Abstract
Apamin-sensitive small-conductance calcium-activated potassium (SK) current (IKAS) plays an important role in cardiac repolarization under a variety of physiological and pathological conditions. The regulation of cardiac IKAS relies on SK channel expression, intracellular Ca2+ and interaction between SK channel and intracellular Ca2+. IKAS activation participates in multiple types of arrhythmias, including atrial fibrillation, ventricular tachyarrhythmias and automaticity and conduction abnormality. Recently, sex dimorphisms in autonomic control have been noticed in IKAS activation, resulting in sex differentiated action potential morphology and arrhythmogenesis. This review provides an update on the Ca2+-dependent regulation of cardiac IKAS and the role of IKAS on arrhythmias, with a special focus on sex differences in IKAS activation. We propose that sex dimorphism in autonomic control of IKAS may play a role in J wave syndrome.
Keywords: sex difference, sex dimorphism, J wave syndrome, Brugada syndrome, early repolarization, ion channel, autonomic control
1. Introduction
K+ currents, such as transient outward K+ current (Ito), inward rectifier K+ current (IK1), ultrarapid delayed rectifier K+ current (IKur) and slow component of the delayed rectifier K+ current (IKs), seem to be significantly, although not necessarily directly, modulated by intracellular Ca2+ concentration ([Ca2+]i) [29]. The importance of Ca2+ in modulating neuronal and cardiac potassium currents have been intensively studied since at least the 1980s [34,23,46]. Kohler et al [45] first identified the small conductance Ca2+-activated K+ (SK) channel in the brain and found it to be responsible for afterhyperpolarization of the neurons. The same channel was later found to be important in cardiac repolarization in animal models and in humans [97]. Unlike voltage-gated and other ligand-gated K+ channels, the SK channel is gated solely by changes in intracellular Ca2+ [1,96]. Hence, intracellular Ca2+ provides a direct and critical link between SK channel and the forming of functional SK current [1,96]. Study of SK channel is facilitated by the use of apamin, an active neurotoxin discovered more than 70 years ago [38]. Apamin is a highly specific SK channel inhibitor in both neuronal and cardiac tissues [38,104]. The 3 subtypes of SK channels (SK1, SK2 and SK3) exhibit differential sensitivity to apamin[45,30]. Among them, SK2 is most sensitive while SK1 is least sensitive and may not be blocked by up to 100 nM of apamin [38]. Because not all SK currents are sensitive to apamin, our laboratory generally used the term apamin-sensitive SK current (IKAS) to describe the current we were studying. All 3 isoforms of SK channels are expressed in animal and human hearts, and are capable of forming homomeric or heteromeric channels [45,84,62,91,98]. Other agents, including Ikas blocker and positive and negative modulators, are also widely adopted to investigate the effects of IKAS inhibition or activation on cardiac electrophysiology and arrhythmias.
2. Regulation of cardiac IKAS
The regulation of cardiac IKAS relies on (1) SK protein expression, (2) intracellular Ca2+ concentration and (3) interaction between Ca2+ and SK channel. This review attempts to provide an overview of the literature on the 3 aspects of cardiac IKAS regulation and the role of IKAS in arrhythmogenesis. In addition, we focus on the sex differences of IKAS activation in multiple experimental conditions.
2.1. SK channel expression regulates IKAS
The SK channels are not uniformly distributed throughout the hearts, with higher mRNA and protein expression levels in atria than ventricles in both animals and humans [91,98]. One exception is equal mRNA levels were reported between chambers in horses [33]. Both patch clamp at cellular level and optical mapping in whole hearts found prominent blocking effects of apamin in atria which were functionally dormant in normal ventricles, indicating more pronounced IKAS in atria than ventricles [91,98,10,13]. In addition, SK channel expression and corresponding IKAS are abundant in cells with automaticity, such as sinoatrial node [11,87], atrioventricular node [111], Purkinje networks [72,93] and pulmonary veins [70,11,68]. The abundant expression of SK channel in Purkinje cells may mediate the increased intracellular Ca2+-membrane potential coupling gain in Purkinje cells [59,72]. In accordance with low expression of SK channels in ventricles of normal hearts, IKAS is minimally activated at baseline condition that apamin prolongs APD by less than 5% [10,13]. However, ventricular IKAS can still be activated at slow heart rate and by pharmacologic interventions, including IKAS or ICa,L activators and β-adrenergic stimulation, suggesting the capability of SK channel forming functional IKAS in ventricles of normal hearts [6,10,9]. Of note, our previous study found higher SK2 protein expressions in ventricles of female than male rabbits [10]. Whether or not sex differentiated expressions of SK channels are present in other species remain unclear.
Multiple pathological conditions, such as atrial fibrillation (AF) and heart failure (HF), might alter the expression of SK channels, contributing to the regulation of IKAS. In a rabbit model of short-term atrial burst pacing, SK2 mRNA, protein and corresponding IKAS were increased in the pulmonary veins, involving in the early electrical remodeling [68]. In contrast, mRNA and proteins of SK channels were downregulated in patients with chronic AF, suggesting an opposite regulatory direction in the chronic remodeling process [51,108,81,27]. In streptozotocin-induced Type 1 diabetic mice, the expression of both SK2 and SK3 were reduced in atrium [101]. However, SK2 expression in atria was downregulated whereas SK3 was upregulated in a Type 2 diabetic mice model [53]. While the downregulation of SK channels led to decreased IKAS in most AF studies [51,81,108], intriguingly increase of IKAS could be possible via the enhanced Ca2+ and Ca2+-SK interaction [27]. On the other hand, upregulation of SK channels expression was commonly observed in HF. In patients with end-stage HF, SK2 protein expression was upregulated in ventricles and SK3 protein also exhibited an increasing tendency [7,3]. In canine HF ventricles, SK3 channel was significantly upregulated while SK2 channel showed a tendency towards increased expression, albeit not significantly [3]. In a rat HF model, mRNA and protein expressions of SK1 and SK3 channels were upregulated while SK2 expressions remained similar with normal controls [65]. In contrast, SK2 mRNA levels were similar between failing and normal rabbit ventricles [13].
In addition to the change of total expression, SK channel expression may develop heightened heterogeneity, thus predisposing transmural and regional differentiated regulation of IKAS. In failing human ventricles, IKAS exhibited higher density in epicardium and endocardium compared with midmyocardium, with some hearts exhibiting M-cell islands [7,106]. The transmural gradient of IKAS was attributed, at least in part, to heterogeneous SK channel expression. The regional differences of SK channel expression were also reported. In chronically paced but not failing rabbit hearts, mRNA levels of SK2 and SK3 channel were significantly higher at the distal compared to the proximal pacing sites [102]. In normal rabbit ventricles, the CyPPA-induced activation of IKAS exhibited more pronounced heterogeneity in right than left ventricles, suggesting a more subtle regional differentiated expression [9]. In contrast, the mRNA and protein expressions of 3 subtypes of SK channels did not alter in animal models of acute or chronic myocardial infarction, suggesting the contributions of SK channel expressions on IKAS upregulation are limited [31]. Other factors, such as Ca2+ and Ca2+-SK interaction, might be important.
SK channels were not only expressed in the plasma membrane, but also were recently discovered in the inner mitochondrial membrane of cardiomyocytes [83,41]. The activation of mitochondrial SK channels attenuated Ca2+-dependent arrhythmia in hypertrophic hearts by reducing the mito-ROS-dependent oxidation of RyR2 channels [41]. Therefore, by contributing to intracellular Ca2+ homeostasis, mitochondrial SK channels might affect IKAS activation on the plasma membrane indirectly. Nevertheless, only channels expressing on the membrane surface of cardiomyocytes were capable to form IKAS directly, consequently shaping action potential and affecting arrhythmogenesis. In rabbit pulmonary vein, 3-hour intermittent burst pacing, which mimicking ectopic pulmonary vein foci, induced SK2 channel trafficking from perinucleus to the membrane, leading to increased IKAS [68]. The trafficking of SK channel to membrane was regulated by multiple factors, in a Ca2+-dependent or independent ways. Filamin A, α-actinin2 and junctophilin type 2 (JP2) were necessary for the Ca2+-dependent trafficking of SK2 channels in atrial myocytes [114,26,71], whereas vesicle-associated membrane protein type 2 (VAMP2)-mediated membranization of SK2 channel was Ca2+ independent [50]. While the binding with wildtype calmodulin (CaM) showed normal trafficking process, SK3 channel binding with CaMN541, a catecholaminergic polymorphic ventricular tachycardia associated variant, showed increased retention in the intracellular compartments, thus leading to decreased IKAS [74]. In addition, SK membrane localization might be modulated by its crosstalk with L-type calcium channel (LTCC). Ablation of Cav1.3 channels resulted in increased membrane localization of SK2 channels [54].
In rat, rabbit, dog and human, ventricular myocytes from failing hearts showed transmurally heterogeneously upregulation of IKAS densities compared with ventricular myocytes from normal hearts [3,13,7,65]. Similar Ikas upregulation was demonstrated in infarcted ventricles of human and animal model [7,31,48] and protect the heart against ischemia-reperfusion injury [83]. Ventricular pacing results in Ca2+ elevation in late activation sites [39], which in turn upregulates Ikas to shorten the action potential duration (APD). This sequence of events predisposes to the development of the short-term cardiac memory in paced ventricles [5]. IKAS upregulation was also observed in chronically paced rabbit ventricles, contributing to the formation of long-term cardiac memory [102].
2.2. Intracellular Ca2+ regulates Ikas
SK channels were gated solely by changes in intracellular Ca2+, hence, intracellular Ca2+ provided a direct link between SK channel and the forming of functional IKAS [1,96]. During the Ca2+ induced Ca2+ release (CICR) process, the Ca2+ influx through LTCCs raises the local concentration from 0.1 to 10 μM, and subsequent Ca2+ release through RyR2 further increased the cleft Ca2+ concentration to ~100 μM, even though the global intracellular Ca2+ concentration only reached ~1 μM. SK channels were very sensitive to local subsarcolemmal Ca2+ and were activated by submicromolar concentrations of intracellular Ca2+ with apparent Kd of ~0.5 μM. Therefore, the sequential activation of LTCC and RyR2 regulated the activation of IKAS. Cardiac SK2 channels coupled with LTCC through a physical bridge, α-actinin2 [55]. The activation of IKAS were critically dependent on the normal expression of LTCC in atrial myocytes, that null deletion of Cav1.3 channel resulted in reduced IKAS, leading to APD prolongation and atrial arrhythmias [55]. BayK 8644, which increased influx of Ca2+ through LTCC, activated dormant IKAS in normal ventricles [10]. On the other hand, the necessity and sufficiency of RyR2-mediated SR Ca2+ release in IKAS activation has been established in SK2-overexpressed rat ventricular myocytes [86]. Inhibition or knockdown of RyR2 or depletion of SR Ca2+ store significantly reduced IKAS in mouse atrial myocytes [63]. Further studies suggest both LTCC and RyR2 were critical in IKAS activation. β-adrenergic receptor stimulation by isoproterenol, which increased both ICa,L and RyR2-mediated Ca2+ releases, prominently activated IKAS in normal ventricles [10]. The super-resolution imaging revealed the physical distances of LTCCs and RyR2 from SK2 channels were within hundreds of nanometers in cardiomyocytes [112]. The spatial proximity of the 3 molecules enabled the optimal IKAS activation by precise control of the SK channel gating on the beat-to-beat basis by integrating the local Ca2+ signaling. Other [Ca2+]i regulator, such as sodium-calcium exchanger (NCX), SR Ca2+-ATPase, sarcolemmal Ca2+-ATPase and mitochondrial Ca2+ uniporter, may also exert effects on IKAS indirectly. For instance, NCX knockout mice, a model of cellular Ca2+ overload, exhibited higher IKAS than wildtype mice in sinoatrial (SA) node [87].
