This meta-analysis conducts a matching-adjusted indirect comparison of teprotumumab vs intravenous methylprednisolone vs placebo in patients with moderate-to-severe thyroid eye disease.
Key Points
Question
Is teprotumumab more efficacious than intravenous methylprednisolone (IVMP) for proptosis and diplopia?
Findings
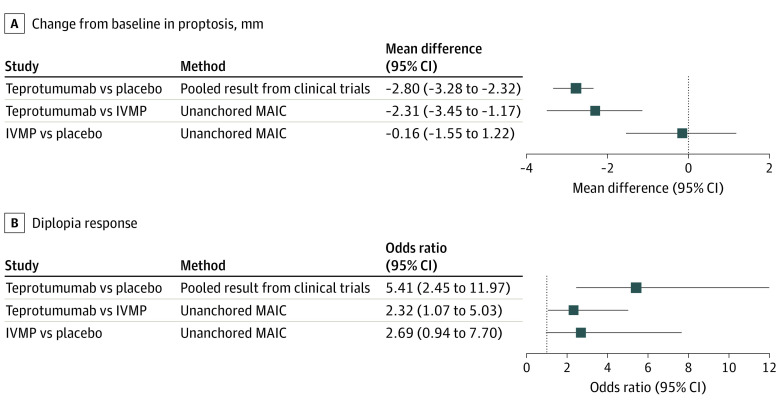
This meta-analysis and matching-adjusted indirect comparison showed an association with small improvements in proptosis from baseline for IVMP vs placebo (−0.16 mm); associated proptosis improvements were statistically significantly greater with teprotumumab vs IVMP (treatment difference, −2.31 mm). For diplopia response, IVMP was not favored over placebo while teprotumumab was favored over IVMP.
Meaning
Improvements in proptosis and diplopia with IVMP vs placebo may be small/not clinically relevant; in this meta-analysis, teprotumumab was associated with greater improvements in proptosis and diplopia vs IVMP, but clinical trials are needed to confirm the clinical relevance of this finding.
Abstract
Importance
Thyroid eye disease can be a debilitating autoimmune disorder characterized by progressive proptosis or diplopia. Teprotumumab has been compared with placebo in randomized clinical trials, but not with intravenous methylprednisolone (IVMP), which sometimes is used in clinical practice for this condition.
Objective
To conduct a matching-adjusted indirect comparison of teprotumumab vs IVMP vs placebo.
Data Sources
Deidentified patient-level data from teprotumumab trials and aggregate-level data from literature on the most recommended regimen of IVMP.
Study Selection
PubMed and Embase were searched for randomized/observational studies using key terms and controlled vocabulary. Full texts of eligible articles were reviewed and cataloged.
Data Extraction and Synthesis
Conducted by 1 reviewer (R.A.Q.) and 1 verifier (R.B.), including study characteristics, eligibility criteria, baseline characteristics, and outcomes.
Main Outcomes and Measures
Changes in proptosis by millimeter and diplopia response (percentage with ≥1 grade reduction) from baseline to week 12 in patients receiving IVMP and placebo, and to week 24 in patients receiving teprotumumab.
Results
The search identified 1019 records, and 6 through manual searches, alerts, and secondary references. After excluding duplicates and screening full-text records, 12 IVMP studies were included in the matching-adjusted indirect comparison (11 for proptosis change [n = 419], 4 for diplopia response [n = 125], and 2 teprotumumab [n = 79] and placebo [n = 83] comparator studies). Treatment with IVMP resulted in a proptosis difference of −0.16 mm (95% CI, −1.55 to 1.22 mm) from baseline to week 12 vs placebo. The proptosis treatment difference between IVMP and teprotumumab of −2.31 mm (95% CI, −3.45 to −1.17 mm) favored teprotumumab. Treatment with IVMP (odds ratio, 2.69; 95% CI, 0.94-7.70) was not favored over placebo in odds of diplopia response; however, teprotumumab was favored over IVMP (odds ratio, 2.32; 95% CI, 1.07-5.03).
Conclusions and Relevance
This meta-analysis suggests that use of IVMP is associated with a small, typically not clinically relevant, change from baseline in proptosis vs placebo, with modest changes in diplopia. While this nonrandomized comparison suggests that use of teprotumumab, compared with IVMP, is associated with greater improvements in proptosis and may be twice as likely to have a 1 grade or higher reduction in diplopia, randomized trials comparing these 2 treatments would be warranted to determine if 1 treatment is superior to the other to a clinically relevant degree.
