Abstract
RATIONALE:
Inhaled hypertonic saline (HS) is an effective hydrating therapy for CF lung disease. However, the mechanism of action and principles pertinent to HS administration remain unclear.
OBEJECTIVE:
To assess the kinetics of airway surface liquid (ASL) and cell volume changes in response to HS aerosol in human bronchial epithelial cultures.
METHODS:
An in vitro system aerosolized HS to epithelial cells at rates comparable to in vivo conditions. ASL volume and cell height responses were measured by confocal microscopy under normal and “CF-like” hyperconcentrated mucus states.
MAIN RESULTS:
Aerosolized HS produced a rapid increase in ASL height and decrease in cell height. Added ASL volume was quickly reabsorbed following termination of nebulization, though cell height did not recover for four hours after excess ASL reabsorption. Addition of a sodium channel blocker significantly increased the magnitude and duration of action of HS. ASL volume responses to repeated HS administrations were blunted but could be restored by a hypotonic saline bolus interposed between HS administrations. HS-induced ASL hydration was prolonged with “CF-like” hyperconcentrated mucus on the airway surface, with more modest reductions in cell volume (14%).
CONCLUSIONS:
Aerosolized HS produced transient increases in ASL height that were limited by active Na+ absorption and cell volume-induced reductions in cell water permeability. Addition of sodium channel blockers prolonged the duration of action of HS. Mucus on airway surfaces prolonged the effect of HS via mucus dependent osmotic forces, suggesting that the duration of action of HS is increased in CF patients with concentrated mucus.
Keywords: aerosolization, ASL, epithelium, hypertonic saline, cell volume
At a glance commentary:
The mechanism of action of hypertonic saline has previously been poorly elucidated. This in vitro model system using cultured human bronchial epithelium and nebulized hypertonic saline provides data that suggests that the effect of hypertonic saline on ASL hydration is transient, dependent on mucus concentration, and can be prolonged with ENaC blockers.
Introduction
Cystic fibrosis (CF) lung disease is characterized by mucus accumulation, airways obstruction, and, bronchiectasis (1). Under normal circumstances, airway epithelial cells have the capacity to absorb and secrete ions via active ion transport, with movement of water governed by generated osmotic gradients. The net effect of this balance between secretion and absorption is to maintain mucus layer hydration at levels adequate to promote efficient mucus clearance (1). Cystic fibrosis transmembrane conductance regulator (CFTR) is an ion channel that normally functions to regulate transepithelial ion and water fluxes. In the absence of functional CFTR, deficient chloride secretion, coupled with unopposed sodium absorption, produces airway surface dehydration with a hyperconcentrated mucus layer covering the airway surface. The thickened mucus layer heterogeneously compresses cilia and abolishes mucociliary clearance (MCC) (2), limits the ability of neutrophils to penetrate mucus (3), and serves as a nidus for persistent bacterial infection and inflammation (4).
Aerosolized hypertonic saline (HS) is an effective therapy in adults with CF, producing improvements in MCC, forced expiratory volume in one second (FEV1), quality of life, and a decreased frequency of pulmonary exacerbations (5, 6). Despite its clinical utility, the mechanism of action of HS to improve lung health remains speculative. Accelerating MCC via electrostatic interactions with mucins (7), expanding ASL volume (hydration) (8), or inhibiting the epithelial sodium channel (ENaC) (9) have all been proposed as mechanisms. Most mechanistic studies deposited large volumes of HS onto HBE surfaces and have not mimicked in vivo aerosol deliveries or rates of HS deposition. Further, most studies using cultured HBE were performed without endogenous normal (2% solids) or CF-like (~8% solids) mucus on HBE surfaces.
As prescribed for clinical use, HS is aerosolized and delivered to the lower airway surfaces in small (nanoliter/cm2/min) volumes over protracted intervals (~15 min). In this study, an in vitro aerosolization system was developed to mimic in vivo aerosol delivery rates to airway epithelia that exhibited a range of mucus concentrations, spanning “normal” to mucus-rich “CF-like” conditions. The primary goal was to characterize the mechanisms and kinetics of HS effects on airway surface hydration. The ancillary goal was to identify strategies to improve the efficacy of HS in the treatment of lung disease. Because of the great diversity of nebulizers and HS strengths used in clinical practice, and the fact that the osmotic effectiveness of HS depends on the rate of NaCl delivery to airway surfaces, our studies investigating HS administrations on ASL expansion are described in μg NaCl deposited per cm2 per min, rather than % HS at a given deposition volume. Some of this data has been previously reported in the form of an abstract. (9–12)
Methods:
A full detail of the methods is provided in the Supplement.
HBE Cultures and Apical Mucus Content:
Primary HBE cells extracted from non-CF explanted lungs were maintained at an air-liquid interface (ALI) until fully differentiated (3–6 weeks). Because mucus is not cleared from airway surfaces in culture, airway surface mucus concentrations can be controlled by varying culture-washing intervals. Culture surfaces were washed daily to produce HBE cultures with small amounts of residual mucus that mimicked normal epithelia, whereas HBE preparations with the mucus layer left unwashed for two weeks were generated to produce mucus compositions more like CF airways in vivo. For studies of ENaC blockers, CF HBE cultures were used that were washed immediately prior to study to isolate Na+ transport effects from the effects of mucus concentration.
Confocal Measurements of ASL:
Primary HBE cells were labeled with 0.5 μl calcein-AM (Invitrogen) basolaterally. ASL was visualized by adding a small volume (5μl) of phosphate buffered saline (PBS) containing Texas Red Dextran (TRD) (Invitrogen, 70,000 MW) luminally. HS (7%) solution was aerosolized utilizing a vibrating mesh nebulizer (Aeroneb Lab; Aerogen), modified to deliver small volumes (nanoliters per minute) directly onto HBE surfaces. This system was mounted in an environmental chamber that regulated temperature to 37°C, humidity at >50%, and CO2 at 5%, interfaced to a scanning confocal microscope (SP5, Leica). The cell and ASL heights were measured simultaneously by high-speed X-Z confocal scanning. Following 2 minutes of baseline imaging, 7% HS was nebulized to deposit HS onto the apical surface of HBE cells at the prescribed rate and measurements were made serially at 30-second intervals.
Mathematical Modeling:
The airway fluid transport model of Warren et al. (13) was utilized to construct an integrative model of 3D human airway ion and fluid transport by Wu (14). This model solves an ordinary differential equation system for membrane potentials (Va, Vb), intracellular ion concentrations ([Na+]i, [Cl−]i, [K+]i), extracellular ion concentrations ([Na+]e, [Cl−]e, [K+]e), and extracellular and intracellular fluid volumes (We, Wi). For the HS simulations, water with a high concentration of NaCl was “added” to the apical surface for designated times, rates, and volumes.
Statistical Analysis:
All data analyses and graphing were performed in SigmaPlot (Systat). Mean values were compared via Student’s t-tests. Area under the curves (AUCs) of the ASL height data were calculated as an index of “hydration activity” of the nebulized solution.
Results:
The first series of studies focused on the effect of HS on HBE cultures with “normal” (i.e., non-CF) mucus concentrations (1.9 ± 1.7% solids) on apical surfaces [see Methods]. HS was delivered to HBE cultures for 15 minutes at a rate of 8μg NaCl/cm2/min (7% HS delivered at 110 nl/cm2/min) to mimic a standard jet nebulizer delivery rate for human subjects (see Supplement). A rapid and significant increase in ASL height was observed, reflecting the osmotic-driven transepithelial fluid flow in response to deposited HS (15) (Figure 1). Net reabsorption of the osmotically expanded ASL began immediately after nebulization was terminated, and ASL height returned to baseline height within 60 minutes after aerosol initiation.
