Abstract
Nonalcoholic fatty liver disease (NAFLD), which has been renamed metabolic dysfunction-associated fatty liver disease, is a growing global medical problem. The incidence of NAFLD and its associated end-stage liver disease is increasing each year, and many research advancements have been achieved to date. This review focuses on the current knowledge of the sex differences in NAFLD and does not elaborate on areas without differences. Studies have revealed significant sex differences in the prevalence, influencing factors, pathophysiology, complications and therapies of NAFLD. Men have a higher incidence than women. Compared with women, men exhibit increased visceral fat deposition, are more susceptible to leptin resistance, lack estrogen receptors, and tend to synthesize fatty acids into fat storage. Male patients will experience more severe hepatic fibrosis and a higher incidence of liver cancer. However, once NAFLD occurs, women show a faster progression of liver fibrosis, higher levels of liver cell damage and inflammation and are less likely to undergo liver transplantation than men. In general, men have more risk factors and more severe pathophysiological reactions than women, whereas the development of NAFLD is faster in women, and the treatments for women are more limited than those for men. Thus, whether sex differences should be considered in the individualized prevention and treatment of NAFLD in the future is worth considering.
Keywords: Nonalcoholic fatty liver disease, Metabolic dysfunction-associated fatty liver disease, Sex differences, Estrogen, Steatosis, Cirrhosis
Core Tip: Nonalcoholic fatty liver disease (NAFLD) is a sexual dimorphic disease, and its prevalence worldwide is increasing each year. However, our understanding of sex differences in NAFLD remains insufficient. The incidence in males is significantly higher than that in females, and studies have also revealed significant sex differences in influencing factors, pathophysiology, complications and therapies. This review summarizes the current research progress on sex differences in NAFLD and indicates that whether sex differences in NAFLD can be considered in future research, treatment and prevention is worth exploring.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), which has been renamed metabolic dysfunction-associated fatty liver disease, affects approximately a quarter of adults worldwide[1]. In the past, NAFLD was considered a Western disease, but with improvements in living standards, the prevalence of this disease in Asia is increasing each year, particularly in China[2], where the incidence has exceeded that in Europe and North America. NAFLD should be regarded as a global disease. Moreover, NAFLD, which manifests as nonalcoholic fatty liver or nonalcoholic steatohepatitis (NASH), is a growing worldwide cause of chronic liver disease, which may gradually lead to severe liver disease, such as liver cirrhosis, hepatocellular carcinoma, and even death. The increasing prevalence of this disease and the serious complications it may cause pose a substantial medical and economic burden to the whole world[3]. With the development of research on NAFLD, an increasing number of findings have revealed significant sex differences regarding this disease. This review focuses on the current knowledge of sex differences in NAFLD and does not elaborate on the areas without differences. The review mainly focuses on the following aspects: Epidemiology, influencing factors, pathophysiology, complications and treatments.
EPIDEMIOLOGY
The prevalence of NAFLD is increasing annually and has exceeded 25% of the global population. The increasing trend of NAFLD is closely related to the increasing standard of life and the increasing prevalence of obesity[4]. A large number of studies have proven that obesity is an important risk factor for hepatic steatosis and promotes the formation of NAFLD[5-7]. Considering the continuing increase in NAFLD, this disease will be the main cause of cirrhosis and hepatocellular carcinoma (HCC) and is the fastest growing cause of orthotopic liver transplantation[8].
Previous epidemiological studies revealed a significant sex difference in the incidence rate of NAFLD, which is strongly related to age. Overall, the prevalence of NAFLD in men is higher than that in women[7,9-11]. Interestingly, studies in pediatric populations have also found that the prevalence of NAFLD is higher in boys than in girls[12]. A study performed in Korea using data from 6648 subjects found that the prevalence of NAFLD in women increased with age, and the prevalence increased sharply with age among women older than 50 years, particularly after the perimenopausal period. However, the prevalence in men shows minimal differences according to age. The prevalence among men under 50 years of age is significantly higher than that among women (22.6% vs 6.8%), whereas the sex difference is not significant among participants over 50 years of age (23.6% vs 24.2%)[7]. Similar conclusions have been reached by studies conducted in Shanghai and Japan[10,11]. Compared with men and postmenopausal women, premenopausal women are at a significantly lower risk for NAFLD. Moreover, hormone replacement therapy can reduce the prevalence of NAFLD in postmenopausal women, which suggests that estrogen is protective against NAFLD[13]. Men are more susceptible to NAFLD at a younger age than women, which is a problem worthy of attention. Men are exposed to high metabolic risk for a longer period of time.
INFLUENCING FACTORS
Adipose distribution, adipocytokines and lipid metabolism
The main sex differences in adipose distribution are well recognized: Men store more adipose tissue in their intra-abdominal depots, whereas women tend to have enlarged peripheral adipose tissue, and these differences are associated with the deleterious metabolic consequences of men and the lower cardiometabolic risk of women[14]. Estrogen promotes and maintains typical female fat by reducing the lipolysis of subcutaneous fat without affecting visceral fat[15]. Peripheral adipocytes exhibit a lower lipolytic response[16]. Heine et al[17] found that estrogen regulates the amount of adipose tissue. The absence of estrogen receptor α (ERα) causes adipocyte hyperplasia and hypertrophy. Postmenopausal women exhibit increased central fat deposition[18]. For a given waist circumference or body mass index, women have higher levels of subcutaneous adipose tissue than men[19]. Sex also influences lipid storage within the liver and muscle. Fat can be stored in adipose tissue, liver and skeletal muscle as triglycerides (TGs). Excessive liver TG storage leads to NAFLD. Studies have found that men have higher levels of TGs stored in the liver than women, and women store more intramyocellular lipids than men, which explains the higher prevalence of NAFLD in men[20,21]. Skeletal muscle is one of the major organs responsible for peripheral glucose disposal, and a higher intramyocellular lipid content is associated with insulin resistance, which decreases the skeletal muscle glucose uptake[22]. However, the increased levels of intramyocellular lipids in women are not related to a higher risk of diabetes, which may be related to the mechanism through which lipids are stored and metabolized in muscles[20].
Adipose tissue releases a multitude of secretory products, which are collectively called adipocytokines. The related sex differences are mainly reflected by the levels of leptin, adiponectin and prohibitin. Leptin is a metabolic regulator that can reduce food intake and inhibit the synthesis of lipids, and its secretion is proportional to the fat mass. However, in some cases, hyperleptinemia can lead to insulin resistance and participates in hepatic steatosis. Studies have repeatedly shown that adiponectin enhances insulin sensitivity and increases lipolysis, which is inversely correlated with the fat mass[23-25]. Many studies have proven that the levels of leptin and adiponectin in males are lower than those in females[26,27]. The serum leptin levels in men and women with NAFLD are higher than those in individuals without NAFLD, whereas the opposite trend has been found for adiponectin. A previous study showed that the leptin level is correlated with the severity of steatosis in men and women, whereas the serum adiponectin level is inversely correlated with the severity of steatosis in men (P < 0.01) but not in women (P = 0.4)[26]. Prohibitin plays a sex-dimorphic role in adipose tissue functions[28]. Its overexpression induces the upregulation of mitochondrial organisms, which leads to obesity and impairments in glucose homeostasis and insulin sensitivity, but this issue is specific to males.