The remodeling of LTCC properties may contribute to altered dynamics of IKAS under pathological conditions. During AF, the expression of LTCCs and current density of ICa,L were reduced [16]. AF was also associated with increased RyR2 diastolic leak, increased sarcoplasmic/endoplasmic reticulum Ca2+ ATPase function and t-tubule loss [16]. Such Ca2+ dynamics, together with SK channel downregulation, led to reduced peak IKAS density during atrial repolarization, but activation during late repolarization phases and diastolic afterdepolarization. On the other hand, T-tubule loss, LTCC dislocation and the impaired communication between LTCC and RyR2 were found in HF. However, constitutive phosphorylation of LTCCs by cytoplasmic CaMKII led to an increased LTCC open probability, predisposing spontaneous secondary [Ca2+]i rising during repolarization phases of AP [6,75]. Under such circumstances, Ikas might be amplified by elevated microdomain [Ca2+]i and during the secondary [Ca2+]i rising [6]. Together with upregulated SK channel expression and increased sensitivity to [Ca2+]i, those three factors contributed to the augmented IKAS in ventricles during HF [7,13]. Other conditions causing [Ca2+]i overload also elicited IKAS activation, such as hypokalemia and slow ventricular rate [5]. The effects of apamin on APD prolongation and CaiTD-APD shortening were more prominent at slow ventricular rate in both normal and failing ventricles[5,106,6], suggesting a more profound activation of IKAS as longer diastolic intervals allowing persistent Ca2+ inflow. Similarly, ventricular IKAS was prominently activated at sites remote from the pacing site where abundant cytosolic Ca2+ accumulation, which attenuated APD prolongation and maintained repolarization reserve [5].
The assemble of SK channels might also affect its binding to LTCC, which consequently affects IKAS. A previous study proposed that homomeric SK channels did not contribute to the IKAS activation because the homomeric SK channels were not within a suitable microdomain containing LTCC [32]. Only SK2-SK3 heteromultimers were activated, which was likely the result of the colocalization of LTCC and heteromeric SK channels within microdomains [90,55]. SK channels preferentially co-assembled to form heteromeric channels and heteromeric channels might predominate in cells expressing multiple SK channel subunits [14].
On the other hand, the effects of Ikas activation on action potential provided a feedback mechanism in driving Ca2+ transient, leading to negative coupling between Ca2+ and membrane potentials [112]. For instance, ICa,L and subsequent CICR process were amplified during isoproterenol infusion, which activated IKAS at early repolarization phases where Cai was more abundant [10]. IKAS activation reversely shortened APD more prominently at the beats with large Cai transient and at phase 2 repolarization, leading to AP triangulation and negative Cai-Vm coupling [10]. Such IKAS mediated negative coupling were also observed during hypothermia [9]. Low body temperature decreased peak ICa,L, reduced Ca2+-dependent ICa,L inactivation and prolonged Cai transient, which in turn increased sarcoplasmic Ca2+ stores and release. The differentiated IKAS activation between larger and small Ca transients and between proximal and distal sites from pacing site led to spatially and electromechanically discordant alternans [9]. IKAS as a negative coupler between intracellular Ca2+ to membrane voltage was further verified by in silico modeling [40].
2.3. Sensitivity of SK channel to Ca2+ regulates Ikas
The sensitivity of SK channel to Ca2+ also contributed to IKAS modulation. During heart failure, in addition to upregulation of SK channel expression, the sensitivity of IKAS to intracellular Ca2+ was also increased, leading to increased IKAS [13,65]. In contrast, the steady-state Ca2+ response of IKAS was shifted rightwards in failing myocytes treated with β-blocker compared with nontreated myocytes, contributing to downregulated IKAS [65].
The affinity of SK channels to Ca2+ was modulated by multiple cofactors. SK channels were activated exclusively by Ca2+-bound calmodulin (CaM). Arrhythmogenic CaM variants causing LQTS and CPVT, including CaMN54I, CaMD96V, CaMD130G, and CaMF142L, significantly down-regulated IKAS mediated by SK3 [74]. Phosphorylation of CaM, when complexed with the channel, casein kinase 2 (CK2) inhibited SK channels, while protein phosphatase 2A (PP2A) reversed the effect of CK2 [110]. During heart failure, the interaction between casein kinase 2 (CK2) and SK channel was decreased in HF while protein phosphatase 2A (PP2A) was increased, upregulating the sensitivity of IKAS to intracellular Ca2+ [99]. The sex-specific IKAS response to isoproterenol was also partially attributed to different CK2/SK2 ratio between females and males [10]. IKAS was increased via the Ca2+/calmodulin-dependent protein kinase II (CaMKII)-dependent pathway in hypertrophic ventricles in rats [61]. Similarly in AF patients, CaMKII activation by autophosphorylation at Thr287 increased calcium sensitivity of IKAS and consequently induced increased IKAS [27].
3. Ikas and cardiac arrhythmias
3.1. Ikas and atrial fibrillation
The importance of SK channels in atrial arrhythmogenesis has been supported by genome-wide association studies in humans. The Caucasian patients with lone atrial fibrillation (AF) have identified genomic regions associated with AF on chromosomes 1q21 (rs13376333), which is intronic to KCNN3 (SK3) [25,24]. The association between the single nucleotide polymorphism (SNP) rs13376333 and AF was replicated in Asian cohorts[57,8], and was further linked to structural AF [100,8]. Therefore, SNP rs13376333 was considered as one of the top AF-susceptibility SNPs. SNP rs13376333 has been incorporated into a risk assessment model to identify subjects at the highest risk of developing AF [64]. However, it failed to predict AF recurrence after catheter ablation [12]. Another synonymous KCNN3 SNP rs1131820 was detected in association with lone AF [66]. In addition, variants of KCNN2 (SK2, rs13184658 and rs337711) also showed relevance with AF [105,36]. These results suggested KCNN variants are associated with AF. SK channels also participate in the atrial electrical remodeling. While mRNA and proteins of SK channels were downregulated in patients with chronic AF, inconsistent results appeared regarding IKAS regulation [51,108,81,27]. IKAS may decrease due to the low channel protein expressions [81,108], or intriguingly increase via the enhanced activation of CaMKII[27].
The SK channel-associated atrial electrical remodeling was also observed in animal models, such as diabetic mice [101,53,52]. However, discrepancies were noticed among studies. In streptozotocin-induced Type 1 diabetic mice, the expression of both SK2 and SK3 were reduced in atrium, which promoted the atrial arrhythmogenesis [101]. However, in a Type 2 diabetic mice model, SK2 expression was downregulated whereas SK3 was upregulated [53].
The mechanisms of SK channel in atrial arrhythmogenesis has been investigated in multiple animal studies. However, the results varied among different experimental models and study protocols, as summarized in Table 1. Of note, a recent study reported that IKAS inhibitors NS8593 and UCL1684 prevented AF via INa inhibition with little effect on IKAS [4].
Table 1.
The effects of IKAS blockade/activation on atrial arrhythmogenesis in animal models
| AF model | Species | Sex | Agents | Experimental settings | Ca2+ (mmol/L)* | K+ (mmol/L)* | Effects of IKAS blockade on atrial electrophysiology | Effects of IKAS blockade on atrial arrhythmogenesis | Effects on ventricular electrophysiology | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Acutely induced AF | Rat | Male | Apamin, NS8593, UCL1684 or ICA | In vivo, isolated heart, cardiomyocytes | 1.8 | 4.0 | Prolong aERP, aAPD, slow conduction velocity | Antiarrhythmic: shorten AF duration | NA | [80,82,79] |
| Induced AF in hypertension-related remodeled atria | Aging, spontaneously hypertensive rats | Male | NS8593 or UCL1684 | In vivo | Normal | Normal | Prolong aERP | Antiarrhythmic: shorten AF duration | NA | [19] |
| Acutely induced AF | Guinea pig, rabbit, rat | Female: guinea pig, rabbit Male: rat |
NS8593, UCL1684 or ICA | In vivo, isolated heart | Normal | Normal | Prolong aERP | Antiarrhythmic: prevent and terminate AF | No effect on QT interval | [21] |
| Acutely induced AF | Guinea pig | Female | ICA, combined with ranolazine, flecainide or lidocaine | Isolated heart | 2.5 | 4.0 | ** Prolong aERP | ** Antiarrhythmic: shorten AF duration | **No effect on QT interval, no AP triangulation | [43] |
| Acutely induced AF | Guinea pig | NA | ICA, combined with dofetilide or amiodarone | Isolated heart | 2.5 | 4.0 | **Prolong aERP without significance | **Antiarrhythmic: shorten AF duration | **No effect on QT interval, no AP triangulation | [42] |
| Acutely induced AF | Homozygous Dct knockout mouse | Female, male | Apamin | Isolated heart | 1.3 | 4.5 | Prolong aAPD | Antiarrhythmic: reduce AF inducibility | NA | [88] |
| Acutely induced AF | Horse | Female and gelding male | NS8593 | In vivo | Normal | Normal | Prolong aERP | Antiarrhythmic: shorten AF duration, reduce AF vulnerability score | No effect on QT interval | [33] |
| Induced AF in remodeled atria by 7-day tachypacing | Dog | NA | NS8593 | In vivo, cardiomyocytes | Normal | Normal | Prolong aAPD and aERP | Antiarrhythmic: shorten AF duration | No effect on vERP | [70] |
| Sustained vernakalant-resistant AF by chronic atrial tachypacing | Pig | NA | AP14145 | In vivo | Normal | Normal | Prolong aERP | Antiarrhythmic: prevent and terminate AF | No effect on vERP | [20] |
| Sustained vernakalant-resistant AF by chronic atrial tachypacing | Pig | Female | AP30663 | In vivo | Normal | Normal | Prolong aERP | Antiarrhythmic: prevent and terminate AF | No effect on QT interval | [18] |
| Acutely induced AF | Dog | Male | Apamin or UCL1684 | Isolated heart | 1.8 | 4.5 | Prolong aAPD with increased heterogeneity | Proarrhythmic: increase AF inducibility | NA | [37] |
| Acutely induced AF | SK2 null mutant mouse | NA | - | In vivo, cardiomyocytes | Normal | Normal | Prolong aAPD | Proarrhythmic: increase AF inducibility | NA | [49] |
| Acutely induced AF | SK3 overexpression mouse | NA | - | In vivo, cardiomyocytes | Normal | Normal | Shorten aAPD | Proarrhythmic: increase AF inducibility | No effect on QT interval | [113] |
Ion concentration in the Tyrode’s solution used in the ex vivo whole heart experiments.
