Abstract
The BACTEC MYCO/F Lytic blood culture bottle (Becton Dickinson Diagnostic Instrument Systems, Sparks, Md.) is designed to optimize the recovery of fungi and mycobacteria; however, this bottle also supports the growth of most aerobic bacteria. We compared the MYCO/F Lytic bottle with two other BACTEC bottles and the Isolator system for the recovery of bacteria as well as fungi and mycobacteria from blood. A total of 6,108 blood culture sets were inoculated with blood obtained from adult patients. Twenty-five to 28 ml of blood collected by a phlebotomy team for each blood culture set was randomly distributed into each of four blood culture receptacles: the Isolator tube (Wampole Laboratories, Cranbury, N.J.) and three BACTEC bottles: the MYCO/F Lytic bottle, the BACTEC Plus Aerobic/F bottle, and the BACTEC Anaerobic Lytic/10 bottle. The sediment from the Isolator tube was inoculated onto chocolate agar (CA), brain heart infusion agar (BHI), and Sabouraud dextrose agar (SDA) and into a BACTEC 13A bottle. Incubation durations were as follows: MYCO/F Lytic bottle, 42 days; Plus Aerobic/F bottle, 5 days; Anaerobic Lytic/10 bottle, 5 days; sediment from Isolator tube on CA, 3 days; sediment from Isolator tube on BHI, 30 days; sediment from Isolator tube on SDA, 30 days; and sediment from Isolator tube in a BACTEC 13A bottle, 42 days. Two isolates of Histoplasma capsulatum were recovered from the Isolator tube only. Three isolates of Mycobacterium tuberculosis complex were recovered: two isolates from the MYCO/F Lytic bottle only and one isolate from the Isolator tube (whose sediment was inoculated into the BACTEC 13A bottle) only. Two isolates of Cryptococcus neoformans were recovered: one from the MYCO/F Lytic bottle only and the other from the MYCO/F Lytic bottle and the Isolator tube (whose sediment was inoculated into the BACTEC 13A bottle). For potential pathogens overall, there was a statistical difference in recovery that favored the Isolator system over the MYCO/F Lytic bottle (P = 0.0015), including statistically significant differences for Staphylococcus aureus (P = 0.0001) and Streptococcus pneumoniae (P = 0.0313). However, there was no statistically significant difference between the two blood culture systems when detection of bloodstream infection was considered. The time to detection for all potential pathogens combined was less for the MYCO/F Lytic bottle than for the Isolator system (P = 0.0004). Overall, the potential pathogen recovery was greater for the BACTEC Plus Aerobic/F bottle than for either the Isolator system (P = 0.0003) or the MYCO/F Lytic bottle (P = 0.0001). However, the BACTEC Plus Aerobic/F bottle did not recover M. tuberculosis, H. capsulatum, or C. neoformans isolates. The combination of the Isolator system and MYCO/F Lytic bottle may be useful as a selective blood culture method to optimize the recovery of fungi and mycobacteria from blood. Compared with the manual Isolator system, the MYCO/F Lytic system has the advantage of less preanalytic processing and continuous automated monitoring of bottles for growth by the BACTEC 9240 instrument.
The Isolator system (Wampole Laboratories, Cranbury, N.J.) is used in our laboratory to recover aerobic bacteria and fungi from blood. Additionally, the sediment from the Isolator tube is injected into BACTEC 13A bottles, which are then monitored for growth of mycobacteria by the BACTEC 460 instrument (Becton Dickinson Diagnostic Instrument Systems, Sparks, Md.). Recently, a blood culture bottle for the recovery of fungi and mycobacteria, the BACTEC MYCO/F Lytic bottle, has been developed for use with the automated BACTEC 9240 blood culture system (Becton Dickinson Diagnostic Instrument Systems). Replacement of the Isolator system with an automated culture method like the MYCO/F Lytic bottle would significantly decrease labor requirements in our laboratory.
We compared the MYCO/F Lytic bottle of the BACTEC 9240 automated blood culture system with the Isolator system for the recovery of microorganisms from the blood of adult patients. Although the MYCO/F Lytic bottle is designed for the recovery of fungi and mycobacteria, we were also interested in its ability to recover aerobic bacteria. The frequencies of detection and the times to detection of bloodstream microorganisms and the frequencies of detection of bloodstream infections (septic episodes) for these two aerobic blood culture systems were compared. Each blood culture set for the study also included a BACTEC Aerobic/F bottle and a BACTEC Anaerobic Lytic/10 bottle. As secondary evaluations, overall pathogen recovery for the MYCO/F Lytic bottle was compared independently with overall pathogen recovery for the BACTEC Plus Aerobic/F bottle and the BACTEC Anaerobic Lytic/10 bottle. Additionally, overall pathogen recovery for the BACTEC Plus Aerobic/F bottle was compared with that for the Isolator system.
(This study was presented in part at the 100th General Meeting of the American Society for Microbiology [E. A. Vetter, C. A. Torgerson, J. G. Hughes, S. Harmsen, C. D. Schleck, C. D. Horstmeier, G. D. Roberts, and F. R. Cockerill, Abstr. 100th Gen. Meet. Am. Soc. Microbiol. 2000, abstr. C-262, p. 191, 2000].)
