Abstract
Multimodality ophthalmic imaging systems aim to enhance the contrast, resolution, and functionality of existing technologies to improve disease diagnostics and therapeutic guidance. These systems include advanced acquisition and post-processing methods using optical coherence tomography (OCT), combined scanning laser ophthalmoscopy and OCT systems, adaptive optics, surgical guidance, and photoacoustic technologies. Here, we provide an overview of these ophthalmic imaging systems and their clinical and basic science applications.
Keywords: adaptive optics, multimodal, ophthalmic imaging, optical coherence tomography, scanning laser ophthalmoscopy
Introduction
Since the first demonstration of fundus photography, technological advancements have allowed for increasingly high-resolution and high-contrast imaging of the anterior and posterior eye. Development of scanning laser ophthalmoscopy (SLO) in 1980 allowed for en face retinal imaging with significantly reduced light exposure compared with conventional indirect ophthalmoscopy, with recent advancements focused on providing an ultra-wide field of view (FOV).1–3 The introduction of optical coherence tomography (OCT) in 1991 enabled depth-resolved volumetric imaging, further facilitating ophthalmic disease diagnosis by providing access to subsurface features. 4 Adaptive optics (AO) was first introduced for fundoscopy in 1997 and has since been applied to both OCT and SLO to achieve cellular-resolution ophthalmic imaging by correcting aberrations of the eye. 5 Recent advances have focused on supplementing structural contrast with functional modalities, such as OCT-based quantification of the metabolic and biomechanical properties of ocular tissue and multimodality systems such as OCT-SLO, surgical microscope–integrated OCT, and multimodal photoacoustic microscopy (PAM). Here, we provide an overview of these ophthalmic imaging systems and their clinical and basic science applications (see Table 1).
Table 1.
Summary of multimodal ophthalmic imaging technologies.
| Technology | Application | In vivo ophthalmic human demonstration | Commercially available clinical system | Clinical or research focus |
|---|---|---|---|---|
| OCT angiography | Vascular imaging | Yes | Yes | Clinical |
| Polarization-sensitive OCT | Tissue depolarization and birefringence | Yes | No | Research |
| Optical coherence elastography Phase-decorrelation OCT |
Tissue biomechanics | Yes | No | Research |
| Photothermal OCT Pump-probe OCT |
Molecular contrast | No | No | Research |
| OCT + Scanning laser ophthalmoscopy | Motion-tracking | Yes | Yes | Clinical |
| Surgical visualization | Surgical imaging | Yes | Yes | Clinical |
| Photoacoustic imaging | Vascular imaging | No | No | Research |
OCT, optical coherence tomography.
OCT-derived contrast mechanisms
OCT is the clinical standard for diagnosing and monitoring ophthalmic diseases and enables subsurface visualization of tissue scattering. A combination of system modifications and post-processing algorithms can be applied to achieve a variety of complementary imaging modes that probe additional contrast mechanisms. Examples of these multimodal OCT techniques include optical coherence tomographic angiography (OCTA), polarization-sensitive OCT (PS-OCT), optical coherence elastography (OCE), phase-decorrelation OCT (PhD-OCT), photothermal OCT (PT-OCT), and pump-probe OCT (PP-OCT).
Optical coherence tomographic angiography
Changes to retinal vascular density and perfusion accompany many ophthalmic diseases, including glaucoma, age-related macular degeneration (AMD), and diabetic retinopathy (DR). 6 Fluorescein angiography (FA) and indocyanine green angiography (ICGA) have traditionally been used to detect vascular leakage, neovascularization, and occlusions. 7 However, these techniques require injection of exogenous contrast and lack depth selectivity for specific retinal layers. 8 Several different OCT acquisition and post-processing methods, collectively termed OCTA, have emerged as non-invasive alternatives for volumetric imaging of retinal vascular perfusion. Doppler OCT uses phase differences between repeated OCT acquisitions to estimate Doppler frequency shift and the relative velocity of flowing scatterers within perfused vessels. 9 However, phase-based angiography techniques are highly susceptible to bulk motion, which reduces sensitivity to blood flow. Power-Doppler methods, including optical micro-angiography (OMAG),10,11 phase variance, 12 and Doppler variance angiography, 13 trade off flow velocity resolution for reduced sensitivity to bulk motion. These methods have demonstrated benefits for high resolution in vivo imaging of retinal and choroidal perfusion. Intensity-based angiography methods, such as split-spectrum amplitude-decorrelation angiography (SSADA) 14 and speckle decorrelation, 15 use intensity changes between repeated OCT cross sections as surrogate measures of blood flow and have been translated for clinical imaging of changes in optic disk perfusion in glaucoma and choroidal neovascularization in AMD patients.16–19 Angiography methods that take advantage of both the amplitude and phase of the OCT signal further enhance robustness against phase instability and include complex differential variance (CDV)20,21 and eigenvalue decomposition. 22 SSADA and OMAG have been integrated into commercially available systems (Optovue AngioVue and Zeiss AngioPlex, respectively), enabling high-quality clinical imaging and quantification of retinal perfusion.23,24
Handheld OCTA
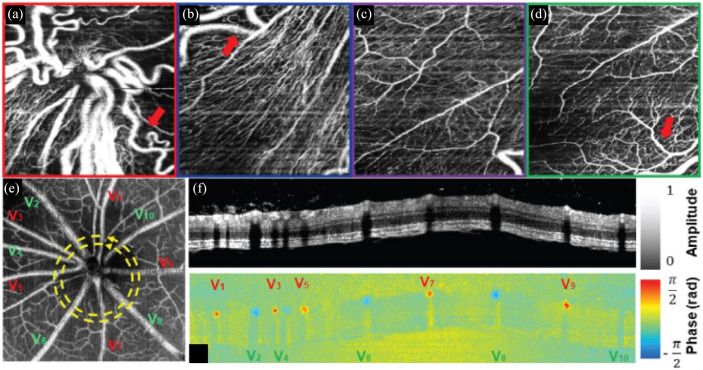
Despite commercialization of OCTA, the majority of these systems require patients be imaged upright, thus precluding imaging in supine or bedridden patients. To address these limitations, several handheld OCT probes have been developed for point-of-care imaging,25,26 including in pediatric patients. 27 Commercially available handheld OCT devices, such as the iVue (Optovue, Inc, Fremont, CA, USA) and Envisu C2300 (Leica Microsystems, GmbH, Wetzlar, DE), have relatively slow imaging speeds that limit sampling density and OCTA sensitivity. Research prototypes optimized for OCTA imaging28–30 increase speeds by an order of magnitude compared with current-generation commercial systems by using 100–400 kHz swept sources. Clinical imaging of retinopathy of prematurity (ROP) patients using these systems has provided in vivo images of the foveal avascular zone and retinal capillary complex in neonates (Figure 1(a)–(d)).31,32
Figure 1.
(a)–(d) Handheld OCTA of ROP at the (a) optic nerve head, (b) peripapillary region, (c) perifoveal region, and (d) margin of the fovea. Visible-light OCT in a rodent model showing (e) OCTA projection with delineation of arteries (red) and veins (green), and circumpapillary (f) retinal structure and (g) Doppler blood flow cross section.
Source: Reprinted with permission from Viehland and colleagues 31 and Pi and colleagues. 43
OCT, optical coherence tomography; OCTA, optical coherence tomographic angiography; ROP, retinopathy of prematurity.
Visible-light OCTA
Ophthalmic OCT has conventionally been performed using near-infrared wavelengths because of the availability of light sources and benefits in reduced scattering and increased penetration depths. Commercial availability of broadband supercontinuum light sources has enabled OCT imaging at visible wavelengths, which provides access to more endogenous and exogenous molecular contrast mechanisms.33–38 When combined with OCTA maps of retinal vascular perfusion, visible-light OCT can provide complementary contrast for quantifying blood oxygenation as a surrogate for quantifying metabolic changes associated with DR and AMD (Figure 1(e)–(g)).36,38–45 In vivo visible-light OCT retinal imaging has been demonstrated, and development of robust clinical systems is ongoing.36,46–48
Clinical utility and challenges
Ongoing clinical OCTA research focuses on imaging in AMD, DR, and diabetic macular edema (DME) and vascular response to therapy or surgery.49–52 Wide-field OCTA systems have also been developed and are capable of imaging a 100° FOV, which benefits imaging of the peripheral retina.53,54 Despite significant advantages over FA and ICGA, the clinical utility of OCTA is limited by several key factors. Engineering advancements in OCTA algorithms require physician education for proper interpretation of angiograms. 55 OCTA algorithms also require repeated imaging and dense sampling over small FOVs, making them susceptible to bulk motion during long acquisitions. Handheld OCTA imaging is further complicated by combined patient and photographer motion, both of which degrade vascular contrast and resolution, distort anatomic features, and preclude robust quantitative measurements.31,56 Additional image artifacts, such as shadowing from large retinal vessels, axially smeared vessel cross sections as a result of scattering, and out-of-focus OCTA projections, can significantly impact vessel segmentation algorithms and quantitative analysis of retinal vascularity.57–60 Motion-compensation methods, such as novel scanning or eye-tracking technologies, are actively being studied to overcome these limitations.61–64 Furthermore, vessel enhancement techniques, such as the complex continuous wavelet transform and multiple en face registration and averaging, can be applied to improve the accuracy of vessel segmentation.65–68 Despite these methods, various commercial systems employ different post-processing and segmentation algorithms, making interpretation by clinicians challenging. 7 While visible-light OCT provides complementary endogenous functional contrast, clinical translation of the technology is challenging because visible wavelengths are distracting and make fixation extremely difficult. 48 In addition, visible-light OCT images routinely require dense averaging to overcome low image quality that results from higher laser noise and significantly lower maximum permission exposure compared with near-infrared sources. 69
Polarization-sensitive OCT
PS-OCT measures changes in polarization states between incident and back-reflected light, which can provide additional tissue-specific contrast. 70 The amplitude and relative phase delay between orthogonal polarization states can be used to compute parameters such as tissue birefringence and degree of polarization uniformity.71–77 These methods enable quantitative in vivo imaging of changes in tissue properties resulting from disease progression and treatment.78,79
Anterior segment
PS-OCT is particularly well suited for corneal imaging because the collagen stroma exhibits strong birefringence. 80 Previous works have shown that corneal birefringence is related to fibril orientation in the lamellae, and quantitative metrics, such as phase retardation and slow axis orientation, have been used to distinguish healthy from diseased corneas.81,82 Keratoconus, which is characterized by thinning and distortion of the central cornea, exhibits increases in phase retardation near the rim of corneal thinning and changes in slow axis orientation resulting from rearrangement of stromal collagen. 83 PS-OCT can also enhance visualization of the trabecular meshwork, ciliary body, and iris, which can be used to study the progression of diseases such as glaucoma.84–86 Excess buildup of aqueous humor because of glaucoma can require surgical intervention, commonly trabeculectomy, which allows for aqueous flow into the subconjunctival space. Post-operative PS-OCT has characterized intra-bleb fibrosis and associated complications to benefit clinical management after trabeculectomy.87,88 Improvements in OCT speed and resolution have benefited PS-OCT by enabling quantitative measurement of corneal layer thicknesses and enhanced visualization of Bowman’s membrane and the sub-basal nerve plexus.89–92
Posterior segment
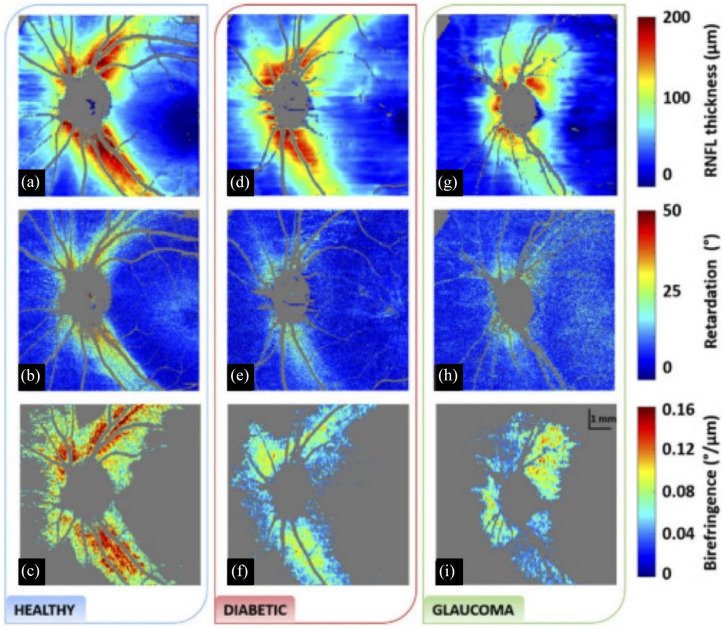
PS-OCT in the posterior segment has traditionally measured retinal nerve fiber layer (RNFL) birefringence and thickness.81,93–95 Similar to the cornea, bundles of parallel cylindrical axons in the RNFL exhibit high birefringence, and decreases in RNFL birefringence can potentially be used as a surrogate measure for ganglion cell and axonal atrophy in glaucoma. 96 Similar changes in phase retardation and birefringence resulting from RNFL thinning have been observed in diabetic eyes (Figure 2).97,98 PS-OCT characterization of the macula has shown that the retinal pigment epithelium (RPE) is highly depolarizing, scrambling the polarization of backscattered light from the RPE and choroid.99,100 Studies have quantified the degree of polarization uniformity (DOPU) from PS-OCT images and used it to aid RPE segmentation and thickness mapping, which can benefit quantitative monitoring of drusen and geographic atrophy in AMD.71,101–105 PS-OCT has also been used to detect and segment subretinal fibrosis, which is common in neovascular AMD and difficult to differentiate from other hyperreflective tissues.106,107
Figure 2.
