Abstract
Background
Radiotherapy-associated secondary cancer is an important issue for the treatment of breast cancer (BCa). This study aimed to investigate the molecular mechanism and genetic risk factors for radiation-associated secondary diseases in BCa.
Methods
The differentially expressed genes (DEGs) between preradiation and postradiation BCa samples in the GSE65505 dataset were obtained. The pathways related to the radiation-associated DEGs in the protein–protein interaction (PPI) network modules were identified. miRNAs targeted to the key genes in the PPI network were identified, and their association with BCa prognosis was analyzed.
Results
A total of 136 radiation-associated DEGs preradiation and postradiation BCa samples were screened out. The PPI network consisted of a significant module that consisted of 21 upregulated DEGs that were associated with “hsa04512: ECM–receptor interaction,” “hsa04151: PI3K-Akt signaling pathway,” and “hsa04115: p53 signaling pathway.” Sixteen DEGs, including three collagen genes collagen type I alpha 1 chain (COL1A1), COL3A1, and COL1A2, were enriched in 17 radiation-associated pathways. The three genes were upregulated in BCa tissues compared with controls and were also elevated by radiation. They were targeted by hsa-miR-29a/c, and the expression levels of hsa-miR-29a/c were associated with a poor prognosis of BCa.
Conclusions
The upregulation of COL1A1, COL3A1, and COL1A2 might be genetic risk factors for radiation-associated secondary diseases in BCa.
Keywords: radiotherapy, secondary cancer, breast cancer, Type I collagen, Type III collagen
1. Introduction
Breast cancer (BCa) is the most commonly diagnosed malignant cancer and the leading cause of cancer-related premature mortality in women worldwide [1]. About 2.1 million newly diagnosed cases of BCa were estimated in 2018 [1]. Epidemiologic studies have well established a series of risk factors for BCa, including family history, race, physical inactivity, obesity, genetic variants, and instability [2,3]. However, the treatment of BCa is still an important issue due to the high recurrence and mortality of this disease.
Adjuvant radiotherapy is the standard treatment for BCa patients that are undergoing breast-conservation surgery or mastectomy. Radiotherapy reduces BCa recurrence and death significantly [4,5,6]. Overall, radiotherapy reduces the 10 year risk of first recurrence from 35.0 to 19.3% and decreases the 15 year risk of death from 25.2 to 21.4% [4]. However, there are increasing evidence showing that radiotherapy causes secondary cancers and heart diseases decades later [7,8,9,10]. For instance, patients exposed to radiotherapy for prostate cancer and BCa had an over-time increased risk of bladder, colorectal, lung, and thyroid cancers compared with patients unexposed to radiotherapy [8,10]. In addition, the chest wall symptoms in BCa patients exposed to radiotherapy were worse than that of patients unexposed to radiotherapy [9]. Hodgkin lymphoma patients that were treated with chest radiotherapy have a high risk of BCa [11]. However, the molecular mechanisms of the pathogenesis of radiation-associated secondary cancers are not clear until now.
Research has pointed out that the smoking habit is a risk factor for radiation-associated secondary primary cancer [12]. In addition, there is evidence showing genetic susceptibility to radiation-associated secondary BCa [11]. Hodgkin lymphoma patients that had a high risk of radiation-associated secondary BCa had a higher polygenic risk score that was composed of the features of nine single-nucleotide polymorphisms [11]. For radiation-associated secondary cancers in patients with BCa, however, the molecular mechanism has not been reported until now.
For filling this gap, we performed this study to identify the radiation-associated genes in BCa. The identification of radiation-related genetic susceptibility and genetic risk factors would be of great value for better understanding the molecular mechanisms underlying the radiation-associated secondary cancers in patients with BCa.
2. Materials and methods
2.1. Microarray data
The mRNA expression dataset GSE65505 was downloaded from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/). GSE65505 (GPL17586 platform, [HTA-2_0] Affymetrix Human Transcriptome Array 2.0 [transcript (gene) version]) consisted of 62 BCa tumor samples, including 29 paired preradiation and postradiation tumor samples: 6, 8, and 15 patients treated with 15 Gy, 18 Gy, and 21 Gy intensity-modulated radiotherapy. BCa samples were extracted from women (aged >55 years old) that had clinically node negative, estrogen receptors (ER)+ and/or PR+, Human epidermal growth factor receptor-2 (HER2−), T1 invasive carcinomas or low-intermediate grade in situ disease (≤2 cm).
2.2. Data processing and identification of radiation-associated genes
The series matrix files from paired preradiation and postradiation tumor samples (n = 58) were downloaded and preprocessed using the Limma package (version 3.34.0; https://bioconductor.org/packages/release/bioc/html/limma.html). Then the differentially expressed genes (DEGs) between paired preradiation and postradiation tumor samples were identified using the Limma package with the criteria of p < 0.05 and |log2(fold change, FC)| ≥ 0.585 (|FC| ≥ 1.5). The sample hierarchical clustering analysis was performed for DEGs using the pheatmap package (version 1.0.8; https://cran.r-project.org/package=pheatmap). The genes common to 15, 18, and 21 Gy radiotherapy-induced DEGs were identified using the Venn diagram analysis and were regarded as radiation-associated genes in BCa.
2.3. Enrichment analysis of radiation-associated genes
To illuminate the radiation-induced molecular functional changes in BCa, the Gene Ontology biological processes and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways that were associated with the radiation-associated genes DEGs were identified. Functional enrichment analysis was performed using the clusterprofiler package (https://github.com/GuangchuangYu/clusterProfiler). The threshold for selecting the significant biological processes and pathways were set at adjusted p value <0.05 and gene count ≥2.
