Roche Molecular Biochemicals; reagent 1), 2.4 μl of MgCl2 stock solution (25 mM; Roche Molecular Biochemicals; reagent 2), 2 pmol of sense primer, 8 pmol of antisense primer, 4 pmol of each probe, and 5 μl of target DNA. Each mixture was made up to 20 μl using sterile PCR-grade water (Roche Molecular Biochemicals; reagent 3). Besides a positive control, each test run included three no-target controls consisting of 15 μl of reaction mixture and 5 μl of PCR-grade water. Reaction capillaries were capped, centrifuged, and placed into the carousel of the LightCycler instrument. The LC-PCR protocol included the following parameters: an initial 10 min of incubation at 95°C for FastStart Taq DNA polymerase activation followed by 55 cycles of denaturation at 95°C for 10 s, annealing at 55°C for 10 s, and extension at 72°C for 20 s. The data were obtained during the annealing period in the “single” mode with the channel setting F2/F1. The fluorescence settings were set to 1 for F1 gain and 15 for F2 gain. F3 gain was not used in the analysis of the data.
LightCycler melting curve analysis for JCV and BKV differentiation.
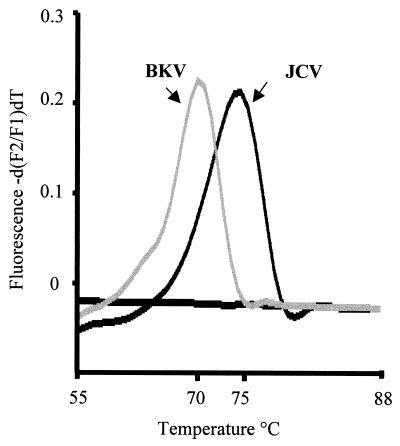
The LightCycler hybridization probes were designed for JCV, and genomic sequence differences between JCV and BKV were detected by melting curve analysis. Essentially both hybridization probes had 100% homology to the JCV sequence, but each probe had a single base mismatch compared to the BKV sequence. Melting curve analysis was performed following PCR amplification using LightCycler software. Briefly, the analysis was commenced at 55°C, and the temperature was raised to 95°C at a rate of 0.1°C/s. The difference between the sequence of the BKV PCR product and the probes resulted in a shift in melting temperature (−5°C) compared to the results for the JCV sequence (Fig. 1).
FIG. 1.
LightCycler melting curve analysis for the differentiation of JCV and BKV.
Conventional PCR amplification.
The conventional PCR was performed using primers PoE1s and PoE2as, which are homologous to a region of sequence of the VP1 gene of JCV and BKV, resulting in a 434-bp product (Table 1) (D. M. Whiley et al., unpublished data). The assay was done with a 50-μl reaction volume containing 2 U of Platinum Taq DNA polymerase (Life Technology); 5 μl of 10× PCR buffer (Life Technology); 2.5 mM MgCl2 solution (Life Technology); 10 pmol of each primer; 200 μM (each) dATP, dGTP, and dCTP (Promega); 400 μM dUTP (Promega); 0.1 nmol of digoxigenin-11-dUTP (Roche Molecular Biochemicals); and 5 μl of specimen extract or controls. The reaction volume was adjusted to 50 μl with PCR-grade water. PCR amplification was performed with a Perkin-Elmer 2400 thermal cycler (Applied Biosystems) using the following conditions: initial denaturation of DNA and activation of Platinum Taq DNA polymerase at 94°C for 2 min followed by 45 cycles at 94°C for 20 s, 55°C for 20 s, and 72°C for 20 s. These steps were followed by an additional primer extension step of 7 min at 72°C.
Detection of amplification products.