Introduction
Thyroid eye disease (TED), or Graves ophthalmopathy, is an autoimmune disorder characterized by progressive inflammation and damage to orbital and ocular tissues.1,2 Age-adjusted prevalence in the US is estimated at 0.25%.3 Thyroid eye disease causes expansion of retro-orbital fat and extraocular muscle, thought to be mediated primarily by the upregulation of the insulin like growth factor 1 receptor on orbital fibroblasts.1 Patients may develop considerable disfiguring facial changes owing to proptosis, disabling diplopia, and in severe cases, vision loss.1
Currently there are limited noninvasive treatment options that improve proptosis and diplopia. The most recent European Group on Graves’ Orbitopathy (EUGOGO) guidelines recommend a cumulative dosage of 4.5 to 5.0 g of intravenous methylprednisolone (IVMP) over 12 weeks for most patients with moderate to severe active TED.4 Although data demonstrate that IVMP is associated with reduced inflammation, the dose, timing of administration, and duration of therapy vary in the literature, making it challenging to compare the clinical results, particularly on the progressive outcomes of proptosis and diplopia. A 2-mm reduction in proptosis and a 1-grade improvement in diplopia have been considered clinically meaningful in prior TED clinical trials.
On January 21, 2020, teprotumumab became the first US Food and Drug Administration–approved treatment for TED.5,6 Teprotumumab, a fully human, monoclonal antibody, inhibits insulin like growth factor 1 receptor activity and reduces downstream pathogenic signaling in TED. A total of 2 placebo-controlled, double-masked, randomized clinical trials (RCTs) of patients with moderate to severe TED demonstrated that teprotumumab was associated with clinically significant reductions in inflammation, proptosis, and diplopia over 24 weeks.7,8
To our knowledge, there are currently no studies directly comparing the efficacy of the most recommended dose of IVMP with teprotumumab or placebo; as such, matching-adjusted indirect comparisons (MAICs) simulating direct comparisons between treatments can be used to estimate comparative treatment effects. The objectives of this study are to (1) to evaluate improvements in proptosis and diplopia with the most recommended treatment regimen of IVMP as reported in the literature and (2) to compare these results with teprotumumab and placebo in patients with moderate to severe active TED using MAICs.
Methods
Patients Receiving Teprotumumab and Placebo
Data sources included deidentified patient-level data for teprotumumab or placebo from the phase 2 (NCT01868997) and 3 (NCT03298867) trials and published aggregate-level data for IVMP (4.5-5 g over 12 weeks). Data for patients receiving teprotumumab or placebo were obtained from 2 published trials: a phase 2 trial that included 43 patients and 45 patients in the teprotumumab and placebo groups, respectively, and a phase 3 trial that included 41 patients and 42 patients in the teprotumumab and placebo groups.7,8 Given the similar inclusion and exclusion criteria, data were pooled to obtain treatment arms with 84 randomized patients and 87 randomized patients for teprotumumab and placebo, respectively.
Literature Review for IVMP
A literature review was conducted to identify existing published literature assessing the most commonly recommended dose of IVMP among patients with moderate to severe active TED.9 PubMed and Embase were searched for relevant RCTs and observational studies from database inception to date of search (October 5, 2020) using a search strategy that included key terms and controlled vocabulary (eg, “intravenous steroid,” “Graves’ orbitopathy,” “thyroid eye disease,” “Graves’ ophthalmopathy”) (search strategy presented in eAppendix 1 in the Supplement). Results were filtered to include only studies conducted in humans. Regular alerts were established to capture any recent studies until April 1, 2021.
Screening and Selection Criteria
Study inclusion was based on PICOS (population, intervention, comparator, outcomes, and study design) criteria established a priori. Briefly, only studies including patients with moderate to severe active TED receiving treatment with IVMP at a dosage of 4.5 g to 5 g over 12 weeks and reporting at least 1 of the 2 outcomes of interest (ie, change from baseline in proptosis in millimeters and/or Bahn-Gorman diplopia score) were included.10 Two reviewers (R.A.Q. and R.B.) independently reviewed each title and abstract to identify eligible studies. Full texts of eligible studies were also examined for inclusion criteria and then reviewed to catalog the results.
Data Extraction
Data were extracted by a single reviewer (R.A.Q.) and verified for accuracy by a second reviewer (R.B.). Data extraction was completed using a standardized form and included study characteristics (eg, authors, study design), eligibility criteria (ie, inclusion and exclusion criteria), patient baseline characteristics (eg, sample sizes, sex, age, smoking status), and trial outcomes (eg, change from baseline in proptosis).
Outcome Measures
Change from baseline in proptosis and diplopia (among patients with diplopia) responses were evaluated. Diplopia responses were defined as a 1-grade or higher reduction in diplopia from baseline, using the Bahn-Gorman scale,10 with the following grades: 0 = no diplopia, 1 = intermittent, 2 = inconstant, and 3 = constant. Patients without diplopia at baseline were excluded. To align with the approved or recommended treatment durations of the comparators, these outcomes were assessed at week 24 for teprotumumab and week 12 for IVMP and placebo.9
Data Synthesis and Meta-analyses
Meta-analyses using the DerSimonian-Laird random-effects models were conducted for the 2 outcomes of interest to obtain pooled estimates for the MAICs.11 Heterogeneity across studies was assessed qualitatively prior to conducting meta-analyses based on study design, inclusion/exclusion criteria, baseline characteristics, and outcome definitions. Sensitivity analyses were also conducted, stratifying by percentage of smokers and study design (RCT/non-RCT).