Figure 1:
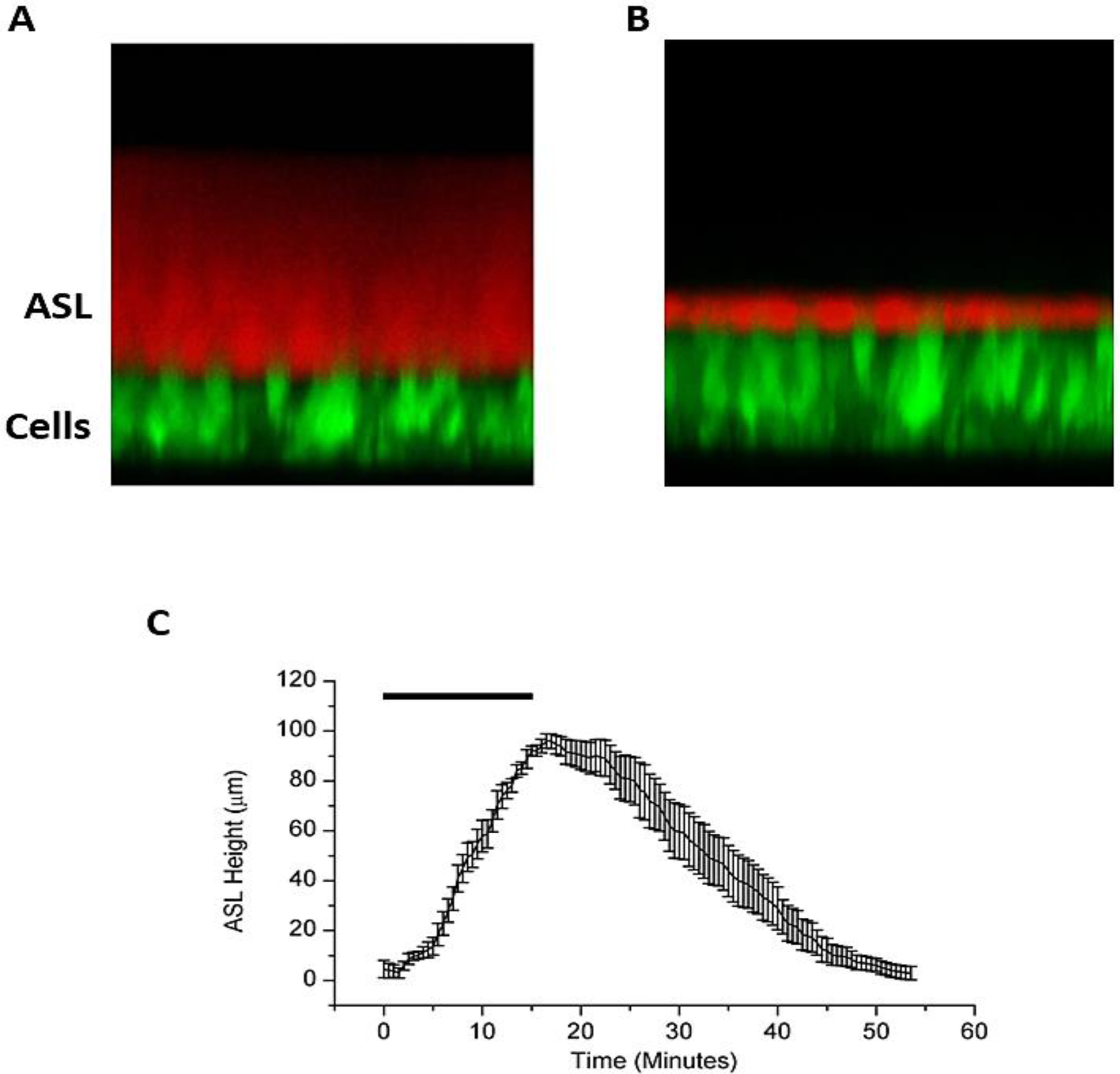
(A) XZ-confocal image of a cross section of airway epithelial cells stained with calcein, a fluorescent green dye, and ASL stained with Texas Red Dextran, a cell impermeable red dye. (B) After administration of an osmotic stimulus (HS), there is an increase in ASL height along with a decline in cell height. (C) HS aerosolized to the surface of HBE cultures results in a significant but transient increase in ASL height, lasting under an hour. N=8. Rectangle represents duration of HS administration (8μg NaCl/cm2/min for 15 min).
In clinical practice, jet nebulizers deliver HS in a typical 4ml dose in approximately 15 minutes, whereas vibrating mesh nebulizers deliver the same volume in about 5 minutes. To address whether HS delivery rates affect ASL volume responses, a constant total mass of salt was nebulized onto the HBE surface at varying nebulization rates and, hence, durations of nebulization. The rates selected for study were designed to a mimic delivery of 7% HS via jet nebulizer (8 μg NaCl/cm2/min), a vibrating mesh nebulizer (18 μg NaCl/cm2/min), and an arbitrarily defined “slow” nebulizer (3 μg/cm2/min) (Figure 2A). Both the 8 and 18 μg NaCl/cm2/min rates produced rapid and significant increases in ASL height. The response of cultures to the 3 μg NaCl/cm2/min rate was considerably smaller with respect to ASL volume expansion, but longer due to the extended nebulization duration. All cultures, irrespective of nebulization rate, rapidly absorbed the added volume from the ASL once nebulization was terminated.
Figure 2:
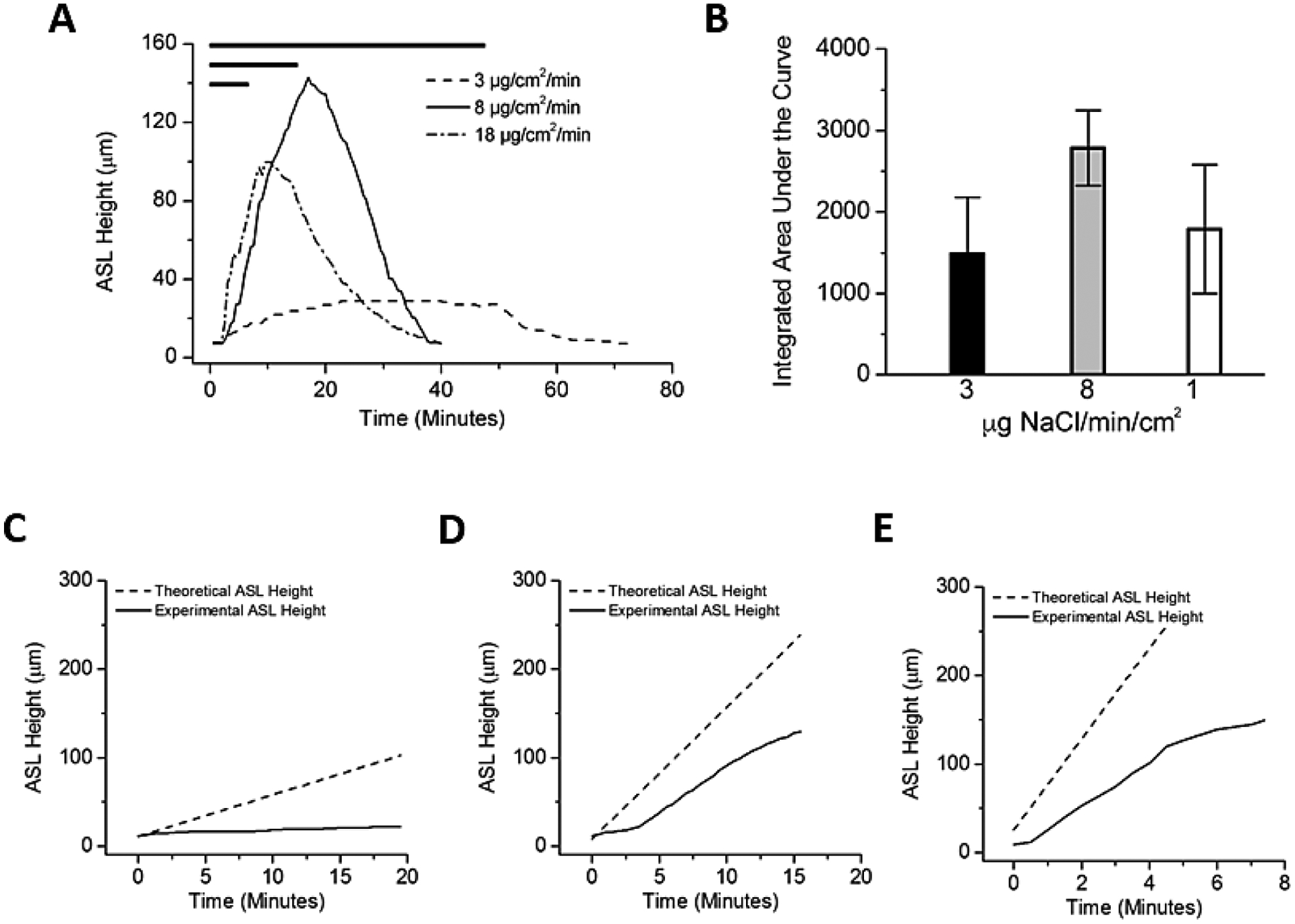
Effects of variation in HS delivery rates on ASL volumes responses. (A) Comparison of ASL heights achieved with three rates of nebulized HS (low, 3μg NaCl/cm2/min; moderate, 8μg NaCl/cm2/min; and high, 18 μg NaCl/cm2/min) with duration varied to hold constant total delivered mass of salt (demonstrated by length of bar at top). (B) Both absolute height change and total hydration (integrated area over time) favored the mid-range dosing regimen but did not reach statistical significance (A; P=0.388). (C,D,E) Dashed lines indicates the expected rise in ASL height if all salt deposited resulted in an equimolar flux of water into the ASL. Solid black lines indicate the actual change in ASL height observed during experiments. The failure of these two lines to overlay indicates an inhibition of the transport of water from the epithelial cells into the ASL. (C) Low dose nebulization (3μg NaCl/cm2/min). Theoretical slope 4.72 μm/min versus experimental slope of 0.45 μm/min, p<0.0001. (D) Medium dose nebulization (8μg NaCl/cm2/min). Theoretical slope 14.92 μm/min versus experimental slope of 8.66 μm/min, p=0.0002. (E) High dose nebulization (18μg NaCl/cm2/min). Theoretical slope 51.49 μm/min versus experimental slope of 20.78 μm/min, p=0.0004. N=4 per experimental condition.
