Key Points
Question
What is the contemporary pattern of evidence-based pharmacotherapy use among a real-world population of US patients with type 2 diabetes and atherosclerotic cardiovascular disease?
Findings
In this cohort study of 324 706 patients from health systems across the US, 58.6% of patients were receiving a statin (and a total of 26.8% of patients were receiving a high-intensity formation), 45.5% of patients were receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, 3.9% of patients were prescribed a glucagon-like peptide-1 receptor agonist, and 2.8% of patients were receiving a sodium glucose cotransporter-2 inhibitors.
Meaning
These findings suggest that multifaceted interventions are needed to overcome the large gaps in evidence-based pharmacotherapy use among this increasing population of patients at high risk of adverse outcomes.
This cohort study examines the prevalence of evidence-based cardiovascular prescribing among patients with type 2 diabetes and atherosclerotic cardiovascular disease in the US.
Abstract
Importance
Based on contemporary estimates in the US, evidence-based therapies for cardiovascular risk reduction are generally underused among patients with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD).
Objective
To determine the use of evidence-based cardiovascular preventive therapies in a broad US population with diabetes and ASCVD.
Design, Setting, and Participants
This multicenter cohort study used health system–level aggregated data within the National Patient-Centered Clinical Research Network, including 12 health systems. Participants included patients with diabetes and established ASCVD (ie, coronary artery disease, cerebrovascular disease, and peripheral artery disease) between January 1 and December 31, 2018. Data were analyzed from September 2020 until January 2021.
Exposures
One or more health care encounters in 2018.
Main Outcomes and Measures
Patient characteristics by prescription of any of the following key evidence-based therapies: high-intensity statin, angiotensin-converting enzyme inhibitor (ACEI) or angiotensin-receptor blocker (ARB) and sodium glucose cotransporter-2 inhibitors (SGLT2I) or glucagon-like peptide-1 receptor agonist (GLP-1RA).
Results
The overall cohort included 324 706 patients, with a mean (SD) age of 68.1 (12.2) years and 144 169 (44.4%) women and 180 537 (55.6%) men. A total of 59 124 patients (18.2% ) were Black, and 41 470 patients (12.8%) were Latinx. Among 205 885 patients with specialized visit data from the prior year, 17 971 patients (8.7%) visited an endocrinologist, 54 330 patients (26.4%) visited a cardiologist, and 154 078 patients (74.8%) visited a primary care physician. Overall, 190 277 patients (58.6%) were prescribed a statin, but only 88 426 patients (26.8%) were prescribed a high-intensity statin; 147 762 patients (45.5%) were prescribed an ACEI or ARB, 12 724 patients (3.9%) were prescribed a GLP-1RA, and 8989 patients (2.8%) were prescribed an SGLT2I. Overall, 14 918 patients (4.6%) were prescribed all 3 classes of therapies, and 138 173 patients (42.6%) were prescribed none. Patients who were prescribed a high-intensity statin were more likely to be men (59.9% [95% CI, 59.6%-60.3%] of patients vs 55.6% [95% CI, 55.4%-55.8%] of patients), have coronary atherosclerotic disease (79.9% [95% CI, 79.7%-80.2%] of patients vs 73.0% [95% CI, 72.8%-73.3%] of patients) and more likely to have seen a cardiologist (40.0% [95% CI, 39.6%-40.4%] of patients vs 26.4% [95% CI, 26.2%-26.6%] of patients).
Conclusions and Relevance
In this large cohort of US patients with diabetes and ASCVD, fewer than 1 in 20 patients were prescribed all 3 evidence-based therapies, defined as a high-intensity statin, either an ACEI or ARB, and either an SGLT2I and/or a GLP-1RA. These findings suggest that multifaceted interventions are needed to overcome barriers to the implementation of evidence-based therapies and facilitate their optimal use.
Introduction
Up to two-thirds of individuals with type 2 diabetes will develop atherosclerotic cardiovascular disease (ASCVD) in their lifetimes.1,2,3 In individuals with diabetes, ASCVD is more extensive, less amenable to treatment, and associated with worse outcomes compared with the general population.4 Fortunately, a number of secondary prevention therapies have been shown to reduce the morbidity and mortality of ASCVD in individuals with diabetes; yet, for a variety of reasons, they are underused in clinical practice.5
Estimates from studies evaluating individuals with diabetes and ASCVD demonstrate a wide variation in the use of key preventive pharmacotherapies, namely high-intensity statins (from 24.7%-45.4%), angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-II receptor blockers (ARBs) (from 53.1%-72.0%), and antihyperglycemic agents with proven cardiovascular benefit, such as sodium glucose cotransporter-2 inhibitors (SGLT2I) and glucagon-like peptide-1 receptor agonists (GLP-1RA) (from 2.5% to 17.6%).6,7,8,9,10,11,12,13 While these data consistently demonstrate concerning gaps in the use of evidence-based therapy, there is significant variation in the magnitude of these estimates owing to differences in data source (eg, registries vs trials vs single-site studies), evolving prescribing trends, and selected patient characteristics. Thus, there is considerable uncertainty as to how representative these findings are for most individuals with diabetes and ASCVD, and we hypothesize that a real-world estimate of evidence-based therapy use will be considerably lower.
The objective of this study was to describe the contemporary use of lipid-, blood pressure–, and glucose-lowering pharmacotherapy among a large, national and representative cohort of individuals with diabetes and ASCVD in the US. Accurately determining the patterns and gaps in evidence-based therapy in this high-risk and increasing population will more precisely inform ongoing implementation programs aimed to increase adoption and improve outcomes.
Methods
For this cohort study, participating sites obtained formal determination from their local institutional review board that this study was not human participants research and thus waiver for informed consent was provided. Because of specifications in the data-use agreements that prohibit release of health system–level data, it is not possible to provide data generated from sites participating in this study. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Source and Study Design
This was a multicenter cohort study performed using data from the US National Patient-Centered Clinical Research Network (PCORnet). PCORnet is composed of multiple clinical data research networks (CDRNs), which in turn comprise 1 or more datamarts (eTable 1 in the Supplement). Datamarts are collections of data that participating health systems generate and store using the PCORnet Common Data Model (CDM) version 5.1 and include demographics, vital signs, diagnoses, encounters, clinician types, procedures, prescription orders, and laboratory results.14 These datamarts enable multisite research by housing individual patient-level data that can be queried and analyzed by site and then returned in aggregate and standardized form.
Cohort Identification
A study period from January 1, 2018, until December 31, 2018, was defined, with an eligible patient’s most recent inpatient or outpatient encounter during this period considered their index date. As previously described,15 International Classification of Diseases, Ninth Revision (ICD-9), International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), and Current Procedural Terminology codes were used to select patients aged 18 years and older with evidence of diabetes and ASCVD during the 5-year period preceding the index date (ie, January 1, 2014, to December 31, 2018). All code lists are available in the eTable 2 in the Supplement. Briefly, ASCVD was defined as patients with coronary artery disease (eg, obstructive coronary atherosclerosis, prior myocardial infarction, prior percutaneous coronary intervention, or coronary artery bypass grafts), peripheral arterial disease (eg, vascular claudication, prior peripheral percutaneous or open revascularization, or amputation from poor circulation), or cerebrovascular disease (eg, carotid atherosclerosis, ischemic stroke, or prior percutaneous or open cerebrovascular revascularization).