The retention of TGs within the liver is a prerequisite for the development of NAFLD. The synthesis of fatty acids (FAs) in the liver is an important determinant of the development of hepatic steatosis[29]. The oxidation of FAs removes TGs from the liver[30]. To some extent, the occurrence of NAFLD depends on the imbalance between liver FA synthesis and oxidation. Individuals with a similar age, BMI, and liver fat content were assessed using metabolic substrates labeled with stable isotope tracers, and the results revealed clear differences in hepatic FA partitioning. Specifically, females tended to favor oxidation pathways, and increased levels of 13C in breath CO2 and plasma 3-hydroxybutyrate are found in females, whereas males tend to favor synthetic pathways. In both men (rs = 0.75, P < 0.05) and women (rs = 0.79, P < 0.01), de novo lipogenesis (DNL) is positively correlated with the plasma very low-density lipoprotein (VLDL) cholesterol concentration[31]. As many studies have revealed, premenopausal women have better lipid profiles than men, as demonstrated by higher high-density lipoprotein cholesterol levels and lower low-density lipoprotein cholesterol, VLDL cholesterol and total plasma TG levels[32,33]. The disposal of FAs via the oxidation pathway may play an important role in preventing the accumulation of TG in the liver, which may partially explain the sex difference in the prevalence of NAFLD.
In addition, glucocorticoids exert certain effects on the human body during the process of lipid metabolism. Excessive glucocorticoids are related to the pathogenesis of NAFLD, which can cause the decomposition of adipose tissue, hyperlipidemia, visceral fat generation and insulin resistance[34]. One study found hepatic steatosis in 20% of patients with Cushing’s syndrome[35]. It is important to note that gender differences remain during the process. Among mice administered high levels of cortisol, male mice exhibit more severe insulin resistance, and female mice show more protective adaptations to adipose tissue, such as increased adiponectin levels[36].
Estrogen and hepatic ERα
Based on the abovementioned differences in prevalence between men and women and the findings that women of postmenopausal age are at increased risk of developing NAFLD, that hormone replacement therapy is protective against NAFLD after menopause[13], and that women are at increased risk of developing NAFLD after using an anti-estrogen drug or undergoing surgical ovariectomy[37,38], we can assume that the resistance to NAFLD in premenopausal women depends on estrogen. A large number of studies have shown that estrogen regulates almost all steps of lipid metabolism. Estrogen reduces the lipolysis of subcutaneous adipocytes by upregulating alpha2A-adrenergic receptors[15] and thereby reduce the delivery of FAs to the liver. Estrogen regulates liver lipid metabolism through ERα. Estrogen signaling reduces DNL to prevent hepatic steatosis. The lack of estrogen signaling will reduce the VLDL output, promote the accumulation of TGs in the liver, and lead to hepatic insulin resistance[39]. In female mammals, estrogen receptor is highly expressed in the liver, but this finding has rarely been observed in males. This receptor plays an important role in the regulation of the synthesis of receptors for cholesterol uptake, cholesterol transport proteins, and enzymes for lipoprotein remodeling[40]. A recent study found that hepatic ERα shows opposite lipid metabolism regulation in men and women that consume diets high in lipids: the male liver exceeds its compensatory capacity, and liver ERα promotes the accumulation of liver lipids by stimulating the input and synthesis of lipids. While the female liver can handle the excess lipids, and the ERα in the female liver is able to reduce lipid synthesis and absorption and promote FA oxidation[41]. In general, hepatic ERα plays an important role in the sex difference in NAFLD.
Androgen
Polycystic ovary syndrome (PCOS) is a female endocrine disease characterized by hyperandrogenemia. Many studies have documented a higher incidence of NAFLD in women with PCOS. However, only patients with PCOS and high androgen levels are associated with a higher risk of NAFLD[42]. It can be inferred that high androgen levels play an important role in this process. Biological studies have shown that androgen can induce cell cycle arrest and initiate hepatocyte apoptosis[43]. High androgen levels can promote inflammation by activating mononuclear cells[44]. Increased androgen levels increase the visceral fat mass by decreasing the activation of adenosine 5' and monophosphate-activated protein kinase and increasing the expression of lipogenic genes in visceral fat[45]. In men, however, decreased androgen levels are independently associated with NAFLD[46]. Low serum testosterone levels increase visceral fat accumulation and inflammation, and these effects lead to insulin resistance and hepatic steatosis[47-49]. Therefore, further studies may be needed to clarify the mechanism related to androgen and NAFLD.
Mitochondria and liver pyruvate kinase
Mitochondrial dysfunction contributes to the development of NAFLD, which predates insulin dysfunction and hepatic steatosis[50]. Exercise enhances mitochondrial function[51]. In general, females exhibited an improved mitochondrial quality than males. Men often need to exercise to maintain a stronger mitochondrial respiratory function, whereas women can maintain this function even without exercise[52]. A recent study reported a close relationship between liver pyruvate kinase (LPK) and NAFLD. As demonstrated by mouse experiments, LPK affects liver lipids, mitochondrial respiration, glucose metabolism and insulin sensitivity. LPK expression is increased in men under the influence of testosterone. LPK overexpression aggravates insulin resistance, increases the plasma cholesterol levels and exacerbates liver steatosis by changing liver mitochondrial respiration, whereas LPK silencing attenuates these effects. Studies have also found a positive correlation between hepatic LPK expression and the liver TG levels in males but not in females, which shows that LPK is only slightly involved in the development of steatosis in females. Researchers have performed liver biopsies in patients with NASH and observed a strong positive correlation between liver LPK expression and the NAFLD activity score in men but no correlation in women[53]. LPK overexpression exerts a male-specific effect on NAFLD.
Vitamin D deficiency
Vitamin D deficiency can increase hepatic fat accumulation and mildly reduce insulin sensitivity[54]. Studies have found that low serum levels of 25-hydroxyvitamin D are associated with dyslipidemia and cardiovascular disease[55,56]. Vitamin D increases intestinal calcium absorption, and this finding is more obvious in males than in females[57]. Calcium supplements can improve blood lipids[58]. Estrogen can also affect calcium absorption[59]. A sex disparity has been found for the association of vitamin D deficiency with NAFLD. A cross-sectional study revealed that vitamin D deficiency is positively associated with NAFLD in men, whereas no significant interaction has been observed in women. Vitamin D deficiency is an independent risk factor for NAFLD in men and may be associated with testosterone levels[60]. Women may respond differently to vitamin D supplementation than men, and women show improvements in blood lipids in response to this supplementation[61].
Serum uric acid
Elevated serum uric acid (SUA) is a risk factor for NAFLD[62,63]. The mechanism may be related to the insulin resistance induced by high SUA levels[64]. According to basic studies, SUA can directly induce and regulate hepatic steatosis and stimulate hepatic fat accumulation[65-67]. Many population-based studies have revealed a sex difference in the association between SUA and NAFLD: The correlation between SUA and NAFLD is significantly higher in women than in men[68,69]. However, a study of patients with type 2 diabetes mellitus (T2DM) revealed that although SUA is associated with NAFLD, an increase in the SUA level is independently associated with a higher risk of NAFLD only in male patients[70]. Therefore, whether diabetes is one of the causes of the inconsistent results is worth further study.
Fructose and dietary intake, sleep quality and the gut microbiota
Regarding food choices, women tend to eat more fruits, vegetables and grains, whereas men tend to choose more meat products, eggs and certain types of poultry[71]. Studies have shown that women tend to eat better-quality diets. The negative correlation between diet quality and obesity is similar in both men and women[72]. However, the diet-induced increases in serum TGs are more significant in females[73]. With a high consumption of fructose, the role of fructose in inducing NAFLD has become increasingly important. Fructose can increase insulin resistance and induce an increase in plasma TGs. In a study of a high-fructose diet, female mice that were fed this diet for a long time showed extensive steatosis and ballooning, whereas males showed only a slight increase in hepatic steatosis[73]. These findings suggest that after the long-term consumption of high-fructose foods, women are more likely than men to develop NAFLD/NASH.