Comparison of (a), (d), (g) RNFL thickness, (b), (e), (h) phase retardation, and (c), (f), (i) birefringence between healthy, diabetic, and glaucoma subjects. Reduced retardance is seen in both diabetic and glaucoma subjects compared with healthy subjects. In addition, there is significantly reduced birefringence in diabetic patients.
Source: Reprinted with permission from Desissaire and colleagues. 98
RNFL, retinal nerve fiber layer.
Clinical utility and challenges
The majority of PS-OCT research has focused on quantifying changes in tissue properties resulting from ophthalmic diseases. Polarization-sensitive OCTA has also been demonstrated for ophthalmic imaging using Jones matrix-based CDV. 21 The use of polarization-based angiography has advantages over conventional OCTA by improving vessel contrast and increasing image signal; four OCTA images (two incident and two detected polarization states) can be generated and averaged for increased SNR. 108 Despite the utility of PS-OCT in differentiating birefringent and depolarizing tissues in the eye, there are no commercially available PS-OCT systems routinely used in clinical ophthalmology. Challenges in clinical translation include increased cost and system complexity over conventional OCT systems. Many PS-OCT systems use free-space optics, which are sensitive to misalignment, 109 whereas fiber-based PS-OCT systems are more robust but are limited by birefringence and polarization mode dispersion of fiber-optic components. 110 One limitation of using depolarization maps for RPE segmentation is distinguishing it from the choroidal stroma. However, by combining PS-OCT with OCTA, the vessel-rich choroidal stroma can be separated from the vessel-free RPE and can be used to evaluate damage in the RPE layer due to serous RPE detachments.111,112
OCT elastography
OCE uses OCT imaging to detect micron-scale displacements caused by an external mechanical stimulus to extract biomechanical properties of tissue.113,114 PhD-OCT is an alternate method for measuring tissue biomechanics that uses the decorrelation of scattered light from Brownian motion as a surrogate measure of tissue viscosity.115–117 Initial OCE demonstrations used OCT speckle tracking of axial displacements from a static loading force to quantify tissue strain and derived Young’s modulus from the linear stress–strain relationship.118,119 OCE can also be used to measure Young’s and shear moduli by combining dynamic loading forces, such as steady-state harmonic loading and transient excitation sources, with advanced wave propagation models. 120 These dynamic OCE methods have been used to non-invasively measure biomechanical properties of the human cornea in vivo, showing the potential for clinical translation and utility. 121
Cornea
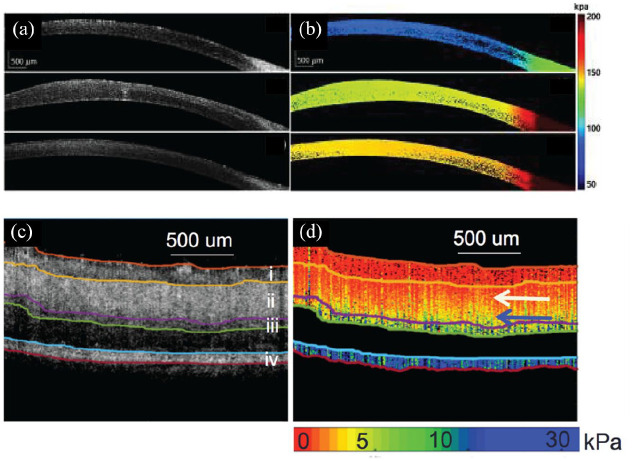
Corneal elasticity can be a useful direct measure for diagnosing keratoconus and corneal ectasia and monitoring corneal collagen crosslinking (CXL) treatment.122–125 The corneal elastic modulus changes with increased intraocular pressure (IOP) and can be used as an indirect measure for diagnosing glaucoma. 126 OCE has shown increased corneal stiffness in in vivo rabbit eyes after CXL treatments with an air puff as the external mechanical stimulus (Figure 3(a) and (b)).127–129 OCE studies using air-coupled ultrasound excitation, which is non-contact and more applicable for clinical translation, have successfully shown quantitative 4-dimensional (4D) visualization of corneal stiffness.130–133 More recent OCE demonstrations have used acoustic radiation force (ARF) loading with an ultrasound transducer, which has a faster response time as compared with air puff loading. 134 Additional OCE stimuli include passive mechanical perturbations caused by the heartbeat 135 or using a pulsed laser to induce mechanical waves, 136 but these methods have yet to be demonstrated in vivo. PhD-OCT has successfully identified changes in corneal biomechanics after CXL in vivo without the need for external stimuli. 117
Figure 3.
OCE imaging of the (a), (b), cornea and (c), (d) retina. (a) Structural OCT and (b) OCE elastogram cross sections of in vivo rabbit cornea pre-, post-, and 1 week after CXL treatment (top to bottom, respectively). (c) Structural OCT and (d) OCE elastogram cross sections of ex vivo porcine retina showing differences in retinal layer stiffness.
Source. Reprinted with permission from Zhou and colleagues 127 and Qu and colleagues. 142
CXL, corneal collagen crosslinking; OCE, optical coherence elastography; OCT, optical coherence tomography.
Lens
OCE can offer a quantitative, non-invasive method for early detection and monitoring of changes in lens biomechanics associated with cataracts and aging. 137 ARF-OCE has shown higher Young’s modulus in cataract lenses compared with healthy lenses in ex vivo rabbit eyes and significant increases in lens stiffness with aging in vivo. 138 OCE imaging has also been combined with Brillouin microscopy, which measures material stiffness using differences in Brillouin light scattering, to show a correlation between Young’s and Brillouin moduli and provide a more complete mapping of lens stiffness in ex vivo porcine eyes. 139 Finally, OCE has also been used to investigate changes in lens elasticity as a function of IOP. 140
Retina
Cellular changes in AMD can alter the elasticity of retinal tissue, making OCE a potential technology for early disease diagnosis. 141 ARF-OCE studies have shown distinct elasticity differences in retinal layers in in vivo rabbit and ex vivo porcine models (Figure 3(c) and (d)).142,143 Decreased retinal stiffness observed in in vivo rabbit AMD eyes was hypothesized to result from lymphocyte infiltration, but initial results did not show statistical significance. 143 OCE studies have also shown that increased optic nerve head Young’s modulus and posterior scleral stiffness are correlated with increasing IOP, which suggests that OCE can also be used to monitor progression of glaucoma.144,145
Clinical utility and challenges
Both OCE and PhD-OCT technologies are in the early stages of clinical translation, and current research is focused on improving imaging speed and phase stability. 146 Repeated images are used to compute differential phase in OCE, but this increases total imaging time and is susceptible to motion artifacts. Recent advances in swept-source OCT technology may overcome these limitations by enabling OCE imaging at megahertz rates. 147 Currently, there are no commercial OCE systems available for clinical use. The broad clinical adoption of OCT in ophthalmology may benefit clinical translation of multimodal OCE technologies pending successful identification of optimal mechanical stimulus mechanisms and clinical applications.
Molecular contrast methods
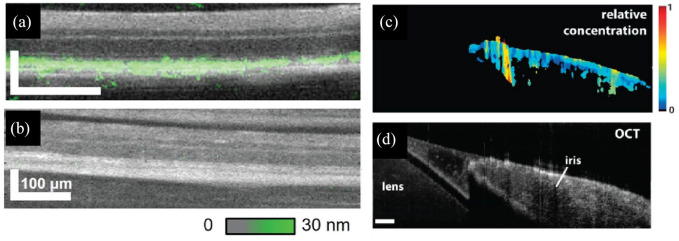
OCT sensitivity to changes in optical pathlength can be increased by leveraging phase information and used to probe additional mechanisms of contrast. As an example, light absorption by endogenous or exogenous contrast agents induces local temperature gradients and index of refraction changes. PT-OCT uses these index changes as a complementary contrast mechanism for detecting functional cellular and subcellular changes that could enable earlier disease detection. 148 PT-OCT has been demonstrated using exogenous gold nanoparticles and endogenous melanin in the RPE and choroid (Figure 4(a) and (b)) in in vivo and ex vivo animal models.148–151 Most recently, PT-OCT using indocyanine green (ICG) was demonstrated as a method for distinguishing the inner limiting membrane, which is of interest for macular surgery applications. 152 PP-OCT is another technique for adding molecular contrast to OCT imaging. PP-OCT excites ground-state electrons using a modulated excitation (pump) and detects the transient absorption at a lower energy wavelength (probe) to identify specific molecular compounds such as methylene blue, hemoglobin, or melanin.153,154 PP-OCT imaging in ex vivo porcine iris has demonstrated sensitivity to melanin, which may be a useful diagnostic tool for ocular melanoma (Figure 4(c) and (d)). 155
Figure 4.
