Abstract
Cancer stem cells (CSCs), also termed cancer-initiating cells, are a special subset of cells with high self-replicating and self-renewing abilities that can differentiate into various cell types under certain conditions. A number of studies have demonstrated that CSCs have distinct metabolic properties. The reprogramming of energy metabolism enables CSCs to meet the needs of self-renewal and stemness maintenance. Increasing evidence supports the view that alterations in lipid metabolism, including an increase in fatty acid (FA) uptake, de novo lipogenesis, formation of lipid droplets and mitochondrial FA oxidation, are involved in CSC regulation. In the present review, the metabolic characteristics of CSCs, particularly in lipid metabolism, were summarized. In addition, the potential mechanisms of CSC lipid metabolism in treatment resistance were discussed. Given their significance in cancer biology, targeting CSC metabolism may serve an important role in future cancer treatment.
Keywords: cancer stem cells, metabolic reprogramming, lipid metabolism, key modulators, resistant to therapy
1. Introduction
The occurrence and development of tumors is a complicated process involving numerous factors. Cancer stem cells (CSCs) are considered to be the seed of the tumor and are characterized by self-renewal and differentiation (1). CSCs serve an important role in maintaining the proliferation, invasion, drug resistance, metastasis and recurrence of malignant tumors (2). In recent years, the CSC model has received increasing attention.
Energy metabolism serves a significant role in the process of substance metabolism. Metabolic reprogramming is one of the most important hallmarks of cancer (3). Tumor-initiating cells (TICs) and CSCs exhibit different biology behaviors compared with non-stem cancer cells (4). The metabolic regulation of ATP synthesis and biological building block formation in CSCs is different compared with that in differential non-stem cancer cells, but similar to that in normal tissue-derived stem cells (5). In the past decade, the role of metabolism in CSC biology has developed into an active area of research, particularly in lipid metabolism. Previous studies have shown that lipid metabolism serves an important role in maintaining the stemness of CSCs (6) and meeting their energy needs, ultimately leading to cancer growth and invasion. In the present review, the origin and evolution of the CSC model are summarized. In addition, the characteristics and mechanisms of lipid metabolism of CSCs, as well as their role in radiotherapy and chemotherapy resistance are discussed.
2. CSCs
Concept and origin of CSCs
Rudolf Virchow observed similarities between tumor tissues and embryonic tissues ~150 years ago, establishing the Embryonic-Rest hypothesis of tumor formation (7). Subsequently, his student Julius Cohnheim extended this theory, suggesting that tumors originate from stem cells remaining from embryonic development and sustained in the tissues (7). Since the 19th century, significant progress has been achieved in understanding CSC biology and the existence of CSCs has also been confirmed in a variety of solid tumor types, including breast carcinoma (8), ovarian carcinoma (9), colon carcinoma (10), pancreatic carcinoma (11) and liver cancer (12).
CSCs, also termed tumor-initiating cells, display significant self-renewing abilities (13). As well as being responsible for the origin and development of tumors (14), CSCs are resistant to chemotherapy and radiotherapy (15), which allows for recurrence and metastasis (16).
Epigenetics in CSCs
Epigenetic regulation of the genome is associated with tumor progression. A variety of epigenetic pathways can contribute to the development and progression of tumors, especially in CSC maintenance and survival. Abnormal epigenetic changes may transform normal stem cells into CSCs. DNA methylation and histone modifications are two key factors involved in the developmental programming of stem cells to specific cell and tissue differentiation lineages (17). The important role of DNA methylation in maintaining CSC properties has been reported in leukemia, lung and colon stem cells (18,19). DNA methylation serves a critical role in this transformation process in the presence of DNA methyltransferases (20). Increasing evidence indicates that the silencing of tumor suppressor genes and activation of various cancer genes, which contribute to the formation of CSCs, are closely related to DNA hypermethylation (21).
In addition, epigenetic mechanisms regulate a number of key CSC pathways, including the Wnt/β-catenin, Hedgehog (Hh) and Notch signaling pathways. Specifically, the Wnt/β-catenin signaling pathway serves an important role in normal tissue development and maintenance, as well as in the self-renewal and differentiation of CSCs (22,23). The Hh signaling pathway also regulates the proliferation and maintains the stemness of progenitor cells and CSCs in several tissues (24). Notch signaling is an evolutionarily conserved pathway that regulates proliferation and differentiation in a wide range of cell types and different stages of cell lineage progression, as well as in CSC differentiation and self-renewal (25).
Role of CSCs in tumor biology
CSCs are considered to be the seed in tumor initiation, angiogenesis and maintenance. As aforementioned, CSCs are also an important factor in tumor therapy resistance and metastasis (26). Compared with non-stem cancer cells, CSCs are more resistant to radiotherapy and chemotherapy (27). The following characteristics contribute to CSC chemotherapy or radiotherapy resistance (28): Quiescent phenotype, efficient DNA repair, high expression of drug efflux pumps and antiapoptotic protein expression.
The target of radiotherapy and/or chemotherapy is primarily focused on fast growing cells (29). CSCs and normal stem cells are quiescent, thus CSCs may be insensitive to traditional radiotherapy and/or chemotherapy (15). CSCs express a high level of ATP-binding cassette transporters (ABC transporters), which contributes to the efflux of chemotherapeutic agents, leading to multidrug resistance (15,30).