Methods for MAIC Analyses
The MAIC analyses were conducted to compare IVMP and teprotumumab with each other and placebo for change from baseline in proptosis and diplopia response in 4 steps. First, data from the teprotumumab pooled trials7,8 were matched to the patient cohorts in the IVMP studies12,13,14,15,16,17,18,19,20,21,22,23 based on inclusion/exclusion criteria. Second, data were adjusted for outcome-specific prognostic factors (ie, patient characteristics that are associated with the outcomes) and treatment-effect modifiers (ie, patient characteristics that may affect the effectiveness of treatment on outcome) using inverse propensity score weighting. These characteristics were identified based on availability in IVMP studies, review of the literature, and clinical relevance. Mean age, proportion of women, and proportion of tobacco users were included in analyses assessing both outcomes. Third, unanchored indirect comparisons were conducted using the weighted data for patients receiving teprotumumab or placebo and pooled estimates from the meta-analyses for IVMP. This method was used because there was no common comparator arm (ie, placebo) between the samples being compared.24 Fourth, balance after MAICs was assessed using effective sample size, which reflects the proportion of the patients in the teprotumumab/placebo groups who are contributing to the adjusted outcome.24 The effective sample size decreased if there were large differences in the characteristics between the populations. Mean differences (MDs) and corresponding 95% CIs were calculated for change from baseline in proptosis. Odds ratios (ORs) and corresponding 95% CIs were calculated for the proportion of diplopia responders.
Scenario Analyses
Scenario (or sensitivity) analyses were conducted by varying the number of characteristics that were included in analyses to assess the robustness of the results. The primary MAIC (ie, scenario A) for each comparison was adjusted for all selected characteristics (mean age, proportion of women, and proportion of tobacco users), whereas subsequent scenarios removed characteristics 1 at a time.
Results
Literature Review for IVMP Studies
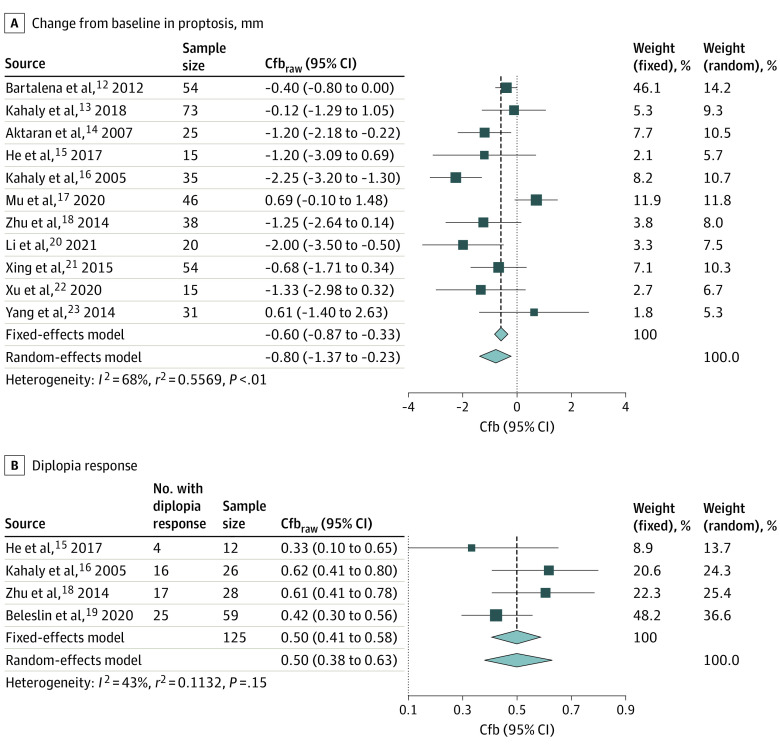
The search strategy identified 1019 records. A total of 6 additional records were identified through manual searches, alerts, and screening references of reviewed articles. After excluding duplicates, the titles and abstracts of 724 records were reviewed, and 680 were excluded. The remaining 44 full-text records were screened, and 32 were excluded for various reasons (eg, irrelevant dosage, time of assessment, or lack of reporting of relevant outcomes). Overall, 12 studies were included: 11 studies reported change from baseline in proptosis and 4 studies reported the proportion of patients with diplopia response. See eAppendix 2 and the eFigure in the Supplement for details.12,13,14,15,16,17,18,19,20,21,22,23
Study Characteristics and Meta-analyses
Study characteristics for the phase 2 and phase 3 teprotumumab and IVMP studies meeting the PICOS criteria are presented in the Table.25 No IVMP studies were placebo-controlled, 7 were RCTs,12,13,14,15,16,17,18 and the rest were observational.19,20,21,22,23 The teprotumumab studies were placebo-controlled, double-masked, multicenter studies conducted in Europe and the US.7,8 Two of the IVMP studies were multicenter and the rest were single center; 5 were conducted in Europe, 1 in Turkey, and 6 in China. Patient inclusion criteria were similar, and all studies included patients 18 years or older with moderate to severe active TED (ie, patients with similar levels of inflammation and with a clinical activity score ≥3).12,13,14,15,16,17,18,19,20,21,22,23 Baseline characteristics were similar across studies: mean age ranged from 35 to 52 years; proportion of women ranged from 35% to 80%; duration of TED symptoms, if reported, was approximately 1 year or less; baseline proptosis ranged from 17 to 24 mm; and proportion of patients with baseline diplopia (any grade) ranged from 35% to 100% (Table).12,13,14,15,16,17,18,19,20,21,22,23 Some differences were noted for the proportion of smokers across the studies, which ranged from 22% to 76% (Table).12,13,14,15,16,17,18,19,20,21,22,23
Table. Characteristics of Teprotumumab/Placebo and IVMP Studies.
