Key Points
Question
What is the optimal approach to breast cancer screening for women with ATM, CHEK2, and PALB2 pathogenic variants?
Findings
This comparative modeling analysis using 2 Cancer Intervention and Surveillance Modeling Network simulation models and risk estimates from the Cancer Risk Estimates Related to Susceptibility Consortium found that annual mammography from age 40 to 74 years was estimated to reduce breast cancer mortality by 36% to 39%; and adding annual magnetic resonance imaging (MRI) starting at age 30 or 35 years was estimated to reduce breast cancer mortality by 54% to 60%. When screening MRI was used, starting mammography before age 40 years did not meaningfully reduce mortality but increased false-positive screenings.
Meaning
This modeling analysis suggests that screening MRI starting at age 30 to 35 years may substantially reduce breast cancer mortality for women with moderate- to high-risk pathogenic variants for breast cancer.
Abstract
Importance
Screening mammography and magnetic resonance imaging (MRI) are recommended for women with ATM, CHEK2, and PALB2 pathogenic variants. However, there are few data to guide screening regimens for these women.
Objective
To estimate the benefits and harms of breast cancer screening strategies using mammography and MRI at various start ages for women with ATM, CHEK2, and PALB2 pathogenic variants.
Design, Setting, and Participants
This comparative modeling analysis used 2 established breast cancer microsimulation models from the Cancer Intervention and Surveillance Modeling Network (CISNET) to evaluate different screening strategies. Age-specific breast cancer risks were estimated using aggregated data from the Cancer Risk Estimates Related to Susceptibility (CARRIERS) Consortium for 32 247 cases and 32 544 controls in 12 population-based studies. Data on screening performance for mammography and MRI were estimated from published literature. The models simulated US women with ATM, CHEK2, or PALB2 pathogenic variants born in 1985.
Interventions
Screening strategies with combinations of annual mammography alone and with MRI starting at age 25, 30, 35, or 40 years until age 74 years.
Main Outcomes and Measures
Estimated lifetime breast cancer mortality reduction, life-years gained, breast cancer deaths averted, total screening examinations, false-positive screenings, and benign biopsies per 1000 women screened. Results are reported as model mean values and ranges.
Results
The mean model-estimated lifetime breast cancer risk was 20.9% (18.1%-23.7%) for women with ATM pathogenic variants, 27.6% (23.4%-31.7%) for women with CHEK2 pathogenic variants, and 39.5% (35.6%-43.3%) for women with PALB2 pathogenic variants. Across pathogenic variants, annual mammography alone from 40 to 74 years was estimated to reduce breast cancer mortality by 36.4% (34.6%-38.2%) to 38.5% (37.8%-39.2%) compared with no screening. Screening with annual MRI starting at 35 years followed by annual mammography and MRI at 40 years was estimated to reduce breast cancer mortality by 54.4% (54.2%-54.7%) to 57.6% (57.2%-58.0%), with 4661 (4635-4688) to 5001 (4979-5023) false-positive screenings and 1280 (1272-1287) to 1368 (1362-1374) benign biopsies per 1000 women. Annual MRI starting at 30 years followed by mammography and MRI at 40 years was estimated to reduce mortality by 55.4% (55.3%-55.4%) to 59.5% (58.5%-60.4%), with 5075 (5057-5093) to 5415 (5393-5437) false-positive screenings and 1439 (1429-1449) to 1528 (1517-1538) benign biopsies per 1000 women. When starting MRI at 30 years, initiating annual mammography starting at 30 vs 40 years did not meaningfully reduce mean mortality rates (0.1% [0.1%-0.2%] to 0.3% [0.2%-0.3%]) but was estimated to add 649 (602-695) to 650 (603-696) false-positive screenings and 58 (41-76) to 59 (41-76) benign biopsies per 1000 women.
Conclusions and Relevance
This analysis suggests that annual MRI screening starting at 30 to 35 years followed by annual MRI and mammography at 40 years may reduce breast cancer mortality by more than 50% for women with ATM, CHEK2, and PALB2 pathogenic variants. In the setting of MRI screening, mammography prior to 40 years may offer little additional benefit.
This decision analytical study uses microsimulation models from the Cancer Intervention and Surveillance Modeling Network to estimate the benefits and harms associated with breast cancer screening strategies using mammography and magnetic resonance imaging at various start ages for women with ATM, CHEK2, and PALB2 pathogenic variants.
Introduction
Genetic testing for breast cancer susceptibility has been an important aspect of cancer prevention since BRCA1 (OMIM 113705) and BRCA2 (OMIM 600185) (BRCA1/2) were identified in 1994 and 1995, respectively.1,2 This discovery facilitated the development of breast cancer screening and risk reduction guidelines for women with BRCA1/2 pathogenic variants (PVs) and their relatives.3,4 More recently, a group of non-BRCA1/2 PVs conferring moderate to high risk of breast cancer has been recognized, the most common of which are ATM (OMIM 607585), CHEK2 (OMIM 604373), and PALB2 (OMIM 610355). Each of these PVs increases breast cancer risk by at least 2-fold, and collectively they are identified in 2% to 3% of women with a diagnosis of breast cancer and in approximately 1% of the population.5,6
Because of the increasing availability and affordability of multigene panel testing,7,8 an increasing number of women are learning that they are carriers of these moderate- to high-risk PVs. The optimal approach to breast cancer screening for these women has not been established. Based on expert opinion and experience with magnetic resonance imaging (MRI) screening for women with BRCA1/2 PVs,9,10,11 the National Comprehensive Cancer Network recommends consideration of annual MRI in addition to mammography for ATM and CHEK2 PV carriers starting at age 40 years and for PALB2 PV carriers at age 30 years.12 Clinical trials comparing multiple approaches with breast cancer screening for women with each PV are not feasible given the prohibitively large sample sizes and follow-up times required.
In the absence of clinical trials, simulation modeling can be used to synthesize available data and compare screening strategies based on the projected effect on screening outcomes. Simulation models from the Cancer Intervention and Surveillance Modeling Network (CISNET) have previously informed cancer screening guidelines for the US Preventive Services Task Force13,14,15 and the American Cancer Society.16,17 For this analysis, we adapted 2 CISNET breast cancer simulation models for women with ATM, CHEK2, and PALB2 PVs using risk estimates from the Cancer Risk Estimates Related to Susceptibility (CARRIERS) Consortium, the largest US consortium of familial- and population-based studies of breast cancer risk due to cancer susceptibility genes.5 Using these models, we evaluated the benefits and harms of screening strategies using MRI and mammography to inform guideline recommendations for carriers of these moderate- to high-risk PVs.