CSCs are inherently resistant to DNA damage. The innate defense system of CSCs protects them against DNA-targeted chemicals and radiotherapy (31). Moreover, even under a radiation dose that causes DNA damage, CSCs can repair the damaged DNA more quickly (26). Indeed, checkpoint kinase (Chk)1 and Chk2, DNA damage and replication Chks, become activated on genotoxic stress to initiate cell cycle arrest and attempt repair or induce apoptosis if the damage is too great (32,33). Chk1 and Chk2 are highly expressed in CSCs. Inhibition of the Chk1/2 kinases with a small molecule inhibitor disrupted the radioresistance of CSCs (34). Moreover, overamplifying apoptotic inhibitor proteins also contributes to CSC treatment resistance. Various CSCs express higher levels of apoptosis protein inhibitors, including X-linked inhibitor of apoptosis protein (XIAP) isoform, which are associated with poor therapeutic responses (35). XIAP can alleviate the radioresistance of CSCs by promoting apoptosis (36).
Numerous other mechanisms also account for CSC resistance to therapy, including the increased production of free-radical scavengers and molecular metabolism mediators (37). Furthermore, certain mutated genes, including tumor suppressors P53, can help to rescue CSCs under stress conditions, including radiation therapy, tissue damage and exposure to toxins (38).
Therefore, to target CSCs more effectively, the molecular mechanisms of CSCs in proliferation and survival require further investigation.
3. Metabolic properties of CSCs
Due to the heterogeneity of tumor cells, the energy metabolism of cancer cells is distinct from that of normal cells and they are heavily dependent on glucose and aerobic oxidation for their energy supply (39). Metabolic reprogramming is one of the hallmarks of cancer cells (3). Cancer cells display a disrupted metabolism; even under oxygen-rich conditions, these cells still depend on glycolysis for energy and survival, a phenomenon termed the Warburg effect (40). Compared to oxidative phosphorylation (OXPHOS), glycolysis results in rapid production of ATP and an increase in metabolic intermediates for anabolic reactions (41). Although the metabolic characteristics of CSCs have been researched in recent years, the exact metabolism of CSCs remains to be elucidated.
A number of studies have reported that CSCs preferentially utilize glycolysis for survival, while others have shown that CSCs may also rely on OXPHOS (42,43). Ciavardelli et al (42) demonstrate that inhibiting the glycolysis of CD44+ CD24− breast CSCs reduces their proliferation, indicating that this population is glycolytic (42). Nasopharyngeal carcinoma, ovarian cancer, osteosarcoma, glioblastoma (GBM) and colon cancer are primarily dependent on mitochondrial OXPHOS for energy (44–48).
Increasing evidence has indicated that non-stem cancer cell population dedifferentiation to CSCs is accompanied by the transformation of metabolic pathways from mitochondrial OXPHOS to glycolysis (49). Therefore, CSCs display highly metabolic heterogeneity and plasticity abilities that allow them to adapt to the changing tumor microenvironment.
4. Lipid metabolism of cancer stem cells
Role of lipid metabolism in cancer cells
Despite the dependence of cancer on glycolysis, glycolytic inhibitors, such as 2-deoxyglucose, exhibit minimal effects on tumor growth inhibition. Therefore, other metabolic pathways are also critical to cancer cell survival, such as lipid metabolism (50). In addition to providing and storing energy as nutrients, lipids also function as the major component of the cell and signal molecules. Phospholipids, including glycerophospholipids and sphingolipids and cholesterol are the main components of the cell membrane (51). Changes in lipid metabolism could directly affect cell membrane synthesis and proliferation.
In addition, various lipid molecules and their metabolic intermediates participate in cell signal transduction, proliferation, cell adhesion and movement, inflammation and vascular regulation (51). The unlimited proliferation of cancer cells requires more fatty acids (FAs) and increased lipid droplet metabolism (52).
Unlike normal cells that preferentially utilize free FAs, cancer cells are dependent on reconstituted FAs, thus display enhanced de novo synthesis of FAs. A series of lipid synthesis enzymes are upregulated in cancer cells, including sterol-regulatory element binding proteins (SREBPs), acetyl-CoA carboxylase (ACC), FA synthase (FASN) and stearoyl-CoA desaturase 1 (SCD1) (53–60). In addition, citrate derived from the citrate (TCA) cycle can be used to produce acetyl-groups for FA synthesis (61). Therefore, lipid metabolism is also critical for the maintenance of cancer cell malignant biological behaviors.
Lipid metabolism of CSCs
In contrast to the dedifferentiation of non-stem cancer cells, increasing studies have reported that lipid metabolism is highly related to the stemness of CSCs (62). In addition to energy generation, biosynthesis and redox homeostasis, FA metabolism has a vital role in determining the fate of CSCs (63–65). FA synthesis and oxidation are essential for the maintenance of CSCs. For example, NANOG, a critical regulator of CSCs, can promote mitochondrial FA oxidation (FAO) to satisfy energy requirements for TICs (66). NANOG promotes the self-renewal abilities, tumor-initiation properties and generation of stem-like TICs, as well as the hepatocellular carcinoma (HCC) oncogenesis of TICs through metabolic reprogramming from OXPHOS to FAO (66). Peroxisomal proliferation-activated receptors (PPARδ) have significant effects on lipid metabolism and are strongly associated with NANOG expression (67). Overexpression of PPARδ or NANOG in TICs increases the probability of FAO occurring (66).
