Key Points
Question
Does capecitabine maintenance therapy improve survival of patients with newly diagnosed metastatic nasopharyngeal carcinoma?
Findings
This phase 3 randomized clinical trial including 104 patients with newly diagnosed metastatic nasopharyngeal carcinoma met its primary end point of improved progression-free survival in favor of capecitabine maintenance therapy plus best supportive care vs best supportive care alone, with manageable toxic effects.
Meaning
This trial provides evidence that capecitabine maintenance therapy may be a promising alternative treatment modality for patients with metastatic nasopharyngeal carcinoma who achieved disease control after capecitabine-containing induction chemotherapy, with a manageable safety profile.
Abstract
Importance
Capecitabine maintenance therapy improves survival outcomes in various cancer types, but data are limited on the efficacy and safety of capecitabine maintenance therapy in metastatic nasopharyngeal carcinoma (NPC).
Objective
To investigate the efficacy and safety of capecitabine maintenance therapy in metastatic NPC.
Design, Setting, and Participants
This randomized phase 3 clinical trial was conducted at Sun Yat-sen University Cancer Center from May 16, 2015, to January 9, 2020, among 104 patients with newly diagnosed metastatic NPC who had achieved disease control after 4 to 6 cycles of induction chemotherapy with paclitaxel, cisplatin, and capecitabine. The final follow-up date was May 30, 2021. All efficacy analyses were conducted in the intention-to-treat population.
Interventions
Eligible patients were randomly assigned (1:1) to receive either capecitabine maintenance therapy (1000 mg/m2 orally twice daily on days 1-14) every 3 weeks plus best supportive care (BSC) (capecitabine maintenance group) or BSC alone after 4 to 6 cycles of induction chemotherapy.
Main Outcomes and Measures
Progression-free survival (PFS). Secondary end points were objective response rate, duration of response, overall survival, and safety.
Results
This study included 104 patients (84 men [80.8%]; median age, 47 years [IQR, 38-54 years]), with 52 assigned to the capecitabine maintenance group and 52 assigned to the BSC group. After a median follow-up of 33.8 months (IQR, 22.9-50.7 months), there were 23 events (44.2%) of progression or death in the capecitabine maintenance group and 37 events (71.2%) of progression or death in the BSC group. Median PFS survival was significantly higher in the capecitabine maintenance group (35.9 months [95% CI, 20.5 months-not reached]) than in the BSC group (8.2 months [95% CI, 6.4-10.0 months]), with a hazard ratio of 0.44 (95% CI, 0.26-0.74; P = .002). Higher objective response rates and longer median duration of response were observed in the capecitabine maintenance group (25.0%; 40.0 months) compared with the BSC group (objective response rate, 25.0% [n = 13] vs 11.5% [n = 6]; and median duration of response, 40.0 months [95% CI, not reached-not reached] vs 13.2 months [95% CI, 9.9-16.5 months]). The most common grade 3 or 4 adverse events during maintenance therapy were anemia (6 of 50 [12.0%]), hand-foot syndrome (5 of 50 [10.0%]), nausea and vomiting (3 of 50 [6.0%]), fatigue (2 of 50 [4.0%]), and mucositis (2 of 50 [4.0%]). No deaths in the maintenance group were deemed treatment-related.
Conclusions and Relevance
In this phase 3 randomized clinical trial, capecitabine maintenance therapy significantly improved PFS for patients with newly diagnosed metastatic NPC who achieved disease control after capecitabine-containing induction chemotherapy. Capecitabine exhibited manageable toxic effects.
Trial Registration
ClinicalTrials.gov Identifier: NCT02460419
This randomized clinical trial investigates the efficacy and safety of capecitabine maintenance therapy for patients with newly diagnosed metastatic nasopharyngeal carcinoma who had achieved disease control after induction chemotherapy.
Introduction
Nasopharyngeal carcinoma (NPC) is a head and neck cancer with unbalanced geographical distribution; it is particularly prevalent in Southern China, East and Southeast Asia, and North Africa.1 Worldwide, approximately 129 000 new cases of NPC and 73 000 deaths attributed to this disease are reported annually.2 The incidence of synchronous metastatic disease is about 5% in newly diagnosed patients. In addition, about 20% of patients with nonmetastatic NPC develop distant failure even after definitive treatments, which remains a main cause of death in these patients.3,4 Taken together, approximately one-fourth of patients with NPC eventually experience metastatic diseases that require systemic therapy.
Guideline-recommended platinum-based chemotherapy is the front-line treatment for patients with metastatic NPC.5,6 Median progression-free survival (PFS) after first-line treatment ranged from 5.0 to 7.0 months.7,8,9 Therefore, treatment strategies with stronger efficacy are needed for those patients. Growing evidence revealed that low-dose maintenance therapy may prevent tumor progression after induction chemotherapy.10 The oral fluoropyrimidine capecitabine was designed to preferentially generate fluorouracil in tumors without complications and inconvenience associated with central venous catheterization. Recently, capecitabine as maintenance therapy has been proven to be effective in prolonging PFS among patients with resected biliary tract cancer, metastatic colorectal cancer, early-stage triple-negative breast cancer, and locoregionally advanced NPC.11,12,13,14 Previous studies have shown that capecitabine is effective as chemotherapy either alone or in combination for patients with metastatic NPC.15,16,17 However, to our knowledge, the efficacy of capecitabine maintenance therapy for patients with metastatic NPC has not been prospectively investigated.
In this open-label, randomized, phase 3 trial, we aimed to evaluate the efficacy and safety of capecitabine maintenance therapy plus best supportive care (BSC) in comparison with BSC alone for patients with metastatic NPC who achieved disease control after induction chemotherapy.