The total airway surface hydration in response to HS administration was calculated as an AUC for each set of experiments. Interestingly, faster salt deposition (18 μg/cm2/min) produced a lower, though not statistically different, total ASL hydration compared to standard jet nebulizer rates (8 μg/cm2/min). Slower aerosol deposition (3 μg/cm2/min), while maintaining a longer period of time above baseline values of ASL height during nebulization, produced trends towards lower peak and AUC values compared to the faster rates (Figure 2B).
Assuming no active sodium-mediated volume absorption or change in apical membrane water permeability during aerosolization, deposition of a constantly accumulating mass of salt to the surface of airway epithelia is predicted to produce a linear increase in ASL volume. For all rates studied, the measured ASL height deviated immediately and consistently from the predicted height (Figures 2C–E). The deviation of measured ASL height from predicted values could reflect active Na+ absorption, reductions in HBE water permeabilities that govern rates of osmotically driven water flow to the HBE surface, or both.
With respect to the role of Na+ transport on ASL volume responses to HS administration, we speculated that Na+ transport would modify the magnitude of HS-induced ASL volume expansion immediately after initiation of HS administration. To experimentally investigate this possibility, ASL volume responses to the low rate of HS administration (3μg NaCl/cm2/min) were measured in the presence and absence of a selective ENaC blocker (VX-371, Vertex Pharmaceuticals). As compared to HS alone, the co-administration of VX-371 (50 μg/mL) produced a more rapid and sustained ASL response during nebulization (Figure 3A).
Figure 3:
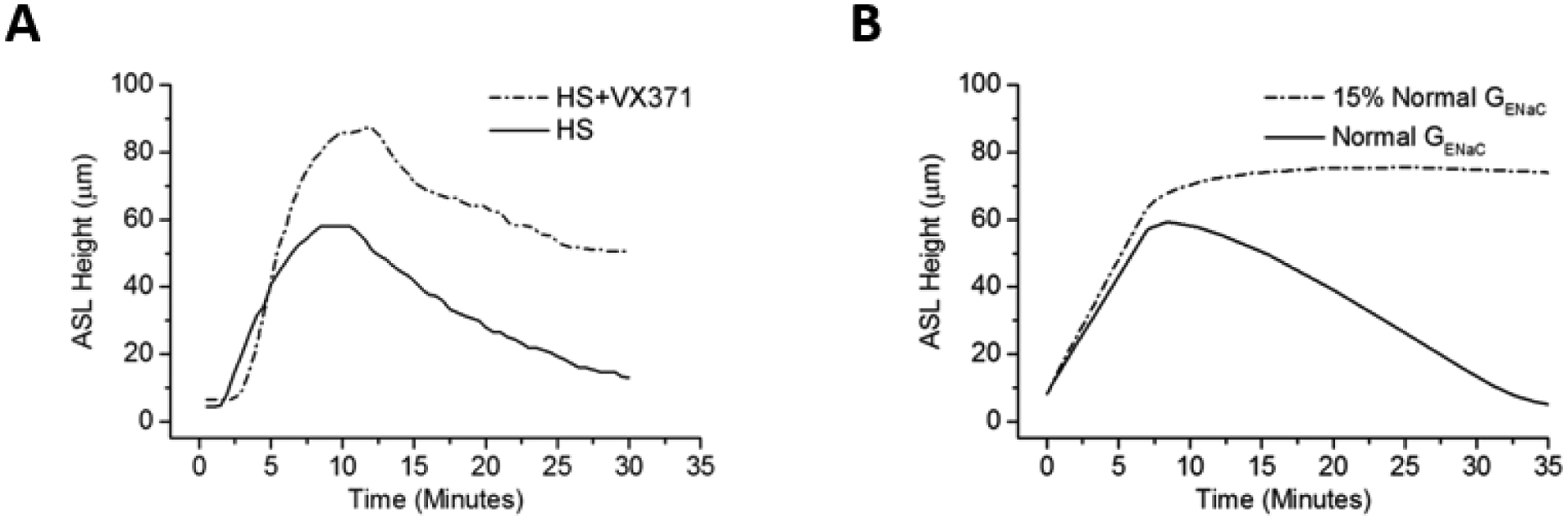
Effect of sodium reabsorption on HS-mediated ASL height. (A) Experimental data of hypertonic saline (at 8μg NaCl/cm2/min) in the absence and presence of a potent sodium channel blocker (VX-371 at 50 μg/ml). (B) Mathematical model predicting the results of HS effect with normal ENaC conductance (solid line, consistent with experimental data) and 15% of ENaC channel normal conductance (dashed line).
A mathematical model of airway epithelial ion and water transport was utilized to quantitatively analyze the relationship between HS-mediated changes to ASL height and active Na+ absorption. The effects on ASL volume of simulated aerosolized HS administration in both the presence and absence of ENaC blockade were both modeled (Figure 3B). The fit of the model to experimental data describing ASL response for HS administration in the absence of ENaC inhibition was achieved by maintaining Na+ transport at basal rates during HS aerosolization. The mathematical model also replicated the effect of ASL kinetics with ENaC inhibition, with a more rapid rate of accumulation and overshoot of ASL, consistent with block of active Na+ absorption during HS administration, and a slower rate of ASL absorption post cessation of HS administration (Figure 3A).