FAs are strictly regulated by CSCs to maintain their self-renewal ability and therapy resistance (65). As a critical intracellular organelle for the storage of excess lipids (68), the content of lipid droplets (LDs) is significantly increased in several solid tumor CSCs, including colorectal, breast, prostate (69–71) and ovarian CSCs (64). Of note, de novo lipogenesis is more active in GBM CSCs compared with that in non-stem cancer cells (72).
Key modulators of lipid metabolism in CSCs
De novo lipogenesis
A number of studies (73,74) have demonstrated higher intracellular lipid accumulation in various CSCs (Table I), which mainly results from de novo lipogenesis activity. The majority of the key regulators of de novo lipogenesis are also critical for CSCs. SCD1, an enzyme that converts saturated FAs into monounsaturated FAs (MUFAs) (75), is expressed at a high level in various tumors and is closely related to the progression and undesirable clinical outcomes of various types of cancer. SCD1 is critical for CSC/TIC generation and stemness maintenance (76) in ovarian (64), breast (77) and liver cancer (78,79). SCD1 overexpression also promotes CSC proliferation and prevents apoptosis (77,80). The enhanced activation of SCD1 and the consequent production of MUFAs could be considered as hallmarks of CSCs.
Table I.
Differences and similarities among bulk cancer cells, CSCs and normal stem cells.
| Pathway | Key molecules | Function | Bulk cancer cells | CSCs | Normal stem cells |
|---|---|---|---|---|---|
| FA synthesis | ACLY | Catalyze citrate converting into acetyl CoA in the cytoplasm | Elevated in gastric adenocarcinoma | Elevated in non-small cell lung carcinoma or breast cancer stem cells | Required for normal stem cells proliferation. Upregulated in mouse neural stem cells and pluripotent cells, in particular ACC and FASN activity. |
| ACC | Carboxylate acetyl-CoA into malonyl-CoA | Upregulated in the breast, gastric, and lung cancers | Elevated in IPSCs | ||
| FASN | De novo lipogenesis | Elevated in liver, prostate, breast, ovarian, endometrial and pancreatic cancers | Overexpressed in IPSCs, NSPCs, GSCs | ||
| SCD | Catalyzes the formation of MUFAs | Higher than non-cancer adjacent tissues | Elevated in the lung, ovarian, breast, and glioblastoma cancer stem cells. SCD-dependent MUFAs could directly regulate CSCs stemness | ||
| SREBP1 | The major transcriptional regulator of lipogenesis | Promotes invasion and migration in breast cancer and colorectal cancer | Regulates stemness through lipogenesis and MUFAs formation by inducing SCD expression | ||
| Mevalonate pathway | HMG-CoAR | The rate-limiting enzyme in the MVA pathway and the popular cholesterol synthesis lowering agents | Integral to tumor growth and progression | Essential for correct membrane anchoring of Rho GTPases which maintains stemness. | Cholesterol biosynthesis mediated by mevalonate pathway is required for NSPC self-renewal and maintenance. |
| FAO | CPT family | The rate-controlling enzyme in FAO | Upregulated in less glycolytic cancer types, such as prostate adenocarcinoma and diffuse large B-cell lymphoma | Fuels multiple CSCs, such as KRAS-mutant lung cancer and MYC-driven triple-negative | Various normal stem cells rely on FAO, such as hematopoietic stem cells, neural stem cells, intestinal stem cells and skeletal muscle stem cells. |
CSCs, cancer stem cells; ACLY, ATP citrate lyase; ACC, acetyl-CoA carboxylase; FASN, FA synthase; SCD, stearoyl-CoA desaturase 1; SREBP1, sterol regulatory element binding protein 1; HMG-CoAR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; MVA, mevalonate; CPT family, carnitine palmitoyltransferase family; FAO, fatty acid; FAO, FA oxidation; IPSCs, induced pluripotent stem cells; NSPCs, neural stem and progenitor cells; GSCs, glioma stem-like cells MUFA, monounsaturated FAs; KRAS, V-Ki-ras2 Κirsten rat sarcoma viral oncogene homolog.
Sterol regulatory element binding protein 1 (SREBP1) belongs to the SREBP transcription factor family and serves an important role in the biosynthesis of FAs and cholesterol (81). SREBP1 is the major transcriptional regulator of lipogenesis and directly regulates several lipogenic enzymes, including ATP citrate lyase (ACLY), ACC1 and FASN (57,81). Overexpression of SREBP1 can promote the growth of various tumors and maintain the stemness of CSCs (81). On the other hand, SREBP1 can also induce the expression of SCD1, which further induces CSC generation and stemness maintenance (82).
FAO
It has been reported that elevated FAO could help nutrient-deficient and hypoxic cancer cells survival, especially those with glycolytic deficiency (83,84). On the one hand, FAO plays a key role in meeting the heightened energy demands of CSCs. On the other hand, FAO could reduce intracellular reactive oxygen species production and maintain an internal steady state (84).