Methods
Study Design and Patient Eligibility
This open-label, randomized phase 3 study was conducted at Sun Yat-sen University Cancer Center (SYSUCC) in China from May 16, 2015, to January 9, 2020. The trial protocol was approved by the institutional review board of SYSUCC (Supplement 1). All patients provided written informed consent. Eligible patients were aged 18 to 65 years, had newly diagnosed metastatic NPC, achieved disease control (including complete response, partial response, and stable disease, assessed by an independent review committee) according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,18 after 4 to 6 cycles of paclitaxel, 150 mg/m2 intravenously on day 1, cisplatin, 60 mg/m2 intravenously on day 1, and capecitabine, 1000 mg/m2 orally twice daily on days 1 to 14 (TPC regimen). More details of the inclusion and exclusion criteria are provided in the trial protocol in Supplement 1. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Randomization and Masking
Block randomization was performed at SYSUCC using a computer-generated random allocation sequence. Patients were randomly assigned (1:1) within 8 to 12 weeks after the last cycle of induction chemotherapy to receive either capecitabine maintenance therapy plus BSC (capecitabine maintenance group) or BSC alone (BSC group). Details of the randomized allocations were contained in sequentially numbered, opaque, sealed envelopes prepared by the statistician. The treatment assignment was unmasked to both patients and clinicians.
Procedures
Patients in the BSC group received only best supportive therapy, defined as measures designed to provide palliation of symptoms and improve quality of life as much as possible, until disease progression. The interval between the capecitabine maintenance treatment and the last cycle of induction chemotherapy was no more than 12 weeks. Patients in the capecitabine maintenance group received BSC plus capecitabine, 1000 mg/m2 orally twice daily on days 1 to 14 every 3 weeks for a maximum of 2 years. The objective assessment of disease status was conducted every 2 cycles of chemotherapy until disease progression. We recorded postprogression survival status and treatment information every 3 months. Tumor response was assessed by an independent review committee, according to RECIST version 1.1, with computed tomography, 18F-fludeoxyglucose–positron emission tomography with computed tomography, and magnetic resonance imaging scans. During the radiologic review process, the independent radiologists were blinded to treatment assignment to avoid potential bias. Patients would continue to receive their allocated treatment until intolerant dose-limited toxic effects occurred, radiologic progression, death, or withdrawal of consent. Once disease progressed, subsequent salvage therapies and reintroduction of TPC or capecitabine maintenance were allowed based on the investigator’s discretion, given a deemed clinical benefit. Locoregional radiotherapy was also permitted for patients with treatment response, and bisphosphonates were allowed for patients with bone metastases. Details of the chemotherapy dose modifications are available in the trial protocol in Supplement 1.
Study End Points
The primary end point was PFS, measured from the date of randomization to the date of disease progression or death from any cause, whichever occurred first. Secondary end points included objective response rate, duration of response, overall survival (OS), and adverse events (AEs). Objective response rate was defined as the proportion of patients who achieved complete or partial response. Duration of response was defined as the interval between initiation of TPC induction chemotherapy and the earliest date of disease progression or death, whichever occurred first (eFigure 1 in Supplement 2). Overall survival was defined as the interval between randomization and death. Adverse events were assessed and graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).19 Additional details concerning end point definitions and AEs are available in the trial protocol in Supplement 1.
Statistical Analysis
The trial used a 2-sided 5% type I error and had 80% power to detect an improvement in PFS from 6 months in the BSC group to 11 months in the capecitabine maintenance group, corresponding to a hazard ratio (HR) of 0.55 in terms of median PFS.7,20 In consideration of these assumptions, the trial design was powered for 98 patients to be randomly assigned in 48.0 months with an additional 12-month follow-up. After considering a 5% dropout rate, we estimated that a total of 104 patients (52 patients in each group) were required.
Continuous variables were expressed as median and IQR and were compared with the t test or Mann-Whitney test. Categorical variables were presented as frequency and percentage and compared with the χ2 test or Fisher exact test. All efficacy analyses were conducted in the intention-to-treat population. Survivals were estimated using the Kaplan-Meier method, and their 95% CIs were calculated using the Brookmeyer-Crowley method. Survival differences were compared using the log-rank test. Cox proportional hazards regression models were used to calculate the stratified HRs and corresponding 95% CIs. The proportional hazard assumption was confirmed based on the Schoenfeld residuals. Treatment effect consistency was measured for the prespecified subgroups and evaluated using an unadjusted Cox proportional hazards regression model. A further interaction analysis was performed to examine whether the therapeutic effect varied in different subgroups. All statistical analyses were conducted using SPSS software, version 24.0 (SPSS Inc) and R, version 4.0.3 (R Group for Statistical Computing). All P values were from 2-sided tests and results were deemed statistically significant at P ≤ .05.