| Source | Study characteristics | Inclusion criteria | Baseline characteristicsa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline sample size | Study design | Single vs multicenter | Age range, y | CAS | Age, y | % | Duration of TED symptoms, mo | Baseline proptosis, mm | Diplopia at baseline, % | ||
| Female | Smokers | ||||||||||
| IV methylprednisolone | |||||||||||
| Bartalena et al,12 2012 EU countriesb |
54 | RCT | Multicenter | 18-75 | ≥3/7 | 50.0 | 57.0 | 74.0 | 12.4 | 22.2 | 74.0 |
| Kahaly et al,13 2018 Germany, Italy |
81 | RCT | Multicenter | 18-75 | ≥3/7 | 50.6 | 79.0 | 51.0 | 8.5 | 21.3 | 64.0 |
| Aktaran et al,14 2007 Turkey |
25 | RCT | Single | ≥18 | ≥3/7 | 44.3 | 56.0 | 40.0 | <6.0 | 22.2 | 44.0 |
| He et al,15 2017 China |
18 | RCT | Single | 18-70 | ≥1/7c | 41.2 | 55.6 | 22.2 | 6.0d | 17.2 | 66.7 |
| Kahaly et al,16 2005 Germany |
35 | RCT | Single | ≥18 | ≥3/7 | 48.0 | 71.0 | 60.0 | 4.0d | 23.8 | 75.0 |
| Mu et al,17 2020 China |
46 | RCT | Single | ≥18 | NRe | 35.2 | 60.9 | 34.8 | 12.6 | 17.1 | 34.8 |
| Zhu et al,18 2014 China |
39 | RCT | Single | ≥18 | ≥3/7 | 45.3 | 61.5 | 25.6 | 13.6 | 22.1 | 71.8 |
| Beleslin et al,19 2020 Serbiaf |
74 | Retrospective | Single | NR | ≥3/7 | 51.0 | 69.0 | 76.0 | 6.0 | 23.1g | 80.0 |
| Li et al,20 2021 China |
20 | Non-RCT | Single | 18-60 | ≥3/7 | 45.5 | 55.0 | 40.0 | 8.0 | 18.9 | 65.0 |
| Xing et al,21 2015 China |
54 | Interventional | Single | ≥18 | ≥3/7 | 49.4 | 35.2 | 50.0 | 7.0 | 22.1 | NR |
| Xu et al,22 2020 China |
15 | Prospective pilot | Single | ≥18 | ≥3/7 | 43.2 | 80.0 | 26.7 | NR | 19.4 | 100 |
| Yang et al,23 2014 the Netherlands |
32 | Retrospective | Single | 18-75 | ≥3/7 | 52.0 | 78.0 | 50.0 | NR | 23.0 | NR |
| Teprotumumab | |||||||||||
| Smith et al,7 2017 Germany, Italy, United Kingdom, US |
42 | RCT | Multicenter | 18-75 | ≥4/7 | 51.6 | 65.1 | 25.6 | 4.7 | 23.4 | 50.0 |
| Douglas et al,8 2020 Germany, Italy, US |
41 | RCT | Multicenter | 18-80 | ≥4/7 | 51.6 | 70.7 | 22.0 | 6.2 | 22.6 | 68.3 |
| Placebo | |||||||||||
| Smith et al,7 2017 Germany, Italy, United Kingdom, US |
45 | RCT | Multicenter | 18-75 | ≥4/7 | 54.2 | 81.8 | 40.9 | 5.2 | 23.1 | 40.0 |
| Douglas et al,8 2020 Germany, Italy, US |
42 | RCT | Multicenter | 18-80 | ≥4/7 | 48.9 | 73.8 | 19.0 | 6.4 | 23.2 | 66.6 |
Abbreviations: CAS, clinical activity score; EU, European Union; IV, intravenous; IVMP, intravenous methylprednisolone; NR, not reported; RCT, randomized clinical trial; TED, thyroid eye disease.