Mevalonate pathway
3-hydroxy-3-methylglutharyl-coenzyme A reductase is the rate-limiting enzyme in the mevalonate pathway and the molecular target of statins (85). The mevalonate pathway represents a metabolic pathway leading to the production of steroid hormones, cholesterol and non-sterol isoprenoids. The mevalonate cascade culminates in the production of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are essential for correct membrane anchoring of Rho family of small guanosine triphosphatases (GTPases). A number of oncogenic receptor tyrosine kinases require Ras proteins for signaling and EGFR signaling is particularly important for CSC maintenance. Furthermore, Rho GTPases maintain stemness by activating the Hippo transducers yes1 associated transcriptional regulator/tafazzin and by promoting the degradation of P27kip, leading to inhibition of retinoblastoma protein activation, which is ultimately conducive to the differentiation of CSCs (86).
5. Lipid metabolism of several solid cancer CSCs
HCC
Liver cancer is the second leading cause of cancer mortality in the world. Among primary liver cancer, HCC is the major histological subtype (87). HCC CSCs are currently considered as a specific subpopulation with significant tumorigenic potential and contribute to the development and recurrence of HCC (88). At present, several special markers have been identified for HCC CSCs, including CD133. Studies have demonstrated that CD133+ HCC CSCs show a significant enhancement of FAO rate and glycolysis, as well as a significant decrease in mitochondrial OXPHOS capacity (66,89).
In addition, inhibiting the prolongation of FAs in HCC CSCs results in a reduction in the content of polyunsaturated FAs, which is critical for HCC CSC ferroptosis resistance (66). NANOG is a critical regulator of HCC CSC lipid metabolic reprogramming. Knockdown of NANOG in HCC CSCs promotes the expression of FASN and ACLY, which is accompanied by increased OXPHOS and inhibition of glycolysis (66). Overexpression of NANOG induces CD133− HCC cancer cell dedifferentiation to CD133+ HCC CSCs and enhanced FAO activity, indicating that NANOG serves a vital role in regulating FA metabolism in liver CSCs (66). Overall, these studies show that liver CSCs suppress OXPHOS. In general, HCC CSCs favor FAO to support their stemness, self-renewal ability and therapy resistance.
Colorectal cancer (CRC)
CRC is the third most frequently diagnosed cancer and one of the most lethal types of cancer in both men and women worldwide (90). CRC CSCs induce tumorigenesis, proliferation, migration and metastasis of CRC. CRC CSCs are defined by a group of cell-surface markers, including CD44, CD133, CD24, epithelial cell adhesion factor molecule, leucine rich repeat containing G protein-coupled receptor 5 and Lin-28 homolog A (91). CRC CSCs also display a special lipid metabolism pathway. Genes associated with FA biosynthesis are downregulated, whereas genes involved in glycolysis, the TCA cycle and one-carbon metabolism pathway are upregulated in CD133+ CRC CSCs, suggesting a strong preference to glycolysis but suppression of FA biosynthesis (92). Tirinato et al (69) found that CD133+ CSCs contain more lipids. The lipid content in cancer cells is also positively correlated with the expression level of CD133 and Wnt/β-catenin pathway activity, which are markers of CSCs (69). CD133high cells possess more LDs compared with CD133low cells. Using the label-free Raman spectroscopy technology, researchers reported that the number of LDs in CRC CSCs was related to their tumorigenicity. The higher the number of LDs, the stronger the tumorigenicity (69). LDs may potentially open a new horizon for more specific ex vivo CRC CSCs diagnostics.
GBM
As the most common primary malignant brain tumor, GBM is extremely aggressive, with a median overall survival of <15 months (93). GBM CSCs contribute to the aggressive behaviors of GBM. GBM CSCs are considered to locate in a special environment, including perivascular, hypoxic and necrotic niches, as well as tumor border regions. Identified by the incorporation of 14 [C]-glucose and 14 [C]-acetate into the lipids, GBM CSCs are reported to have a higher rate of de novo lipogenesis compared with differentiated non-stem cancer cells (72). Increased de novo lipogenesis and extracellular lipid uptake result in LD accumulation in GBM CSCs (94). Moreover, GBM CSCs express a higher level of FASN protein, which facilitates the synthesis of FAs. Increased de novo lipogenesis may also contribute to the upregulation of FASN, thus maintaining the stemness of GBM CSCs (72). Inhibition of FASN expression decreased the expression of stemness markers and inhibited the proliferation and migration of GBM CSCs (72).
Pancreatic cancer
Pancreatic carcinoma is the fourth leading cause of mortality (95). Pancreatic CSCs were identified in 2007 (96). Metabolism reprogramming is reported as a critical factor in pancreatic CSC survival and stemness maintenance (97). Higher FA synthesis and activation of the mevalonate pathway are observed in pancreatic CSCs compared with pancreatic carcinoma non-stem cancer cells (65). Acetoacetyl-CoA transferase (ACAT2) synthesizes acetoacetyl CoA in the mevalonate pathway and leads to the elevation of cholesterol, which is significantly upregulated in pancreatic CSCs (65). Inhibition of FASN and ACAT2 reduces FA and cholesterol synthesis, which further decreases pancreatic CSC viability (65). Furthermore, the prognosis of patients with PDAC with high FASN expression levels is significantly worse and it has been proven that it depends on the induction of EGFR/ERK signaling, which is critical for pancreatic CSC maintenance (98).