Poor sleep affects the production of hormones and increases the risk of metabolic syndrome. However, poor sleep quality can be more detrimental to women than men by increasing the risk of T2DM and cardiovascular disease, and this finding may be related to the higher testosterone levels in men and sex differences in peroxisome proliferator-activated receptor-α[74]. The sex differences in gut microbes may be influenced by age, race, and diet. Intestinal microbiota-dependent metabolites, such as short-chain fatty acids and trimethylamine N-oxide, are involved in the regulation of cholesterol metabolism and insulin sensitivity. Women are more vulnerable to adverse effects[75]. Bile acids are important signaling molecules that activate receptors such as farnesoid X receptor, and these receptors have been shown to promote hepatic steatosis[76]. Intestinal microorganisms can mediate the metabolism of bile acids. The gut microbiota differs according to sex, which leads to differences in the synthesis and metabolism of bile acids and other metabolites between males and females and thus affects the metabolism of liver fat[77].
PATHOPHYSIOLOGY
Hepatic fibrosis
NAFLD includes a spectrum of liver disorders consisting of nonalcoholic fatty liver and NASH, which range from simple hepatic steatosis to inflammation and fibrosis and even progress to cirrhosis. Hepatic stellate cells are one of the primary target cells of hepatic inflammatory stimulation and play a major role during liver repair reactions, including fibrosis[78]. In mice, estrogen inhibits the activation of stellate cells and suppresses the induction of hepatic fibrosis through estrogen receptors[79]. A cross-sectional study revealed that postmenopausal women and men are at a higher risk (60% to 70%) of developing severe fibrosis than premenopausal women. In postmenopausal women, estrogen replacement treatment appears to reduce the risk of advanced fibrosis[80]. A recent systematic review and meta-analysis of 62239 individuals also found that women have a lower risk of developing NAFLD than men pooled risk ratio (RR), 0.81; 95%CI, 0.68-0.97; I2 = 97.5%]. However, after the development of NAFLD, women face a higher risk of developing advanced fibrosis than men (RR, 1.56; 95%CI, 1.36-1.80; I2 = 0), and this finding is particularly obvious among individuals older than 50 years[81]. This finding suggests the protective effect of estrogen on hepatic fibrosis in patients with NASH, and the effect is more pronounced in patients with hepatitis C virus[82]. However, the severity of hepatocyte injury and inflammation in NAFLD shows the opposite trend. Premenopausal women exhibit increased levels of lobular inflammation, hepatocyte ballooning and Mallory-Denk bodies than men and postmenopausal women. Hormone replacement therapy is related to a risk of more severe hepatocyte inflammation in postmenopausal women[83]. This association may be related to an increase in the progesterone levels, but this hypothesis needs further study.
Branched-chain amino acids
Branched-chain amino acids (BCAAs) are amino acids with nonlinear aliphatic side chains and include the essential amino acids leucine, valine and isoleucine. High plasma BCAA levels may contribute to insulin resistance and increase the risks of metabolic syndrome and T2DM[84,85]. Intestinal microorganisms are also related to the synthesis of BCAAs[86]. The level of plasma BCAAs in patients with NAFLD is increased, and its changes show sex dimorphism. Studies have shown that the plasma BCAA concentration is positively correlated with the severity of NAFLD[87]. However, a recent study found that only the female BCAA concentration is positively associated with the level of steatosis and fibrosis in NAFLD, whereas no correlation has been detected in males, as demonstrated by a moderate negative correlation between the plasma valine level and lobular inflammation. Additionally, menopause alone has no significant effect on the plasma BCAA concentration in NAFLD[88]. The mechanism of BCAAs involved in NAFLD remains unclear. BACCs are associated with activation of the mammalian target of rapamycin pathway and liver injury in mice[89-91]. However, BCAA supplementing can reduce further liver injury in patients with liver cirrhosis[92].
Macrophages and inflammation
A large number of experimental and clinical studies have shown that macrophages play a critical role in the development and progression of NAFLD. Liver-resident macrophages, which are also known as Kupffer cells, are important participants in liver metabolism disorders and inflammation. These cells activate the inflammatory response, recruit monocytes into the liver, and then differentiate into proinflammatory macrophages to promote the development of NAFLD. Kupffer cells are closely associated with insulin resistance, FA accumulation, and inflammatory injury to promote the progression of fibrosis[93]. High fructose intake triggers the activation of Kupffer cells, which leads to an inflammatory response. As mentioned above, fructose plays an important role in inducing NAFLD. The main types of macrophages can be divided into proinflammatory and anti-inflammatory subgroups (M1 and M2), and both estrogen and androgen receptors can be found in murine macrophages and promote M2 phenotype differentiation[94]. The effect of sex on the differentiation of hepatic macrophages in patients with NAFLD has not been reported. However, accumulating evidence shows that testosterone reduces the secretion of proinflammatory cytokines by macrophages and exerts anti-inflammatory effects[95].
COMPLICATIONS
HCC
HCC is the fourth leading cause of cancer-related death worldwide, and its morbidity and mortality rates are both increasing[96,97]. With improvements in the prevention, diagnosis and treatment of viral hepatitis, the proportion of end-stage liver disease caused by NAFLD progression is increasing. NAFLD is the most common cause of chronic liver disease in the world. HCC is one of the major complications of NASH-associated cirrhosis[98]. NASH-related liver disease has become the leading indicator of liver transplantation (LT)[99]. The incidence of liver cancer in males is significantly higher than that in females. Premenopausal women are also at a lower risk of NAFLD than men. Some studies have found that estrogen may prevent the occurrence of liver cancer and can play a beneficial biological role once HCC develops[100]. Among patients with nonsurgical liver cancer and patients undergoing surgical resection, the prognosis of women is better than that of men[101,102], but the benefits from LT in patients exhibit no significant gender differences[101]. Moreover, under the current organ allocation system, the proportion of women who receive a LT is lower than that of men. The explanations of this sex difference in LT may include size mismatch and lower creatinine levels in women, which leads to lower Model for End-Stage Liver Disease scores[103,104].
Cardiovascular disease
An increasing number of studies have shown that NAFLD can increase the incidence and prevalence of cardiovascular disease (CVD). In addition, the incidence of CVD presents a sex difference similar to that of NAFLD: Men younger than 50 years are at a higher risk of developing CVD than women, but the incidence of CVD in postmenopausal women is higher[105]. Women are also at a lower risk of death from CVD than men[106]. As mentioned above, women exhibit better blood lipid values than men, and men with NAFLD have worse TG and high-density lipoprotein levels than women[107]. In addition, the incidence of other cardiovascular risk factors in patients with NASH, such as hypertension, renal failure and smoking, is lower in women than in men. Women are more likely to develop obesity and diabetes. A retrospective observational cohort study of 41005 adult patients with NASH conducted by Gayatri Pemmasani found that males have a higher incidence of most CVDs, such as coronary artery disease, myocardial infarction, and heart failure, than females[108]. However, another previous study showed that women with NAFLD lose the protective effect that women have against cardiovascular disease[109]. These researchers found that women and men with NAFLD have a similar risk of CVD and that women with NAFLD develop CVD earlier than women without NAFLD. This finding may be due to the high metabolic burden of patients with NAFLD because these metabolites neutralize the protective effect of estrogen.
T2DM
NAFLD is associated with an increased risk of T2DM, is involved in the pathogenesis of T2DM and promotes insulin resistance. Obesity is a risk factor for NAFLD and T2DM. However, a recent study obtained a novel finding that NAFLD has a hazard ratio of 2.331 for the incidence of diabetes. Among lean patients with NAFLD, the effect appeared to be more pronounced in women, particularly postmenopausal women, than in men (5.53 vs 2.02)[110]. A study conducted in Japan also showed that the female sex is an independent risk factor for T2DM through the follow-up of patients diagnosed with NAFLD[111].