MATERIALS AND METHODS
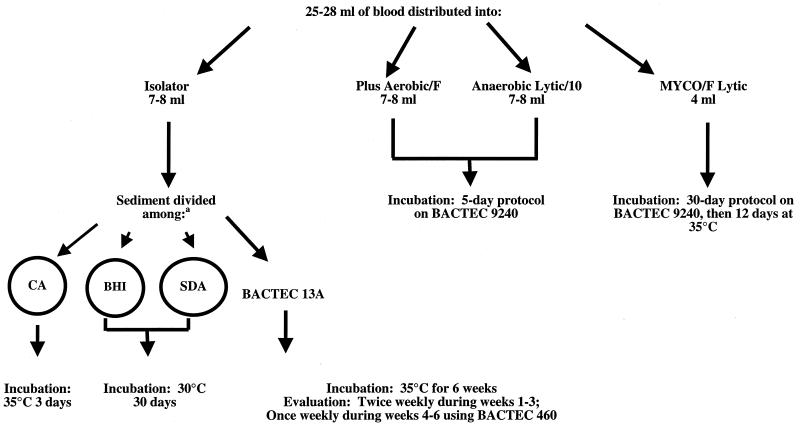
All blood samples for culture were obtained from patients over 16 years of age at the Mayo Medical Center in Rochester, Minn. Samples from patients who declined to provide permission to use their specimens and medical histories for evaluation (Minnesota Statute 144.335) were excluded from the study. The Mayo Medical Center consists of two large teaching hospitals (1,800 beds combined) and a large subspecialty clinic. The same procedures for collection of blood, processing, and detection of microorganisms were used for all patients. Phlebotomists aseptically collected 25 to 28 ml of blood using a needle and a syringe. Inoculation of blood culture receptacles was randomized on the basis of a predetermined randomization schedule. Figure 1 shows how the blood culture receptacles were inoculated, the processing of sediment from the Isolator tube, incubation conditions, and incubation durations. The Isolator tube was processed in the Clinical Microbiology Laboratory according to the manufacturer's instructions. The BACTEC Plus Aerobic/F, BACTEC Anaerobic Lytic/10, and BACTEC MYCO/F Lytic bottles were loaded into the BACTEC 9240 instrument in the computer-assigned position. The BACTEC 9240 instrument was observed by technologists at approximately 4-h intervals for positive signals. The BACTEC 13A bottles were evaluated in the BACTEC 460 instrument at the intervals indicated in Fig. 1.
FIG. 1.
Inoculation, incubation conditions, and incubation durations. a, A total of 1.0 to 1.5 of sediment from the Isolator tube was divided as follows: one-half of the volume was inoculated into a BACTEC 13A bottle, and the remaining one-half of the volume was divided into three equal portions and inoculated on chocolate agar (CA), brain heart infusion agar (BHI), and Sabouraud dextrose agar (SDA) plates.
Microorganisms isolated from positive cultures were identified by standard biochemical techniques. All isolates were evaluated, regardless of what medium they were isolated from. Time to detection was defined as the time that elapsed from the time of collection to the time of a positive staining result (with Gram auramine-rhodamine, or Calcofluor white stain). This was dependent on the examination schedules for both the manual (Isolator) and automated (BACTEC) systems (Fig. 1).
A microorganism isolated from blood was classified as a potential pathogen if, according to its identity, the organism is rarely characterized as a contaminant. If the organisms were either viridans group streptococci or coagulase-negative Staphylococcus spp, they were considered potential pathogens if they were isolated from at least two of the blood culture receptacles, i.e., an Isolator tube, a BACTEC Plus Aerobic F/bottle or a BACTEC Anaerobic Lytic/F bottle, in the blood culture set. For the Isolator tube, growth of the same organism from any of the three agar plates used or the BACTEC 13A bottle (Fig. 1) was considered a single positive receptacle (scored as a single positive result). Bloodstream infections (septic episodes) were defined by criteria modified from those previously published by Kirkley and colleagues (2). To summarize, a bloodstream infection was defined as the initial isolation of a pathogen, the subsequent isolation of a different pathogen, or the isolation of the same pathogen after at least a 7-day interval since the first positive culture with that organism. If more than one pathogen was isolated from a blood culture set, each individual pathogen was counted as a separate bloodstream infection.
For each organism species detected (and overall), comparison of the detection rates of the two systems were assessed by the sign test. Paired comparisons of the time to detection between the two systems were made by using the Wilcoxon signed rank test. All calculated P values were two sided, and P values of ≤0.05 were considered statistically significant.
RESULTS
The results of the study are provided in Tables 1 to 7. A total of 6,108 blood cultures met the criteria for inclusion in the study. These cultures yielded 736 isolates of bacteria, mycobacteria, or fungi. On the basis of our criteria for pathogens and contaminants, 578 of these isolates were considered potential pathogens and 158 were considered potential contaminants.
TABLE 1.
