Abstract
Objective
To describe initial benzodiazepine dosing strategies and factors associated with variation in benzodiazepine dosing in a national cohort of hospitalized patients with alcohol withdrawal syndrome (AWS).
Patients and Methods
This cross-sectional study included adult patients with AWS admitted to medical services and treated with benzodiazepines at 93 Veterans Health Administration hospitals in 2013. Treatment was categorized by initial benzodiazepine dosing strategy—fixed-dose, symptom-triggered, or front-loading. Associations with patient characteristics, facility, and cumulative benzodiazepine exposure, intensive care, and intubation were evaluated.
Results
Among 6938 medical inpatients with AWS, 2909 (41.9%), 2829 (40.8%), and 1200 (17.3%) received treatment with symptom-triggered, fixed-dose, and front-loading benzodiazepines, respectively. The magnitude of differences in initial treatment associated with patient characteristics was small compared with differences associated with the predominant practice at a facility. Compared with fixed-dose therapy, symptom-triggered therapy was associated with higher cumulative benzodiazepine exposure (mean, 208-mg vs 182-mg diazepam equivalents) and higher probability of intensive care and intubation (28.2% vs 21.3% and 4.8% vs 3.5%, respectively).
Conclusion
This study revealed that real-world AWS treatment of medical inpatients was often inconsistent with published guidelines recommending symptom-triggered long-acting benzodiazepines for AWS. The facility where a patient was hospitalized was associated with marked treatment variation. In contrast to prior randomized controlled trials conducted in specialized detoxification units, hospitalized patients who received symptom-triggered therapy in this study had greater cumulative benzodiazepine exposure and higher probability of intensive care and intubation than those receiving fixed-dose therapy.
Abbreviations and Acronyms: AWS, alcohol withdrawal syndrome; BZD, benzodiazepine; CIWA-Ar, Clinical Institute Withdrawal Assessment for Alcohol revised; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICU, intensive care unit; RCT, randomized controlled trial; VHA, Veterans Health Administration
Alcohol withdrawal syndrome (AWS) is common in hospitalized patients and associated with increased intensive care unit (ICU) and hospital length of stay, hospital-acquired infections, sepsis, and in-hospital mortality.1, 2, 3, 4, 5, 6 Treatment with benzodiazepines (BZDs) improves AWS but can also lead to complications in hospitalized patients,7 including somnolence, respiratory depression, delirium, and death, with greater BZD exposure associated with increased risk of adverse outcomes.8, 9, 10, 11, 12, 13 Treatment of AWS with BZDs is typically provided using 1 of 3 dosing strategies: fixed-dose, symptom-triggered, or front-loading (Table 1).14 Clinical guidelines generally recommend treatment of mild to moderate AWS using symptom-triggered dosing of long-acting BZDs.14, 15, 16, 17 For severe AWS, front-loading with large/frequent doses of BZDs is often recommended.14,18,19
Table 1.
Benzodiazepine Dosing Strategies and National Prevalence of Each Strategy Among 6938 Medical Inpatients With Alcohol Withdrawal Syndrome in the Veterans Health Administration During 2013a,b
| Benzodiazepine dosing strategies |
National prevalence in medical inpatients with AWS, No. (%; 95% CI) | |
|---|---|---|
| Clinical definition | Operational definition for this study | |
| Fixed-dose therapy A predetermined dose is administered at fixed, scheduled intervals Dose frequency/amount is tapered over a period of days |
≥2 Doses of the same benzodiazepine administered on a scheduled basis at ≥4-h intervals (eg, every 6-8 h) | 2829 (40.8; 35.5-46.3) |
| Symptom-triggered therapy Patients are monitored using a structured assessment scale (eg, CIWA-Ar) Medications are administered when symptoms cross a given severity threshold (eg, CIWA-Ar ≥8) Different doses of medication are administered for different categories of scores (eg, CIWA-Ar scores 8-15 vs >15) |
≥2 Doses of the same benzodiazepine administered “PRN” (as needed) at ≥4-h intervals (eg, every 6-8 h) | 2909 (41.9; 36.4-47.6) |
| Front-loading therapy Moderate to high doses of medication are administered frequently or continuously early in the course of treatment for rapid control of symptoms |
(1) ≥40-mg diazepam equivalents of benzodiazepine administered as a 1-time dose AND/OR (2) Combination of “PRN” (as needed) and scheduled doses resulting in ≥40-mg diazepam equivalents of benzodiazepine within 4 h AND/OR (3) Any continuous infusion of benzodiazepine |
1200 (17.3; 15.3-19.5) |
AWS, alcohol withdrawal syndrome; CIWA-Ar, Clinical Institute Withdrawal Assessment for Alcohol revised.
Estimated confidence intervals account for intraclass correlations at the hospital level.
Although clinical guidelines support the use of symptom-triggered BZDs for most patients who require pharmacotherapy for AWS,14,16,17 little is known about how BZDs are used to treat AWS in hospitals, where practical barriers may interfere with symptom-triggered dosing strategies. Symptom-triggered therapy involves medication titration using a structured assessment scale—most commonly, the revised Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar).14,15,20,21 While use of the CIWA-Ar was associated with lower total BZD exposure and shorter duration of treatment in alcohol detoxification units,15,21 subsequent studies in hospitals identified challenges with use of the CIWA-Ar in patients who are acutely ill.22, 23, 24, 25, 26 No published research has evaluated whether hospital practices for AWS align with guideline recommendations. Factors associated with the use of different BZD dosing strategies for AWS and associations between BZD dosing strategies and key features of the hospital course (eg, cumulative BZD exposure, ICU care, intubation) are also unknown.