Variables
Demographic information was obtained from the CDM demographic and location tables. We used existing information on race and ethnicity, which was documented in the medical record based on self-report and/or clinician observation. Race and ethnicity data were included in this analysis because of prior work suggesting associations between race and ethnicity and clinical care patterns. Comorbid conditions defined by ICD-9 and ICD-10-CM codes were extracted from the CDM diagnosis table, active smoking status from the CDM vital table, and laboratory results (ie, lipid profile, estimated glomerular filtration rate [eGFR] and hemoglobin A1c [HbA1c]) from the investigations table. Lower- and upper-bound truncation points for biologically plausible measurement ranges were based on a test query to improve data quality prior to aggregation. Medication prescriptions were defined using RxNorm concept unique identifiers from the CDM prescribing table. Patients were considered to be using a medication of interest if it was listed in the prescribing table at any time in the 12 months prior to their index date. Health care resource utilization was obtained from the CDM encounter and diagnosis tables over a 12-month period prior to index date. Clinicians of interest were endocrinologists, cardiologists, and primary care physicians.
Evidence-Based Therapy Composite Score
Evidence-based therapy was defined as the use of a high-intensity statin (atorvastatin 40-80 mg or rosuvastatin 20-40 mg), an ACEI or ARB (or angiotensin-II receptor/neprilysin inhibitor [ARNI]), and either an SGLT2I and/or GLP-1RA. Although simvastatin 80 mg is considered a high-intensity formulation, it is not recommended by the US Food and Drug Administration and does not appear in the American College of Cardiology guidelines; thus, it was not considered an evidence-based therapy. Patients in this cohort were considered to have indications for all 3 components and given a composite score of 0 to 3 reflecting the number of evidence-based therapies prescribed. Patients with HbA1c less than 7% (to convert to proportion of total hemoglobin, multiply by 0.01) with or without metformin were ascribed 1 point for the SGLT2I and/or GLP-1RA domain in the 3-point composite score. Of note, it is now recognized that metformin monotherapy is no longer adequate for these patients with high risk, and while this is reflected in the current guidelines, it was not contemporary guidance during the study period. Although some heterogeneity with regard to effects on specific cardiovascular (CV) and kidney outcomes has been found within the SGLT2I and GLP-1RA classes, for the purposes of the analyses and their interpretation, these medications were considered to exhibit class effects.16,17
Statistical Analysis
Site-level aggregate data were summarized using weighted summary measures to account for sample size from each site. Categorical variables are presented as frequencies (percentages) by summing numerators and denominators for each site. Missing data for categorical variables are presented for each variable as frequencies (percentages) of the expected column total. Continuous variables are presented as pooled means; variances across sites were tested for homogeneity according to Hartley test,18 and pooled SDs are presented. To understand patient and health care characteristics associated with the prescription of the evidence-based therapies of interest, the cohort was dichotomized into low (ie, use of 0 or 1 evidence-based therapy) and high (ie, use of 2 or 3 evidence-based therapies) score groups. Statistical comparison between treatment groups prescribed each medication of interest (ie, high-intensity statin, ACEI or ARB, and SGLT2I and/or GLP-1RA) was not possible, given the lack of mutual exclusivity; however, descriptive comparisons were made on the basis of clinically relevant differences and narrow 95% CIs, generated using pooled SDs for continuous variables and assumed binomial proportions for categorical variables.
All analyses were performed using SAS statistical software version 9.1 (SAS Institute). Data were analyzed from September 2020 to January 2021.
Results
Cohort Assembly
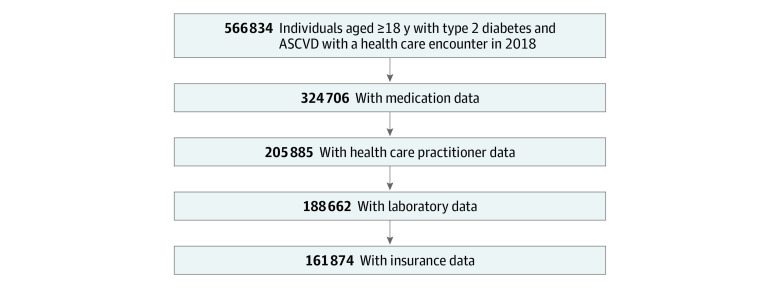
Twelve geographically diverse health systems contributing to 5 CDRNs and 16 datamarts responded within the required timeframe and were able to distribute the query (eTable 1 in the Supplement). Within these participating datamarts, there were 561 259 eligible patients, of whom 324 706 patients (57.9%) had complete medication tables, and among these, 205 885 patients (63.4%) had data on encounters by clinician type, 188 662 patients (58.1%) had laboratory results, and 161 874 patients (49.9%) reported insurance status (Figure 1).
Figure 1. Cohort Inclusion Flowchart.
Study Participants
Among 324 706 patients included in analysis, the overall mean (SD) age was 68.1 (12.2) years, 144 169 (44.4%) were women and 180 537 (55.6%) were men. A total of 9282 patients (2.8%) were Asian, 59 124 patients (18.2%) were Black, 41 470 patients (12.3%) were Latinx, and 207 846 patients (64.0%) were White. Coronary artery disease was present in 237 012 patients (73.0%), 60 125 patients (18.5%) had cerebrovascular disease, and 151 709 patients (46.7%) had peripheral arterial disease. Baseline characteristics are presented overall and by evidence-based therapy in Table 1.
Table 1. Patient Characteristics by Individual Evidence-Based Therapy.
