Abstract
Objective
This study sought to evaluate the association between reported marijuana use and post-PCI in-hospital outcomes.
Background
Marijuana use is increasing as more states in the US legalize its use for recreational and medicinal purposes. Little is known about the frequency of use and relative safety of marijuana among patients presenting for PCI.
Methods
We analyzed the BMC2 PCI registry data between 1/1/2013 and 10/1/2016. We used 1:1 propensity matching and multivariable logistic regression to adjust for differences between patients with or without reported marijuana use and compared rates of post-PCI complications.
Results
Among 113,477 patients, 3,970 reported marijuana use. Compared with those without reported marijuana use, patients with reported marijuana use were likely to be younger (53.9 vs. 65.8 years), use tobacco (73.0% vs. 26.8%), present with STEMI (27.3% vs. 15.9%), and have fewer cardiovascular comorbidities. After matching, compared with patients without reported marijuana use, patients with reported marijuana use experienced significantly higher risks of bleeding (adjusted OR [aOR] 1.54 [95% CI: 1.20–1.97], p<0.001) and cerebrovascular accidents (CVA) (aOR 11.01 [1.32–91.67]; p=0.026), and a lower risk of AKI (aOR 0.61 [0.42–0.87]; p = 0.007). There were no significant differences in the risks of transfusion and death.
Conclusion
A modest fraction of patients undergoing PCI used marijuana. Reported marijuana use was associated with higher risks of CVA and bleeding and a lower risk of AKI after PCI. Clinicians and patients should be aware of the higher risk of post-PCI complications in these patients.
Keywords: Marijuana, Percutaneous Coronary Intervention, Coronary Artery Disease, Myocardial Infarction, Outcomes
Condensed Abstract
Little is known about the frequency and safety of marijuana use among patients undergoing percutaneous coronary intervention (PCI). Using the Blue Cross Blue Shield Michigan Cardiovascular Consortium clinical PCI registry, we evaluated the association between reported marijuana use and post-PCI in-hospital outcomes. We found that approximately 3–4% of patients undergoing PCI reported using marijuana recently. Reported marijuana use was associated with higher risks of bleeding and cerebrovascular accidents, but a lower risk of acute kidney injury after PCI. As marijuana use increases, clinicians and patients should be counseled on the increased risks of post-PCI complications among these patients.
INTRODUCTION
Marijuana use is increasing rapidly in the United States, as more states are legalizing its use for recreational and medicinal purposes (1). Marijuana exerts its biochemical effects through activation of cannabinoid receptors which are expressed on multiple organs, including hematologic, vascular, and myocardial tissues (2). Activation of cannabinoid receptors is implicated in the pathogenesis of atherosclerosis and precipitation of acute coronary syndrome. Marijuana has been shown to cause endothelial injury and modulate both the immune system and platelet function (2–4).
While some of the effects of marijuana on cardiovascular physiology have been described, its effects on clinical cardiovascular risks and outcomes are incompletely elucidated (5–7). Recent observational studies demonstrated that patients who use marijuana presenting with acute myocardial infarction (AMI) are often younger and have fewer traditional cardiovascular risk factors (8–10). However, there remains a dearth of evidence examining the association of marijuana use on cardiovascular outcomes.
In this context, we sought to describe the characteristics of patients with reported marijuana use undergoing percutaneous coronary intervention (PCI) and evaluate the association between reported marijuana use and post-PCI in-hospital outcomes.
METHODS
We performed a retrospective analysis on data collected by the Blue Cross Blue Shield Michigan Cardiovascular Consortium. BMC2 is a multi-center, statewide quality improvement collaborative that maintains a prospective registry of all patients who underwent PCI at 48 non-federal hospitals in Michigan. Detailed descriptions of the registry have been previously described (11–13). For this study, we included patients who underwent PCI from January 1, 2013 to October 1, 2016. Marijuana use was abstracted from the patient’s medical record at the time of PCI and was defined as the use of marijuana in an inhaled form at any time within one month prior to the index PCI. Sites were not specifically instructed to ask patients about marijuana use. Instead, this information was retrospectively abstracted from the patient’s medical records as part of the BMC2 PCI registry. The University of Michigan Institutional Review Board has waived the need for ongoing Institutional Review Board approval on all analyses that are performed using BMC2 data and determined that it met the definition of research not requiring informed consent.