This study evaluated the treatment and hospital course of patients with AWS admitted to medical services in the Veterans Health Administration (VHA) nationwide during 2013. Specifically, this study describes (1) initial BZD dosing strategies for hospitalized patients with AWS, (2) factors associated with the use of different BZD dosing strategies, and (3) associations between BZD dosing strategies and cumulative BZD exposure, ICU care, and intubation.
Patients and Methods
Study Sample and Source of Data
This retrospective cohort included secondary analyses of administrative and clinical data from the VHA in fiscal year 2013 (October 1, 2012, to September 30, 2013).27 Patients were eligible if engaged in VHA care and (1) admitted for 24 or more hours to a medical service, (2) diagnosed as having AWS, and (3) treated with BZDs. The study was limited to 93 facilities with surgical and intensive care capabilities (ie, common characteristics of general hospitals). Most inpatients with AWS in the VHA are admitted to medical services,6 and treatment by other services could reflect other factors.28 Among patients with multiple eligible admissions, one hospitalization per patient was randomly selected. Inpatient AWS was determined by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic/procedure code(s) documented during the hospitalization (Supplemental Box 1, available online at http://www.mayoclinicproceedings.org). Treatment of AWS with BZDs was defined as receipt of 2 or more doses of BZD separated by 14 or more hours during hospital days 0 through 10, the expected time frame for AWS. Demographic data, ICD-9-CM diagnostic and procedure codes, inpatient pharmacy records, and facility data were obtained from the VHA Corporate Data Warehouse. The study received approval and waivers of informed consent and HIPAA (Health Insurance Portability and Accountability Act) authorization from the VA Puget Sound Health Care System and University of Washington institutional review boards.
AWS Treatment—Initial BZD Dosing Strategies for AWS
The initial BZD dosing strategy (Table 1) used for each patient with AWS was defined as the strategy received earliest in the hospital course. Because structured symptom monitoring (eg, using the CIWA-Ar) was inconsistently documented in the VHA at the time of this study, scheduled and “PRN” (as needed) administration of medications were used as proxy measures for fixed-dose and symptom-triggered therapy, respectively. Specifically, fixed dosing was defined as 2 or more doses of the same BZD administered on a scheduled basis at 4-hour or longer intervals. Symptom-triggered dosing was defined as 2 or more doses of the same BZD administered as needed at 4-hour or longer intervals. Front loading was defined as (1) 40-mg or more diazepam equivalents of BZD administered as a 1-time dose and/or (2) a combination of as needed and scheduled doses resulting in 40-mg or more diazepam equivalents of BZD within 4 hours and/or (3) any continuous infusion of BZD.14, 15, 16,18,19,21 All BZDs on the VHA formulary were included and categorized as short- or long-acting. The milligram diazepam equivalents of each BZD were calculated using a standard algorithm (Supplemental Box 2, available online at http://www.mayoclinicproceedings.org). Receipt of alternative (non-BZD) AWS medications (Supplemental Table 1, available online at http://www.mayoclinicproceedings.org) and adjuvant antipsychotic medications were also described for each patient.14
Hospital Course of AWS Treatment
Cumulative BZD exposure was defined as the total BZD (milligram diazepam equivalents) received by patients during hospital days 0 through 10. Intensive care unit treatment was defined by VHA bed codes, and intubation was defined by ICD-9-CM procedure codes during hospital days 0 through 10 (Supplemental Box 1). Although ICU care and intubation were measurable using procedure codes, more granular data regarding the specific reason for intubation or ICU admission were not available.
Covariates
Demographic data were obtained the day of admission or during the prior year. Prior-year AWS and alcohol use disorder were defined using inpatient and outpatient ICD-9-CM diagnosis/procedure codes documented in the 365 days prior to hospital admission (Supplemental Box 1). Nineteen common comorbid inpatient diagnoses were identified using ICD-9-CM codes (Supplemental Table 2, available online at http://www.mayoclinicproceedings.org). Hospitals were defined by unique VHA facility codes.
Statistical Analyses
Initial BZD dosing strategies were identified for each patient, and the national prevalence of each strategy was estimated with 95% CIs, accounting for intraclass correlations at the hospital level. Bivariate analyses characterized the demographic and clinical characteristics of patients who received each BZD dosing strategy and the classes of AWS medications the patients received (eg, short- or long-acting BZDs). We used χ2 tests to assess differences across the 3 dosing strategy groups. The prevalence of each BZD dosing strategy was evaluated by hospital, and hospitals were ranked on the basis of the proportion of patients initially treated with each BZD dosing strategy.