Comparison of BACTEC MYCO/F Lytic bottle to Isolator system
| Potential pathogen(s) or contaminant(s) | No. of isolates with the following result:
|
P valueb | ||||
|---|---|---|---|---|---|---|
| Positive with Isolator system only | Positive with BACTEC MYCO/F Lytic bottle only | Positive with both | Positive with neithera | Total | ||
| Pathogens | ||||||
| All microorganisms | 115 | 71 | 205 | 187 | 578 | 0.0015 |
| Staphylococcus aureus | 36 | 6 | 40 | 19 | 101 | 0.0001 |
| Staphylococcus spp., coagulase negative | 23 | 27 | 36 | 53 | 139 | 0.6718 |
| Streptococcus pneumoniae | 6 | 0 | 7 | 6 | 19 | 0.0313 |
| Viridans group streptococci | 4 | 8 | 7 | 20 | 39 | 0.3877 |
| Enterococcus spp. | 7 | 1 | 5 | 12 | 25 | 0.0703 |
| Enterobacteriaceae family | 20 | 12 | 65 | 28 | 125 | 0.2153 |
| Pseudomonas spp. | 2 | 1 | 13 | 3 | 19 | 1.0 |
| Obligately anaerobic bacteria | 0 | 0 | 3 | 24 | 27 | 1.0 |
| Other bacteria | 7 | 10 | 17 | 17 | 51 | 0.6291 |
| Mycobacterium tuberculosis complex | 1 | 2 | 0 | 0 | 3 | 1.0 |
| Candida spp. | 7 | 3 | 11 | 5 | 26 | 1.0 |
| Cryptococcus neoformans | 0 | 1 | 1 | 0 | 2 | 1.0 |
| Histoplasma capsulatum | 2 | 0 | 0 | 0 | 2 | 0.5 |
| Contaminants | ||||||
| All microorganisms | 133 | 8 | 0 | 17 | 158 | <0.0001 |
| Bacillus spp. | 20 | 2 | 0 | 2 | 24 | 0.0001 |
| Corynebacterium spp., not C. jeikeim | 15 | 5 | 0 | 5 | 25 | 0.0414 |
| Propionibacterium spp. | 1 | 1 | 0 | 4 | 6 | 1.0 |
| Staphylococcus spp., coagulase negative | 74 | 0 | 0 | 6 | 80 | 0.0001 |
| Viridans group streptococci | 6 | 0 | 0 | 0 | 6 | 0.0313 |
| Other bacteria | 17 | 0 | 0 | 0 | 17 | 0.0001 |
Refers to the number of isolates detected with only the BACTEC Plus Aerobic/F bottle or the BACTEC Anaerobic Lytic/10 bottle.
Refer to Materials and Methods section for calculation of P values.
TABLE 7.
Bloodstream infections detected by the Isolator system and BACTEC Plus Aerobic/F bottle
| Potential pathogen(s) | No. of bloodstream infections detected with:
|
P valuea | ||
|---|---|---|---|---|
| Isolator system only | BACTEC Plus Aerobic/F bottle only | Both | ||
| All microorganisms | 54 | 88 | 145 | 0.005 |
| Staphylococcus aureus | 5 | 2 | 30 | 0.453 |
| Staphylococcus spp., coagulase negative | 9 | 34 | 35 | 0.0002 |
| Streptococcus pneumoniae | 0 | 3 | 8 | 0.25 |
| Viridans group streptococci | 1 | 10 | 5 | 0.012 |
| Enterococcus spp. | 7 | 3 | 3 | 0.344 |
| Enterobacteriaceae family | 17 | 16 | 40 | 1.0 |
| Pseudomonas spp. | 1 | 3 | 10 | 0.625 |
| Obligately anaerobic bacteria | 0 | 1 | 1 | 1.0 |
| Other bacteria | 6 | 12 | 8 | 0.24 |
| Mycobacterium tuberculosis complex | 1 | 0 | 0 | 1.0 |
| Candida spp. | 4 | 4 | 5 | 1.0 |
| Cryptococcus neoformans | 1 | 0 | 0 | 1.0 |
| Histoplasma capsulatum | 2 | 0 | 0 | 0.5 |
Refer to Materials and Methods section for calculation of P values.
BACTEC MYCO/F Lytic bottle compared with Isolator system.
As shown in Table 1, there was a statistically significant difference in overall potential pathogen recovery for the Isolator system (n = 294) compared to that for the MYCO/F Lytic bottle (n = 276) (P = 0.0015). When individual pathogens were assessed, the Isolator system detected statistically significantly more Streptococcus pneumoniae (P = 0.0313) and Staphylococcus aureus (P = 0.0001) isolates than the MYCO/F Lytic bottle. However, statistically significant differences were not observed when the total number of bloodstream infections (septic episodes) detected with these two systems were compared (Table 2). Statistically significantly more septic episodes caused by S. aureus were detected (P = 0.0414) with the Isolator system than with MYCO/F Lytic bottles. The Isolator system recovered statistically significantly more contaminants overall than the MYCO/F Lytic bottles (P < 0.0001) (Table 1).
TABLE 2.