| Patient characteristics | Patients, % (95% CI) | ||||
|---|---|---|---|---|---|
| Overall (N = 324 706) | Evidence-based therapy prescription | ||||
| High-intensity statin (n = 88 426) | ACEI/ARB (n = 147 762) | SGLT2I (n = 8989) | GLP-1RA (n = 12 724) | ||
| Age, mean (SD) [95% CI], y | 68.1 (12.2) [68.0-68.1] | 67.0 (10.9) [67.0-67.1] | 68.1 (11.4) [68.0-68.1] | 63.2 (9.9) [63.0-63.4] | 62.9 (10.3) [62.7-63.1] |
| Sex | |||||
| Women | 44.4 (44.2-44.6) | 40.1 (39.7-40.4) | 44.2 (44.0-44.5) | 36.5 (35.5-37.5) | 46.7 (45.8-47.5) |
| Men | 55.6 (55.4-55.8) | 59.9 (59.6-60.3) | 55.8 (55.5-56.0) | 63.5 (62.5-64.5) | 53.3 (52.5-54.2) |
| Race | |||||
| Asian | 2.9 (2.8-2.9) | 3.0 (2.9-3.1) | 2.8 (2.7-2.9) | 3.6 (3.2-4.0) | 2.0 (1.8-2.3) |
| Black | 18.2 (18.1-18.3) | 20.5 (20.2-20.7) | 19.6 (19.4-19.8) | 13.4 (12.7-14.1) | 17.5 (16.8-18.1) |
| White | 64.0 (63.9-64.2) | 61.0 (60.7-61.3) | 63.4 (63.2-63.7) | 66.2 (65.2-67.2) | 64.8 (64.0-65.6) |
| Othera | 14.9 (14.8-15.0) | 15.6 (15.3-15.8) | 14.2 (14.0-14.3) | 16.9 (16.1-17.7) | 15.7 (15.1-16.4) |
| Latinx ethnicity | 12.8 (12.7-12.9) | 14.0 (13.8-14.2) | 13.6 (13.4-13.8) | 13.4 (12.7-14.1) | 13.5 (12.9-14.1) |
| ASCVD | |||||
| Coronary artery disease | 73.0 (72.8-73.2) | 79.9 (79.7-80.2) | 72.3 (72.1-72.6) | 72.2 (71.3-73.2) | 69.5 (68.7-70.3) |
| MI | 24.0 (23.8-24.1) | 33.9 (33.6-34.2) | 25.5 (25.2-25.7) | 20.6 (19.8-21.4) | 19.9 (19.2-20.6) |
| PCI | 21.4 (21.2-21.5) | 30.5 (30.2-30.8) | 22.2 (22.0-22.4) | 21.4 (20.5-22.2) | 19.6 (18.9-20.3) |
| Cerebrovascular disease | 18.5 (18.4-18.7) | 23.5 (23.2-23.8) | 19.6 (19.4-19.8) | 16.1 (15.3-16.9) | 16.4 (15.8-17.1) |
| Stroke | 12.4 (12.3-12.5) | 16.6 (16.4-16.8) | 13.2 (13.0-13.4) | 9.7 (9.1-10.3) | 10.5 (10.0-11.1) |
| Peripheral arterial disease | 46.7 (46.6-46.9) | 47.3 (47.0-47.6) | 50.1 (49.9-50.4) | 47.1 (46.1-48.2) | 51.2 (50.4-52.1) |
| Comorbidities | |||||
| Heart failure | 32.1 (31.9-32.3) | 38.8 (38.5-39.2) | 34.6 (34.3-34.8) | 21.3 (20.5-22.2) | 26.3 (25.5-27.1) |
| Hypertension | 92.1 (92.0-92.2) | 94.8 (94.6-94.9) | 96.9 (96.8-97.0) | 92.1 (91.5-92.6) | 93.5 (93.0-93.9) |
| Dyslipidemia | 82.9 (82.8-83.1) | 90.3 (90.1-90.5) | 87.6 (87.5-87.8) | 89.7 (89.0-90.3) | 89.6 (89.1-90.1) |
| Smoking | 11.6 (11.5-11.7) | 15.1 (14.8-15.3) | 12.8 (12.7-13.) | 9.8 (9.2-10.4) | 10.4 (9.9-10.9) |
| Charlson Comorbidity Index score, mean (SD) [95% CI] | 4.1 (2.8) [4.1-4.1] | 4.5 (2.9) [4.5-4.5] | 4.3 (2.8) [4.2-4.3] | 3.3 (2.4) [3.2-3.3] | 3.8 (2.6) [3.8-3.9] |
| Diabetes complications | |||||
| Diabetic ketoacidosis | 1.7 (1.7-1.8) | 2.4 (2.3-2.5) | 2.0 (1.9-2.0) | 1.6 (1.3-1.9) | 2.4 (2.1-2.7) |
| Retinopathy | 9.4 (9.3-9.5) | 12.8 (12.6-13.0) | 11.4 (11.2-11.6) | 11.6 (11.0-12.3) | 17.0 (16.4-17.7) |
| Neuropathy | 26.3 (26.2-26.5) | 30.4 (30.1-30.7) | 29.6 (29.4-29.9) | 31.0 (30.1-32.0) | 40.1 (39.3-41.0) |
| Diabetic foot | 5.1 (5.0-5.2) | 6.0 (5.8-6.2) | 5.3 (5.2-5.5) | 3.8 (3.4-4.2) | 6.1 (5.7-6.5) |
| SBP, mean (SD) [95% CI], mm Hg | 132 (20) [132-132] | 132 (21) [131-132] | 133 (21) [133-133] | 129 (18) [128-129] | 131 (18) [130-131] |
| BMI, mean (SD) [95% CI] | 31.3 (7.3) [31.3-31.4] | 31.5 (7.1) [31.5-31.6] | 31.9 (7.3) [31.8-31.9] | 32.7 (6.8) [32.6-32.9] | 35.2 (7.6) [35.1-35.3] |
| Laboratory values, mean (SD) [95% CI]b | |||||
| Cholesterol, mg/dL | |||||
| LDL (n = 159 903) | 83.3 (35.6) [83.2-83.5] | 79.3 (37.4) [79.1-79.8] | 82.7 (35.5) [82.4-82.9] | 81.0 (34.6) [80.1-81.9] | 79.5 (34.6) [78.7-80.3] |
| HDL (n = 142 453) | 46.3 (15.2) [46.2-46.4] | 44.0 (14.1) [43.9-44.1] | 45.7 (14.7) [45.6-45.8] | 43.5 (12.9) [43.2-43.9] | 43.2 (13.3) [42.9-43.4] |
| Triglycerides, mg/dL (n = 159 493) | 158.0 (119.4) [157.4-158.6] | 162.5 (129.7) [161.4-163.6] | 160.8 (120.8) [160.1-161.6] | 186.2 (161.2) [182.0-190.5] | 185.5 (137.0) [182.5-188.6] |
| HbA1c, % (n = 188 662) | 7.3 (2.3) [7.2-7.3] | 7.4 (2.0) [7.4-7.5] | 7.3 (1.9) [7.3-7.4] | 8.0 (1.6) [7.9-8.0] | 8.1 (1.8) [8.0-8.1] |
| eGFR, mL/min/1.73 m2 (n = 177 224) | |||||
| <15 | 5.2 (5.1-5.3) | 5.8 (5.6-6.0) | 3.8 (3.7-3.9) | 0.7 (0.5-1.0) | 2.2 (1.9-2.5) |
| 15-29 | 6.5 (6.4-6.6) | 7.2 (7.1-7.4) | 5.3 (5.1-5.4) | 1.1 (0.8-1.4) | 4.3(3.9-4.8) |
| 30-59 | 34.4 (34.2-34.7) | 35.5 (35.1-35.8) | 35.7 (35.4-36.0) | 26.1 (25.0-27.3) | 32.9 (31.9-33.9) |
| ≥60 | 53.6 (53.4-53.8) | 51.3 (51.0-51.7) | 55.0 (54.7-55.3) | 71.7 (70.5-72.9) | 60.3 (59.3-61.4) |
| Missing | 0.2 (0.19-0.23) | 0.2 (0.1-0.2) | 0.2 (0.2-0.2) | 0.4 (0.2-0.5) | 0.2 (0.1-0.3) |
| Geographic region (n = 191 376)b | |||||
| Northeast | 46.1 (45.9-46.3) | 43.1 (42.7-43.5) | 41.6 (41.3-41.9) | 50.1 (48.9-51.4) | 46.1 (45.1-47.2) |
| South | 41.2 (41.0-41.5) | 43.6 (43.2-44.0) | 43.7 (43.4-44.0) | 38.6 (37.4-39.8) | 42.2 (41.1-43.3) |
| West | 7.3 (7.2-7.4) | 8.4 (8.2-8.6) | 10.2 (10.0-10.4) | 5.7 (5.1-6.2) | 6.5 (6.0-7.0) |