Combined effects of IKAS blockade and other antiarrhythmic drugs, compared with baseline.
aAPD, atrial action potential duration; aERP, atrial effective refractory period; AF, atrial fibrillation; vERP: ventricular effective refractory period; ICA: N-(23yridine-2-yl)-4-(23yridine-2-yl)thiazol-2-amine; Dct, dopachrome tautomerase
3.2. Ikas and ventricular arrhythmias
In the past decade, the importance of SK channels in ventricular electrophysiology and arrhythmogenesis has been demonstrated in both human and experimental models. Association between KCNN2 variants (rs13184658 and rs10076582) and ventricular tachycardia has been found in patients with aborted sudden cardiac death or unexplained syncope [105]. The p.F503L KCNN2 variant facilitated the development of drug-induced long QT syndrome in human [44]. In explanted human hearts with non-ischemic dilated cardiomyopathy, reduced mRNA and protein expressions of SK2 channels were identified in subjects with than without VT [69]. Conversely, abundant SK2 protein were found in the intercalated discs of ventricular myocytes from explanted failing hearts [106]. In addition to SK2, upregulation of KCNN3 gene was detected in patients with dilated cardiomyopathy and sustained monomorphic ventricular tachycardia [67]. Consistent with the human data, overexpression of KCNN3 in mice resulted in high incidence of sudden cardiac death [58].
The mechanisms of SK channel in ventricular arrhythmogenesis has been investigated in multiple animal models, as summarized in Table 2.
Table 2.
The role of IKAS blockade on ventricular arrhythmogenesis in animal models
| Model | Baseline rhythm at EPS | Ventricular arrhythmia | agents | Species | Sex | experimental settings | Ca2+ (mmol/L)* | K+ (mmol/L)* | Effects of IKAS blockade on ventricular electrophysiology | Effects of IKAS blockade on ventricular arrhythmogenesis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal heart with hypokalemia | AV block and ventricular pacing | Induced VF | Apamin | Rabbit | NA | Isolated heart, cardiomyocytes | 1.8 | 2.4 | Prolong APD | Proarrhythmic: increase VF inducibility | [5] |
| Heart with chronic ventricular pacing, no HF | AV block and ventricular pacing | Induced VF | Apamin | Rabbit | Female | Isolated heart | 1.8 | 4.5 | Prolong APD, prominent at distal sites from pacing site | Proarrhythmic: increase VF inducibility and duration | [102] |
| Heart hypertrophy by TAB, with isoproterenol and NS309 or CyPPA | Sinus rhythm | PVC, Spontaneous VT or VF | Apamin | Rat | Male | Isolated heart, cardiomyocytes | 1.0 | 5.0 | Prolong APD | Proarrhythmic: VT/VF induced by β-adrenergic stimulation | [41] |
| HF by tachypacing | AV block | PVC, TdP | Apamin | Rabbit | NA | Isolated heart | 1.8 | 4.5 | Prolong QTc and APD | Proarrhythmic: Increased EADs, PVC and TdP | [6] |
| Acute MI, with reperfusion | Sinus rhythm | PVC, spontaneous VT or VF | NS8593 or AP14145 | Pig | Female | In vivo | Normal | Normal | No effect on QTc | Neutral: no effects on the frequency of PVC, VT, or VF | [56] |
| Normal heart with hypercalcemia | Atrial pacing | Induced VF | ICA, NS8593 | Guinea pig | Female | Isolated heart | 2.5 | 4.0 | Prolong APD and ERP | Antiarrhythmic: prevent and terminate VF | [17] |
| Normal heart with isoproterenol | Sinus rhythm | Induced VF | Apamin | Rabbit | Female, male | Isolated heart, cardiomyocytes | 1.8 | 4.7 | Prolong APD in females | Antiarrhythmic: reduce VF inducibility | [10] |
| Normal heart with CyPPA | Sinus rhythm | Spontaneous VF | Apamin | Rabbit | Female, male | Isolated heart | 1.8 | 4.7 | Prolong APD heterogeneously | Antiarrhythmic: Prevent spontaneous VF | [9] |
| Normal heart with hypothermia | Sinus rhythm | Spontaneous VF | Apamin | Rabbit | Female, male | Isolated heart | 1.8 | 4.7 | Prolong APD heterogeneously | Antiarrhythmic: Prevent spontaneous VF | [9] |
| Heart hypertrophy with global hypoxia | Ventricular pacing | Spontaneous VF | Apamin, or UCL1684 | Spontaneous hypertensive rats | Male | Isolated heart | 1.8 | 5.4 | Prolong APD | Antiarrhythmic: prevent VF | [85] |
| HF by tachypacing | Sinus rhythm | Spontaneous VF | Apamin | Rabbit | NA | Isolated heart | 1.8 | 4.5 | Prolong APD, prevent postshock APD shortening | Antiarrhythmic: Prevent spontaneous VF | [13] |
| Acute MI | Sinus rhythm | Spontaneous VF, induced VT | Apamin or UCL1684 | Rat | Male | In vivo | Normal | Normal | Prolonged ERP, prolonged APD in MI area | Antiarrhythmic: Prevent spontaneous VF/VT, reduce VT inducibility | [31] |
| Chronic (5 weeks) MI | Sinus rhythm | NA | Apamin | Rabbit | Female | Isolated heart, cardiomyocytes | 1.8 | 4.5 | Prevented postpacing APD shortening | Antiarrhythmic | [48] |
Ion concentration in the Tyrode’s solution used in the ex vivo whole heart experiments.
APD, action potential duration; CV, conduction velocity; CyPPA, cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine; EPS, electrophysiological study; ERP, effective refractory period; HF, heart failure; MI, myocardial infarction; NA, not available; PVC, premature ventricular complexes; TAB, thoracic aortic banding; TdP, Torsades de pointes; VF: ventricular fibrillation; VT, ventricular tachycardia;
3.3. IKAS and arrhythmias from cardiac conduction systems
The expression of SK2 channel in AV node has been verified by multiple techniques[111]. The functional roles of IKAS in AV node were investigated taking advantage of genetically altered mouse models with SK2 overexpression or null mutation. Overexpression of SK2 channels led to increased automaticity and shortened APD in AV node cells. In contrast, SK2 null mutant mice manifested with decreased spontaneous firing rate and prolonged APD in AV node [111].
In addition, SK channel was found more abundantly expressed in sinoatrial node, pulmonary veins and Purkinje cells than cardiomyocytes [11,87,72,93,70]. Consequently, IKAS played important roles in automaticity and its associated arrhythmogenesis [11,87,72,93,70]. Histological studies in failing human hearts showed that IKAS is abundantly expressed in the intercalated discs. IKAS blockade by apamin significantly reduced the transmural conduction velocity [105]. The functional roles of IKAS in cardiac conduction systems were summarized in Table 3.
Table 3.
The role of IKAS blockade on pulmonary vein and cardiac conduction systems
| Region | Model | Agents | Species | Sex | experimental settings | Ca2+ (mmol/L)* | K+ (mmol/L)* | Effects of IKAS blockade on automaticity | Effects of IKAS on MDP | Effects of IKAS on AP amplitude | Effects of IKAS blockade on APD | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PV | Remodeled atria by 7-day atrial tachypacing | NS8593 | Dog | NA | In vivo, single cells | Normal | Normal | NA | No effect | No effect | Prolong APD | [70] |
| PV | Normal atria with intermittent burst pacing | Apamin | Rabbit | Male | Tissue, single cells | 0.9 | 4.0 | NA | Prolong APD | [68] | ||
| Non-denudated PV | Normal atria | Apamin | Rabbit | NA | Tissue, single cells | 2.7 | 4.0 | Increase automaticity | No effect | No effect | No effect | [11] |
| Denudated PV | Normal atria | Apamin | Rabbit | NA | Tissue, single cells | 2.7 | 4.0 | Decrease automaticity | No effect | No effect | Prolong APD | [11] |
| SA node | Normal atria | Apamin | Rabbit | NA | Tissue, single cells | 2.7 | 4.0 | Decrease automaticity | No effect | No effect | Prolong APD | [11] |
| SA node | atria | Apamin | Wildtype mouse | Female, male | Tissue, single cells | 1.8 | 5.4 | Decrease automaticity | Decrease MDP | NA | Prolong APD | [87] |
| SA node | atria | Apamin | NCX knockout mouse | Female, male | Tissue, single cells | 1.8 | 5.4 | No effect on firing rate, but increase firing regularity | NA | NA | Prolong APD | [87] |
| Purkinje fiber | Normal ventricle with AV block | Apamin | Rabbit | Female, male | Isolated heart and pseudotendon | 1.8 | 4.0 | Increase automaticity | No effect on MDP, but lower the threshold potential for phase 0 depolarization | No effect | Prolong APD | [72,93] |
AV, atrioventricular block; APD, action potential duration; PV, pulmonary vein; MDP, maximal diastolic potential; NCX, sodium-calcium exchanger; SA, sinoatrial
3.4. Autonomic remodeling of neuronal SK channel and cardiac arrhythmias
Cardiac rhythmicity was regulated by autonomic nervous systems. Recently, several methods of neuromodulation were adopted to reduce atrial and ventricular arrhythmic burdens. As SK channel abundantly expressed in neuronal systems, the regulation of neuronal IKAS might modulate cardiac arrhythmias. Low-level vagus nerve stimulation (LL-VNS) reduced stellate ganglion nerve activity and consequently ameliorated atrial arrhythmias [77]. Follow-up study showed that the LL-VNS induced neuronal remodeling was mediated by the increased SK2 proteins expression and membranization in the left stellate ganglion [76]. Therefore, modulating IKAS activity in the stellate ganglion may serve as an upstream pathway to regulate cardiac arrhythmogenesis, and potentially serve as a therapeutic target. Subsequent studies, as summarized in Table 4, reported similar findings that, by stimulating right tragus and spinal cord, SK2 channels were upregulated in stellate ganglion and atrial ganglionated plexi, thus reduce cardiac arrhythmias [76,115,107,94]. The neuronal SK channel upregulation may rely on the increased intracellular Ca2+ induced by rapid electrical stimulation of the nerves, which in turn reduces neuronal discharges by inducing neuron cell death and hyperpolarizing the cell membrane [35].
Table 4.