Bloodstream infections detected with the MYCO/F Lytic bottle and the Isolator systema
| Potential pathogen(s) | No. bloodstream infections detected with:
|
P valuec | ||
|---|---|---|---|---|
| Isolator system only | BACTEC MYCO/F Lytic bottle only | Both | ||
| All microorganisms | 79 | 58 | 122 | 0.0871 |
| Staphylococcus aureus | 15 | 5 | 20 | 0.0414 |
| Staphylococcus spp., coagulase negative | 21 | 24 | 22 | 0.7660 |
| Streptococcus pneumoniae | 4 | 0 | 4 | 0.1250 |
| Viridans group streptococci | 3 | 8 | 3 | 0.2266 |
| Enterococcus spp. | 7 | 1 | 3 | 0.0703 |
| Enterobacteriaceae family | 15 | 8 | 44 | 0.2100 |
| Pseudomonas spp. | 2 | 1 | 9 | 1.0 |
| Obligately anaerobic bacteria | 0 | 0 | 1 | 1.0 |
| Other bacteria | 7 | 8 | 8 | 1.0 |
| Mycobacterium tuberculosis complex | 0 | 1 | 0 | 1.0 |
| Candida spp. | 3 | 2 | 7 | 1.0 |
| Cryptocococus neoformans | 0 | 0 | 1 | 1.0 |
| Histoplasma capsulatum | 2 | 0 | 0 | 0.5000 |
See Materials and Methods for definition of bloodstream infection.
Refer to Materials and Methods section for calculation of P values.
Two isolates of Histoplasma capsulatum were recovered from different patients, each from the Isolator system only. For one patient, a single colony of H. capsulatum was isolated on one plate, the brain heart infusion agar plate, inoculated with sediment from the Isolator tube. The second isolate of H. capsulatum was recovered from both brain heart infusion agar and Sabouraud dextrose agar plates inoculated with sediment from the Isolator tube. Three isolates of Mycobacterium tuberculosis complex were recovered; two isolates from the MYCO/F Lytic bottle only and one isolate from the Isolator tube (whose sediment was subculture into the BACTEC 13A bottle) only. Two isolates of Cryptococcus neoformans were recovered; one from the MYCO/F Lytic bottle only and the other from the MYCO/F Lytic bottle and the Isolator tube (whose sediment was subcultured into the BACTEC 13A bottle).
In blood culture sets which produced growth of the same pathogens in both systems, there was a statistically significant difference in the median time to detection for all pathogens combined for the MYCO/F Lytic bottle compared with that for the Isolator system (Table 3). The mean and median times for recovery of all pathogens combined were 39.6 and 25 h, respectively, for the MYCO/F Lytic bottle, whereas they were 55.2 and 29.5 h, respectively, for the Isolator system.
TABLE 3.
Comparison of times to detection of pathogens for BACTEC MYCO/F Lytic bottle and Isolator system
| Microorganism(s) | Median (mean) detection time (h) with Isolator system | No. of isolates detected with Isolator system | Median (mean) detection time with BACTEC MYCO/F Lytic bottle | No. of isolates detected with BACTEC MYCO/F bottle | Difference in detection time (h) between Isolator system and BACTEC MYCO/F Lytic bottlec median (mean) | P valuea |
|---|---|---|---|---|---|---|
| All potential pathogens | 29.5 (55.2) | 320 | 25.0 (39.6) | 276 | 0 (−5.4) | 0.0004 |
| Staphylococcus aureus | 24 (51.7) | 76 | 22.5 (39.5) | 46 | 0 (−9.53) | 0.7896 |
| Staphylococcus spp., coagulase negative | 34 (40.2) | 59 | 27.0 (31.0) | 63 | 0 (8.0) | 0.0880 |
| Streptococcus pneumoniae | 34.0 (32.8) | 13 | 21.0 (20.4) | 7 | 0 (0.14) | 1.0 |
| Viridans group streptococci | 40.0 (41.4) | 11 | 22.0 (37.5) | 15 | 0 (5.14) | 0.7500 |
| Enterococcus spp. | 39.0 (37.7) | 12 | 27.0 (42.7) | 6 | −11 (−19.4) | 1.0 |
| Enterobacteriaceae family | 24.0 (47.9) | 85 | 21.0 (24.8) | 77 | 0 (19.6) | 0.0001 |
| Pseudomonas spp. | 37.0 (45.9) | 15 | 25.5 (29.4) | 14 | 12 (10.4) | 0.015 |
| Other bacteria | 30.5 (41.3) | 24 | 47.0 (70.6) | 27 | −7 (−35.6) | 0.1722 |
| Anaerobic organisms | 44.0 (37.0) | 3 | 25.0 (25.3) | 3 | 12 (11.67) | 0.5000 |
| Mycobacterium sp.b | 744 (744) | 1 | 564 (564) | 2 | ||
| Candida spp. | 84 (141.8) | 18 | 33.0 (44.9) | 14 | 17 (29.8) | 0.1562 |
| Cryptococcus neoformansb | 168 (168) | 1 | 72 (72) | 2 | 96 (96) | 1.0 |
| Histoplasma capsulatumb | 360 (360) | 2 | 0 |
Refer to Materials and Methods section for calculation of P values.