If not available, mean values were estimated from median.25
The Netherlands, Belgium, France, Italy, Switzerland, and Greece.
Median CAS was 4.0. These patients were classified as active patients.
Median.
Mean CAS was 4.0 with an SD of 1.0.
Authors are associated with clinics in Serbia; however, the country was not explicitly stated.
Average of left eye and right eye; result not used in proptosis evaluation.
For the proptosis and diplopia analyses, 419 and 125 patients receiving IVMP were included, respectively. Results from the meta-analyses for change from baseline in proptosis (mm) and proportion of diplopia responders are shown in Figure 1. These unadjusted analyses demonstrated that IVMP treatment resulted in a reduction of 0.80 mm (95% CI, −1.37 to −0.23 mm) in proptosis and a 50% (95% CI, 38%-63%) diplopia response rate from baseline to week 12.
Figure 1. Meta-analyses to Obtain Pooled Estimates for Intravenous Methylprednisolone (IVMP) for Change From Baseline in Proptosis (A) and Diplopia Response (B).
Cfb indicates change from baseline; Cfbraw, raw change from baseline.
MAIC Results
Change From Baseline in Proptosis
Given the similar inclusion and exclusion criteria for the teprotumumab and IVMP studies, none of the patients in the teprotumumab or placebo groups were excluded during the matching process; however, 5 patients from the teprotumumab group and 3 patients from the placebo group with missing data were removed. As such, 79 and 83 patients in the teprotumumab and placebo groups were included, respectively. There were 419 patients in the aggregate-level data for IVMP. The analysis was adjusted for mean age, proportion of women, and proportion of smokers (eAppendixes 3 and 4 in the Supplement). The MAICs showed that teprotumumab was associated with a statistically significantly greater change from baseline in proptosis compared with IVMP (MD, −2.31 mm; 95% CI, −3.45 to −1.17 mm; effective sample size: 56 [Figure 2]). The difference in proptosis change from baseline in the IVMP group compared with the placebo group was not statistically significant (MD, −0.16 mm; 95% CI, −1.55 to 1.22; effective sample size: 37 [Figure 2]). Results for the unadjusted and scenario analyses were aligned with these primary analyses (eAppendixes 3 and 4 in the Supplement).
Figure 2. Unanchored Matching-Adjusted Indirect Comparisons (MAICs) and Pooled Results From Clinical Trials for Change From Baseline in Proptosis (A) and Diplopia Response (B).
Randomization controls for known/unknown confounders vs matching-adjusted indirect comparison controls for subset of known confounders. IVMP indicates intravenous methylprednisolone.
Diplopia Response
During the matching process, patients with missing data and those without diplopia at baseline were removed (21 in the teprotumumab group and 31 in the placebo group). Overall, the diplopia response analyses included 63 patients for teprotumumab, 56 patients for placebo, and 125 patients in the aggregate-level data for IVMP. After adjusting for baseline characteristics (characteristics before and after matching shown in eAppendixes 3 and 4 in the Supplement), results from the MAICs showed that IVMP was associated with numerically increased odds of diplopia response compared with placebo (OR, 2.69; 95% CI, 0.94- 7.70; effective sample size: 24 [Figure 2]). Furthermore, the teprotumumab diplopia response was greater when compared with IVMP (OR, 2.32; 95% CI, 1.07-5.03; effective sample size: 44 [Figure 2]). Results for the unadjusted and scenario analyses were largely aligned with the primary analyses; however, some changes in statistical significance were noted for the comparisons between IVMP and placebo, potentially because of the small effective sample size (eAppendixes 3 and 4 in the Supplement).
Discussion
Disfigurement and disability caused by proptosis and diplopia may result in a markedly reduced quality of life in patients with TED.26,27 Therefore, effective treatment should aim to improve these outcomes. The recommended treatment regimen of IVMP evaluated here is commonly used to reduce inflammation in TED4; however, the variability in reported improvements in proptosis and diplopia has made the efficacy on these outcomes unclear. Given the low cost and historical use of glucocorticoids to temporize acute inflammation, comparisons with new therapies, such as teprotumumab, are needed to better understand each drug’s place in the TED treatment armamentarium. To our knowledge, this is the first analysis to assess the change from baseline in proptosis and diplopia response with the most used regimen of IVMP compared with teprotumumab and placebo.