Others
A previous study showed that the serum insulin levels are directly correlated with a higher risk of colorectal adenomas (OR, 1.5; 95%CI, 1.1-2.0; P = 0.005) and hyperplastic polyps (OR, 1.3; 95%CI, 1.0-1.7; P = 0.075)[112]. Disorders of insulin and adipocytokine metabolism are now thought to influence the development of colon tumors[113]. Patients with NAFLD always show fat metabolism disorders, insulin resistance, and high insulin levels. A large number of studies have shown that NAFLD is a risk factor for adenomatous polyps and hyperplastic polyps[114,115]. However, the correlation exhibits significant sex differences: NAFLD is associated with an increased risk of colorectal adenomatous and hyperplastic polyps in men (OR = 1.53, 95%CI: 1.18-2.00, P < 0.05; OR = 1.42, 95%CI: 1.04-1.95, P < 0.05) but is not a significant risk factor in women (OR = 0.44, 95%CI: 0.18-1.04, P > 0.05; OR = 1.18, 95%CI: 0.50-2.78, P > 0.05)[115]. The promoting mechanism of NAFLD on colorectal adenoma and hyperplastic polyps is unclear, but some researches believe that this mechanism may be related to the metabolic disorder of adipocytes and the effect of inflammatory cytokines[113]; thus hypothesis needs further exploration.
Abdominal obesity and insulin resistance are risk factors for erosive esophagitis (EO). Metabolic syndrome (MS) and NAFLD are significantly associated with EO. A previous study revealed significant sex differences in the effects of NAFLD and MS on EO: MS (OR 1.26; 95%CI 1.09 to 1.45) shows a greater detrimental effect on EO in males, NAFLD (OR 1.93; 95%CI 1.43 to 2.59) is significantly associated with EO in females, and the relationship between NAFLD and EO is stronger in premenopausal females than in postmenopausal females (51.1% vs 48.9%)[116]. In addition, MS is independently associated with EO through increased serum cytokines. Men exhibit increased visceral obesity deposition than women, and visceral obesity increases the esophageal reflux by increasing the serum cytokine levels[117]. The sex difference between NAFLD and EO may be related to estrogen, which reduces oxidative stress and serum cytokines. Hence, the decrease in estrogen levels found in female patients with NAFLD leads to a decrease in the protective effect on EO.
THERAPY
Very low-carbohydrate ketogenic diets
Obesity is a risk factor for NAFLD. Very low-carbohydrate ketogenic diets (VLCKDs) constitute a new treatment for obesity that functions by reducing the caloric intake and promoting the transformation of energy metabolism from carbohydrates to TGs to reduce weight. Previous studies have suggested that VLCKDs are associated with inducing the activity of lysosomal acid lipase and improving hepatic steatosis, which can benefit patients with NAFLD[118]. Studies have shown that men benefit more from this therapy than women, particularly premenopausal women[119]. This finding may be related to the fact that men have more visceral adipose tissue and exhibit a higher basal energy expenditure.
Inhibition of protein tyrosine phosphatase 1B
Protein tyrosine phosphatase 1B (PTP1B) is an enzyme with multiple functions that can inhibit leptin and insulin signal transduction, which results in abnormal glucose tolerance and hepatic steatosis. PTP1B inhibition may be a potential weight loss therapy that increases energy consumption, weight loss and insulin sensitivity[120]. A study of the role of proopiomelanocortin neuronal-specific PTP1B deficiency in metabolic regulation after consumption of a high-fat diet found that male but not female mice fed this diet exhibit significantly reduced liver lipid accumulation than control mice[121]. This result may indicate that PTP1B is a potential target in the treatment of NAFLD in men.
Others
At present, the treatment of NAFLD remains focused on prevention, as reflected by the control of risk factors, such as weight loss, reduced fat and fructose intake, increased exercise, and vitamin D supplementation. Men lose weight mainly by reducing their visceral adipose tissue and exhibit better histological improvement than women[122,123]. Women are more affected by dietary factors than men. Reducing lipid and fructose intake is more beneficial for female patients. Physical activities are beneficial to the prevention of NAFLD, and exercise can reduce liver enzymes in postmenopausal women[124]. Vitamin D deficiency is an independent risk factor for NAFLD in men, and men should be screened early and administered timely supplementation. Therefore, the optimal prevention of NAFLD may differ by sex, but no consensus has been reached, and further exploration is still needed.
CONCLUSION
As mentioned above, NAFLD exhibits significant sex dimorphism in many aspects, particularly in influencing factors, pathophysiology (Figure 1) and intrahepatic and extrahepatic damage (Figure 2). In general, the higher incidence among males than females is related to adipose distribution, adipose metabolism, differences in estrogen and its receptors, liver metabolism and other factors. The protective effects of estrogen reduce the degree of liver fibrosis in women. Robust evidence shows that NAFLD is closely associated with liver cancer, cardiovascular disease, T2DM and other diseases, and men with NAFLD are at a higher risk of experiencing these complications than women. However, once NAFLD occurs, the inflammation and disease progression is markedly worse among female than male patients, and the treatments for females are more limited than those for men. Although we found a large number of sex differences in NAFLD, the relevant principles are unclear, and further research is needed. Whether sex differences should be considered in future research and whether they can be applied to clinical personalized treatment and prevention are still worth exploring.
Figure 1.
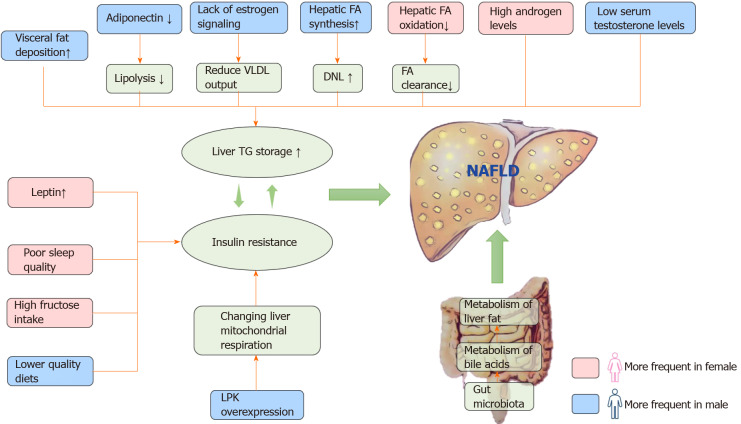
Overview of sex differences in etiology and pathogenesis of nonalcoholic fatty liver disease. Men store more visceral adipose tissue than women. Adipokines mediate fat metabolism, and adiponectin can increase lipolysis; however, excessive leptin can lead to insulin resistance and steatosis. The estrogen receptor plays an important role in the regulation of fat metabolism. Increased androgen levels in women and low testosterone in men are prone to visceral fat accumulation. In the process of fatty acid metabolism, men tend to synthesize, and women tend to oxidize. We found that the overexpression of liver pyruvate kinase can lead to changes in liver mitochondrial function, which leads to the deformation of liver fat. The difference in intestinal microflora between men and women also plays a role in the sex difference in nonalcoholic fatty liver disease (NAFLD). In addition, sleep quality, high sugar intake and diet quality can also affect the formation of NAFLD. VLDL: Very low-density lipoprotein; DNL: De novo lipogenesis; TG: Triglyceride; LPK: Liver pyruvate kinase; FA: Fatty acid; NAFLD: Nonalcoholic fatty liver disease.
Figure 2.