To explore the association between initial BZD dosing strategy and hospital course, 3 outcomes were evaluated using mixed-effects linear or logistic regression: cumulative BZD exposure, ICU care, and intubation. All 3 models included patient demographic and clinical characteristics, inpatient diagnoses, and hospital site as a random effect. In models of ICU care and intubation, cumulative BZD exposure was included as a covariate representing AWS severity, planned a priori, reasoning that greater severity of AWS would require greater amounts of counterbalancing BZD medication. Adjusted probabilities and marginal effects were predicted from the models using the observed values of covariates (as opposed to mean values). All analyses were performed using Stata statistical software, version 16.0 (StataCorp).29
Results
Among 209,151 eligible patients admitted to medical services at 93 hospitals in the VHA in fiscal year 2013, 9727 (4.7%) had a documented diagnosis of inpatient AWS (Supplemental Figure 1, available online at http://www.mayoclinicproceedings.org). Within this group, 2789 (28.7%) received no or minimal BZDs and were excluded from further analyses. Roughly two-thirds of excluded patients (1860 [66.7%]) received no alternative (non-BZD) medications for AWS, suggesting clinically insignificant AWS requiring no or minimal pharmacotherapy.
The study sample included 6938 patients with AWS who received BZD treatment. Patients were on average 57 years old and were predominantly male (6735 patients [97.1%]) and White (5023 patients [72.4%]), and many were hospitalized with acute exacerbations of mental health disorders (3513 patients [50.6%]) or complications related to nutrition, electrolyte, or acid-base disorders (3114 patients [44.9%]). Regarding medications used for AWS in the 6938 patients, lorazepam, a short-acting BZD, was prescribed most often (5518 patients [79.5%]), followed by chlordiazepoxide (2490 patients [35.9%]) and diazepam (1003 patients [14.5%]), both long-acting BZDs (Supplemental Table 3, available online at http://www.mayoclinicproceedings.org). Many patients received more than one BZD. In addition, 2230 patients (32.1%) received a non-BZD AWS medication, most commonly gabapentin, and 1511 (21.8%) received an antipsychotic medication, most commonly haloperidol.14
AWS Treatment—Initial BZD Dosing Strategies for AWS
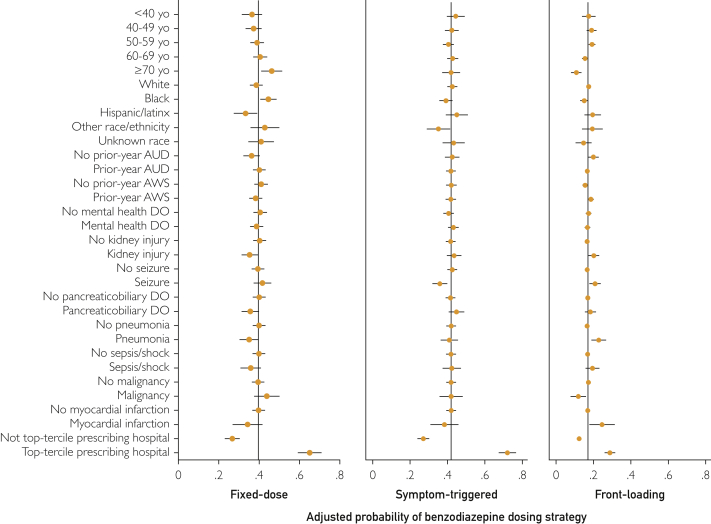
No single initial BZD dosing strategy for AWS predominated among medical inpatients (Table 1): 2829 (40.8%) received fixed-dose therapy, 2909 (41.9%) received symptom-triggered therapy, and 1200 (17.3%) received front-loading therapy. Patients receiving symptom-triggered therapy were less likely to receive long-acting BZDs (1140 patients [39.2%]) than patients receiving fixed-dose (1654 patients [58.5%]) or front-loading therapy (688 patients [57.3%]; P<.001) (Supplemental Table 4, available online at http://www.mayoclinicproceedings.org). While several patient-level characteristics differed across BZD dosing strategies for AWS, the magnitude of these differences was small (Table 2). In the adjusted models (Supplemental Table 5, available online at http://www.mayoclinicproceedings.org), fixed-dose therapy was positively associated with age 70 years or older (vs age <40 years), Black race (vs White race), and prior-year alcohol use disorder and inversely associated with prior-year AWS, kidney injury, pancreaticobiliary disease, and pneumonia. Symptom-triggered therapy was positively associated with mental health disorders and inversely associated with Black and “Other” race (vs White race) and seizure. Front-loading therapy was positively associated with prior-year AWS, kidney injury, seizure, pneumonia, and myocardial infarction and inversely associated with age 70 years or older (vs age <40 years), Black race (vs White race), and malignancy.
Table 2.