6. Summary
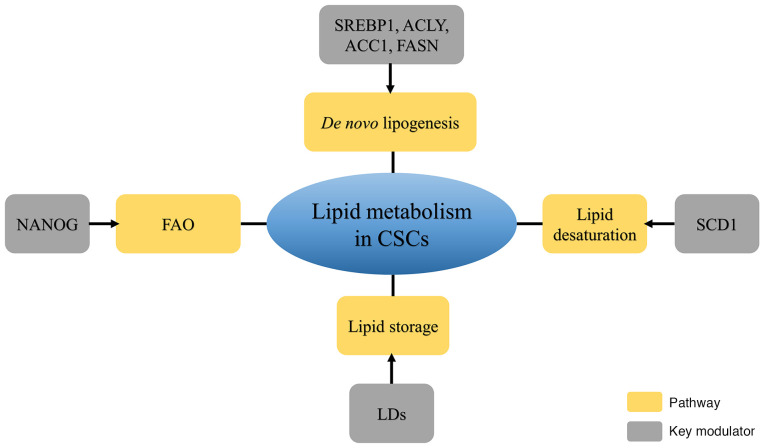
CSCs, with their self-renewal and tumor-initiating abilities, serve an important role in metastatic dissemination, radioresistance, chemoresistance and recurrence. A review on metabolomics demonstrated the contribution of lipid metabolism to the generation and maintenance of CSCs (76). Lipid metabolism reprogramming, including de novo lipogenesis and the formation of LDs and FAO, is involved in CSC generation and stemness maintenance (Fig. 1).
Figure 1.
Diagram of four key pathways with related modulators of cancer stem cells. Increasing de novo lipogenesis, lipid desaturation, FAO and lipid storage participate in maintaining the properties of cancer stem cells. FAO, fatty acid oxidation; NANOG, Nanog Homeobox; LDs, lipid droplets; SCD1, stearoyl-CoA desaturase 1; SREBP1, sterol regulatory element-binding protein 1; ACLY, ATP citrate lyase; ACC1, acetyl-CoA carboxylase; FASN, fatty acid synthase; CSCs, cancer stem cells.
Thus, understanding the mechanisms underlying CSC lipid metabolism reprogramming, as well as identifying the differences in lipid metabolism between CSCs and non-stem cancer cells will be of significance for improving the current clinical treatment of cancer. Several therapeutic targets of lipid metabolism have been developed to enhance antitumor effects (99). For instance, SCD1 inhibitors, CAY10566 and A939572, targeting FA desaturation process effectively suppress cancer stemness and tumor progression (64). As CSCs have been widely investigated, the development of effective agents targeting lipid metabolism and ameliorating radioresistance or chemoresistance is important.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by grants from the Postgraduate Innovation Project of Jiangsu Province (grant nos. SJCX20_1435 and SJCX20_1436).
Availability of data and materials
Data sharing is not applicable.
Authors' contributions
YL and JG designed the structure of the review. LS revised the manuscript critically for important intellectual content. HL and ZZ wrote and reviewed the article. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung Y, Kim WY. Cancer stem cell targeting: Are we there yet? Arch Pharm Res. 2015;38:414–422. doi: 10.1007/s12272-015-0570-2. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Dando I, Dalla Pozza E, Biondani G, Cordani M, Palmieri M, Donadelli M. The metabolic landscape of cancer stem cells. IUBMB Life. 2015;67:687–693. doi: 10.1002/iub.1426. [DOI] [PubMed] [Google Scholar]
- 5.Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer. 2016;114:1305–1312. doi: 10.1038/bjc.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: A therapeutic perspective. Nat Rev Clin Oncol. 2017;14:11–31. doi: 10.1038/nrclinonc.2017.1. [DOI] [PubMed] [Google Scholar]
- 7.Capp JP. Cancer stem cells: From historical roots to a new perspective. J Oncol. 2019;2019:5189232. doi: 10.1155/2019/5189232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharmacol Sin. 2013;34:732–740. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 16.Dalla Pozza E, Dando I, Biondani G, Brandi J, Costanzo C, Zoratti E, Fassan M, Boschi F, Melisi D, Cecconi D, et al. Pancreatic ductal adenocarcinoma cell lines display a plastic ability to bidirectionally convert into cancer stem cells. Int J Oncol. 2015;46:1099–1108. doi: 10.3892/ijo.2014.2796. [DOI] [PubMed] [Google Scholar]
- 17.Toh TB, Lim JJ, Chow EK. Epigenetics in cancer stem cells. Mol Cancer. 2017;16:29. doi: 10.1186/s12943-017-0596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bröske AM, Vockentanz L, Kharazi S, Huska MR, Mancini E, Scheller M, Kuhl C, Enns A, Prinz M, Jaenisch R, et al. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat Genet. 2009;41:1207–1215. doi: 10.1038/ng.463. [DOI] [PubMed] [Google Scholar]
- 19.Morita R, Hirohashi Y, Suzuki H, Takahashi A, Tamura Y, Kanaseki T, Asanuma H, Inoda S, Kondo T, Hashino S, et al. DNA methyltransferase 1 is essential for initiation of the colon cancers. Exp Mol Pathol. 2013;94:322–329. doi: 10.1016/j.yexmp.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Wongtrakoongate P. Epigenetic therapy of cancer stem and progenitor cells by targeting DNA methylation machineries. World J Stem Cells. 2015;7:137–148. doi: 10.4252/wjsc.v7.i1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteller M. Epigenetic gene silencing in cancer: The DNA hypermethylome. Hum Mol Genet. 2007;16:R50–R59. doi: 10.1093/hmg/ddm018. Spec No: 1. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, Hein K, Vogt R, Kemler R. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science. 2012;336:1549–1554. doi: 10.1126/science.1218370. [DOI] [PubMed] [Google Scholar]