The role SK channels in neuromodulation of cardiac arrhythmias
| Intervention | Anatomical target of stimulation | Protocol of intervention | Animal model | Species | Sex | Cardiac effects | *Intervention related SK channel regulation | Disease of interest | reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Region | SK2 expression | |||||||||
| Vagal nerve stimulation | Left cervical vagal nerve | 1 week, low level | Normal heart | Dog | Male | NA | LSG | Upregulation and redistribution to the cell membrane | Arrhythmia | [76] |
| Vagal nerve stimulation | Right tragus | 3 hours | Normal heart | Dog | NA | Decrease LF and LF/HF ratio; attenuate sympathetically induced sinus rate acceleration | RSG | Upregulation | Inappropriate sinus tachycardia | [115] |
| Vagal nerve stimulation | Right tragus | 2 months, intermittent, low-level | Post-MI | Dog | NA | Reduce ventricular arrhythmia inducibility | LSG | Upregulation | Post-MI ventricular arrhythmias | [107] |
| Spinal cord stimulation | T1-T5 level | 6 hours | rapid atrial pacing-induced AF | Dog | NA | Reduce AF inducibility | LSG, ARGP | Upregulation, | AF | [94] |
Compared with non-intervention group of the same model
AF, atrial fibrillation; ARGP, anterior right ganglionated plexus; HF, high frequency component of heart rate variability; LF, low frequency component of heart rate variability; LSG, left stellate ganglion; MI, myocardial infarction; RSG, right stellate ganglion
4. Sex differences in IKAS
Sex dimorphisms exert striking effects in almost all aspects of human cardiac arrhythmias, including the prevalence, symptoms, diagnosis, risk stratification, treatment, drug sensitivity and outcomes [22]. The most notable examples are long QT syndrome (LQTS) and Brugada syndrome, where prominent sex disparities in clinical expressivity exist. Women are at higher risk than men of Torsade de Pointes with LQTS type 1 and type 2 [73]. Conversely, male is predominant over female in the prevalence and arrhythmic events in J wave syndrome, including both Brugada syndrome and early repolarization syndrome [60,2]. The cellular basis of these sex-related distinctions in human cardiac arrhythmogenesis is not completely resolved due to the limited availability to human cardiac tissues. However, the importance of cardiac ion currents in sex differences of cardiac electrophysiology and dysrhythmia susceptibility have been demonstrated by animal experiments and reproduced by computer simulations. The densities of Ica,L and IK,ATP are higher, while Ito, IKr and IKur are lower in female than male [92]. Other currents, including INa, IKs, IK1 and INCX are equal between sexes [92]. These sex differences in ion current densities conjunctionally contributed to the longer action potential (AP) duration (APD) in cardiomyocytes of female compared with male under physiological conditions. In conjunction with other factors such as transmural and regional heterogeneities, heterotypic cell-cell coupling and abnormal intracellular calcium handling, the dysregulation of ion current densities led to altered automaticity, triggered activities and reentrant, thus predisposing cardiac arrhythmias.
However, the sex differences of SK channel and IKAS have not been investigated until recently. In Table 1–4, we listed a total of 36 studies regarding IKAS and cardiac arrhythmias. Among them, 14 studies did not mention the sexes of the animals, 10 studies only used male animals and 7 studies only used female animals. Only 5 studies were conducted in both sexes.
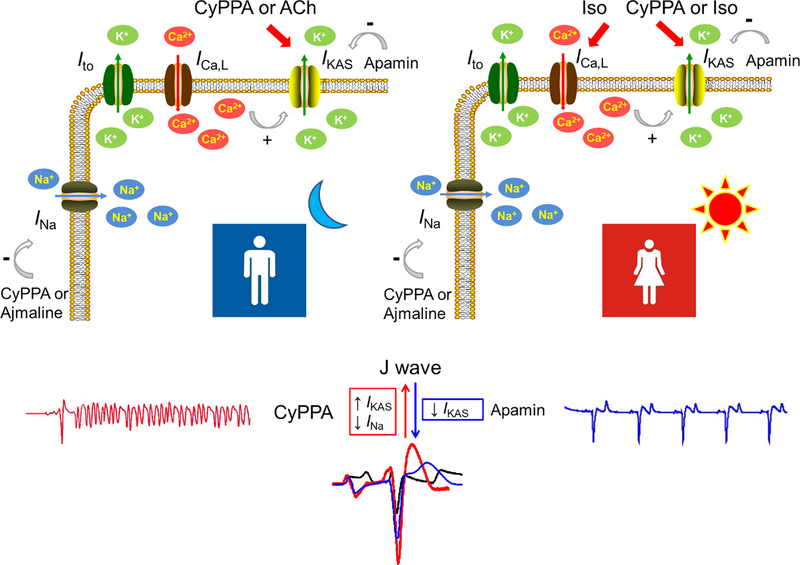
In normal rabbit ventricles, β-adrenergic stimulation by isoproterenol activated ventricular IKAS in females to a much greater extent than in males, suggesting a sex specific activation of IKAS [10]. The more prominent IKAS activation in ventricles of female rabbits was attributed to more abundant expression of SK2 channel, larger ICa,L and lower CK2/SK2 ratio in females. IKAS activation in females induced negative Ca2+ -voltage coupling, promoted electromechanically discordant phase 2 repolarization alternans and facilitated ventricular fibrillation, which were not observed in males [10]. In addition, IKAS was abundantly activated increased in female but not in male ventricles with drug-induced long QT syndrome. Increased IKAS helped to preserve the repolarization reserve in female ventricles treated with IKs and IKr blockers or INaL activators [95]. However, a subsequent study found that concomitant IKAS activation and INa inhibition by cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine (CyPPA) recapitulated J wave syndrome in rabbits and exhibited more prominent effects in males than females [9]. In this CyPPA model, males had significantly higher J wave amplitude and more episodes of spontaneous ventricular fibrillation than females. Electrical storm only developed in males. β-adrenergic stimulation mainly activated SK2 rather than SK3 [10]. As CyPPA was a subtype-selective potentiator of SK3 channels, the effects of CyPPA were mainly attributed to SK3 rather SK2 activation[9]. While isoproterenol activates IKAS in female more than male ventricles, acetylcholine activates IKAS more in male than in female ventricles [28]. Activation by acetylcholine with concomitant INa inhibition by ajmaline also induced J-wave elevation and facilitated the induction of ventricular arrhythmias more in male than female ventricles [28]. Despite binding to M2 receptor reduces protein kinase A activity, acetylcholine binding to M1 receptor may gate Ca2+ release from intracellular stores and facilitate voltage-dependent refilling of Ca2+ stores, thereby maintaining the SK-mediated fidelity of inhibitory cholinergic signaling in pyramidal neurons [15]. As M2 receptor is dominant in the heart, the mechanism of acetylcholine activate IKAS is not fully understood. However, as only low level of acetylcholine-sensitive K+ current (IK.Ach) is present in the ventricles, the electrophysiological effects of acetylcholine in ventricles may largely rely on the activation of IKAS. Taken together, these findings suggest that during the day time, sympathetic tone activates IKAS in females but that outward current was counterbalanced by the increased inward current (ICa,L). Therefore, in spite of IKAS activation, females do not exhibit J point elevation during the day. Males are less liable to J wave syndrome during daytime because of absence of IKAS activation by the high sympathetic tone. However, at night, IKAS in males is activated by the heightened parasympathetic tone without concomitant activation of ICa,L, leading to J point elevation, ventricular arrhythmia and sudden cardiac death. The absence of IKAS activation by acetylcholine in females protects them against the ventricular arrhythmias at night. Therefore, sex differences in IKAS may be used the explain the nocturnal occurrences of sudden death in males but not females. The schematic (Figure 1) shows the sex differentiated activation of IKAS in arrhythmogenesis of J wave syndrome.
Figure 1.
IKAS activation and J wave syndrome. Concomitant IKAS activation (by CyPPA or acetylcholine) and INa inhibition (by CyPPA or ajmaline) lead to J point elevation and facilitate ventricular arrhythmias. J point elevation and ventricular arrhythmias are attenuated by IKAS inhibition (by apamin). Acetylcholine activates IKAS more prominently in men while isoproterenol activates IKAS more prominently in women. We propose that IKAS activation by sympathetic tone during the daytime is counterbalanced by the increased inward current (ICa,L) in females. Therefore, in spite of IKAS activation, females do not exhibit J point elevation during the daytime. At night, IKAS in males is activated by the heightened parasympathetic tone without concomitant activation of ICa,L, leading to J point elevation, ventricular arrhythmia and sudden cardiac death. The sex differences in IKAS may explain the mechanisms of J wave syndrome, including both the Brugada syndrome and the early repolarization syndrome. Ach, acetylcholine; CyPPA, cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine; Iso, isoproterenol.
We do not yet have data to compare the relative importance of IKAS and Ito in the generation of J wave syndrome. However, a major consequence of Ito activation is to increase Ca2+ entry through the already opened LTCC during phase 1 of the action potential. Colocalization of IKAS channels with LTCC in the subsarcolemmal or junctional space may result in a spiky IKAS, which can promote the development of J wave syndrome and ventricular arrhythmias in computer simulation studies [47]. Therefore, it is possible that a collaboration between Ito, LTCC and IKAS is needed to generate the J wave syndrome.
5. Clinical perspectives
As IKAS participated in cardiac arrhythmias, IKAS has been considered as a new target of antiarrhythmic therapy in both experimental and clinical settings. E4031 and chromanol 293B were historically considered as specific blocker of IKr (IC50 10–30 μM) and IKs (IC50 400 nM), respectively. However, E4031 at 500 nM and chromanol 293B at 100 mM inhibited 37% and 38% IKAS, respectively [89]. Therefore, it should be noticed that the effects of E4031 and chromanol 293B on cardiac electrophysiology may combine the effects of IKr or IKs blockade and IKAS inhibition.
Several IKAS specific blockers, such as apamin, are neurotoxins and cannot be used for antiarrhythmic therapy [38]. Although not specific blockers, several clinically used antiarrhythmic drugs might also target Ikas. A previous study suggested that dofetilide and propafenone inhibited IKAS with no subtype selectivity [78]. Their inhibition may have minimal clinical importance for antiarrhythmic effect due to much higher IC50 values than the effective free therapeutic plasma concentration of the drugs when used for AF treatment [78]. Amiodarone, disopyramide, dronedarone, flecainide, ibutilide, quinidine, sotalol and vernakalant had no effect on the IKAS conducted by SK3 [78]. However, other studies suggested that amiodarone and dronedarone inhibited IKAS and dronedarone provided a greater degree of IKAS inhibition than amiodarone in atrial myocytes from chronic atrial fibrillation [89,109].
Non-antiarrhythmic drugs may also have potential antiarrhythmic effects by targeting IKAS. Ondansetron, a 5-HT3 receptor antagonist, blocked IKAS, while did not inhibit IKs or IKr at therapeutic concentrations [44,103]. As IKAS activation recapitulated J wave syndrome phenotype in the experimental model, iKAS blockade by ondansetron may have the potential to prevent ventricular arrhythmias in Brugada or early repolarization syndromes [9]. Additionally, as IKAS activation relied on intracellular Ca2+, drugs targeting Ca2+ and Ca2+- IKAS interaction, such as LTCC, RyR2 and calmodulin, may have indirect effects on IKAS, subsequently contributed to their antiarrhythmic or proarrhythmic mechanisms. On the translational level, the discovery and use of IKAS blockers in the treatment of atrial fibrillation and ventricular tachyarrhythmias require further investigations, with specific consideration on sex differences.