Results
Patients
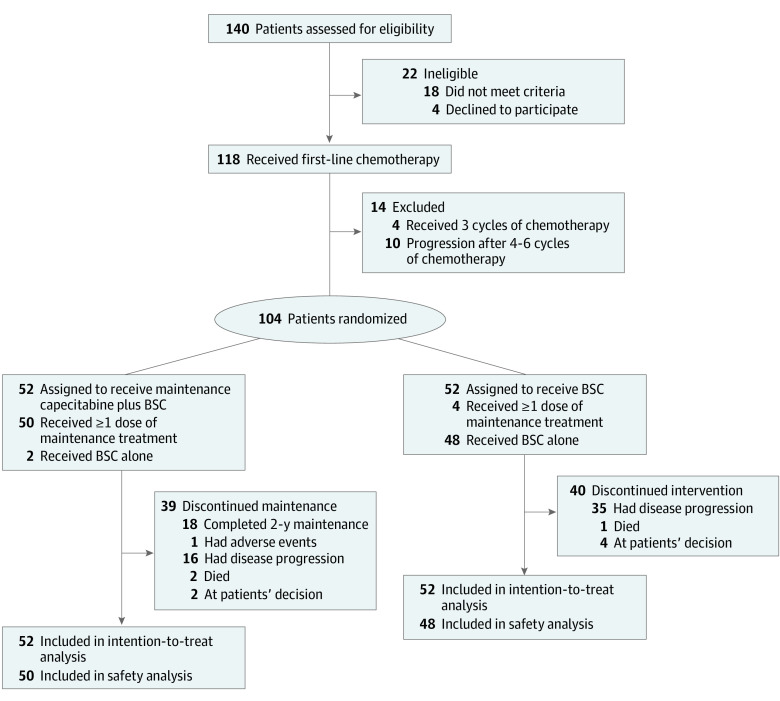
Between May 16, 2015, and January 9, 2020, 140 patients with newly diagnosed metastatic NPC were screened. After the exclusion of 36 patients for ineligibility, 104 patients (84 men [80.8%]; median age, 47 years [IQR, 38-54 years]) were randomly assigned to the capecitabine maintenance group (n = 52) or BSC group (n = 52; Figure 1). Baseline characteristics of the study population are shown in Table 1.21 Almost all patients (100 [96.2%]) had undifferentiated nonkeratinizing carcinoma and more than half of patients (61 [58.7%]) presented with synchronous metastatic NPC. A total of 89 patients (85.6%) had received 6 cycles of TPC induction chemotherapy and 15 patients (14.4%) received 4 to 5 cycles of TPC induction chemotherapy. Response to induction chemotherapy is shown in eTable 1 in Supplement 2.
Figure 1. Trial Profile.
BSC indicates best supportive care.
Table 1. Baseline Demographics and Disease Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| Capecitabine + BSC (n = 52) | BSC alone (n = 52) | |
| Age, median (IQR), y | 45 (38-51) | 49 (38-55) |
| Age group, y | ||
| ≤45 | 29 (55.8) | 24 (46.2) |
| >45 | 23 (44.2) | 28 (53.8) |
| Sex | ||
| Male | 43 (82.7) | 41 (78.8) |
| Female | 9 (17.3) | 11 (21.2) |
| ECOG performance status | ||
| 0 | 13 (25.0) | 11 (21.2) |
| 1 | 39 (75.0) | 41 (78.8) |
| Histologic characteristicsa | ||
| Non-keratinizing | ||
| Undifferentiated (type III) | 51 (98.1) | 49 (94.2) |
| Differentiated (type II) | 1 (1.9) | 0 |
| Poorly differentiated | 0 | 3 (5.8) |
| Smoking status | ||
| Yes | 10 (19.2) | 15 (28.8) |
| No | 42 (80.8) | 37 (71.2) |
| Stage | ||
| Primary metastases | 33 (63.5) | 28 (53.8) |
| Asynchronous metastases | 19 (36.5) | 24 (46.2) |
| Metastasis type | ||
| Oligometastasis | 16 (30.8) | 15 (28.8) |
| Polymetastases | 36 (69.2) | 37 (71.2) |
| Site of metastasis | ||
| Lung | 17 (32.7) | 20 (38.5) |
| Bone | 33 (63.5) | 30 (57.7) |
| Liver | 20 (38.5) | 11 (21.2) |
| No. of first-line chemotherapy cycles | ||
| 4-5 | 6 (11.5) | 9 (17.3) |
| 6 | 46 (88.5) | 43 (82.7) |
| Treatment response to first-line chemotherapy | ||
| Complete or partial response | 43 (82.7) | 42 (80.8) |
| Stable disease | 9 (17.3) | 10 (19.2) |
| EBV DNA, copies/mL | ||
| ≤4000 | 21 (40.4) | 25 (48.1) |
| >4000 | 31 (59.6) | 27 (51.9) |
| Local consolidative therapy | ||
| Yes | 10 (19.2) | 11 (21.2) |
| No | 42 (80.8) | 41 (78.8) |
Abbreviations: BSC, best supportive care; EBV, Epstein-Barr virus; ECOG, Eastern Cooperative Oncology Group.
Histologic characteristics were categorized according to the World Health Organization classification of tumors.21
Compliance with treatment was good, with a median of 24 cycles (range, 2-34) of capecitabine administered. As of May 30, 2021, 39 patients discontinued capecitabine maintenance therapy for the following reasons: completed 2-year capecitabine maintenance therapy (n = 18), intolerable AEs (n = 1), disease progression (n = 16), death (n = 2), and withdrawal of consent (n = 2). The 2 deaths leading to treatment discontinuation were not related to treatment, and the patients died without radiologically confirmed disease progression. In the BSC group, 40 patients discontinued for the following reasons: disease progression (n = 35), death (n = 1), and withdrawal of consent (n = 4).
Subsequent salvage therapies were administered to 17 of 23 patients (73.9%) from the capecitabine maintenance group with disease progression and 27 of 37 patients (73.0%) from the BSC group. Platinum-based chemotherapy was the most frequently used second-line chemotherapy regimen, administered to 14 of 23 patients (60.9%) in the capecitabine maintenance group and 21 of 37 patients (56.8%) in the BSC group. The TPC chemotherapy followed by capecitabine maintenance regimen was administered to 9 of 37 patients (24.3%) in the BSC group and reintroduced in 4 of 23 patients (17.4%) in the capecitabine maintenance group. In addition, immune checkpoint inhibitors were administered to 13 of the 60 patients (21.7%) in the 2 groups. The details of concomitant therapy or medications and subsequent salvage therapies are summarized in eTable 2 in Supplement 2.