An enzyme-linked amplicon hybridization assay (ELAHA) developed in our laboratory was used for amplicon detection (18). This method included two hybridization reactions for each sample, one using a JCV-specific probe, PoEJP2, and one using a second probe, PoEBP1, which was BKV specific. Both probes were labeled at the 5′ terminus with biotin during manufacture (Table 1). Following amplification, 10 μl of reaction mixture was added to each of two reaction tubes (200 μl) containing 10 ng of either probe. Forty microliters of 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate) was added to each tube, giving a final reaction volume of 50 μl. The tubes were placed into a thermal cycler (Applied Biosystems) and kept at 94°C for 5 min to denature the amplicon, followed by rapid cooling to 4°C. Following denaturation, the contents of the tubes were transferred to streptavidin-coated wells (Labsystems) and incubated for 20 min at 37°C. The wells were washed four times with 1× SSC. Antidigoxigenin-peroxidase conjugate solution (Roche Diagnostics) was prepared by diluting the conjugate in phosphate-buffered saline (pH 7.2) to a final concentration of 150 mU/ml. Fifty microliters of diluted conjugate was added to each well and incubated for 20 min at 37°C. The wells were washed four times with 1× phosphate-buffered saline containing 0.5% Tween 20. Finally, 100 μl of tetramethylbenzidine substrate (Elisa Systems; contains tetramethylbenzidine at 2.08 mmol per liter; pH 3.3) was added to each well and incubated at room temperature for 10 min. The reaction was stopped by the addition of 100 μl of 1 M HCl to each well. The absorbance was recorded using a plate spectrophotometer (Murex Diagnostics) at a wavelength of 450 nm, with 690 nm as a reference. A positive result was indicated by an absorbance of 0.2 or greater.
Determination of LC-PCR assay sensitivity relative to that of PCR-ELAHA.
JCV and BKV DNA specimens previously extracted from a urine specimen that tested positive for these viruses by conventional PCR were diluted in serial 10-fold dilutions ranging from 10−1 to 10−7 in PCR-grade water. These dilutions were tested with both the LC-PCR assay and the conventional PCR-ELAHA using the conditions described above. The detection limit of each assay was determined as the lowest dilution returning a positive reaction.
DNA sequencing.
DNA sequencing of amplification products was performed using an ABI Prism dye terminator cycle sequencing kit (Applied Biosystems). Essentially, PCR products were electrophoresed through a 1% agarose gel (Sigma). The polyomavirus VP1-specific amplification product was excised and purified using a QIAquick gel extraction kit (Qiagen). For the sequencing reaction, a 20-μl reaction mixture was used, containing 4 μl of ABI Prism terminator ready reaction mix (Applied Biosystems), 1 μl of VP1 sense primer (2 pmol/μl), 10 μl of PCR-grade water, and 5 μl of purified amplicon. The reaction mixture was placed into a Perkin-Elmer 2400 thermal cycler for 35 cycles at 94°C for 30 s, 50°C for 15 s, and 60°C for 4 min and a final hold at 4°C. An ethanol precipitation step was performed according to the protocol described by the kit manufacturer (Applied Biosystems). Samples were analyzed on an ABI 377 DNA sequencer (Applied Biosystems).
RESULTS
Human polyomavirus DNA was detected by both the LC-PCR assay and the PCR-ELAHA in 49 (40.2%) of the 122 urine specimens tested. Negative results were obtained for 73 specimens. The LC-PCR-positive specimens were further investigated by melting curve analysis to determine the polyomavirus type present (JCV or BKV).
In the 49 polyomavirus-positive samples, a single virus type was detected in 44 specimens by both the LC-PCR assay and the PCR-ELAHA. The LC-PCR detected JCV in 33 specimens and BKV in 14 specimens, with an additional 2 specimens being positive for both JCV and BKV. In these two specimens, both viruses were detected by both assays, whereas the PCR-ELAHA detected both viruses in 3 of the 49 specimens in which the LC-PCR detected JCV alone. In all, the LC-PCR and PCR-ELAHA detected JCV DNA in 35 specimens (28.7%), the LC-PCR detected BKV DNA in 16 specimens (13.1%) (Table 2), and the PCR-ELAHA detected BKV DNA in 19 specimens (15.6%). Further examination of results in relation to specimen population showed that the positivity rates for both JCV and BKV were lowest in the pediatric population (11.5 and 0%, respectively), whereas the positivity rates were highest in samples from immunocompromised subjects (45.8% for JCV and 37.5% for BKV) (Table 2).