2.4. Construction of protein–protein interaction network (PPI) of radiation-associated DEGs
The interactions between the products of radiation-associated DEGs in the GSE65505 dataset were predicted in the STRING database (Version: 10.0, http://www.string-db.org/) with the default parameter (score >0.4). Then the PPI network of radiation-associated genes was constructed using the Cytoscape software (version 3.4.0, http://chianti.ucsd.edu/cytoscape-3.4.0/). The important module in the PPI network (score >5) was identified using the MCODE plugin in Cytoscape. Radiation-associated genes that were included in the important modules were subjected to the functional enrichment analysis using the clusterprofiler package. The significant pathways that were associated with the genes in important module were selected using the same criteria as mentioned above.
2.5. Identification of BCa-associated pathways
To select the key pathways related to the radiation-associated DEGs in the important module, the BCa-associated pathways in Comparative Toxicogenomics Database (CTD, http://ctdbase.org/) were identified using the searching term of “breast neoplasm.” The items overlapped between the CTD database and the KEGG pathways were regarded as the key pathways in BCa, and the enriched genes were regarded as the key radiation-associated DEGs in BCa.
2.6. Construction of miRNA–target network
The miRNA–target pairs of the aforementioned key radiation-associated DEGs in BCa were predicted using the Webgestal online tool [13] using the overrepresentation enrichment analysis methods. Then the miRNA–target pairs with the p value of less than 0.05 were selected and used for the construction of miRNA–target network. The network was visualized using the Cytoscape software.
2.7. Identification of prognosis-associated genes and miRNAs in BCa
To investigate the association of genes and miRNAs with the prognosis of BCa, the correlation of aforementioned radiation-associated genes and miRNAs in the miRNA–target network with the overall survival (OS) of patients with breast invasive carcinoma (BRCA) in the Cancer Genome Atlas (TCGA) were analyzed using the user-friendly and interactive web resource of UALCAN (http://ualcan.path.uab.edu/index.html). Significantly difference was identified using the criterion of logRank p < 0.05.
3. Results
3.1. Identification of radiation-associated genes in BCa in GSE65505
A total of 405, 204, and 325 DEGs were identified in BCa tumor samples receiving 15 Gy, 18 Gy, and 21 Gy intensity-modulated radiotherapy in the GSE65505 dataset, respectively (Figure 1a). Hierarchical clustering analysis demonstrated the distinct expression profiles of these DEGs in samples preradiation and postradiation (Figure 1b). Venn diagram analysis showed that common 133 DEGs were upregulated by the three radiotherapies, and only three DEGs were commonly downregulated (Figure 1c). Then, 136 radiation-associated DEGs in BCa were identified in this study (Table A1).
Figure 1.
The statistics and clustering of the DEGs in BCa preradiation and postradiation: (a) the volcano plot of the DEGs in BCa samples between preradiotherapy and postradiotherapy (15 Gy, 18 Gy, and 21 Gy), (b) the hierarchical clustering analysis of DEGs, and (c) the Venn diagram of the upregulated and downregulated radiation-associated genes in BCa in the GSE65505 dataset.
3.2. Enrichment analysis of upregulated radiation-associated DEGs in BCa
The functional enrichment analysis of the 136 radiation-associated DEGs indicated that these genes were enriched into 563 biological processes that were associated with extracellular matrix (ECM) organization, inflammatory response, response to metal ion, neutrophil-mediated immunity, and cell chemotaxis, and 38 KEGG pathways including advanced glycation end-receptor for dvanced glycation end products (AGE-RAGE) signaling pathway in diabetic complication, p53 signaling pathway, PI3K-Akt signaling pathway, and ECM–receptor interaction. The top ten biological processes and KEGG pathways are shown in Table A2.
3.3. PPI network of radiation-associated DEGs in BCa
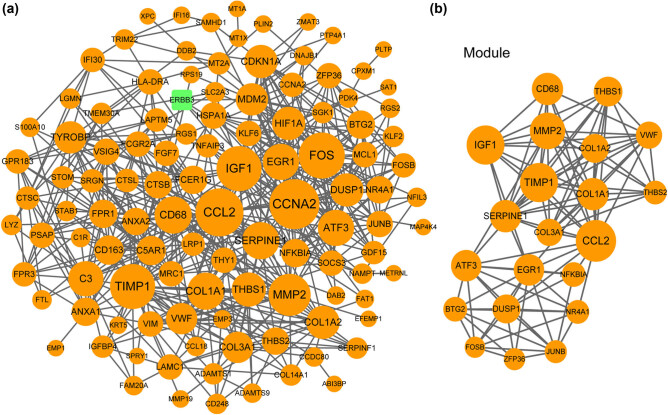
The PPI network that derived from the 136 radiation-associated genes was composed of 112 nodes (products of genes) and 529 lines (interaction pairs; Figure 2a). One module of a score of 9.9 and 21 upregulated radiation-associated DEGs was identified from the PPI network (Figure 2b). The interaction degree of these nodes is shown in Table 1. The insulin like growth factor 1 (IGF1), matrix metallopeptidase 2 (MMP2), collagen type I alpha 1 chain (COL1A1), COL1A2, and COL3A1 gene had the interaction degrees of 30, 27, 22, 18, and 16, respectively.
Figure 2.
The PPI network and the important module of the radiation-associated genes in BCa: (a) the PPI network consisted of 111 upregulated and 1 downregulated radiation-associated genes in BCa and (b) the important module (score = 9.9, 21 nodes) identified using MCODE. Node size indicates the interaction degree. The higher the degree, the larger the node size is. The one downregulated radiation-associated gene is presented by blue square.
Table 1.