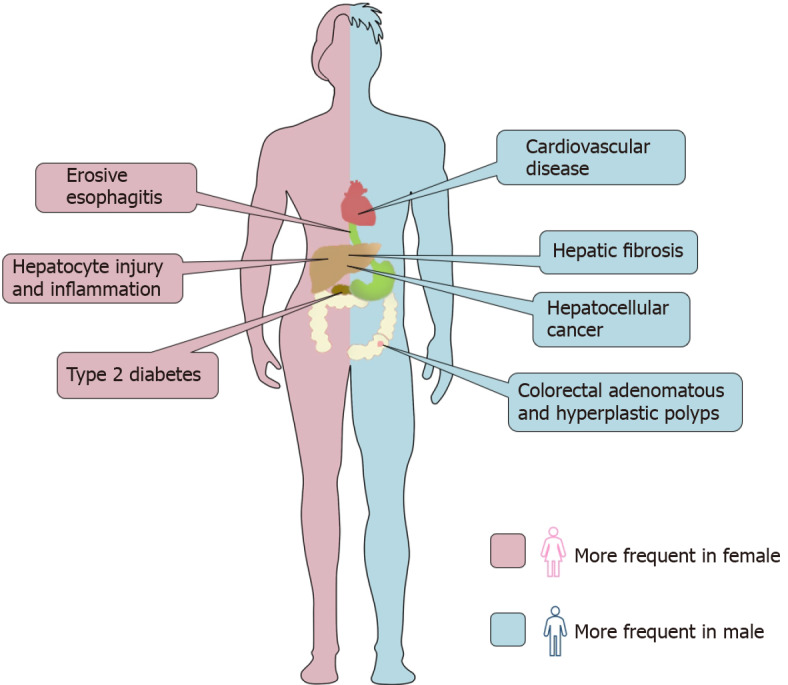
Sex differences in intrahepatic and extrahepatic outcomes in nonalcoholic fatty liver disease. As a metabolic disorder, nonalcoholic fatty liver disease (NAFLD) is related not only to liver injury but also to a variety of extrahepatic diseases. The picture summarizes the differences between men and women in this respect. Without the protection of estrogen, men have more serious liver fibrosis than women and are more likely to develop liver cancer. The incidence of cardiovascular events and colorectal adenoma in men with NAFLD is higher than that in women. However, female patients have more severe hepatocyte injury and inflammation than male patients and have a higher risk of erosive esophagitis and type 2 diabetes.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest associated with this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 5, 2021
First decision: November 15, 2021
Article in press: December 31, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Machado M, Tarantino G, Ulasoglu C S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
Contributor Information
Xing-Yu Chen, The Second Affiliated Hospital, Chongqing Medical University, Chongqing 404100, China.
Cong Wang, The Second Affiliated Hospital, Chongqing Medical University, Chongqing 404100, China.
Yi-Zhou Huang, The Second Affiliated Hospital, Chongqing Medical University, Chongqing 404100, China.
Li-Li Zhang, The Second Affiliated Hospital, Chongqing Medical University, Chongqing 404100, China. zhanglili.jl@foxmail.com.
References
- 1.Eslam M, Sanyal AJ, George J International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Unexpected Rapid Increase in the Burden of NAFLD in China From 2008 to 2018: A Systematic Review and Meta-Analysis. Hepatology. 2019;70:1119–1133. doi: 10.1002/hep.30702. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 4.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Festi D, Colecchia A, Sacco T, Bondi M, Roda E, Marchesini G. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev. 2004;5:27–42. doi: 10.1111/j.1467-789x.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 6.Morita S, Neto Dde S, Morita FH, Morita NK, Lobo SM. Prevalence of Non-alcoholic Fatty Liver Disease and Steatohepatitis Risk Factors in Patients Undergoing Bariatric Surgery. Obes Surg. 2015;25:2335–2343. doi: 10.1007/s11695-015-1696-5. [DOI] [PubMed] [Google Scholar]
- 7.Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Keum DK, Kim BI. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138–143. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- 8.Shingina A, DeWitt PE, Dodge JL, Biggins SW, Gralla J, Sprague D, Bambha K. Future Trends in Demand for Liver Transplant: Birth Cohort Effects Among Patients With NASH and HCC. Transplantation. 2019;103:140–148. doi: 10.1097/TP.0000000000002497. [DOI] [PubMed] [Google Scholar]
- 9.Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T JSG-NAFLD. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 10.Fang JG, Zhu J, Li XJ, Li R, Dai F, Song XM, Chen L, Li F, Chen SY. [Epidemiological survey of prevalence of fatty liver and its risk factors in a general adult population of Shanghai] Zhonghua Gan Zang Bing Za Zhi. 2005;13:83–88. [PubMed] [Google Scholar]
- 11.Hamaguchi M, Kojima T, Ohbora A, Takeda N, Fukui M, Kato T. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World J Gastroenterol. 2012;18:237–243. doi: 10.3748/wjg.v18.i3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenzie J, Fisher BM, Jaap AJ, Stanley A, Paterson K, Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clin Endocrinol (Oxf) 2006;65:40–44. doi: 10.1111/j.1365-2265.2006.02543.x. [DOI] [PubMed] [Google Scholar]
- 14.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J Clin Endocrinol Metab. 2004;89:1869–1878. doi: 10.1210/jc.2003-031327. [DOI] [PubMed] [Google Scholar]
- 16.Leibel RL, Edens NK, Fried SK. Physiologic basis for the control of body fat distribution in humans. Annu Rev Nutr. 1989;9:417–443. doi: 10.1146/annurev.nu.09.070189.002221. [DOI] [PubMed] [Google Scholar]
- 17.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambikairajah A, Walsh E, Tabatabaei-Jafari H, Cherbuin N. Fat mass changes during menopause: a metaanalysis. Am J Obstet Gynecol. 2019;221:393–409.e50. doi: 10.1016/j.ajog.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, Ravussin E, Ryan DH, Smith SR, Katzmarzyk PT. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19:402–408. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaudry KM, Devries MC. Sex-based differences in hepatic and skeletal muscle triglyceride storage and metabolism 1. Appl Physiol Nutr Metab. 2019;44:805–813. doi: 10.1139/apnm-2018-0635. [DOI] [PubMed] [Google Scholar]
- 21.Høeg L, Roepstorff C, Thiele M, Richter EA, Wojtaszewski JF, Kiens B. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J Appl Physiol (1985) 2009;107:824–831. doi: 10.1152/japplphysiol.91382.2008. [DOI] [PubMed] [Google Scholar]
- 22.van Loon LJ. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. J Appl Physiol (1985) 2004;97:1170–1187. doi: 10.1152/japplphysiol.00368.2004. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 26.Ayonrinde OT, Olynyk JK, Beilin LJ, Mori TA, Pennell CE, de Klerk N, Oddy WH, Shipman P, Adams LA. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology. 2011;53:800–809. doi: 10.1002/hep.24097. [DOI] [PubMed] [Google Scholar]
- 27.Valencak TG, Osterrieder A, Schulz TJ. Sex matters: The effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 2017;12:806–813. doi: 10.1016/j.redox.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ande SR, Nguyen KH, Padilla-Meier GP, Wahida W, Nyomba BL, Mishra S. Prohibitin overexpression in adipocytes induces mitochondrial biogenesis, leads to obesity development, and affects glucose homeostasis in a sex-specific manner. Diabetes. 2014;63:3734–3741. doi: 10.2337/db13-1807. [DOI] [PubMed] [Google Scholar]
- 29.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodson L, Frayn KN. Hepatic fatty acid partitioning. Curr Opin Lipidol. 2011;22:216–224. doi: 10.1097/MOL.0b013e3283462e16. [DOI] [PubMed] [Google Scholar]
- 31.Pramfalk C, Pavlides M, Banerjee R, McNeil CA, Neubauer S, Karpe F, Hodson L. Sex-Specific Differences in Hepatic Fat Oxidation and Synthesis May Explain the Higher Propensity for NAFLD in Men. J Clin Endocrinol Metab. 2015;100:4425–4433. doi: 10.1210/jc.2015-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magkos F, Mittendorfer B. Gender differences in lipid metabolism and the effect of obesity. Obstet Gynecol Clin North Am. 2009;36:245–265, vii. doi: 10.1016/j.ogc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Johnson JL, Slentz CA, Duscha BD, Samsa GP, McCartney JS, Houmard JA, Kraus WE. Gender and racial differences in lipoprotein subclass distributions: the STRRIDE study. Atherosclerosis. 2004;176:371–377. doi: 10.1016/j.atherosclerosis.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Papanastasiou L, Fountoulakis S, Vatalas IA. Adrenal disorders and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42:151–163. doi: 10.23736/S0391-1977.16.02583-9. [DOI] [PubMed] [Google Scholar]
- 35.Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP, Besser GM, Grossman AB, Reznek RH. Hepatic steatosis in Cushing's syndrome: a radiological assessment using computed tomography. Eur J Endocrinol. 2003;149:543–548. doi: 10.1530/eje.0.1490543. [DOI] [PubMed] [Google Scholar]