Methods
Model Overview
The CISNET models used in this analysis were Model E (Erasmus Medical Center, Rotterdam, the Netherlands) and Model W-H (University of Wisconsin–Madison, Madison; and Harvard Medical School, Boston, Massachusetts). Full details regarding the development and validation of these models have been described previously18,19,20,21 and can be found online.22 These models were independently developed using different structures, assumptions, and methods to implement unobservable parameters for the natural history of breast cancer.23 The use of 2 separate models therefore provides a plausible range of results given the inherent uncertainty of unobservable parameters related to the natural history of breast cancer. The models also share some common data elements, including non–breast cancer mortality risk, screening performance, and treatment effectiveness.23 Both models have been previously validated and reproduce age-specific Surveillance, Epidemiology, and End Results Program incidence rates and mortality rates in the US population.24,25 This modeling analysis was determined not to be human participants research by the institutional review boards of Erasmus Medical Center, University of Wisconsin–Madison, and Harvard Medical School; therefore, this study was exempt from institutional review board approval and did not require informed consent.
The models simulate lifetime horizons of individual women and background US breast cancer incidence (including ductal carcinoma in situ and invasive breast cancer) in the absence of screening and treatment based on age-period-cohort models.26 Breast cancer survival is dependent on age and tumor size and/or stage at diagnosis, estrogen receptor status, ERBB2 status, and treatment effectiveness. When screening is performed, cancers can be diagnosed at earlier size and stage than with clinical detection, potentially reducing mortality. Women in the models can die of breast cancer or noncancer causes.
Population and Model Input Parameters
We modeled US women with ATM, CHEK2, or PALB2 PVs born in 1985 (the youngest birth cohort for whom intensive breast cancer screening could potentially be recommended in 2010-2020) and observed from the age of 25 years for their lifetimes. Input parameters for cancer risk and for incidence, subtype, screening performance, and treatment effectiveness are summarized in Table 1.5,9,27,28,29,30,31
Table 1. Model Input Parameters.
| Parameter | ATM | PALB2 a | CHEK2 a | Source |
|---|---|---|---|---|
| Breast cancer risk and subtype | ||||
| Odds ratio of breast cancer | 1.82 | 3.67 | 2.36 | CARRIERS Consortium5 (age-specific odds ratios available in eTable 1 in Supplement 1) |
| Subtype distributions, % | CARRIERS Consortium5 | |||
| ER positive and ERBB2 negative | 70 | 47 | 67 | |
| ER positive and ERBB2 positive | 22 | 13 | 22 | |
| ER negative and ERBB2 positive | 4 | 1 | 5 | |
| ER negative and ERBB2 negative | 4 | 39 | 7 | |
| MMG | MRI | MMG plus MRI | ||
| Screening performance | ||||
| Sensitivity, % | Overall, 40.8 | 90.8 (84.7) | 96.0 (92.2) | Chiarelli et al,9 with age-specific adjustments for MMGb,c |
| At age 30-39 y, 40.0 | ||||
| At age 40-49 y, 40.4 | ||||
| At age 50-69 y, 41.9 | ||||
| Specificity, % | Chiarelli et al9 | |||
| Initial screening | 88.0 | 79.7 (78.8 to 80.6) | 72.2 (71.2 to 73.1) | |
| With DBT | 89.6 | NA | 73.8 | Conant et al27d |
| Second or later screening | 92.5 | 90.5 (89.9 to 91.0) | 84.5 (83.8 to 85.2) | |
| With DBT | 94.1 | NA | 85.5 | Conant et al27d |
| False-positive screenings with biopsy performed, % | Chiarelli et al9 | |||
| Initial screening | 19 | 36 | 28 | |
| Second or later screening | 13 | 38 | 26 | |
| AJCC stage (screening-detected cancers), % | Chiarelli et al,9 adjusted for missing stage of cancers treated with NAC28 | |||
| DCIS | 22 | 22 | 23 | |
| I | 48 | 58 | 57 | |
| II | 24 | 15 | 15 | |
| III | 6 | 4 | 4 | |
| Treatment and mortality | ||||
| Treatment receipt | Guideline treatment by age, stage, and receptor status | NCCN29 | ||
| Treatment effectiveness | Estimated from meta-analyses of randomized trials | Peto et al30 | ||
| Nonbreast cancer mortality | Age-specific and birth cohort–specific all-cause mortality | Gangnon et al31 | ||
Abbreviations: AJCC, American Joint Committee on Cancer; BCSC, Breast Cancer Surveillance Consortium; CARRIERS, Cancer Risk Estimates Related to Susceptibility; DBT, digital breast tomosynthesis; DCIS, ductal carcinoma in situ; ER, estrogen receptor; MMG, mammography; MRI, magnetic resonance imaging; NA, not applicable; NAC, neoadjuvant chemotherapy; NCCN, National Comprehensive Cancer Network.
Values shown in parentheses were used in sensitivity analyses.
In the study by Chiarelli et al,9 all women received MMG and MRI performed concurrently, and sensitivity calculations for each modality included cancers detected by the other modality as false-negative screenings. The models were therefore calibrated with MMG and MRI performed concurrently, adjusting the individual performance of each modality until the model output matched the observed data.
Age-specific MMG sensitivity was derived from the overall sensitivity reported in the study by Chiarelli et al,9 by adjusting for differences in breast density by age based on data from the BCSC.
Specificity of MMG and MMG plus MRI were adjusted by decreasing false-positive screenings due to MMG by 15%.
Risk of Breast Cancer
Parameters for incidence and subtype of breast cancer for each PV were derived from data provided by the CARRIERS Consortium.5 We used aggregated data for 32 247 cases and 32 544 controls in 12 population-based studies.32,33,34,35,36,37,38,39,40,41,42,43 Population-based studies of breast cancer risk are more generalizable than studies of women accrued after clinical genetic testing, which is often performed because of a strong family history of cancer or early age at diagnosis. Our use of the population-based subset of the CARRIERS Consortium data thus ensures that the models’ results are more broadly relevant across the population. Overall breast cancer risk estimates from the CARRIERS Consortium for 28 cancer predisposition genes have been previously published.5 For this analysis, age-specific odds ratios of breast cancer for women with ATM, CHEK2, or PALB2 PVs were separately estimated using logistic regression models adjusted for study, first-degree family history of breast cancer, race and ethnicity, age, and an interaction of age and PV. These odds ratios were then applied to the derived background age-specific, cohort-specific, and period-specific breast cancer incidence for the 1985 birth cohort of the US population (eTable 1 in Supplement 1).5 Estrogen receptor and ERBB2 breast cancer subtype distributions for each PV were also calculated and incorporated into the simulation models (Table 1).5,9,27,28,29,30,31
Screening Performance and Breast Cancer Stage
Screening performance (ie, sensitivity, specificity, and rates of benign biopsies) for mammography and MRI was derived from published estimates from the High Risk Ontario Breast Screening Program (OBSP).9 The OBSP is an organized screening program for women at high risk for breast cancer receiving annual mammography and MRI screening because of various risk factors (including genetic risk, family history, and history of prior radiotherapy to the chest). Because the performance of mammography in the OBSP is calculated for mammography performed in conjunction with MRI (with cancers detected only by MRI counting as false-negative screenings), we calibrated screening performance by simulating joint mammography and MRI screening and calculating the sensitivity of each modality in the same fashion. We also adjusted OBSP estimates of mammography sensitivity by age using data from the Breast Cancer Surveillance Consortium to account for the higher prevalence of dense breasts among young women.44 The distributions of breast cancer stage by mode of detection were estimated based on OBSP data,9 with adjustments for missing pathologic stage information for women treated with neoadjuvant chemotherapy based on published estimates.28 We assumed equal screening performance across PVs.