Acknowledgments
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81900288], Shanghai Sailing Program, Shanghai, China [grant number 19YF1431900], the National Institutes of Health USA grants R01HL139829 and OT2OD028190 and the Burns and Allen Chair in Cardiology Research of the Cedars-Sinai Medical Center, Los Angeles, California, USA.
Footnotes
6. Declarations
Conflicts of interest: none.
This article is published as part of the Special Issue on Fairytales of Calcium Signal Dynamics in Cardiac Myocytes and Fibroblasts: Mechanisms and Therapeutics
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Adelman JP, Maylie J, Sah P (2012) Small-conductance Ca2+-activated K+ channels: form and function. Annual review of physiology 74:245–269. doi: 10.1146/annurev-physiol-020911-153336 [DOI] [PubMed] [Google Scholar]
- 2.Antzelevitch C, Yan GX, Ackerman MJ, Borggrefe M, Corrado D, Guo J, Gussak I, Hasdemir C, Horie M, Huikuri H, Ma C, Morita H, Nam GB, Sacher F, Shimizu W, Viskin S, Wilde AA (2016) J-Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. Heart Rhythm 13:e295–324. doi: 10.1016/j.hrthm.2016.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonilla IM, Long VP 3rd, Vargas-Pinto P, Wright P, Belevych A, Lou Q, Mowrey K, Yoo J, Binkley PF, Fedorov VV, Gyorke S, Janssen PM, Kilic A, Mohler PJ, Carnes CA (2014) Calcium-activated potassium current modulates ventricular repolarization in chronic heart failure. PLoS One 9:e108824. doi: 10.1371/journal.pone.0108824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burashnikov A, Barajas-Martinez H, Hu D, Robinson VM, Grunnet M, Antzelevitch C (2020) The SK Channel Inhibitors NS8593 and UCL1684 Prevent the Development of Atrial Fibrillation via Atrial-selective Inhibition of Sodium Channel Activity. J Cardiovasc Pharmacol. doi: 10.1097/FJC.0000000000000855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan YH, Tsai WC, Ko JS, Yin D, Chang PC, Rubart M, Weiss JN, Everett THt, Lin SF, Chen PS (2015) Small-Conductance Calcium-Activated Potassium Current Is Activated During Hypokalemia and Masks Short-Term Cardiac Memory Induced by Ventricular Pacing. Circulation 132:1377–1386. doi: 10.1161/CIRCULATIONAHA.114.015125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang PC, Hsieh YC, Hsueh CH, Weiss JN, Lin SF, Chen PS (2013) Apamin induces early afterdepolarizations and torsades de pointes ventricular arrhythmia from failing rabbit ventricles exhibiting secondary rises in intracellular calcium. Heart rhythm 10:1516–1524. doi: 10.1016/j.hrthm.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang PC, Turker I, Lopshire JC, Masroor S, Nguyen BL, Tao W, Rubart M, Chen PS, Chen Z, Ai T (2013) Heterogeneous upregulation of apamin-sensitive potassium currents in failing human ventricles. Journal of the American Heart Association 2:e004713. doi: 10.1161/JAHA.112.004713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang SH, Chang SN, Hwang JJ, Chiang FT, Tseng CD, Lee JK, Lai LP, Lin JL, Wu CK, Tsai CT (2012) Significant association of rs13376333 in KCNN3 on chromosome 1q21 with atrial fibrillation in a Taiwanese population. Circ J 76:184–188. doi: 10.1253/circj.cj-11-0525 [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Xu DZ, Wu AZ, Guo S, Wan J, Yin D, Lin SF, Chen Z, Rubart-von der Lohe M, Everett THt, Qu Z, Weiss JN, Chen PS (2018) Concomitant SK current activation and sodium current inhibition cause J wave syndrome. JCI insight 3. doi: 10.1172/jci.insight.122329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M, Yin D, Guo S, Xu DZ, Wang Z, Chen Z, Rubart-von der Lohe M, Lin SF, Everett Iv TH, Weiss JN, Chen PS (2018) Sex-specific activation of SK current by isoproterenol facilitates action potential triangulation and arrhythmogenesis in rabbit ventricles. The Journal of physiology 596:4299–4322. doi: 10.1113/JP275681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen WT, Chen YC, Lu YY, Kao YH, Huang JH, Lin YK, Chen SA, Chen YJ (2013) Apamin modulates electrophysiological characteristics of the pulmonary vein and the Sinoatrial Node. Eur J Clin Invest 43:957–963. doi: 10.1111/eci.12125 [DOI] [PubMed] [Google Scholar]
- 12.Choi EK, Park JH, Lee JY, Nam CM, Hwang MK, Uhm JS, Joung B, Ko YG, Lee MH, Lubitz SA, Ellinor PT, Pak HN (2015) Korean Atrial Fibrillation (AF) Network: Genetic Variants for AF Do Not Predict Ablation Success. J Am Heart Assoc 4:e002046. doi: 10.1161/JAHA.115.002046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua SK, Chang PC, Maruyama M, Turker I, Shinohara T, Shen MJ, Chen Z, Shen C, Rubart-von der Lohe M, Lopshire JC, Ogawa M, Weiss JN, Lin SF, Ai T, Chen PS (2011) Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circulation research 108:971–979. doi: 10.1161/CIRCRESAHA.110.238386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Church TW, Weatherall KL, Correa SA, Prole DL, Brown JT, Marrion NV (2015) Preferential assembly of heteromeric small conductance calcium-activated potassium channels. Eur J Neurosci 41:305–315. doi: 10.1111/ejn.12789 [DOI] [PubMed] [Google Scholar]
- 15.Dasari S, Hill C, Gulledge AT (2017) A unifying hypothesis for M1 muscarinic receptor signalling in pyramidal neurons. The Journal of physiology 595:1711–1723. doi: 10.1113/JP273627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denham NC, Pearman CM, Caldwell JL, Madders GWP, Eisner DA, Trafford AW, Dibb KM (2018) Calcium in the Pathophysiology of Atrial Fibrillation and Heart Failure. Front Physiol 9:1380. doi: 10.3389/fphys.2018.01380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diness JG, Kirchhoff JE, Sheykhzade M, Jespersen T, Grunnet M (2015) Antiarrhythmic Effect of Either Negative Modulation or Blockade of Small Conductance Ca2+-activated K+ Channels on Ventricular Fibrillation in Guinea Pig Langendorff-perfused Heart. J Cardiovasc Pharmacol 66:294–299. doi: 10.1097/FJC.0000000000000278 [DOI] [PubMed] [Google Scholar]
- 18.Diness JG, Kirchhoff JE, Speerschneider T, Abildgaard L, Edvardsson N, Sorensen US, Grunnet M, Bentzen BH (2020) The KCa2 Channel Inhibitor AP30663 Selectively Increases Atrial Refractoriness, Converts Vernakalant-Resistant Atrial Fibrillation and Prevents Its Reinduction in Conscious Pigs. Front Pharmacol 11:159. doi: 10.3389/fphar.2020.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diness JG, Skibsbye L, Jespersen T, Bartels ED, Sorensen US, Hansen RS, Grunnet M (2011) Effects on atrial fibrillation in aged hypertensive rats by Ca(2+)-activated K(+) channel inhibition. Hypertension 57:1129–1135. doi: 10.1161/HYPERTENSIONAHA.111.170613 [DOI] [PubMed] [Google Scholar]
- 20.Diness JG, Skibsbye L, Simo-Vicens R, Santos JL, Lundegaard P, Citerni C, Sauter DRP, Bomholtz SH, Svendsen JH, Olesen SP, Sorensen US, Jespersen T, Grunnet M, Bentzen BH (2017) Termination of Vernakalant-Resistant Atrial Fibrillation by Inhibition of Small-Conductance Ca(2+)-Activated K(+) Channels in Pigs. Circulation Arrhythmia and electrophysiology 10. doi: 10.1161/CIRCEP.117.005125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diness JG, Sorensen US, Nissen JD, Al-Shahib B, Jespersen T, Grunnet M, Hansen RS (2010) Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circulation Arrhythmia and electrophysiology 3:380–390. doi: 10.1161/CIRCEP.110.957407 [DOI] [PubMed] [Google Scholar]
- 22.Ehdaie A, Cingolani E, Shehata M, Wang X, Curtis AB, Chugh SS (2018) Sex Differences in Cardiac Arrhythmias: Clinical and Research Implications. Circulation Arrhythmia and electrophysiology 11:e005680. doi: 10.1161/CIRCEP.117.005680 [DOI] [PubMed] [Google Scholar]
- 23.Eisner DA, Vaughan-Jones RD (1983) Do calcium-activated potassium channels exist in the heart? Cell Calcium 4:371–386. doi: 10.1016/0143-4160(83)90015-5 [DOI] [PubMed] [Google Scholar]
- 24.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, Arking DE, Muller-Nurasyid M, Krijthe BP, Lubitz SA, Bis JC, Chung MK, Dorr M, Ozaki K, Roberts JD, Smith JG, Pfeufer A, Sinner MF, Lohman K, Ding J, Smith NL, Smith JD, Rienstra M, Rice KM, Van Wagoner DR, Magnani JW, Wakili R, Clauss S, Rotter JI, Steinbeck G, Launer LJ, Davies RW, Borkovich M, Harris TB, Lin H, Volker U, Volzke H, Milan DJ, Hofman A, Boerwinkle E, Chen LY, Soliman EZ, Voight BF, Li G, Chakravarti A, Kubo M, Tedrow UB, Rose LM, Ridker PM, Conen D, Tsunoda T, Furukawa T, Sotoodehnia N, Xu S, Kamatani N, Levy D, Nakamura Y, Parvez B, Mahida S, Furie KL, Rosand J, Muhammad R, Psaty BM, Meitinger T, Perz S, Wichmann HE, Witteman JC, Kao WH, Kathiresan S, Roden DM, Uitterlinden AG, Rivadeneira F, McKnight B, Sjogren M, Newman AB, Liu Y, Gollob MH, Melander O, Tanaka T, Stricker BH, Felix SB, Alonso A, Darbar D, Barnard J, Chasman DI, Heckbert SR, Benjamin EJ, Gudnason V, Kaab S (2012) Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet 44:670–675. doi: 10.1038/ng.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellinor PT, Lunetta KL, Glazer NL, Pfeufer A, Alonso A, Chung MK, Sinner MF, de Bakker PI, Mueller M, Lubitz SA, Fox E, Darbar D, Smith NL, Smith JD, Schnabel RB, Soliman EZ, Rice KM, Van Wagoner DR, Beckmann BM, van Noord C, Wang K, Ehret GB, Rotter JI, Hazen SL, Steinbeck G, Smith AV, Launer LJ, Harris TB, Makino S, Nelis M, Milan DJ, Perz S, Esko T, Kottgen A, Moebus S, Newton-Cheh C, Li M, Mohlenkamp S, Wang TJ, Kao WH, Vasan RS, Nothen MM, MacRae CA, Stricker BH, Hofman A, Uitterlinden AG, Levy D, Boerwinkle E, Metspalu A, Topol EJ, Chakravarti A, Gudnason V, Psaty BM, Roden DM, Meitinger T, Wichmann HE, Witteman JC, Barnard J, Arking DE, Benjamin EJ, Heckbert SR, Kaab S (2010) Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet 42:240–244. doi: 10.1038/ng.