TABLE 2.
Distribution of JCV and BKV in urine specimens tested by LC-PCR and classified by patient type (n = 122)
| Patient group | No. of patients tested | Distributiona of:
|
Total % positive | |||
|---|---|---|---|---|---|---|
| JCV
|
BKV
|
|||||
| No. | % | No. | % | |||
| Sexual health clinic | 22 | 6 | 27.2 | 1 | 4.5 | 31.7 |
| Pediatric clinic | 26 | 3 | 11.5 | 0 | 0 | 11.5 |
| Antenatal clinic | 20 | 4 | 20.0 | 3 | 15.0 | 35.0 |
| Indigenous health clinic | 10 | 3 | 33.3 | 0 | 0 | 33.3 |
| General adult medical wards | 20 | 8 | 40.0 | 3 | 15.0 | 55.0 |
| Immunocompromised | 24 | 11b | 45.8 | 9b | 37.5 | 83.3 |
| Total | 122 | 35 | 16 | 40.2 | ||
Positive results.
Two samples were positive for both JCV and BKV.
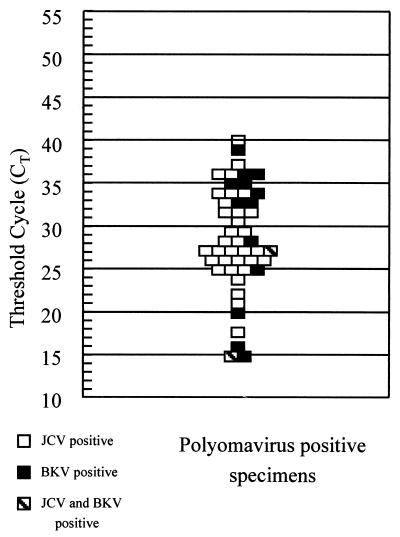
In the LC-PCR, the threshold cycle (CT) was determined as the cycle number at which the sample quantification curve became exponential. The CT values ranged from 21 to 40 cycles for JCV-positive specimens and from 15 to 39 cycles for BKV-positive samples (Fig. 2). No significant difference was observed for the two sample populations, including those positive for both viruses.
FIG. 2.
Detection of JCV and BKV in urine by LC-PCR. The diagram shows the CT for positive polyomavirus samples. Negative samples did not cross the threshold during the 55 PCR cycles.
Testing of 10-fold serial dilutions of BKV and JCV DNAs showed that the LC-PCR could detect dilutions equal to or less than 10−4, whereas the PCR-ELAHA was able to detect dilutions equal to or less than 10−5. This represented a 10-fold difference in relative sensitivity between the two assays, with the PCR-ELAHA proving the more sensitive.
Probes designed to detect nucleotide polymorphisms in two base pairs of the BKV LC-PCR product correctly differentiated JCV from BKV in all LC-PCR-positive specimens by melting curve analysis. The melting temperatures for all JCV and BKV amplification products were 75 and 70°C, respectively, consistent with theoretical melting temperatures.
JCV amplification products obtained in the PCR-ELAHA were sequenced, and the JCV subtypes present were identified as 1A, 1B, 2A, 2B, 2C, and 4. The LC-PCR was able to detect each of these six JCV subtypes in the sample population. Melting curve analysis of the LC-PCR amplification product for each JCV subtype produced identical melting temperatures (75°C).
DISCUSSION
The LightCycler is a newly developed, commercially available system designed to decrease the time of the PCR by monitoring the amplification of target sequences in real time by FRET analysis. This technology represents a significant breakthrough in PCR amplification and amplicon detection compared to the open and contamination-susceptible methods used in conventional PCR, and its benefits over conventional PCR have been documented (13, 14, 22, 23). For our study, this technology offered some significant benefits in diagnosing JCV and BKV infections. The LC-PCR assay could generate results within 1.5 h of receipt of the specimen into the laboratory, significantly faster than the 5 to 6 h required for PCR-ELAHA, and hands-on time was significantly reduced because of the elimination of the need for the additional detection step required by PCR-ELAHA.