Treatment and Mortality
To isolate the effects of breast cancer screening on mortality, we assumed that all women with a diagnosis of breast cancer received guideline-concordant age-specific, stage-specific, and cancer subtype–specific therapy.12 Treatment effectiveness was based on meta-analyses of clinical trials.30 Risk of non–breast cancer mortality was based on age-specific and birth cohort–specific all-cause mortality rates.31
Statistical Analysis
We evaluated 5 primary screening strategies for each PV: annual mammography alone starting at age 40 years and annual mammography starting at age 40 years with annual MRI starting at age 40, 35, 30, and 25 years. Annual mammography starting at age 40 years was chosen because it is the least-intensive screening mammography strategy recommended in the setting of elevated risk for breast cancer.4,29 In a secondary analysis, we examined 2 additional strategies testing the effect of earlier start ages for mammography (35 and 30 years) with MRI screening at age 30 years. For all strategies, we assumed that screening was continued until age 74 years. To project the efficacy of screening, we assumed a 100% screening participation rate. Simulations were continued until all women died of either breast cancer or non–breast cancer causes, and individual events were tracked and aggregated as lifetime population metrics.
Outcomes included lifetime screening benefits with screening vs no screening per 1000 women screened, including breast cancer mortality reduction (expressed as the percentage relative reduction in total breast cancer deaths), absolute breast cancer deaths averted, and life-years gained (LYG). We assessed cumulative lifetime screening resources and harms (total screenings, false-positive screenings, and benign biopsies) per 1000 women screened. Finally, we calculated incremental ratios of false-positive screenings and benign biopsies per LYG for each strategy relative to the next least-intensive screening strategy. Outcomes were reported as mean values and ranges across models.
Sensitivity Analyses
We varied parameters related to breast cancer risk and screening performance to evaluate the effect of parameter uncertainty with the robustness of results. We assumed higher and lower breast cancer risk for each PV by adding and subtracting the SE from the age-specific risk estimates provided by the CARRIERS Consortium (eTable 1 in Supplement 1). We varied the sensitivity of the performance of MRI alone and the performance of mammography with MRI using the lower bounds of the 95% CIs9 and MRI specificity across the upper and lower CIs in the OBSP (Table 1).5,9,27,28,29,30,31 To account for potential differences in screening specificity by age, we evaluated outcomes using alternative screening specificity estimates stratified by age and screening round provided by the Breast Cancer Surveillance Consortium (eTable 2 in Supplement 1). Finally, we considered a scenario with digital breast tomosynthesis used for mammography screening; for this scenario, we assumed a 15% reduction in mammography false-positive screenings (Table 1)5,9,27,28,29,30,31 with no change in overall sensitivity based on prior work.27
Results
Breast Cancer Incidence and Mortality
Results are reported as model mean values and ranges. The mean model-estimated projections for cumulative lifetime breast cancer risk in the absence of screening was 20.9% (range across models, 18.1%-23.7%) for women with the ATM PVs, 27.6% (23.4%-31.7%) for women with the CHEK2 PVs, and 39.5% (35.6%-43.3%) for women with the PALB2 PVs. Cumulative mean lifetime risks of breast cancer death in the absence of screening were 3.4% (2.4%-4.5%) for women with the ATM PVs, 4.6% (3.1%-6.1%) for women with the CHEK2 PVs, and 7.7% (6.4%-9.1%) for women with the PALB2 PVs.
Screening Benefits and Harms by Screening Strategy
Across PVs, lifetime mortality benefits and harms with screening increased with multimodality screening and with younger screening ages compared with mammography alone starting at age 40 years. Per 1000 women screened, annual mammography alone starting at age 40 years compared with no screening was estimated to reduce breast cancer mortality by a mean of 36.4% (34.6%-38.2%) to 38.5% (37.8%-39.2%) and resulted in 13.3 (9.0-17.6) to 29.7 (22.0-37.4) breast cancer deaths averted (Table 2), 2092 (2085-2099) to 2224 (2222-2227) false-positive screenings, and 279 (278-280) to 296 (296-297) benign biopsies across PVs (Table 3). Annual mammography and MRI starting at age 40 years was estimated to reduce breast cancer mortality by 52.3% (51.4%-53.1%) to 53.6% (52.9%-54.3%) and resulted in 18.4 (12.5-24.4) to 42.4 (32.7-52.2) breast cancer deaths averted (Table 2), 4233 (4213-4252) to 4569 (4555-4583) false-positive screenings, and 1109 (1104-1114) to 1196 (1193-1200) benign biopsies compared with no screening (Table 3). The most intensive strategy (annual MRI alone from 25-39 years and annual mammography and MRI from 40-74 years) reduced breast cancer mortality by 55.7% (55.5%-55.8%) to 60.2% (58.9%-61.2%) and averted 20.5 (14.5-26.4) to 45.0 (35.4-54.5) breast cancer deaths (Table 2), and resulted in 5592 (5563-5621) to 5932 (5907-5957) false-positive screenings and 1637 (1629-1645) to 1725 (1718-1732) benign biopsies per 1000 women (Table 3).
Table 2. Estimated Lifetime Benefits of MRI Screening Strategies With Annual Mammography From Age 40 to 74 Years Alone and With Annual MRI at Varying Start Ages for Modeled Women With ATM, CHEK2, and PALB2 Pathogenic Variants.
| Start age | Breast cancer mortality reduction, mean (range), %a | Life-years gained per 1000 women, mean (range)a | Breast cancer deaths averted per 1000 women, mean (range)a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ATM | CHEK2 | PALB2 | ATM | CHEK2 | PALB2 | ATM | CHEK2 | PALB2 | |
| Annual mammography at 40 y | 38.5 (37.8-39.2) | 38.4 (38.0-38.8) | 36.4 (34.6-38.2) | 291 (263-319) | 370 (330-409) | 621 (559-684) | 13.3 (9.0-17.6) | 17.4 (11.6-23.1) | 29.7 (22.0-37.4) |
| Plus MRI at 40 y | 53.6 (52.9-54.3) | 53.6 (53.3-53.9) | 52.3 (51.4-53.1) | 420 (388-452) | 533 (489-577) | 921 (876-967) | 18.4 (12.5-24.4) | 24.2 (16.4-32.1) | 42.4 (32.7-52.2) |
| Plus MRI at 35 y | 57.6 (57.2-58.0) | 57.0 (56.3-57.7) | 54.4 (54.2-54.7) | 473 (447-498) | 591 (555-627) | 992 (959-1025) | 19.7 (13.7-25.7) | 25.6 (17.7-33.5) | 44.0 (34.4-53.7) |
| Plus MRI at 30 y | 59.5 (58.5-60.4) | 58.4 (57.2-59.6) | 55.4 (55.3-55.4) | 501 (478-523) | 620 (587-652) | 1025 (998-1051) | 20.3 (14.3-26.2) | 26.2 (18.3-34.1) | 44.7 (35.2-54.3) |
| Plus MRI at 25 y | 60.2 (58.9-61.2) | 58.9 (57.5-60.3) | 55.7 (55.5-55.8) | 510 (489-531) | 630 (599-661) | 1037 (1013-1061) | 20.5 (14.5-26.4) | 26.4 (18.5-34.2) | 45.0 (35.4-54.5) |
Abbreviation: MRI, magnetic resonance imaging.