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan HK, Luo TX, Zhao WD, Mu YH, Yang Y, Guo WJ, Tu HY, Zhang Q (2018) Functional interaction of Junctophilin 2 with small- conductance Ca(2+) -activated potassium channel subtype 2(SK2) in mouse cardiac myocytes. Acta Physiol (Oxf) 222. doi: 10.1111/apha.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan X, Yu Y, Lan H, Ou X, Yang L, Li T, Cao J, Zeng X, Li M (2018) Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) Increases Small-Conductance Ca2+-Activated K+ Current in Patients with Chronic Atrial Fibrillation. Med Sci Monit 24:3011–3023. doi: 10.12659/MSM.909684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fei YD, Chen M, Guo S, Ueoka A, Chen Z, Rubart-von der Lohe M, Everett THt, Qu Z, Weiss JN, Chen PS (2020) Simultaneous activation of the small conductance calcium activated potassium current by acetylcholine and inhibition of sodium current by ajmaline cause J-wave syndrome in Langendorff-perfused rabbit ventricles. Heart rhythm. doi: 10.1016/j.hrthm.2020.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grandi E, Sanguinetti MC, Bartos DC, Bers DM, Chen-Izu Y, Chiamvimonvat N, Colecraft HM, Delisle BP, Heijman J, Navedo MF, Noskov S, Proenza C, Vandenberg JI, Yarov-Yarovoy V (2017) Potassium channels in the heart: structure, function and regulation. The Journal of physiology 595:2209–2228. doi: 10.1113/JP272864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grunnet M, Jensen BS, Olesen SP, Klaerke DA (2001) Apamin interacts with all subtypes of cloned small-conductance Ca2+-activated K+ channels. Pflugers Arch 441:544–550. doi: 10.1007/s004240000447 [DOI] [PubMed] [Google Scholar]
- 31.Gui L, Bao Z, Jia Y, Qin X, Cheng ZJ, Zhu J, Chen QH (2013) Ventricular tachyarrhythmias in rats with acute myocardial infarction involves activation of small-conductance Ca2+-activated K+ channels. Am J Physiol Heart Circ Physiol 304:H118–130. doi: 10.1152/ajpheart.00820.2011 [DOI] [PubMed] [Google Scholar]
- 32.Hancock JM, Weatherall KL, Choisy SC, James AF, Hancox JC, Marrion NV (2015) Selective activation of heteromeric SK channels contributes to action potential repolarization in mouse atrial myocytes. Heart rhythm 12:1003–1015. doi: 10.1016/j.hrthm.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 33.Haugaard MM, Hesselkilde EZ, Pehrson S, Carstensen H, Flethoj M, Praestegaard KF, Sorensen US, Diness JG, Grunnet M, Buhl R, Jespersen T (2015) Pharmacologic inhibition of small-conductance calcium-activated potassium (SK) channels by NS8593 reveals atrial antiarrhythmic potential in horses. Heart rhythm 12:825–835. doi: 10.1016/j.hrthm.2014.12.028 [DOI] [PubMed] [Google Scholar]
- 34.Hill JA Jr., Coronado R, Strauss HC (1988) Reconstitution and characterization of a calcium-activated channel from heart. CircRes 62:411–415 [DOI] [PubMed] [Google Scholar]
- 35.Honrath B, Krabbendam IE, Culmsee C, Dolga AM (2017) Small conductance Ca(2+)-activated K(+) channels in the plasma membrane, mitochondria and the ER: Pharmacology and implications in neuronal diseases. Neurochem Int 109:13–23. doi: 10.1016/j.neuint.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 36.Hsu J, Gore-Panter S, Tchou G, Castel L, Lovano B, Moravec CS, Pettersson GB, Roselli EE, Gillinov AM, McCurry KR, Smedira NG, Barnard J, Van Wagoner DR, Chung MK, Smith JD (2018) Genetic Control of Left Atrial Gene Expression Yields Insights into the Genetic Susceptibility for Atrial Fibrillation. Circ Genom Precis Med 11:e002107. doi: 10.1161/CIRCGEN.118.002107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsueh CH, Chang PC, Hsieh YC, Reher T, Chen PS, Lin SF (2013) Proarrhythmic effect of blocking the small conductance calcium activated potassium channel in isolated canine left atrium. Heart rhythm 10:891–898. doi: 10.1016/j.hrthm.2013.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishii TM, Maylie J, Adelman JP (1997) Determinants of apamin and d-tubocurarine block in SK potassium channels. The Journal of biological chemistry 272:23195–23200. doi: 10.1074/jbc.272.37.23195 [DOI] [PubMed] [Google Scholar]
- 39.Jeyaraj D, Wan X, Ficker E, Stelzer JE, Deschenes I, Liu H, Wilson LD, Decker KF, Said TH, Jain MK, Rudy Y, Rosenbaum DS (2013) Ionic bases for electrical remodeling of the canine cardiac ventricle. Am J Physiol Heart Circ Physiol 305:H410–419. doi: 10.1152/ajpheart.00213.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy M, Bers DM, Chiamvimonvat N, Sato D (2017) Dynamical effects of calcium-sensitive potassium currents on voltage and calcium alternans. The Journal of physiology 595:2285–2297. doi: 10.1113/JP273626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim TY, Terentyeva R, Roder KH, Li W, Liu M, Greener I, Hamilton S, Polina I, Murphy KR, Clements RT, Dudley SC Jr., Koren G, Choi BR, Terentyev D (2017) SK channel enhancers attenuate Ca2+-dependent arrhythmia in hypertrophic hearts by regulating mito-ROS-dependent oxidation and activity of RyR. Cardiovascular research 113:343–353. doi: 10.1093/cvr/cvx005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirchhoff JE, Diness JG, Abildgaard L, Sheykhzade M, Grunnet M, Jespersen T (2016) Antiarrhythmic effect of the Ca(2+)-activated K(+) (SK) channel inhibitor ICA combined with either amiodarone or dofetilide in an isolated heart model of atrial fibrillation. Pflugers Arch 468:1853–1863. doi: 10.1007/s00424-016-1883-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirchhoff JE, Diness JG, Sheykhzade M, Grunnet M, Jespersen T (2015) Synergistic antiarrhythmic effect of combining inhibition of Ca(2)(+)-activated K(+) (SK) channels and voltage-gated Na(+) channels in an isolated heart model of atrial fibrillation. Heart rhythm 12:409–418. doi: 10.1016/j.hrthm.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 44.Ko JS, Guo S, Hassel J, Celestino-Soper P, Lynnes TC, Tisdale JE, Zheng JJ, Taylor SE, Foroud T, Murray MD, Kovacs RJ, Li X, Lin SF, Chen Z, Vatta M, Chen PS, Rubart M (2018) Ondansetron blocks wild-type and p.F503L variant small-conductance Ca(2+)-activated K(+) channels. Am J Physiol Heart Circ Physiol 315:H375–H388. doi: 10.1152/ajpheart.00479.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP (1996) Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273:1709–1714 [DOI] [PubMed] [Google Scholar]
- 46.Lancaster B, Nicoll RA (1987) Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. The Journal of physiology 389:187–203. doi: 10.1113/jphysiol.1987.sp016653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landaw J, Zhang Z, Song Z, Liu MB, Olcese R, Chen PS, Weiss JN, Qu Z (2020) Small-conductance Ca(2+)-activated K(+) channels promote J-wave syndrome and phase 2 reentry. Heart Rhythm. doi: 10.1016/j.hrthm.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee YS, Chang PC, Hsueh CH, Maruyama M, Park HW, Rhee KS, Hsieh YC, Shen C, Weiss JN, Chen Z, Lin SF, Chen PS (2013) Apamin-sensitive calcium-activated potassium currents in rabbit ventricles with chronic myocardial infarction. Journal of cardiovascular electrophysiology 24:1144–1153. doi: 10.1111/jce.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, Zhang Z, Singapuri A, Albert TR, Rajagopal AV, Bond CT, Periasamy M, Adelman J, Chiamvimonvat N (2009) Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. The Journal of physiology 587:1087–1100. doi: 10.1113/jphysiol.2008.167718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li T, Fan X, Yu Y, Chen L, Huang W, Yang Y, Cao J, Zeng X, Tan X (2018) Synaptobrevin-2 facilitates the trafficking and function of atrial SK2 channel. Sci China Life Sci 61:599–603. doi: 10.1007/s11427-017-9139-7 [DOI] [PubMed] [Google Scholar]
- 51.Ling TY, Wang XL, Chai Q, Lau TW, Koestler CM, Park SJ, Daly RC, Greason KL, Jen J, Wu LQ, Shen WF, Shen WK, Cha YM, Lee HC (2013) Regulation of the SK3 channel by microRNA-499--potential role in atrial fibrillation. Heart rhythm 10:1001–1009. doi: 10.1016/j.hrthm.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ling TY, Yi F, Lu T, Wang XL, Sun X, Willis MS, Wu LQ, Shen WK, Adelman JP, Lee HC (2019) F-box protein-32 down-regulates small-conductance calcium-activated potassium channel 2 in diabetic mouse atria. The Journal of biological chemistry 294:4160–4168. doi: 10.1074/jbc.RA118.003837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu CH, Hua N, Fu X, Pan YL, Li B, Li XD (2018) Metformin regulates atrial SK2 and SK3 expression through inhibiting the PKC/ERK signaling pathway in type 2 diabetic rats. BMC Cardiovasc Disord 18:236. doi: 10.1186/s12872-018-0950-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu L, Sirish P, Zhang Z, Woltz RL, Li N, Timofeyev V, Knowlton AA, Zhang XD, Yamoah EN, Chiamvimonvat N (2015) Regulation of gene transcription by voltage-gated L-type calcium channel, Cav1.3. The Journal of biological chemistry 290:4663–4676. doi: 10.1074/jbc.M114.586883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA, Chiamvimonvat N (2007) Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res 100:112–120. doi:01.RES.0000253095.44186.72 [pii] 10.1161/01.RES.0000253095.44186.72 [DOI] [PubMed] [Google Scholar]
- 56.Lubberding AF, Sattler SM, Grunnet M, Sorensen US, Tfelt-Hansen J, Jespersen T (2019) Arrhythmia development during inhibition of small-conductance calcium-activated potassium channels in acute myocardial infarction in a porcine model. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 21:1584–1593. doi: 10.1093/europace/euz223 [DOI] [PubMed] [Google Scholar]