Previous studies have demonstrated that HS can produce a reduction in cell volume (16, 17), which can then inhibit cell water permeabilities (17–19). Accordingly, we measured HBE cell volume responses to HS aerosol administration to test whether reductions in cell water permeabilities blunt ASL responses to aerosolized HS. Figure 4 shows that cell height (a surrogate for cell volume) was reduced during and after HS nebulization by ~ 20% from initial cell heights.
Figure 4:
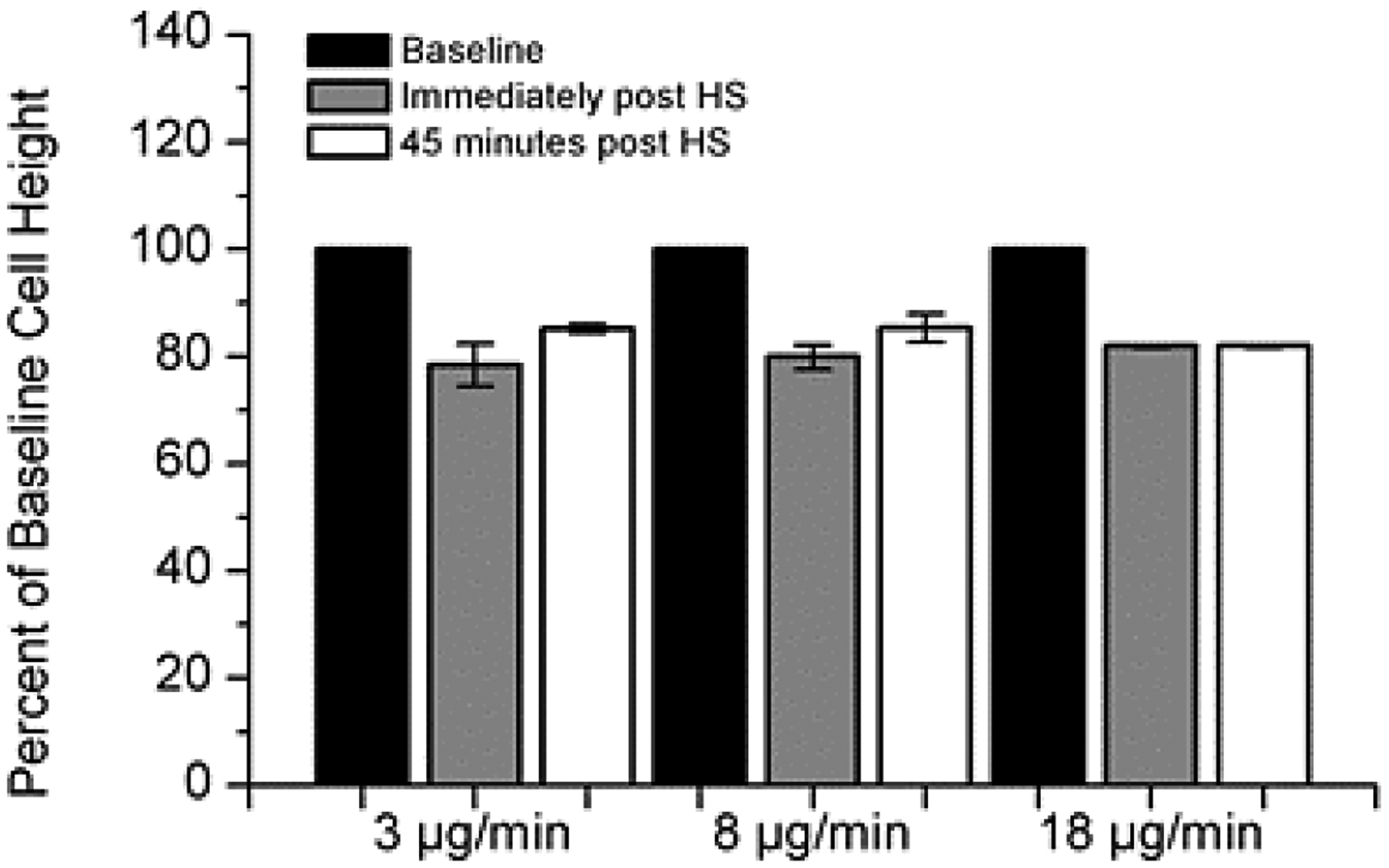
Cell volume changes in response to osmotic stimulus of HS at varying HS deposition rates. A 20% decrease in cell height in response to the hypertonic stimulus was observed at all rates. Cell height did not recover during the time frame of the experimentation (60 minutes). Cell volume reduction was a consistent finding regardless of rate of HS administration. (n=4 for each condition).
The contribution of cellular water permeability to ASL volume responses to aerosolized HS was investigated further using a pharmacologic approach. Because mercury-sensitive water channels (aquaporins; AQPs) have been identified in the apical and basolateral membranes of HBE epithelia (20, 21), the role of these channels on HS-mediated changes in ASL volume responses was evaluated. As shown in Figure 5A, both apical and basolateral HgCl2 administration significantly reduced ASL volume responses to aerosolized HS. Inhibition of apical AQPs resulted in a smaller decrease in cell height compared to basolateral AQP inhibition. These data suggest that in the setting of aerosolized HS, cell volume homeostasis is dominated by the apical water permeability (Figure 5B). The modeling studies that simulated HS-induced ASL responses basally and with selective apical versus basolateral AQP inhibition mimicked the experimental data (Figure 5C).
Figure 5:
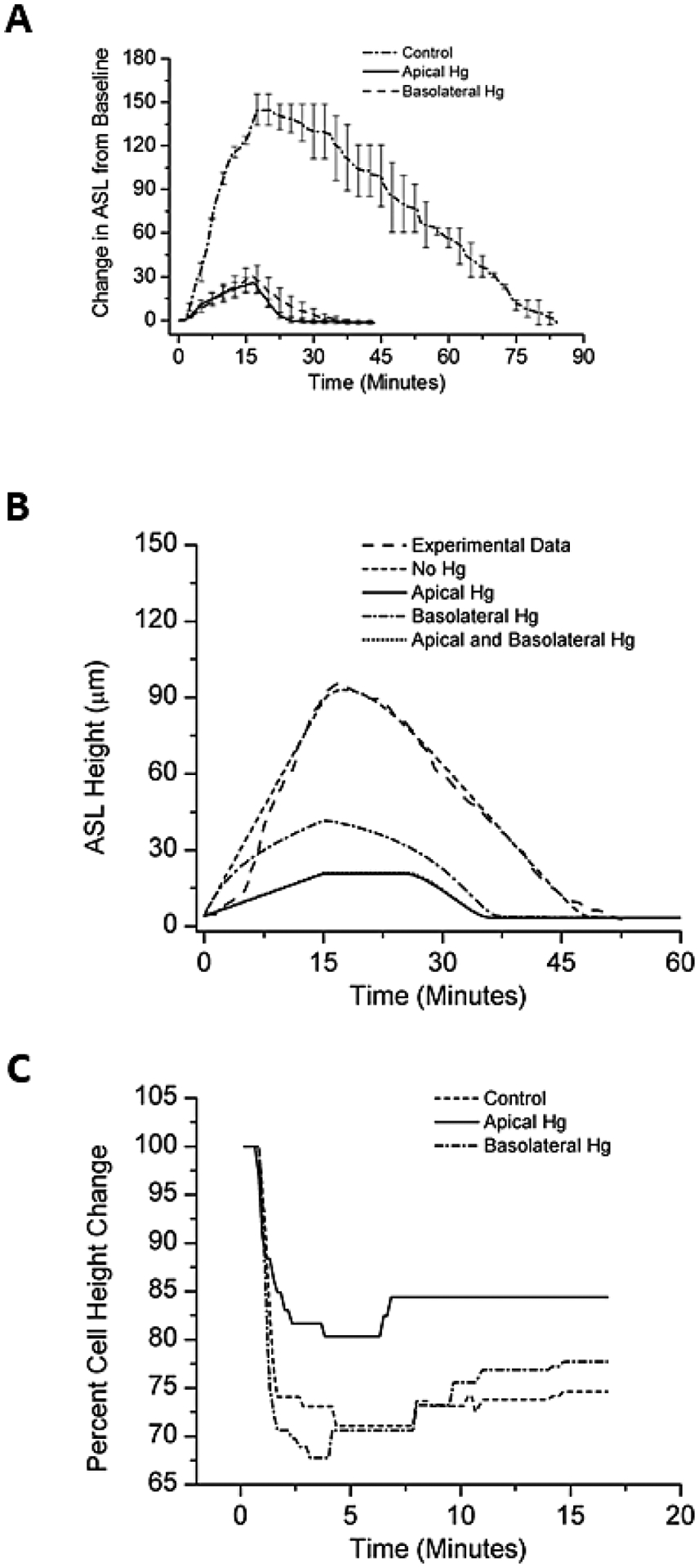
Incubation with mercury chloride apically or basolaterally resulted in a diminished ASL response to aerosolized HS (at 8μg NaCl/cm2/min) compared with native cultures. (A) Experimental data. (B) Mathematical model of experimental data. N=8 for each condition. (C) Cell height change to aerosolized HS in the absence (control) or presence of selective block of the apical or basolateral cell membrane with mercury chloride.