Characteristics of Medical Inpatients Stratified by Benzodiazepine Dosing Strategy for AWS in the Veterans Health Administration During 2013a,b,c
| Variable | Fixed dose (N=2829) | Symptom triggered (N=2909) | Front loading (N=1200) | P value |
|---|---|---|---|---|
| Age (y) | <.001 | |||
| <40 | 154 (5.4) | 213 (7.3) | 75 (6.2) | |
| 40-49 | 354 (12.5) | 414 (14.2) | 174 (14.5) | |
| 50-59 | 1049 (37.1) | 1035 (35.6) | 482 (40.2) | |
| 60-69 | 1055 (37.3) | 1056 (36.3) | 413 (34.4) | |
| ≥70 | 217 (7.7) | 191 (6.6) | 56 (4.7) | |
| Male | 2757 (97.5) | 2811 (96.6) | 1167 (97.2) | .17 |
| Race | <.001 | |||
| White | 1951 (69.0) | 2206 (75.8) | 865 (72.1) | |
| Black | 602 (21.3) | 402 (13.8) | 189 (15.7) | |
| Hispanic/Latinx | 107 (3.8) | 114 (3.9) | 69 (5.7) | |
| Other race/ethnicity | 81 (2.9) | 77 (2.6) | 38 (3.2) | |
| Unknown | 88 (3.1) | 110 (3.8) | 39 (3.2) | |
| Single | 2220 (78.5) | 2337 (80.3) | 947 (78.9) | .20 |
| Homeless | 864 (30.5) | 920 (31.6) | 363 (30.2) | .57 |
| Prior-year AWS and/or AUD | ||||
| AWS | 1355 (47.9) | 1500 (51.6) | 650 (54.2) | <.001 |
| AUD | 2483 (87.8) | 2554 (87.8) | 1023 (85.2) | .06 |
| AWS/AUD | 2528 (89.4) | 2600 (89.4) | 1051 (87.6) | .20 |
| Comorbid inpatient diagnosesd | ||||
| Mental health disorder | 1381 (48.8) | 1555 (53.4) | 577 (48.1) | <.001 |
| Nutrition, electrolyte, or acid-base disorder | 1293 (45.7) | 1252 (43.0) | 569 (47.4) | .02 |
| Kidney injury | 341 (12.1) | 366 (12.6) | 202 (16.8) | <.001 |
| Seizure | 289 (10.2) | 229 (7.9) | 151 (12.6) | <.001 |
| Pancreaticobiliary disease | 232 (8.2) | 291 (10.0) | 127 (10.6) | .02 |
| Pneumonia | 173 (6.1) | 189 (6.5) | 124 (10.3) | <.001 |
| Sepsis/shock | 148 (5.2) | 194 (6.7) | 111 (9.2) | <.001 |
| Malignancy | 117 (4.1) | 87 (3.0) | 28 (2.3) | <.01 |
| Myocardial infarction | 49 (1.7) | 54 (1.9) | 41 (3.4) | <.01 |
AUD, alcohol use disorder; AWS, alcohol withdrawal syndrome.
Data are presented as No. (percentage) of patients.
χ2 Tests were used to assess differences across groups.
Inpatient diagnoses that did not differ by benzodiazepine dosing strategy: gastrointestinal tract disorder, liver injury, musculoskeletal or soft tissue disorder, chronic obstructive pulmonary disease, other substance use condition, cardiac dysrhythmia, diabetes mellitus, trauma, congestive heart failure, and cerebrovascular disease.
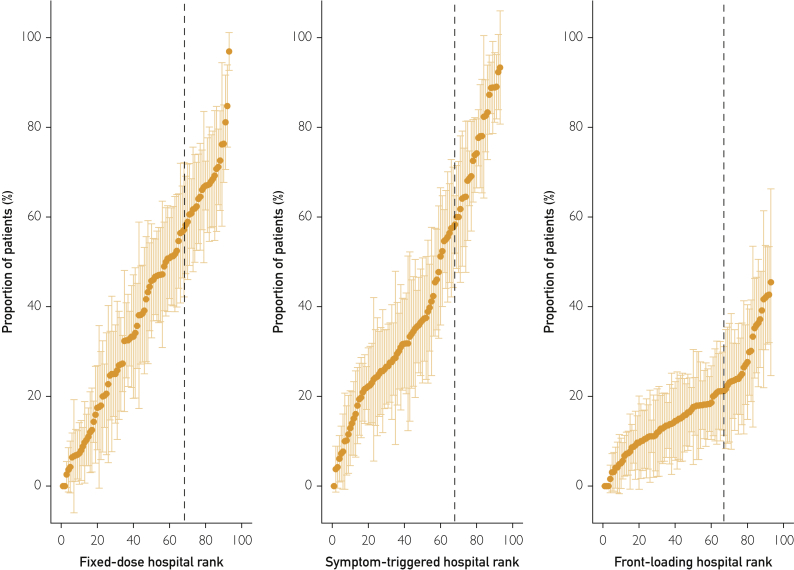
The proportion of patients initially treated with each BZD dosing strategy varied substantially across the 93 hospitals (Figure 1). This difference was especially true for fixed-dose and symptom-triggered strategies, which—depending on the facility—were the initial treatment strategies for between 0% and more than 90% of patients (Figure 1). At hospitals ranked in the top third for prescribing fixed-dose or symptom-triggered therapy (to the right of dashed lines in Figure 1), upwards of 60% of patients received that strategy. The hospital where patients were admitted thus appeared to be a strong predictor of initial AWS treatment. To evaluate this hypothesis, 3 binary variables were constructed to identify hospitals with a predominant AWS treatment strategy, defined as being in the top tercile for prescribing each BZD dosing strategy (vs not). These binary indicators were added to 3 mixed-effects logistic regression models that also included patient characteristics (one model for each strategy). The adjusted relative odds and predicted probability of each dosing strategy across patient and hospital characteristics revealed the magnitude of differences associated with hospital prescribing pattern (Figure 2 and Supplemental Table 5). Specifically, AWS treatment at hospitals in the top tercile for prescribing fixed-dose therapy was associated with a 38.3% absolute increase in probability of receiving this strategy compared with treatment at other hospitals (65.0% vs 26.7%). AWS treatment at hospitals in the top tercile for prescribing symptom-triggered therapy was associated with a 44.9% absolute increase in probability of receiving this strategy (72.0% vs 27.1%). The association was weaker at facilities in the top tercile for prescribing front-loading BZDs, with a 16.5% absolute increase in probability of receiving this dosing strategy (28.8% vs. 12.3%).