| Other | 0.2 (0.2-0.2) | 0.3 (0.2-0.3) | 0.2 (0.2-0.3) | 0.2 (0.1-0.3) | 0.2 (0.1-0.3) |
| Missing | 4.0 (4.0-4.1) | 2.9 (2.8-3.0) | 2.9 (2.8-3.0) | 4.5 (4.0-5.0) | 3.4 (3.0-3.8) |
| Insurance coverage (n = 161 874)b | |||||
| Medicare | 46.5 (46.2-46.7) | 39.3 (38.8-39.7) | 40.7 (40.3-41.1) | 23.9 (22.7-25.2) | 32.4 (31.2-33.7) |
| Medicaid | 3.4 (3.3-3.5) | 5.2 (5.0-5.5) | 4.2 (4.0-4.4) | 2.0 (1.6-2.4) | 2.8 (2.4-3.2) |
| Military health care | 1.3 (1.3-1.4) | 1.8 (1.7-1.9) | 1.3 (1.3-1.4) | 2.0 (1.6-2.4) | 1.8 (1.5-2.2) |
| Private | 12.0 (11.9-12.2) | 8.8 (8.4-9.0) | 9.5 (9.3-9.7) | 17.1 (16.0-18.2) | 15.5 (14.5-16.5) |
| State-specific | 0.2 (0.2-0.3) | 0.4 (0.3-0.4) | 0.3 (0.25-0.3) | 0.4 (0.2-0.5) | 0.4 (0.2-0.6) |
| Self-pay | 1.0 (1.0-1.1) | 1.8 (1.6-1.9) | 1.5 (1.4-1.6) | 0.7 (0.4-0.9) | 0.6 (0.4-0.9) |
| Other | 11.9 (11.8-12.1) | 16.9 (15.8-16. 6) | 15.4 (15.1-15.7) | 17.0 (15.9-18.1) | 19.9 (18.8-21.0) |
| Missing | 23.6 (23.4-23.8) | 26.7 (26.2-27.1) | 27.1 (26.8-27.4) | 37.0 (35.7-38.5) | 26.5 (25.4-27.7) |
| Physicians (n = 205 885)b | |||||
| Endocrinologist | 8.7 (8.6-8.9) | 9.8 (9.5-10.1) | 10.0 (9.8-10.1) | 28.4 (27.2-29.7) | 30.5 (29.5-31.6) |
| Cardiologist | 26.4 (26.2-26.6) | 40.0 (39.6-40.4) | 34.1 (33.8-34.4) | 32.1 (30.8-33.4) | 32.5 (31.4-33.5) |
| Primary care | 74.8 (74.7-75.0) | 81.0 (80.6-81.3) | 82.1 (81.9-82.4) | 76.5 (75.3-77.7) | 79.7 (78.8-80.6) |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.029; HbA1c to proportion of total hemoglobin, multiply by 0.01; triglycerides to millimoles per liter, multiply by 0.0113.
Other race refers to American Indian, Alaskan Native, Native Hawaiian or other Pacific Islander, or some other nonlisted race that is not Asian, Black, or White.
Completeness varied for specific data elements; therefore the analytic population size is presented where the full cohort was smaller.
Lipid-, Blood Pressure–, and Glucose-Lowering Therapies
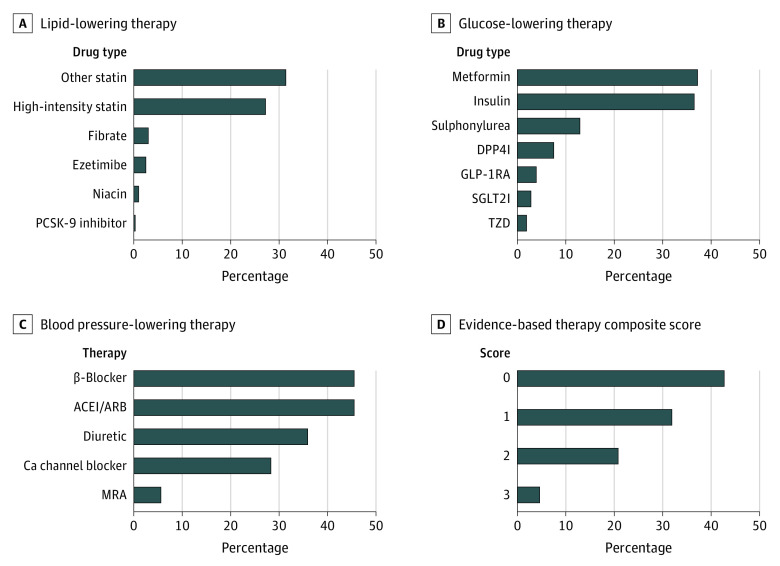
Use of lipid-, blood pressure–, and glucose-lowering therapies are presented in Figure 2. Overall, 190 346 patients (58.6%) were prescribed a statin, but only 87 160 patients (26.8%) were prescribed a high-intensity statin. Use of nonstatin low-density cholesterol–lowering therapies was low, with 8161 patients (2.5%) prescribed ezetimibe and 1055 patients (0.3%) prescribed a PCSK9 inhibitor. ACEIs or ARBs were prescribed in 147 762 patients (45.5%). Of the antihyperglycemic medications, metformin was prescribed in 120 821 patients (37.2%), sulfonylureas in 42 027 patients (12.9%), and insulin in 118 508 patients (36.5%). Use of glucose-lowering drugs with proven CV benefit was low, with 12 724 patients (3.9%) of patients prescribed a GLP-1RA and 8989 patients (2.8%) prescribed an SGLT2I.
Figure 2. Proportions of Patients Receiving Evidence-Based Therapies for Diabetes and Atherosclerotic Cardiovascular Disease.
D, Evidence-based therapy composite score was scored as 0, no evidence-based therapies; 1, 1 evidence-based therapy; 2, 2 evidence-based therapies; and 3, 3 evidence-based therapies.
Receipt of Evidence-Based Therapy
Compared with the overall cohort, patients prescribed a high-intensity statin were more likely to be men (59.9% [95% CI, 59.6%-60.3%] of patients vs 55.6% [95% CI, 55.4%-55.8%] of patients), more likely to have coronary (79.9% [95% CI, 79.7%-80.2%] of patients vs 73.0% [95% CI, 72.8%-73.3%] of patients) or cerebrovascular (23.5% [95% CI, 23.2%-23.8%] of patients vs 18.5% [95% CI, 18.4%-18.7%] of patients) disease, and more likely to have seen a cardiologist (40.0% [95% CI, 39.6%-40.4%] of patients vs 26.4% [95% CI, 26.2%-26.6%] of patients). Patients prescribed a high-intensity statin, compared with the overall cohort, had a greater burden of heart failure (38.8% [95% CI, 38.5%-39.2%] of patients vs 32.1% [95% CI, 31.9%-32.3%] of patients), cigarette smoking (15.1% [95% CI, 14.8%-15.3%] of patients vs 11.6% [95% CI, 11.5%-11.7%] of patients) and dyslipidemia (90.3% [95% CI, 90.1%-90.5%] of patients vs 82.9% [95% CI, 82.8%-83.1%] of patients).
The demographics of participants prescribed an ACEI or ARB did not differ significantly from the overall cohort. However, patients receiving an ACEI or ARB, compared with the overall cohort, were more likely to have peripheral artery disease (50.1% [95% CI, 49.9%-50.4%] of patients vs 46.7% [95% CI, 46.6%-46.9%] of patients), hypertension (96.9% [95% CI, 96.8%-97.0%] of patients vs 92.1% [95% CI, 92.0%-92.2%] of patients), and dyslipidemia (87.6% [95% CI, 87.5%-87.8%] of patients vs 82.9% [95% CI, 82.8%-83.1%] of patients). Those prescribed an ACEI or ARB were also more likely to have seen a primary care physician (82.1% [95% CI, 81.9%-82.4%] of patients vs 74.8% [95% CI, 74.7%-75.0%] of patients) or a cardiologist (34.1% [95% CI, 33.8%-34.4%] of patients vs 26.4% [95% CI, 26.6%-26.6%] of patients) in the prior 12 months (Table 1).