We hypothesized that persistent reduction in cell volumes observed after HS administration would limit the ability of HBEs to respond to repeated administrations of HS. This hypothesis was tested by exposing HBE cultures to sequential HS administrations (8 μg NaCl/cm2/min), with the second administration delivered 15 minutes after ASL height had returned to baseline following the first HS dose. ASL height and AUC of the second administration was significantly reduced compared to initial HS administration (Figure 6A).
Figure 6:
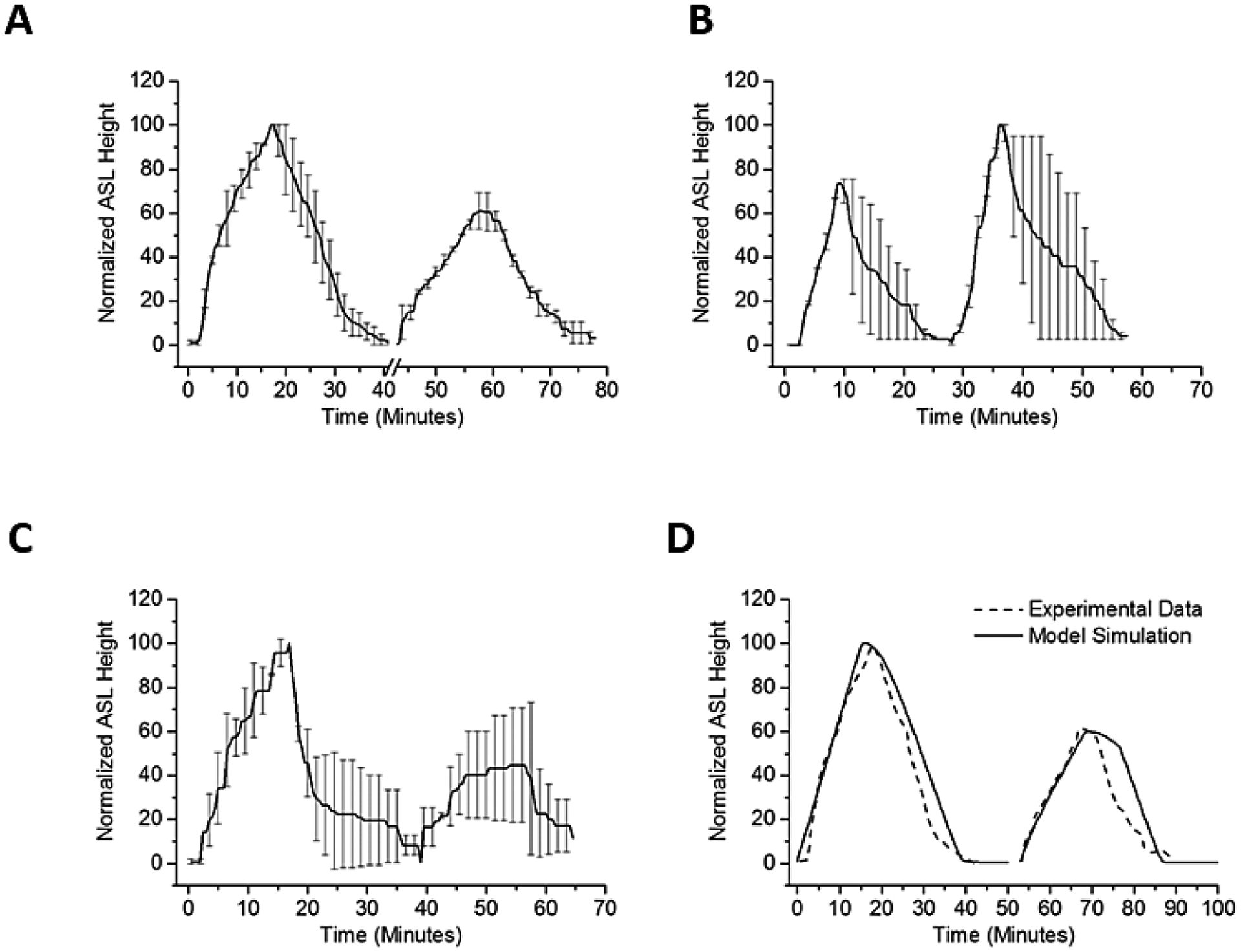
Sequential ASL volume responses to aerosolized 7% HS for 15 min (8μg NaCl/cm2/min). (A) Sequential ASL volume responses to aerosolized HS. A smaller ASL response to a second administration of aerosolized HS was observed when two identical HS doses were separated by 15 minutes. Break in x-axis indicate time between two doses. For each dose, HS was nebulized at 8 μg NaCl/cm2/min, administered continuously for 15 minutes. The ASL height of the second peak was about 60% of the first peak. (B) A hypotonic saline rinse was interposed between HS administration. The hypotonic solution administration produced cellular swelling associated with improved ASL volume responses to a subsequent administration of HS (~140% of first dose). (C) Interposition of an isotonic rinse between the first and second HS administration. The same second dose effect is not seen when doses of HS were separated by an isotonic saline bolus. ASL height of second peak persisted ~50% of the first peak. (D) In model simulation, the water permeability of the HBE apical membrane during the second dose was reduced to 10% of the basal water permeability. N=4 for each condition.
We next directly tested the hypothesis that the reduction in the effectiveness of the second HS administration reflected reduced HBE cell water permeabilities consequent with cell height (volume) reduction, rather than persistent acceleration of Na+ transport. To test this notion, a small volume of hypotonic saline (0.63% NaCl, 10 μl) was transiently added (3 minutes) to the apical surface of HBE’s following completion of the first aerosolized HS administration. Application of the hypotonic saline bolus resulted in cell swelling (increase in height 32 ± 9 μm, n=4), that restored cell height to baseline levels. The interposition of the hypotonic bolus was associated with a statistically larger response in ASL and AUC to the second HS administration (Figure 6B). In contrast, applying a bolus dose of isotonic saline (0.9% NaCl, 10 μl) produced neither cell swelling nor a recovery in responsiveness to the subsequent HS challenge (Figure 6C). Modeling this phenomenon predicted that reduction in apical water permeability to about 10% of baseline levels would produce the AUC decrement with the second HS administration observed experimentally (Figure 6D).