Efficacy
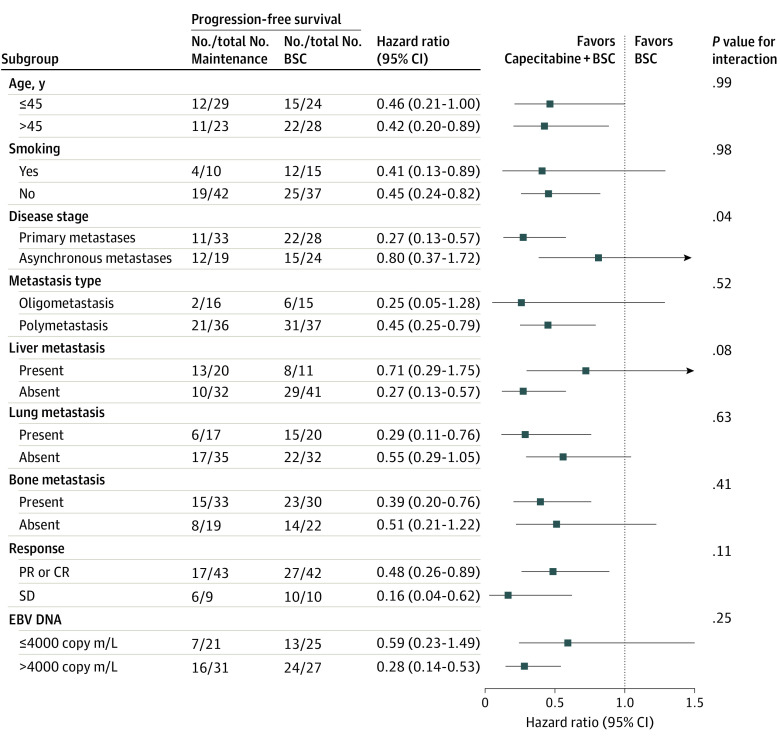
As of May 30, 2021, the median follow-up duration after randomization was 33.8 months (IQR, 22.9-50.7 months) for the intention-to-treat population. A total of 23 of 52 patients (44.2%) in the capecitabine maintenance group experienced disease progression or died, compared with 37 of 52 patients (71.2%) in the BSC group. Median PFS in the capecitabine maintenance group was 35.9 months (95% CI, 20.5 months-not reached), significantly longer than median PFS in the BSC group (8.2 months [95% CI, 6.4-10.0 months]) (HR, 0.44 [95% CI, 0.26-0.74]; P = .002; Figure 2A). The objective response rate was 25.0% (n = 13) in the capecitabine maintenance group and 11.5% (n = 6) in the BSC group (eTable 3 in Supplement 2). The median duration of response in the capecitabine maintenance group was 40.0 months (95% CI, not reached-not reached) vs 13.2 months (95% CI, 9.9-16.5 months) in the BSC group (HR, 0.44 [95% CI, 0.26-0.75]; P = .002; Figure 2B). A post hoc subgroup analysis showed the relative consistency of capecitabine’s treatment effect on PFS across all patient subgroups (Figure 3). The only significant heterogeneity was seen among patients with synchronous metastatic NPC. During follow-up, 14 of 52 patients (26.9%) in the capecitabine maintenance group died and 23 of 52 patients (44.2%) in the BSC group died, and the OS was immature in both groups. Median OS was not reached in the capecitabine maintenance group and was 41.5 months (IQR, not reached-not reached) in the BSC group. No significant difference was detected between the capecitabine maintenance group and BSC group for OS (HR, 0.59 [95% CI, 0.30-1.16]; P = .13; eFigure 2 in Supplement 2). After excluding the patients who received crossover treatment, a significant OS difference was observed between the BSC group and capecitabine maintenance group (eFigure 3 in Supplement 2).
Figure 2. Kaplan-Meier Plot in the Intention-to-Treat Population.