Results are shown as mean values of cumulative lifetime outcomes per 1000 women screened across Model E (Erasmus Medical Center, Rotterdam, the Netherlands) and Model W-H (University of Wisconsin–Madison, Madison; Harvard Medical School, Boston, Massachusetts).
Table 3. Total Screenings, False-positive Screenings, and Benign Biopsies for Screening Strategies With Annual Mammography at Age 40 to 74 Years Alone and With Annual MRI.
| Start age | Total screenings, mean (range)a | False-positive screenings, mean (range)a,b | Benign biopsies, mean (range)a,b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ATM | CHEK2 | PALB2 | ATM | CHEK2 | PALB2 | ATM | CHEK2 | PALB2 | |
| Annual mammography at 40 y | 29 182 (29 148-29 215) | 28 505 (28 582-28 527) | 27 412 (27 321-27 503) | 2224 (2222-2227) | 2174 (2172-2175) | 2092 (2085-2099) | 296 (296-297) | 290 (290-290) | 279 (278-280) |
| Plus MRI at 40 y | 57 173 (57 050-57 296) | 55 511 (55 463-55 559) | 52 814 (52 664-52 964) | 4569 (4555-4583) | 4441 (4438-4443) | 4233 (4213-4252) | 1196 (1193-1200) | 1163 (1162-1164) | 1109 (1104-1114) |
| Plus MRI at 35 y | 61 789 (61 568-62 010) | 60 104 (60 058-60 150) | 57 392 (57 149-57 636) | 5001 (4979-5023) | 4871 (4861-4880) | 4661 (4635-4688) | 1368 (1362-1374) | 1334 (1331-1337) | 1280 (1272-1287) |
| Plus MRI at 30 y | 66 100 (65 867-66 333) | 64 403 (63 988-64 818) | 61 694 (61 474-61 913) | 5415 (5393-5437) | 5284 (5249-5319) | 5075 (5057-5093) | 1528 (1517-1538) | 1493 (1479-1508) | 1439 (1429-1449) |
| Plus MRI at 25 y | 71 507 (71 247-71 767) | 69 819 (69 731-69 906) | 67 100 (66 827-67 373) | 5932 (5907-5957) | 5802 (5789-5815) | 5592 (5563-5621) | 1725 (1718-1732) | 1691 (1687-1695) | 1637 (1629-1645) |
Abbreviation: MRI, magnetic resonance imaging.
Results are shown as model mean values of cumulative lifetime outcomes per 1000 women screened across Model E (Erasmus Medical Center, Rotterdam, the Netherlands) and Model W-H (University of Wisconsin–Madison, Madison; Harvard Medical School, Boston, Massachusetts).
Total false-positive screenings and benign biopsies exceed the number of women screened because women can experience multiple false-positive screenings and/or benign biopsies during their lifetimes.
Screening Efficiency
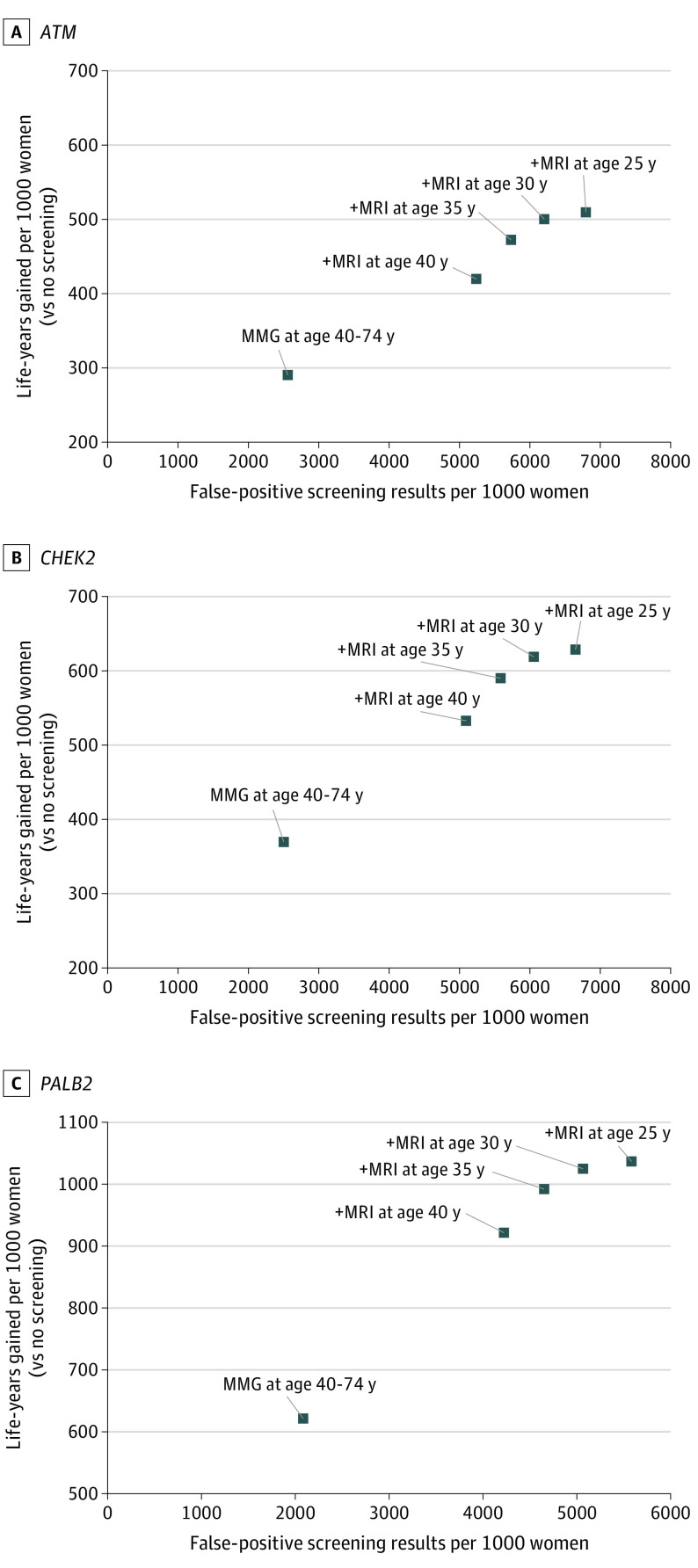
Compared with mammography alone from age 40 to 74 years, annual MRI screening from age 35 to 39 years followed by mammography and MRI screening from age 40 to 74 years was more efficient (fewer false-positive screenings and benign biopsies per LYG) than annual mammography and MRI from age 40 to 74 years for all PVs (Figure; eTable 3 in Supplement 1). This strategy resulted in 7.0 (6.4-7.6) to 15.3 (14.9-15.6) additional false-positive screenings and 2.7 (2.5-3.0) to 5.9 (5.8-6.0) benign biopsies per LYG relative to mammography alone from age 40 to 74 years. Starting MRI at age 30 vs 35 years similarly resulted in 12.8 (11.6-14.0) to 15.2 (14.9-15.5) additional false-positive screenngs and 4.9 (4.5-5.4) to 5.9 (5.8-6.0) benign biopsies per LYG, making it another efficient strategy. However, starting MRI at age 25 years was considerably less efficient than starting at age 30 years, resulting in 47.0 (32.6-61.3) to 57.9 (43.5-72.3) additional false-positive screenings and 18.0 (12.5-23.4) to 22.2 (16.7-27.7) benign biopsies per LYG. Results were similar when comparing false-positive screenings and benign biopsies per breast cancer death averted (eFigure 1 in Supplement 1).