Isolates were recovered from one system only, so a comparison of detection times cannot be done.
Differences in detection times for the same organism isolated from both the Isolator system and the BACTEC MYCO/F Lytic bottle for the same blood culture set.
BACTEC MYCO/F Lytic bottle compared with BACTEC Plus Aerobic/F bottle.
As demonstrated in Table 4, the BACTEC Plus Aerobic/F bottle detected statistically significantly more potential pathogens overall (n = 373) than the BACTEC MYCO/F Lytic bottle (n = 276) (P = 0.0001). The BACTEC Plus Aerobic/F bottle also detected statistically significantly more isolates of S. aureus (P = 0.0001), potentially pathogenic coagulase-negative Staphylococcus spp. (P = 0.0018), and S. pneumoniae (P = 0.0020). The BACTEC Plus Aerobic/F bottle did not detect the two isolates of M. tuberculosis and two isolates of C. neoformans that the MYCO/F Lytic detected. Both the BACTEC MYCO/F Lytic bottle and the BACTEC Plus Aerobic/F bottle had similar frequencies of isolation of contaminants overall.
TABLE 4.
Comparison of BACTEC MYCO/F Lytic bottle to BACTEC Plus Aerobic/F bottle
| Potential pathogen(s) or contaminant(s) | No. of isolates with the following result:
|
P valueb | ||||
|---|---|---|---|---|---|---|
| Positive with BACTEC Plus Aerobic/F bottle only | Positive with BACTEC MYCO/F Lytic bottle only | Positive with both bottles | Positive with neither bottlea | Total | ||
| Pathogens | ||||||
| All microorganisms | 174 | 77 | 199 | 128 | 578 | 0.0001 |
| Staphylococcus aureus | 46 | 6 | 40 | 9 | 101 | 0.0001 |
| Staphylococcus spp., coagulase negative | 49 | 22 | 41 | 27 | 139 | 0.0018 |
| Streptococcus pneumoniae | 10 | 0 | 7 | 2 | 19 | 0.0020 |
| Viridans group streptococci | 14 | 6 | 9 | 10 | 39 | 0.1153 |
| Enterococcus spp. | 5 | 1 | 5 | 14 | 25 | 0.2188 |
| Enterobacteriaceae family | 27 | 20 | 57 | 21 | 125 | 0.3817 |
| Pseudomonas spp. | 4 | 1 | 13 | 1 | 19 | 0.3750 |
| Obligately anaerobic bacteria | 1 | 0 | 3 | 23 | 27 | 1.0 |
| Other bacteria | 12 | 12 | 15 | 12 | 51 | 1.0 |
| Mycobacterium tuberculosis complex | 0 | 2 | 0 | 1 | 3 | 0.5000 |
| Candida spp. | 6 | 5 | 9 | 6 | 26 | 1.0 |
| Cryptococcus neoformans | 0 | 2 | 0 | 0 | 2 | 0.5000 |
| Histoplasma capsulatum | 0 | 0 | 0 | 2 | 2 | 1.0 |
| Contaminants | ||||||
| All microorganisms | 9 | 8 | 0 | 141 | 158 | 1.0 |
| Bacillus spp. | 0 | 2 | 0 | 22 | 24 | 0.5000 |
| Corynebacterium spp., not C. jeikeim | 4 | 5 | 0 | 16 | 25 | 1.0 |
| Propionibacterium spp. | 1 | 1 | 0 | 4 | 6 | 1.0 |
| Staphylococcus spp., coagulase negative | 4 | 0 | 0 | 76 | 80 | 0.1250 |
| Viridans group streptococci | 0 | 0 | 0 | 6 | 6 | 1.0 |
| Other bacteria | 0 | 0 | 0 | 17 | 17 | 1.0 |
Refers to the number of isolates detected with only the Isolator system or the BACTEC Anaerobic Lytic/10 bottle.
Refer to Materials and Methods section for calculation of P values.
BACTEC MYCO/F Lytic bottle compared with BACTEC Anaerobic Lytic/10 bottle.
As shown in Table 5, there was no statistically significant difference in overall potential pathogen recovery for the MYCO/F Lytic bottle (n = 276) compared to that for the BACTEC Anaerobic Lytic/10 bottle (n = 298). As expected, the BACTEC Anaerobic Lytic/10 bottle recovered statistically significantly more isolates of obligately anaerobic bacteria than the BACTEC MYCO/F Lytic bottle (P = 0.0001). Also, as expected, the MYCO/F Lytic bottle detected statistically significantly more Pseudomonas spp. (P = 0.0002) and Candida spp. (P = 0.0074). The BACTEC Anaerobic Lytic/10 bottle also isolated statistically significantly more S. pneumoniae (P = 0.0039) and statistically significantly more potentially pathogenic viridans group streptococci (P = 0.0118) and Enterococcus spp. (P = 0.0063). Both of these systems recovered comparable numbers of potential contaminants overall.
TABLE 5.