Molecular contrast imaging. In vivo PT-OCT comparing (a) pigmented to (b) albino mouse retina with computed melanin concentration (green) overlaid on OCT cross sections. PP-OCT image of ex vivo porcine iris showing the (c) relative concentration of melanin and (d) corresponding OCT cross section.
Source: Reprinted with permission from Lapierre-Landry and colleagues 150 and Jacob and colleagues. 155
OCT, optical coherence tomography; PP-OCT, pump-probe OCT; PT-OCT, photothermal OCT.
Clinical utility and challenges
PT-OCT and PP-OCT are promising multimodal techniques that supplement OCT contrast, but neither have been demonstrated in in vivo human applications nor have commercialized clinical systems. Tissue heating and high excitation power levels are two safety concerns that need to be addressed. Acquisition speed is an additional limiting factor because of the need to critically sample the modulation frequency of the pulsed light source and slow heating response of absorbers. 152
OCT-SLO
The inherent orthogonal priority acquisition orientations between OCT and SLO provide complementary depth-resolved and en face information that makes combined OCT-SLO an ideal multimodality technology. These systems can leverage fundus features for motion-tracking and volumetric imaging of anatomic structures and pathologies.
Tracking SLO
SLO has been used extensively for aiming and motion compensation of ophthalmic OCT.156–159 Tracking SLO has been particularly critical for OCTA, which requires dense sampling over small FOVs and is, therefore, difficult to target ROIs and highly susceptible to saccadic and bulk motion artifacts. 62 Many current generation combined OCT-SLO systems use shared optics and scanners to relay light into the eye to reduce overall system complexity and provide pixel-level co-registration between corresponding OCT and SLO images.160–163 SLO motion-tracking precision is related to both spatial and temporal resolution, and development of line-scanning SLO (LSLO) has overcome frame-rate limitations of conventional point-scanning SLO systems at the expense of full confocality, allowing imaging of fast dynamics up to several hundred frames per second.163–166
Handheld OCT-SLO
Multimodal OCT-SLO systems have traditionally been integrated with slit lamps that required patients to sit upright during imaging. The development of compact OCT-SLO handheld probes has benefited ophthalmic disease diagnostics in pediatric and bedridden patients.27,167 When combined with a white-light supercontinuum light source, additional spectroscopic information can be extracted, including color SLO images that can be used to detect early signs of fundus discoloration in AMD. 168 Novel extended source LSLO illumination and detection schemes, such as those using spectral encoding, have replaced complex free-space relays from source to detector with fiber optics, and handheld prototypes using these technologies have recently been used for motion-corrected in vivo ophthalmic OCT and OCTA.30,169,170
Adaptive optics
Lateral resolution in ophthalmic imaging is fundamentally limited by wavefront aberrations of the eye. AO overcomes this limitation by actively compensating for these aberrations using a deformable mirror, enabling cellular-resolution in vivo imaging.5,171 Combined AO-SLO and AO-OCT systems are capable of imaging individual photoreceptors with up to two to three times higher transverse resolution than conventional SLO and OCT.172–175 When combined with hardware- and software-based retinal-tracking technologies, 176 AO systems can be used to quantify retinal photoreceptor distributions across large FOVs and study cellular changes associated with retinal disease progression (Figure 5(a)–(f)).103,177–207 In addition to structural imaging, AO systems have also demonstrated benefits for functional imaging, including visualizing perfusion in the retinal microvasculature and phase dynamics from photoreceptor photostimulation.208–210
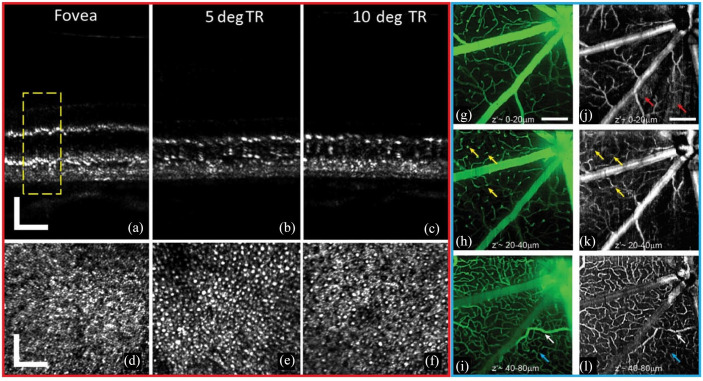
Figure 5.
In vivo human (a)–(c) AO-OCT and (d)–(f) AO-SLO showing photoreceptors at (a), (d) the fovea, (b), (e) 5° TR, and (c), (f) 10° TR. Scale bar = 50 µm. (g)–(i) oSLO fluorescence projections and (j)–(l) corresponding OCTA projections showing murine retinal perfusion. Scale bar = 200 µm.
Source: Reprinted with permission from Wells-Gray and colleagues 201 and Zhang and colleagues. 218
AO-OCT, adaptive optics OCT; AO-SLO, adaptive optics SLO; OCTA, optical coherence tomographic angiography; oSLO, oblique scanning laser ophthalmoscopy; TR, temporal retina.
Fluorescence SLO
The combination of SLO and fluorescence contrast is uniquely suited for high-specificity functional imaging in clinical ophthalmology and basic science. Traditional clinical applications have included fundus autofluorescence to detect endogenous fluorophores and exogenous fluorescein or ICG to visualize retinal vascular perfusion and leakage. 211 In animal models, combined OCT and fluorescence SLO systems enabled longitudinal imaging of retinal structure and characterization of function and cell populations in transgenic models expressing fluorescent proteins in serum albumin, microglia, and photoreceptors.212,213 Animal models also provide an opportunity to study the effects of retinal injury, including laser-induced choroidal neovascularization, retinal vascular occlusion, laser lesioning, and image-guided intraocular drug-delivery.214–217 One major limitation of fluorescence SLO is its limited depth-sectioning and volumetric imaging capability. Oblique SLO (oSLO) addresses this limitation by using obliquely oriented excitation and detection paths to acquire fluorescence cross sections and volumes (Figure 5(g)–(l)). 218 Fluorescence SLO can also be combined with AO to improve depth-sectioning and lateral resolution to enhance visualization of individual photoreceptors, ganglion cells, and RPE cells in animal models.219–224
Clinical utility and challenges
Combined OCT-SLO technology has been integrated into commercial systems, including the Heidelberg Spectralis, Optos Silverstone, and Zeiss PLEX Elite, for active motion-tracking and high-resolution en face and cross-sectional imaging of the fundus. While several AO systems, such as the Imagine Eyes rtx1 and Physical Sciences compact AO retinal imager (CAORI), are commercially available, broad adoption of the technology is limited because of system complexity, the need for long imaging and post-processing times, and lack of standardized processing and analysis algorithms. 225 Sensorless AO systems have been demonstrated in research prototypes and eliminate the need for physical wavefront sensors, which significantly reduces system complexity.226–230 These technologies have been recently integrated into handheld probes for AO imaging in supine and pediatric patients, which may further motivate broad clinical adoption.231,232 Fluorescence angiography and fundus autofluorescence are well established in clinical ophthalmology. More recently, autofluorescence lifetimes have been used as a surrogate for retinal metabolism that provides additional contrast for identifying ophthalmic and systemic diseases.233–235
Multimodal surgical visualization
Integration of OCT with ophthalmic surgical microscopy can provide additional high-resolution depth-resolved volumetric visualization of tissue microstructures that benefits surgical decision-making. 236 Initial demonstrations of intraoperative OCT (iOCT) were performed with handheld probes during surgery,237–239 and the technology has since advanced to microscope-integrated systems that allow for OCT imaging concurrent with ophthalmic surgery.240,241 Recent iOCT research has focused on improving speed and ergonomics, such as automated instrument-tracking and enhanced visualization. The combination of high-speed swept-source OCT systems with real-time volumetric rendering methods has allowed for 4D visualization of surgical dynamics (Figure 6(b)).242–244 OCTA imaging has also been explored as a method for integrating vascular contrast to further enhance intraoperative visualization.245,246
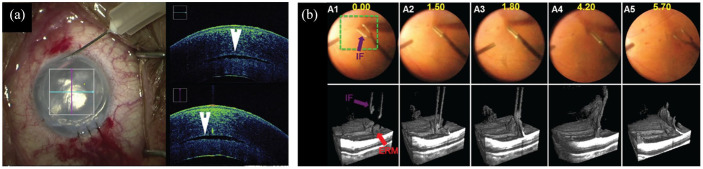
Figure 6.
iOCT visualization during (a) anterior and (b) posterior segment surgery. (a) iOCT showing graft placement during corneal transplant showing a persistent fluid interface (arrowhead). (b) 4D iOCT imaging of forces peeling epiretinal membrane.
Source: Reprinted with permission from Carrasco-Zevallos and colleagues 243 and Ehlers and colleagues. 249
4D, 4-dimensional; iOCT, intraoperative optical coherence tomography.
Clinical utility and challenges
Multiple iOCT systems are commercially available, including the Haag-Streit Surgical iOCT, Zeiss RESCAN 700, and Leica EnFocus. 247 Clinical studies have shown that iOCT can benefit surgical decision-making for donor graft positioning during corneal transplant (Figure 6(a)) and during membrane peeling and retinal detachment repair procedures.248,249 One major challenge in iOCT imaging has been shadowing from optically opaque surgical instruments. However, semi-transparent, OCT-compatible surgical instruments have been explored to overcome these limitations. 250 While preliminary studies have shown the benefits of iOCT for providing real-time surgical feedback, additional improvements in the technology are being developed, and studies demonstrating clinical utility are ongoing.
Photoacoustic imaging
Photoacoustic imaging detects acoustic waves generated by thermoelastic expansion from the absorption of pulsed laser illumination and reconstructs a volume of absorbers.251,252 In ophthalmology, PAM primarily visualizes blood absorption and has advantages over FA and ICGA by not requiring exogenous contrast and OCTA by being sensitive to vascular leakage. PAM also has higher depth penetration compared with OCT and can achieve imaging depths exceeding 10 mm. 253 PAM demonstrations in animal models include visualization of deep retinal and choroidal vasculature, subretinal injections, and neovascularization. 254 Multimodal PAM and OCT imaging provides complementary structural and vascular contrast and has been used to visualize neovascularization in animal models of DR and wet-AMD.255–259 Combined PAM and OCT has also shown advantages for visualizing vascular and structural changes associated with retinal vein occlusion (Figure 7) and choroidal vascular occlusion over FA.260,261 Finally, PAM and OCT can be used to quantify retinal oxygen metabolism rates by combining blood flow measurements from Doppler OCT with hemoglobin oxygen saturation measured using multiwavelength PAM that may benefit early diagnosis of glaucoma, DR, and AMD.39,262
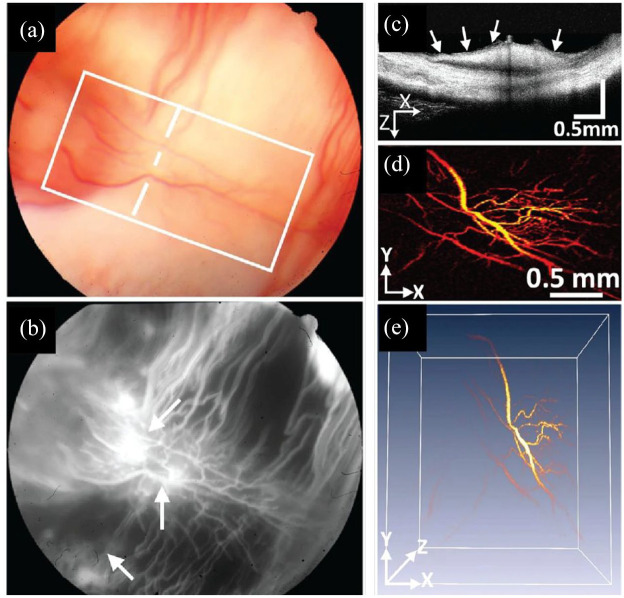
Figure 7.