The list and interaction degree of the 21 upregulated radiation-associated genes in the module of the PPI network of breast cancer
| Nodes | Regulation | Degree | Nodes | Regulation | Degree |
|---|---|---|---|---|---|
| CCL2 | UP | 33 | DUSP1 | UP | 18 |
| TIMP1 | UP | 30 | COL3A1 | UP | 16 |
| IGF1 | UP | 30 | VWF | UP | 16 |
| MMP2 | UP | 27 | BTG2 | UP | 13 |
| COL1A1 | UP | 22 | NFKBIA | UP | 13 |
| CD68 | UP | 22 | THBS2 | UP | 12 |
| SERPINE1 | UP | 22 | NR4A1 | UP | 12 |
| EGR1 | UP | 21 | JUNB | UP | 11 |
| ATF3 | UP | 21 | ZFP36 | UP | 10 |
| THBS1 | UP | 19 | FOSB | UP | 9 |
| COL1A2 | UP | 18 |
CCL2: C-C motif chemokine ligand 2; MMP2: matrix metallopeptidase 2; COL1A1: collagen type I alpha 1 Chain; SERPINE1: serpin family E member 1; EGR1: early growth response 1; COL3A1: collagen type III alpha 1 chain; THBS1: thrombospondin 1; VWF: von Willebrand factor; THBS2: thrombospondin 2; NFKBIA: nuclear factor kappa B inhibitor alpha; FOSB: FosB proto-oncogene, AP-1 transcription factor subunit; DUSP1: dual specificity phosphatase 1.
3.4. Pathways that related to radiation-associated genes
The 21 upregulated radiation-associated DEGs in the module were associated with 19 KEGG pathways such as “hsa04933: AGE-RAGE signaling pathway in diabetic complications,” “hsa04512: ECM–receptor interaction,” “hsa04151: PI3K-Akt signaling pathway,” and “hsa04115: p53 signaling pathway” (Table 2). The function properties of the 21 radiation-associated genes were basically in line with the 136 radiation-associated DEGs in BCa. Among the 19 pathways, 17 pathways that were common to BCa-related pathways in the CTD database were regarded as the key radiation-associated pathways in BCa. Moreover, 16 upregulated radiation-associated DEGs (including IGF1, MMP2, COL1A1, COL3A1, and ZFP36) were involved in the 17 key radiation-associated pathways (Table 2).
Table 2.
The list of top 10 biological processes and KEGG pathways related to the radiation-associated genes in the module of the PPI network of breast cancer
| ID | Description | Count | p value | p. adjust | Gene symbol |
|---|---|---|---|---|---|
| *hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 7 | 2.53 × 10−9 | 2.71 × 10−7 | CCL2, MMP2, COL1A1, SERPINE1, EGR1, COL1A2, and COL3A1 |
| *hsa04512 | ECM–receptor interaction | 5 | 1.91 × 10−6 | 1.02 × 10−4 | COL1A1, THBS1, COL1A2, VWF, and THBS2 |
| *hsa04510 | Focal adhesion | 6 | 6.19 × 10−6 | 2.21 × 10−4 | IGF1, COL1A1, THBS1, COL1A2, VWF, and THBS2 |
| hsa04926 | Relaxin signaling pathway | 5 | 1.26 × 10−5 | 3.05 × 10−4 | MMP2, COL1A1, COL1A2, COL3A1, and NFKBIA |
| *hsa04151 | PI3K-Akt signaling pathway | 7 | 1.43 × 10−5 | 3.05 × 10−4 | IGF1, COL1A1, THBS1, COL1A2, VWF, THBS2, and NR4A1 |
| *hsa05205 | Proteoglycans in cancer | 5 | 1.17 × 10−4 | 2.08 × 10−3 | IGF1, MMP2, COL1A1, THBS1, and COL1A2 |
| *hsa04611 | Platelet activation | 4 | 2.16 × 10−4 | 3.20 × 10−3 | COL1A1, COL1A2, COL3A1, and VWF |
| *hsa05144 | Malaria | 3 | 2.40 × 10−4 | 3.20 × 10−3 | CCL2, THBS1, and THBS2 |
| *hsa04115 | p53 signaling pathway | 3 | 7.04 × 10−4 | 8.37 × 10−3 | IGF1, SERPINE1, and THBS1 |
| hsa05165 | Human papillomavirus infection | 5 | 1.05 × 10-3 | 1.13 × 10-2 | COL1A1, THBS1, COL1A2, VWF, and THBS2 |
| *hsa04657 | IL-17 signaling pathway | 3 | 1.53 × 10−3 | 1.40 × 10−2 | CCL2, NFKBIA, and FOSB |
| *hsa04974 | Protein digestion and absorption | 3 | 1.58 × 10−3 | 1.40 × 10−2 | COL1A1, COL1A2, and COL3A1 |
| *hsa05142 | Chagas disease (American trypanosomiasis) | 3 | 1.93 × 10−3 | 1.48 × 10−2 | CCL2, SERPINE1, and NFKBIA |
| *hsa05146 | Amoebiasis | 3 | 1.93 × 10−3 | 1.48 × 10−2 | COL1A1, COL1A2, and COL3A1 |
| *hsa04066 | HIF-1 signaling pathway | 3 | 2.34 × 10−3 | 1.67 × 10−2 | TIMP1, IGF1, and SERPINE1 |
| *hsa04668 | TNF signaling pathway | 3 | 2.53 × 10−3 | 1.69 × 10−2 | CCL2, NFKBIA, and JUNB |
| *hsa04380 | Osteoclast differentiation | 3 | 3.69 × 10−3 | 2.32 × 10−2 | NFKBIA, JUNB, and FOSB |
| *hsa05219 | Bladder cancer | 2 | 4.55 × 10−3 | 2.62 × 10−2 | MMP2 and THBS1 |
| *hsa05418 | Fluid shear stress and atherosclerosis | 3 | 4.65 × 10−3 | 2.62 × 10−2 | CCL2, MMP2, and DUSP1 |
*Notes the overlapping KEGG pathways between the comparative toxicogenomics database and that related to the 21 radiation-sensitive genes.