- 23.Myant KB, Cammareri P, McGhee EJ, Ridgway RA, Huels DJ, Cordero JB, Schwitalla S, Kalna G, Ogg EL, Athineos D, et al. ROS production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761–773. doi: 10.1016/j.stem.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 25.Andersson ER, Sandberg R, Lendahl U. Notch signaling: Simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 26.Eyler CE, Rich JN. Survival of the fittest: Cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou HM, Zhang JG, Zhang X, Li Q. Targeting cancer stem cells for reversing therapy resistance: Mechanism, signaling, and prospective agents. Signal Transduct Target Ther. 2021;6:62. doi: 10.1038/s41392-020-00430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 29.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold CR, Mangesius J, Skvortsova II, Ganswindt U. The role of cancer stem cells in radiation resistance. Front Oncol. 2020;10:164. doi: 10.3389/fonc.2020.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Jaarsveld MTM, Deng D, Ordoñez-Rueda D, Paulsen M, Wiemer EAC, Zi Z. Cell-type-specific role of CHK2 in mediating DNA damage-induced G2 cell cycle arrest. Oncogenesis. 2020;9:35. doi: 10.1038/s41389-020-0219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patil M, Pabla N, Dong Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell Mol Life Sci. 2013;70:4009–4021. doi: 10.1007/s00018-013-1307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hambardzumyan D, Squatrito M, Holland EC. Radiation resistance and stem-like cells in brain tumors. Cancer Cell. 2006;10:454–456. doi: 10.1016/j.ccr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 35.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 36.Morrison R, Schleicher SM, Sun Y, Niermann KJ, Kim S, Spratt DE, Chung CH, Lu B. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J Oncol. 2011;2011:941876. doi: 10.1155/2011/941876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo M, Wicha MS. Targeting cancer stem cell redox metabolism to enhance therapy responses. Semin Radiat Oncol. 2019;29:42–54. doi: 10.1016/j.semradonc.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gealy R, Zhang L, Siegfried JM, Luketich JD, Keohavong P. Comparison of mutations in the p53 and K-ras genes in lung carcinomas from smoking and nonsmoking women. Cancer Epidemiol Biomarkers Prev. 1999;8:297–302. [PubMed] [Google Scholar]
- 39.Liberti MV, Locasale JW. The Warburg effect: How does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 41.Guppy M, Greiner E, Brand K. The role of the Crabtree effect and an endogenous fuel in the energy metabolism of resting and proliferating thymocytes. Eur J Biochem. 1993;212:95–99. doi: 10.1111/j.1432-1033.1993.tb17637.x. [DOI] [PubMed] [Google Scholar]
- 42.Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, De Cola A, Scavo E, Carollo R, D'Agostino D, et al. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis. 2014;5:e1336. doi: 10.1038/cddis.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasto A, Bellio C, Pilotto G, Ciminale V, Silic-Benussi M, Guzzo G, Rasola A, Frasson C, Nardo G, Zulato E, et al. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget. 2014;5:4305–4319. doi: 10.18632/oncotarget.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen YA, Wang CY, Hsieh YT, Chen YJ, Wei YH. Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle. 2015;14:86–98. doi: 10.4161/15384101.2014.974419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB, Morrison CD, Odunsi K. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One. 2014;9:e84941. doi: 10.1371/journal.pone.0084941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palorini R, Votta G, Balestrieri C, Monestiroli A, Olivieri S, Vento R, Chiaradonna F. Energy metabolism characterization of a novel cancer stem cell-like line 3AB-OS. J Cell Biochem. 2014;115:368–379. doi: 10.1002/jcb.24671. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y, Zhou Y, Shingu T, Feng L, Chen Z, Ogasawara M, Keating MJ, Kondo S, Huang P. Metabolic alterations in highly tumorigenic glioblastoma cells: Preference for hypoxia and high dependency on glycolysis. J Biol Chem. 2011;286:32843–32853. doi: 10.1074/jbc.M111.260935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song IS, Jeong YJ, Han J. Mitochondrial metabolism in cancer stem cells: A therapeutic target for colon cancer. BMB Rep. 2015;48:539–540. doi: 10.5483/BMBRep.2015.48.10.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snaebjornsson MT, Janaki-Raman S, Schulze A. Greasing the wheels of the cancer machine: The Role of lipid metabolism in cancer. Cell Metab. 2020;31:62–76. doi: 10.1016/j.cmet.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Luo X, Cheng C, Tan Z, Li N, Tang M, Yang L, Cao Y. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. 2017;16:76. doi: 10.1186/s12943-017-0646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 53.Rohrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 54.Geng F, Cheng X, Wu X, Yoo JY, Cheng C, Guo JY, Mo X, Ru P, Hurwitz B, Kim SH. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-Mediated lipogenesis. Clin Cancer Res. 2016;22:5337–5348. doi: 10.1158/1078-0432.CCR-15-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gopal K, Grossi E, Paoletti P, Usardi M. Lipid composition of human intracranial tumors: A biochemical study. Acta Neurochir (Wien) 1963;11:333–347. doi: 10.1007/BF01402012. [DOI] [PubMed] [Google Scholar]