In vivo PAM showing neovascularization in rabbit retina. (a) Color fundus, (b) FA, (c) cross-sectional OCT, (d) PAM maximum amplitude projection, and (e) PAM 3D reconstruction of neovascularization (arrows).
Source: Reprinted with permission from Nguyen and colleagues. 260
FA, fluorescein angiography; OCT, optical coherence tomography; PAM, photoacoustic microscopy; 3D, 3-dimensional.
Clinical utility and challenges
Although there are commercial PAM systems such as the Vevo LAZR-X for cardiac and neuroimaging, PAM imaging systems for ophthalmic applications are not currently available. 263 Barriers to clinical translation of PAM include system complexity, slow imaging speeds, and the need for a contact-PAM transducer. Multimodal PAM and OCT requires dedicated laser sources for PAM excitation and OCT imaging, a transducer for PAM detection, and an OCT engine. Methods to share a laser source between PAM and OCT to reduce system complexity have been proposed. However, hemoglobin absorption is low in the near-infrared, which limits PAM sensitivity at OCT wavelengths. 264
Conclusion
Multimodal ophthalmic imaging spans multiple optical and acoustic technologies. Many of these methods, such as OCTA, PS-OCT, OCE, PhD-OCT, PT-OCT, and PP-OCT, aim to add additional contrast to conventional OCT images. The complementary en face and cross-sectional information from combined SLO and OCT systems has benefited motion-tracking and aiming in clinical ophthalmology, and the addition of AO and fluorescence methods has further improved resolution and imaging specificity. Integration of OCT with surgical microscopy has benefited real-time surgical feedback and surgical decision-making. Finally, multimodal photoacoustic imaging systems enable quantitative imaging of blood perfusion and in vivo hemodynamics. Overall, multimodal ophthalmic imaging developments have led to improvements in clinical imaging and novel basic science research with new possibilities for clinical translation and improvements in disease diagnosis and therapeutic monitoring.
Acknowledgments
Morgan J. Ringel and Eric M. Tang contributed equally.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Vanderbilt University, the Vanderbilt Institute for Surgery and Engineering (VISE), and the US National Institutes of Health Grant No. R01-EY030490, R01-EY031769 and T32-EB021937. The content was solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iD: Yuankai K. Tao  https://orcid.org/0000-0002-6135-6241
https://orcid.org/0000-0002-6135-6241
Contributor Information
Morgan J. Ringel, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, USA
Eric M. Tang, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, USA
Yuankai K. Tao, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37235, USA.
References
- 1. Webb RH, Hughes GW, Pomerantzeff O. Flying spot TV ophthalmoscope. Appl Opt 1980; 19: 2991–2997. [DOI] [PubMed] [Google Scholar]
- 2. Webb RH, Hughes GW. Scanning laser ophthalmoscope. IEEE Trans Biomed Eng 1981; 28: 488–492. [DOI] [PubMed] [Google Scholar]
- 3. Patel SN, Shi A, Wibbelsman TD, et al. Ultra-widefield retinal imaging: an update on recent advances. Ther Adv Ophthalmol 2020; 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991; 254: 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang J, Williams DR, Miller DT. Supernormal vision and high-resolution retinal imaging through adaptive optics. J Opt Soc Am A Opt Image Sci Vis 1997; 14: 2884–2892. [DOI] [PubMed] [Google Scholar]
- 6. de Carlo TE, Romano A, Waheed NK, et al. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous 2015; 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spaide RF, Fujimoto JG, Waheed NK, et al. Optical coherence tomography angiography. Prog Retin Eye Res 2018; 64: 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spaide RF, Klancnik JM, Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 2015; 133: 45–50. [DOI] [PubMed] [Google Scholar]
- 9. Makita S, Hong Y, Yamanari M, et al. Optical coherence angiography. Opt Express 2006; 14: 7821–7840. [DOI] [PubMed] [Google Scholar]
- 10. Wang RK, Jacques SL, Ma Z, et al. Three dimensional optical angiography. Opt Express 2007; 15: 4083–4097. [DOI] [PubMed] [Google Scholar]
- 11. An L, Wang RK. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt Express 2008; 16: 11438–11452. [DOI] [PubMed] [Google Scholar]
- 12. Fingler J, Zawadzki RJ, Werner JS, et al. Volumetric microvascular imaging of human retina using optical coherence tomography with a novel motion contrast technique. Opt Express 2009; 17: 22190–22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu L, Chen Z. Doppler variance imaging for three-dimensional retina and choroid angiography. J Biomed Opt 2010; 15: 016029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012; 20: 4710–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mariampillai A, Standish BA, Moriyama EH, et al. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt Lett 2008; 33: 1530–1532. [DOI] [PubMed] [Google Scholar]
- 16. Jia Y, Morrison JC, Tokayer J, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express 2012; 3: 3127–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014; 121: 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jia Y, Baileya ST, Hwanga TS, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci USA 2015; 112: E2395–E2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu G, Jia Y, Pechauer AD, et al. Split-spectrum phase-gradient optical coherence tomography angiography. Biomed Opt Express 2016; 7: 2943–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nam AS, Chico-Calero I, Vakoc BJ. Complex differential variance algorithm for optical coherence tomography angiography. Biomed Opt Express 2014; 5: 3822–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braaf B, Donner S, Nam AS, et al. Complex differential variance angiography with noise-bias correction for optical coherence tomography of the retina. Biomed Opt Express 2018; 9: 486–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yousefi S, Zhi Z, Wang RK. Eigendecomposition-based clutter filtering technique for optical microangiography. IEEE Trans Biomed Eng 2011; 58: 2316–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. An L, Shen TT, Wang RK. Using ultrahigh sensitive optical microangiography to achieve comprehensive depth resolved microvasculature mapping for human retina. J Biomed Opt 2011; 16: 106013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chung CS, Nesper PL, Park JJ, et al. Comparison of Zeiss cirrus and Optovue RTVue OCT angiography systems: a quantitative and qualitative approach examining the three capillary networks in diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retin 2018; 49: E198–E205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jung W, Kim J, Jeon M, et al. Handheld optical coherence tomography scanner for primary care diagnostics. IEEE Trans Biomed Eng 2011; 58: 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu CD, Kraus MF, Potsaid B, et al. Handheld ultrahigh speed swept source optical coherence tomography instrument using a MEMS scanning mirror. Biomed Opt Express 2014; 5: 293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larocca F, Nankivil D, Dubose T, et al. In vivo cellular-resolution retinal imaging in infants and children using an ultracompact handheld probe. Nat Photonics 2016; 10: 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang J, Liu L, Campbell JP, et al. Handheld optical coherence tomography angiography. Biomed Opt Express 2017; 8: 2287–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campbell JP, Nudleman E, Yang J, et al. Handheld optical coherence tomography angiography and ultra-wide-field optical coherence tomography in retinopathy of prematurity. JAMA Ophthalmol 2017; 135: 977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malone JD, El-Haddad MT, Yerramreddy SS, et al. Handheld spectrally encoded coherence tomography and reflectometry for motion-corrected ophthalmic optical coherence tomography and optical coherence tomography angiography. Neurophotonics 2019; 6: 041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viehland C, Chen X, Tran-Viet D, et al. Ergonomic handheld OCT angiography probe optimized for pediatric and supine imaging. Biomed Opt Express 2019; 10: 2623–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song S, Zhou K, Xu JJ, et al. Development of a clinical prototype of a miniature hand-held optical coherence tomography probe for prematurity and pediatric ophthalmic imaging. Biomed Opt Express 2019; 10: 2383–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yi J, Li X. Estimation of oxygen saturation from erythrocytes by high-resolution spectroscopic optical coherence tomography. Opt Lett 2010; 35: 2094–2096. [DOI] [PubMed] [Google Scholar]
- 34. Robles FE, Wilson C, Grant G, et al. Molecular imaging true-colour spectroscopic optical coherence tomography. Nat Photonics 2011; 5: 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yi J, Wei Q, Liu W, et al. Visible-light optical coherence tomography for retinal oximetry. Opt Lett 2013; 38: 1796–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yi J, Chen S, Shu X, et al. Human retinal imaging using visible-light optical coherence tomography guided by scanning laser ophthalmoscopy. Biomed Opt Express 2015; 6: 3701–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chong SP, Merkle CW, Leahy C, et al. Quantitative microvascular hemoglobin mapping using visible light spectroscopic optical coherence tomography. Biomed Opt Express 2015; 6: 1429–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chong SP, Bernucci M, Radhakrishnan H, et al. Structural and functional human retinal imaging with a fiber-based visible light OCT ophthalmoscope. Biomed Opt Express 2017; 8: 323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu DY, Cringle SJ. Retinal degeneration and local oxygen metabolism. Exp Eye Res 2005; 80: 745–751. [DOI] [PubMed] [Google Scholar]
- 40. Nesper PL, Soetikno BT, Zhang HF, et al. OCT angiography and visible-light OCT in diabetic retinopathy. Vision Res 2017; 139: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen S, Yi J, Zhang HF. Measuring oxygen saturation in retinal and choroidal circulations in rats using visible light optical coherence tomography angiography. Biomed Opt Express 2015; 6: 2840–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pi S, Camino A, Zhang M, et al. Angiographic and structural imaging using high axial resolution fiber-based visible-light OCT. Biomed Opt Express 2017; 8: 4595–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pi S, Camino A, Wei X, et al. Rodent retinal circulation organization and oxygen metabolism revealed by visible-light optical coherence tomography. Biomed Opt Express 2018; 9: 5851–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pi S, Hormel TT, Wei X, et al. Retinal capillary oximetry with visible light optical coherence tomography. Proc Natl Acad Sci USA 2020; 117: 11658–11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen S, Shu X, Nesper PL, et al. Retinal oximetry in humans using visible-light optical coherence tomography [Invited]. Biomed Opt Express 2017; 8: 1415–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chong SP, Zhang T, Kho A, et al. Ultrahigh resolution retinal imaging by visible light OCT with longitudinal achromatization. Biomed Opt Express 2018; 9: 1477–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rubinoff I, Beckmann L, Wang Y, et al. Speckle reduction in visible-light optical coherence tomography using scan modulation. Neurophotonics 2019; 6: 041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shu X, Beckmann L, Wang Y, et al. Designing visible-light optical coherence tomography towards clinics. Quant Imaging Med Surg 2019; 9: 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Russell JF, Shi Y, Hinkle JW, et al. Longitudinal wide-field swept-source OCT angiography of neovascularization in proliferative diabetic retinopathy after panretinal photocoagulation. Ophthalmol Retina 2019; 3: 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen H, Chi W, Cai X, et al. Macular microvasculature features before and after vitrectomy in idiopathic macular epiretinal membrane: an OCT angiography analysis. Eye 2019; 33: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sacconi R, Tomasso L, Corbelli E, et al. Early response to the treatment of choroidal neovascularization complicating central serous chorioretinopathy: a OCT-angiography study. Eye 2019; 33: 1809–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vujosevic S, Toma C, Villani E, et al. Diabetic macular edema with neuroretinal detachment: OCT and OCT-angiography biomarkers of treatment response to anti-VEGF and steroids. Acta Diabetol 2020; 57: 287–296. [DOI] [PubMed] [Google Scholar]