Because of the frequency of infection and the risk of disseminated disease in the immunologically compromised host, rapid laboratory detection of polyomavirus has become an important issue in diagnostic virology; in addition, identification of the polyomavirus present has significance for patient management. Traditional methods of diagnosing polyomavirus infections, such as isolation or serologic analysis, are not widely practiced in the routine diagnostic laboratory because of technical limitations or the lack of commercial reagents. Nucleic acid amplification techniques, particularly PCR, have demonstrated superior sensitivity compared to all other diagnostic methods for the detection of polyomaviruses, but the routine implementation of PCR in the clinical laboratory has been impeded by problems of amplicon carryover contamination and time-consuming amplicon detection techniques (14, 24).
In this study, the LC-PCR detected JCV in samples from all patient groups, with the lowest prevalence in the pediatric population and the highest in the immunocompromised population. The high rate of polyomavirus reactivation in immunocompromised patients is well documented, particularly in transplant recipients and AIDS patients (5, 6, 10), which formed the majority of our sample group. Similarly, the prevalence of BKV was highest in our samples from immunocompromised subjects, but this virus was not detected in the pediatric and indigenous health clinic samples. The low prevalence of JCV and BKV in our pediatric population was not surprising, as the subjects in this group were predominantly younger than 4 years, when community exposure to these viruses is limited. In contrast, the low prevalence of BKV in samples from the indigenous health clinic group was probably due to the small number of samples tested, and conclusions about these results are premature. In the antenatal clinic and general adult medical ward sample populations, both viruses were detected at relatively high frequencies, consistent with previous reports of polyomavirus reactivation in pregnant women and in older patients (2, 3).
These results show that the LC-PCR used in this study allows the accurate detection and characterization of polyomaviruses in urine specimens from a diverse patient population. The results of this assay were in complete agreement with the results obtained for the detection of polyomaviruses by the PCR-ELAHA, showing that the LC-PCR is a suitable alternative to conventional PCR. However, we did establish a difference in the limit of detection between the two assays. In an effort to resolve this anomaly, various parameters of the LC-PCR assay were reexamined and optimized. It was found that asymmetric addition of the primers produced optimal results. The addition of the antisense primer in a fourfold excess resulted in a significant increase in fluorescence emitted during the reaction. This procedure also improved the differentiation of amplicon DNA during melting curve analysis. Testing a dilution series of positive DNA samples showed that the LC-PCR remained less sensitive than the conventional PCR by a factor of 10. This result was somewhat surprising but might represent a limitation in the use of hybridization probes for FRET detection of amplicons. The difference in sensitivity could also have been due to thermodynamic differences inherent in the primers and/or probes used in the assay. However, the primer sets for the LC-PCR and the conventional PCR were selected for their similar melting temperatures and thus should have had similar thermodynamic characteristics. Alternatively, differences in sensitivity might have been related to the secondary structure of the target DNA at the primer or probe binding sites.
In characterizing the polyomavirus type present, the LC-PCR failed to identify BKV in three of the five specimens in which both viruses were detected by PCR-ELAHA. This result may have been due to differences in PCR sensitivity between the assays or to competition for hybridization probes because of differences in the relative load of each virus in the clinical specimen. In the three samples in question, the LC-PCR did detect JCV, further suggesting that perhaps JCV was detected in preference to BKV as a result of the hybridization probes having greater sequence homology and therefore greater affinity for the JCV amplicon. The extent to which this limitation applies would depend on the relative loads of JCV and BKV in the sample. This means that the chances of identifying a dual infection would be greatly reduced in circumstances where the concentration of JCV DNA is in significant excess over that of BKV DNA.