Comparison of BACTEC MYCO/F Lytic bottle to BACTEC Anaerobic Lytic/10 bottle
| Potential pathogen(s) or contaminant(s) | No. of isolates with the following result:
|
P valueb | ||||
|---|---|---|---|---|---|---|
| Positive with BACTEC Anaerobic Lytic/10 bottle only | Positive with BACTEC MYCO/F Lytic bottle only | Positive with both bottles | Positive with neither bottlea | Total | ||
| Pathogens | ||||||
| All microorganisms | 125 | 103 | 173 | 177 | 578 | 0.1643 |
| Staphylococcus aureus | 7 | 6 | 40 | 48 | 101 | 1.0 |
| Staphylococcus spp., coagulase negative | 30 | 28 | 35 | 46 | 139 | 0.8957 |
| Streptococcus pneumoniae | 9 | 0 | 7 | 3 | 19 | 0.0039 |
| Viridans group streptococci | 16 | 4 | 11 | 8 | 39 | 0.0018 |
| Enterococcus spp. | 11 | 1 | 5 | 8 | 25 | 0.0063 |
| Enterobacteriaceae family | 18 | 15 | 62 | 30 | 125 | 0.7283 |
| Pseudomonas spp. | 0 | 13 | 1 | 5 | 19 | 0.0002 |
| Obligately anaerobic bacteria | 24 | 3 | 0 | 0 | 27 | 0.0001 |
| Other bacteria | 8 | 16 | 11 | 16 | 51 | 0.1516 |
| Mycobacterium tuberculosis complex | 0 | 2 | 0 | 1 | 3 | 0.5000 |
| Candida spp. | 2 | 13 | 1 | 10 | 26 | 0.0074 |
| Cryptococcus neoformans | 0 | 2 | 0 | 0 | 2 | 0.5000 |
| Histoplasma capsulatum | 0 | 0 | 0 | 2 | 2 | 1.0 |
| Contaminants | ||||||
| All microorganisms | 8 | 7 | 1 | 142 | 158 | 1.0 |
| Bacillus spp. | 2 | 2 | 0 | 20 | 24 | 1.0 |
| Corynebacterium spp., not C. jeikeim | 1 | 5 | 0 | 19 | 25 | 0.2188 |
| Propionibacterium spp. | 3 | 0 | 1 | 2 | 6 | 0.2500 |
| Staphylococcus spp., coagulase negative | 2 | 0 | 0 | 78 | 80 | 0.5000 |
| Other bacteria | 0 | 0 | 0 | 17 | 17 | 1.0 |
Refers to the number of isolates detected with only the Isolator system or the BACTEC Plus Aerobic/F bottle.
Refer to Materials and Methods section for calculation of P values.
BACTEC Plus Aerobic/F bottle compared with Isolator system.
As displayed in Table 6, the BACTEC Plus Aerobic/F bottle detected statistically significantly more potential pathogens overall (n = 373) than the Isolator system (n = 320) (P = 0.0003). The BACTEC Plus Aerobic/F bottle also detected statistically significantly more isolates of potentially pathogenic coagulase-negative Staphylococcus spp. (P = 0.0001) and potentially pathogenic viridans group streptococci (P = 0.002). There was no statistically significant difference in the recovery of Candida spp. for the two blood culture systems. However, two isolates of H. capsulatum, one isolate of C. neoformans, and one isolate of M. tuberculosis were recovered by the Isolator system but not by the BACTEC Plus Aerobic/F bottle. The Isolator system recovered statistically significantly more contaminants overall than the BACTEC Plus Aerobic/F bottle (P = 0.0001) (Table 6).
TABLE 6.
Comparison of Isolator system to BACTEC Plus Aerobic/F bottle
| Potential pathogen(s) or contaminant(s) | No. of isolates with the following result:
|
P valueb | ||||
|---|---|---|---|---|---|---|
| Positive with Isolator system only | Positive with BACTEC Plus Aerobic/F bottle only | Positive with both | Positive with neithera | Total | ||
| Pathogens | ||||||
| All microorganisms | 75 | 128 | 245 | 130 | 578 | 0.0003 |
| Staphylococcus aureus | 7 | 17 | 69 | 8 | 101 | 0.0639 |
| Staphylococcus spp., coagulase negative | 10 | 41 | 49 | 39 | 139 | 0.0001 |
| Streptococcus pneumoniae | 0 | 4 | 13 | 2 | 19 | 0.125 |
| Viridans group streptococci | 1 | 13 | 10 | 15 | 39 | 0.002 |
| Enterococcus spp. | 7 | 5 | 5 | 8 | 25 | 0.774 |
| Enterobacteriaceae family | 27 | 26 | 58 | 14 | 125 | 1.0 |
| Pseudomonas spp. | 1 | 3 | 14 | 1 | 19 | 0.625 |
| Obligately anaerobic bacteria | 0 | 1 | 3 | 23 | 27 | 1.0 |
| Other bacteria | 10 | 13 | 14 | 14 | 51 | 0.678 |
| Mycobacterium tuberculosis complex | 1 | 0 | 0 | 2 | 3 | 1.0 |
| Candida spp. | 8 | 5 | 10 | 3 | 26 | 0.581 |
| Cryptococcus neoformans | 1 | 0 | 0 | 1 | 2 | 1.0 |
| Histoplasma capsulatum | 2 | 0 | 0 | 0 | 2 | 0.50 |
| Contaminants | ||||||
| All microorganisms | 133 | 9 | 0 | 16 | 158 | 0.0001 |
| Bacillus spp. | 20 | 0 | 0 | 4 | 24 | 0.0001 |
| Corynebacterium spp., not C. jeikeim | 15 | 4 | 0 | 6 | 25 | 0.019 |
| Propionibacterium spp. | 1 | 1 | 0 | 4 | 6 | 1.0 |
| Staphylococcus spp., coagulase negative | 74 | 4 | 0 | 2 | 80 | 0.0001 |
| Viridans group streptococci | 6 | 0 | 0 | 0 | 6 | 0.031 |
| Other bacteria | 17 | 0 | 0 | 0 | 17 | 0.0001 |
Refers to the number of isolates detected with only the BACTEC MYCO/F Lytic bottle or the BACTEC Anaerobic Lytic/10 bottle.