Patients prescribed an SGLT2I or GLP-1RA, compared with the overall cohort, were younger (mean age: SGLT2I, 63.2 [95% CI, 63.0-63.4] years; GLP-1RA: 62.9 [95% CI, 62.7-63.1] years; overall: 68.1 [95% CI, 68.0-68.1] years), were more likely to have private insurance (SGLT2I: 17.1% [95% CI, 16.0%-18.2%] of patients; GLP-1RA: 15.5% [95% CI, 14.5%-16.5%] of patients; overall: 12.0% [95% CI, 11.9%-12.2%] of patients), and had fewer medical comorbidities (mean Charlson comorbidity index score: SGLT2I: 3.3 [95% CI, 3.2-3.3]; GLP-1RA: 3.8 [95% CI, 3.8-3.9]; overall: 4.1 [95% CI, 4.1-4.1]), and lower prevalence of heart failure (SGLT2I: 21.3% [95% CI, 20.5%-22.2%] of patients; GLP-1RA: 26.3% [95% CI, 25.5%-27.1%] of patients; overall: 32.1% [95% CI, 31.9%-32.3%] of patients) and atrial fibrillation (SGLT2I: 13.8% [95% CI, 13.1%-14.5%] of patients; GLP-1RA: 14.8% [95% CI, 14.2%-15.4%] of patients; overall: 21.3% [95% CI, 21.2%-21.5%] of patients). Patients prescribed either an SGLT2I or a GLP-1RA had similar rates of end-organ diabetes complications yet were more likely to have visited an endocrinologist in the prior 12 months compared with the overall population (SGLT2I: 28.4% [95% CI, 27.2%-29.7%] of patients; GLP-1RA: 30.5% [95% CI, 29.5%-31.6%] of patients; overall: 8.7% [95% CI, 8.6%-8.9%] of patients). Patients prescribed an SGLT2I, compared with those prescribed a GLP-1RA, were less likely to be women (36.5% [95% CI, 35.5%-37.5%] of patients vs 46.7% [95% CI, 45.8%-47.5%] of patients) and less likely to be Black (13.4% [95% CI, 12.7%-14.1%] of patients vs 17.5% [95% CI, 16.8%-18.1%] of patients). Patients prescribed an SGLT2I had fewer diabetes end-organ complications compared with those prescribed a GLP-1RA (neuropathy: 31.0% [95% CI, 30.1%-32.0%] of patients vs 40.1% [95% CI, 39.3%-41.0%] of patients; retinopathy: 11.6% [95% CI, 11.2%-11.6%] of patients vs 17.0% [95% CI, 16.4%-17.7%] of patients; DKA: 1.6% [95% CI, 1.3%-1.9%] of patients vs 2.4% [95% CI, 2.1%-2.7%] of patients; diabetic foot: 3.8% [95% CI, 3.4%-4.2%] of patients vs 6.1% [95% CI, 5.7%-6.5%] of patients). Of note, heart failure (26.3% [95% CI, 25.5%-27.1%] of patients vs 21.3% [95% CI, 20.5%-22.2%] of patients) and mild kidney dysfunction (eGFR 30-59 mL/min/m2: 32.9% [95% CI, 31.9%-33.9%] of patients vs 26.1% [95% CI, 25.0%-27.3%] of patients) were more common among those prescribed a GLP-1RA than those prescribed an SGLT2I.
Extent of Evidence-Based Therapy Use
Overall, 138 173 patients (42.6%) were prescribed no evidence-based CV-risk mitigating medications, 103 420 patients (31.9%) were prescribed 1 medication, 68 195 patients (21.0%) were prescribed 2 medications, and only 14 918 patients (4.6%) were prescribed 3 evidence-based medications.
Groups of patients with low (0 or 1 points) and high (2 or 3 points) composite medication scores are presented in Table 2. Patients with high evidence-based therapy scores, compared with those with low scores, had similar racial and ethnic characteristics but were less likely to be women (41.5% [95% CI, 41.2%-41.8%] of patients vs 45.4% [95% CI, 45.2%-45.6%] of patients). Patients with high composite scores, compared with those with low scores, had a higher burden of hypertension (96.4% [95% CI, 96.2%-96.5%] of patients vs 90.7% [95% CI, 90.6%-90.8%] of patients) and dyslipidemia (90.6% [95% CI, 90.4%-90.8%] of patients vs 80.3% [95% CI, 80.2%-80.3%] of patients) and a higher prevalence of end-organ diabetes complications (neuropathy: 31.3% [95% CI, 30.9%-31.6%] of patients vs 24.6% [95% CI, 24.4%-24.8%] of patients; retinopathy: 12.4% [95% CI, 12.2%-12.6%] of patients vs 8.3% [95% CI, 8.2%-8.5%] of patients; DKA: 2.1% [95% CI, 2.1%-2.2%] of patients vs 1.6% [95% CI, 1.5%-1.6%] of patients). Regional and payer variation in care was observed, with lower scores more common than high scores among patients in the Northeast (48.3% [95% CI, 48.1%-48.6%] of patients vs 40.7% [95% CI, 40.3%-41.1%] of patients) and patients with Medicare coverage (49.5% [95% CI, 49.2%-49.8%] of patients vs 35.7% [95% CI, 35.2%-36.2%] of patients). Patients with high scores, compared with those with low scores, were more likely to have seen a cardiologist (39.2% [95% CI, 38.8%-39.6%] of patients vs 22.3% [95% CI, 22.1%-22.5%] of patients) or primary care physician (83.4% [95% CI, 83.1%-83.7%] of patients vs 72.1% [95% CI, 71.9%-72.3%] of patients) in the prior 12 months.
Table 2. Patient Characteristic by Overall Evidence-Based Composite Score.