- 53. Zhang Q, Rezaei KA, Saraf SS, et al. Ultra-wide optical coherence tomography angiography in diabetic retinopathy. Quant Imaging Med Surg 2018; 8: 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Couturier A, Rey PA, Erginay A, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti–vascular endothelial growth factor. Ophthalmology 2019; 126: 1685–1694. [DOI] [PubMed] [Google Scholar]
- 55. Hagag AM, Gao SS, Jia Y, et al. Optical coherence tomography angiography: technical principles and clinical applications in ophthalmology. Taiwan J Ophthalmol 2017; 7: 115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Munk MR, Giannakaki-Zimmermann H, Berger L, et al. OCT-angiography: a qualitative and quantitative comparison of 4 OCT-A devices. PLoS ONE 2017; 12: e0177059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bernucci MT, Merkle CW, Srinivasan VJ. Investigation of artifacts in retinal and choroidal OCT angiography with a contrast agent. Biomed Opt Express 2018; 9: 1020–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Camino A, Jia Y, Yu J, et al. Automated detection of shadow artifacts in optical coherence tomography angiography. Biomed Opt Express 2019; 10: 1514–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Choi WJ, Paulson B, Yu S, et al. Mean-subtraction method for de-shadowing of tail artifacts in cerebral OCTA images: a proof of concept. Materials 2020; 13: 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Holmen IC, Konda MS, Pak JW, et al. Prevalence and severity of artifacts in optical coherence tomographic angiograms. JAMA Ophthalmol 2020; 138: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ferguson RD, Hammer DX, Paunescu LA, et al. Tracking optical coherence tomography. Opt Lett 2004; 29: 2139–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Braaf B, Vienola KV, Sheehy CK, et al. Real-time eye motion correction in phase-resolved OCT angiography with tracking SLO. Biomed Opt Express 2013; 4: 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen S, Lou Z, Chen D, et al. An artificial flexible visual memory system based on an UV-motivated memristor. Adv Mater 2018; 30: 1705400. [DOI] [PubMed] [Google Scholar]
- 64. Wei X, Hormel TT, Guo Y, et al. High-resolution wide-field OCT angiography with a self-navigation method to correct microsaccades and blinks. Biomed Opt Express 2020; 11: 3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fathi A, Naghsh-Nilchi AR. Automatic wavelet-based retinal blood vessels segmentation and vessel diameter estimation. Biomed Signal Process Control 2013; 8: 71–80. [Google Scholar]
- 66. Eladawi N, Elmogy M, Helmy O, et al. Automatic blood vessels segmentation based on different retinal maps from OCTA scans. Comput Biol Med 2017; 89: 150–161. [DOI] [PubMed] [Google Scholar]
- 67. Uji A, Balasubramanian S, Lei J, et al. Multiple enface image averaging for enhanced optical coherence tomography angiography imaging. Acta Ophthalmol 2018; 96: e820–e827. [DOI] [PubMed] [Google Scholar]
- 68. Chu Z, Zhou H, Cheng Y, et al. Improving visualization and quantitative assessment of choriocapillaris with swept source OCTA through registration and averaging applicable to clinical systems. Sci Rep 2018; 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. ANSI Z136.1-1993. American national standard for the safe use of lasers. [Google Scholar]
- 70. Hee MR, Swanson EA, Fujimoto JG, et al. Polarization-sensitive low-coherence reflectometer for birefringence characterization and ranging. J Opt Soc Am B 1992; 9: 903–908. [Google Scholar]
- 71. Götzinger E, Pircher M, Geitzenauer W, et al. Retinal pigment epithelium segmentation by polarization sensitive optical coherence tomography. Opt Express 2008; 16: 16410–16422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Götzinger E, Pircher M, Baumann B, et al. Three-dimensional polarization sensitive OCT imaging and interactive display of the human retina. Opt Express 2009; 17: 4151–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. de Boer JF, Milner TE, van Gemert MJC, et al. Two-dimensional birefringence imaging in biological tissue by polarization-sensitive optical coherence tomography. Opt Lett 1997; 22: 934–936. [DOI] [PubMed] [Google Scholar]
- 74. de Boer JF, Milner TE, Nelson JS. Determination of the depth-resolved Stokes parameters of light backscattered from turbid media by use of polarization-sensitive optical coherence tomography. Opt Lett 1999; 24: 300–302. [DOI] [PubMed] [Google Scholar]
- 75. Saxer CE, de Boer JF, Park BH, et al. High-speed fiber–based polarization-sensitive optical coherence tomography of in vivo human skin. Opt Lett 2000; 25: 1355–1357. [DOI] [PubMed] [Google Scholar]
- 76. Jiao S, Yao G, Wang LV. Depth-resolved two-dimensional Stokes vectors of backscattered light and Mueller matrices of biological tissue measured with optical coherence tomography. Appl Opt 2000; 39: 6318–6324. [DOI] [PubMed] [Google Scholar]
- 77. Jiao S, Wang LV. Two-dimensional depth-resolved Mueller matrix of biological tissue measured with double-beam polarization-sensitive optical coherence tomography. Opt Lett 2002; 27: 101–103. [DOI] [PubMed] [Google Scholar]
- 78. de Boer J, Srinivas S, Malekafzali A, et al. Imaging thermally damaged tissue by Polarization Sensitive Optical Coherence Tomography. Opt Express 1998; 3: 212–218. [DOI] [PubMed] [Google Scholar]
- 79. Schoenenberger K, Colston BW, Maitland DJ, et al. Mapping of birefringence and thermal damage in tissue by use of polarization-sensitive optical coherence tomography. Appl Opt 1998; 37: 6026–6036. [DOI] [PubMed] [Google Scholar]
- 80. Maurice DM. The structure and transparency of the cornea. J Physiol 1957; 136: 263–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ducros MG, De Boer JF, Huang HE, et al. Polarization sensitive optical coherence tomography of the rabbit eye. IEEE J Sel Top Quantum Electron 1999; 5: 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Götzinger E, Pircher M, Sticker M, et al. Measurement and imaging of birefringent properties of the human cornea with phase-resolved, polarization-sensitive optical coherence tomography. J Biomed Opt 2004; 9: 94–102. [DOI] [PubMed] [Google Scholar]
- 83. Götzinger E, Pircher M, Dejaco-Ruhswurm I, et al. Imaging of birefringent properties of keratoconus corneas by polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci 2007; 48: 3551–3558. [DOI] [PubMed] [Google Scholar]
- 84. Pircher M, Goetzinger E, Leitgeb R, et al. Transversal phase resolved polarization sensitive optical coherence tomography. Phys Med Biol 2004; 49: 1257–1263. [DOI] [PubMed] [Google Scholar]
- 85. Miyazawa A, Yamanari M, Makita S, et al. Tissue discrimination in anterior eye using three optical parameters obtained by polarization sensitive optical coherence tomography. Opt Express 2009; 17: 17426–17440. [DOI] [PubMed] [Google Scholar]
- 86. Yasuno Y, Yamanari M, Kawana K, et al. Visibility of trabecular meshwork by standard and polarization-sensitive optical coherence tomography. J Biomed Opt 2010; 15: 061705. [DOI] [PubMed] [Google Scholar]
- 87. Yasuno Y, Yamanari M, Kawana K, et al. Investigation of post-glaucoma-surgery structures by three-dimensional and polarization sensitive anterior eye segment optical coherence tomography. Opt Express 2009; 17: 3980–3996. [DOI] [PubMed] [Google Scholar]
- 88. Fukuda S, Beheregaray S, Kasaragod D, et al. Noninvasive evaluation of phase retardation in blebs after glaucoma surgery using anterior segment polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci 2014; 55: 5200–5206. [DOI] [PubMed] [Google Scholar]
- 89. Beer F, Wartak A, Pircher N, et al. Mapping of corneal layer thicknesses with polarization-sensitive optical coherence tomography using a conical scan pattern. Invest Ophthalmol Vis Sci 2018; 59: 5579–5588. [DOI] [PubMed] [Google Scholar]
- 90. Beer F, Patil RP, Sinha-Roy A, et al. Ultrahigh resolution polarization sensitive optical coherence tomography of the human cornea with conical scanning pattern and variable dispersion compensation. Appl Sci 2019; 9: 4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wartak A, Schenk MS, Bühler V, et al. Micro-optical coherence tomography for high-resolution morphologic imaging of cellular and nerval corneal micro-structures. Biomed Opt Express 2020; 11: 5920–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pircher N, Beer F, Holzer S, et al. Large field of view corneal epithelium and Bowman’s layer thickness maps in keratoconic and healthy eyes. Am J Ophthalmol 2020; 209: 168–177. [DOI] [PubMed] [Google Scholar]
- 93. Ducros MG, Marsack JD, Rylander HG, 3rd, et al. Primate retina imaging with polarization-sensitive optical coherence tomography. J Opt Soc Am A Opt Image Sci Vis 2001; 18: 2945–2956. [DOI] [PubMed] [Google Scholar]
- 94. Cense B, Chen TC, Park BH, et al. Thickness and birefringence of healthy retinal nerve fiber layer tissue measured with polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci 2004; 45: 2606–2612. [DOI] [PubMed] [Google Scholar]
- 95. Cense B, Chen TC, Park BH, et al. In vivo birefringence and thickness measurements of the human retinal nerve fiber layer using polarization-sensitive optical coherence tomography. J Biomed Opt 2004; 9: 121–125. [DOI] [PubMed] [Google Scholar]
- 96. Zhou Q, Knighton RW. Light scattering and form birefringence of parallel cylindrical arrays that represent cellular organelles of the retinal nerve fiber layer. Appl Opt 1997; 36: 2273–2285. [DOI] [PubMed] [Google Scholar]
- 97. Yamanari M, Miura M, Makita S, et al. Phase retardation measurement of retinal nerve fiber layer by polarization-sensitive spectral-domain optical coherence tomography and scanning laser polarimetry. J Biomed Opt 2008; 13: 014013. [DOI] [PubMed] [Google Scholar]
- 98. Desissaire S, Pollreisz A, Sedova A, et al. Analysis of retinal nerve fiber layer birefringence in patients with glaucoma and diabetic retinopathy by polarization sensitive OCT. Biomed Opt Express 2020; 11: 5488–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pircher M, Götzinger E, Leitgeb R, et al. Imaging of polarization properties of human retina in vivo with phase resolved transversal PS-OCT. Opt Express 2004; 12: 5940–5951. [DOI] [PubMed] [Google Scholar]
- 100. Pircher M, Götzinger E, Findl O, et al. Human macula investigated in vivo with polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci 2006; 47: 5487–5494. [DOI] [PubMed] [Google Scholar]
- 101. Baumann B, Götzinger E, Pircher M, et al. Measurements of depolarization distribution in the healthy human macula by polarization sensitive OCT. J Biophotonics 2009; 2: 426–434. [DOI] [PubMed] [Google Scholar]