- 56.Currie E, Schulze A, Zechner R, Walther TC, Farese RV., Jr Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimano H, Sato R. SREBP-regulated lipid metabolism: Convergent physiology-divergent pathophysiology. Nat Rev Endocrinol. 2017;13:710–730. doi: 10.1038/nrendo.2017.91. [DOI] [PubMed] [Google Scholar]
- 58.Schlosser HA, Drebber U, Urbanski A, Haase S, Baltin C, Berlth F, Neiss S, von Bergwelt-Baildon M, Fetzner UK, Warnecke-Eberz U, et al. Glucose transporters 1, 3, 6, and 10 are expressed in gastric cancer and glucose transporter 3 is associated with UICC stage and survival. Gastric Cancer. 2017;20:83–91. doi: 10.1007/s10120-015-0577-x. [DOI] [PubMed] [Google Scholar]
- 59.Sharen G, Peng Y, Cheng H, Liu Y, Shi Y, Zhao J. Prognostic value of GLUT-1 expression in pancreatic cancer: Results from 538 patients. Oncotarget. 2017;8:19760–19767. doi: 10.18632/oncotarget.15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun HW, Yu XJ, Wu WC, Chen J, Shi M, Zheng L, Xu J. GLUT1 and ASCT2 as predictors for prognosis of hepatocellular carcinoma. PLoS One. 2016;11:e0168907. doi: 10.1371/journal.pone.0168907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams NC, O'Neill LAJ. A Role for the krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front Immunol. 2018;9:141. doi: 10.3389/fimmu.2018.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mancini R, Noto A, Pisanu ME, De Vitis C, Maugeri-Saccà M, Ciliberto G. Metabolic features of cancer stem cells: The emerging role of lipid metabolism. Oncogene. 2018;37:2367–2378. doi: 10.1038/s41388-018-0141-3. [DOI] [PubMed] [Google Scholar]
- 63.Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, et al. JAK/STAT3-Regulated fatty Acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27:136–150.e5. doi: 10.1016/j.cmet.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, Matei D, Cheng JX. Lipid Desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell. 2017;20:303–314.e5. doi: 10.1016/j.stem.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brandi J, Dando I, Pozza ED, Biondani G, Jenkins R, Elliott V, Park K, Fanelli G, Zolla L, Costello E, et al. Proteomic analysis of pancreatic cancer stem cells: Functional role of fatty acid synthesis and mevalonate pathways. J Proteomics. 2017;150:310–322. doi: 10.1016/j.jprot.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Chen CL, Uthaya Kumar DB, Punj V, Xu J, Sher L, Tahara SM, Hess S, Machida K. NANOG metabolically reprograms tumor-initiating Stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab. 2016;23:206–219. doi: 10.1016/j.cmet.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, Pandolfi PP. A PML-PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. doi: 10.1038/oncsis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tirinato L, Liberale C, Di Franco S, Candeloro P, Benfante A, La Rocca R, Potze L, Marotta R, Ruffilli R, Rajamanickam VP, et al. Lipid droplets: A new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells. 2015;33:35–44. doi: 10.1002/stem.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Gonzalo-Calvo D, López-Vilaró L, Nasarre L, Perez-Olabarria M, Vázquez T, Escuin D, Badimon L, Barnadas A, Lerma E, Llorente-Cortés V. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: A molecular and clinicopathological study. BMC Cancer. 2015;15:460. doi: 10.1186/s12885-015-1469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X, Ratliff TL, Cheng JX. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, Ogata M, Katsuyama Y, Sadahiro H, Suzuki M, Owada Y. Inhibition of fatty acid synthase decreases expression of stemness markers in glioma stem cells. PLoS One. 2016;11:e0147717. doi: 10.1371/journal.pone.0147717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H, Feng Z, He ML. Lipid metabolism alteration contributes to and maintains the properties of cancer stem cells. Theranostics. 2020;10:7053–7069. doi: 10.7150/thno.41388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clémot M, Sênos Demarco R, Jones DL. Lipid mediated regulation of adult stem cell behavior. Front Cell Dev Biol. 2020;8:115. doi: 10.3389/fcell.2020.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castro LF, Wilson JM, Goncalves O, Galante-Oliveira S, Rocha E, Cunha I. The evolutionary history of the stearoyl-CoA desaturase gene family in vertebrates. BMC Evol Biol. 2011;11:132. doi: 10.1186/1471-2148-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yi M, Li J, Chen S, Cai J, Ban Y, Peng Q, Zhou Y, Zeng Z, Peng S, Li X, et al. Emerging role of lipid metabolism alterations in Cancer stem cells. J Exp Clin Cancer Res. 2018;37:118. doi: 10.1186/s13046-018-0826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colacino JA, McDermott SP, Sartor MA, Wicha MS, Rozek LS. Transcriptomic profiling of curcumin-treated human breast stem cells identifies a role for stearoyl-coa desaturase in breast cancer prevention. Breast Cancer Res Treat. 2016;158:29–41. doi: 10.1007/s10549-016-3854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai KKY, Kweon SM, Chi F, Hwang E, Kabe Y, Higashiyama R, Qin L, Yan R, Wu RP, Lai K, et al. Stearoyl-CoA desaturase promotes liver fibrosis and tumor development in mice via a wnt positive-signaling loop by stabilization of low-density lipoprotein-receptor-related proteins 5 and 6. Gastroenterology. 