3.5. Construction of the radiation-associated miRNA–target network in BCa
The miRNA–target pairs of the aforementioned 16 radiation-associated DEGs in BCa were predicted, and the corresponding radiation-associated miRNA–target network was constructed (Figure 3). This miRNA–target network consisted of ten radiation-associated DEGs, including IGF1, MMP2, COL1A1, COL3A1, and ZFP36, and nine miRNAs, including hsa-miR-29a, hsa-miR-29b, hsa-miR-29c, hsa-miR-196a, and hsa-miR-196b. In this network, five radiation-associated DEGs, including IGF1, MMP2, COL1A1, COL3A1, and ZFP36, were simultaneously regulated by hsa-miR-29a, hsa-miR-29b, and hsa-miR-29c. hsa-miR-196a and hsa-miR-196b both targeted to three collagen genes, including COL1A1, COL3A1, and COL1A2. The other four radiation-associated DEGs, including JunB proto-oncogene (JUNB), BTG antiproliferation factor 2 (BTG2), FosB proto-oncogene, AP-1 transcription factor subunit (FOSB), and nuclear receptor subfamily 4 group A member 1 (NR4A1), were regulated by one or two miRNAs, respectively (Figure 3).
Figure 3.

The miRNA–target regulatory network involving the radiation-associated genes in the key pathways related to BCa. Node size indicates the interaction degree. The higher the degree, the larger the node size is. The miRNA–target interaction pairs were identified using the Webgestal online tool. The miRNA–target pairs with a p < 0.05 are used for the construction of the miRNA–target network.
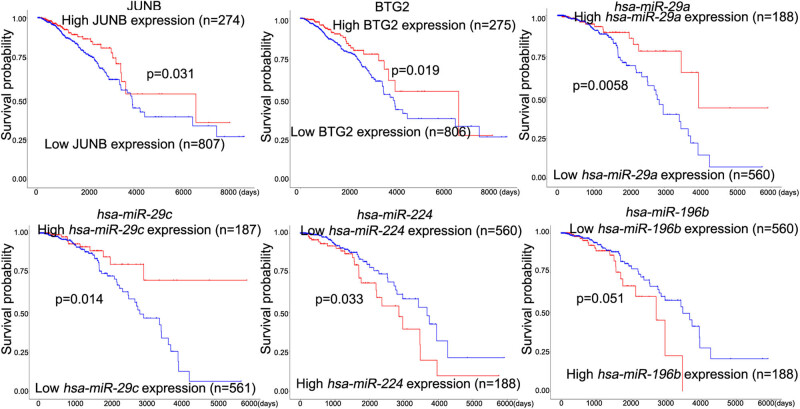
3.6. The association of radiation-associated genes with the prognosis of BCa
Based on the analysis in the UALCAN database, we found that two genes, BTG2 and JUNB, and four miRNAs, including hsa-miR-29a, hsa-miR-29c, hsa-miR-224, and hsa-miR-196b, were significantly associated with the prognosis in BCa (Figure 4). The high expression levels of JUNB (p = 0.031), BTG2 (p = 0.019), hsa-miR-29a (p = 0.0058), and hsa-miR-29c (p = 0.014) as well as the low expression levels of hsa-miR-224 (p = 0.033) and hsa-miR-196b (p = 0.051) were associated with a low survival probability in the TCGA BRCA patients (Figure 4).
Figure 4.
The survival analysis of genes and miRNAs in the miRNA–target regulatory network. Survival analysis was performed using the UALCAN online tool and based on the TCGA cohort (breast invasive carcinoma).
Besides, we found three genes, including COL1A1, COL3A1, and COL1A2, were significantly upregulated in TCGA BRCA tumor samples compared with normal controls (Figure 5), whereas the other seven genes were not significantly changed in BCa tumor samples (data now shown).
Figure 5.
The expression of the COL1A1, COL3A1, and COL1A2 genes in the TCGA breast cancer tumor and normal control samples. The statistical differences were analyzed using the UALCAN online tool. Data was expressed as median and range (minimum to maximum).
4. Discussion
The increasing incidence of radiation-associated secondary cancers is challenging the treatment strategies for human solid cancers. Based on the tumorigenicity [11], we speculated that the mechanisms responsible for radiation-associated secondary cancers involve the dysregulation of a cluster of genetic factors. As expected, we identified that the upregulation of three collagen genes (COL1A1, COL3A1, and COL1A2), BTG2, and JUNB might be responsible for the radiation-associated secondary cancers in patients with BCa.
COL1A1 and COL3A1, respectively, encodes the α1 chain of Type I and III collagen, both of which are the fibril-forming collagens [14,15]. Type I and Type III procollagens are crucial components of ECM, which is important for tumor microenvironment [14]. They act important roles in the proliferation and/or metastasis of cancer cells. Previous study showed that the Type I and Type III procollagens were elevated in human scirrhous carcinoma of breast with a spatial specificity [16]. In situ hybridization showed that the expression levels of the Type I and Type III procollagens were decreased with the increased distance between the fibroblasts and tumor cells [16]. The upregulation and association of COL1A1, COL3A1, and COL1A2 with collagen prolyl-4-hydroxylase α subunit 2 (P4HA2) had been reported in BCa [17,18,19]. In addition, the other factors that related to ECM or collagens, including MMP2, CCL2, and tissue inhibitor of metalloproteinase-1 (TIMP1), were also identified to be upregulated in BCa tissues compared with control [18]. Here, in our present study, we identified that the exposure to radiotherapy elevated the expression of COL1A1, COL3A1, and COL1A2 in BCa tissues rather than decreased them. According to the above studies, we assumed that the consistent upregulation of the three genes might of great value for exploring the development of radiation-associated secondary nonbreast diseases in BCa.