Teprotumumab is associated with statistically significant improvements in change from baseline in proptosis and diplopia response compared with IVMP. There were no major drops in effective sample size (71% and 70% of the original sample retained for proptosis and diplopia response, respectively), reinforcing that these are stable estimates that can be used for interpretation.24 In addition, results were consistent across scenario analyses accounting for age, sex, and smoking status.
This study allowed for the efficacy of IVMP to be estimated vs placebo, which is important as, to our knowledge, there are no placebo-controlled trials with the IVMP regimen evaluated that account for natural disease progression or improvement. Treatment with IVMP was associated with marginal improvements in proptosis, which was not significantly or clinically different from placebo. Though the diplopia response was different from placebo, the magnitude of that response was not as robust as that reported with teprotumumab vs placebo.28 Furthermore, there were substantial drops in effective sample size (only 45% and 43% of the original sample retained in proptosis and diplopia response, respectively), indicating that these results should be interpreted with caution.
Although there are few studies comparing TED treatments, these results are supported by previous analyses with other steroid regimens. A meta-analysis by Tu et al29 showed that glucocorticoids did not result in proptosis improvement vs other monotherapies (eg, mycophenolate mofetil) among patients with TED (MD, 0.42; 95% CI, 0.00-0.85). Additionally, only 1 published study30 has compared IVMP (6 randomized to receive 6 g over 4 months) with placebo (n = 9) and did not meet its recruitment target. Analyses performed 2.5 years after enrollment demonstrated a statistically significantly greater response rate in the primary outcome (a composite outcome) for IVMP vs placebo among patients with moderate-to-severe active TED (risk ratio, 7.50; 95% CI, 1.14-49.26). However, the proptosis response was not statistically significantly different from placebo with a mean change of 0.6 mm (risk ratio, 1.5; 95% CI, 0.3-7.9; P = .68). Diplopia improvements were also not statistically significant (2 of 4 patients receiving IVMP improved vs 0 of 5 patients receiving placebo; P = .07).30,31
Published studies report a wide range of IVMP doses and regimens for the treatment of TED. The recent recommendation from EUGOGO include a cumulative dose of 4.5 to 5 g for most patients with moderate to severe active disease, with 7.5 g recommended for patients with more severe disease and those with constant/inconstant diplopia.4 Although a multicenter RCT conducted by EUGOGO using 3 cumulative doses of IVMP (2.25, 4.98, or 7.47 g over 12 weeks) in patients with moderate to severe active TED observed the greatest improvements in a composite ophthalmic index, inflammation, and quality of life with the 7.47-g dose, none of the doses showed considerable improvements in proptosis or diplopia. Furthermore, the 7.47-g dose was associated with slightly more major adverse events than the lower doses.12 There are 2 other trials of the 7.5-g dose that also did not show statistically significant changes in proptosis or diplopia.12,32,33 Thus, the 4.5- to 5-g cumulative dose remains the most recommended steroid treatment for most moderate to severe TED, although data supporting the recommendation for the higher dose in patients with constant/inconstant diplopia are limited.4
The variability of reported proptosis and diplopia improvements across IVMP studies could be because of differences in study populations with respect to factors such as smoking, sex, and disease severity, which may influence treatment response.34 We adjusted for some of these differences when comparing the efficacy of IVMP with teprotumumab.
Limitations
Although we used standard methods for assessing indirect comparisons and adjusted for clinically relevant characteristics, comparing results across different studies is fraught with potential bias. First, unanchored MAICs were conducted because there was no common comparator arm between the studies. The unanchored MAIC method assumes that all prognostic factors and treatment-effect modifiers are well balanced across studies being compared; this assumption may not be met, as characteristics considered in the MAICs are limited to those reported in the included studies. However, we used the most relevant characteristics based on data availability and literature review. Second, for the comparison between IVMP and placebo, the effective sample size was reduced on adjusting for relevant characteristics, increasing the likelihood of differences between the studies being compared, resulting in broad CIs and possibly confounding the results. Third, there are insufficient long-term outcome studies for both IV steroids and teprotumumab to assess comparative durability and reactivation rates at this time, and the present analyses are limited to the formal study times for both drugs. Additionally, no studies reviewed included patients with compressive optic neuropathy, nor other therapies used to treat TED, including orbital radiotherapy, rituximab, or tocilizumab.
In the absence of head-to-head trials of IVMP vs teprotumumab and IVMP vs placebo, these MAICs provide an estimate of their comparative efficacy. Teprotumumab was associated with larger improvements in proptosis and diplopia than IVMP. The improvements in proptosis with IVMP alone from baseline were not different vs placebo in this analysis and are supported by the only placebo-controlled published trial.30 Compared with IVMP, teprotumumab was associated with meaningful improvement in proptosis with a 2 times greater odds of diplopia response.