Figure. False-positive Screenings and Life-Years Gained for Screening Strategies for Women With Pathogenic Variants in ATM, CHEK2, and PALB2.
Results are shown as mean values of cumulative lifetime outcomes per 1000 women screened across Model E (Erasmus Medical Center, Rotterdam, the Netherlands) and Model W-H (University of Wisconsin–Madison, Madison, Wisconsin; Harvard Medical School, Boston, Massachusetts). In all strategies, mammography (MMG) is performed annually from age 40 to 74 years; the start age of magnetic resonance imaging (MRI) screening varies by strategy.
Strategies With Earlier Mammography
With annual MRI screening from age 30 to 39 years, starting mammography earlier than 40 years increased false-positive screenings and benign biopsies but had little effect on mortality reduction or LYG (Table 4). For example, with annual MRI starting at age 30 years, adding mammography at age 30 vs 40 years decreased mortality by only 0.1% (0.1%-0.2%) to 0.3% (0.2%-0.3%) and increased LYG by 3 (3-4) to 5 (5-5) per 1000 women screened but resulted in 649 (602-695) to 650 (603-696) additional false-positive screenings and 58 (41-76) to 59 (41-76) additional biopsies per 1000 women screened.
Table 4. Estimated Impact of Starting Mammography at Earlier Ages for Strategies With MRI Screening Starting at Age 30 Years in Modeled Women With ATM, CHEK2, and PALB2 Pathogenic Variants.
| Start age | Breast cancer mortality reduction, mean (range), %a | Life-years gained, mean (range)a | False-positive screenings, mean (range)a,b | Benign biopsies, mean (range)a,b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATM | CHEK2 | PALB2 | ATM | CHEK2 | PALB2 | ATM | CHEK2 | PALB2 | ATM | CHEK2 | PALB2 | |
| MRI at 30 y, annual mammography at 40 y vs no screening | 59.5 (58.5-60.4) | 58.4 (57.2-59.6) | 55.4 (55.3-55.4) | 501 (478-523) | 620 (587-652) | 1025 (998-1051) | 5415 (5437-5393) | 5284 (5249-5319) | 5075 (5057-5093) | 1528 (1517-1538) | 1493 (1479-1508) | 1439 (1429-1449) |
| MRI at 30 y, annual mammography at 35 vs 40 y | 0.2 (0.1-0.2) | 0.1 (0.1-0.2) | 0.1 (0.1-0.1) | 2 (2-2) | 3 (2-3) | 3 (3-3) | 338 (291-386) | 339 (291-387) | 338 (291-385) | 37 (20-55) | 38 (20-55) | 37 (20-55) |
| MRI at 30 y, annual mammography at 30 vs 40 y | 0.3 (0.2-0.3) | 0.2 (0.1-0.2) | 0.1 (0.1-0.2) | 3 (3-4) | 4 (4-5) | 5 (5-5) | 650 (603-696) | 650 (603-696) | 649 (602-695) | 59 (41-76) | 59 (41-76) | 58 (41-76) |
Abbreviation: MRI, magnetic resonance imaging.
Results are shown as model mean values of cumulative lifetime outcomes per 1000 women screened across Model E (Erasmus Medical Center, Rotterdam, the Netherlands) and Model W-H (University of Wisconsin–Madison, Madison; Harvard Medical School, Boston, Massachusetts).
Total false-positive screenings and benign biopsies exceed the number of women screened because women can experience multiple false-positive screenings and/or benign biopsies during their lifetimes.
Sensitivity Analyses
Sensitivity analyses varying breast cancer risk (eTables 4 and 5 and eFigure 2 in Supplement 1), MRI sensitivity (eTable 6 in Supplement 1), and screening specificity (eTables 7-10 and eFigure 3A-C in Supplement 1) did not meaningfully change conclusions regarding the relative efficiency of the screening strategies, although absolute benefits and harms varied by scenario. Using lower and higher breast cancer risk estimates, mean lifetime breast cancer risk ranged from 17.6% (15.6%-19.6%) to 24.9% (21.3%-28.4%) for ATM, 24.5% (20.5%-28.4%) to 31.0% (26.9%-35.1%) for CHEK2, and 33.4% (28.3%-38.5%) to 46.8% (43.9%-49.6%) for PALB2. With annual MRI screening from age 30 to 39 years and mammography and MRI from age 40 to 74 years, breast cancer mortality reduction ranged from a mean of 54.7% (54.2%-55.1%) to 59.5% (58.8%-60.3%) across the range of breast cancer risk considered and only decreased to 51.6% (50.1%-53.1%) to 55.5% (54.9%-56.0%) with lower MRI sensitivity (eTable 6 in Supplement 1). When MRI specificity varied, false-positive screenings per 1000 women ranged from a mean of 4841 (4824-4858) to 5165 (5145-5186) with the best specificity to 5318 (5298-5337) to 5673 (5649-5696) with the lowest specificity based on OBSP data (eTables 7 and 8 in Supplement 1) and 5106 (5073-5138) to 5375 (5338-5412) false-positive screenings using age-specific specificity from the Breast Cancer Surveillance Consortium (eTable 9 in Supplement 1). When mammography specificity was adjusted for digital breast tomosynthesis screening, false-positive screenings decreased to a mean of 4800 (4780-4819) to 5411 (5093-5140) per 1000 women (eTable 10 in Supplement 1).
Discussion
To our knowledge, this is the first study to use comparative modeling to evaluate breast cancer screening strategies for women with ATM, CHEK2, and PALB2 PVs. We found that, for the modeled women with these PVs, combined annual MRI and mammography screening was estimated to reduce breast cancer mortality by more than 50% for all strategies considered. Based on our results, annual MRI screening starting at age 30 to 35 years followed by combined annual MRI and mammography at age 40 years likely offers the best balance of screening benefits and harms. Starting mammography earlier than 40 years increased false-positive screenings and benign biopsies but added little benefit for women receiving MRI.