Figure 1.
Proportion of medical inpatients with alcohol withdrawal syndrome receiving fixed-dose, symptom-triggered, and front-loading benzodiazepines by hospital (N=93 sites) in the Veterans Health Administration during 2013. Hospitals to the right of the dashed lines are in the top tercile for prescribing each dosing strategy.
Figure 2.
Adjusted probability of fixed-dose (n=2829), symptom-triggered (n=2909), or front-loading (n=1200) benzodiazepine dosing strategies across patient characteristics and hospital prescribing pattern. Each panel shows results from a separate mixed-effects logistic regression model (one for each benzodiazepine dosing strategy) including all demographic and clinical factors, all inpatient diagnoses, and hospital as a random effect, using margins to estimate the adjusted predicted probability (Supplemental Table 5). Variables (all binary) in the model but not depicted (due to no significant association with a dosing strategy): sex (male/female), single relationship status, homelessness, nutrition/electrolyte/acid-base disorder (DO), gastrointestinal tract DO, liver injury, musculoskeletal or soft tissue DO, chronic obstructive pulmonary disease, other substance use condition, cardiac dysrhythmia, diabetes mellitus, trauma, congestive heart failure. See Supplemental Table 5 for full model with results for all variables. AUD, alcohol use disorder; AWS, alcohol withdrawal syndrome; yo, years old.
Hospital Course of AWS Treatment
Cumulative BZD exposure, ICU care, and intubation during hospital days 0 through 10 differed based on initial BZD dosing strategy for AWS (Table 3; Supplemental Table 6, available online at http://www.mayoclinicproceedings.org). Symptom-triggered therapy was associated with higher cumulative BZD exposure than fixed-dose therapy (adjusted predicted mean, 208 vs 182 mg diazepam equivalents). The probabilities of both ICU care and intubation were also greater for patients receiving symptom-triggered therapy than those receiving fixed-dose therapy (28.2% vs 21.3% adjusted probability of ICU care and 4.8% vs 3.5% adjusted probability of intubation). Front-loading therapy was associated with greater cumulative BZD exposure (adjusted predicted mean, 351 mg diazepam equivalents), need for ICU care (31.9% adjusted probability), and need for intubation (10.5% adjusted probability) than either fixed-dose or symptom-triggered therapy.
Table 3.
Associations Between Benzodiazepine Dosing Strategy for Alcohol Withdrawal Syndrome and Cumulative Benzodiazepine Exposure, Intensive Care, and Intubation, Unadjusted and Adjusted for Patient Characteristics in the Veterans Health Administration during 2013a, b, c
| Strategy | Cumulative benzodiazepine |
ICU care |
Intubation |
|||
|---|---|---|---|---|---|---|
| Unadj (95% CI) |
Adj (95% CI) |
Unadj (95% CI) |
Adj (95% CI) |
Unadj (95% CI) |
Adj (95% CI) |
|
| Coefficient | Odds ratio | Odds ratio | ||||
| Fixed-dose | Reference | Reference | Reference | Reference | Reference | Reference |
| Symptom-triggered | 30.5 (13.5-47.5)d | 26.4 (9.4-43.4)d | 1.7 (1.5-2.0)d | 1.7 (1.4-2.0)d | 1.6 (1.2-2.2)d | 1.5 (1.1-2.1)d |
| Front-loading | 175.9 (155.3-196.4)d | 168.8 (148.2-189.4)d | 3.0 (2.6-3.6)d | 2.2 (1.8-2.6)d | 5.6 (4.2-7.4)d | 4.9 (3.5-6.9)d |
| Predicted mean mg diazepam equivalents | Predicted probability | Predicted probability | ||||
|---|---|---|---|---|---|---|
| Fixed-dose | 179 (161-197) | 182 (164-200) | 19.9 (16.9-22.9) | 21.3 (18.6-24.0) | 3.2 (2.3-4.0) | 3.5 (2.8-4.2) |
| Symptom-triggered | 210 (192-227) | 208 (190-226) | 28.8 (25.2-32.4) | 28.2 (25.0-31.3) | 4.9 (3.8-6.0) | 4.8 (4.0-5.6) |
| Front-loading | 355 (333-377) | 351 (329-372) | 40.1 (35.7-44.6) | 31.9 (28.2-35.6) | 14.7 (12.0-17.3) | 10.5 (8.9-12.1) |
eFor complete results of each model, including adjusted coefficients and odds ratios for each covariate, see Supplemental Table 6.
Adj, adjusted; ICU, intensive care unit; Unadj, unadjusted.
All adjusted models included all patient demographic and clinical characteristics, all inpatient diagnoses, and hospital site as a random effect. In models of ICU care and intubation, cumulative benzodiazepine exposure was also included as a covariate representing alcohol withdrawal syndrome severity planned a priori—ie, greater severity of alcohol withdrawal syndrome will require greater amounts of counterbalancing medications.