- 36.Kaikaew K, Steenbergen J, van Dijk TH, Grefhorst A, Visser JA. Sex Difference in Corticosterone-Induced Insulin Resistance in Mice. Endocrinology. 2019;160:2367–2387. doi: 10.1210/en.2019-00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishino M, Hayakawa K, Nakamura Y, Morimoto T, Mukaihara S. Effects of tamoxifen on hepatic fat content and the development of hepatic steatosis in patients with breast cancer: high frequency of involvement and rapid reversal after completion of tamoxifen therapy. AJR Am J Roentgenol. 2003;180:129–134. doi: 10.2214/ajr.180.1.1800129. [DOI] [PubMed] [Google Scholar]
- 38.Matsuo K, Gualtieri MR, Cahoon SS, Jung CE, Paulson RJ, Shoupe D, Muderspach LI, Wakatsuki A, Wright JD, Roman LD. Surgical menopause and increased risk of nonalcoholic fatty liver disease in endometrial cancer. Menopause. 2016;23:189–196. doi: 10.1097/GME.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmisano BT, Zhu L, Stafford JM. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv Exp Med Biol. 2017;1043:227–256. doi: 10.1007/978-3-319-70178-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Della Torre S, Mitro N, Fontana R, Gomaraschi M, Favari E, Recordati C, Lolli F, Quagliarini F, Meda C, Ohlsson C, Crestani M, Uhlenhaut NH, Calabresi L, Maggi A. An Essential Role for Liver ERα in Coupling Hepatic Metabolism to the Reproductive Cycle. Cell Rep. 2016;15:360–371. doi: 10.1016/j.celrep.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meda C, Barone M, Mitro N, Lolli F, Pedretti S, Caruso D, Maggi A, Della Torre S. Hepatic ERα accounts for sex differences in the ability to cope with an excess of dietary lipids. Mol Metab. 2020;32:97–108. doi: 10.1016/j.molmet.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramezani-Binabaj M, Motalebi M, Karimi-Sari H, Rezaee-Zavareh MS, Alavian SM. Are women with polycystic ovarian syndrome at a high risk of non-alcoholic Fatty liver disease; a meta-analysis. Hepat Mon. 2014;14:e23235. doi: 10.5812/hepatmon.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai R, Yan D, Li J, Chen S, Liu Y, Chen R, Duan C, Wei M, Li H, He T. Activation of PKR/eIF2α signaling cascade is associated with dihydrotestosterone-induced cell cycle arrest and apoptosis in human liver cells. J Cell Biochem. 2012;113:1800–1808. doi: 10.1002/jcb.24051. [DOI] [PubMed] [Google Scholar]
- 44.González F, Nair KS, Daniels JK, Basal E, Schimke JM. Hyperandrogenism sensitizes mononuclear cells to promote glucose-induced inflammation in lean reproductive-age women. Am J Physiol Endocrinol Metab. 2012;302:E297–E306. doi: 10.1152/ajpendo.00416.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McInnes KJ, Corbould A, Simpson ER, Jones ME. Regulation of adenosine 5',monophosphate-activated protein kinase and lipogenesis by androgens contributes to visceral obesity in an estrogen-deficient state. Endocrinology. 2006;147:5907–5913. doi: 10.1210/en.2006-0879. [DOI] [PubMed] [Google Scholar]
- 46.Kim S, Kwon H, Park JH, Cho B, Kim D, Oh SW, Lee CM, Choi HC. A low level of serum total testosterone is independently associated with nonalcoholic fatty liver disease. BMC Gastroenterol. 2012;12:69. doi: 10.1186/1471-230X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord. 2000;24:485–491. doi: 10.1038/sj.ijo.0801183. [DOI] [PubMed] [Google Scholar]
- 48.Tarantino G, Savastano S, Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J Gastroenterol. 2010;16:4773–4783. doi: 10.3748/wjg.v16.i38.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27:861–868. doi: 10.2337/diacare.27.4.861. [DOI] [PubMed] [Google Scholar]
- 50.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52:727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fletcher JA, Meers GM, Linden MA, Kearney ML, Morris EM, Thyfault JP, Rector RS. Impact of various exercise modalities on hepatic mitochondrial function. Med Sci Sports Exerc. 2014;46:1089–1097. doi: 10.1249/MSS.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellissimo CA, Perry CGR. Sex differences in the regulation of hepatic mitochondrial turnover following physical activity: do males need more quality control than females? J Physiol. 2018;596:6125–6126. doi: 10.1113/JP276896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chella Krishnan K, Floyd RR, Sabir S, Jayasekera DW, Leon-Mimila PV, Jones AE, Cortez AA, Shravah V, Péterfy M, Stiles L, Canizales-Quinteros S, Divakaruni AS, Huertas-Vazquez A, Lusis AJ. Liver Pyruvate Kinase Promotes NAFLD/NASH in Both Mice and Humans in a Sex-Specific Manner. Cell Mol Gastroenterol Hepatol. 2021;11:389–406. doi: 10.1016/j.jcmgh.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giblin RJ, Bennett EJ, Zosky GR, Dwyer RM. The Impact of Sex and 25(OH)D Deficiency on Metabolic Function in Mice. Nutrients. 2017;9 doi: 10.3390/nu9090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 56.Karhapää P, Pihlajamäki J, Pörsti I, Kastarinen M, Mustonen J, Niemelä O, Kuusisto J. Diverse associations of 25-hydroxyvitamin D and 1,25-dihydroxy-vitamin D with dyslipidaemias. J Intern Med. 2010;268:604–610. doi: 10.1111/j.1365-2796.2010.02279.x. [DOI] [PubMed] [Google Scholar]
- 57.Uhland-Smith A, DeLuca HF. 1,25-dihydroxycholecalciferol analogs cannot replace vitamin D in normocalcemic male rats. J Nutr. 1993;123:1777–1785. doi: 10.1093/jn/123.11.1777. [DOI] [PubMed] [Google Scholar]
- 58.Major GC, Alarie F, Doré J, Phouttama S, Tremblay A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. 2007;85:54–59. doi: 10.1093/ajcn/85.1.54. [DOI] [PubMed] [Google Scholar]
- 59.Dong XL, Zhang Y, Wong MS. Estrogen deficiency-induced Ca balance impairment is associated with decrease in expression of epithelial Ca transport proteins in aged female rats. Life Sci. 2014;96:26–32. doi: 10.1016/j.lfs.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 60.Park D, Kwon H, Oh SW, Joh HK, Hwang SS, Park JH, Yun JM, Lee H, Chung GE, Ze S, Bae Y, Lee A. Is Vitamin D an Independent Risk Factor of Nonalcoholic Fatty Liver Disease? J Korean Med Sci. 2017;32:95–101. doi: 10.3346/jkms.2017.32.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharifi N, Amani R, Hajiani E, Cheraghian B. Women may respond different from men to vitamin D supplementation regarding cardiometabolic biomarkers. Exp Biol Med (Maywood) 2016;241:830–838. doi: 10.1177/1535370216629009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu C, Yu C, Xu L, Miao M, Li Y. High serum uric acid increases the risk for nonalcoholic Fatty liver disease: a prospective observational study. PLoS One. 2010;5:e11578. doi: 10.1371/journal.pone.0011578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shih MH, Lazo M, Liu SH, Bonekamp S, Hernaez R, Clark JM. Association between serum uric acid and nonalcoholic fatty liver disease in the US population. J Formos Med Assoc. 2015;114:314–320. doi: 10.1016/j.jfma.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu Y, Hu Y, Huang T, Zhang Y, Li Z, Luo C, Luo Y, Yuan H, Hisatome I, Yamamoto T, Cheng J. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem Biophys Res Commun. 2014;447:707–714. doi: 10.1016/j.bbrc.2014.04.080. [DOI] [PubMed] [Google Scholar]