There are tremendous evidence showing that COL1A1, COL3A1, COL1A2, or P4HA2 promotes the metastasis of BCa and contributes to a poor survival in patients with ER+ BCa [14,18]. By contrast, the inhibition of COL1A1 or P4HA2 counteracted the metastasis and suppressed the proliferation of the BCa cells [14,18]. The study by Srour et al. [20] showed that MMP2, COL1A1, COL3A1, and COL1A2 had lower expression levels in lymph node metastasis compared with triple-negative BCa (TNBC). This finding was in line with the fact that from Gan. [16] who showed that the expression of the Type I and Type III procollagens were decreased with the increased distance between fibroblasts and tumor cells. The interesting evidence was that the elevated COL1A1 expression in BCa had been reported to be correlated with a better response to cisplatin-based chemotherapy [14]. A recent study by Liu et al. [21] showed that there was an opposite conclusion that COL1A1 expression was negatively correlated with radiosensitivity in cervical cancer. Liu et al. [21] reported that the COL1A1 activation could inhibit X-ray radiation-induced apoptosis in cervical cancer. Our present study showed that COL1A1 and COL3A1 were targeted by hsa-miR-29a/b/c that were decreased in radio-resistant nasopharyngeal carcinoma cells [22]. The expression of COL1A1 exerted a radio-resistance effect in nasopharyngeal carcinoma cells [22]. Accordingly, we suspected that the consistent radiation-associated upregulation of the COL1A1, COL3A1, and COL1A2 genes in BCa tissues might be used as genetic susceptibility and risk factors for radio-resistance. This might be related to the radiation-associated secondary nonbreast diseases in patients with BCa.
The association of hsa-miR-29 family members with tumorigenesis and drug-resistance in BCa cells had been proven previously [23,24,25]. hsa-miR-29a acts an oncogenic role [23,24,26], whereas the hsa-miR-29c exhibits a tumor suppressor role in BCa [25,26]. However, we found that the low expression levels of hsa-miR-29a/c were associated with a good survival outcome of BCa. Also, both of them targeted to the upregulated COL1A1 and COL3A1 genes. These results suggested that the predicted miRNA–mRNA regulatory networks, including hsa-miR-29a/c–COL1A1/COL3A1, might have crucial roles in the progression of BCa and the development of radiation-associated secondary diseases.
5. Conclusion
In summary, this present study showed that radiotherapy significantly upregulated the expression of COL1A1, COL3A1, and COL1A2 genes in BCa tumor tissues. The upregulation of them might be risk factors for radiation-associated secondary nonbreast diseases in patients with BCa. However, the association of COL1A1, COL3A1, and COL1A2 upregulation with radiation-associated secondary diseases should be validated using large cohort trials.
Abbreviations
- BCa
breast cancer
- BRCA
breast invasive carcinoma
- CTD
comparative Toxicogenomics Database
- DEGs
differentially expressed genes
- ECM
extracellular matrix
- GEO
gene expression omnibus
- IGF1
insulin like growth factor 1
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MMP2
matrix metallopeptidase 2
- OS
overall survival
- PPI
protein–protein interaction
- TIMP1
tissue inhibitor of metalloproteinase-1
- TNBC
triple-negative breast cancer
Appendix
Table A1.
The list of the radiation-associated genes in breast cancer (BCa) in the GSE65505 dataset
| Gene symbol | |||
|---|---|---|---|
| EGR1 | HSPA1A | COL1A2 | FCER1G |
| ZFP36 | S100A10 | SNORD99 | C1R |
| FOS | HIF1A | SNORD114-3 | COL1A1 |
| MDM2 | PTP4A1 | CD68 | SGK1 |
| FOSB | MMP2 | COL14A1 | CTSC |
| CCL2 | SOCS3 | LGMN | PSAP |
| MT1A | PDK4 | JUN | VWF |
| LYZ | RGS2 | COL3A1 | XPC |
| ANXA1 | MT1M | ABI3BP | IGF1 |
| RASD1 | NAMPT | THBS2 | FCGR2C |
| TNFAIP3 | ADAMTS9 | MAP4K4 | TMEM30A |
| MT2A | GEM | NR4A1 | ATF3 |
| CCN1 | HLA-DRA | LRP1 | SAMHD1 |
| SERPINE1 | SNORD92 | EMP3 | FPR1 |
| MT1X | MT1JP | IL6-AS1 | TYROBP |
| KLF6 | LAPTM5 | MMP19 | C5AR1 |
| SLC2A3 | MCL1 | SERPINF1 | CPXM1 |
| SRGN | PLTP | CTSL | FGF7 |
| KRT5 | ANXA2 | ZMAT3 | H19 |
| DUSP1 | FAT1 | DAB2 | SLIT3 |
| NFKBIA | NFIL3 | IFI30 | VGLL3 |
| GPR183 | CD163 | XGY2 | SNORD114-21 |
| EMP1 | VIM | KLF2 | THY1 |
| THBS1 | TRIM22 | PTCSC1 | EDA2R |
| CCDC80 | EFEMP1 | GDF15 | PLIN2 |
| YBX3 | CD248 | SPRY1 | METRNL |
| STOM | FTL | FAM20A | VSIG4 |
| SNORD114-14 | CDKN1A | ADAMTS1 | DRAM1 |
| IFI16 | SNORD114-12 | ECSCR | CTSB |
| RPS19 | DNAJB1 | C3 | STAB1 |
| SAT1 | RGS1 | IGFBP4 | CCL18 |
| BTG2 | SNORD114-1 | FPR3 | *POTEKP |
| NEAT1 | JUNB | LAMC1 | *ERBB3 |
| DDB2 | MRC1 | TIMP1 | *CYP4X1 |
*The three downregulated radiation-sensitive genes.