| Patient characteristics | Evidence-based therapy score, % (95% CI) | |
|---|---|---|
| 0-1 (n = 241 593) | 2-3 (n = 83 113) | |
| Age, mean (SD) [95% CI], y | 68.5 (12.6) [68.5-68.6] | 66.7 (10.7) [66.7-66.8] |
| Sex | ||
| Women | 45.4 (45.2-45.6) | 41.5 (41.2-41.8) |
| Men | 54.6 (54.4-54.8) | 58.5 (58.2-58.9) |
| Race | ||
| Asian | 2.8 (2.8-2.9) | 2.9 (2.8-3.1) |
| Black | 17.6 (17.5-17.8) | 19.9 (19.7-20.2) |
| White | 64.5 (64.4-64.7) | 62.5 (62.1-62.8) |
| Othera | 15.0 (14.9-15.2) | 14.7 (14.4-14.9) |
| Latinx ethnicity | 12.3 (12.2-12.5) | 14.0 (13.8-14.3) |
| ASCVD | ||
| Coronary artery disease | 72.1 (71.9-72.2) | 75.7 (75.4-76.0) |
| MI | 22.0 (21.8-22.1) | 29.8 (29.5-30.2) |
| PCI | 19.6 (19.4-19.7) | 26.7 (26.37-26.97) |
| Cerebrovascular disease | 17.6 (17.4-17.7) | 21.2 (20.96-21.51) |
| Stroke | 11.6 (11.5-11.8) | 14.7 (14.46-14.94) |
| Peripheral arterial disease | 45.9 (45.7-46.1) | 49.2 (48.90-49.58) |
| Comorbidities | ||
| Heart failure | 31.0 (30.8-31.2) | 35.3 (35.02-35.67) |
| Atrial fibrillation | 21.7 (21.6-21.9) | 20.3 (20.0-20.5) |
| Hypertension | 90.7 (90.6-90.8) | 96.4 (96.2-96.5) |
| Dyslipidemia | 80.3 (80.2-80.5) | 90.6 (90.4-90.8) |
| Smoking | 10.6 (10.4-10.7) | 14.6 (14.4-14.9) |
| Charlson Comorbidity Index score, mean (SD) [95% CI] | 4.1 (2.8) [4.1-4.1] | 4.3 (2.8) [4.2-4.3] |
| Diabetes complications | ||
| Diabetic ketoacidosis | 1.6 (1.5-1.6) | 2.1 (2.1-2.2) |
| Retinopathy | 8.3 (8.2-8.5) | 12.4 (12.2-12.6) |
| Neuropathy | 24.6 (24.4-24.8) | 31.3 (30.9-31.6) |
| Diabetic foot | 5.0 (4.9-5.1) | 5.4 (5.3-5.6) |
| SBP, mm Hg (n = 303 859)b | 132 (20) [132-132] | 132 (20) [132-132] |
| BMI (n = 295 725)b | 31.0 (7.2) [31.0-31.1] | 32.1 (7.3) [32.1-32.2] |
| Laboratory values, mean (SD) [95% CI]b | ||
| Cholesterol, mg/dL | ||
| LDL (n = 159 903) | 85.0 (35.3) [84.8-85.2] | 80.1 (35.7) [79.8-80.4] |
| HDL (n = 142 453) | 47.3 (15.7) [47.2-47.4] | 44.6 (13.9) [44.4-44.7] |
| Triglycerides, mg/dL (n = 159 493) | 155.2 (116.1) [154.5-155.9] | 163.3 (125.2) [162.3-164.4] |
| HbA1c, % (n = 188 662) | 7.2 (1.7) [7.2-7.2] | 7.3 (1.7) [7.2-7.3] |
| eGFR, mL/min/1.73m2 (n = 177 224) | ||
| <15 | 6.0 (5.9-6.1) | 3.7 (3.6-3.9) |
| 15-29 | 7.5 (7.3-7.6) | 4.7 (4.5-4.8) |
| 30-59 | 34.6 (34.3-34.9) | 34.1 (33.7-34.5) |
| ≥60 | 51.7 (51.4-52.0) | 57.3 (56.9-57.7) |
| Missing | 0.2 (0.2-0.3) | 0.2 (0.1-0.2) |
| Geographic region (n = 191 376)b | ||
| Northeast | 48.3 (48.1-48.6) | 40.7 (40.3-41.1) |
| South | 40.0 (39.8-40.3) | 44.2 (43.8-44.6) |
| West | 6.0 (5.9-6.2) | 10.4 (10.2-10.7) |
| Other | 0.2 (0.1-0.2) | 0.3 (0.2-0.3) |
| Missing | 4.7 (4.6-4.8) | 2.5 (2.4-2.6) |
| Insurance coverage (n = 161 874)b | ||
| Medicare | 49.5 (49.2-49.8) | 35.7 (35.2-36.2) |
| Medicaid | 2.9 (2.8-3.0) | 5.1 (4.9-5.4) |
| Military health care | 1.2 (1.2-1.3) | 1.6 (1.4-1.7) |
| Private | 12.9 (12.7-13.1) | 9.0 (8.7-9.3) |
| State-specific | 0.2 (0.2-0.2) | 0.4 (0.3-0.4) |
| Self-pay | 0.8 (0.8-0.9) | 1.8 (1.7-2.0) |
| Other | 10.3 (10.1-10.4) | 17.9 (17.5-18.3) |
| Missing | 22.2 (22.0-22.4) | 28.5 (28.1-29.0) |
| Physicians (n = 205 885)b | ||
| Endocrinologist | 8.0 (7.9-8.1) | 11.0 (10.7-11.3) |
| Cardiologist | 22.3 (22.1-22.5) | 39.2 (38.8-39.6) |
| Primary care | 72.1 (71.9-72.3) | 83.4 (83.1-83.71) |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.029; HbA1c to proportion of total hemoglobin, multiply by 0.01; triglycerides to millimoles per liter, multiply by 0.0113.
Other race refers to American Indian, Alaskan Native, Native Hawaiian or other Pacific Islander, or some other nonlisted race that is not Asian, Black, or White.
Completeness varied for specific data elements; therefore the analytic population size is presented where the full cohort was smaller.
Discussion
This cohort study including more than 300 000 patients across multiple health systems represents a large contemporary landscape evaluation of the real-world cardiometabolic care patterns of patients in the US with both diabetes and ASCVD. The study has a number of important findings. First, more than one-third of patients were receiving none of the 3 key evidence-based therapies associated with significant CV benefit, and fewer than 1 in 20 patients were receiving all 3. Second, more than one-quarter of patients were prescribed a guideline-recommended dose of statin, less than half were prescribed an ACEI or ARB, and fewer than 1 in 15 patients were prescribed an antihyperglycemic agent with CV benefit. Third, while endocrinologist encounters were more common among those receiving either an SGLT2I or GLP-1RA, they were infrequent care episodes and reinforce the need for other physicians, such as cardiologists and primary care physicians, to assist with adoption of these agents.
These data suggest that previously described gaps in the use of evidence-based therapies for individuals with diabetes and ASCVD in selected environments extend to this large, distributed network of health systems across the US. The finding that only 58.6% of patients in this study were prescribed a statin is considerably lower than a recently published estimate of 74.6% from a database of commercially insured patients in the US.9 Notably, the rate of overall statin use in this study was similar to findings from a comparable Medical Expenditure Panel Survey population from 2013, which reported that only 52.7% of patients with diabetes and ASCVD were receiving a statin.19 In this context, these new data raise concerns that despite strengthening of guideline recommendations in the years prior to our study window,20 there has been minimal progress in increasing the use of these widely available, cost-effective, safe, and proven medications in the general population.
A plethora of data support the role of ACEIs and ARBs in diabetes with and without chronic kidney disease,21,22,23,24 ASCVD with or without diabetes25,26,27 and as first-line agents in hypertension with or without diabetes.28 Thus, our cohort had at least 1 indication for either an ACEI or an ARB, and yet only 45% of patients were prescribed one. This estimate is lower than other current national estimates from survey (55%29) and health system (IQR, 51%-69%30) data evaluating similar populations and lower than that reported in contemporary registry (72%6) and clinical trial (80%31) cohorts. While higher use may be observed among registry and trial cohorts owing to their enrichment for patients whose medical histories are less complex and who are more adherent with medical instruction, there were no significant differences in the prevalence of chronic kidney disease, CV and non-CV comorbidity, or diabetes complications among patients receiving ACEIs or ARBs vs those not receiving either drug in this cohort, making the risk-treatment paradox a less obvious explanation in this study. Given their potential role in mitigating the increasing prevalence of chronic kidney disease32 among patients with diabetes and that treatment benefits from established and emerging diabetes therapies (eg, SGLT2I,33 GLP-1RA,34 fineronone35) were observed at high levels of background ACEI or ARB therapy, further enquiry into the barriers preventing the use of these inexpensive and well-tolerated medications is urgently required.