- 102. Baumann B, Gotzinger E, Pircher M, et al. Segmentation and quantification of retinal lesions in age-related macular degeneration using polarization-sensitive optical coherence tomography. J Biomed Opt 2010; 15: 061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cense B, Gao W, Brown JM, et al. Retinal imaging with polarization-sensitive optical coherence tomography and adaptive optics. Opt Express 2009; 17: 21634–21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schlanitz FG, Sacu S, Baumann B, et al. Identification of Drusen characteristics in age-related macular degeneration by polarization-sensitive optical coherence tomography. Am J Ophthalmol 2015; 160: 335–344.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cense B, Miller DT, King BJ, et al. Measuring polarization changes in the human outer retina with polarization-sensitive optical coherence tomography. J Biophotonics 2018; 11: e201700134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Roberts P, Sugita M, Deák G, et al. Automated identification and quantification of subretinal fibrosis in neovascular age-related macular degeneration using polarization-sensitive OCT. Invest Ophthalmol Vis Sci 2016; 57: 1699–1705. [DOI] [PubMed] [Google Scholar]
- 107. Gräfe MGO, van de Kreeke JA, Willemse J, et al. Subretinal fibrosis detection using polarization sensitive optical coherence tomography. Transl Vis Sci Technol 2020; 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gong P, Li Q, Wang Q, et al. Jones matrix-based speckle-decorrelation angiography using polarization-sensitive optical coherence tomography. J Biophotonics 2020; 13: e202000007. [DOI] [PubMed] [Google Scholar]
- 109. de Boer JF, Hitzenberger CK, Yasuno Y. Polarization sensitive optical coherence tomography—a review [Invited]. Biomed Opt Express 2017; 8: 1838–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Baumann B. Polarization sensitive optical coherence tomography: a review of technology and applications. Appl Sci 2017; 7: 474. [Google Scholar]
- 111. Azuma S, Makita S, Miyazawa A, et al. Pixel-wise segmentation of severely pathologic retinal pigment epithelium and choroidal stroma using multi-contrast Jones matrix optical coherence tomography. Biomed Opt Express 2018; 9: 2955–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Miura M, Makita S, Azuma S, et al. Evaluation of focal damage in the retinal pigment epithelium layer in serous retinal pigment epithelium detachment. Sci Rep 2019; 9: 3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lerner RM, Parker KJ, Holen J, et al. Sono-elasticity: medical elasticity images derived from ultrasound signals in mechanically vibrated targets. In: Kessler LW. (eds) Acoustical imaging. Boston, MA: Springer, 1988, pp. 317–327. [Google Scholar]
- 114. Kennedy BF, Kennedy KM, Sampson DD. A review of optical coherence elastography: fundamentals, techniques and prospects. IEEE J Sel Top Quantum Electron 2014; 20: 272–288. [Google Scholar]
- 115. Chhetri RK, Kozek KA, Johnston-Peck AC, et al. Imaging three-dimensional rotational diffusion of plasmon resonant gold nanorods using polarization-sensitive optical coherence tomography. Phys Rev E Stat Nonlin Soft Matter Phys 2011; 83: 040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Blackmon RL, Sandhu R, Chapman BS, et al. Imaging extracellular matrix remodeling in vitro by diffusion-sensitive optical coherence tomography. Biophys J 2016; 110: 1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Blackburn BJ, Gu S, Ford MR, et al. Noninvasive assessment of corneal crosslinking with phase-decorrelation optical coherence tomography. Invest Ophthalmol Vis Sci 2019; 60: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schmitt JM. OCT elastography: imaging microscopic deformation and strain of tissue. Opt Express 1998; 3: 199–211. [DOI] [PubMed] [Google Scholar]
- 119. Chen EJ, Novakofski J, Kenneth Jenkins W, et al. Young’s modulus measurements of soft tissues with application to elasticity imaging. IEEE Trans Ultrason Ferroelectr Freq Control 1996; 43: 191–194. [Google Scholar]
- 120. Liang X, Crecea V, Boppart SA. Dynamic optical coherence elastography: a review. J Innov Opt Health Sci 2010; 3: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ramier A, Eltony AM, Chen YT, et al. In vivo measurement of shear modulus of the human cornea using optical coherence elastography. Sci Rep 2020; 10: 17366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol 2018; 66: 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Roberts CJ, Dupps WJ., Jr. Biomechanics of corneal ectasia and biomechanical treatments. J Cataract Refract Surg 2014; 40: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hong CW, Sinha-Roy A, Schoenfield L, et al. Collagenase-mediated tissue modeling of corneal ectasia and collagen cross-linking treatments. Invest Ophthalmol Vis Sci 2012; 53: 2321–2327. [DOI] [PubMed] [Google Scholar]
- 125. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 2003; 135: 620–627. [DOI] [PubMed] [Google Scholar]
- 126. Dascalescu D, Corbu C, Vasile P, et al. The importance of assessing corneal biomechanical properties in glaucoma patients care—a review. Rom J Ophthalmol 2016; 60: 219–225. [PMC free article] [PubMed] [Google Scholar]
- 127. Zhou Y, Wang Y, Shen M, et al. In vivo evaluation of corneal biomechanical properties by optical coherence elastography at different cross-linking irradiances. J Biomed Opt 2019; 24: 105001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Singh M, Li J, Vantipalli S, et al. Noncontact elastic wave imaging optical coherence elastography for evaluating changes in corneal elasticity due to crosslinking. IEEE J Sel Top Quantum Electron 2016; 22: 6801911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Han Z, Li J, Singh M, et al. Analysis of the effect of the fluid-structure interface on elastic wave velocity in cornea-like structures by OCE and FEM. Laser Phys Lett 2016; 13: 035602. [Google Scholar]
- 130. Ambroziński Ł, Pelivanov I, Song S, et al. Air-coupled acoustic radiation force for non-contact generation of broadband mechanical waves in soft media. Appl Phys Lett 2016; 109: 043701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jin ZI, Khazaeinezhad R, Zhu J, et al. In-vivo 3D corneal elasticity using air-coupled ultrasound optical coherence elastography. Biomed Opt Express 2019; 10: 6272–6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ambroziński Ł, Song S, Yoon SJ, et al. Acoustic micro-tapping for non-contact 4D imaging of tissue elasticity. Sci Rep 2016; 6: 38967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pitre JJ, Kirby MA, Li DS, et al. Nearly-incompressible transverse isotropy (NITI) of cornea elasticity: model and experiments with acoustic micro-tapping OCE. Sci Rep 2020; 10: 12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Qu Y, Ma T, He Y, et al. Acoustic radiation force optical coherence elastography of corneal tissue HHS public access. IEEE J Sel Top Quantum Electron 2016; 22: 6803507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nair A, Singh M, Aglyamov SR, et al. Heartbeat OCE: corneal biomechanical response to simulated heartbeat pulsation measured by optical coherence elastography. J Biomed Opt 2020; 25: 055001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Li C, Guan G, Huang Z, et al. Noncontact all-optical measurement of corneal elasticity. Opt Lett 2012; 37: 1625–1627. [DOI] [PubMed] [Google Scholar]
- 137. Tabandeh H, Thompson GM, Heyworth P, et al. Water content, lens hardness and cataract appearance. Eye 1994; 8: 125–129. [DOI] [PubMed] [Google Scholar]
- 138. Li Y, Zhu J, Chen JJ, et al. Simultaneously imaging and quantifying in vivo mechanical properties of crystalline lens and cornea using optical coherence elastography with acoustic radiation force excitation. APL Photonics 2019; 4: 106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ambekar YS, Singh M, Zhang J, et al. Multimodal quantitative optical elastography of the crystalline lens with optical coherence elastography and Brillouin microscopy. Biomed Opt Express 2020; 11: 2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhang H, Larin KV, Aglyamov SR, et al. Quantifying lens elastic properties with optical coherence elastography as a function of intraocular pressure. In: Larin KV, Scarcelli G. (eds) Optical elastography and tissue biomechanics VI. Bellingham, WA: SPIE, 2019, p. 60. [Google Scholar]
- 141. Krishnan L, Hoying JB, Nguyen H, et al. Interaction of angiogenic microvessels with the extracellular matrix. Am J Physiol Circ Physiol 2007; 293: H3650–H3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Qu Y, He Y, Zhang Y, et al. Quantified elasticity mapping of retinal layers using synchronized acoustic radiation force optical coherence elastography. Biomed Opt Express 2018; 9: 4054–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Qu Y, He Y, Saidi A, et al. In vivo elasticity mapping of posterior ocular layers using acoustic radiation force optical coherence elastography. Invest Ophthalmol Vis Sci 2018; 59: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Du Z, Li R, Qian X, et al. Quantitative confocal optical coherence elastography for evaluating biomechanics of optic nerve head using Lamb wave model. Neurophotonics 2019; 6: 041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Singh M, Nair A, Aglyamov SR, et al. Noncontact optical coherence elastography of the posterior porcine sclera in situ as a function of IOP. In: Manns F, Söderberg PG, Ho A. (eds) Ophthalmic technologies XXVII. Bellingham, WA: SPIE, 2017, p. 1004524. [Google Scholar]
- 146. Kirby MA, Pelivanov I, Song S, et al. Optical coherence elastography in ophthalmology. J Biomed Opt 2017; 22: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Singh M, Wu C, Liu C-H, et al. Phase-sensitive optical coherence elastography at 15 million A-Lines per second. Opt Lett 2015; 40: 2588–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gordon AY, Lapierre-Landry M, Skala MC, et al. Photothermal optical coherence tomography of anti-angiogenic treatment in the mouse retina using gold nanorods as contrast agents. Transl Vis Sci Technol 2019; 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Adler DC, Huang S-W, Huber R, et al. Photothermal detection of gold nanoparticles using phase-sensitive optical coherence tomography. Opt Express 2008; 16: 4376–4393. [DOI] [PubMed] [Google Scholar]
- 150. Lapierre-Landry M, Gordon AY, Penn JS, et al. In vivo photothermal optical coherence tomography of endogenous and exogenous contrast agents in the eye. Sci Rep 2017; 7: 9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lapierre-Landry M, Huckenpahler AL, Link BA, et al. Imaging melanin distribution in the zebrafish retina using photothermal optical coherence tomography. Transl Vis Sci Technol 2018; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lapierre-Landry M, Connor TB, Carroll J, et al. Photothermal optical coherence tomography of indocyanine green in ex vivo eyes. Opt Lett 2018; 43: 2470–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Rao KD, Choma MA, Yazdanfar S, et al. Molecular contrast in optical coherence tomography by use of a pump-probe technique. Opt Lett 2003; 28: 340–342. [DOI] [PubMed] [Google Scholar]