Table A2.
The top 15 gene ontology biological processes and the KEGG pathways related to the upregulated radiation-associated genes in breast cancer (BCa)
| ID | Description | Count | P value | P adjusted | Gene symbol |
|---|---|---|---|---|---|
| GO:0030198 | Extracellular matrix organization | 18 | 1.18 × 10−10 | 2.15 × 10−7 | SERPINE1, THBS1, MMP2, COL1A2, TIMP1, COL1A1, et al. |
| GO:0043062 | Extracellular structure organization | 19 | 1.42 × 10−10 | 2.15 × 10−7 | SERPINE1, THBS1, MMP2, COL1A2, TIMP1, COL1A1, et al. |
| GO:0071674 | Mononuclear cell migration | 9 | 1.46 × 10−8 | 1.48 × 10−5 | SERPINE1, DUSP1, THBS1, RPS19, C5AR1, CCL18, et al. |
| GO:0050727 | Regulation of inflammatory response | 17 | 5.81 × 10−8 | 4.41 × 10−5 | SERPINE1, NEAT1, SOCS3, SERPINF1, IGF1, CCL18, et al. |
| GO:0070997 | Neuron death | 14 | 1.77 × 10−7 | 7.54 × 10−5 | EGR1, FOS, CCL2, BTG2, LRP1, SERPINF1, ERBB3, et al. |
| GO:0002683 | Negative regulation of immune system process | 16 | 1.78 × 10−7 | 7.54 × 10−5 | ZFP36, THBS1, COL3A1, FCER1G, THY1, VSIG4, et al. |
| GO:0071276 | Cellular response to cadmium ion | 6 | 2.16 × 10−7 | 7.54 × 10−5 | FOS, MT1A, MT2A, MT1X, MT1M, JUN |
| GO:0031589 | Cell-substrate adhesion | 14 | 2.18 × 10−7 | 7.54 × 10−5 | SERPINE1, THBS1, COL3A1, MAP4K4, COL1A1, et al. |
| GO:0030099 | Myeloid cell differentiation | 15 | 2.58 × 10−7 | 7.54 × 10−5 | ZFP36, THBS1, HIF1A, JUNB, KLF2, FCER1G, et al. |
| GO:0010038 | Response to metal ion | 14 | 3.06 × 10−7 | 7.54 × 10−5 | FOSB, THBS1, HIF1A, JUNB, JUN, SERPINF1, et al. |
| hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 8 | 1.03 × 10−5 | 9.25 × 10−4 | EGR1, CCL2, SERPINE1, MMP2, COL1A2, JUN, et al. |
| hsa04115 | p53 signaling pathway | 7 | 1.05 × 10−5 | 9.25 × 10−4 | MDM2, SERPINE1, THBS1, DDB2, IGF1, et al. |
| hsa04151 | PI3K-Akt signaling pathway | 14 | 2.02 × 10−5 | 1.18 × 10−3 | THBS1, COL1A2, THBS2, COL1A1, IGF1, ERBB3, et al. |
| hsa05205 | Proteoglycans in cancer | 10 | 6.11 × 10−5 | 2.03 × 10−3 | THBS1, MMP2, COL1A1, IGF1, ERBB3, et al. |
| hsa04380 | Osteoclast differentiation | 8 | 6.22 × 10−5 | 2.03 × 10−3 | FOS, FOSB, JUNB, JUN, FCGR2C, TYROBP, et al. |
| hsa05150 | Staphylococcus aureus infection | 7 | 6.92 × 10−5 | 2.03 × 10−3 | FPR3, C1R, FCGR2C, FPR1, C5AR1, et al. |
| hsa05140 | Leishmaniasis | 6 | 1.62 × 10−4 | 3.58 × 10−3 | FOS, NFKBIA, HLA-DRA, JUN, C3, FCGR2C |
| hsa04612 | Antigen processing and presentation | 6 | 1.74 × 10−4 | 3.58 × 10−3 | HSPA1A, HLA-DRA, LGMN, CTSL, IFI30, CTSB |
| hsa04668 | TNF signaling pathway | 7 | 1.83 × 10−4 | 3.58 × 10−3 | FOS, CCL2, SOCS3, JUNB, JUN, et al. |
| hsa04145 | Phagosome | 8 | 2.07 × 10−4 | 3.64 × 10−3 | THBS1, THBS2, CTSL, C3, C1R, FCGR2C, et al. |
Footnotes
Funding information: This study is supported by Zhejiang Provincial Natural Science Foundation & Zhejiang Society for Mathematical Medicine (LSY19H160002).
Author contributions: Yao GR: conception, study design, data acquisition, analysis and important interpretation, manuscript drafting, and approval of final manuscript; Zhao KY: study design, data acquisition, and analysis; Bao KK: data acquisition and analysis; Bu LY: conception, data analysis and interpretation, manuscript revision, and approval of final manuscript; and Li J: manuscript revision and data acquisition.
Conflict of interest: The authors declare that they have no competing interests.