Our study adds to the growing body of literature supporting the use of MRI for women at elevated risk of breast cancer. Prior modeling analyses using CISNET models have estimated that MRI screening reduces breast cancer mortality by 38% to 62% for women with BRCA1/2 PVs45,46 and 56% to 71% for women with a history of radiotherapy to the chest.47 We estimated a 52% to 60% reduction in breast cancer mortality with MRI among women with PVs in ATM, CHEK2, or PALB2, suggesting that MRI has important benefits even in the setting of moderate risk due to genetic susceptibility. Women with PALB2 PVs experienced the most benefit associated with MRI screening, with the most breast cancer deaths averted and greatest life expectancy gains for all strategies, although the incremental benefits of starting MRI screening at age 30 vs 35 years were similar to those among women with CHEK2 and ATM PVs. This larger benefit is expected, given the higher risk of breast cancer overall and for estrogen receptor–negative breast cancers with PALB2 PVs, which have a poorer prognosis.5
We examined incremental benefits and harms for MRI screening starting at age 25 to 40 years to quantify relative benefits of earlier screening. Although there is no established threshold of screening benefits to harms for women with a moderate or high risk of breast cancer, our models suggest that MRI screening prior to age 30 years considerably increases false-positive screenings and benign biopsies, with little effect on mortality or life expectancy. In other settings, efficiency ratios of procedures per LYG have been used to guide cancer screening policies, such as 40 colonoscopies per LYG for colorectal cancer screening.16 Based on our results, starting MRI screening at age 35 years and starting MRI screening at age 30 years were estimated to result in approximately 3 to 6 and 5 to 6 additional benign biopsies per LYG, respectively, suggesting that the tradeoffs of starting MRI screening in this age range are likely acceptable.
Another finding of our analysis is that mammography screening prior to age 40 years may offer little benefit when women were undergoing annual MRI screening but increased harms. The value of screening mammography for women younger than 40 years undergoing MRI has been questioned,9,48,49 as it is uncommon for mammography to detect a cancer missed on MRI in young women,49,50,51 and the few cancers not detected on MRI are typically more indolent, including low-grade ductal carcinoma in situ.52 Our models suggest that earlier mammography in this setting also has disadvantages, substantially increasing false-positive screenings and benign biopsies. Young women are also potentially more susceptible to risks of radiation-induced cancers from mammography screening,53 potentially further reducing the benefit and increasing harms of early mammography. For this reason, the use of MRI screening starting at age 25 years followed by combined mammography and MRI screening starting at age 30 years is recommended for women with BRCA1/2 PVs.29 Our results suggest that, when screening with MRI, mammography could be delayed until age 40 years for women with ATM, CHEK2, and PALB2 PVs.
Strengths and Limitations
This study has many strengths, including the use of 2 well-established CISNET models, consistent results across models, and the use of population-based breast cancer risk estimates from the largest US study of genetic breast cancer risk. This study also has several limitations. First, our model outcomes are population-level metrics that do not account for all factors that should be considered when selecting a screening strategy for an individual woman, including family history. Second, we considered only annual MRI screening intervals because longer screening intervals for women with genetic susceptibility to breast cancer have not been evaluated in clinical trials or used in clinical practice. Outcomes for biennial and triennial MRI intervals, age-specific MRI intervals, and novel combinations of alternating MRI and mammography should be evaluated in future analyses intended to guide clinical trial design. Third, we did not consider costs or quality of life in this analysis, and future analyses are warranted to evaluate cost-effectiveness. Fourth, we did not estimate screening-related overdiagnosis, given the paucity of data on cancers overdiagnosed by MRI screening. Although MRI screening detects more breast cancers than mammography, MRI may preferentially detect more biologically significant cancers52 and the proportion of cancers overdiagnosed may be lower than with mammography. Fifth, we did not evaluate alternative screening modalities such as whole breast ultrasonography or contrast-enhanced mammography. To date, however, MRI is the only imaging modality shown to decrease advanced breast cancers in women at elevated risk of breast cancer.11 Additional data on cancer outcomes with other screening modalities for women with genetic susceptibility to breast cancer are needed to inform guidelines for their use.
Conclusions
This comparative modeling analysis supports the use of MRI screening for women with moderate to high risk of breast cancer due to ATM, CHEK2, and PALB2 PVs. Annual MRI screening starting at age 30 to 35 years followed by annual MRI and mammography starting at age 40 years was estimated to reduce breast cancer mortality by more than 50% in these women, whereas additional mammography prior to age 40 years may have little benefit.
eTable 1. Age-Specific Odds Ratios for Breast Cancer Used in Base Case Analysis and Sensitivity Analyses
eTable 2. Specificity of Screening With Mammography Alone, MRI Alone, and Mammography Combined With MRI Stratified by Age Group and Screening Round
eTable 3. Incremental Screening Harms per Life Year Gained for Screening Strategies With Varying Start Age of Magnetic Resonance Imaging
eFigure 1. False-Positive Exams and Breast Cancer Deaths Averted for Screening Strategies for Women With Pathogenic Variants in ATM, CHEK2, and PALB2
eTable 4. Sensitivity Analysis of Screening Outcomes Assuming Higher Breast Cancer Risk
eTable 5. Sensitivity Analysis of Screening Outcomes Assuming Lower Breast Cancer Risk
eFigure 2. False-Positive Screens Versus Life Years Gained for Screening Strategies for Women With Pathogenic Variants in ATM, CHEK2, and PALB2, Varying Breast Cancer Risk ± 1 Standard Error Based on CARRIERS Data
eTable 6. Sensitivity Analysis of Screening Outcomes Assuming the Lower Confidence Limit of MRI Sensitivity
eTable 7. Sensitivity Analysis of Screening Outcomes Assuming the Lower Confidence Limit of the MRI Specificity
eTable 8. Sensitivity Analysis of Screening Outcomes Assuming the Upper Confidence Limit of the MRI Specificity
eTable 9. Sensitivity Analysis of Screening Outcomes Using Age-Specific MRI Specificity from the Breast Cancer Surveillance Consortium
eTable 10. Sensitivity Analysis of Screening Outcomes Assuming the Use of Digital Breast Tomosynthesis for Mammography Screening
eFigure 3A. False-Positive Screens Versus Life Years Gained for Screening Strategies for Women With Pathogenic Variants in ATM Under Varying Assumptions for Screening Specificity