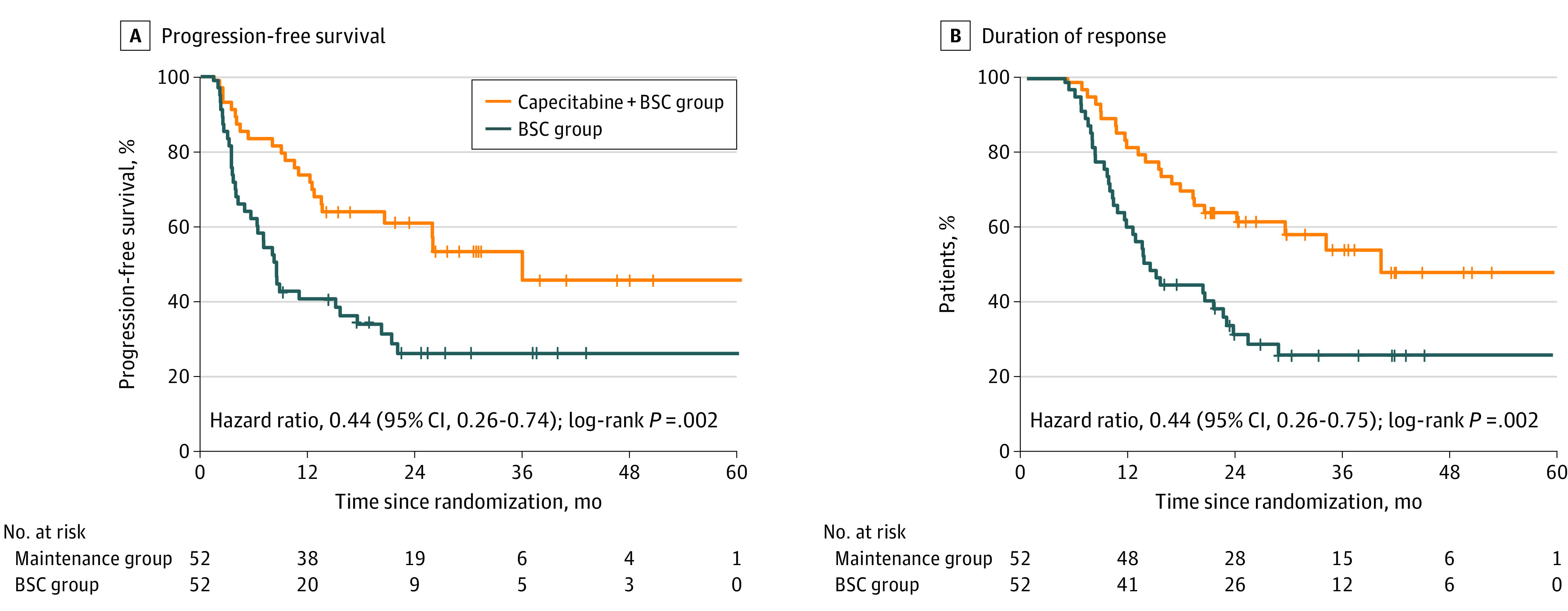
A, Progression-free survival since randomization. B, Duration of response since induction chemotherapy. BSC indicates best supportive care.
Figure 3. Subgroup Analysis of Progression-Free Survival.
Effect of treatment on progression-free survival in subgroups of the intention-to-treat population defined according to prespecified factors and baseline characteristics. BSC indicates best supportive care; CR, complete response; EBV, Epstein-Barr virus; PR, partial response; and SD, stable disease.
Safety
Adverse effects were recorded for 50 patients in the capecitabine maintenance group and 48 patients in the BSC group who received the allocated treatment. The most common grade 1 or 2 AEs were anemia, neutropenia, hand-foot syndrome, nausea and vomiting, and mucositis. However, the overall incidence of grade 3 or 4 AEs was low. The incidence of hand-foot syndrome, nausea and vomiting, and mucositis was higher in the capecitabine maintenance group compared with the BSC group. Grade 3 or 4 AEs in the capecitabine maintenance group included anemia (6 [12.0%]), hand-foot syndrome (5 [10.0%]), nausea and vomiting (3 [6.0%]), fatigue (2 [4.0%]), mucositis (2 [4.0%]), neutropenia (1 [2.0%]), and thrombocytopenia (1 [2.0%]). Maintenance therapy was discontinued in 1 patient owing to fatigue and nausea and vomiting. Reasons for dose modifications in the 6 patients were neutropenia (n = 1), fatigue (n = 1), nausea and vomiting (n = 2), and hand-foot syndrome (n = 3). No treatment-related deaths were observed. The overall safety profile during the TPC induction chemotherapy is in eTable 4 in Supplement 2 and during maintenance therapy is in Table 2.19
Table 2. Treatment-Emergent Adverse Events During Maintenance Therapya.
| Event | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Capecitabine + BSC (n = 50) | BSC alone (n = 48) | |||||||
| Any grade | Grade 1-2 | Grade 3 | Grade 4 | Any grade | Grade 1-2 | Grade 3 | Grade 4 | |
| Hematologic toxic effects | ||||||||
| Anemia | 29 (58.0) | 23 (46.0) | 5 (10.0) | 1 (2.0) | 32 (66.7) | 31 (64.6) | 0 | 1 (2.1) |
| Neutropenia | 24 (48.0) | 23 (46.0) | 1 (2.0) | 0 | 19 (39.6) | 19 (39.6) | 0 | 0 |
| Thrombocytopenia | 8 (16.0) | 7 (14.0) | 1 (2.0) | 0 | 4 (8.3) | 4 (8.3) | 0 | 0 |
| Nonhematologic toxic effects | ||||||||
| Hand-foot syndrome | 23 (46.0) | 18 (36.0) | 4 (8.0) | 1 (2.0) | 4 (8.3) | 4 (8.3) | 0 | 0 |
| Nausea and vomiting | 15 (30.0) | 12 (24.0) | 3 (6.0) | 0 | 4 (8.3) | 4 (8.3) | 0 | 0 |
| Mucositis | 13 (26.0) | 11 (22.0) | 2 (4.0) | 0 | 1 (2.1) | 1 (2.1) | 0 | 0 |
| Fatigue | 7 (14.0) | 5 (10.0) | 2 (4.0) | 0 | 1 (2.1) | 1 (2.1) | 0 | 0 |
| Diarrhea | 3 (6.0) | 3 (6.0) | 0 | 0 | 1 (2.1) | 1 (2.1) | 0 | 0 |
Abbreviation: BSC, best supportive care.
Grade 1-2 adverse events occurring in 10% or more of patients and all the grade 3-4 events are listed according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.19 No treatment-related death occurred in either group.
Discussion
To our knowledge, this is the first phase 3 randomized clinical trial investigating the efficacy of capecitabine maintenance therapy in addition to BSC among patients with newly diagnosed metastatic NPC who achieved disease control after 4 to 6 cycles of TPC induction chemotherapy. We demonstrated that capecitabine maintenance therapy plus BSC was associated with improved PFS compared with BSC alone, with manageable toxic profiles.