- 154. Carrasco-Zevallos O, Shelton RL, Kim W, et al. In vivo pump-probe optical coherence tomography imaging in Xenopus laevis. J Biophotonics 2015; 8: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Jacob D, Shelton RL, Applegate BE. Fourier domain pump-probe optical coherence tomography imaging of melanin. In: 2009 conference on lasers and electro-optics and 2009 conference on quantum electronics and laser science conference (CLEO/QELS 2009), Baltimore, MD, 2–4 June 2009, p. 12399. Washington, DC: Optical Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Pircher M, Baumann B, Götzinger E, et al. Simultaneous SLO/OCT imaging of the human retina with axial eye motion correction. Opt Express 2007; 15: 16922–16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Pircher M, Götzinger E, Sattmann H, et al. In vivo investigation of human cone photoreceptors with SLO/OCT in combination with 3D motion correction on a cellular level. Opt Express 2010; 18: 13935–13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Vienola KV, Braaf B, Sheehy CK, et al. Real-time eye motion compensation for OCT imaging with tracking SLO. Biomed Opt Express 2012; 3: 2950–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Schwarzhans F, Desissaire S, Steiner S, et al. Generating large field of view en-face projection images from intra-acquisition motion compensated volumetric optical coherence tomography data. Biomed Opt Express 2020; 11: 6881–6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Podoleanu AG, Jackson DA. Combined optical coherence tomograph and scanning laser ophthalmoscope. Electron Lett 1998; 34: 1088–1090. [Google Scholar]
- 161. Podoleanu AG, Dobre GM, Cucu RG, et al. Sequential optical coherence tomography and confocal imaging. Opt Lett 2004; 29: 364–366. [DOI] [PubMed] [Google Scholar]
- 162. Hammer DX, Iftimia NV, Ustun TE, et al. Dual OCT/SLO imager with three-dimensional tracker. In: Manns F, Soederberg PG, Ho A. (eds) Ophthalmic technologies XV. Bellingham, WA: SPIE, 2005, p. 33. [Google Scholar]
- 163. Iftimia NV, Hammer DX, Bigelow CE, et al. Hybrid retinal imager using line-scanning laser ophthalmoscopy and spectral domain optical coherence tomography. Opt Express 2006; 14: 12909–12914. [DOI] [PubMed] [Google Scholar]
- 164. Hammer DX, Ferguson RD, Ustun TE, et al. Line-scanning laser ophthalmoscope. J Biomed Opt 2006; 11: 041126. [DOI] [PubMed] [Google Scholar]
- 165. Malone JD, El-Haddad MT, Bozic I, et al. Simultaneous multimodal ophthalmic imaging using swept-source spectrally encoded scanning laser ophthalmoscopy and optical coherence tomography. Biomed Opt Express 2017; 8: 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. El-Haddad MT, Bozic I, Tao YK. Spectrally encoded coherence tomography and reflectometry: simultaneous en face and cross-sectional imaging at 2 gigapixels per second. J Biophotonics 2018; 11: e201700268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Larocca F, Nankivil D, Farsiu S, et al. Handheld simultaneous scanning laser ophthalmoscopy and optical coherence tomography system. Biomed Opt Express 2013; 4: 2307–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. LaRocca F, Nankivil D, Farsiu S, et al. True color scanning laser ophthalmoscopy and optical coherence tomography handheld probe. Biomed Opt Express 2014; 5: 3204–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Tao YK, Izatt JA. Spectrally encoded confocal scanning laser ophthalmoscopy. Opt Lett 2010; 35: 574–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Tao YK, Farsiu S, Izatt JA. Interlaced spectrally encoded confocal scanning laser ophthalmoscopy and spectral domain optical coherence tomography. Biomed Opt Express 2010; 1: 431–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Pircher M, Zawadzki RJ. Review of adaptive optics OCT (AO-OCT): principles and applications for retinal imaging [Invited]. Biomed Opt Express 2017; 8: 2536–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Roorda A, Romero-Borja F, Donnelly WJ, III, et al. Adaptive optics scanning laser ophthalmoscopy. Opt Express 2002; 10: 405–412. [DOI] [PubMed] [Google Scholar]
- 173. Miller DT, Qu J, Jonnal RS, et al. Coherence gating and adaptive optics in the eye. In: Tuchin VV, Izatt JA, Fujimoto JG. (eds) Coherence domain optical methods and optical coherence tomography in biomedicine VII. Bellingham, WA: SPIE, 2003, p. 65. [Google Scholar]
- 174. Hermann B, Fernández EJ, Unterhuber A, et al. Adaptive-optics ultrahigh-resolution optical coherence tomography. Opt Lett 2004; 29: 2142–2144. [DOI] [PubMed] [Google Scholar]
- 175. Dubra A, Sulai Y. Reflective afocal broadband adaptive optics scanning ophthalmoscope. Biomed Opt Express 2011; 2: 1757–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Mujat M, Ferguson RD, Patel AH, et al. High resolution multimodal clinical ophthalmic imaging system. Opt Express 2010; 18: 11607–11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Stevenson SB, Roorda A. Correcting for miniature eye movements in high-resolution scanning laser ophthalmoscopy. In: Manns F, Soederberg PG, Ho A. (eds) Ophthalmic technologies XV. Bellingham, WA: SPIE, 2005, p. 12. [Google Scholar]
- 178. Vogel CR, Arathorn DW, Roorda A, et al. Retinal motion estimation in adaptive optics scanning laser ophthalmoscopy. Opt Express 2006; 14: 487–497. [DOI] [PubMed] [Google Scholar]
- 179. Cunefare D, Huckenpahler AL, Patterson EJ, et al. RAC-CNN: multimodal deep learning based automatic detection and classification of rod and cone photoreceptors in adaptive optics scanning light ophthalmoscope images. Biomed Opt Express 2019; 10: 3815–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wang Y, Bensaid N, Tiruveedhula P, et al. Human foveal cone photoreceptor topography and its dependence on eye length. Elife 2019; 8: e47148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Duncan JL, Zhang Y, Gandhi J, et al. High-resolution imaging with adaptive optics in patients with inherited retinal degeneration. Invest Ophthalmol Vis Sci 2007; 48: 3283–3291. [DOI] [PubMed] [Google Scholar]
- 182. Genead MA, Fishman GA, Rha J, et al. Photoreceptor structure and function in patients with congenital achromatopsia. Invest Ophthalmol Vis Sci 2011; 52: 7298–7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Song H, Rossi EA, Latchney L, et al. Cone and rod loss in Stargardt disease revealed by adaptive optics scanning light ophthalmoscopy. JAMA Ophthalmol 2015; 133: 1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Sun LW, Johnson RD, Langlo CS, et al. Assessing photoreceptor structure in retinitis pigmentosa and usher syndrome. Invest Ophthalmol Vis Sci 2016; 57: 2428–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Bennett J, Wellman J, Marshall KA, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet 2016; 388: 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Zhang Y, Rha J, Jonnal RS, et al. Adaptive optics parallel spectral domain optical coherence tomography for imaging the living retina. Opt Express 2005; 13: 4792–4811. [DOI] [PubMed] [Google Scholar]
- 187. Zawadzki RJ, Jones SM, Olivier SS, et al. Adaptive-optics optical coherence tomography for high-resolution and high-speed 3D retinal in vivo imaging. Opt Express 2005; 13: 8532–8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Zhang Y, Cense B, Rha J, et al. High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography. Opt Express 2006; 14: 4380–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hammer DX, Ferguson RD, Bigelow CE, et al. Adaptive optics scanning laser ophthalmoscope for stabilized retinal imaging. Opt Express 2006; 14: 3354–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Fernández EJ, Hermann B, Považay B, et al. Ultrahigh resolution optical coherence tomography and pancorrection for cellular imaging of the living human retina. Opt Express 2008; 16: 11083–11094. [DOI] [PubMed] [Google Scholar]
- 191. Torti C, Považay B, Hofer B, et al. Adaptive optics optical coherence tomography at 120,000 depth scans/s for non-invasive cellular phenotyping of the living human retina. Opt Express 2009; 17: 19382–19400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Kocaoglu OP, Lee S, Jonnal RS, et al. Imaging cone photoreceptors in three dimensions and in time using ultrahigh resolution optical coherence tomography with adaptive optics. Biomed Opt Express 2011; 2: 748–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Kocaoglu OP, Cense B, Jonnal RS, et al. Imaging retinal nerve fiber bundles using optical coherence tomography with adaptive optics. Vision Res 2011; 51: 1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Hammer DX, Iftimia NV, Ferguson RD, et al. Foveal fine structure in retinopathy of prematurity: an adaptive optics Fourier domain optical coherence tomography study. Invest Ophthalmol Vis Sci 2008; 49: 2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Choi SS, Zawadzki RJ, Lim MC, et al. Evidence of outer retinal changes in glaucoma patients as revealed by ultrahigh-resolution in vivo retinal imaging. Br J Ophthalmol 2011; 95: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Panorgias A, Zawadzki RJ, Capps AG, et al. Multimodal assessment of microscopic morphology and retinal function in patients with geographic atrophy. Invest Ophthalmol Vis Sci 2013; 54: 4372–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Nadler Z, Wang B, Schuman JS, et al. In vivo three-dimensional characterization of the healthy human lamina cribrosa with adaptive optics spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2014; 55: 6459–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Dong ZM, Wollstein G, Wang B, et al. Adaptive optics optical coherence tomography in glaucoma. Prog Retin Eye Res 2017; 57: 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Ferguson RD, Zhong Z, Hammer DX, et al. Adaptive optics scanning laser ophthalmoscope with integrated wide-field retinal imaging and tracking. J Opt Soc Am A Opt Image Sci Vis 2010; 27: A265–A277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Felberer F, Kroisamer J-S, Baumann B, et al. Adaptive optics SLO/OCT for 3D imaging of human photoreceptors in vivo. Biomed Opt Express 2014; 5: 439–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Wells-Gray EM, Choi SS, Zawadzki RJ, et al. Volumetric imaging of rod and cone photoreceptor structure with a combined adaptive optics-optical coherence tomography-scanning laser ophthalmoscope. J Biomed Opt 2018; 23: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Stevenson SB, Roorda A, Kumar G. Eye tracking with the adaptive optics scanning laser ophthalmoscope. In: Eye tracking research and applications symposium (ETRA). New York: ACM Press, 2010, pp. 195–198. [Google Scholar]
- 203. Sheehy CK, Tiruveedhula P, Sabesan R, et al. Active eye-tracking for an adaptive optics scanning laser ophthalmoscope. Biomed Opt Express 2015; 6: 2412–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Chui TY, Song H, Burns SA. Adaptive-optics imaging of human cone photoreceptor distribution. J Opt Soc Am A Opt Image Sci Vis 2008; 25: 3021–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Dubra A, Sulai Y, Norris JL, et al. Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed Opt Express 2011; 2: 1864–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Doble N, Choi SS, Codona JL, et al. In vivo imaging of the human rod photoreceptor mosaic. Opt Lett 2011; 36: 31–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Cunefare D, Langlo CS, Patterson EJ, et al. Deep learning based detection of cone photoreceptors with multimodal adaptive optics scanning light ophthalmoscope images of achromatopsia. Biomed Opt Express 2018; 9: 3740–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Felberer F, Rechenmacher M, Haindl R, et al. Imaging of retinal vasculature using adaptive optics SLO/OCT. Biomed Opt Express 2015; 6: 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Liu Z, Tam J, Saeedi O, et al. Trans-retinal cellular imaging with multimodal adaptive optics. Biomed Opt Express 2018; 9: 4246–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Zhang F, Kurokawa K, Lassoued A, et al. Cone photoreceptor classification in the living human eye from photostimulation-induced phase dynamics. Proc Natl Acad Sci USA 2019; 116: 7951–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Seeliger MW, Beck SC, Pereyra-Muñoz N, et al. In vivo confocal imaging of the retina in animal models using scanning laser ophthalmoscopy. Vision Res 2005; 45: 3512–3519. [DOI] [PubMed] [Google Scholar]