We next investigated whether the presence of hyperconcentrated mucus associated with CF airways (22) alters the kinetics of HS delivery. In these experiments, HBE cultures exhibiting hyperconcentrated mucus (12 ± 4.3% solids) were utilized as a mimic of the concentrated mucus observed in CF airways. Pre-HS administration, ASL height in the hyperconcentrated mucus cultures was ~ 3-fold higher than the normal mucus cultures, and the changes in ASL height in hyperconcentrated mucus cultures were subsequently increased with HS administration (Figure 7A). Although both normal and hyperconcentrated mucus cultures absorbed the increased ASL volume immediately after cessation of HS administration, the rate of reabsorption was significantly slower in hyperconcentrated versus normal mucus cultures (5.5 vs. 2.8 μm/min, p=0.01, Figure 7B). In the hyperconcentrated mucus cultures, the total duration of HS-induced ASL volume expansion was approximately double that of “normal” mucus cultures. Further, in repetitive HS administration protocols, the second administration of HS produced an ASL response similar to the first administration (p=0.988). Pertinent to this observation, cell heights in HBEs covered by hyperconcentrated mucus decreased less in response to HS (15 ± 4.7% vs. 23 ± 5.7%) than in normal cultures, though this result did not achieve statistical significance.
Figure 7:
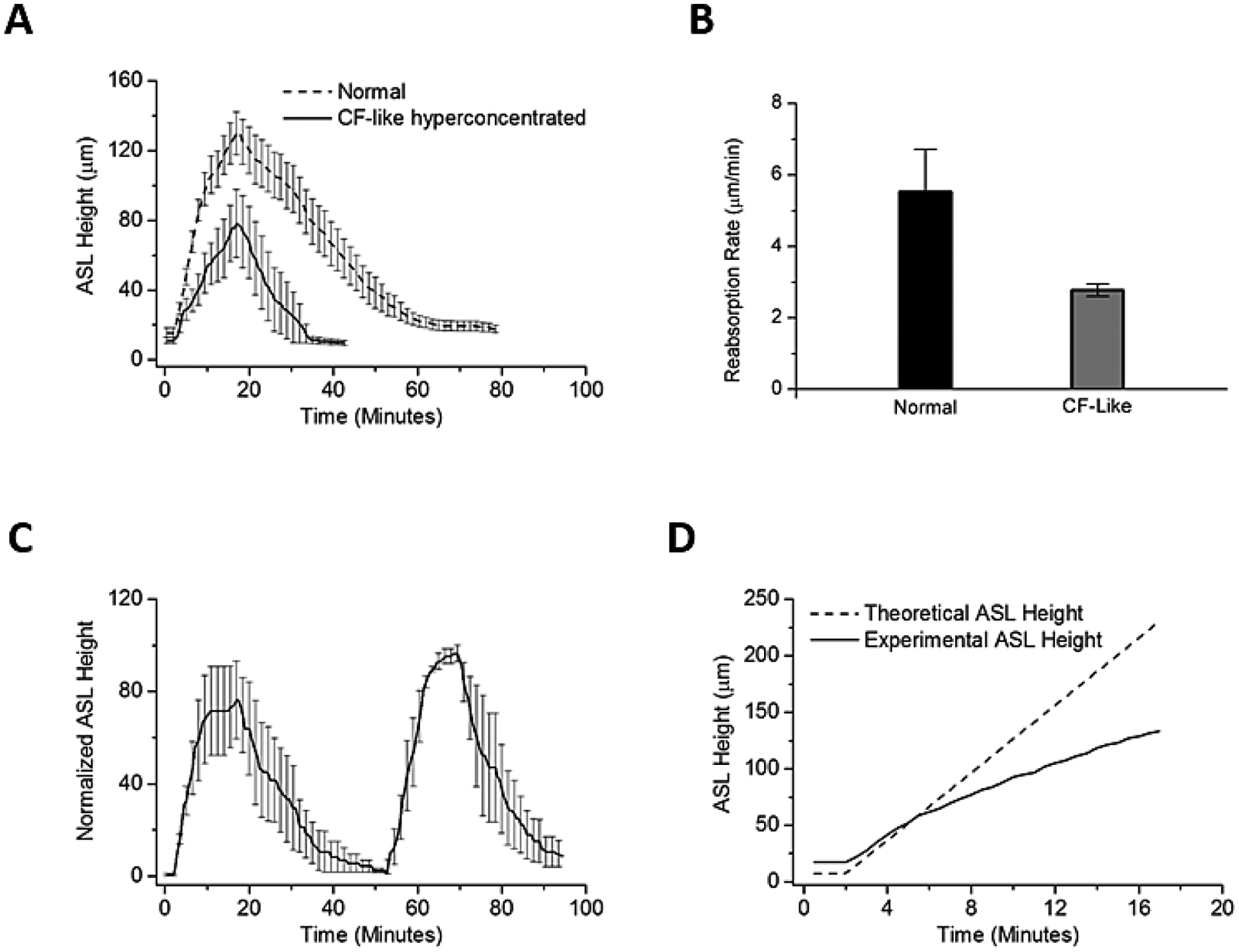
The effects of hyperconcentrated mucus on HBE surfaces on nebulized HS (at 8μg NaCl/cm2/min) in inducing ASL volume responses. (A) In the normal (2% mucus) state (solid line), there was a 3-fold increase in ASL volume during nebulization. HBE cultures with hyperconcentrated mucus (12%) (Dashed line) exhibited a 7-fold increase in ASL above baseline ASL. The duration of ASL being increased above basal levels was also increased in hyperconcentrated mucus HBE cultures. (B) Rates of reabsorption (time to baseline ASL, in μm/min) were significantly different between the normal and the CF-like hyperconcentrated mucus cultures. (C) Two sequential doses of HS administered to hyperconcentrated cultures. A similar response in ASL height was noted for the two HS administrations. (D) Dashed line indicates the expected rise in ASL height if all salt deposited remained on the surface and resulted in an equimolar flux of water into the ASL. Solid line indicates the actual change in ASL height observed during experiments. Compare to Figures 2C–E: In the presence of an intact mucus layer (12% solids), actual and expected ASL height more closely approximated each other early in the HS delivery interval. Theoretical slope 14.48 μm/min versus experimental 7.61 μm/min, p=0.03.
The larger peak ASL response and slower absorption of NaCl and water in the hyperconcentrated versus normal mucus cultures suggested an additional force was governing water flux in the hyperconcentrated mucus system. To generate an index of the magnitude of this effect, the predicted versus measured ASL heights during HS administration were compared (Figure 7D). The measured early administration values were significantly closer to the predicted value for hyperconcentrated compared with normal mucus cultures (see Figures 2C–E), suggesting that the presence of concentrated mucus generated additional mucus-related osmotic forces that retarded fluid absorption (22).
As a test of strategies independent of mucus concentration to extend the duration HS-mediated airway surface hydration, the effects of combined administration of HS with an ENaC blocker was tested. Mathematical modeling predicted that the addition of an ENaC blocker would greatly extend the duration of ASL expansion following HS administration to airway surfaces (Figure 8A). To test this, a sparse sampling ASL measurement technique was used to measure the ASL height on CF HBE after aerosolyzed HS (delivered at 8 μg NaCl/cm2/min) containing VX-371 (50 μg/ml) in cultures with normal (2%) mucus. As shown in Figure 8B, the effect of HS alone was so transient that it effectively had waned by the first sampling point at one hour (refer to Figure 1A). In contrast, HS+VX-371 generated a larger and more prolonged increase in ASL. Estimating hydrating activity from the AUC of ASL heights expansion over 8h, HS+VX-371 produced an ~8X increase in hydration over HS alone (Figure 8C).