Nasopharyngeal carcinoma is sensitive to platinum-based chemotherapy, with a response rate of 40% to 65%.1 The extension of full-dose chemotherapy after disease control may not be feasible owing to cumulative toxic effects and diminishing benefits. However, in the absence of further interventions, most patients with responsive metastatic NPC will experience disease progression soon after first-line treatment. Compared with full-dose chemotherapy, low-dose maintenance chemotherapy has good tolerability and could be an appealing strategy to further improve outcomes.10 Prior to our randomized clinical trial, 2 retrospective studies reported the promising role of maintenance chemotherapy using a fluorouracil analogue after systemic chemotherapy for patients with metastatic NPC.22,23 In this trial, we prospectively confirmed the efficacy of capecitabine maintenance therapy in addition to induction chemotherapy for patients with metastatic NPC. This strategy could be a user-friendly, easily accessible, and cost-effective treatment.
The present study results showed that median PFS was significantly improved in the capecitabine maintenance group compared with the BSC group (35.2 vs 8.2 months). Several underlying mechanisms may explain the effect of maintenance capecitabine on PFS and the rationale of using capecitabine as maintenance therapy rather than subsequent salvage therapy. First, primary systemic therapy leading to disease control leaves residual malignant cells that are possibly to be eradicated by immediate subsequent maintenance therapy.24 A second possibility is that capecitabine maintenance therapy would postpone the growth of distant micrometastatic disease by reducing the tumor burden and also eliminate distant micrometastatic lesions.25 Third, low-dose maintenance chemotherapy may be able to induce angiogenic dormancy by inhibiting tumor angiogenesis and enhancing immune response against tumor-associated antigens.10,26 Recently, 3 randomized multicenter studies showed that anti–programmed cell death protein 1 (anti-PD-1) in combination with standard gemcitabine plus cisplatin (GP) chemotherapy had satisfactory efficacy and tolerable toxic effects among patients with newly diagnosed metastatic NPC.27,28,29 Whether TPC chemotherapy combined with anti-PD-1 has the same efficacy is also worth further exploration. In addition, the recently updated results of KEYNOTE-122 indicated that median OS was 17.2 months with pembrolizumab monotherapy and 15.3 months with chemotherapy, but with fewer toxic effects for patients with platinum-pretreated recurrent or metastatic NPC.30,31 The results demonstrated that anti-PD-1 may also be a promising maintenance option for recurrent or metastatic NPC. The efficacy and safety of capecitabine maintenance chemotherapy combined with immune checkpoint inhibitors for metastatic NPC deserves further investigation.
In a landmark 2016 phase 3 study of recurrent or metastatic NPC, Zhang et al9 reported that PFS and OS were superior with GP compared with fluorouracil plus cisplatin, establishing GP as a preferred first-line regimen. However, the median PFS with GP was only 7.0 months. In our study, selection of a TPC regimen instead of a GP regimen as first-line treatment was based on several rationales. First, several clinical trials have demonstrated that a taxane added to cisplatin and fluorouracil (TPF) significantly prolonged survival with manageable toxic effects for patients with advanced head and neck cancer.32,33,34 A TPF regimen was also proven to be effective in metastatic and/or recurrent NPC, with an objective response rate of 74%.7 Second, a retrospective study comparing different first-line regimens found no significant differences between GP and TPF regimens for metastatic NPC.35 Third, replacing fluorouracil with capecitabine in a TPF regimen led to a median PFS of 8.0 months and tolerable toxic effects for patients with refractory and relapsed NPC.17 Fourth, administration of a maintenance agent that had been shown to be effective and well tolerated during the induction period combines the advantage of continuing a beneficial therapy with the improved safety of treatment with a single agent. Given the promising antitumor activity of a TPC regimen, a controlled, randomized phase 3 study of TPC vs a standard GP regimen for patients with metastatic NPC is ongoing (ChiCTR1900027112).
Patients in the capecitabine maintenance group experienced more AEs than those in the BSC group. Four grade 3 to 4 AEs (anemia, hand-foot syndrome, nausea and vomiting, and mucositis) occurred more frequently in the capecitabine maintenance group than the BSC group. As shown in previous studies,12,36,37 capecitabine administration was associated with hand-foot syndrome, which was the most common treatment-emergent AE, with a 10% incidence of grade 3 or higher events in maintenance treatment. The grade 3 or 4 anemia (12.0%), hand-foot syndrome (10.0%), and nausea and vomiting (6.0%) were the only AEs that occurred in more than 5% of patients in the capecitabine maintenance group. Nevertheless, treatment-emergent AEs in the capecitabine maintenance group were manageable in most patients with dose modifications and no treatment-related death occurred. The safety profile of capecitabine appeared to be generally acceptable among patients receiving maintenance therapy for metastatic NPC.
Over the past decades, maintenance therapy administered for 12 or 24 months after induction treatment has been assessed for several cytotoxic agents.13,14,38,39,40 Given the fact that 80% to 90% of patients with metastatic NPC would experience disease progression within 2 years after induction chemotherapy, a maximum duration of 2 years was selected for the current study.7,17 To ensure tolerability during the maintenance treatment, the protocol-defined daily dose of capecitabine (2000 mg/m2) was slightly lower than the standard dose (2500 mg/m2). Up to the last follow-up, 18 patients had completed 2 years of capecitabine maintenance therapy with acceptable toxic effects. However, some unsolved problems still need to be further explored. The optimal duration of capecitabine maintenance remains unclear. Furthermore, the potential associations between the activities of the studied regimens and tumor biomarkers remains inconclusive. Prospective studies are needed to define the optimal duration of capecitabine maintenance and identify the individuals who might benefit more from maintenance therapy.
