Abstract
Introduction
Unilateral lymphadenopathy, an adverse effect of the COVID-19 vaccines, has posed a diagnostic challenge in oncologic imaging (1–8). We sought to determine the outcomes of axillary lymphadenopathy after COVID-19 vaccination on breast imaging examinations.
Material and Methods
In this institutional review board–approved and Health Insurance Portability and Accountability Act–compliant retrospective study, we identified patients who received the COVID-19 vaccine and underwent breast imaging between December 30, 2020, and April 12, 2021, at 17 sites across one institution. Follow-up examinations were recorded through December 10, 2021. Seven radiologists extracted clinical-pathologic data and vaccine information from the medical records. The original radiology reports were used for imaging characteristics. Long-axis lymph node measurements were recorded. Statistics were calculated in Microsoft Excel and Mann-Whitney U tests in SPSS software (version 25, SPSS).
Results
Among the 1217 patients who received the COVID-19 vaccine and underwent breast imaging, 537 (44%) had lymphadenopathy identified during at least one imaging examination. A total of 823 patients (68%) presented for screening (Table E1 [online]). A total of 334 patients underwent mammography and sonography on the same day: 29 (9%) patients had lymphadenopathy identified with mammography alone, 203 (61%) patients with US alone, and 102 (30%) patients with both mammography and US. In patients with lymphadenopathy, the average node measured 1.8 cm (range, 0.5–4.2 cm). Charts were reviewed for imaging and biopsy follow-up for 273 days (range, 253–348 days).
Among the patients with known vaccine manufacturer information, 459 patients received the Moderna vaccine (46% with lymphadenopathy) and 505 patients received the Pfizer vaccine (38% with lymphadenopathy) (P = .007). Eighteen patients received the Johnson & Johnson vaccine (39% with lymphadenopathy) (Table E1 [online]).
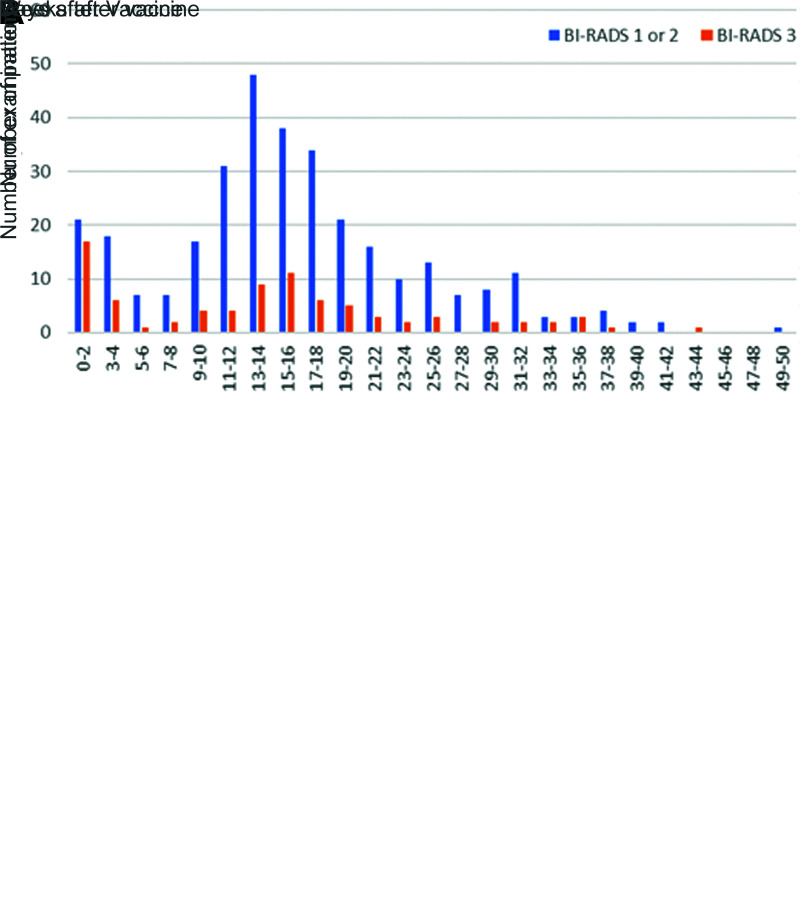
Patients demonstrated lymphadenopathy as early as 1 day following the first dose and as late as 71 days following the second dose. The timing was different between the groups with and without lymphadenopathy. Lymphadenopathy was more likely to be seen within 14 days after vaccination and was rare after 50 days after the second dose (Fig 1A).
Figure 1:
Graphs compare (A) patients with and without lymphadenopathy (LAD) at initial breast imaging after COVID-19 vaccination. Lymphadenopathy is seen most commonly in the first 2 weeks after vaccination, but can also persist at least 10 weeks. (B) Graphs compare patients with LAD and follow-up imaging. Bars show the percent of examinations assigned Breast Imaging Reporting and Data System (BI-RADS) category 1 or 2 (negative or benign findings) versus BI-RADS 3 (probably benign finding; short-term follow-up is recommended) recommendations by time after the vaccination. Twenty-five percent of examinations performed at 0–12 weeks were given BI-RADS 3 recommendations, and none of these patients were subsequently diagnosed with a new malignancy.