- 65.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, Schreiner G, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Sautin YY, Johnson RJ. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wan X, Xu C, Lin Y, Lu C, Li D, Sang J, He H, Liu X, Li Y, Yu C. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J Hepatol. 2016;64:925–932. doi: 10.1016/j.jhep.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 67.Choi YJ, Shin HS, Choi HS, Park JW, Jo I, Oh ES, Lee KY, Lee BH, Johnson RJ, Kang DH. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab Invest. 2014;94:1114–1125. doi: 10.1038/labinvest.2014.98. [DOI] [PubMed] [Google Scholar]
- 68.Wu SJ, Zhu GQ, Ye BZ, Kong FQ, Zheng ZX, Zou H, Shi KQ, Lin L, Braddock M, Huang WJ, Chen YP, Zheng MH. Association between sex-specific serum uric acid and non-alcoholic fatty liver disease in Chinese adults: a large population-based study. Medicine (Baltimore) 2015;94:e802. doi: 10.1097/MD.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hwang IC, Suh SY, Suh AR, Ahn HY. The relationship between normal serum uric acid and nonalcoholic fatty liver disease. J Korean Med Sci. 2011;26:386–391. doi: 10.3346/jkms.2011.26.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fan N, Zhang L, Xia Z, Peng L, Wang Y, Peng Y. Sex-Specific Association between Serum Uric Acid and Nonalcoholic Fatty Liver Disease in Type 2 Diabetic Patients. J Diabetes Res. 2016;2016:3805372. doi: 10.1155/2016/3805372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiferaw B, Verrill L, Booth H, Zansky SM, Norton DM, Crim S, Henao OL. Sex-based differences in food consumption: Foodborne Diseases Active Surveillance Network (FoodNet) Population Survey, 2006-2007. Clin Infect Dis. 2012;54 Suppl 5:S453–S457. doi: 10.1093/cid/cis247. [DOI] [PubMed] [Google Scholar]
- 72.Maskarinec G, Namatame LA, Kang M, Buchthal SD, Ernst T, Monroe KR, Shepherd JA, Wilkens LR, Boushey CJ, Marchand LL, Lim U. Differences in the association of diet quality with body fat distribution between men and women. Eur J Clin Nutr. 2020;74:1434–1441. doi: 10.1038/s41430-020-0563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hyer MM, Dyer SK, Kloster A, Adrees A, Taetzsch T, Feaster J, Valdez G, Neigh GN. Sex modifies the consequences of extended fructose consumption on liver health, motor function, and physiological damage in rats. Am J Physiol Regul Integr Comp Physiol. 2019;317:R903–R911. doi: 10.1152/ajpregu.00046.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008;22:960–968. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Razavi AC, Potts KS, Kelly TN, Bazzano LA. Sex, gut microbiome, and cardiovascular disease risk. Biol Sex Differ. 2019;10:29. doi: 10.1186/s13293-019-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie G, Wang X, Zhao A, Yan J, Chen W, Jiang R, Ji J, Huang F, Zhang Y, Lei S, Ge K, Zheng X, Rajani C, Alegado RA, Liu J, Liu P, Nicholson J, Jia W. Sex-dependent effects on gut microbiota regulate hepatic carcinogenic outcomes. Sci Rep. 2017;7:45232. doi: 10.1038/srep45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kobold D, Grundmann A, Piscaglia F, Eisenbach C, Neubauer K, Steffgen J, Ramadori G, Knittel T. Expression of reelin in hepatic stellate cells and during hepatic tissue repair: a novel marker for the differentiation of HSC from other liver myofibroblasts. J Hepatol. 2002;36:607–613. doi: 10.1016/s0168-8278(02)00050-8. [DOI] [PubMed] [Google Scholar]
- 79.Zhang B, Zhang CG, Ji LH, Zhao G, Wu ZY. Estrogen receptor β selective agonist ameliorates liver cirrhosis in rats by inhibiting the activation and proliferation of hepatic stellate cells. J Gastroenterol Hepatol. 2018;33:747–755. doi: 10.1111/jgh.13976. [DOI] [PubMed] [Google Scholar]
- 80.Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, Suzuki A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406–1414. doi: 10.1002/hep.26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balakrishnan M, Patel P, Dunn-Valadez S, Dao C, Khan V, Ali H, El-Serag L, Hernaez R, Sisson A, Thrift AP, Liu Y, El-Serag HB, Kanwal F. Women Have a Lower Risk of Nonalcoholic Fatty Liver Disease but a Higher Risk of Progression vs Men: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2021;19:61–71.e15. doi: 10.1016/j.cgh.2020.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Codes L, Asselah T, Cazals-Hatem D, Tubach F, Vidaud D, Paraná R, Bedossa P, Valla D, Marcellin P. Liver fibrosis in women with chronic hepatitis C: evidence for the negative role of the menopause and steatosis and the potential benefit of hormone replacement therapy. Gut. 2007;56:390–395. doi: 10.1136/gut.2006.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang JD, Abdelmalek MF, Guy CD, Gill RM, Lavine JE, Yates K, Klair J, Terrault NA, Clark JM, Unalp-Arida A, Diehl AM, Suzuki A Nonalcoholic Steatohepatitis Clinical Research Network. Patient Sex, Reproductive Status, and Synthetic Hormone Use Associate With Histologic Severity of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2017;15:127–131.e2. doi: 10.1016/j.cgh.2016.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bloomgarden Z. Diabetes and branched-chain amino acids: What is the link? J Diabetes. 2018;10:350–352. doi: 10.1111/1753-0407.12645. [DOI] [PubMed] [Google Scholar]
- 86.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Doré J, Mattila I, Plichta DR, Pöhö P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jørgensen T, Holm JB, Trošt K MetaHIT Consortium, Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 87.Lake AD, Novak P, Shipkova P, Aranibar N, Robertson DG, Reily MD, Lehman-McKeeman LD, Vaillancourt RR, Cherrington NJ. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids. 2015;47:603–615. doi: 10.1007/s00726-014-1894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grzych G, Vonghia L, Bout MA, Weyler J, Verrijken A, Dirinck E, Chevalier Curt MJ, Van Gaal L, Paumelle R, Francque S, Tailleux A, Haas JT, Staels B. Plasma BCAA Changes in Patients With NAFLD Are Sex Dependent. J Clin Endocrinol Metab. 2020;105 doi: 10.1210/clinem/dgaa175. [DOI] [PubMed] [Google Scholar]
- 89.Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, Fürnsinn C, Promintzer M, Anderwald C, Bischof M, Roden M. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56:1600–1607. doi: 10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- 90.Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Zhang F, Zhao S, Yan W, Xia Y, Chen X, Wang W, Zhang J, Gao C, Peng C, Yan F, Zhao H, Lian K, Lee Y, Zhang L, Lau WB, Ma X, Tao L. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine. 2016;13:157–167. doi: 10.1016/j.ebiom.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, Kawaguchi T, Kurosaki M, Sakaida I, Shimizu M, Taniai M, Terai S, Nishikawa H, Hiasa Y, Hidaka H, Miwa H, Chayama K, Enomoto N, Shimosegawa T, Takehara T, Koike K. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J Gastroenterol. 2021;56:593–619. doi: 10.1007/s00535-021-01788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 94.Becerra-Díaz M, Strickland AB, Keselman A, Heller NM. Androgen and Androgen Receptor as Enhancers of M2 Macrophage Polarization in Allergic Lung Inflammation. J Immunol. 2018;201:2923–2933. doi: 10.4049/jimmunol.1800352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corcoran MP, Meydani M, Lichtenstein AH, Schaefer EJ, Dillard A, Lamon-Fava S. Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. J Endocrinol. 