eFigure 3B. False-Positive Screens Versus Life Years Gained for Screening Strategies for Women With Pathogenic Variants in CHEK2 Under Varying Assumptions for Screening Specificity
eFigure 3C. False-Positive Screens Versus Life Years Gained for Screening Strategies for Women With Pathogenic Variants in PALB2 Under Varying Assumptions for Screening Specificity
eAppendix. Additional Funding and Acknowledgements for Carriers
Nonauthor Collaborators
References
- 1.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66-71. doi: 10.1126/science.7545954 [DOI] [PubMed] [Google Scholar]
- 2.Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789-792. doi: 10.1038/378789a0 [DOI] [PubMed] [Google Scholar]
- 3.Lynch HT, Snyder C, Lynch J. Hereditary breast cancer: practical pursuit for clinical translation. Ann Surg Oncol. 2012;19(6):1723-1731. doi: 10.1245/s10434-012-2256-z [DOI] [PubMed] [Google Scholar]
- 4.Saslow D, Boetes C, Burke W, et al. ; American Cancer Society Breast Cancer Advisory Group . American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75-89. doi: 10.3322/canjclin.57.2.75 [DOI] [PubMed] [Google Scholar]
- 5.Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384(5):440-451. doi: 10.1056/NEJMoa2005936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorling L, Carvalho S, Allen J, et al. ; Breast Cancer Association Consortium . Breast cancer risk genes—association analysis in more than 113,000 women. N Engl J Med. 2021;384(5):428-439. doi: 10.1056/NEJMoa1913948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurian AW, Ward KC, Hamilton AS, et al. Uptake, results, and outcomes of germline multiple-gene sequencing after diagnosis of breast cancer. JAMA Oncol. 2018;4(8):1066-1072. doi: 10.1001/jamaoncol.2018.0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stadler ZK, Schrader KA, Vijai J, Robson ME, Offit K. Cancer genomics and inherited risk. J Clin Oncol. 2014;32(7):687-698. doi: 10.1200/JCO.2013.49.7271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiarelli AM, Blackmore KM, Muradali D, et al. Performance measures of magnetic resonance imaging plus mammography in the High Risk Ontario Breast Screening Program. J Natl Cancer Inst. 2020;112(2):136-144. doi: 10.1093/jnci/djz079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakker MF, de Lange SV, Pijnappel RM, et al. ; DENSE Trial Study Group . Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381(22):2091-2102. doi: 10.1056/NEJMoa1903986 [DOI] [PubMed] [Google Scholar]
- 11.Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664-1669. doi: 10.1200/JCO.2009.27.0835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology: genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 1.2022-August 11, 2021. Accessed November 17, 2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf [DOI] [PubMed]
- 13.Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different U.S. breast cancer screening strategies. Ann Intern Med. 2016;164(4):215-225. doi: 10.7326/M15-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meza R, Jeon J, Toumazis I, et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: modeling study for the US Preventive Services Task Force. JAMA. 2021;325(10):988-997. doi: 10.1001/jama.2021.1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315(23):2595-2609. doi: 10.1001/jama.2016.6828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterse EFP, Meester RGS, Siegel RL, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society Colorectal Cancer Screening Guideline. Cancer. 2018;124(14):2964-2973. doi: 10.1002/cncr.31543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meester RGS, Peterse EFP, Knudsen AB, et al. Optimizing colorectal cancer screening by race and sex: microsimulation analysis II to inform the American Cancer Society Colorectal Cancer Screening Guideline. Cancer. 2018;124(14):2974-2985. doi: 10.1002/cncr.31542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Broek JJ, van Ravesteyn NT, Heijnsdijk EA, de Koning HJ. Simulating the impact of risk-based screening and treatment on breast cancer outcomes with MISCAN-Fadia. Med Decis Making. 2018;38(1_suppl):54S-65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alagoz O, Berry DA, de Koning HJ, et al. Introduction to the Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer models. Med Decis Making. 2018;38(1_suppl):3S-8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Broek JJ, van Ravesteyn NT, Mandelblatt JS, et al. Comparing CISNET breast cancer incidence and mortality predictions to observed clinical trial results of mammography screening from ages 40 to 49. Med Decis Making. 2018;38(1_suppl):140S-150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alagoz O, Ergun MA, Cevik M, et al. The University of Wisconsin Breast Cancer Epidemiology Simulation Model: an update. Med Decis Making. 2018;38(1_suppl):99S-111S. doi: 10.1177/0272989X17711927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute. CISNET model registry. Accessed November 11, 2021. https://resources.cisnet.cancer.gov/registry
- 23.Mandelblatt JS, Near AM, Miglioretti DL, et al. Common model inputs used in CISNET collaborative breast cancer modeling. Med Decis Making. 2018;38(1_suppl):9S-23S. doi: 10.1177/0272989X17700624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fryback DG, Stout NK, Rosenberg MA, Trentham-Dietz A, Kuruchittham V, Remington PL. The Wisconsin Breast Cancer Epidemiology Simulation model. J Natl Cancer Inst Monogr. 2006;(36):37-47. doi: 10.1093/jncimonographs/lgj007 [DOI] [PubMed] [Google Scholar]
- 25.Tan SY, van Oortmarssen GJ, de Koning HJ, Boer R, Habbema JD. The MISCAN-Fadia continuous tumor growth model for breast cancer. J Natl Cancer Inst Monogr. 2006;(36):56-65. doi: 10.1093/jncimonographs/lgj009 [DOI] [PubMed] [Google Scholar]
- 26.Gangnon RE, Sprague BL, Stout NK, et al. The contribution of mammography screening to breast cancer incidence trends in the United States: an updated age-period-cohort model. Cancer Epidemiol Biomarkers Prev. 2015;24(6):905-912. doi: 10.1158/1055-9965.EPI-14-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conant EF, Barlow WE, Herschorn SD, et al. ; Population-based Research Optimizing Screening Through Personalized Regimen (PROSPR) Consortium . Association of digital breast tomosynthesis vs digital mammography with cancer detection and recall rates by age and breast density. JAMA Oncol. 2019;5(5):635-642. doi: 10.1001/jamaoncol.2018.7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergquist JR, Murphy BL, Storlie CB, Habermann EB, Boughey JC. Incorporation of treatment response, tumor grade and receptor status improves staging quality in breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2017;24(12):3510-3517. doi: 10.1245/s10434-017-6010-4 [DOI] [PubMed] [Google Scholar]
- 29.Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(1):77-102. doi: 10.6004/jnccn.2021.0001 [DOI] [PubMed] [Google Scholar]