Hospital intraclass correlation coefficient for cumulative benzodiazepine, 0.05 (95% CI, 0.03-0.07); ICU care, 0.20 (95% CI, 0.15-0.26); intubation, 0.06 (95% CI, 0.03-0.13).
P<.01.
Discussion
This study of almost 7000 medical inpatients with AWS, treated with BZDs at 93 hospitals nationwide, identified important variation in initial treatment strategies used for inpatient AWS. Although symptom-triggered therapy using long-acting BZDs is generally recommended,14, 15, 16, 17 this study of routine hospital practice found that symptom-triggered therapy and fixed-dose therapy were used about equally (41.9% vs 40.8%), and most patients received short-acting BZDs (79.5% received lorazepam). Benzodiazepine dosing strategies were weakly associated with individual patient characteristics. Rather, the predominant dosing strategy used at the hospital where patients received their care was strongly associated with initial AWS treatment. As expected, patients treated with front-loading therapy, recommended for severe AWS,14,18,19 had the highest cumulative BZD exposure and were most likely to need ICU care and/or intubation. Unexpectedly, patients initially treated with symptom-triggered therapy also had higher cumulative BZD exposure and probabilities of ICU care and intubation than patients receiving fixed-dose therapy.
A majority of hospitalized patients with AWS did not receive symptom-triggered dosing of BZDs, although this strategy is recommended by guidelines.14, 15, 16, 17 While some hospitals appeared to routinely implement symptom-triggered therapy, using it for more than 80% of medical inpatients with AWS, many other hospitals appeared to favor fixed-dose therapy (Figure 1; Supplemental Figure 2, available online at http://www.mayoclinicproceedings.org). This finding may reflect barriers to use of symptom-triggered BZDs in acutely ill patients.23,25 Symptom-triggered BZD protocols for AWS require substantial investment in physician and nursing education, as well as time at the bedside, which may not be feasible at all facilities.
Certain patient characteristics were associated with lower likelihood of symptom-triggered therapy, including Black or “Other” race. Likewise, a small study in ICU patients found that CIWA-Ar assessments were completed less often in patients treated for AWS who identified as Black.26 These associations could reflect differences in care associated with racism and/or other biases.30, 31, 32 Confounding variables are also important to consider, particularly given the apparent influence of hospital factors on AWS treatment (eg, if regional variation in treatment strategies exists and fixed-dose strategies are predominantly used at facilities in regions where Black patients are more commonly hospitalized).
Front-loading therapy is recommended for severe AWS and often requires management in an ICU.14,16, 17, 18 Among patients who received front-loading therapy in this study, nearly 1 in 3 received ICU care and 1 in 10 required intubation during hospital days 0 through 10—2- to 3-fold higher than patients receiving fixed-dose or symptom-triggered therapy. These findings are perhaps not surprising but offer a point of reference for future research. Recently published guidelines suggest very large doses of BZDs may be needed to control the manifestations of severe AWS, acknowledging the associated risks of oversedation and respiratory depression,14 and also suggest phenobarbital be considered as an alternative or adjuvant treatment for AWS.14
No prior studies have evaluated BZD dosing strategies in hospitalized patients with acute illness. In contrast to the findings of prior randomized controlled trials (RCTs) conducted in specialized detoxification units,15,21 medical inpatients with AWS receiving symptom-triggered therapy had greater cumulative BZD exposure and higher likelihood of ICU care and intubation than those receiving fixed-dose therapy. The RCTs comparing symptom-triggered and fixed-dose therapy found shorter treatment duration, reduced cumulative BZD exposure, and no differences in withdrawal severity or adverse events among patients randomized to symptom-triggered therapy,15,21 but these studies were in patients without acute comorbidities. Our cross-sectional study’s finding of greater cumulative BZD exposure associated with symptom-triggered therapy should be interpreted with caution and may reflect complex, bidirectional relationships between patient-level factors (eg, demographic characteristics, comorbid medical/surgical diagnoses, severity of AWS), hospital structures (eg, care protocols, electronic order sets, nurse to patient ratio), and BZD dosing.
This study’s finding that nearly 80% of hospitalized patients with AWS received short-acting BZDs highlights a potentially important gap between clinical guidelines recommending long-acting BZDs and actual hospital practice.14,16,17,19 A Cochrane review suggested that chlordiazepoxide (a long-acting BZD) was associated with slightly better treatment performance than other BZDs with respect to prevention of AWS seizures, adverse events, and treatment dropout.7 In practice, however, hospital physicians may hesitate to use long-acting BZDs in patients with acute illness.8, 9, 10, 11, 12, 13 Importantly, most research supporting long-acting BZDs for AWS has been conducted in specialized detoxification units rather than general hospital settings.7,33
Our study had limitations. The study relied on secondary VHA administrative and clinical data. Use of ICD-9-CM diagnosis/procedure codes to identify patients with AWS may result in underidentification. Initial BZD dosing strategies were defined using electronic pharmacy data, including scheduled vs “as needed” orders to distinguish fixed-dose and symptom-triggered BZDs. Misclassification of initial dosing strategies is possible, and crossover was not evaluated. This study also lacked commonly used measures of AWS severity (eg, CIWA-Ar), adverse effects of BZDs (eg, sedation), and a global/composite measure of acute illness severity. The CIWA-Ar scores were not consistently recorded in the VHA in fiscal year 2013. While hospital site was associated with significant variation in BZD dosing strategies, the granular factors responsible for this variation could not be evaluated using the available secondary data. Because the study was cross-sectional, temporal relationships between BZD dosing, ICU care, and intubation could not be evaluated (eg, ICU admission could lead to symptom-triggered BZD dosing if staffing was inadequate elsewhere in the hospital). The study data set included mostly male, older-age, White patients engaged in VHA care in 2013. Treatment may have evolved since the time of data collection, although no RCTs have been reported in hospitalized patients with AWS since 2013 and we therefore expect that notable practice variation persists.