- 212. Zawadzki RJ, Zhang P, Zam A, et al. Adaptive-optics SLO imaging combined with widefield OCT and SLO enables precise 3D localization of fluorescent cells in the mouse retina. Biomed Opt Express 2015; 6: 2191–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Zhang P, Zam A, Jian Y, et al. In vivo wide-field multispectral scanning laser ophthalmoscopy–optical coherence tomography mouse retinal imager: longitudinal imaging of ganglion cells, microglia, and Müller glia, and mapping of the mouse retinal and choroidal vasculature. J Biomed Opt 2015; 20: 126005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Gong Y, Li J, Sun Y, et al. Optimization of an image-guided laser-induced choroidal neovascularization model in mice. PLoS ONE 2015; 10: e0132643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Soetikno BT, Shu X, Liu Q, et al. Optical coherence tomography angiography of retinal vascular occlusions produced by imaging-guided laser photocoagulation. Biomed Opt Express 2017; 8: 3571–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Tao YK, Benavides OR, Terrones BD, et al. Multimodality optical coherence tomography and fluorescence confocal scanning laser ophthalmoscopy for image-guided feedback of intraocular injections in mouse models In: Manns F, Söderberg PG, Ho A. (eds) Ophthalmic technologies XXVIII. Bellingham, WA: SPIE, 2018, p. 51. [Google Scholar]
- 217. Malone JD, Levine EM, Tao YK. OCT and fluorescence SLO for guided laser delivery and longitudinal imaging in a murine model of targeted retinal laser injury. In: Manns F, Söderberg PG, Ho A. (eds) Ophthalmic technologies XXX. Bellingham, WA: SPIE, 2020, p. 49. [Google Scholar]
- 218. Zhang L, Song W, Shao D, et al. Volumetric fluorescence retinal imaging in vivo over a 30-degree field of view by oblique scanning laser ophthalmoscopy (oSLO). Biomed Opt Express 2018; 9: 25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Gray DC, Merigan W, Wolfing JI, et al. In vivo fluorescence imaging of primate retinal ganglion cells and retinal pigment epithelial cells. Opt Express 2006; 14: 7144–7158. [DOI] [PubMed] [Google Scholar]
- 220. Biss DP, Sumorok D, Burns SA, et al. In vivo fluorescent imaging of the mouse retina using adaptive optics. Opt Lett 2007; 32: 659–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Hunter JJ, Masella B, Dubra A, et al. Images of photoreceptors in living primate eyes using adaptive optics two-photon ophthalmoscopy. Biomed Opt Express 2011; 2: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Sharma R, Yin L, Geng Y, et al. In vivo two-photon imaging of the mouse retina. Biomed Opt Express 2013; 4: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Palczewska G, Dong Z, Golczak M, et al. Noninvasive two-photon microscopy imaging of mouse retina and retinal pigment epithelium through the pupil of the eye. Nat Med 2014; 20: 785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Qin Z, He S, Yang C, et al. Adaptive optics two-photon microscopy enables near-diffraction-limited and functional retinal imaging in vivo. Light Sci Appl 2020; 9: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Wynne N, Carroll J, Duncan JL. Promises and pitfalls of evaluating retinal diseases with adaptive optics scanning light ophthalmoscopy (AOSLO). Prog Retin Eye Res 2020; 2020: 100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Hofer H, Sredar N, Queener H, et al. Wavefront sensorless adaptive optics ophthalmoscopy in the human eye. Opt Express 2011; 19: 14160–14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Jian Y, Xu J, Gradowski MA, et al. Wavefront sensorless adaptive optics optical coherence tomography for in vivo retinal imaging in mice. Biomed Opt Express 2014; 5: 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Polans J, Keller B, Carrasco-Zevallos OM, et al. Wide-field retinal optical coherence tomography with wavefront sensorless adaptive optics for enhanced imaging of targeted regions. Biomed Opt Express 2017; 8: 16–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Wahl DJ, Ng R, Ju MJ, et al. Sensorless adaptive optics multimodal en-face small animal retinal imaging. Biomed Opt Express 2019; 10: 252–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Camino A, Zang P, Athwal A, et al. Sensorless adaptive-optics optical coherence tomographic angiography. Biomed Opt Express 2020; 11: 3952–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. DuBose T, Nankivil D, LaRocca F, et al. Handheld adaptive optics scanning laser ophthalmoscope. Optica 2018; 5: 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Hagan K, DuBose T, Cunefare D, et al. Multimodal handheld adaptive optics scanning laser ophthalmoscope. Opt Lett 2020; 45: 4940–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Schweitzer D, Hammer M, Schweitzer F, et al. In vivo measurement of time-resolved autofluorescence at the human fundus. J Biomed Opt 2004; 9: 1214–1222. [DOI] [PubMed] [Google Scholar]
- 234. Dysli C, Wolf S, Berezin MY, et al. Fluorescence lifetime imaging ophthalmoscopy. Prog Retin Eye Res 2017; 60: 120–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Feeks JA, Hunter JJ. Adaptive optics two-photon excited fluorescence lifetime imaging ophthalmoscopy of exogenous fluorophores in mice. Biomed Opt Express 2017; 8: 2483–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Carrasco-Zevallos OM, Viehland C, Keller B, et al. Review of intraoperative optical coherence tomography: technology and applications [Invited]. Biomed Opt Express 2017; 8: 1607–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Boppart SA, Bouma BE, Pitris C, et al. Forward-imaging instruments for optical coherence tomography. Opt Lett 1997; 22: 1618–1620. [DOI] [PubMed] [Google Scholar]
- 238. Radhakrishnan S, Rollins AM, Roth JE, et al. Real-time optical coherence tomography of the anterior segment at 1310 nm. Arch Ophthalmol 2001; 119: 1179–1185. [DOI] [PubMed] [Google Scholar]
- 239. Dayani PN, Maldonado R, Farsiu S, et al. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina 2009; 29: 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Tao YK, Ehlers JP, Toth CA, et al. Intraoperative spectral domain optical coherence tomography for vitreoretinal surgery. Opt Lett 2010; 35: 3315–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. El-Haddad MT, Tao YK. Advances in intraoperative optical coherence tomography for surgical guidance. Curr Opin Biomed Eng 2017; 3: 37–48. [Google Scholar]
- 242. Carrasco-Zevallos OM, Viehland C, Keller B, et al. Constant linear velocity spiral scanning for near video rate 4D OCT ophthalmic and surgical imaging with isotropic transverse sampling. Biomed Opt Express 2018; 9: 5052–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Carrasco-Zevallos OM, Keller B, Viehland C, et al. Live volumetric (4D) visualization and guidance of in vivo human ophthalmic surgery with intraoperative optical coherence tomography. Sci Rep 2016; 6: 31689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. El-Haddad MT, Tao YK. Automated stereo vision instrument tracking for intraoperative OCT guided anterior segment ophthalmic surgical maneuvers. Biomed Opt Express 2015; 6: 3014–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Lu CD, Witkin AJ, Waheed NK, et al. Ultrahigh speed ophthalmic surgical OCT for intraoperative OCT angiography and widefield imaging. Invest Ophthalmol Vis Sci 2016; 57: 466. [Google Scholar]
- 246. Viehland C, Vajzovic L, Chen X, et al. Intraoperative microscope integrated optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2017; 58: 3123. [Google Scholar]
- 247. Posarelli C, Sartini F, Casini G, et al. What is the impact of intraoperative microscope-integrated OCT in ophthalmic surgery? Relevant applications and outcomes a systematic review. J Clin Med 2020; 9: 1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Ehlers JP, Dupps WJ, Kaiser PK, et al. The prospective intraoperative and perioperative ophthalmic imaging with optical CoherEncE TomogRaphy (PIONEER) study: 2-year results. Am J Ophthalmol 2014; 158: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Ehlers JP, Modi YS, Pecen PE, et al. The DISCOVER study 3-year results: feasibility and usefulness of microscope-integrated intraoperative OCT during ophthalmic surgery. Ophthalmology 2018; 125: 1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Ehlers JP, Srivastava SK, Feiler D, et al. Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: microscope integration, surgical instrumentation, and heads-up display surgeon feedback. PLoS ONE 2014; 9: e105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Rosencwaig A, Gersho A. Theory of the photoacoustic effect with solids. J Appl Phys 1976; 47: 64–69. [Google Scholar]
- 252. Billeh YN, Liu M, Buma T. Spectroscopic photoacoustic microscopy using a photonic crystal fiber supercontinuum source. Opt Express 2010; 18: 18519–18524. [DOI] [PubMed] [Google Scholar]
- 253. Drexler W, Liu M, Kumar A, et al. Optical coherence tomography today: speed, contrast, and multimodality. J Biomed Opt 2014; 19: 071412. [DOI] [PubMed] [Google Scholar]
- 254. Hu Z, Wang X, Liu Q, et al. Photoacoustic imaging in ophthalmology. Int J Ophthalmol Eye Res 2015; 3: 126–132. [Google Scholar]
- 255. Jiao S, Jiang M, Hu J, et al. Photoacoustic ophthalmoscopy for in vivo retinal imaging. Opt Express 2010; 18: 3967–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Penn JS, Madan A, Caldwell RB, et al. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 2008; 27: 331–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Zhang W, Li Y, Nguyen VP, et al. High-resolution, in vivo multimodal photoacoustic microscopy, optical coherence tomography, and fluorescence microscopy imaging of rabbit retinal neovascularization. Light Sci Appl 2018; 7: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Nguyen VP, Li Y, Aaberg M, et al. In vivo 3D imaging of retinal neovascularization using multimodal photoacoustic microscopy and optical coherence tomography imaging. J Imaging 2018; 4: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Li Y, Zhang W, Nguyen VP, et al. Real-time OCT guidance and multimodal imaging monitoring of subretinal injection induced choroidal neovascularization in rabbit eyes. Exp Eye Res 2019; 186: 107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Nguyen VP, Li Y, Zhang W, et al. High-resolution multimodal photoacoustic microscopy and optical coherence tomography image-guided laser induced branch retinal vein occlusion in living rabbits. Sci Rep 2019; 9: 10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Nguyen VP, Li Y, Henry J, et al. High resolution multimodal photoacoustic microscopy and optical coherence tomography visualization of choroidal vascular occlusion. Int J Mol Sci 2020; 21: 6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Song W, Wei Q, Liu W, et al. A combined method to quantify the retinal metabolic rate of oxygen using photoacoustic ophthalmoscopy and optical coherence tomography. Sci Rep 2014; 4: 6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Fatima A, Kratkiewicz K, Manwar R, et al. Review of cost reduction methods in photoacoustic computed tomography. Photoacoustics 2019; 15: 100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Liu X, Liu T, Wen R, et al. Optical coherence photoacoustic microscopy for in vivo multimodal retinal imaging. Opt Lett 2015; 40: 1370–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]