Limitations
This study has some limitations. First, OS events were not mature in this study. However, we already observed that there was a trend of an OS advantage in this population. The absolute number of deaths was higher in the BSC group than the capecitabine maintenance group (23 vs 14). A longer follow-up is needed to assess whether the PFS benefit observed with maintenance therapy could translate into an OS benefit. Second, this was a single-center study; these findings should be confirmed by multicenter study. Third, this trial excluded patients older than 65 years in view of their ability to tolerate treatment; therefore, our results may not be generalizable to older patients.
Conclusions
In this phase 3 randomized clinical trial, capecitabine maintenance therapy plus BSC demonstrated superior PFS vs BSC alone for patients with metastatic NPC who achieved disease control after capecitabine-containing induction chemotherapy. Capecitabine also exhibited manageable toxic effects.
Trial Protocol
eTable 1. Response to the Induction Chemotherapy
eTable 2. Summary of Concomitant Therapy or Medications and Subsequent Therapies for Progressive Patients
eTable 3. Pattern of Failure and Disease Status at Last Assessment and Best Tumor Response to Maintenance Therapy
eTable 4. Treatment-Emergent Adverse Events During TPC Induction Chemotherapy
eFigure 1. (A) Treatment Scheme. (B) Definition of Duration of Response.
eFigure 2. Kaplan-Meier Analysis of Overall Survival in the Intention-to-Treat Population
eFigure 3. Kaplan-Meier Analysis of Overall Survival Between Maintenance Group and BSC Group Excluded Crossover Treatment
Data Sharing Statement
References
- 1.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi: 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 2.Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70(4):313. doi: 10.3322/caac.21609 [DOI] [PubMed] [Google Scholar]
- 3.Li AC, Xiao WW, Shen GZ, et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of IMRT: long-term results and benefits of chemotherapy. Oncotarget. 2015;6(27):24511-24521. doi: 10.18632/oncotarget.4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong KCW, Hui EP, Lo K-W, et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol. 2021;18(11):679-695. doi: 10.1038/s41571-021-00524-x [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network . Head and neck cancers, version 1.2015. Accessed May 12, 2015. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.2015 [DOI] [PMC free article] [PubMed]
- 6.National Comprehensive Cancer Network . Head and neck cancers, version 3.2021. April 27, 2021. Accessed June 23, 2021. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 7.Jin Y, Shi YX, Cai XY, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;138(10):1717-1725. doi: 10.1007/s00432-012-1219-x [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Li J, Hong X, et al. Retrospective case series of gemcitabine plus cisplatin in the treatment of recurrent and metastatic nasopharyngeal carcinoma. Oral Oncol. 2008;44(5):464-470. doi: 10.1016/j.oraloncology.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388(10054):1883-1892. doi: 10.1016/S0140-6736(16)31388-5 [DOI] [PubMed] [Google Scholar]
- 10.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7(8):455-465. doi: 10.1038/nrclinonc.2010.82 [DOI] [PubMed] [Google Scholar]
- 11.Primrose JN, Fox RP, Palmer DH, et al. ; BILCAP study group . Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663-673. doi: 10.1016/S1470-2045(18)30915-X [DOI] [PubMed] [Google Scholar]
- 12.Luo HY, Li YH, Wang W, et al. Single-agent capecitabine as maintenance therapy after induction of XELOX (or FOLFOX) in first-line treatment of metastatic colorectal cancer: randomized clinical trial of efficacy and safety. Ann Oncol. 2016;27(6):1074-1081. doi: 10.1093/annonc/mdw101 [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Wang SS, Huang H, et al. ; South China Breast Cancer Group (SCBCG) . Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial. JAMA. 2021;325(1):50-58. doi: 10.1001/jama.2020.23370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YP, Liu X, Zhou Q, et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet. 2021;398(10297):303-313. doi: 10.1016/S0140-6736(21)01123-5 [DOI] [PubMed] [Google Scholar]
- 15.Chua D, Wei WI, Sham JS, Au GK. Capecitabine monotherapy for recurrent and metastatic nasopharyngeal cancer. Jpn J Clin Oncol. 2008;38(4):244-249. doi: 10.1093/jjco/hyn022 [DOI] [PubMed] [Google Scholar]
- 16.Li YH, Wang FH, Jiang WQ, et al. Phase II study of capecitabine and cisplatin combination as first-line chemotherapy in Chinese patients with metastatic nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 2008;62(3):539-544. doi: 10.1007/s00280-007-0641-2 [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Huang HQ, Bai B, Cai QC, Wang XX, Cai QQ. Treatment outcome of docetaxel, capecitabine and cisplatin regimen for patients with refractory and relapsed nasopharyngeal carcinoma who failed previous platinum-based chemotherapy. Expert Opin Pharmacother. 2014;15(2):163-171. doi: 10.1517/14656566.2014.866652 [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute . Common Terminology Criteria for Adverse Events. Accessed June 14, 2010. https://evs.nci.nih.gov/ftp1/CTCAE