- 57.Luo Z, Yan C, Zhang W, Shen X, Zheng W, Chen F, Cao X, Yang Y, Lin X, Wang Z, Huang M (2014) Association between SNP rs13376333 and rs1131820 in the KCNN3 gene and atrial fibrillation in the Chinese Han population. Clin Chem Lab Med 52:1867–1873. doi: 10.1515/cclm-2014-0491 [DOI] [PubMed] [Google Scholar]
- 58.Mahida S, Mills RW, Tucker NR, Simonson B, Macri V, Lemoine MD, Das S, Milan DJ, Ellinor PT (2014) Overexpression of KCNN3 results in sudden cardiac death. Cardiovascular research 101:326–334. doi: 10.1093/cvr/cvt269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maruyama M, Joung B, Tang L, Shinohara T, On YK, Han S, Choi EK, Kim DH, Shen MJ, Weiss JN, Lin SF, Chen PS (2010) Diastolic intracellular calcium-membrane voltage coupling gain and postshock arrhythmias: role of purkinje fibers and triggered activity. Circulation research 106:399–408. doi: 10.1161/CIRCRESAHA.109.211292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milman A, Gourraud JB, Andorin A, Postema PG, Sacher F, Mabo P, Conte G, Giustetto C, Sarquella-Brugada G, Hochstadt A, Kim SH, Juang JJM, Maeda S, Takahashi Y, Kamakura T, Aiba T, Leshem E, Michowitz Y, Rahkovich M, Mizusawa Y, Arbelo E, Huang Z, Denjoy I, Wijeyeratne YD, Napolitano C, Brugada R, Casado-Arroyo R, Champagne J, Calo L, Tfelt-Hansen J, Priori SG, Takagi M, Veltmann C, Delise P, Corrado D, Behr ER, Gaita F, Yan GX, Brugada J, Leenhardt A, Wilde AAM, Brugada P, Kusano KF, Hirao K, Nam GB, Probst V, Belhassen B (2018) Gender differences in patients with Brugada syndrome and arrhythmic events: Data from a survey on arrhythmic events in 678 patients. Heart rhythm 15:1457–1465. doi: 10.1016/j.hrthm.2018.06.019 [DOI] [PubMed] [Google Scholar]
- 61.Mizukami K, Yokoshiki H, Mitsuyama H, Watanabe M, Tenma T, Takada S, Tsutsui H (2015) Small-conductance Ca2+-activated K+ current is upregulated via the phosphorylation of CaMKII in cardiac hypertrophy from spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 309:H1066–1074. doi: 10.1152/ajpheart.00825.2014 [DOI] [PubMed] [Google Scholar]
- 62.Monaghan AS, Benton DC, Bahia PK, Hosseini R, Shah YA, Haylett DG, Moss GW (2004) The SK3 subunit of small conductance Ca2+-activated K+ channels interacts with both SK1 and SK2 subunits in a heterologous expression system. The Journal of biological chemistry 279:1003–1009. doi: 10.1074/jbc.M308070200 [DOI] [PubMed] [Google Scholar]
- 63.Mu YH, Zhao WC, Duan P, Chen Y, Zhao WD, Wang Q, Tu HY, Zhang Q (2014) RyR2 modulates a Ca2+-activated K+ current in mouse cardiac myocytes. PloS one 9:e94905. doi: 10.1371/journal.pone.0094905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muse ED, Wineinger NE, Spencer EG, Peters M, Henderson R, Zhang Y, Barrett PM, Rivera SP, Wohlgemuth JG, Devlin JJ, Shiffman D, Topol EJ (2018) Validation of a genetic risk score for atrial fibrillation: A prospective multicenter cohort study. PLoS Med 15:e1002525. doi: 10.1371/journal.pmed.1002525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ni Y, Wang T, Zhuo X, Song B, Zhang J, Wei F, Bai H, Wang X, Yang D, Gao L, Ma A (2013) Bisoprolol reversed small conductance calcium-activated potassium channel (SK) remodeling in a volume-overload rat model. Mol Cell Biochem 384:95–103. doi: 10.1007/s11010-013-1785-5 [DOI] [PubMed] [Google Scholar]
- 66.Olesen MS, Jabbari J, Holst AG, Nielsen JB, Steinbruchel DA, Jespersen T, Haunso S, Svendsen JH (2011) Screening of KCNN3 in patients with early-onset lone atrial fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 13:963–967. doi: 10.1093/europace/eur007 [DOI] [PubMed] [Google Scholar]
- 67.Ortega A, Tarazon E, Rosello-Lleti E, Gil-Cayuela C, Lago F, Gonzalez-Juanatey JR, Cinca J, Jorge E, Martinez-Dolz L, Portoles M, Rivera M (2015) Patients with Dilated Cardiomyopathy and Sustained Monomorphic Ventricular Tachycardia Show Up-Regulation of KCNN3 and KCNJ2 Genes and CACNG8-Linked Left Ventricular Dysfunction. PloS one 10:e0145518. doi: 10.1371/journal.pone.0145518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozgen N, Dun W, Sosunov EA, Anyukhovsky EP, Hirose M, Duffy HS, Boyden PA, Rosen MR (2007) Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovascular research 75:758–769. doi: 10.1016/j.cardiores.2007.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parajuli N, Valtuille L, Basu R, Famulski KS, Halloran PF, Sergi C, Oudit GY (2015) Determinants of ventricular arrhythmias in human explanted hearts with dilated cardiomyopathy. Eur J Clin Invest 45:1286–1296. doi: 10.1111/eci.12549 [DOI] [PubMed] [Google Scholar]
- 70.Qi XY, Diness JG, Brundel BJ, Zhou XB, Naud P, Wu CT, Huang H, Harada M, Aflaki M, Dobrev D, Grunnet M, Nattel S (2014) Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation 129:430–440. doi: 10.1161/CIRCULATIONAHA.113.003019 [DOI] [PubMed] [Google Scholar]
- 71.Rafizadeh S, Zhang Z, Woltz RL, Kim HJ, Myers RE, Lu L, Tuteja D, Singapuri A, Bigdeli AA, Harchache SB, Knowlton AA, Yarov-Yarovoy V, Yamoah EN, Chiamvimonvat N (2014) Functional interaction with filamin A and intracellular Ca2+ enhance the surface membrane expression of a small-conductance Ca2+-activated K+ (SK2) channel. Proceedings of the National Academy of Sciences of the United States of America 111:9989–9994. doi: 10.1073/pnas.1323541111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reher TA, Wang Z, Hsueh CH, Chang PC, Pan Z, Kumar M, Patel J, Tan J, Shen C, Chen Z, Fishbein MC, Rubart M, Boyden P, Chen PS (2017) Small-Conductance Calcium-Activated Potassium Current in Normal Rabbit Cardiac Purkinje Cells. J Am Heart Assoc 6. doi: 10.1161/JAHA.117.005471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salama G, Bett GC (2014) Sex differences in the mechanisms underlying long QT syndrome. Am J Physiol Heart Circ Physiol 307:H640–648. doi: 10.1152/ajpheart.00864.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saljic A, Muthukumarasamy KM, la Cour JM, Boddum K, Grunnet M, Berchtold MW, Jespersen T (2019) Impact of arrhythmogenic calmodulin variants on small conductance Ca(2+) -activated K(+) (SK3) channels. Physiol Rep 7:e14210. doi: 10.14814/phy2.14210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanchez-Alonso JL, Bhargava A, O’Hara T, Glukhov AV, Schobesberger S, Bhogal N, Sikkel MB, Mansfield C, Korchev YE, Lyon AR, Punjabi PP, Nikolaev VO, Trayanova NA, Gorelik J (2016) Microdomain-Specific Modulation of L-Type Calcium Channels Leads to Triggered Ventricular Arrhythmia in Heart Failure. Circulation research 119:944–955. doi: 10.1161/CIRCRESAHA.116.308698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen MJ, Hao-Che C, Park HW, George Akingba A, Chang PC, Zheng Z, Lin SF, Shen C, Chen LS, Chen Z, Fishbein MC, Chiamvimonvat N, Chen PS (2013) Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart rhythm 10:910–915. doi: 10.1016/j.hrthm.2013.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shen MJ, Shinohara T, Park HW, Frick K, Ice DS, Choi EK, Han S, Maruyama M, Sharma R, Shen C, Fishbein MC, Chen LS, Lopshire JC, Zipes DP, Lin SF, Chen PS (2011) Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation 123:2204–2212. doi: 10.1161/CIRCULATIONAHA.111.018028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simo-Vicens R, Sauter DRP, Grunnet M, Diness JG, Bentzen BH (2017) Effect of antiarrhythmic drugs on small conductance calcium - activated potassium channels. Eur J Pharmacol 803:118–123. doi: 10.1016/j.ejphar.2017.03.039 [DOI] [PubMed] [Google Scholar]
- 79.Skibsbye L, Bengaard AK, Uldum-Nielsen AM, Boddum K, Christ T, Jespersen T (2018) Inhibition of Small Conductance Calcium-Activated Potassium (SK) Channels Prevents Arrhythmias in Rat Atria During beta-Adrenergic and Muscarinic Receptor Activation. Front Physiol 9:510. doi: 10.3389/fphys.2018.00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skibsbye L, Diness JG, Sorensen US, Hansen RS, Grunnet M (2011) The duration of pacing-induced atrial fibrillation is reduced in vivo by inhibition of small conductance Ca(2+)-activated K(+) channels. J Cardiovasc Pharmacol 57:672–681. doi: 10.1097/FJC.0b013e318217943d [DOI] [PubMed] [Google Scholar]
- 81.Skibsbye L, Poulet C, Diness JG, Bentzen BH, Yuan L, Kappert U, Matschke K, Wettwer E, Ravens U, Grunnet M, Christ T, Jespersen T (2014) Small-conductance calcium-activated potassium (SK) channels contribute to action potential repolarization in human atria. Cardiovascular research 103:156–167. doi: 10.1093/cvr/cvu121 [DOI] [PubMed] [Google Scholar]
- 82.Skibsbye L, Wang X, Axelsen LN, Bomholtz SH, Nielsen MS, Grunnet M, Bentzen BH, Jespersen T (2015) Antiarrhythmic Mechanisms of SK Channel Inhibition in the Rat Atrium. J Cardiovasc Pharmacol 66:165–176. doi: 10.1097/FJC.0000000000000259 [DOI] [PubMed] [Google Scholar]
- 83.Stowe DF, Gadicherla AK, Zhou Y, Aldakkak M, Cheng Q, Kwok WM, Jiang MT, Heisner JS, Yang M, Camara AK (2013) Protection against cardiac injury by small Ca(2+)-sensitive K(+) channels identified in guinea pig cardiac inner mitochondrial membrane. Biochim Biophys Acta 1828:427–442. doi: 10.1016/j.bbamem.2012.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strassmaier T, Bond CT, Sailer CA, Knaus HG, Maylie J, Adelman JP (2005) A novel isoform of SK2 assembles with other SK subunits in mouse brain. The Journal of biological chemistry 280:21231–21236. doi: 10.1074/jbc.M413125200 [DOI] [PubMed] [Google Scholar]