The human polyomavirus genome displays a great deal of sequence variation, not only between JCV and BKV but also within the various subtypes of JCV (1). This fact presents considerable difficulties in designing primers and probes for the detection of JCV and BKV by LC-PCR. In this study, we identified conserved binding sites and evaluated PCR primers and hybridization probes for application in a sensitive and specific LC-PCR assay for JCV and BKV. The LC-PCR and PCR-ELAHA targeted different gene sequences on the human polyomavirus genome, yet the results showed good agreement, thereby adding further confidence to the specificity of the assay. Also, the LC-PCR primer and probe target binding sites for JCV proved to be highly conserved, as demonstrated by the ability of the assay to detect a range of JCV subtypes (1A, 1B, 2A, 2B, 2C, and 4). In addition, all BKV and JCV isolates gave the expected melting temperatures on melting curve analysis.
In recent years, many PCR protocols for the detection of infectious agents in the clinical diagnostic laboratory have been described. The advent of real-time PCR using the LightCycler has created new potential to expand the use of PCR in disease diagnosis. The sensitivity and specificity of our human polyomavirus LC-PCR assay, combined with the added benefits of real-time detection, make it particularly suitable for the detection and differentiation of JCV and BKV in a clinical laboratory. Our aims are to implement the polyomavirus LC-PCR as a routine diagnostic assay and to further expand the use of LightCycler technology as a test platform in the diagnostic laboratory.
ACKNOWLEDGMENTS
This work was supported by Royal Children's Hospital Foundation grant 1322-034, which is sponsored by the Woolworth's “Care for Kids” campaign.
We thank Melanie Syrmis for assistance in editing the manuscript.
REFERENCES
- 1.Agostini H T, Ryschkewitsch C F, Stoner G L. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J Clin Microbiol. 1996;34:159–204. doi: 10.1128/jcm.34.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews C A, Daniel R W, Shah K V. Serologic studies of papovavirus infections in pregnant women and renal transplant recipients. Prog Clin Biol Res. 1983;145:133–141. [PubMed] [Google Scholar]
- 3.Andrews C A, Shah K V. Occurrence and significance of papovaviruses BK and JC in the urine. Prog Med Virol. 1989;36:42–61. [PubMed] [Google Scholar]
- 4.Arthur R R, Dagostin S, Shah K V. Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J Clin Microbiol. 1989;27:1214–1219. doi: 10.1128/jcm.27.6.1174-1179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthur R R, Shah K V, Charache P, Saral R. BK and JC virus infections in recipients of bone marrow transplants. J Infect Dis. 1988;158:563–569. doi: 10.1093/infdis/158.3.563. [DOI] [PubMed] [Google Scholar]
- 6.Boubenider S, Hiesse C, Marchand S, Hafi A, Kriaa F, Charpentier B. Post-transplantation polyomavirus infections. J Nephrol. 1999;12:24–29. [PubMed] [Google Scholar]
- 7.Chang D, Wang M, Ou W C, Tsai R T, Fung C Y, Hwang Y J. A simple method for detecting human polyomavirus DNA in urine by the polymerase chain reaction. J Virol Methods. 1996;58:131–136. doi: 10.1016/0166-0934(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 8.Chesters P M, Heritage J, McCance D J. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983;147:676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- 9.Coleman D V, Mackenzie E F, Gardner S D, Poulding J M, Amer B, Russell W J. Human polyomavirus (BK) infection and ureteric stenosis in renal allograft recipients. J Clin Pathol. 1978;31:338–347. doi: 10.1136/jcp.31.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degener A M, Pietropaolo V, Di Taranto C, Rizzuti V, Ameglio F, Cordiali Fei P, Caprilli F, Capitanio B, Sinibaldi L, Orsi N. Detection of JC and BK viral genome in specimens of HIV-1 infected subjects. New Microbiol. 1997;20:115–122. [PubMed] [Google Scholar]