Figure 8:
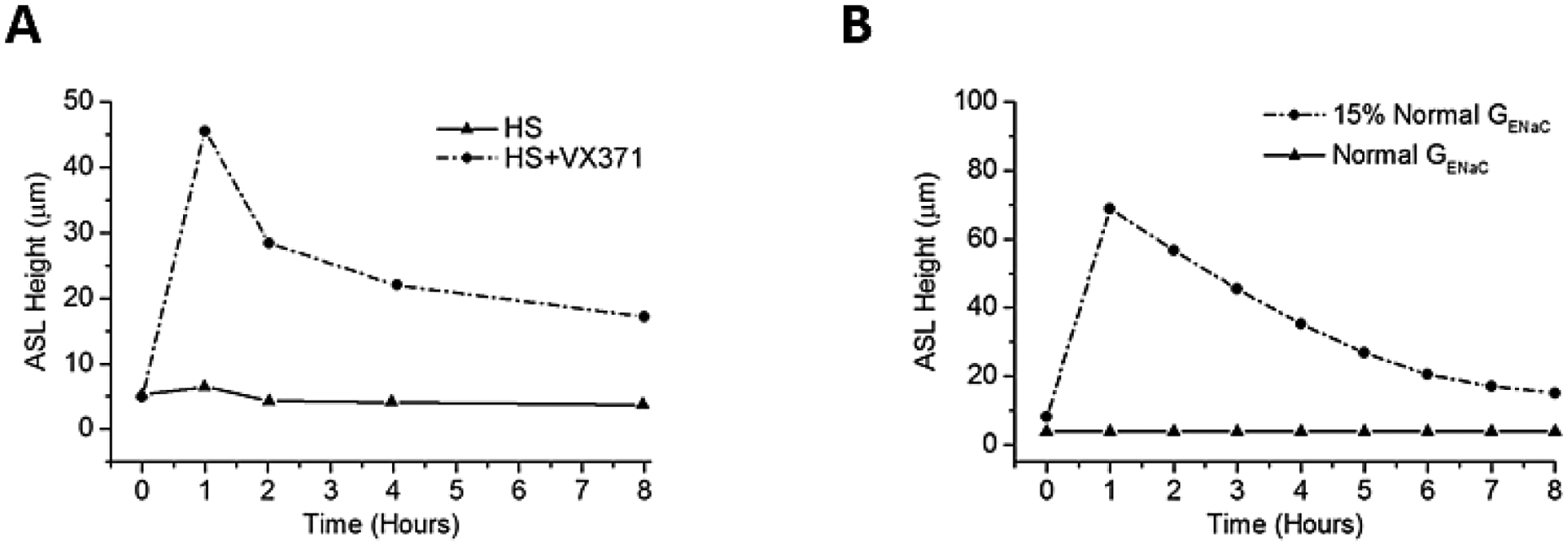
Increasing the durability of HS delivery. (A) Experimental data showing the effect of HS alone (8 μg NaCl/cm2/min) or in the presence of a long-term sodium channel blocker (VX-371) over 8 hours. (B) Mathmatical modeling shown the effect of inhibiting 85% of ENaC current (i.e. 15% residual activity) on ASL height over 8 hours. Note that the effect of HS alone was so transient that the increase in ASL height (seen at 30 minutes; refer to Figure 1B) was completed by the first sampling point at one hour.
Discussion:
The mechanism of action of HS in improving mucus clearance has been controversial. Some studies have suggested HS produces electrostatic effects on mucus rheology to improve clearance, whereas others have focused on salt-mediated ENaC inhibition (23), or dilution of mucus concentration by osmotically-driven ASL volume expansion. Our in vitro studies of clinically-relevant doses of aerosolized HS delivered to HBE cultures with “normal” 2% mucus solids revealed a rapid expansion of ASL volume. This ASL expansion in response to HS aerosols is similar to that reported in vivo in mice measured by synchrotron-based tomography (24). Further, acute aerosolization of HS has been reported to reduce airway mucus concentrations in COPD subjects (25). Thus, the ASL expansion findings, juxtaposed to our findings that the osmolarity of ASL during HS nebulization does not exceed 370 mOsm/L (see below), argues that the major effect of HS on mucus clearance is via ASL volume expansion and mucus dilution, not electrostatic interactions with mucus or chronic effects on ENaC.
The HS-induced ASL volume response was mediated by water flux in response to the deposition of osmotically active NaCI on HBE surfaces. Previous studies have demonstrated the contribution of aquaporins to airway epithelial water fluxes. Two observations suggest that aquaporin-mediated transepithelial water fluxes dominated the ASL response to aerosolized HS. First, the flux of water to the lumen in response to HS was accompanied by a reduction in cell height/volume, suggesting intracellular water moved into ASL. This notion is consistent with previous studies (17) that reported the aquaporin-dominated apical membrane water permeability of HBE was ~ 10 fold higher than the basolateral membrane. Note, this configuration that mediates loss of water from the cell in response to luminal HS allows use of cell volume changes as an “osmometer”. The cell volume responses to HS aerosols suggest that ASL achieved osmolalities of ~370mOsm during HS administration, far less than achieved by direct HS additions (7% HS given at 7.7 μg/NaCl/cm2/min)]. The second observation was that the ASL responses to aerosolized HS were blocked by mercury chloride, an inhibitor of AQP 3–5 known to be expressed in HBEs. These data, plus the cell modeling data quantitating the effects of cell water permeabilities on ASL volume responses, strongly suggest that aquaporin-mediated cellular water permeabilities participate in the ASL volume responses to aerosolized HS (Figure 5).
The effects of aerosolized HS on cell volume reduction and transepithelial Na+ transport likely explain the discrepancy between the predicted ASL responses to deposition of HS on HBE surfaces and measured responses (Figure 2). First, reductions in cell volume were associated with reductions in cell water permeabilities, limiting water fluxes toward a hypertonic lumen. Second, as evidenced by a greater maximal response to HS in the presence of an ENaC blocker, active Na+ transport removed a component of the deposited NaCI during aerosol administration. An interesting observation that emerged from the modeling of active Na+ transport responses to HS was that an increase in Na+ transport rates was required to mimic the rate of ASL volume absorption post cessation of aerosol. This effect likely reflects dilution of local extra-cellular inhibitors of ENaC, e.g. ATP, that accelerated the rate of absorption during HS administration (26).
A key issue with respect to clinical responses to HS relates to the concentration of mucus on airway surfaces. The presence of hyperconcentrated mucus on HBE surfaces was associated with increased ASL heights/volumes, longer duration of ASL hydration to a single HS administration, and larger responses to repetitive doses of HS. We postulate that mucus acts as a “sponge”, providing a concentration-dependent polymer gel-mediated osmotic driving force, added to that of HS-induced small molecule (salt) osmotic gradients, and modulates maximal mucus/ASL heights on the HBE surface. The increased durability of the ASL expansion in response to aerosolized HS in hyperconcentrated versus normal mucus cultures parallels the longer duration of action of HS in CF as compared to normal subjects observed in in vivo MCC studies (5, 27).
Our studies were also designed to identify strategies to increase the effectiveness of aerosolized HS to expand ASL hydration, including studies of HS delivery rates, repetitive dosing, and combined use with ENaC blockers. For example, very slow rates of HS delivery, below that of clinical nebulizers, were relatively ineffective. We speculate that this result reflects the fact that NaCl deposition rates were similar to rates of endogenous active transepithelial Na+ absorption (28). Delivery of HS at rates approximating a jet nebulizer were effective in increasing ASL hydration but a further increase in efficacy was not observed with faster rates mimicking vibrating mesh nebulizers. The absolute decrease in AUC and the slowing of ASL volume expansion observed towards the end of rapid HS administration (18μg NaCl/cm2/min) were consistent with large reductions in transepithelial water permeabilities.
An important observation pertinent to HS dosing frequency was that HBE cell heights did not return to baseline levels for more than 4 hours following HS administration. We speculate that the absence of cell volume regulation mechanisms reflected decreased water permeability. The delay in return of apical water permeability after HS likely accounted for the blunted response to second HS administrations on HBE cultures with normal mucus (Figure 6). Importantly, epithelial cell swelling induced by application of hypotonic saline to the apical HBE surface restored second HS responses to those similar to the first HS administration. These data suggest that novel strategies may be required to optimize the effectiveness of repetitive HS dosing in patients with milder lung disease. In contrast, repetitive dosing in subjects with severe (12% mucus solids) disease may be an effective strategy.