Data availability statement: The microarray dataset is available at the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/; accession number GSE65505.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed]; Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- [2].Paz M, de Alencar M, Gomes JA, Da CMK, Islam MT, Ali ES, et al. Correlations between risk factors for breast cancer and genetic instability in cancer patients-a clinical perspective study. Front Genet. 2017;8:236. [DOI] [PMC free article] [PubMed]; Paz M, de Alencar M, Gomes JA, Da CMK, Islam MT, Ali ES. et al. Correlations between risk factors for breast cancer and genetic instability in cancer patients-a clinical perspective study. Front Genet. 2017;8:236. doi: 10.3389/fgene.2017.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Coughlin SS. Epidemiology of breast cancer in women. Adv Exp Med Biol. 2019;1152:9–29. [DOI] [PubMed]; Coughlin SS. Epidemiology of breast cancer in women. Adv Exp Med Biol. 2019;1152:9–29. doi: 10.1007/978-3-030-20301-6_2. [DOI] [PubMed] [Google Scholar]
- [4].Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10 year recurrence and 15 year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–16. [DOI] [PMC free article] [PubMed]; Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M. et al. Effect of radiotherapy after breast-conserving surgery on 10 year recurrence and 15 year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–16. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang SL, Fang H, Song YW, Wang WH, Hu C, Liu YP, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019;20(3):352–60. [DOI] [PubMed]; Wang SL, Fang H, Song YW, Wang WH, Hu C, Liu YP. et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019;20(3):352–60. doi: 10.1016/S1470-2045(18)30813-1. [DOI] [PubMed] [Google Scholar]
- [6].Klein J, Tran W, Watkins E, Vesprini D, Wright FC, Look HN, et al. Locally advanced breast cancer treated with neoadjuvant chemotherapy and adjuvant radiotherapy: a retrospective cohort analysis. BMC Cancer. 2019;19(1):306. [DOI] [PMC free article] [PubMed]; Klein J, Tran W, Watkins E, Vesprini D, Wright FC, Look HN. et al. Locally advanced breast cancer treated with neoadjuvant chemotherapy and adjuvant radiotherapy: a retrospective cohort analysis. BMC Cancer. 2019;19(1):306. doi: 10.1186/s12885-019-5499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Taylor C, Correa C, Duane FK, Aznar MC, Anderson SJ, Bergh J, et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35(15):1641–9. [DOI] [PMC free article] [PubMed]; Taylor C, Correa C, Duane FK, Aznar MC, Anderson SJ, Bergh J. et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35(15):1641–9. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wallis CJ, Mahar AL, Choo R, Herschorn S, Kodama RT, Shah PS, et al. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ. 2016;352:i851. [DOI] [PMC free article] [PubMed]; Wallis CJ, Mahar AL, Choo R, Herschorn S, Kodama RT, Shah PS. et al. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ. 2016;352:i851. doi: 10.1136/bmj.i851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Velikova G, Williams LJ, Willis S, Dixon JM, Loncaster J, Hatton M, et al. Quality of life after postmastectomy radiotherapy in patients with intermediate-risk breast cancer (SUPREMO): 2 year follow-up results of a randomised controlled trial. Lancet Oncol. 2018;19(11):1516–29. [DOI] [PubMed]; Velikova G, Williams LJ, Willis S, Dixon JM, Loncaster J, Hatton M. et al. Quality of life after postmastectomy radiotherapy in patients with intermediate-risk breast cancer (SUPREMO): 2 year follow-up results of a randomised controlled trial. Lancet Oncol. 2018;19(11):1516–29. doi: 10.1016/S1470-2045(18)30515-1. [DOI] [PubMed] [Google Scholar]
- [10].Grantzau T, Overgaard J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: a systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother Oncol. 2016;121(3):402–13. [DOI] [PubMed]; Grantzau T, Overgaard J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: a systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother Oncol. 2016;121(3):402–13. doi: 10.1016/j.radonc.2016.08.017. [DOI] [PubMed] [Google Scholar]
- [11].Opstal-van WA, de Haan HG, Hauptmann M, Schmidt MK, Broeks A, Russell NS, et al. Genetic susceptibility to radiation-induced breast cancer after Hodgkin lymphoma. Blood. 2019;133(10):1130–9. [DOI] [PMC free article] [PubMed]; Opstal-van WA, de Haan HG, Hauptmann M, Schmidt MK, Broeks A, Russell NS. et al. Genetic susceptibility to radiation-induced breast cancer after Hodgkin lymphoma. Blood. 2019;133(10):1130–9. doi: 10.1182/blood-2018-07-862607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].DiMarzio P, Peila R, Dowling O, Timony DM, Balgobind A, Lee LN, et al. Smoking and alcohol drinking effect on radiotherapy associated risk of second primary cancer and mortality among breast cancer patients. Cancer Epidemiol. 2018;57:97–103. [DOI] [PubMed]; DiMarzio P, Peila R, Dowling O, Timony DM, Balgobind A, Lee LN. et al. Smoking and alcohol drinking effect on radiotherapy associated risk of second primary cancer and mortality among breast cancer patients. Cancer Epidemiol. 2018;57:97–103. doi: 10.1016/j.canep.2018.10.002. [DOI] [PubMed] [Google Scholar]
- [13].Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33(Web Server issue):W741–8. [DOI] [PMC free article] [PubMed]; Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33(Web Server issue):W741–8. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu J, Shen JX, Wu HT, Li XL, Wen XF, Du CW, et al. Collagen 1A1 (COL1A1) promotes metastasis of breast cancer and is a potential therapeutic target. Discov Med. 2018;25(139):211–23. [PubMed]; Liu J, Shen JX, Wu HT, Li XL, Wen XF, Du CW. et al. Collagen 1A1 (COL1A1) promotes metastasis of breast cancer and is a potential therapeutic target. Discov Med. 2018;25(139):211–23. [PubMed] [Google Scholar]