- 11.De Santis R, Azzi A. Duplex polymerase chain reaction for the simultaneous detection of the human polyomavirus BK and JC DNA. Mol Cell Probes. 1996;14:325–330. doi: 10.1006/mcpr.1996.0044. [DOI] [PubMed] [Google Scholar]
- 12.Echavarria M, Forman M, Ticehurst J, Dumler J S, Charache P. PCR method for detection of adenovirus in urine of healthy and human immunodeficiency virus-infected individuals. J Clin Microbiol. 1998;36:3323–3326. doi: 10.1128/jcm.36.11.3323-3326.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espy M J, Teo R, Ross T K, Svien K A, Wold A D, Uhl J R, Smith T F. Diagnosis of varicella-zoster virus infections in the clinical laboratory by LightCycler PCR. J Clin Microbiol. 2000;38:3187–3189. doi: 10.1128/jcm.38.9.3187-3189.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espy M J, Uhl J R, Mitchell P S, Thorvilson J N, Svien K A, Wold A D, Smith T F. Diagnosis of herpes simplex virus infections in the clinical laboratory by LightCycler PCR. J Clin Microbiol. 2000;38:795–799. doi: 10.1128/jcm.38.2.795-799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giraldo G, Beth E, Lee J, de Harven E, Chernesky M. Solid-phase immune electron microscopy–double-antibody technique for rapid detection of papovaviruses. J Clin Microbiol. 1982;15:517–521. doi: 10.1128/jcm.15.3.517-521.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holman R C, Janssen R S, Buehler J W, Zelasky M T, Hooper W C. Epidemiology of progressive multifocal leukoencephalopathy in the United States: analysis of national mortality and AIDS surveillance data. Neurology. 1991;41:1733–1736. doi: 10.1212/wnl.41.11.1733. [DOI] [PubMed] [Google Scholar]
- 17.Knowles W A, Woodroof M, Porter A A, Gardner S D. An indirect immunofluorescence method for detection of infectious BK virus in urine. J Virol Methods. 1989;26:351–354. doi: 10.1016/0166-0934(89)90118-3. [DOI] [PubMed] [Google Scholar]
- 18.Mackay I M, Metharom P, Sloots T P, Wei M Q. Quantitative PCR-ELAHA for the determination of retroviral vector transduction efficiency. Mol Ther. 2001;3:801–808. doi: 10.1006/mthe.2001.0320. [DOI] [PubMed] [Google Scholar]
- 19.Major E O, Amemiya K, Tornatore C S, Houff S A, Berger J R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCance D J. Persistence of animal and human papovaviruses in renal and nervous tissues. Prog Clin Biol Res. 1983;145:343–357. [PubMed] [Google Scholar]
- 21.Nilsen I, Flaegstad T, Traavik T. Detection of specific IgA antibodies against BK virus by ELISA. J Med Virol. 1991;33:89–94. doi: 10.1002/jmv.1890330205. [DOI] [PubMed] [Google Scholar]
- 22.Ohyashiki J H, Suzuki A, Aritaki K, Nagate A, Shoji N, Ohyashiki K, Ojima T, Abe K, Yamamoto K. Use of real-time PCR to monitor human herpesvirus 6 reactivation after allogeneic bone marrow transplantation. Int J Mol Med. 2000;6:427–432. doi: 10.3892/ijmm.6.4.427. [DOI] [PubMed] [Google Scholar]
- 23.Schaade L, Kockelkorn P, Ritter K, Kleines M. Detection of cytomegalovirus DNA in human specimens by LightCycler PCR. J Clin Microbiol. 2000;38:4006–4009. doi: 10.1128/jcm.38.11.4006-4009.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Udaykumar J, Epstein S, Hewlett I K. A novel method employing UNG to avoid carry-over contamination in RNA-PCR. Nucleic Acids Res. 1993;21:3917–3918. doi: 10.1093/nar/21.16.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittwer C T, Herrmann M G, Moss A A, Rasmussen R P. Continuous fluorescence monitoring of rapid cycle DNA amplification. BioTechniques. 1997;22:130–138. doi: 10.2144/97221bi01. [DOI] [PubMed] [Google Scholar]