Despite these limitations, this study has important strengths. The sample included 6938 patients with AWS, admitted to 93 hospitals nationwide. By comparison, all 14 high-quality studies in a recent clinical review of inpatient AWS combined included only 1355 patients with AWS, and only 27 patients with AWS from general hospital samples (as opposed to samples drawn from specialized detoxification units).34,35 This is the first study to describe variation in BZD dosing strategies used in routine hospital care and their association with patient characteristics and hospital prescribing patterns. By also evaluating the associations between initial BZD dosing strategies and key features of the hospital treatment course (ie, cumulative BZD exposure, ICU care, and intubation), this study generates important hypotheses for future research.36
Conclusion
This study describes routine management of AWS in a national sample of hospitalized patients, providing novel insights regarding the actual treatment and hospital course of medical inpatients with AWS. The results revealed large variation in the use of different BZD dosing strategies across hospitals and treatment practices that predominantly do not reflect published expert recommendations.14, 15, 16, 17 The study suggests that BZD dosing strategies for AWS, previously studied in specialized detoxification units, may be associated with different patient outcomes when used routinely in general hospital settings. Our results highlight the need for further research to establish best practices for inpatient treatment of AWS.
Acknowledgments
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the US Department of Veterans Affairs, the US government, the National Institutes of Health, or the National Institute on Alcohol Abuse and Alcoholism.
All authors had access to the data supporting this study and a role in writing the submitted manuscript.
Footnotes
Grant Support: Drs Steel and Hawkins were supported by the VA Puget Sound Health Care System Research and Development Associate Chief of Staff Pilot Grant Program; Drs Malte and Hawkins were supported by the Center of Excellence for Substance Abuse Treatment and Education; and Dr Bradley was supported by grant K24AA022128 from the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism.
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Moss M., Burnham E.L. Alcohol abuse in the critically ill patient. Lancet. 2006;368(9554):2231–2242. doi: 10.1016/S0140-6736(06)69490-7. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien J.M., Jr., Lu B., Ali N.A., et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35(2):345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- 3.McKeon A., Frye M.A., Delanty N. The alcohol withdrawal syndrome. J Neurology Neurosurg Psychiatry. 2008;79(8):854–862. doi: 10.1136/jnnp.2007.128322. [DOI] [PubMed] [Google Scholar]
- 4.de Wit M., Jones D.G., Sessler C.N., Zilberberg M.D., Weaver M.F. Alcohol-use disorders in the critically ill patient. Chest. 2010;138(4):994–1003. doi: 10.1378/chest.09-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monte R., Rabuñal R., Casariego E., López-Agreda H., Mateos A., Pértega S. Analysis of the factors determining survival of alcoholic withdrawal syndrome patients in a general hospital. Alcohol Alcohol. 2010;45(2):151–158. doi: 10.1093/alcalc/agp087. [DOI] [PubMed] [Google Scholar]
- 6.Steel T.L., Malte C.A., Bradley K.A., Lokhandwala S., Hough C.L., Hawkins E.J. Prevalence and variation of clinically recognized inpatient alcohol withdrawal syndrome in the Veterans Health Administration. J Addict Med. 2020;14(4):300–304. doi: 10.1097/ADM.0000000000000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amato L., Minozzi S., Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst Rev. 2011;2011(6):CD008537. doi: 10.1002/14651858.CD008537.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elie M., Cole M.G., Primeau F.J., Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13(3):204–212. doi: 10.1046/j.1525-1497.1998.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouimet S., Kavanagh B.P., Gottfried S.B., Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intens Care Med. 2007;33(1):66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 10.Van Rompaey B., Elseviers M.M., Schuurmans M.J., Shortridge-Baggett L.M., Truijen S., Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13(3):R77. doi: 10.1186/cc7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasilevskis E.E., Han J.H., Hughes C.G., Ely E.W. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287. doi: 10.1016/j.bpa.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta S., Cook D., Devlin J.W., et al. SLEAP Investigators; Canadian Critical Care Trials Group. Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med. 2015;43(3):557–566. doi: 10.1097/CCM.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 13.Lyons P.G., Snyder A., Sokol S., Edelson D.P., Mokhlesi B., Churpek M.M. Association between opioid and benzodiazepine use and clinical deterioration in ward patients. J Hosp Med. 2017;12(6):428–434. doi: 10.12788/jhm.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The ASAM clinical practice guideline on alcohol withdrawal management [published correction appears in J Addict Med. 2020;14(5):e280] J Addict Med. 2020;14(3S, suppl 1):1–72. doi: 10.1097/ADM.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 15.Saitz R., Mayo-Smith M.F., Roberts M.S., Redmond H.A., Bernard D.R., Calkins D.R. Individualized treatment for alcohol withdrawal: a randomized double-blind controlled trial. JAMA. 1994;272(7):519–523. [PubMed] [Google Scholar]