- [15].Kuivaniemi H, Tromp G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene. 2019;707:151–71. [DOI] [PMC free article] [PubMed]; Kuivaniemi H, Tromp G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene. 2019;707:151–71. doi: 10.1016/j.gene.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gan YB. Expression of type I and type III procollagen genes in human scirrhous carcinoma of breast by in situ hybridization. Zhonghua Zhong Liu Za Zhi. 1992;14(5):348–50. [PubMed]; Gan YB. Expression of type I and type III procollagen genes in human scirrhous carcinoma of breast by in situ hybridization. Zhonghua Zhong Liu Za Zhi. 1992;14(5):348–50. [PubMed] [Google Scholar]
- [17].Wang R, Fu L, Li J, Zhao D, Zhao Y, Yin L. Microarray analysis for differentially expressed genes between stromal and epithelial cells in development and metastasis of invasive breast cancer. J Comput Biol. 2020;27(12):1631–43. [DOI] [PubMed]; Wang R, Fu L, Li J, Zhao D, Zhao Y, Yin L. Microarray analysis for differentially expressed genes between stromal and epithelial cells in development and metastasis of invasive breast cancer. J Comput Biol. 2020;27(12):1631–43. doi: 10.1089/cmb.2019.0154. [DOI] [PubMed] [Google Scholar]
- [18].Xiong G, Deng L, Zhu J, Rychahou PG, Xu R. Prolyl-4-hydroxylase α subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer. 2014;14:1. [DOI] [PMC free article] [PubMed]; Xiong G, Deng L, Zhu J, Rychahou PG, Xu R. Prolyl-4-hydroxylase α subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer. 2014;14:1. doi: 10.1186/1471-2407-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen Y, Pan Y, Ji Y, Sheng L, Du X. Network analysis of differentially expressed smoking-associated mRNAs, lncRNAs and miRNAs reveals key regulators in smoking-associated lung cancer. Exp Ther Med. 2018;16(6):4991–5002. [DOI] [PMC free article] [PubMed]; Chen Y, Pan Y, Ji Y, Sheng L, Du X. Network analysis of differentially expressed smoking-associated mRNAs, lncRNAs and miRNAs reveals key regulators in smoking-associated lung cancer. Exp Ther Med. 2018;16(6):4991–5002. doi: 10.3892/etm.2018.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Srour MK, Gao B, Dadmanesh F, Carlson K, Qu Y, Deng N, et al. Gene expression comparison between primary triple-negative breast cancer and paired axillary and sentinel lymph node metastasis. Breast J. 2020;26(5):904–10. [DOI] [PMC free article] [PubMed]; Srour MK, Gao B, Dadmanesh F, Carlson K, Qu Y, Deng N. et al. Gene expression comparison between primary triple-negative breast cancer and paired axillary and sentinel lymph node metastasis. Breast J. 2020;26(5):904–10. doi: 10.1111/tbj.13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu S, Liao G, Li G. Regulatory effects of COL1A1 on apoptosis induced by radiation in cervical cancer cells. Cancer Cell Int. 2017;17:73. [DOI] [PMC free article] [PubMed]; Liu S, Liao G, Li G. Regulatory effects of COL1A1 on apoptosis induced by radiation in cervical cancer cells. Cancer Cell Int. 2017;17:73. doi: 10.1186/s12935-017-0443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guo Y, Zhai J, Zhang J, Ni C, Zhou H. Improved radiotherapy sensitivity of nasopharyngeal carcinoma cells by miR-29-3p targeting COL1A1 3′-UTR. Med Sci Monit. 2019;25:3161–9. [DOI] [PMC free article] [PubMed]; Guo Y, Zhai J, Zhang J, Ni C, Zhou H. Improved radiotherapy sensitivity of nasopharyngeal carcinoma cells by miR-29-3p targeting COL1A1 3′-UTR. Med Sci Monit. 2019;25:3161–9. doi: 10.12659/MSM.915624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shen H, Li L, Yang S, Wang D, Zhong S, Zhao J, et al. MicroRNA-29a contributes to drug-resistance of breast cancer cells to adriamycin through PTEN/AKT/GSK3β signaling pathway. Gene. 2016;593(1):84–90. [DOI] [PubMed]; Shen H, Li L, Yang S, Wang D, Zhong S, Zhao J. et al. MicroRNA-29a contributes to drug-resistance of breast cancer cells to adriamycin through PTEN/AKT/GSK3β signaling pathway. Gene. 2016;593(1):84–90. doi: 10.1016/j.gene.2016.08.016. [DOI] [PubMed] [Google Scholar]
- [24].Li ZH, Xiong QY, Xu L, Duan P, Yang QO, Zhou P, et al. miR-29a regulated ER-positive breast cancer cell growth and invasion and is involved in the insulin signaling pathway. Oncotarget. 2017;8(20):32566–75. [DOI] [PMC free article] [PubMed]; Li ZH, Xiong QY, Xu L, Duan P, Yang QO, Zhou P. et al. miR-29a regulated ER-positive breast cancer cell growth and invasion and is involved in the insulin signaling pathway. Oncotarget. 2017;8(20):32566–75. doi: 10.18632/oncotarget.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wu SY, Wu AT, Yuan KS, Liu SH. Brown seaweed fucoidan inhibits cancer progression by dual regulation of mir-29c/ADAM12 and miR-17-5p/PTEN axes in human breast cancer cells. J Cancer. 2016;7(15):2408–19. [DOI] [PMC free article] [PubMed]; Wu SY, Wu AT, Yuan KS, Liu SH. Brown seaweed fucoidan inhibits cancer progression by dual regulation of mir-29c/ADAM12 and miR-17-5p/PTEN axes in human breast cancer cells. J Cancer. 2016;7(15):2408–19. doi: 10.7150/jca.15703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li W, Yi J, Zheng X, Liu S, Fu W, Ren L, et al. miR-29c plays a suppressive role in breast cancer by targeting the TIMP3/STAT1/FOXO1 pathway. Clin Epigenetics. 2018;10:64. [DOI] [PMC free article] [PubMed]; Li W, Yi J, Zheng X, Liu S, Fu W, Ren L. et al. miR-29c plays a suppressive role in breast cancer by targeting the TIMP3/STAT1/FOXO1 pathway. Clin Epigenetics. 2018;10:64. doi: 10.1186/s13148-018-0495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]