Refer to Materials and Methods section for calculation of P values.
Statistically significant differences which favored the BACTEC Plus Aerobic/F bottle over the Isolator system for detection of bloodstream infections included the following: overall pathogen detection (P = 0.005), potentially pathogenic coagulase-negative Staphylococcus spp. (P = 0.0002), and viridans group streptococci (n = 0.012) (Table 7).
DISCUSSION
Other investigators have demonstrated that the BACTEC MYCO/F Lytic bottle is an effective selective blood culture system for the recovery of both fungi and mycobacteria (3, 4). Waite and Woods (4) simultaneously compared the performance of the MYCO/F Lytic bottle to that of the Isolator system for the recovery of fungi and to that of the ESPII system (AccuMed International, Westlake, Ohio) for the recovery of mycobacteria. Their evaluation was selective, in that blood specimens were obtained from AIDS patients with suspected fungemia or mycobacteremia, or both; therefore, an accurate assessment of the reliabilities of these three systems to detect fungi like Candida species or aerobic and anaerobic bacteria was not possible. As a result, assessments were primarily limited to C. neoformans, H. capsulatum, and Mycobacterium spp. Waite and Woods (4) observed that the Isolator system detected more isolates of H. capsulatum than the MYCO/F Lytic bottle (14 versus 7 isolates, respectively). Those investigators also noted that more isolates of C. neoformans were detected with the MYCO/F Lytic bottle than with the Isolator system (10 versus 5 isolates, respectively). These findings are in agreement with the results of our study.
Waite and Woods (4) also observed that the MYCO/F Lytic bottle detected statistically significantly more Mycobacteria spp. than the ESPII system (60 versus 44 isolates, respectively) and detected these isolates statistically significantly sooner. Although we detected only three isolates of M. tuberculosis in our study, two of the isolates were recovered with the MYCO/F Lytic bottle. It should be noted, however, that the methods used for mycobacterial culture in the two studies varied. We subcultured 0.5 to 0.75 ml of the sediment from the Isolator tube into a BACTEC 13A bottle; Waite and Woods (4) subcultured 0.5 ml of the sediment from an Isolator tube into an ESPII bottle.
Our study was not limited to any specific patient population. As such, we had proportionately fewer H. capsulatum isolates and Mycobacteria sp. isolates among the overall pathogens recovered. Nevertheless, our findings for H. capsulatum, C. neoformans, and Mycobacteria spp. were remarkedly similar to those of the study of Waite and Woods (4). In addition, we were able to determine the utility of the MYCO/F Lytic bottle for the isolation of Candida spp. and bacteria other than mycobacteria.
Fuller and colleagues (3) recently reported data from a comparison of the MYCO/F Lytic bottle to the Isolator system. In contrast to our study and the study by Waite and Woods (4), all of the sediment from the Isolator tube was subcultured onto fungal isolation plates. Despite this relatively larger inoculum for isolation of fungi for the Isolator system, Fuller and colleagues (3) noted comparable recoveries of H. capsulatum for both blood culture systems. Fuller and colleagues (3) inoculated 3 to 5 ml of blood directly into a BACTEC 13A bottle. As discussed above, we inoculated one-half of the Isolator tube sediment into a BACTEC 13A bottle and Waite and colleagues (4) inoculated 0.5 ml of Isolator tube sediment into an ESPII bottle. As in our study, Fuller and colleagues (3) observed that the MYCO/F Lytic medium recovered more Mycobacteria sp. isolates than the Isolator system.
The lower levels of recovery of H. capsulatum with the MYCO/F Lytic bottles compared with those for the Isolator system in our study may relate, in part, to specimen sampling. One of the two isolates of H. capsulatum produced only one colony on the brain heart infusion agar inoculated with the Isolator tube sediment. In this case, the assumption can be made that there was 1 CFU for ∼4 ml of blood specimen. Therefore, the lack of recovery of H. capsulatum with the MYCO/F Lytic bottle may, in this instance, be related to the sampling of specimens.