Finally, consistent with model predictions, the addition of an ENaC blocker to HS increased both the initial magnitude as well as the duration of the ASL response to HS aerosols. HS versus HS/ENaC blocker regimens are now in clinical testing to assess whether in vitro ASL models predict clinical efficacy.
In conclusion, the ASL of HBE cultures with normal mucus concentrations increased in response to nebulized HS delivered at rates similar to those delivered by jet nebulizers, but reabsorption began immediately with termination of nebulization. Nebulizing at rates faster than those delivered by jet nebulizers did not improve ASL hydration, nor did nebulization at very low rates for prolonged periods of time. The addition of an ENaC blocker, however, prolonged ASL hydration responses to HS. Importantly, the effects of aerosolized HS were more pronounced and prolonged in HBE cultures with CF-like hyper-concentrated mucus, likely reflecting the increased gel-based osmotic forces generated by concentrated mucus. These data predict greater efficacy and a prolonged duration of HS action in CF patients with hyper-concentrated mucus typical of more severe lung disease.
Supplementary Material
What this study adds to the field:
Hypertonic saline is increasingly being used in mucoobstructive diseases, including cystic fibrosis, COPD, and bronchiolitis. This study provides insight into the effect of hypertonic saline on airway epithelial cells in the setting of normal mucus as well as hyperconcentrated mucus. These results may help guide appropriate patient selection for this osmotic therapy.
Acknowledgements:
The authors would like to acknowledge the UNC Tissue Culture Core (NIH P30 DK0655988 and CFF RDP BOUCHE15R0). This project was funded by the CFF Leroy Matthews Physician Scientist Award (CFF GORALS12LO) as well as BUTTON07XX0, NIH R01HL125280-01A1, and NIH P30DK065988-11.
Abbreviation List
- ALI
air-liquid interface
- AQP
aquaporin
- ASL
airway surface liquid
- AUC
area under the ASL height curve
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CO2
carbon dioxide
- ENaC
epithelial sodium channel
- FEV1
forced expiratory volume in 1 second
- HBE
human bronchial epithelium
- HS
hypertonic saline
- MCC
mucociliary clearance
- NaCl
sodium chloride
- PBS
phosphate buffered saline
- TRD
Texas Red Dextran
- UNC
University of North Carolina at Chapel Hill
Footnotes
Descriptor number: 9.16 Cystic Fibrosis: Basic Studies
References:
- 1.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261(1):5–16. [DOI] [PubMed] [Google Scholar]
- 2.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337(6097):937–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsui H, Verghese MW, Kesimer M, Schwab UE, Randell SH, Sheehan JK, et al. Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J Immunol. 2005;175(2):1090–9. [DOI] [PubMed] [Google Scholar]
- 4.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109(3):317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354(3):241–50. [DOI] [PubMed] [Google Scholar]
- 6.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354(3):229–40. [DOI] [PubMed] [Google Scholar]
- 7.Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126(3):879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarran R, Grubb BR, Parsons D, Picher M, Hirsh AJ, Davis CW, et al. The CF salt controversy: in vivo observations and therapeutic approaches. Mol Cell. 2001;8(1):149–58. [DOI] [PubMed] [Google Scholar]
- 9.Goralski JLaB B Mucus Concentration Affects HBE Response to Nebulized Hypertonic Saline. Pediatric Pulmonology. 2014;38:182. [Google Scholar]
- 10.Goralski JLaB B Nebulized Hypertonic Saline Yields Disparate ASL Heights with Sequential Doses. Pediatric Pulmonology. 2013;36:107. [Google Scholar]
- 11.Goralski JLaB B CF Cells do not Hyperabsorb Sodium in the Setting of Nebulized Hypertonic Saline. Pediatric Pulmonology. 2012;34:105. [Google Scholar]
- 12.Goralski JLaB B An In Vitro Study of the Kinetics of Hypertonic Saline on ASL Height. Pediatric Pulmonology. 2010;33:205. [Google Scholar]
- 13.Warren NJ, Tawhai MH, Crampin EJ. A mathematical model of calcium-induced fluid secretion in airway epithelium. J Theor Biol. 2009;259(4):837–49. [DOI] [PubMed] [Google Scholar]
- 14.Wu D, Miyawaki S, Tawhai MH, Hoffman EA, Lin CL. A Numerical Study of Water Loss Rate Distributions in MDCT-Based Human Airway Models. Ann Biomed Eng. 2015;43(11):2708–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackmon RL, Kreda SM, Sears PR, Chapman BS, Hill DB, Tracy JB, et al. Direct monitoring of pulmonary disease treatment biomarkers using plasmonic gold nanorods with diffusion-sensitive OCT. Nanoscale. 2017;9(15):4907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischbarg J Fluid transport across leaky epithelia: central role of the tight junction and supporting role of aquaporins. Physiol Rev. 2010;90(4):1271–90. [DOI] [PubMed] [Google Scholar]
- 17.Willumsen NJ, Davis CW, Boucher RC. Selective response of human airway epithelia to luminal but not serosal solution hypertonicity. Possible role for proximal airway epithelia as an osmolality transducer. J Clin Invest. 1994;94(2):779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasgado-Flores H, Krishna Mandava V, Siman H, Van Driessche W, Pilewski JM, Randell SH, et al. Effect of apical hyperosmotic sodium challenge and amiloride on sodium transport in human bronchial epithelial cells from cystic fibrosis donors. Am J Physiol Cell Physiol. 2013;305(11):C1114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herschlag G, Garcia GJ, Button B, Tarran R, Lindley B, Reinhardt B, et al. A mechanochemical model for auto-regulation of lung airway surface layer volume. J Theor Biol. 2013;325:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yukutake Y, Tsuji S, Hirano Y, Adachi T, Takahashi T, Fujihara K, et al. Mercury chloride decreases the water permeability of aquaporin-4-reconstituted proteoliposomes. Biol Cell. 2008;100(6):355–63. [DOI] [PubMed] [Google Scholar]
- 21.Kuwahara M, Gu Y, Ishibashi K, Marumo F, Sasaki S. Mercury-sensitive residues and pore site in AQP3 water channel. Biochemistry. 1997;36(46):13973–8. [DOI] [PubMed] [Google Scholar]
- 22.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124(7):3047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Astrand AB, Hemmerling M, Root J, Wingren C, Pesic J, Johansson E, et al. Linking increased airway hydration, ciliary beating, and mucociliary clearance through ENaC inhibition. Am J Physiol Lung Cell Mol Physiol. 2015;308(1):L22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan KS, Donnelley M, Farrow N, Fouras A, Yagi N, Suzuki Y, et al. In vivo X-ray imaging reveals improved airway surface hydration after a therapy designed for cystic fibrosis. American journal of respiratory and critical care medicine. 2014;190(4):469–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson MAG, Bennett PWD, Zeman PKL, Wu PJ, Gladman MC, Fuller RF, et al. Effects of Inhaled Hypertonic Saline on Mucociliary Clearance and Clinical Outcomes in Patients with Chronic Bronchitis. 2017. [Google Scholar]
- 26.Sandefur CI, Boucher RC, Elston TC. Mathematical model reveals role of nucleotide signaling in airway surface liquid homeostasis and its dysregulation in cystic fibrosis. Proc Natl Acad Sci U S A. 2017;114(35):E7272–E81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett WD, Wu J, Fuller F, Balcazar JR, Zeman KL, Duckworth H, et al. Duration of action of hypertonic saline on mucociliary clearance in the normal lung. J Appl Physiol (1985). 2015;118(12):1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Locke LW, Myerburg MM, Markovetz MR, Parker RS, Weber L, Czachowski MR, et al. Quantitative imaging of airway liquid absorption in cystic fibrosis. Eur Respir J. 2014;44(3):675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, et al. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. American journal of respiratory and critical care medicine. 2015;192(2):182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsui H, Wagner VE, Hill DB, Schwab UE, Rogers TD, Button B, et al. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci U S A. 2006;103(48):18131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