Eight percent (43 of 537) of patients underwent a biopsy; 34 (79%) had benign results (Fig 2) and nine (21%) had malignant results (Table E2 [online]). Four patients were diagnosed with metastatic breast cancer (Fig E1 [online]); all had suspicious concurrent mammographic findings in the ipsilateral breast. Four patients were diagnosed with lymphoma; three patients already had known diagnoses, and the fourth patient presented with bilateral lymphadenopathy (Fig E2 [online]). One patient with a known history of lung cancer was diagnosed with lung cancer metastatic to an axillary lymph node.
Figure 2:
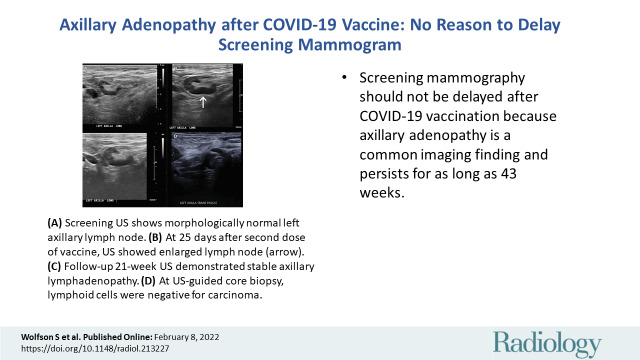
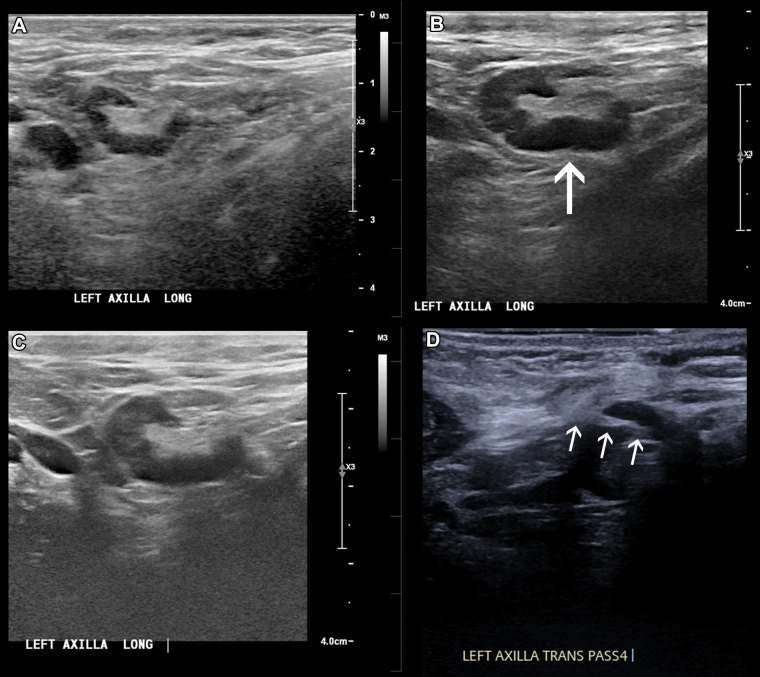
In a 46-year-old patient with a strong family history of breast cancer, (A) a screening US prior to COVID-19 vaccination demonstrated a morphologically normal left axillary lymph node. (B) Twenty-five days following the second dose of the COVID-19 vaccine, the patient presented with a palpable lump in the left axilla and US demonstrated enlarged lymph nodes with cortex measuring up to 6 mm in thickness (arrow). (C) Follow-up US 21 weeks following demonstrated stable axillary lymphadenopathy. (D) A US-guided core biopsy was then recommended and pathologic analysis demonstrated lymphoid cells negative for carcinoma. Arrows indicate the path of the needle.
In 387 (72%) patients with lymphadenopathy, there were 407 follow-up examinations, averaging 15.7 weeks after the initial examinations (range, 0.2–49 weeks). At follow-up imaging, 323 of 407 (79.4%) studies demonstrated decreased lymph node size and were assessed as benign. Eighty-four (20.6%) demonstrated stable findings and were assessed as probably benign with recommendation to undergo additional follow-up (Fig 1B). Among 135 patients with follow-up examinations within 12 weeks, 25% were given Breast Imaging Reporting and Data System, or BI-RADS, category 3 recommendations. No patients in the follow-up group were diagnosed with a subsequent malignancy. Persistent axillary lymphadenopathy was seen up to 43 weeks after vaccination.