More than one-third of patients were receiving none of the evidence-based therapies, with just one-quarter achieving a higher composite score of 2 or more evidence-based therapies. While interpretation is limited by univariable comparison, there was no obvious evidence of risk-treatment paradox,36,37 with patients with higher composite evidence-based therapy scores having similar comorbidity scores and risk profiles. Furthermore while other studies have described marked disparity in preventive care patterns by race and sex,38,39,40,41 these were less apparent in our cohort, with only a modest difference in high-intensity statin use favoring men compared with women. Given that our cohort was restricted to those with a recent encounter, patients with less access to care would have been more likely to be excluded; as Black and Latinx patients continue to suffer from inequitable health system access,42,43 disparate patterns among these groups may have been attenuated and deserve further, dedicated enquiry.
Only 6.7% of patients in the cohort were prescribed either an SGLT2I or GLP-1RA, which is considerably lower than other contemporary estimates of 9.9% from an insured population9 and 17% from a diabetes registry.6 Some potential barriers to the optimal use of SGLT2I and GLP-1RA are cost and insurance formulary preferences, as evidenced by the greater proportion of patients receiving these drugs in this study having private insurance. Since the acquisition of these data, a number of consensus documents and guidelines have emerged calling on cardiologists to embrace these agents as key tenets of cardiovascular risk reduction44,45,46,47,48,49; the impact of these publications on adoption of SGLT2I and GLP-1RA remains to be seen.
Limitations
There are several limitations to this study. The use of aggregate data meant that multivariable analyses for factors associated with individual therapy prescription or high vs low composite score were not possible. Furthermore, a granular understanding of the contributors to missingness of relevant data are also not possible with an aggregated data set. In this context, there was an obligate loss in sample size, as data completeness in the CDM varied among datamarts, particularly with respect to insurance status and laboratory values. Medication use was discerned from prescribing information available in PCORnet, and actual use or dispensing was not observed. A more nuanced analysis comparing medication prescription, dispensing, and actual use is not currently possible in the PCORnet environment and would require linkage with individual pharmacy dispensing records and claims data. Prescriptions administered outside of the PCORnet health system are also not captured. In contrast, patients who abandon their prescription or discontinue without informing their physician would be considered as prescribed and thus our estimate may overall be optimistic. While these functions of the data set contribute to variations, definitions, and estimates of clinical use (ie, a composite of prescription, dispensing, adherence, and continuation) the presence of a medication in the electronic health record represents a real-world assessment of treatment status. Our assessment of evidence-based therapy prescription must only be considered an estimate, as access to patient-level data was not available and thus it was not possible to consider the impact of relative or absolute contraindications. The intent was to generate an inclusive cohort of patients without removing any from the denominator; thus, while every patient had a potential evidence-based therapy score of 3, this is a broad generalization, since a number of patients could never be prescribed all 3 medications owing to contraindications, allergies, or intolerance (eg, dialysis, prior rhabdomyolysis). However, there are significant strengths of this type of analysis; namely, the large and unselected nature of this data set encompasses not only geographic variation but also patient, physician, and practice diversity, which strengthen the generalizability of the present findings.
Conclusions
In this cohort study of more than 300 000 patients with diabetes and ASCVD in contemporary clinical practice from the US, more than one-third of patients were not receiving any guideline-directed, evidence-based, CV risk–mitigating therapies (or doses), and fewer than 1 in 20 patients were receiving all 3 therapies. It is particularly concerning that only one-quarter were prescribed a high-intensity statin and less than half an ACE or ARB, treatments that are inexpensive and well tolerated. These estimates of evidence-based therapy prescription are considerably lower than those observed in other recent analyses in selected populations. These findings amplify the need to close these critical gaps between evidence generation and clinical practice for most patients in the US with diabetes and ASCVD.
eTable 1. Datamarts and Respective Health System Participants
eTable 2. Code Lists for Comorbidities and Qualifying ASCVD
References
- 1.Bancks MP, Ning H, Allen NB, et al. Long-term absolute risk for cardiovascular disease stratified by fasting glucose level. Diabetes Care. 2019;42(3):457-465. doi: 10.2337/dc18-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB Sr. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham Heart Study. Diabetes Care. 2008;31(8):1582-1584. doi: 10.2337/dc08-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791-798. doi: 10.1161/CIRCULATIONAHA.105.548206 [DOI] [PubMed] [Google Scholar]
- 4.Nelson AJ, Peterson ED, Pagidipati NJ. Atherosclerotic cardiovascular disease and heart failure: determinants of risk and outcomes in patients with diabetes. Prog Cardiovasc Dis. 2019;62(4):306-314. doi: 10.1016/j.pcad.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 5.Perel P, Avezum A, Huffman M, et al. Reducing premature cardiovascular morbidity and mortality in people with atherosclerotic vascular disease: the World Heart Federation roadmap for secondary prevention of cardiovascular disease. Glob Heart. 2015;10(2):99-110. doi: 10.1016/j.gheart.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 6.Arnold SV, de Lemos JA, Rosenson RS, et al. ; GOULD Investigators . Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140(7):618-620. doi: 10.1161/CIRCULATIONAHA.119.041730 [DOI] [PubMed] [Google Scholar]
- 7.Arnold SV, Inzucchi SE, Tang F, et al. Real-world use and modeled impact of glucose-lowering therapies evaluated in recent cardiovascular outcomes trials: an NCDR research to practice project. Eur J Prev Cardiol. 2017;24(15):1637-1645. doi: 10.1177/2047487317729252 [DOI] [PubMed] [Google Scholar]
- 8.Hamid A, Vaduganathan M, Oshunbade AA, et al. Antihyperglycemic therapies with expansions of US Food and Drug Administration indications to reduce cardiovascular events: prescribing patterns within an academic medical center. J Cardiovasc Pharmacol. 2020;76(3):313-320. doi: 10.1097/FJC.0000000000000864 [DOI] [PubMed] [Google Scholar]
- 9.Nelson AJ, Ardissino M, Haynes K, et al. Gaps in evidence-based therapy use in insured patients in the United States with type 2 diabetes mellitus and atherosclerotic cardiovascular disease. J Am Heart Assoc. 2021;10(2):e016835. doi: 10.1161/JAHA.120.016835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantalone KM, Misra-Hebert AD, Hobbs TM, et al. Antidiabetic treatment patterns and specialty care utilization among patients with type 2 diabetes and cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):54. doi: 10.1186/s12933-018-0699-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumarsono A, Buckley LF, Machado SR, et al. Medicaid expansion and utilization of antihyperglycemic therapies. Diabetes Care. 2020;43(11):2684-2690. doi: 10.2337/dc20-0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaduganathan M, Sathiyakumar V, Singh A, et al. Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol. 2018;72(25):3370-3372. doi: 10.1016/j.jacc.2018.08.2202 [DOI] [PubMed] [Google Scholar]