- 16.Mayo-Smith M.F. Pharmacological management of alcohol withdrawal: a meta-analysis and evidence-based practice guideline. JAMA. 1997;278(2):144–151. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- 17.Royal College of Physicians . The National Clinical Guideline Centre; London, UK: 2010. Alcohol Use Disorders: Diagnosis and Clinical Management of Alcohol-Related Physical Complications.https://www.ncbi.nlm.nih.gov/books/NBK65581/ Published 2010. Accessed February 10, 2022. [PubMed] [Google Scholar]
- 18.Gold J.A., Rimal B., Nolan A., Nelson L.S. A strategy of escalating doses of benzodiazepines and phenobarbital administration reduces the need for mechanical ventilation in delirium tremens. Crit Care Med. 2007;35(3):724–730. doi: 10.1097/01.CCM.0000256841.28351.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayo-Smith M.F., Beecher L.H., Fischer T.L., et al. Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium: an evidence-based practice guideline [published correction appears in Arch Intern Med. 2004;164(18):2068] Arch Intern Med. 2004;164(13):1405–1412. doi: 10.1001/archinte.164.13.1405. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan J.T., Sykora K., Schneiderman J., Naranjo C.A., Sellers E.M. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) Br J Addict. 1989;84(11):1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 21.Daeppen J.-B., Gache P., Landry U., et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117–1121. doi: 10.1001/archinte.162.10.1117. [DOI] [PubMed] [Google Scholar]
- 22.Bostwick J.M., Lapid M.I. False positives on the Clinical Institute Withdrawal Assessment for Alcohol—Revised: is this scale appropriate for use in the medically ill? Psychosomatics. 2004;45(3):256–261. doi: 10.1176/appi.psy.45.3.256. [DOI] [PubMed] [Google Scholar]
- 23.Hecksel K.A., Bostwick J.M., Jaeger T.M., Cha S.S. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274–279. doi: 10.4065/83.3.274. [DOI] [PubMed] [Google Scholar]
- 24.Bostwick J.M. Poor care, not poor protocols, for alcohol withdrawal [reply 1] Mayo Clin Proc. 2008;83(6):728–730. [PubMed] [Google Scholar]
- 25.Eloma A.S., Tucciarone J.M., Hayes E.M., Bronson B.D. Evaluation of the appropriate use of a CIWA-Ar alcohol withdrawal protocol in the general hospital setting. Am J Drug Alcohol Abuse. 2018;44(4):418–425. doi: 10.1080/00952990.2017.1362418. [DOI] [PubMed] [Google Scholar]
- 26.Steel T.L., Giovanni S.P., Katsandres S.C., et al. Should the CIWA-Ar be the standard monitoring strategy for alcohol withdrawal syndrome in the intensive care unit? Addict Sci Clin Pract. 2021;16(1):21. doi: 10.1186/s13722-021-00226-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley K.A., Williams E.C., Achtmeyer C.E., Volpp B., Collins B.J., Kivlahan D.R. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care. 2006;12(10):597–606. [PubMed] [Google Scholar]
- 28.Butterfield M., Thorne-Humphrey L., Suzuki J., Herschenhous N. Evaluation of a novel protocol for assessment and treatment of alcohol withdrawal syndrome in psychiatric inpatients. Am J Addict. 2020;29(6):500–507. doi: 10.1111/ajad.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12(2):308–331. [Google Scholar]
- 30.Largent E.A. Public health, racism, and the lasting impact of hospital segregation. Public Health Rep. 2018;133(6):715–720. doi: 10.1177/0033354918795891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsan M., Garrick O., Graziani G. Does diversity matter for health? experimental evidence from Oakland. Am Econ Rev. 2019;109(12):4071–4111. [Google Scholar]
- 32.Seear K. Addressing alcohol and other drug stigma: where to next? Drug Alcohol Rev. 2020;39(2):109–113. doi: 10.1111/dar.13028. [DOI] [PubMed] [Google Scholar]
- 33.Amato L., Minozzi S., Vecchi S., Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;3:CD005063. doi: 10.1002/14651858.CD005063.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Wood E., Albarqouni L., Tkachuk S., et al. Will this hospitalized patient develop severe alcohol withdrawal syndrome? the Rational Clinical Examination Systematic Review [published correction appears in JAMA. 2019;322(4):369] JAMA. 2018;320(8):825–833. doi: 10.1001/jama.2018.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maldonado J.R., Sher Y., Das S., et al. Prospective validation study of the Prediction of Alcohol Withdrawal Severity Scale (PAWSS) in medically ill inpatients: a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol Alcohol. 2015;50(5):509–518. doi: 10.1093/alcalc/agv043. [DOI] [PubMed] [Google Scholar]
- 36.Steel T.L., Afshar M., Edwards S., et al. Research needs for inpatient management of severe alcohol withdrawal syndrome: an official American Thoracic Society research statement. Am J Respir Crit Care. 2021;204(7):e61–e87. doi: 10.1164/rccm.202108-1845ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.