2017;152:1477–1491. doi: 10.1053/j.gastro.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bansal S, Berk M, Alkhouri N, Partrick DA, Fung JJ, Feldstein A. Stearoyl-CoA desaturase plays an important role in proliferation and chemoresistance in human hepatocellular carcinoma. J Surg Res. 2014;186:29–38. doi: 10.1016/j.jss.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mason P, Liang B, Li L, Fremgen T, Murphy E, Quinn A, Madden SL, Biemann HP, Wang B, Cohen A, et al. SCD1 inhibition causes cancer cell death by depleting mono-unsaturated fatty acids. PLoS One. 2012;7:e33823. doi: 10.1371/journal.pone.0033823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pandey PR, Xing F, Sharma S, Watabe M, Pai SK, Iiizumi-Gairani M, Fukuda K, Hirota S, Mo YY, Watabe K. Elevated lipogenesis in epithelial stem-like cell confers survival advantage in ductal carcinoma in situ of breast cancer. Oncogene. 2013;32:5111–5122. doi: 10.1038/onc.2012.519. [DOI] [PubMed] [Google Scholar]
- 82.Sun Y, He W, Luo M, Zhou Y, Chang G, Ren W, Wu K, Li X, Shen J, Zhao X, Hu Y. SREBP1 regulates tumorigenesis and prognosis of pancreatic cancer through targeting lipid metabolism. Tumour Biol. 2015;36:4133–4141. doi: 10.1007/s13277-015-3047-5. [DOI] [PubMed] [Google Scholar]
- 83.Raulien N, Friedrich K, Strobel S, Rubner S, Baumann S, von Bergen M, Körner A, Krueger M, Rossol M, Wagner U. Fatty acid oxidation compensates for lipopolysaccharide-induced warburg effect in glucose-deprived monocytes. Front Immunol. 2017;8:609. doi: 10.3389/fimmu.2017.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: Fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16:718–731. doi: 10.1038/nrc.2016.76. [DOI] [PubMed] [Google Scholar]
- 86.Ginestier C, Monville F, Wicinski J, Cabaud O, Cervera N, Josselin E, Finetti P, Guille A, Larderet G, Viens P, et al. Mevalonate metabolism regulates Basal breast cancer stem cells and is a potential therapeutic target. Stem Cells. 2012;30:1327–1337. doi: 10.1002/stem.1122. [DOI] [PubMed] [Google Scholar]
- 87.Mak D, Kramvis A. Epidemiology and aetiology of hepatocellular carcinoma in Sub-Saharan Africa. Hepatoma Res. 2021;7:39. [Google Scholar]
- 88.Afify SM, Sanchez Calle A, Hassan G, Kumon K, Nawara HM, Zahra MH, Mansour HM, Khayrani AC, Alam MJ, Du J, et al. A novel model of liver cancer stem cells developed from induced pluripotent stem cells. Br J Cancer. 2020;122:1378–1390. doi: 10.1038/s41416-020-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song K, Kwon H, Han C, Zhang J, Dash S, Lim K, Wu T. Active glycolytic metabolism in CD133(+) hepatocellular cancer stem cells: Regulation by MIR-122. Oncotarget. 2015;6:40822–40835. doi: 10.18632/oncotarget.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vázquez-Iglesias L, Barcia-Castro L, Rodríguez-Quiroga M, Páez de la Cadena M, Rodríguez-Berrocal J, Cordero OJ. Surface expression marker profile in colon cancer cell lines and sphere-derived cells suggests complexity in CD26+ cancer stem cells subsets. Biology Open. 2019;8:bio041673. doi: 10.1242/bio.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen KY, Liu X, Bu P, Lin CS, Rakhilin N, Locasale JW, Shen X. A metabolic signature of colon cancer initiating cells. Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:4759–4762. doi: 10.1109/EMBC.2014.6944688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang B, Li X, Li Y, Zhang J, Zong Z, Zhang H. Current immunotherapies for glioblastoma multiforme. Front Immunol. 2021;11:603911. doi: 10.3389/fimmu.2020.603911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Menard JA, Christianson HC, Kucharzewska P, Bourseau-Guilmain E, Svensson KJ, Lindqvist E, Indira Chandran V, Kjellén L, Welinder C, Bengzon J, et al. Metastasis stimulation by hypoxia and acidosis-induced extracellular lipid uptake is mediated by proteoglycan-dependent endocytosis. Cancer Res. 2016;76:4828–4840. doi: 10.1158/0008-5472.CAN-15-2831. [DOI] [PubMed] [Google Scholar]
- 95.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World J Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 97.Arasanz H, Hernández C, Bocanegra A, Chocarro L, Zuazo M, Gato M, Ausin K, Santamaría E, Fernández-Irigoyen J, Fernandez G, et al. Profound reprogramming towards stemness in pancreatic cancer cells as adaptation to AKT inhibition. Cancers. 2020;12:2181. doi: 10.3390/cancers12082181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bian Y, Yu Y, Wang S, Li L. Up-regulation of fatty acid synthase induced by EGFR/ERK activation promotes tumor growth in pancreatic cancer. Biochem Biophys Res Commun. 2015;463:612–617. doi: 10.1016/j.bbrc.2015.05.108. [DOI] [PubMed] [Google Scholar]
- 99.Visweswaran M, Arfuso F, Warrier S, Dharmarajan A. Aberrant lipid metabolism as an emerging therapeutic strategy to target cancer stem cells. Stem Cells. 2020;38:6–14. doi: 10.1002/stem.3101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable.