Abstract
The efficacy of chemotherapeutic drugs for the treatment of brain metastasis may be compromised by the blood–brain barrier (BBB) and blood–tumor barrier (BTB). P-glycoprotein (P-gp) is a multidrug resistance protein that potentially limits the penetration of chemotherapeutics through the BBB and BTB. 5-Fluorouracil (5-FU) is widely used to treat cancer. Bioactive constituents of medicinal herbs, such as borneol and tetrandrine, potentially improve drug penetration through the BBB and BTB. We hypothesized that borneol and tetrandrine might modulate the BBB and BTB to enhance 5-FU penetration into the brain. To investigate this, in vitro and in vivo models were developed to explore the modulatory effects of borneol and tetrandrine on 5-FU penetration through the BBB and BTB. In the in vitro models, barrier integrity, cell viability, barrier penetration, P-gp activity, and NF-κB expression were assessed. In the in vivo brain metastasis models, cancer cells were injected into the internal carotid artery to evaluate tumor growth. The experimental results demonstrated that borneol and borneol + tetrandrine reduced BBB integrity. The efflux pump function of P-gp was partially inhibited by tetrandrine and borneol + tetrandrine. In the in vivo experiment, borneol + tetrandrine effectively prolonged survival without compromising body weight. In conclusion, BBB and BTB integrity was modulated by borneol and borneol + tetrandrine. The combination of borneol and tetrandrine could be used to improve the chemotherapeutic control of brain metastasis.
Keywords: blood–brain barrier, blood–tumor barrier, p-glycoprotein, brain metastasis, synergistic effect
Introduction
The blood–brain barrier (BBB) is a specialized barrier that protects the brain microenvironment against toxins and pathogens in the circulation and maintains brain homeostasis. This barrier is composed of endothelial cells of brain capillaries, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. The barrier function of these cells results from the tight intercellular junctions and efflux transporters expressed on the plasma membrane. P-glycoprotein (P-gp) expression at the BBB prevents the entry of unwanted bloodborne toxins and signaling molecules into the brain. 1 P-gp plays a critical role in cross-resistance mediated by the BBB and blood–tumor barrier (BTB).2,3 The BBB remains intact until small tumor cell colonies coalesce to form large tumor masses; accumulating evidence indicates that BBB permeability varies among different experimental brain metastases and that its function is related to the growth pattern and size of the lesions.4,5 The debate on the “openness” of the BTB, a tumor-disrupted form of the BBB formed by brain metastases or primary brain tumors, remains ongoing. The phenotypes of BTB vary according to cancer type as well as cancer genotype.6,7 Most data indicate that the BTB is not as intact as the BBB and is not sufficiently open for chemotherapeutics to penetrate. 8
Metastatic brain tumors are the most common brain malignancies and have a poor prognosis. The limited ability of chemotherapeutics to penetrate the BBB and BTB might require modification to improve the efficacy of treatments for brain metastasis. A phase I study concluded that lapatinib combined with the 5-FU prodrug capecitabine for HER2-positive breast cancer patients with central nervous system metastases is a feasible combination. 9 Capecitabine is an oral fluoropyrimidine carbamate preferentially converted to the cytotoxic 5-FU in tumor and plasma. 10 Meanwhile, brain metastases from colorectal cancer are rare, but there is no treatment guideline for patients suffering from brain metastases. Although 5-fluorouracil (5-FU) is the adjuvant treatment for primary and metastatic colorectal cancer,11-13 the chemotherapy guideline for brain metastases is still absent. 14 To prove the concept that penetration of chemotherapeutics through BBB could be modulated to treat brain metastasis, we used 5-FU as a candidate drug for the experimental model.
In a murine model of primary glioma, borneol was found to be capable of increasing BTB permeability by augmenting the expression levels of tight junction-associated proteins. 15 However, agents that can effectively enhance BTB permeability in brain metastases have not yet been developed. Therefore, scientists are seeking potential drugs to increase the permeability of the BBB and BTB. Borneol (Figure 1A) is a bioactive ingredient found in several species of plants, such as Heterotheca subaxillaris, 16 Artemisia princeps, 17 Callicarpa rubella, 18 Blumea balsamifera, 19 and Kaempferia galanga. 20 Borneol has been reported to enhance drug penetration through the BBB. 21 Moreover, it has been identified as a potentially promising neuroprotective agent for treating cerebral ischemic injury, largely by alleviating BBB disruption. 22 Tetrandrine (Figure 1B) is a bis-benzylisoquinoline alkaloid isolated from Stephania tetrandra, which has been reported to inhibit the function of P-gp. 23
Figure 1.
Chemical structures of (A) borneol and (B) tetrandrine.
To our knowledge, little is known about whether borneol and tetrandrine could be used together to play a therapeutic role in metastatic brain cancer. We hypothesized that borneol and tetrandrine could be used together in combination with 5-FU to exert a synergistic therapeutic effect in metastatic brain cancer. Borneol could be used to enhance BBB/BTB permeability, and tetrandrine could be used to inhibit P-gp expression. Both effects could cooperatively provide therapeutic benefits for metastatic brain cancer. To investigate this hypothesis, multiple experimental models were developed to evaluate aspects such as cell viability, BBB/BTB integrity, and the effect of the P-gp inhibitors borneol and tetrandrine on BBB/BTB permeability in vitro and on chemotherapeutic control of brain metastasis in vivo. The pharmacodynamic interaction of the herbal drugs borneol and tetrandrine in improving the BBB/BTB penetration ability of 5-FU was further discussed.
Materials and Methods
Cell Culture
The murine colorectal adenocarcinoma cell line CT26, murine melanoma cell line B16F10, and murine brain endothelial cell (MBEC) line bEnd3 were obtained from the American Type Culture Collection and maintained in RPMI 1640 medium and Dulbecco’s modified Eagle’s medium, respectively. All culture media contained 10% heat-inactivated fetal calf serum (HyClone, Logan, UT, USA), L-glutamine (200 mM), NaHCO3 (10 nM), HEPES (20 mM), and geneticin (G418; Sigma, St. Louis, MO, USA). Cells (5 × 104/mL) were maintained in the exponential growth phase before the experiments and were incubated in a humidified atmosphere containing 95% air and 5% CO2 at 37°C. The murine colorectal carcinoma cell line CT26 has been widely used as a syngeneic tumor model to study immunotherapies. 24
Establishment of the BBB and BTB Models With Permeability Calibration
An in vitro model of the BBB was established by monolayer culture of bEnd3 MBECs in a Transwell apparatus (Corning 3401: 12 mm × 0.4 μm polycarbonate 12-well Transwell, USA). The in vitro BTB model was established by sequential seeding of MBECs (4 × 105/mL) for 48 h in the upper chambers and then tumor cells (CT26) (6.67 × 104/mL) were added in the lower chambers for 24 h. The upper and lower chambers were separated by a permeable membrane to further observe the impact of tumor cells on BBB integrity. To test and calibrate the BBB permeability of 5-FU in vitro, 4 materials of different sizes, including fluorescein sodium (Na-F 376 Da) and FITC-dextran (4000, 20 000, and 70 000 Da), were evaluated in Transwell culture. The permeability calibration data collected for the 4 materials in increasing order of size were 61%, 24%, 21%, and 19%, indicating size-dependent permeability.
MTT Assay of Cell Viability
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay is a colorimetric assay that evaluates cell metabolic activity to characterize cell viability. MTT solution (500 ng/mL in culture medium) was added to the harvested cells at 100 μL/well and incubated at 37°C for 3 h. After incubation, the MTT solution was removed, and 100 µL of dimethyl sulfoxide was added to each well and incubated for 15 min. An ELISA plate reader was used to determine the ratio of the absorbance at 570 and 630 nm. First, to determine the direct effect of borneol (#15598, Sigma-AldrichTM, Merck KGaA, Darmstadt, Germany) and tetrandrine (#T2695, Sigma-AldrichTM, Merck KGaA, Darmstadt, Germany) on tumor cell viability in the absence of BBB/BTB and 5-FU, tumor cells were treated with drugs at different concentrations. The concentration of borneol ranged from 0 to 300 μg/mL, and that of tetrandrine ranged from 0 to 10 μg/mL. After 24 h, the half-maximal inhibitory concentration (IC50) values (ie, the concentration at which cell viability was reduced by approximately 50%) in CT26 cells were determined. The combined effect of borneol and tetrandrine was evaluated using the Chou-Talalay method. 25 Second, tumor cells were treated with borneol (300 μg/mL) and tetrandrine (10 μg/mL) in the BTB model without 5-FU. These results could facilitate the understanding of the effect of borneol and tetrandrine on tumor cells in the BTB. In the BTB model, we next tested the effect on CT26 cell viability of 5-FU with or without borneol/tetrandrine, alone or in combination. The control group cells were treated with 5-FU (10 μg/mL) alone. The effects of 5-FU on the viability of CT26 cells with or without borneol (300 μg/mL), tetrandrine (10 μg/mL), and borneol (300 μg/mL) + tetrandrine (10 μg/mL) were observed. In the BTB model, CT26 cells seeded in the lower chamber were collected and subjected to MTT assay. To obtain further data on the integrity of BBB/BTB, the effects of borneol, tetrandrine, and borneol + tetrandrine on the integrity of the BTB were examined in subsequent experiments.
Transendothelial Electrical Resistance (TEER) Assay of BBB Integrity
Changes in BBB integrity can be addressed in vitro by determining the permeability of a cell monolayer to different solutes and measuring the TEER,26,27 which reflects the ionic conductance of the paracellular pathway in the epithelial monolayer. The TEER method is widely applied because it is noninvasive and can be used to monitor live cells. The device we use to measure TEER is Epithelial Volt/Ohm (TEER) Meter made by WPI (World Precision Instruments, Sarasota, FL, USA). TEER values were determined by measuring the resistance of the cell layer between 2 electrodes positioned on either side of the barrier. The measured resistance value before the addition of cells was subtracted from subsequent measurement values; specifically, the TEER was calculated using the following equation: TEER = [Resistanceexp(Ω) – Resistanceblank(Ω)] × bottom area (cm2). A lower TEER indicates higher permeability. The TEER method was used to evaluate the effect of co-cultured CT26 and B16F10 cells on BBB integrity. CT26 cells significantly influenced the integrity of the BBB, indicating that they can be used to establish an informative BTB model. The integrity of the BBB in the BTB model was influenced by borneol (300 μg/mL), tetrandrine (10 μg/mL), and their combination. To obtain further data on the activity of P-gp, the effects of borneol, tetrandrine, and borneol + tetrandrine on P-gp activity were analyzed in subsequent experiments.
Flow Cytometric Analysis of P-gp Expression
P-gp activity was estimated by flow cytometry using the fluorescent substrate rhodamine 123, which was purchased from Thermo Fisher Scientific (Pittsburgh, PA, USA). The bEnd3 cells were seeded in the upper chamber of the Transwell apparatus on day 1. The tumor cells were seeded in the bottom chamber of the Transwell apparatus on day 3. The different groups were treated with 5-FU, borneol, and tetrandrine on day 4. On day 5, the cells were harvested and incubated with 20 μM rhodamine 123 solution for 45 min. Cells were then washed twice with PBS and analyzed (10 000 cells/sample) for the accumulation of rhodamine 123 using a FACSCalibur flow cytometer (excitation at 515 nm and emission at 545 nm; Becton Dickinson, San Jose, CA, USA). Data were analyzed using CellQuest software (Becton Dickinson). 7-Aminoactinomycin D (7-AAD, 0.25 μg/mL) was used to differentiate dead cells for exclusion. The concentrations used were as follows: borneol (300 μg/mL), tetrandrine (10 μg/mL), and 5-FU (10 μg/mL). 5-FU was added to all groups. In some experiments, SN50 (trifluoroacetate salt ≥95% (HPLC-grade), Sigma), a peptide capable of inhibiting the nuclear translocation of the active NF-κB complex, was used to inhibit the function of NF-κB. In our experiments, SN50 was used at a concentration of 20 μM. To investigate the potential mechanism of borneol, tetrandrine, and P-gp, western blot analysis of NF-κB was performed.
Western Blot Analysis of NF-κB Expression
P-gp-mediated drug resistance is associated with the overexpression of MDR1 in cells. 28 The MDR1 promoter sequence contains a κB site recognized by NF-κB. 29 Therefore, downregulation of NF-κB can reverse the MDR mechanism in tumor cells. 30 Cells were treated with borneol (300 μg/mL) or tetrandrine (10 μg/mL) for 24 h. The cells were then collected, and whole-cell lysates were prepared. Forty micrograms of protein extract was used for the experiments. The membrane was blocked with 5% skim milk and incubated with primary antibodies against P-gp and β-actin (Sigma-Aldrich, Eugene, OR, USA) at 4°C overnight. Then, a goat anti-mouse secondary antibody (Merck Millipore, Darmstadt, Germany) was added. A MultiGel-21 enhanced chemiluminescence system (TopBio Inc. Taipei, Taiwan) was used for final protein detection. Similar procedures to NF-κB were performed, but with antibodies against NF-κB. The effects of 5-FU, borneol, and tetrandrine on mice were observed in subsequent experiments.
Experimental Animal Model of Internal Carotid Artery Guided Brain Metastasis and Treatment
Male BALB/c mice (4-5 weeks old) were obtained from the National Laboratory Animal Center (Taipei, Taiwan). Mice were bred and maintained in a specific pathogen-free area at our animal facility. Mice were housed, 5 per cage, and provided sterilized laboratory chow (LabDiet 5001, PMI Nutrition International, LLC, MO) and water ad libitum. The virological and bacteriological status of the mice was monitored routinely. The animal room was automatically maintained on a 12-h light/dark cycle at 22 ± 1°C for acclimatization. All the experiments were performed under sterile conditions. The animal experimental protocols were conducted in accordance with the regulations of the Institutional Guide for the Care and Use of Laboratory Animals, and the experimental protocol was approved by the Experimental Animal Committee of Mackay Memorial Hospital (MMH-A-S-106-37). Mice were anesthetized by intraperitoneal injection of ketamine hydrochloride (120 mg/kg). A skin incision was made over the right side of the neck, and the vessels over the neck were identified at the positions of the common carotid artery, external carotid artery, and internal carotid artery. The external carotid artery was ligated, and CT26 cancer cells were injected into the common carotid artery to circulate into the internal carotid artery. The skin was closed after the cancer cells were injected. The success of internal carotid artery injection was evaluated using an in vivo imaging system and acquisition of both dorsal and ventral images, which showed a systemic distribution of bioluminescence throughout the animal. Subsequent metastasis was monitored by optical imaging twice weekly for 60 days.
After initiation of tumor growth by common carotid artery injection as described below, mice were treated with no medication (control), 5-FU (10 mg/kg/day) alone, borneol (300 mg/kg/day) + 5-FU (10 mg/kg/day), tetrandrine (10 mg/kg/day) + 5-FU (10 mg/kg/day), or borneol (300 mg/kg/day) + tetrandrine (10 mg/kg/day) + 5-FU (10 mg/kg/day) for 5 consecutive days. Intraperitoneal injection was used to administer all medications. Each group contained 3 mice for the survival observation. The day of administration was defined as study day 1, and the mice were observed for 60 days. Tumors were measured weekly using an in vivo imaging system (IVIS Instrument, Spectrum, 120V, Andor C-124262/Perkinelmer, USA), and measurement data were expressed as the luciferase intensity within the region of interest (ROI). Hair was shaved from the head before using IVIS. The use of IVIS could decrease the requirement of sacrifice and organ collection. 31
Statistical Analysis
Differences between 2 groups were compared using an independent Student’s t-test. Differences between more than 2 groups were compared using one-way ANOVA followed by Dunnett’s post hoc test, as specified in the figure legends. The Mann–Whitney U test or Kruskal–Wallis test followed by Dunn’s test was applied to compare the difference when the distribution of data did not follow the normality assumption (Shapiro–Wilk test), as specified in the figure legends. The Brown–Forsythe test followed by Dunnett’s T3 test was applied to compare the difference when the distribution of data did not follow the assumption of equal variances. Survival time was estimated using Kaplan–Meier curves and log-rank tests. A linear mixed model was used to compare repeated measures of body weight and tumor size across time points among the different treatment groups. Data are presented as mean ± standard deviation. Statistical significance was set at P < .05. IBM SPSS Statistics 21 (IBM Co., Armonk, NY, USA) was used for statistical analysis.
Results and Discussion
Effect of Borneol and Tetrandrine on Cultured Tumor Cells
To investigate the cytotoxicity of borneol and tetrandrine on CT26 tumor cell viability in the absence of 5-FU and BBB/BTB, cells were treated with borneol at concentrations ranging from 0 to 300 μg/mL (0, 1, 5, 10, 50, 100, and 300 μg/mL) and tetrandrine at concentrations ranging from 0 to 10 μg/mL (0, 2.5, 5, and 10 μg/mL) for 24 h. In this study, the IC50 values for borneol and tetrandrine were 240 and 7 μg/mL, respectively. The IC50 values for borneol + tetrandrine at 2 concentrations (expressed as borneol IC50/tetrandrine IC50) were 300/4 and 150/5 μg/mL. The effect of the combination of borneol and tetrandrine was more significant than that of the other treatments. These results showed that tetrandrine had a greater inhibitory effect than borneol on the viability of CT26 tumor cells. The combination of borneol and tetrandrine profoundly suppressed cell viability, especially at higher concentrations.
To compare the combination index (CI) of borneol and tetrandrine in terms of CT26 tumor cell viability, The Chou–Talalay method, which is based on the median-effect equation derived from the mass-action law principle, was used. 25 This theory provides a common link between a single entity and multiple entities and between first-order and higher-order dynamics. The resulting combination index (CI) theorem of Chou–Talalay offers quantitative definitions for additive effects (CI = 1), synergism (CI < 1), and antagonism (CI > 1) in drug combinations. 25
According to the Chou–Talalay method, the CI values of borneol + tetrandrine at CI50, CI75, CI90, and CI95 were 0.42, 0.24, 0.14, and 0.10, respectively. These CI values were less than 1, suggesting a synergistic effect of the borneol + tetrandrine combination. This is the first time that borneol has been combined with tetrandrine for the treatment of cancer cells.
Effect of Borneol and Tetrandrine on Tumor Cells With a BTB
To explore the effect of borneol and tetrandrine on tumor cells in the presence of BTB, in the absence of 5-FU, tumor cells were treated with borneol (300 μg/mL) and tetrandrine (10 μg/mL) in the BTB model. The CT26 cell viability rates were 0.68 ± 0.39, 0.76 ± 2.60, and 0.26 ± 0.43 after treatment with borneol (300 μg/mL), tetrandrine (10 μg/mL) and borneol (300 μg/mL) + tetrandrine (10 μg/mL), respectively. No significant inhibition of tumor cell viability was observed in the BTB model. These results suggest that borneol, tetrandrine, and borneol + tetrandrine might have a comparatively lower effect on the inhibition of tumor cell viability in the BTB model. Borneol is a promising candidate for central nervous system drug delivery, mainly through mediation of multitargeted effects that increase BBB permeability 32 ; moreover, tetrandrine has a potential therapeutic effect in cancer. 33 These observations and lower toxicity on the inhibition of tumor cell viability in the BTB model might provide synergistic treatment with chemotherapy medicine on the viability of CT26 cells.
Modulation of the Effect of 5-FU on Tumor Cells With a BTB by Borneol, Tetrandrine and Borneol + Tetrandrine
To investigate the modulatory effects of borneol and tetrandrine on 5-FU treatment of tumor cells in the BTB, 300 μg/mL borneol, 10 μg/mL tetrandrine, and 10 μg/mL 5-FU were used, and CT26 cell viability was assessed in the BTB experimental model. The control group was treated with 5-FU alone (10 μg/mL). The CT26 cell viability rates were 0.49 ± 0.46, 0.38 ± 0.58, and 0.017 ± 0.015 after treatment with borneol (300 μg/mL), tetrandrine (10 μg/mL) and borneol (300 μg/mL) + tetrandrine (10 μg/mL), respectively, demonstrating that CT26 cell viability after treatment with 5-FU (10 μg/mL) was significantly reduced in the group treated with borneol (300 μg/mL) + tetrandrine (10 μg/mL) (Figure 2). These results suggest that the penetration of 5-FU through the BTB was significantly enhanced by the combination of borneol and tetrandrine. According to a previous report, P-gp inhibition may disrupt the BBB or BTB to enhance the efficacy of chemotherapeutic drugs on brain tumors.34,35 In addition, our results indicate that borneol regulates BBB permeability in experimental models of ischemic stroke. 22 Moreover, physical methods, such as pulsed high-intensity focused ultrasound with an ultrasound contrast agent, have been used to disrupt the BBB and BTB for chemotherapeutic applications. 36
Figure 2.

Modulation of 5-FU effect by borneol and tetrandrine on tumor cells with BTB.
Data are presented as mean ± standard deviation. The concentrations used were BN (300 μg/mL), tetrandrine (10 μg/mL), and 5-FU (10 μg/mL) in the BTB model. N = 3 for each group. *P < .001 (Brown–Forsythe test followed by Dunnett’s T3 test). Borneol (300 μg/mL) and tetrandrine (10 μg/mL), especially in combination (borneol + tetrandrine), enhanced the effect of 5-FU on the viability of CT26 cells.
Abbreviations: BN, borneol; TT, tetrandrine; BN + TT, borneol combined with tetrandrine; BTB, blood–tumor barrier.
Effect of Borneol, Tetrandrine and Borneol + Tetrandrine on BTB Integrity
To evaluate the effect of tumor cell co-culture on BBB integrity without chemotherapy medications, we used the BTB model and TEER assay. In the BTB model, endothelial bEnd3 and tumor CT26 cells were seeded in the upper and lower chambers of the transwell apparatus. Because electrical resistance can reflect ionic conductance, TEER has been widely used to investigate the integrity of the BBB and BTB. As shown in Figure 3, CT26 cells significantly impaired BBB integrity. The BBB (bEnd3) and CT26-BTB (bEnd3 + CT26) TEER values were significantly different at 18.55 ± 4.77 and 7.00 ± 2.60, respectively. To investigate the effect of borneol, tetrandrine, and borneol + tetrandrine on the integrity of the BTB, the herbal ingredients borneol, tetrandrine, and borneol + tetrandrine were used to assess the TEER of the BTB. The electrical resistance in the BBB and BTB models was significantly reduced by treatment with borneol (300 μg/mL), tetrandrine (10 μg/mL), and borneol (300 μg/mL) + tetrandrine (10 μg/mL). The CT26-BTB TEER values were 19.09 ± 7.53, 4.82 ± 1.43, 8.55 ± 2.95, and 3.03 ± 2.10 after treatment with control, borneol (300 μg/mL), tetrandrine (10 μg/mL) and borneol (300 μg/mL) + tetrandrine (10 μg/mL) (Figure 4). These results imply that borneol markedly influences the integrity of BTB. The TEER values in the borneol + tetrandrine group were significantly lower than those in the control group, suggesting a synergistic effect of the combination of borneol and tetrandrine. Both borneol and tetrandrine are natural products that exhibit multiple biological activities. For example, a previous report indicated that borneol synergistically enhanced the anticancer efficacy of cisplatin in human glioma cells. 37
Figure 3.
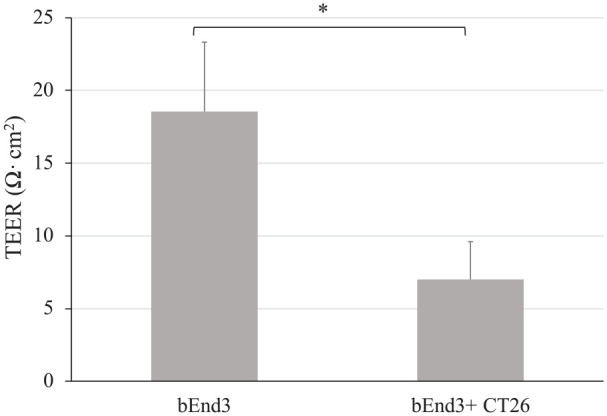
Integrity of BBB affected by tumor cells in the BTB model.
Data are presented as mean ± standard deviation. CT26 cells showed significantly impaired BBB integrity. n = 3 per group. *P < .05, t-test indicates a significant difference compared to the control group.
Abbreviations: BBB, blood–brain barrier; BTB, blood–tumor barrier.
Figure 4.

Effect of borneol and tetrandrine on BBB/BTB integrity.
Data are presented as mean ± standard deviation. These results imply that borneol may influence the integrity of BTB. The TEER values in the borneol + tetrandrine group were significantly (up to 80%) lower than those in the control group, suggesting a synergistic effect of the combination of borneol and tetrandrine. *P < 0.05 (Kruskal–Wallis test followed by Dunn’s test) indicates a significant difference compared to the control group. Borneol and tetrandrine were used at concentrations of 300 and 10 μg/mL, respectively. n = 3 per group.
Abbreviations: BN, borneol; TT, tetrandrine; BN + TT, borneol combined with tetrandrine; BBB, blood–brain barrier; BTB, blood–tumor barrier.
Effect of Borneol, Tetrandrine and Borneol + Tetrandrine on P-gp Activity
To investigate the activity of the P-gp transporter, rhodamine 123 was used and analyzed by flow cytometry. The materials increase the cellular accumulation of rhodamine 123 in P-gp–expressing cells by inhibiting its efflux. The use of rhodamine 123 to evaluate the inhibitory potential of P-gp on drug retention has been previously reported. 38 The expression of P-gp in the CT26-BTB model was examined using flow cytometry. The performance of P-gp-mediated transport was evaluated using flow cytometry after rhodamine 123 staining. The relative fluorescence intensity of rhodamine 123 in drug-treated cells was compared with that in the 5-FU (10 μg/mL) group, which was set to 1, and 5-FU (10 μg/mL) was added to all groups. The values of rhodamine 123 intensity in the borneol (300 μg/mL), tetrandrine (10 μg/mL), and borneol (300 μg/mL) + tetrandrine (10 μg/mL) groups were 1.05 ± 0.18, 6.81 ± 1.35, and 8.11 ± 1.60, respectively. These results suggest that resistance to P-gp-mediated transport was significantly enhanced by treatment with borneol (300 μg/mL) and tetrandrine (10 μg/mL), but was not affected by treatment with borneol (300 μg/mL) or tetrandrine (10 μg/mL) alone (Figure 5). Borneol showed a tissue-specific BBB-opening effect. Moreover, borneol has been shown to increase the delivery of rhodamine 123 in the hippocampus and hypothalamus. 39 Rhodamine-123 is a substrate for both P-gp and the organic cation carrier systems in cell lines such as the kidney LLC-PK1 cell line. 40
Figure 5.

Effect of borneol and tetrandrine on P-gp activity in the BTB model.
Data are presented as mean ± standard deviation. The concentrations of the analytes borneol, tetrandrine, and 5-FU used in this experiment were 300, 10, and 10 μg/mL, respectively. n = 3 per group. *P < .05 (Kruskal–Wallis test followed by Dunn’s test) indicates a significant difference compared to the control group (10 μg/mL 5-FU).
Abbreviations: BN, borneol; TT, tetrandrine; BN + TT, borneol combined with tetrandrine; BTB, blood–tumor barrier.
P-gp, an efflux transporter protein expressed in cerebral microvascular endothelial cells, is present in both the BBB and BTB, 1 implying that compounds that inhibit P-gp-mediated transport may modulate drug efflux through the BBB and BTB to promote the retention of penetrated drugs, rendering a net effect of enhanced permeability. Our results were consistent with those of a previous report, which indicated that borneol increased the permeability of the physiological BBB. 41 The BTB is highly heterogeneous and characterized by numerous distinct features, including nonuniform permeability and active efflux of molecules. 42 The potential cause of the high failure rates of drug permeability is the BBB and BTB, which limit the access of potentially effective chemotherapeutics to metastatic lesions. Strategies to overcome these barriers include new small molecules capable of crossing into the brain parenchyma, novel formulations of existing chemotherapeutics, and barrier disruption techniques. 43 Our results indicate that resistance to P-gp-mediated transport was significantly increased by treatment with tetrandrine in combination with borneol (Figure 5).
Effect of NF-κB on P-gp Activity by Western Blot Analysis
To investigate the possible mechanism by which the potential herbal ingredients borneol and tetrandrine inhibit P-gp activity, the relationships between the herbal ingredients and NF-κB were evaluated. In brain endothelial cells, the BBB function can be influenced by NF-κB. 44 In this study, the P-gp proteion expression level in the 5-FU (10 μg/mL) reference group was set to 1, and the relative expression levels of P-gp in the borneol (300 μg/mL), tetrandrine (10 μg/mL), and borneol (300 μg/mL) + tetrandrine (10 μg/mL) treatment groups were 0.76 ± 0.20, 0.76 ± 0.19, and 0.68 ± 0.2. Borneol, tetrandrine, and borneol + tetrandrine may suppress the expression level of P-gp, but no significant differences were observed.
In the present study, the relationship between the herbal ingredients and the expression of NF-κB was explored using western blot analysis. The NF-κB expression level in the 5-FU (10 μg/mL) reference group was set to 1, and the relative expression levels of NF-κB in the borneol (300 μg/mL), tetrandrine (10 μg/mL), and borneol (300 μg/mL) + tetrandrine (10 μg/mL) treatment groups were 1.04 ± 0.4, 1.08 ± 0.24, and 1.02 ± 0.43, respectively, suggesting that NF-κB expression levels were not significantly different among the groups. Thus, the expression of P-gp might be downregulated by borneol and tetrandrine, but NF-κB might not be influenced by borneol and tetrandrine in the CT26-BTB model.
Furthermore, to explore the relationships among the herbal ingredients, NF-κB activity, and P-gp activity, the activity of P-gp was evaluated through flow cytometric analysis of rhodamine 123 in the CT26-BTB model. SN50 is a peptide capable of inhibiting the nuclear translocation of the active NF-κB complex. To further examine the effect of NF-κB activity on P-gp activity, SN50 was used as an inhibitor of NF-κB activity. The P-gp activity rate in the 5-FU (10 μg/mL) group was set to 1, and the relative P-gp activity levels in the borneol (300 μg/mL), tetrandrine (10 μg/mL), and borneol (300 μg/mL) + tetrandrine (10 μg/mL) groups, as determined by flow cytometric analysis of rhodamine 123 retention, were 0.86 ± 0.05, 4.78 ± 2.30, and 4.70 ± 2.11, respectively, in the absence of SN50 (Figure 6A), and 0.74 ± 0.07, 4.63 ± 2.32, and 4.24 ± 1.48, respectively, with SN50 (Figure 6B). Both the 5-FU + TT and 5-FU + borneol + tetrandrine groups were significantly different from the control group, which was independent of the addition of SN50. To compare Figure 6A and B, these results suggested that the activity of P-gp was not significantly different among the groups, further indicating that tetrandrine, either alone or in combination with borneol, does not modulate the activity of P-gp through altering the expression of NF-κB. These results are different from those reported by Fan et al. 30 In our study, the permeability of the physiological BBB was increased by borneol, and borneol did not influence the activity of P-gp (Figure 5).
Figure 6.
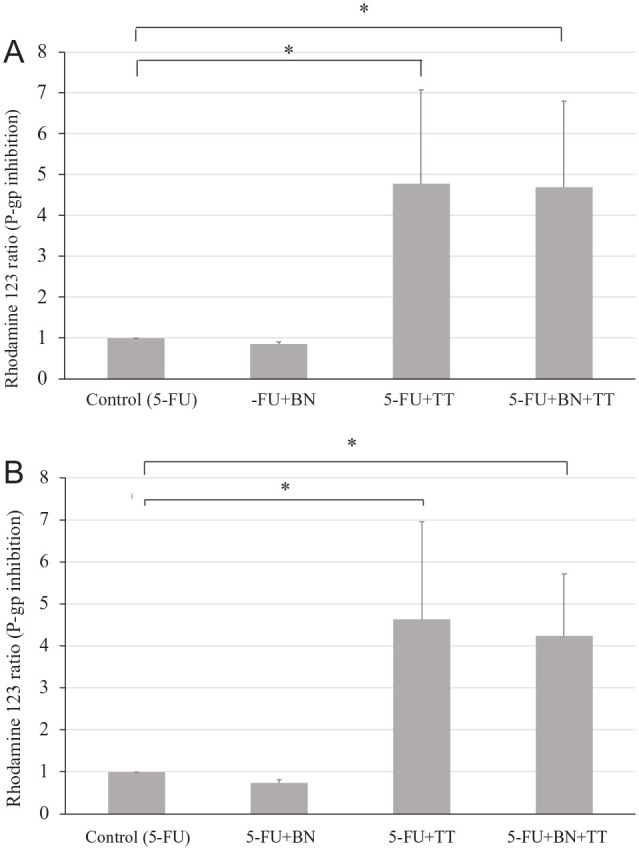
Effect of the NF-κB inhibitor on the modulatory activity of BN and TT in the BTB model. (A) Without SN50. (B) With SN50.
Data are presented as mean ± standard deviation. (A) Effect of BN and TT in the BTB model without the NF-κB inhibitor (SN50) treatment. (B) Effect of BN and TT in the BTB model following treatment with the NF-κB inhibitor SN50 (20 μM). The concentrations of the analytes borneol, tetrandrine, and 5-FU were 300, 10, and 10 μg/mL, respectively. *P < .05 (ANOVA followed by Dunnett’s test) indicates a significant difference compared to the control group (10 μg/mL 5-FU). Both the 5-FU + TT and 5-FU + BN + TT groups showed significant changes compared to the control group, which was independent of the addition of SN50. On comparing the differences between Figure 6A and B by flow cytometry, the activity of P-gp was not significantly different among the groups. According to this result, the 5-FU + TT and 5-FU + BN + TT groups could influence the function of P-gp through another pathway.
Abbreviations: 5-FU + BN, 5-FU combined with borneol; 5-FU + TT, 5-FU combined with tetrandrine; 5-FU + BN + TT, 5-FU combined with borneol and tetrandrine; BTB, blood–tumor barrier.
Survival Time and Body Weight Analysis
To investigate the efficacy and wellbeing effects of 5-FU, 5-FU + borneol, 5-FU + tetrandrine, and 5-FU + borneol + tetrandrine treatment in mice, the survival time and body weight of mice injected with CT26 tumor cells were monitored. Mice were injected with CT26 cancer cells into the common carotid artery with ligation of the external carotid artery, from which they circulated into the internal carotid artery. To ensure metastasis, an in vivo imaging system was used to acquire both the dorsal and ventral images. The images showed a systemic distribution of bioluminescence throughout the animals. The survival times in the control, 5-FU, 5-FU + borneol, 5-FU + tetrandrine and 5-FU + borneol + tetrandrine groups were 19.5 ± 3.1, 18 ± 4.4, 24 ± 3.5, 21.7 ± 7.1, and 31 ± 1.0 days, respectively. Borneol combined with tetrandrine significantly prolonged the survival of 5-FU-treated mice with brain metastasis (Figure 7). Moreover, the borneol + tetrandrine + 5-FU-treated mice survived longer than mice in the untreated control group. No acute toxicity or obvious changes in body weight were observed in any of the mice. These results suggest that borneol + tetrandrine may be a useful antimetastatic agent and can be used as an adjunct to chemotherapeutics to treat brain metastasis, as a synergistic effect of the herbal ingredients with 5-FU was observed. Our study is consistent with a previous report indicating that adjuvant chemotherapy may prolong the survival of patients with cancer. 45 Although the augmentation of 5-FU effect by borneol + tetrandrine against brain metastasis might be through modulation of BBB/BTB in vivo, as noted in the in vitro model, the causal relationship remains to be elucidated.
Figure 7.
The effect of various treatments on the survival time of mice with brain metastasis.
Data are presented as mean ± standard deviation. Borneol combined with tetrandrine significantly prolonged the survival of 5-FU-treated mice with brain metastasis. Moreover, the borneol + tetrandrine + 5-FU-treated mice survived longer than mice in the untreated control group. Doses of 10 mg/kg 5-FU, 300 mg/kg borneol, and 10 mg/kg tetrandrine were used in the experiment. *P < .05, log-rank test indicates a significant difference compared to each group.
Abbreviations: 5-FU + BN: 5-FU combined with borneol; 5-FU + TT, 5-FU combined with tetrandrine; 5-FU + BN + TT, 5-FU combined with borneol and tetrandrine.
Conclusion
In vitro assays showed that the bioactive constituents borneol and tetrandrine potentially improved the penetration of 5-FU through the BBB and BTB models. Studies in the in vivo brain metastasis model indicated that the combination of borneol and tetrandrine can be used as an adjunct to chemotherapeutics to treat brain metastasis. Both in vitro and in vivo experiments demonstrated the synergistic effect of these herbal ingredients with 5-FU. Our study provides the first demonstration that borneol + tetrandrine combined with 5-FU can potentially be used to treat brain metastases of tumor cells.
Footnotes
Animal Welfare Statements: All animal experiment protocols were approved by the International Animal Care and Use Committee (IACUC) of Mackay Memorial Hospital (approval number MMH-A-S-106-37, grant numbers MMH-E-110-13), Taipei, Taiwan.
Author Contributions: J.F. Lin, Y.S. Liu, Y.C. Huang, C.W. Chi, and C.C. Tsai performed the experiments, analyzed the data and prepared the manuscript. T.H. Tsai and Y.J. Chen designed the experiments, edited the paper and secured funding. All authors read and agreed to the published version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by MacKay Memorial Hospital (grant numbers MMH-E-109-13, MOST 104-2623-E-195-001-NU, and MMH-106-140) of Taiwan.
ORCID iD: Yu-Jen Chen  https://orcid.org/0000-0001-9794-8938
https://orcid.org/0000-0001-9794-8938
References
- 1. Dewanjee S, Dua T, Bhattacharjee N, et al. Natural products as alternative choices for P-glycoprotein (P-gp) inhibition. Molecules. 2017;22:871. doi: 10.3390/molecules22060871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adkins CE, Mittapalli RK, Manda VK, et al. P-glycoprotein mediated efflux limits substrate and drug uptake in a preclinical brain metastases of breast cancer model. Front Pharmacol. 2013;4:136. doi: 10.3389/fphar.2013.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aryal M, Fischer K, Gentile C, Gitto S, Zhang YZ, McDannold N. Effects on P-glycoprotein expression after blood-brain barrier disruption using focused ultrasound and microbubbles. PLoS One. 2017;12:e0166061. doi: 10.1371/journal.pone.0166061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang RD, Price JE, Fujimaki T, Bucana CD, Fidler IJ. Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am J Pathol. 1992;141:1115-1124. [PMC free article] [PubMed] [Google Scholar]
- 5. Whelan R, Hargaden GC, Knox AJS. Modulating the blood-brain barrier: a comprehensive review. Pharmaceutics. 2021;13:1980. doi: 10.3390/pharmaceutics13111980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phoenix TN, Patmore DM, Boop S, et al. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell. 2016;29:508-522. doi: 10.1016/j.ccell.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Griveau A, Seano G, Shelton SJ, et al. A glial signature and Wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell. 2018;33:874-889.e7. doi: 10.1016/j.ccell.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyle LT, Lockman PR, Adkins CE, et al. Alterations in pericyte subpopulations are associated with elevated blood-tumor barrier permeability in experimental brain metastasis of breast cancer. Clin Cancer Res. 2016;22:5287-5299. doi: 10.1158/1078-0432.CCR-15-1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morikawa A, de Stanchina E, Pentsova E, et al. Phase I study of intermittent high-dose lapatinib alternating with capecitabine for HER2-positive breast cancer patients with central nervous system metastases. Clin Cancer Res. 2019;25:3784-3792. doi: 10.1158/1078-0432.CCR-18-3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40:85-104. doi: 10.2165/00003088-200140020-00002 [DOI] [PubMed] [Google Scholar]
- 11. André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. New Engl J Med. 2004;350:2343-2351. doi: 10.1056/NEJMoa032709 [DOI] [PubMed] [Google Scholar]
- 12. Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465-1471. doi: 10.1200/JCO.2010.33.6297 [DOI] [PubMed] [Google Scholar]
- 14. Müller S, Köhler F, Hendricks A, et al. Brain metastases from colorectal cancer: a systematic review of the literature and meta-analysis to establish a guideline for daily treatment. Cancers (Basel). 2021;13:21. doi: 10.3390/cancers13040900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duan M, Xing Y, Guo J, Chen H, Zhang R. Borneol increases blood-tumour barrier permeability by regulating the expression levels of tight junction-associated proteins. Pharm Biol. 2016;54:3009-3018. doi: 10.1080/13880209.2016.1199044 [DOI] [PubMed] [Google Scholar]
- 16. Morimoto M, Cantrell CL, Libous-Bailey L, Duke SO. Phytotoxicity of constituents of glandular trichomes and the leaf surface of camphorweed, Heterotheca subaxillaris. Phytochemistry. 2009;70:69-74. doi: 10.1016/j.phytochem.2008.09.026 [DOI] [PubMed] [Google Scholar]
- 17. Chung MS. Antiviral activities of Artemisia princeps var. orientalis essential oil and its α-thujone against norovirus surrogates. Food Sci Biotechnol. 2017;26:1457-1461. doi: 10.1007/s10068-017-0158-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung NH, Huong LT, Chung NT, et al. Callicarpa species from central Vietnam: essential oil compositions and mosquito larvicidal activities. Plants (Basel). 2020;9:16. doi: 10.3390/plants9010113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang MY, Khine AA, Liu JW, et al. Resolution of isoborneol and its isomers by GC/MS to identify “synthetic” and “semi-synthetic” borneol products. Chirality. 2018;30:1233-1239. doi: 10.1002/chir.23017 [DOI] [PubMed] [Google Scholar]
- 20. Liu XC, Liang Y, Shi WP, Liu QZ, Zhou L, Liu ZL. Repellent and insecticidal effects of the essential oil of Kaempferia galanga rhizomes to Liposcelis bostrychophila (Psocoptera: Liposcelidae). J Econ Entomol. 2014;107:1706-1712. doi: 10.1603/ec13491 [DOI] [PubMed] [Google Scholar]
- 21. Wu T, Zhang A, Lu H, Cheng Q. The role and mechanism of borneol to open the blood-brain barrier. Integr Cancer Ther. 2018;17:806-812. doi: 10.1177/1534735418767553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen ZX, Xu QQ, Shan CS, et al. Borneol for regulating the permeability of the blood-brain barrier in experimental ischemic stroke: preclinical evidence and possible mechanism. Oxid Med Cell Longev. 2019;2019:2936737. doi: 10.1155/2019/2936737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei N, Sun H, Wang F, Liu G. H1, a novel derivative of tetrandrine reverse P-glycoprotein-mediated multidrug resistance by inhibiting transport function and expression of P-glycoprotein. Cancer Chemother Pharmacol. 2011;67:1017-1025. doi: 10.1007/s00280-010-1397-7 [DOI] [PubMed] [Google Scholar]
- 24. Wang M, Bronte V, Chen PW, et al. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154:4685-4692. [PMC free article] [PubMed] [Google Scholar]
- 25. Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440-446. doi: 10.1158/0008-5472.CAN-09-1947 [DOI] [PubMed] [Google Scholar]
- 26. Czupalla CJ, Liebner S, Devraj K. In vitro models of the blood-brain barrier. Methods Mol Biol. 2014;1135:415-437. doi: 10.1007/978-1-4939-0320-7_34 [DOI] [PubMed] [Google Scholar]
- 27. Kriaučiūnaitė K, Kaušylė A, Pajarskienė J, et al. Immortalised hippocampal astrocytes from 3xTG-AD mice fail to support BBB integrity in vitro: role of extracellular vesicles in glial-endothelial communication. Cell Mol Neurobiol. 2021;41:551-562. doi: 10.1007/s10571-020-00871-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu JM, Chen Y, Chen JC, Lin TY, Tseng SH. Tetrandrine induces apoptosis and growth suppression of colon cancer cells in mice. Cancer Lett. 2010;287:187-195. doi: 10.1016/j.canlet.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 29. Loaiza B, Hernández-Gutierrez S, Montesinos JJ, Valverde M, Rojas E. Nuclear transcription factor kappa B downregulation reduces chemoresistance in bone marrow-derived cells through P-glycoprotein modulation. Arch Med Res. 2016;47:78-88. doi: 10.1016/j.arcmed.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 30. Fan X, Chai L, Zhang H, Wang Y, Zhang B, Gao X. Borneol depresses P-glycoprotein function by a NF-κB signaling mediated mechanism in a blood brain barrier in vitro model. Int J Mol Sci. 2015;16:27576-27588. doi: 10.3390/ijms161126051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poussard A, Patterson M, Taylor K, et al. In vivo imaging systems (IVIS) detection of a neuro-invasive encephalitic virus. J Vis Exp. 2012;70:e4429. doi: 10.3791/4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng Q, Chen ZX, Xu MB, et al. Borneol, a messenger agent, improves central nervous system drug delivery through enhancing blood-brain barrier permeability: a preclinical systematic review and meta-analysis. Drug Deliv. 2018;25:1617-1633. doi: 10.1080/10717544.2018.1486471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang KH, Liao HF, Chang HH, et al. Inhibitory effect of tetrandrine on pulmonary metastases in CT26 colorectal adenocarcinoma-bearing BALB/c mice. Am J Chin Med. 2004;32:863-872. doi: 10.1142/s0192415x04002478 [DOI] [PubMed] [Google Scholar]
- 34. van der Sandt IC, Gaillard PJ, Voorwinden HH, de Boer AG, Breimer DD. P-glycoprotein inhibition leads to enhanced disruptive effects by anti-microtubule cytostatics at the in vitro blood-brain barrier. Pharm Res. 2001;18:587-592. doi: 10.1023/a:1011016923346 [DOI] [PubMed] [Google Scholar]
- 35. Harun MSR, Marsh V, Elsaied NA, Webb KF, Elsheikha HM. Effects of Toxoplasma gondii infection on the function and integrity of human cerebrovascular endothelial cells and the influence of verapamil treatment in vitro. Brain Res. 2020;1746:147002. doi: 10.1016/j.brainres.2020.147002 [DOI] [PubMed] [Google Scholar]
- 36. Yang FY, Lin GL, Horng SC, et al. Pulsed high-intensity focused ultrasound enhances the relative permeability of the blood–tumor barrier in a glioma-bearing rat model. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:964-970. doi: 10.1109/tuffc.2011.1897 [DOI] [PubMed] [Google Scholar]
- 37. Cao WQ, Zhai XQ, Ma JW, et al. Natural borneol sensitizes human glioma cells to cisplatin-induced apoptosis by triggering ROS-mediated oxidative damage and regulation of MAPKs and PI3K/AKT pathway. Pharm Biol. 2020;58:72-79. doi: 10.1080/13880209.2019.1703756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jouan E, Le Vée M, Mayati A, Denizot C, Parmentier Y, Fardel O. Evaluation of P-glycoprotein inhibitory potential using a rhodamine 123 accumulation assay. Pharmaceutics. 2016;8:12. doi: 10.3390/pharmaceutics8020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu B, Ruan M, Dong X, Yu Y, Cheng H. The mechanism of the opening of the blood-brain barrier by borneol: a pharmacodynamics and pharmacokinetics combination study. J Ethnopharmacol. 2013;150:1096-1108. [PubMed] [Google Scholar]
- 40. van der Sandt IC, Blom-Roosemalen MC, de Boer AG, Breimer DD. Specificity of doxorubicin versus rhodamine-123 in assessing P-glycoprotein functionality in the LLC-PK1, LLC-PK1:MDR1 and Caco-2 cell lines. Eur J Pharm Sci. 2000;11:207-214. doi: 10.1016/s0928-0987(00)00097-x [DOI] [PubMed] [Google Scholar]
- 41. Wang HJ, Wu JJ, Xue Q, Zhang JF, Xu XY. [Effect factors and mechanisms of borneol’s bidirectional regulation on blood-brain barrier permeability]. Zhongguo Zhong Yao Za Zhi. 2017;42:2200-2207. doi: 10.19540/j.cnki.cjcmm.2017.0100 [DOI] [PubMed] [Google Scholar]
- 42. Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20:26-41. doi: 10.1038/s41568-019-0205-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sprowls SA, Arsiwala TA, Bumgarner JR, et al. Improving CNS delivery to brain metastases by blood-tumor barrier disruption. Trends Cancer. 2019;5:495-505. doi: 10.1016/j.trecan.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu C, Argyropoulos G, Zhang Y, Kastin AJ, Hsuchou H, Pan W. Neuroinflammation activates Mdr1b efflux transport through NFkappaB: promoter analysis in BBB endothelia. Cell Physiol Biochem. 2008;22:745-756. doi: 10.1159/000185558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gao P, Huang XZ, Song YX, et al. Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: a population-based study. BMC Cancer. 2018;18:234. doi: 10.1186/s12885-018-4138-7 [DOI] [PMC free article] [PubMed] [Google Scholar]