Conclusions
Indirect treatment comparisons are routinely used in the absence of head-to-head studies to obtain efficacy estimates for relevant treatments. In the absence of masked RCTs, which provide the best evidence to weigh the risks and benefits between therapies, these insights into comparative efficacy may assist in clinical decisions and future trials. In this meta-analysis, findings suggest that IVMP is associated with a small, typically not clinically relevant, change from baseline in proptosis vs placebo, with modest changes in diplopia. While this nonrandomized comparison suggests that teprotumumab, as compared with IVMP, is associated with greater improvements in proptosis and may be twice as likely as to have a 1 grade or higher reduction in diplopia, RCTs comparing these 2 treatments would be warranted to determine if 1 treatment is superior to the other to a clinically relevant degree.
Given the efficacy of teprotumumab and the limited efficacy of IVMP on proptosis and diplopia, it would be unethical to examine these outcomes in a placebo/IVMP trial. Therefore, future studies directly comparing new treatments to IVMP should monitor clinical changes that may occur spontaneously and ensure that the patient populations are equally balanced for prognostic factors that may contribute to improvement or worsening as seen in the natural history of the disease.
eFigure. Baseline proptosis across studies used for the meta-analysis
eAppendix 1. Search strategy
eAppendix 2. PRISMA 2009 Flow Diagram
eAppendix 3. MAICs for Teprotumumab versus IVMP
eAppendix 4. MAICs for IVMP versus Placebo
References
- 1.Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726-738. doi: 10.1056/NEJMra0905750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ugradar S, Goldberg RA, Rootman DB. Bony orbital volume expansion in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2019;35(5):434-437. doi: 10.1097/IOP.0000000000001292 [DOI] [PubMed] [Google Scholar]
- 3.Lazarus JH. Epidemiology of Graves’ Orbitopathy (GO) and relationship with thyroid disease. Best Pract Res Clin Endocrinol Metab. 2012;26(3):273-279. doi: 10.1016/j.beem.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 4.Bartalena L, Kahaly GJ, Baldeschi L, et al. ; EUGOGO † . The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(4):G43-G67. doi: 10.1530/EJE-21-0479 [DOI] [PubMed] [Google Scholar]
- 5.Ting M, Ezra DG. Teprotumumab: a disease modifying treatment for Graves’ orbitopathy. Thyroid Res. 2020;13:12. doi: 10.1186/s13044-020-00086-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piantanida E, Bartalena L. Teprotumumab: a new avenue for the management of moderate-to-severe and active Graves’ orbitopathy? J Endocrinol Invest. 2017;40(8):885-887. doi: 10.1007/s40618-017-0717-8 [DOI] [PubMed] [Google Scholar]
- 7.Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748-1761. doi: 10.1056/NEJMoa1614949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341-352. doi: 10.1056/NEJMoa1910434 [DOI] [PubMed] [Google Scholar]
- 9.Bartalena L, Baldeschi L, Boboridis K, et al. ; European Group on Graves’ Orbitopathy (EUGOGO) . The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J. 2016;5(1):9-26. doi: 10.1159/000443828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahn RS, Gorman CA. Choice of therapy and criteria for assessing treatment outcome in thyroid-associated ophthalmopathy. Endocrinol Metab Clin North Am. 1987;16(2):391-407. doi: 10.1016/S0889-8529(18)30485-7 [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 12.Bartalena L, Krassas GE, Wiersinga W, et al. ; European Group on Graves’ Orbitopathy . Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(12):4454-4463. doi: 10.1210/jc.2012-2389 [DOI] [PubMed] [Google Scholar]
- 13.Kahaly GJ, Riedl M, König J, et al. ; European Group on Graves’ Orbitopathy (EUGOGO) . Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol. 2018;6(4):287-298. doi: 10.1016/S2213-8587(18)30020-2 [DOI] [PubMed] [Google Scholar]
- 14.Aktaran S, Akarsu E, Erbağci I, Araz M, Okumuş S, Kartal M. Comparison of intravenous methylprednisolone therapy vs. oral methylprednisolone therapy in patients with Graves’ ophthalmopathy. Int J Clin Pract. 2007;61(1):45-51. doi: 10.1111/j.1742-1241.2006.01004.x [DOI] [PubMed] [Google Scholar]
- 15.He Y, Mu K, Liu R, Zhang J, Xiang N. Comparison of two different regimens of intravenous methylprednisolone for patients with moderate to severe and active Graves’ ophthalmopathy: a prospective, randomized controlled trial. Endocr J. 2017;64(2):141-149. doi: 10.1507/endocrj.EJ16-0083 [DOI] [PubMed] [Google Scholar]