- 20.Hong RL, Sheen TS, Ko JY, Hsu MM, Wang CC, Ting LL. Induction with mitomycin C, doxorubicin, cisplatin and maintenance with weekly 5-fluorouracil, leucovorin for treatment of metastatic nasopharyngeal carcinoma: a phase II study. Br J Cancer. 1999;80(12):1962-1967. doi: 10.1038/sj.bjc.6690627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H-Y, Chang Y-L, To K-F, et al. A new prognostic histopathologic classification of nasopharyngeal carcinoma. Chin J Cancer. 2016;35:41. doi: 10.1186/s40880-016-0103-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun XS, Liu SL, Liang YJ, et al. The role of capecitabine as maintenance therapy in de novo metastatic nasopharyngeal carcinoma: a propensity score matching study. Cancer Commun (Lond). 2020;40(1):32-42. doi: 10.1002/cac2.12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Q, Chen M, Xu H, et al. Oral maintenance chemotherapy using S-1/capecitabine in metastatic nasopharyngeal carcinoma patients after systemic chemotherapy: a single-institution experience. Cancer Manag Res. 2020;12:1387-1396. doi: 10.2147/CMAR.S234271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi: 10.1056/NEJMoa1612645 [DOI] [PubMed] [Google Scholar]
- 25.Epstein RJ. Maintenance therapy to suppress micrometastasis: the new challenge for adjuvant cancer treatment. Clin Cancer Res. 2005;11(15):5337-5341. doi: 10.1158/1078-0432.CCR-05-0437 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Sun M, Huang G, et al. Maintenance of antiangiogenic and antitumor effects by orally active low-dose capecitabine for long-term cancer therapy. Proc Natl Acad Sci U S A. 2017;114(26):E5226-E5235. doi: 10.1073/pnas.1705066114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Qu S, Li J, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2021;22(8):1162-1174. doi: 10.1016/S1470-2045(21)00302-8 [DOI] [PubMed] [Google Scholar]
- 28.Mai HQ, Chen QY, Chen D, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. 2021;27(9):1536-1543. doi: 10.1038/s41591-021-01444-0 [DOI] [PubMed] [Google Scholar]
- 29.Ruan X, Liang J-H, Pan Y, et al. Apatinib for the treatment of metastatic or locoregionally recurrent nasopharyngeal carcinoma after failure of chemotherapy: A multicenter, single-arm, prospective phase 2 study. Cancer. 2021;127(17):3163-3171. doi: 10.1002/cncr.33626 [DOI] [PubMed] [Google Scholar]
- 30.Ng QS, Spreafico A, Lee V, et al. KEYNOTE-122: phase 2 study of pembrolizumab versus standard-of-care chemotherapy in platinum-pretreated, recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol. 2016;114(suppl 8):VIII15. doi: 10.1093/annonc/mdw525.48 [DOI] [Google Scholar]
- 31.Chan AT, Lee VHF, Hong RL, et al. 858O Results of KEYNOTE-122: a phase III study of pembrolizumab (pembro) monotherapy vs chemotherapy (chemo) for platinum-pretreated, recurrent or metastatic (R/M) nasopharyngeal carcinoma (NPC). Ann Oncol. 2021;32(suppl 5):S786. doi: 10.1016/j.annonc.2021.08.1268 [DOI] [Google Scholar]
- 32.Vermorken JB, Remenar E, van Herpen C, et al. ; EORTC 24971/TAX 323 Study Group . Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695-1704. doi: 10.1056/NEJMoa071028 [DOI] [PubMed] [Google Scholar]
- 33.Posner MR, Hershock DM, Blajman CR, et al. ; TAX 324 Study Group . Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705-1715. doi: 10.1056/NEJMoa070956 [DOI] [PubMed] [Google Scholar]
- 34.Hitt R, López-Pousa A, Martínez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23(34):8636-8645. doi: 10.1200/JCO.2004.00.1990 [DOI] [PubMed] [Google Scholar]
- 35.Ma SX, Zhou T, Huang Y, et al. The efficacy of first-line chemotherapy in recurrent or metastatic nasopharyngeal carcinoma: a systematic review and meta-analysis. Ann Transl Med. 2018;6(11):201. doi: 10.21037/atm.2018.05.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li YH, Luo HY, Wang FH, et al. Phase II study of capecitabine plus oxaliplatin (XELOX) as first-line treatment and followed by maintenance of capecitabine in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol. 2010;136(4):503-510. doi: 10.1007/s00432-009-0682-5 [DOI] [PubMed] [Google Scholar]
- 37.Cunningham D, Lang I, Marcuello E, et al. ; AVEX study investigators . Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14(11):1077-1085. doi: 10.1016/S1470-2045(13)70154-2 [DOI] [PubMed] [Google Scholar]
- 38.Lee SM, Rudd R, Woll PJ, et al. Randomized double-blind placebo-controlled trial of thalidomide in combination with gemcitabine and Carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(31):5248-5254. doi: 10.1200/JCO.2009.21.9733 [DOI] [PubMed] [Google Scholar]
- 39.Vergote IB, Jimeno A, Joly F, et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: a European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. J Clin Oncol. 2014;32(4):320-326. doi: 10.1200/JCO.2013.50.5669 [DOI] [PubMed] [Google Scholar]
- 40.Shinagawa K, Yanada M, Sakura T, et al. Tamibarotene as maintenance therapy for acute promyelocytic leukemia: results from a randomized controlled trial. J Clin Oncol. 2014;32(33):3729-3735. doi: 10.1200/JCO.2013.53.3570 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Response to the Induction Chemotherapy
eTable 2. Summary of Concomitant Therapy or Medications and Subsequent Therapies for Progressive Patients
eTable 3. Pattern of Failure and Disease Status at Last Assessment and Best Tumor Response to Maintenance Therapy
eTable 4. Treatment-Emergent Adverse Events During TPC Induction Chemotherapy
eFigure 1. (A) Treatment Scheme. (B) Definition of Duration of Response.
eFigure 2. Kaplan-Meier Analysis of Overall Survival in the Intention-to-Treat Population
eFigure 3. Kaplan-Meier Analysis of Overall Survival Between Maintenance Group and BSC Group Excluded Crossover Treatment
Data Sharing Statement