2010;206:217–224. doi: 10.1677/JOE-10-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 97.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, Anderson RN, Ma J, Ly KN, Cronin KA, Penberthy L, Kohler BA. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khan FZ, Perumpail RB, Wong RJ, Ahmed A. Advances in hepatocellular carcinoma: Nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J Hepatol. 2015;7:2155–2161. doi: 10.4254/wjh.v7.i18.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, Setiawan VW, Tran T, Ayoub WS, Lu SC, Klein AS, Sundaram V, Nissen NN. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol. 2018;113:1649–1659. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang W, Lu Y, Xu Y, Xu L, Zheng W, Wu Y, Li L, Shen P. Estrogen represses hepatocellular carcinoma (HCC) growth via inhibiting alternative activation of tumor-associated macrophages (TAMs) J Biol Chem. 2012;287:40140–40149. doi: 10.1074/jbc.M112.348763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang D, Hanna DL, Usher J, LoCoco J, Chaudhari P, Lenz HJ, Setiawan VW, El-Khoueiry A. Impact of sex on the survival of patients with hepatocellular carcinoma: a Surveillance, Epidemiology, and End Results analysis. Cancer. 2014;120:3707–3716. doi: 10.1002/cncr.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang R, Liu Y, Sun H, Wang T, Li C, Fan J, Wang Z. Estradiol is significantly associated with prognosis in non-surgical liver cancer patients: from bench to bedside. Aging (Albany NY) 2021;13:3483–3500. doi: 10.18632/aging.202280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mindikoglu AL, Regev A, Seliger SL, Magder LS. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl. 2010;16:1147–1157. doi: 10.1002/lt.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mindikoglu AL, Emre SH, Magder LS. Impact of estimated liver volume and liver weight on gender disparity in liver transplantation. Liver Transpl. 2013;19:89–95. doi: 10.1002/lt.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wells GL. Cardiovascular Risk Factors: Does Sex Matter? Curr Vasc Pharmacol. 2016;14:452–457. doi: 10.2174/1570161114666160722113116. [DOI] [PubMed] [Google Scholar]
- 106.Kim C, Cushman M, Khodneva Y, Lisabeth LD, Judd S, Kleindorfer DO, Howard VJ, Safford MM. Risk of Incident Coronary Heart Disease Events in Men Compared to Women by Menopause Type and Race. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Du T, Sun X, Yuan G, Zhou X, Lu H, Lin X, Yu X. Sex differences in the impact of nonalcoholic fatty liver disease on cardiovascular risk factors. Nutr Metab Cardiovasc Dis. 2017;27:63–69. doi: 10.1016/j.numecd.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 108.Pemmasani G, Yandrapalli S, Aronow W. Sex differences in cardiovascular diseases and associated risk factors in non-alcoholic steatohepatitis. Am J Cardiovasc Dis. 2020;10:362–366. [PMC free article] [PubMed] [Google Scholar]
- 109.Allen AM, Therneau TM, Mara KC, Larson JJ, Watt KD, Hayes SN, Kamath PS. Women With Nonalcoholic Fatty Liver Disease Lose Protection Against Cardiovascular Disease: A Longitudinal Cohort Study. Am J Gastroenterol. 2019;114:1764–1771. doi: 10.14309/ajg.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei L, Cheng X, Luo Y, Yang R, Lei Z, Jiang H, Chen L. Lean non-alcoholic fatty liver disease and risk of incident diabetes in a euglycaemic population undergoing health check-ups: A cohort study. Diabetes Metab. 2021;47:101200. doi: 10.1016/j.diabet.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 111.Akuta N, Kawamura Y, Arase Y, Saitoh S, Fujiyama S, Sezaki H, Hosaka T, Kobayashi M, Suzuki Y, Suzuki F, Ikeda K, Kumada H. Hepatocellular carcinoma is the most common liver-related complication in patients with histopathologically-confirmed NAFLD in Japan. BMC Gastroenterol. 2018;18:165. doi: 10.1186/s12876-018-0900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yoshida I, Suzuki A, Vallée M, Matano Y, Masunaga T, Zenda T, Shinozaki K, Okada T. Serum insulin levels and the prevalence of adenomatous and hyperplastic polyps in the proximal colon. Clin Gastroenterol Hepatol. 2006;4:1225–1231. doi: 10.1016/j.cgh.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 113.Saxena A, Chumanevich A, Fletcher E, Larsen B, Lattwein K, Kaur K, Fayad R. Adiponectin deficiency: role in chronic inflammation induced colon cancer. Biochim Biophys Acta. 2012;1822:527–536. doi: 10.1016/j.bbadis.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hwang ST, Cho YK, Park JH, Kim HJ, Park DI, Sohn CI, Jeon WK, Kim BI, Won KH, Jin W. Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. J Gastroenterol Hepatol. 2010;25:562–567. doi: 10.1111/j.1440-1746.2009.06117.x. [DOI] [PubMed] [Google Scholar]
- 115.Chen QF, Zhou XD, Sun YJ, Fang DH, Zhao Q, Huang JH, Jin Y, Wu JS. Sex-influenced association of non-alcoholic fatty liver disease with colorectal adenomatous and hyperplastic polyps. World J Gastroenterol. 2017;23:5206–5215. doi: 10.3748/wjg.v23.i28.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hung WC, Wu JS, Sun ZJ, Lu FH, Yang YC, Chang CJ. Gender differences in the association of non-alcoholic fatty liver disease and metabolic syndrome with erosive oesophagitis: a cross-sectional study in a Taiwanese population. BMJ Open. 2016;6:e013106. doi: 10.1136/bmjopen-2016-013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chung SJ, Kim D, Park MJ, Kim YS, Kim JS, Jung HC, Song IS. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut. 2008;57:1360–1365. doi: 10.1136/gut.2007.147090. [DOI] [PubMed] [Google Scholar]
- 118.Ministrini S, Calzini L, Nulli Migliola E, Ricci MA, Roscini AR, Siepi D, Tozzi G, Daviddi G, Martorelli EE, Paganelli MT, Lupattelli G. Lysosomal Acid Lipase as a Molecular Target of the Very Low Carbohydrate Ketogenic Diet in Morbidly Obese Patients: The Potential Effects on Liver Steatosis and Cardiovascular Risk Factors. J Clin Med. 2019;8 doi: 10.3390/jcm8050621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.D'Abbondanza M, Ministrini S, Pucci G, Nulli Migliola E, Martorelli EE, Gandolfo V, Siepi D, Lupattelli G, Vaudo G. Very Low-Carbohydrate Ketogenic Diet for the Treatment of Severe Obesity and Associated Non-Alcoholic Fatty Liver Disease: The Role of Sex Differences. Nutrients. 2020;12 doi: 10.3390/nu12092748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aberdein N, Dambrino RJ, do Carmo JM, Wang Z, Mitchell LE, Drummond HA, Hall JE. Role of PTP1B in POMC neurons during chronic high-fat diet: sex differences in regulation of liver lipids and glucose tolerance. Am J Physiol Regul Integr Comp Physiol. 2018;314:R478–R488. doi: 10.1152/ajpregu.00287.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Doucet E, St-Pierre S, Alméras N, Imbeault P, Mauriège P, Pascot A, Després JP, Tremblay A. Reduction of visceral adipose tissue during weight loss. Eur J Clin Nutr. 2002;56:297–304. doi: 10.1038/sj.ejcn.1601334. [DOI] [PubMed] [Google Scholar]
- 123.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367–78.e5; quiz e14. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 124.Barsalani R, Riesco E, Lavoie JM, Dionne IJ. Effect of exercise training and isoflavones on hepatic steatosis in overweight postmenopausal women. Climacteric. 2013;16:88–95. doi: 10.3109/13697137.2012.662251. [DOI] [PubMed] [Google Scholar]