- 16.Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90(9):5234-5240. doi: 10.1210/jc.2005-0148 [DOI] [PubMed] [Google Scholar]
- 17.Mu PW, Tang XX, Wang YN, et al. Comparison of two regimens for patients with thyroid-associated ophthalmopathy receiving intravenous methyl prednisolone: a single center prospective randomized trial. Exp Ther Med. 2020;20(6):153. doi: 10.3892/etm.2020.9282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu W, Ye L, Shen L, et al. A prospective, randomized trial of intravenous glucocorticoids therapy with different protocols for patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2014;99(6):1999-2007. doi: 10.1210/jc.2013-3919 [DOI] [PubMed] [Google Scholar]
- 19.Beleslin BN, Ciric J, Stojkovic M, et al. Comparison of efficacy and safety of parenteral versus parenteral and oral glucocorticoid therapy in Graves’ orbitopathy. Int J Clin Pract. 2020;74(11):e13608. doi: 10.1111/ijcp.13608 [DOI] [PubMed] [Google Scholar]
- 20.Li HX, Zhao XH, Song Y, et al. Changes in ocular biomechanics after treatment for active Graves’ orbitopathy. J Endocrinol Invest. 2021:44(3):453-458. doi: 10.1007/s40618-020-01322-5 [DOI] [PubMed] [Google Scholar]
- 21.Xing L, Ye L, Zhu W, et al. Smoking was associated with poor response to intravenous steroids therapy in Graves’ ophthalmopathy. Br J Ophthalmol. 2015;99(12):1686-1691. doi: 10.1136/bjophthalmol-2014-306463 [DOI] [PubMed] [Google Scholar]
- 22.Xu N, Cui Y, Fu D, Sun F. Tear inflammatory cytokines and ocular surface changes in patients with active thyroid eye disease treated with high-dose intravenous glucocorticoids. J Endocrinol Invest. 2020;43(7):901-910. doi: 10.1007/s40618-019-01174-8 [DOI] [PubMed] [Google Scholar]
- 23.Yang M, Wiersinga WM, Soeters MR, Mourits MP. What is the aim of immunosuppressive treatment in patients with Graves’ orbitopathy? Ophthalmic Plast Reconstr Surg. 2014;30(2):157-161. doi: 10.1097/IOP.0000000000000036 [DOI] [PubMed] [Google Scholar]
- 24.Phillippo D, Ades T, Dias S, Palmer S, Abrams KR, Welton N. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE. Accessed January 4, 2022. https://research-information.bris.ac.uk/en/publications/nice-dsu-technical-support-document-18-methods-for-population-adj
- 25.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruscolini A, Sacchetti M, La Cava M, et al. Quality of life and neuropsychiatric disorders in patients with Graves’ orbitopathy: current concepts. Autoimmun Rev. 2018;17(7):639-643. doi: 10.1016/j.autrev.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 27.Ponto KA, Pitz S, Pfeiffer N, Hommel G, Weber MM, Kahaly GJ. Quality of life and occupational disability in endocrine orbitopathy. Dtsch Arztebl Int. 2009;106(17):283-289. doi: 10.3238/arztebl.2009.0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahaly GJ, Douglas RS, Holt RJ, Sile S, Smith TJ. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021;9(6):360-372. doi: 10.1016/S2213-8587(21)00056-5 [DOI] [PubMed] [Google Scholar]
- 29.Tu X, Dong Y, Zhang H, Su Q. Corticosteroids for Graves’ ophthalmopathy: systematic review and meta-analysis. Biomed Res Int. 2018;2018:4845894. doi: 10.1155/2018/4845894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Geest RJ, Sasim IV, Koppeschaar HP, et al. Methylprednisolone pulse therapy for patients with moderately severe Graves’ orbitopathy: a prospective, randomized, placebo-controlled study. Eur J Endocrinol. 2008;158(2):229-237. doi: 10.1155/2018/4845894 [DOI] [PubMed] [Google Scholar]
- 31.Zhao LQ, Yu DY, Cheng JW. Intravenous glucocorticoids therapy in the treatment of Graves’ ophthalmopathy: a systematic review and meta-analysis. Int J Ophthalmol. 2019;12(7):1177-1186. doi: 10.18240/ijo.2019.07.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvi M, Vannucchi G, Currò N, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015;100(2):422-431. doi: 10.1210/jc.2014-3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vannucchi G, Covelli D, Campi I, et al. The therapeutic outcome to intravenous steroid therapy for active Graves’ orbitopathy is influenced by the time of response but not polymorphisms of the glucocorticoid receptor. Eur J Endocrinol. 2013;170(1):55-61. doi: 10.1530/EJE-13-0611 [DOI] [PubMed] [Google Scholar]
- 34.Eckstein A, Quadbeck B, Mueller G, et al. Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. Br J Ophthalmol. 2003;87(6):773-776. doi: 10.1136/bjo.87.6.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Baseline proptosis across studies used for the meta-analysis
eAppendix 1. Search strategy
eAppendix 2. PRISMA 2009 Flow Diagram
eAppendix 3. MAICs for Teprotumumab versus IVMP
eAppendix 4. MAICs for IVMP versus Placebo