As in our study, Fuller and colleagues (3) also compared the MYCO/F Lytic bottle to the BACTEC Plus Aerobic/F bottle and the Isolator system for the recovery of bacterial pathogens. In that comparison the MYCO/F Lytic bottle was equivalent to the Plus Aerobic/F bottle; however, the MYCO/F Lytic bottle isolated statistically significantly more aerobic bacteria than the Isolator system (3). Our results showed that for the recovery of potential pathogens overall, including aerobic and anaerobic bacteria, mycobacteria, and fungi, there was a statistically significant difference that favored the Isolator system over the MYCO/F Lytic bottle.
For the present study, the BACTEC Plus Aerobic/F bottle isolated statistically significantly more potential pathogens overall than the Isolator system. Also, there was no statistically significant difference in the recovery of Candida spp. for the two systems. This was anticipated, as in a previous study, we showed the same results for the BACTEC Plus Aerobic/F bottle compared with those for the Isolator system (1). The BACTEC Plus Aerobic/F bottle also recovered statistically significantly more potential pathogens overall than the BACTEC MYCO/F Lytic bottle. This was expected, as 4 ml of blood was inoculated into the MYCO/F Lytic bottle, whereas 7 to 8 ml was inoculated into the Plus Aerobic/F bottle. Nevertheless, using similar volumes, Fuller and colleagues (3) did not observe this difference. For the present study, the MYCO/F Lytic bottle performed poorly for the recovery of obligately anaerobic bacteria compared with the performance of the BACTEC Anaerobic/10 bottle. This was expected, as this bottle is not optimized for recovery of obligately anaerobic bacteria, and again, less blood (4 ml) was inoculated into the MYCO/F Lytic bottle than into the Anaerobic/10 bottle (7 to 8 ml).
Because the numbers of fungi (other than Candida spp.) and mycobacteria were limited in our study, we cannot recommend the use of the MYCO/F Lytic system over the Isolator system or vice versa. Therefore, we believe that the combination of the Isolator system and the MYCO/F Lytic bottle may be useful as a selective blood culture method to optimize the recovery of fungi and mycobacteria from blood. The current practice in our laboratory is to offer separate orderable blood cultures, one designated the “bacteria-candida blood culture” and the other designated the “fungus-mycobacteria blood culture.” The bacteria-candida blood culture set consists of 30 ml of blood distributed equally among two BACTEC Plus Aerobic/F bottles and one BACTEC Anaerobic Lytic/10 bottle. The fungus-mycobacteria blood culture set consists of 16 ml of blood; 8 ml is inoculated into an Isolator tube and 4 ml is inoculated into two separate MYCO/F Lytic bottles.
Because of personnel requirements and cost issues, the use of the MYCO/F Lytic system alone may be adequate, especially for microbiology laboratories located in geographic areas where H. capsulatum is not endemic. Further studies are required to determine whether larger specimen volumes for the MYCO/F Lytic system (i.e., 4 ml inoculated into two separate MYCO/F Lytic bottles) will improve the recovery of H. capsulatum for the MYCO/F Lytic system compared to that for the Isolator system. If equivalency is noted with these studies, the MYCO/F Lytic system could serve as a stand-alone system for the recovery of fungi and mycobacteria from blood. Compared with the manual Isolator system, the BACTEC MYCO/F Lytic system has the advantage of less preanalytic processing and continuous automated monitoring of bottles for growth by the BACTEC 9240 instrument.
ACKNOWLEDGMENTS
We thank Roberta Kondert for efforts in preparation of the manuscript, the technologists in the Clinical Microbiology Laboratory and the phlebotomists for contributions to the evaluation, and Becton Dickinson for providing the BACTEC 9240 and 460 instruments and the BACTEC MYCO/F Lytic and 13A blood culture bottles.
REFERENCES
- 1.Cockerill F R, III, Reed G S, Hughes J G, Torgerson C A, Vetter E A, Harmsen W S, Dale J C, Roberts G D, Ilstrup D M, Henry N K. Clinical comparison of BACTEC 9240 Plus Aerobic/F Resin bottles and the Isolator Aerobic culture system for detection of bloodstream infections. J Clin Microbiol. 1997;35:1469–1472. doi: 10.1128/jcm.35.6.1469-1472.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkley B A, Easley K A, Washington J A. Controlled clinical evaluation of Isolator and ESP aerobic blood culture systems for detection of bloodstream infections. J Clin Microbiol. 1994;32:1547–1549. doi: 10.1128/jcm.32.6.1547-1549.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuller D, Davis T E, Denys G A, York M K. Evaluation of BACTEC MYCO/F Lytic medium for recovery of mycobacteria, fungi, and bacteria from blood. J Clin Microbiol. 2001;39:2933–2936. doi: 10.1128/JCM.39.8.2933-2936.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waite R T, Woods G T. Evaluation of BACTEC MYCO/F Lytic medium for recovery of mycobacteria and fungi from blood. J Clin Microbiol. 1998;36:1176–1179. doi: 10.1128/jcm.36.5.1176-1179.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]