Discussion
This is the largest study, to our knowledge, to evaluate axillary lymphadenopathy, with long-term follow-up of 6 months following COVID-19 vaccination. Adenopathy is a common adverse effect identified within 44% of our patients, with persistent lymphadenopathy seen up to 43 weeks after vaccination. Our findings suggest that patients should not delay their screening mammogram because they were recently vaccinated. The Moderna vaccine was significantly more associated with lymphadenopathy compared with Pfizer, consistent with a recent study (7) that evaluated axillary adenopathy on chest CT images (7). Prior to the COVID-19 vaccine era, axillary adenopathy in women with an otherwise normal mammogram was reported in 0.02%–0.04% of screening mammograms (4).
A minority of patients (8%) were recommended for biopsy, only nine (21%) with malignant results. All patients diagnosed with axillary metastases had suspicious imaging findings in the ipsilateral breast. Additional malignancies were detected either in patients with known cancer history or in patients presenting with bilateral lymphadenopathy. These results support the European Society of Breast Imaging guidelines (5) that recommend benign or probably benign assessment for patients without breast cancer history and no suspicious breast imaging findings, as well as the Radiology scientific expert panel guidelines (3) that recommend no default follow-up imaging in patients at low risk of axillary nodal metastases.
In our large cohort with extended follow-up, there were no new diagnoses of malignancy during the follow-up period. The time to resolution of reactive lymphadenopathy was variable, with persistent lymphadenopathy seen up to 43 weeks after vaccination. This is even longer than a case of persistent fluorodeoxyglucose PET/CT uptake at 70 days after the second dose of COVID-19 vaccination (8). Given the extended period of time to resolution, no short-term follow-up imaging should be recommended. This differs from current guidelines, which recommend a short-term follow-up examination in 4–12 weeks following the second vaccine dose (4).
Other studies with lower reported incidence of lymphadenopathy (3%–16%) are based on self-reported symptoms or detection solely on mammography; in contrast, our study had an incidence of 44%. This difference reflects our practice pattern that includes axillary adenopathy seen with either mammography or breast US in both the screening and diagnostic setting (2).
In conclusion, there should be no delay in screening mammograms due to recent vaccination and there is variable time to resolution of reactive lymphadenopathy. Lymphadenopathy should be interpreted in the context of patient risk factors with vigilance in patients with concurrent suspicious mammographic findings in the ipsilateral breast.
Footnotes
From the 2021 RSNA Annual Meeting.
Disclosures of conflicts of interest: S.W. Disclosed no relevant relationships. E.K. Member of the Radiology In Training editorial board. A.P. Disclosed no relevant relationships. R.B. Disclosed no relevant relationships. R.D.S. Disclosed no relevant relationships. N.S. Disclosed no relevant relationships. D.A. Disclosed no relevant relationships. M.M.S. Disclosed no relevant relationships. H.B.T. Disclosed no relevant relationships. L.M. Member of the Radiology editorial board. B.R. Co-president of the New York Breast Imaging Society.
References
- 1. Özütemiz C , Krystosek LA , Church AL , et al . Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients . Radiology 2021. ; 300 ( 1 ): E296 – E300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson KA , Maimone S , Gococo-Benore DA , Li Z , Advani PP , Chumsri S . Incidence of Axillary Adenopathy in Breast Imaging After COVID-19 Vaccination . JAMA Oncol 2021. ; 7 ( 9 ): 1395 – 1397 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Becker AS , Perez-Johnston R , Chikarmane SA , et al . Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: Radiology Scientific Expert Panel . Radiology 2021. ; 300 ( 2 ): E323 – E327 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grimm L , Destounis S , Dogan B , et al . SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination . Society of Breast Imaging website . https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf. Accessed December 16, 2021 .
- 5. Schiaffino S , Pinker K , Magni V , et al . Axillary lymphadenopathy at the time of COVID-19 vaccination: ten recommendations from the European Society of Breast Imaging (EUSOBI) . Insights Imaging 2021. ; 12 ( 1 ): 119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lehman CD , Lamb LR , D'Alessandro HA . Mitigating the Impact of Coronavirus Disease (COVID-19) Vaccinations on Patients Undergoing Breast Imaging Examinations: A Pragmatic Approach . AJR Am J Roentgenol 2021. ; 217 ( 3 ): 584 – 586 . [DOI] [PubMed] [Google Scholar]
- 7. Nishino M , Hatabu H , Ricciuti B , Vaz V , Michael K , Awad MM . Axillary Lymphadenopathy After Coronavirus Disease 2019 Vaccinations in Patients With Thoracic Malignancy: Incidence, Predisposing Factors, and Imaging Characteristics . J Thorac Oncol 2022. ; 17 ( 1 ): 154 – 159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eshet Y , Tau N , Alhoubani Y , Kanana N , Domachevsky L , Eifer M . Prevalence of Increased FDG PET/CT Axillary Lymph Node Uptake Beyond 6 Weeks after mRNA COVID-19 Vaccination . Radiology 2021. ; 300 ( 3 ): E345 – E347 . [DOI] [PMC free article] [PubMed] [Google Scholar]