- 85.Tenma T, Mitsuyama H, Watanabe M, Kakutani N, Otsuka Y, Mizukami K, Kamada R, Takahashi M, Takada S, Sabe H, Tsutsui H, Yokoshiki H (2018) Small-conductance Ca(2+)-activated K(+) channel activation deteriorates hypoxic ventricular arrhythmias via CaMKII in cardiac hypertrophy. Am J Physiol Heart Circ Physiol 315:H262–H272. doi: 10.1152/ajpheart.00636.2017 [DOI] [PubMed] [Google Scholar]
- 86.Terentyev D, Rochira JA, Terentyeva R, Roder K, Koren G, Li W (2014) Sarcoplasmic reticulum Ca(2)(+) release is both necessary and sufficient for SK channel activation in ventricular myocytes. Am J Physiol Heart Circ Physiol 306:H738–746. doi: 10.1152/ajpheart.00621.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Torrente AG, Zhang R, Wang H, Zaini A, Kim B, Yue X, Philipson KD, Goldhaber JI (2017) Contribution of small conductance K(+) channels to sinoatrial node pacemaker activity: insights from atrial-specific Na(+) /Ca(2+) exchange knockout mice. The Journal of physiology 595:3847–3865. doi: 10.1113/JP274249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsai WC, Chan YH, Hsueh CH, Everett THt, Chang PC, Choi EK, Olaopa MA, Lin SF, Shen C, Kudela MA, Rubart-von der Lohe M, Chen Z, Jadiya P, Tomar D, Luvison E, Anzalone N, Patel VV, Chen PS (2016) Small conductance calcium-activated potassium current and the mechanism of atrial arrhythmia in mice with dysfunctional melanocyte-like cells. Heart rhythm 13:1527–1535. doi: 10.1016/j.hrthm.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turker I, Yu CC, Chang PC, Chen Z, Sohma Y, Lin SF, Chen PS, Ai T (2013) Amiodarone inhibits apamin-sensitive potassium currents. PloS one 8:e70450. doi: 10.1371/journal.pone.0070450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tuteja D, Rafizadeh S, Timofeyev V, Wang S, Zhang Z, Li N, Mateo RK, Singapuri A, Young JN, Knowlton AA, Chiamvimonvat N (2010) Cardiac small conductance Ca2+-activated K+ channel subunits form heteromultimers via the coiled-coil domains in the C termini of the channels. Circulation research 107:851–859. doi: 10.1161/CIRCRESAHA.109.215269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, Xu Y, Nie L, Vazquez AE, Young JN, Glatter KA, Chiamvimonvat N (2005) Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol 289:H2714–2723. doi:00534.2005 [pii] 10.1152/ajpheart.00534.2005 [DOI] [PubMed] [Google Scholar]
- 92.Verkerk AO, Wilders R, de Geringel W, Tan HL (2006) Cellular basis of sex disparities in human cardiac electrophysiology. Acta Physiol (Oxf) 187:459–477. doi: 10.1111/j.1748-1716.2006.01586.x [DOI] [PubMed] [Google Scholar]
- 93.Wan J, Chen M, Wang Z, Everett THt, Rubart-von der Lohe M, Shen C, Qu Z, Weiss JN, Boyden PA, Chen PS (2019) Small-conductance calcium-activated potassium current modulates the ventricular escape rhythm in normal rabbit hearts. Heart rhythm 16:615–623. doi: 10.1016/j.hrthm.2018.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang S, Zhou X, Huang B, Wang Z, Zhou L, Chen M, Yu L, Jiang H (2016) Spinal cord stimulation suppresses atrial fibrillation by inhibiting autonomic remodeling. Heart rhythm 13:274–281. doi: 10.1016/j.hrthm.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 95.Wu AZ, Chen M, Yin D, Everett THt, Ch0en Z, Rubart M, Weiss JN, Qu Z, Chen PS (2020) Sex-specific IKAS activation in rabbit ventricles with drug-induced QT prolongation. Heart rhythm. doi: 10.1016/j.hrthm.2020.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP (1998) Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395:503–507. doi: 10.1038/26758 [DOI] [PubMed] [Google Scholar]
- 97.Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, Nie L, Tuxson HR, Young JN, Glatter KA, Vazquez AE, Yamoah EN, Chiamvimonvat N (2003) Molecular identification and functional roles of a Ca(2+)-activated K+ channel in human and mouse hearts. J Biol Chem 278:49085–49094 [DOI] [PubMed] [Google Scholar]
- 98.Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, Nie L, Tuxson HR, Young JN, Glatter KA, Vazquez AE, Yamoah EN, Chiamvimonvat N (2003) Molecular identification and functional roles of a Ca(2+)-activated K+ channel in human and mouse hearts. The Journal of biological chemistry 278:49085–49094. doi: 10.1074/jbc.M307508200 [DOI] [PubMed] [Google Scholar]
- 99.Yang D, Wang T, Ni Y, Song B, Ning F, Hu P, Luo L, Wang Y, Ma A (2015) Apamin-Sensitive K+ Current Upregulation in Volume-Overload Heart Failure is Associated with the Decreased Interaction of CK2 with SK2. J Membr Biol 248:1181–1189. doi: 10.1007/s00232-015-9839-0 [DOI] [PubMed] [Google Scholar]
- 100.Yao JL, Zhou YF, Yang XJ, Qian XD, Jiang WP (2015) KCNN3 SNP rs13376333 on Chromosome 1q21 Confers Increased Risk of Atrial Fibrillation. Int Heart J 56:511–515. doi: 10.1536/ihj.15-133 [DOI] [PubMed] [Google Scholar]
- 101.Yi F, Ling TY, Lu T, Wang XL, Li J, Claycomb WC, Shen WK, Lee HC (2015) Down-regulation of the small conductance calcium-activated potassium channels in diabetic mouse atria. The Journal of biological chemistry 290:7016–7026. doi: 10.1074/jbc.M114.607952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yin D, Chen M, Yang N, Wu AZ, Xu D, Tsai WC, Yuan Y, Tian Z, Chan YH, Shen C, Chen Z, Lin SF, Weiss JN, Chen PS, Everett THt (2018) Role of apamin-sensitive small conductance calcium-activated potassium currents in long-term cardiac memory in rabbits. Heart rhythm 15:761–769. doi: 10.1016/j.hrthm.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yin D, Yang N, Tian Z, Wu AZ, Xu D, Chen M, Kamp NJ, Wang Z, Shen C, Chen Z, Lin SF, Rubart-von der Lohe M, Chen PS, Everett THt (2020) Effects of ondansetron on apamin-sensitive small conductance calcium-activated potassium currents in pacing-induced failing rabbit hearts. Heart rhythm 17:332–340. doi: 10.1016/j.hrthm.2019.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu CC, Ai T, Weiss JN, Chen PS (2014) Apamin does not inhibit human cardiac Na+ current, L-type Ca2+ current or other major K+ currents. PloS one 9:e96691. doi: 10.1371/journal.pone.0096691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu CC, Chia-Ti T, Chen PL, Wu CK, Chiu FC, Chiang FT, Chen PS, Chen CL, Lin LY, Juang JM, Ho LT, Lai LP, Yang WS, Lin JL (2016) KCNN2 polymorphisms and cardiac tachyarrhythmias. Medicine (Baltimore) 95:e4312. doi: 10.1097/MD.0000000000004312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu CC, Corr C, Shen C, Shelton R, Yadava M, Rhea IB, Straka S, Fishbein MC, Chen Z, Lin SF, Lopshire JC, Chen PS (2015) Small conductance calcium-activated potassium current is important in transmural repolarization of failing human ventricles. Circulation Arrhythmia and electrophysiology 8:667–676. doi: 10.1161/CIRCEP.114.002296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu L, Wang S, Zhou X, Wang Z, Huang B, Liao K, Saren G, Chen M, Po SS, Jiang H (2016) Chronic Intermittent Low-Level Stimulation of Tragus Reduces Cardiac Autonomic Remodeling and Ventricular Arrhythmia Inducibility in a Post-Infarction Canine Model. JACC Clin Electrophysiol 2:330–339. doi: 10.1016/j.jacep.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 108.Yu T, Deng C, Wu R, Guo H, Zheng S, Yu X, Shan Z, Kuang S, Lin Q (2012) Decreased expression of small-conductance Ca2+-activated K+ channels SK1 and SK2 in human chronic atrial fibrillation. Life Sci 90:219–227. doi: 10.1016/j.lfs.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 109.Yu Y, Luo D, Li Z, Zhang J, Li F, Qiao J, Yu F, Li M (2020) Inhibitory Effects of Dronedarone on Small Conductance Calcium Activated Potassium Channels in Patients with Chronic Atrial Fibrillation: Comparison to Amiodarone. Med Sci Monit 26:e924215. doi: 10.12659/MSM.924215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang M, Meng XY, Cui M, Pascal JM, Logothetis DE, Zhang JF (2014) Selective phosphorylation modulates the PIP2 sensitivity of the CaM-SK channel complex. Nat Chem Biol 10:753–759. doi: 10.1038/nchembio.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Q, Timofeyev V, Lu L, Li N, Singapuri A, Long MK, Bond CT, Adelman JP, Chiamvimonvat N (2008) Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circ Res 102:465–471. doi:CIRCRESAHA.107.161778 [pii] 10.1161/CIRCRESAHA.107.161778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang XD, Coulibaly ZA, Chen WC, Ledford HA, Lee JH, Sirish P, Dai G, Jian Z, Chuang F, Brust-Mascher I, Yamoah EN, Chen-Izu Y, Izu LT, Chiamvimonvat N (2018) Coupling of SK channels, L-type Ca(2+) channels, and ryanodine receptors in cardiomyocytes. Sci Rep 8:4670. doi: 10.1038/s41598-018-22843-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang XD, Timofeyev V, Li N, Myers RE, Zhang DM, Singapuri A, Lau VC, Bond CT, Adelman J, Lieu DK, Chiamvimonvat N (2014) Critical roles of a small conductance Ca(2)(+)-activated K(+) channel (SK3) in the repolarization process of atrial myocytes. Cardiovascular research 101:317–325. doi: 10.1093/cvr/cvt262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Z, Ledford HA, Park S, Wang W, Rafizadeh S, Kim HJ, Xu W, Lu L, Lau VC, Knowlton AA, Zhang XD, Yamoah EN, Chiamvimonvat N (2017) Distinct subcellular mechanisms for the enhancement of the surface membrane expression of SK2 channel by its interacting proteins, alpha-actinin2 and filamin A. The Journal of physiology 595:2271–2284. doi: 10.1113/JP272942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou X, Zhou L, Wang S, Yu L, Wang Z, Huang B, Chen M, Wan J, Jiang H (2016) The Use of Noninvasive Vagal Nerve Stimulation to Inhibit Sympathetically Induced Sinus Node Acceleration: A Potential Therapeutic Approach for Inappropriate Sinus Tachycardia. Journal of cardiovascular electrophysiology 27:217–223. doi: 10.1111/jce.12859 [DOI] [PubMed] [Google Scholar]