- 13.Weng W, Tian Y, Kong SX, et al. The prevalence of cardiovascular disease and antidiabetes treatment characteristics among a large type 2 diabetes population in the United States. Endocrinol Diabetes Metab. 2019;2(3):e00076. doi: 10.1002/edm2.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleurence RL, Curtis LH, Califf RM, Platt R, Selby JV, Brown JS. Launching PCORnet, a national patient-centered clinical research network. J Am Med Inform Assoc. 2014;21(4):578-582. doi: 10.1136/amiajnl-2014-002747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiese AD, Roumie CL, Buse JB, et al. Performance of a computable phenotype for identification of patients with diabetes within PCORnet: the Patient-Centered Clinical Research Network. Pharmacoepidemiol Drug Saf. 2019;28(5):632-639. doi: 10.1002/pds.4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aroda VR. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab. 2018;20(suppl 1):22-33. doi: 10.1111/dom.13162 [DOI] [PubMed] [Google Scholar]
- 17.Caruso I, Cignarelli A, Giorgino F. Heterogeneity and similarities in GLP-1 receptor agonist cardiovascular outcomes trials. Trends Endocrinol Metab. 2019;30(9):578-589. doi: 10.1016/j.tem.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 18.Frey J. Testing for equivalence of variances using Hartley’s ratio. Can J Stat. 2010;38:647-664. doi: 10.1002/cjs.10069 [DOI] [Google Scholar]
- 19.Salami JA, Warraich HJ, Valero-Elizondo J, et al. National trends in nonstatin use and expenditures among the US adult population from 2002 to 2013: insights from Medical Expenditure Panel Survey. J Am Heart Assoc. 2018;7(2):7. doi: 10.1161/JAHA.117.007132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 21.Brenner BM, Cooper ME, de Zeeuw D, et al. ; RENAAL Study Investigators . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861-869. doi: 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 22.Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342(3):145-153. doi: 10.1056/NEJM200001203420301 [DOI] [PubMed] [Google Scholar]
- 23.Lewis EJ, Hunsicker LG, Clarke WR, et al. ; Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851-860. doi: 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 24.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group . The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870-878. doi: 10.1056/NEJMoa011489 [DOI] [PubMed] [Google Scholar]
- 25.Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. 2006;368(9535):581-588. doi: 10.1016/S0140-6736(06)69201-5 [DOI] [PubMed] [Google Scholar]
- 26.Danchin N, Cucherat M, Thuillez C, Durand E, Kadri Z, Steg PG. Angiotensin-converting enzyme inhibitors in patients with coronary artery disease and absence of heart failure or left ventricular systolic dysfunction: an overview of long-term randomized controlled trials. Arch Intern Med. 2006;166(7):787-796. doi: 10.1001/archinte.166.7.787 [DOI] [PubMed] [Google Scholar]
- 27.Evans M, Carrero JJ, Szummer K, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in myocardial infarction patients with renal dysfunction. J Am Coll Cardiol. 2016;67(14):1687-1697. doi: 10.1016/j.jacc.2016.01.050 [DOI] [PubMed] [Google Scholar]
- 28.Palmer AJ, Valentine WJ, Chen R, et al. A health economic analysis of screening and optimal treatment of nephropathy in patients with type 2 diabetes and hypertension in the USA. Nephrol Dial Transplant. 2008;23(4):1216-1223. doi: 10.1093/ndt/gfn082 [DOI] [PubMed] [Google Scholar]
- 29.Chu CD, Powe NR, McCulloch CE, et al. ; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team . Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use among hypertensive US adults with albuminuria. Hypertension. 2021;77(1):94-102. doi: 10.1161/HYPERTENSIONAHA.120.16281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold SV, Goyal A, Inzucchi SE, et al. Quality of Care of the initial patient cohort of the Diabetes Collaborative Registry. J Am Heart Assoc. 2017;6(8):6. doi: 10.1161/JAHA.117.005999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 32.Collaboration GBDCKD; GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709-733. doi: 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cannon CP, Pratley R, Dagogo-Jack S, et al. ; VERTIS CV Investigators . Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425-1435. doi: 10.1056/NEJMoa2004967 [DOI] [PubMed] [Google Scholar]
- 34.Hernandez AF, Green JB, Janmohamed S, et al. ; Harmony Outcomes committees and investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519-1529. doi: 10.1016/S0140-6736(18)32261-X [DOI] [PubMed] [Google Scholar]
- 35.Bakris GL, Agarwal R, Anker SD, et al. ; FIDELIO-DKD Investigators . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219-2229. doi: 10.1056/NEJMoa2025845 [DOI] [PubMed] [Google Scholar]
- 36.Hall M, Bebb OJ, Dondo TB, et al. Guideline-indicated treatments and diagnostics, GRACE risk score, and survival for non-ST elevation myocardial infarction. Eur Heart J. 2018;39(42):3798-3806. doi: 10.1093/eurheartj/ehy517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338(21):1516-1520. doi: 10.1056/NEJM199805213382106 [DOI] [PubMed] [Google Scholar]
- 38.Gamboa CM, Colantonio LD, Brown TM, Carson AP, Safford MM. Race-sex differences in statin use and low-density lipoprotein cholesterol control among people with diabetes mellitus in the Reasons for Geographic and Racial Differences in Stroke Study. J Am Heart Assoc. 2017;6(5):6. doi: 10.1161/JAHA.116.004264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu A, Kamat S, Argulian E. Trends and disparities in statin use and low-density lipoprotein cholesterol levels among US patients with diabetes, 1999-2014. Diabetes Res Clin Pract. 2018;139:1-10. doi: 10.1016/j.diabres.2018.02.019 [DOI] [PubMed] [Google Scholar]
- 40.Nanna MG, Wang TY, Xiang Q, et al. Sex differences in the use of statins in community practice. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005562. doi: 10.1161/CIRCOUTCOMES.118.005562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safford MM, Gamboa CM, Durant RW, et al. Race-sex differences in the management of hyperlipidemia: the Reasons for Geographic and Racial Differences in Stroke study. Am J Prev Med. 2015;48(5):520-527. doi: 10.1016/j.amepre.2014.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nanna MG, Peterson ED. Racial differences in long-term cardiovascular outcomes: the need to move from description to action. JACC Cardiovasc Interv. 2020;13(13):1596-1598. doi: 10.1016/j.jcin.2020.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trivedi AN, Nsa W, Hausmann LR, et al. Quality and equity of care in U.S. hospitals. N Engl J Med. 2014;371(24):2298-2308. doi: 10.1056/NEJMsa1405003 [DOI] [PubMed] [Google Scholar]
- 44.American Diabetes Association . 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(suppl 1):S103-S123. doi: 10.2337/dc19-S010 [DOI] [PubMed] [Google Scholar]
- 45.Bradley SM, Adusumalli S, Amin AP, et al. ; CV-QUIC Collaborators . The Cardiovascular Quality Improvement and Care Innovation Consortium: inception of a multicenter collaborative to improve cardiovascular care. Circ Cardiovasc Qual Outcomes. 2021;14(1):e006753. doi: 10.1161/CIRCOUTCOMES.120.006753 [DOI] [PubMed] [Google Scholar]
- 46.Cosentino F, Grant PJ, Aboyans V, et al. ; ESC Scientific Document Group . 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255-323. doi: 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 47.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72(24):3200-3223. doi: 10.1016/j.jacc.2018.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das SR, Everett BM, Birtcher KK, et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145. doi: 10.1016/j.jacc.2020.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson AJ, Pagidipati NJ, Aroda VR, et al. Incorporating SGLT2i and GLP-1RA for cardiovascular and kidney disease risk reduction: call for action to the cardiology community. Circulation. 2021;144(1):74-84. doi: 10.1161/CIRCULATIONAHA.121.053766 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Datamarts and Respective Health System Participants
eTable 2. Code Lists for Comorbidities and Qualifying ASCVD