- 30.Peto R, Davies C, Godwin J, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432-444. doi: 10.1016/S0140-6736(11)61625-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gangnon RE, Stout NK, Alagoz O, Hampton JM, Sprague BL, Trentham-Dietz A. Contribution of breast cancer to overall mortality for US women. Med Decis Making. 2018;38(1_suppl):24S-31S. doi: 10.1177/0272989X17717981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer JR, Ruiz-Narvaez EA, Rotimi CN, et al. Genetic susceptibility loci for subtypes of breast cancer in an African American population. Cancer Epidemiol Biomarkers Prev. 2013;22(1):127-134. doi: 10.1158/1055-9965.EPI-12-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490-2501. doi: 10.1002/cncr.101970 [DOI] [PubMed] [Google Scholar]
- 34.Patel AV, Jacobs EJ, Dudas DM, et al. The American Cancer Society’s Cancer Prevention Study 3 (CPS-3): recruitment, study design, and baseline characteristics. Cancer. 2017;123(11):2014-2024. doi: 10.1002/cncr.30561 [DOI] [PubMed] [Google Scholar]
- 35.Bernstein L, Allen M, Anton-Culver H, et al. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States). Cancer Causes Control. 2002;13(7):625-635. doi: 10.1023/A:1019552126105 [DOI] [PubMed] [Google Scholar]
- 36.Vachon CM, Li J, Scott CG, et al. No evidence for association of inherited variation in genes involved in mitosis and percent mammographic density. Breast Cancer Res. 2012;14(1):R7. doi: 10.1186/bcr3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346-357. doi: 10.1093/oxfordjournals.aje.a010213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson JE, Sellers TA, Scott CG, et al. The influence of mammogram acquisition on the mammographic density and breast cancer association in the Mayo Mammography Health Study cohort. Breast Cancer Res. 2012;14(6):R147. doi: 10.1186/bcr3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(9):714-724. doi: 10.1016/S2213-8587(18)30137-2 [DOI] [PubMed] [Google Scholar]
- 40.Hirko KA, Chai B, Spiegelman D, et al. Erythrocyte membrane fatty acids and breast cancer risk: a prospective analysis in the Nurses’ Health Study II. Int J Cancer. 2018;142(6):1116-1129. doi: 10.1002/ijc.31133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ambrosone CB, Ciupak GL, Bandera EV, et al. Conducting molecular epidemiological research in the age of HIPAA: a multi-institutional case-control study of breast cancer in African-American and European-American women. J Oncol. 2009;2009:871250. doi: 10.1155/2009/871250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629-642. doi: 10.1001/jama.295.6.629 [DOI] [PubMed] [Google Scholar]
- 43.Trentham-Dietz A, Sprague BL, Hampton JM, et al. Modification of breast cancer risk according to age and menopausal status: a combined analysis of five population-based case-control studies. Breast Cancer Res Treat. 2014;145(1):165-175. doi: 10.1007/s10549-014-2905-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10):dju255. doi: 10.1093/jnci/dju255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plevritis SK, Kurian AW, Sigal BM, et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA. 2006;295(20):2374-2384. doi: 10.1001/jama.295.20.2374 [DOI] [PubMed] [Google Scholar]
- 46.Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458-1468. doi: 10.1158/1055-9965.EPI-11-1196 [DOI] [PubMed] [Google Scholar]
- 47.Yeh JM, Lowry KP, Schechter CB, et al. Clinical benefits, harms, and cost-effectiveness of breast cancer screening for survivors of childhood cancer treated with chest radiation: a comparative modeling study. Ann Intern Med. 2020;173(5):331-341. doi: 10.7326/M19-3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Obdeijn IM, Winter-Warnars GA, Mann RM, Hooning MJ, Hunink MG, Tilanus-Linthorst MM. Should we screen BRCA1 mutation carriers only with MRI? a multicenter study. Breast Cancer Res Treat. 2014;144(3):577-582. doi: 10.1007/s10549-014-2888-8 [DOI] [PubMed] [Google Scholar]
- 49.Obdeijn IM, Mann RM, Loo CCE, et al. ; Hereditary Breast Ovarian Cancer Research Group Netherlands (HEBON) . The supplemental value of mammographic screening over breast MRI alone in BRCA2 mutation carriers. Breast Cancer Res Treat. 2020;181(3):581-588. doi: 10.1007/s10549-020-05642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhl C, Weigel S, Schrading S, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA Trial. J Clin Oncol. 2010;28(9):1450-1457. doi: 10.1200/JCO.2009.23.0839 [DOI] [PubMed] [Google Scholar]
- 51.Narayan AK, Visvanathan K, Harvey SC. Comparative effectiveness of breast MRI and mammography in screening young women with elevated risk of developing breast cancer: a retrospective cohort study. Breast Cancer Res Treat. 2016;158(3):583-589. doi: 10.1007/s10549-016-3912-y [DOI] [PubMed] [Google Scholar]
- 52.Sung JS, Stamler S, Brooks J, et al. Breast cancers detected at screening MR imaging and mammography in patients at high risk: method of detection reflects tumor histopathologic results. Radiology. 2016;280(3):716-722. doi: 10.1148/radiol.2016151419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berrington de Gonzalez A, Berg CD, Visvanathan K, Robson M. Estimated risk of radiation-induced breast cancer from mammographic screening for young BRCA mutation carriers. J Natl Cancer Inst. 2009;101(3):205-209. doi: 10.1093/jnci/djn440 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Age-Specific Odds Ratios for Breast Cancer Used in Base Case Analysis and Sensitivity Analyses
eTable 2. Specificity of Screening With Mammography Alone, MRI Alone, and Mammography Combined With MRI Stratified by Age Group and Screening Round
eTable 3. Incremental Screening Harms per Life Year Gained for Screening Strategies With Varying Start Age of Magnetic Resonance Imaging
eFigure 1. False-Positive Exams and Breast Cancer Deaths Averted for Screening Strategies for Women With Pathogenic Variants in ATM, CHEK2, and PALB2
eTable 4. Sensitivity Analysis of Screening Outcomes Assuming Higher Breast Cancer Risk
eTable 5. Sensitivity Analysis of Screening Outcomes Assuming Lower Breast Cancer Risk
eFigure 2. False-Positive Screens Versus Life Years Gained for Screening Strategies for Women With Pathogenic Variants in ATM, CHEK2, and PALB2, Varying Breast Cancer Risk ± 1 Standard Error Based on CARRIERS Data
eTable 6. Sensitivity Analysis of Screening Outcomes Assuming the Lower Confidence Limit of MRI Sensitivity
eTable 7. Sensitivity Analysis of Screening Outcomes Assuming the Lower Confidence Limit of the MRI Specificity
eTable 8. Sensitivity Analysis of Screening Outcomes Assuming the Upper Confidence Limit of the MRI Specificity
eTable 9. Sensitivity Analysis of Screening Outcomes Using Age-Specific MRI Specificity from the Breast Cancer Surveillance Consortium
eTable 10. Sensitivity Analysis of Screening Outcomes Assuming the Use of Digital Breast Tomosynthesis for Mammography Screening
eFigure 3A. False-Positive Screens Versus Life Years Gained for Screening Strategies for Women With Pathogenic Variants in ATM Under Varying Assumptions for Screening Specificity
eFigure 3B. False-Positive Screens Versus Life Years Gained for Screening Strategies for Women With Pathogenic Variants in CHEK2 Under Varying Assumptions for Screening Specificity
eFigure 3C. False-Positive Screens Versus Life Years Gained for Screening Strategies for Women With Pathogenic Variants in PALB2 Under Varying Assumptions for Screening Specificity
eAppendix. Additional Funding and Acknowledgements for Carriers
Nonauthor Collaborators