In-Hospital Outcomes
We compared post-PCI in-hospital outcomes between patients with and without recent reported marijuana use. All in-hospital outcomes were assessed during the index hospitalization when PCI was performed. The primary in-hospital outcomes included death due to any cause, cerebrovascular accident (CVA), acute kidney injury (AKI), transfusion, and bleeding. Per the National Cardiovascular Data Registry definitions, CVA was defined as loss of neurological function by an ischemic or hemorrhagic event with residual symptoms lasting at least 24 hours after onset or leading to death. Transfusion was defined as any transfusion requiring whole blood or packed red blood cells. Bleeding was defined as any suspected or confirmed bleeding events observed or documented within 72 hours associated with any of the following: 1) a decrease in hemoglobin ≥3g/dL; 2) requirement of transfusion of whole blood or packed red blood cells; or 3) requirement of procedural intervention or surgery to stop, reverse, or correct the bleeding (14). AKI was defined as a post-PCI increase in serum creatinine of ≥0.5 mg/dL above the pre-PCI value. This definition of AKI is more associated with adverse clinical events in patients undergoing PCI (15,16).
We also performed a post-hoc analysis with two additional post-PCI in-hospital outcomes: hemodynamically significant arrhythmias and stent thrombosis. Hemodynamically significant arrhythmia was defined as ventricular tachycardia or ventricular fibrillation requiring pharmacologic and/or mechanical treatment including firing of an implantable cardioverter-defibrillator. Stent thrombosis was defined as angiographic evidence of thrombosis of the stent that was placed during the index admission (i.e. prior to discharge). Furthermore, we examined the association between the location of bleeding (i.e. access site vs non-access site) and reported marijuana use. Access site bleeding was defined as bleeding from the location of percutaneous entry with non-access site bleeding encompassing all other sites.
Statistical Analysis
We compared baseline characteristics between patients with and without reported marijuana use using Pearson chi-square or Fisher’s exact tests for categorical variables and Student’s t tests for continuous variables. We used a Cochran-Armitage test for trend to assess for a temporal trend in reported marijuana use among patients who underwent PCI. For in-hospital outcomes, propensity score matching and multivariable logistic regression techniques were used to adjust for differences in baseline characteristics between the two groups. A list of all clinical variables used to estimate the propensity score can be found in Supplementary Table 1. For propensity score matching, cases with missing values for continuous variables including creatinine (N=2,455), hemoglobin (N=2,359), height (N=344), and weight (N=129) were excluded from the matching process. Variables with greater than 10% missing values were excluded from the logistic regression model used to estimate the propensity score (17). To account for missingness for categorical variables, a separate category for missingness was generated. A total of 3,847 (3.4%) cases were excluded (Supplementary Figure 1). Patients with and without reported marijuana use were matched in a 1:1 fashion without replacement and within a caliper of 0.25 standard deviation of the propensity score. We reported adjusted odds ratios (aORs) for in-hospital outcomes using logistic regression models fitted in the propensity matched cohort, adjusting for all variables included in propensity matching (Supplementary Table 1). We used a 2-sided p value <0.05 to define statistical significance. For statistical analysis, R version 3.6.3 (R Foundation; Vienna, Austria) was used. All code files used for generation of results are available through Milan Seth (mcseth@med.umich.edu).
RESULTS
Among 113,477 patients who underwent PCI, 3,970 (3.5%) reported marijuana use (Central Illustration). After exclusions, a total of 109,630 patients were considered for propensity score matching. Patients with reported marijuana use represented <4.0% of all patients undergoing PCI across all study quarters. However, this proportion increased over the study period from 2.4% in quarter 1 of 2013 to 3.8% in quarter 3 of 2016 (p<0.001) (Supplementary Figure 2).
Central Illustration:
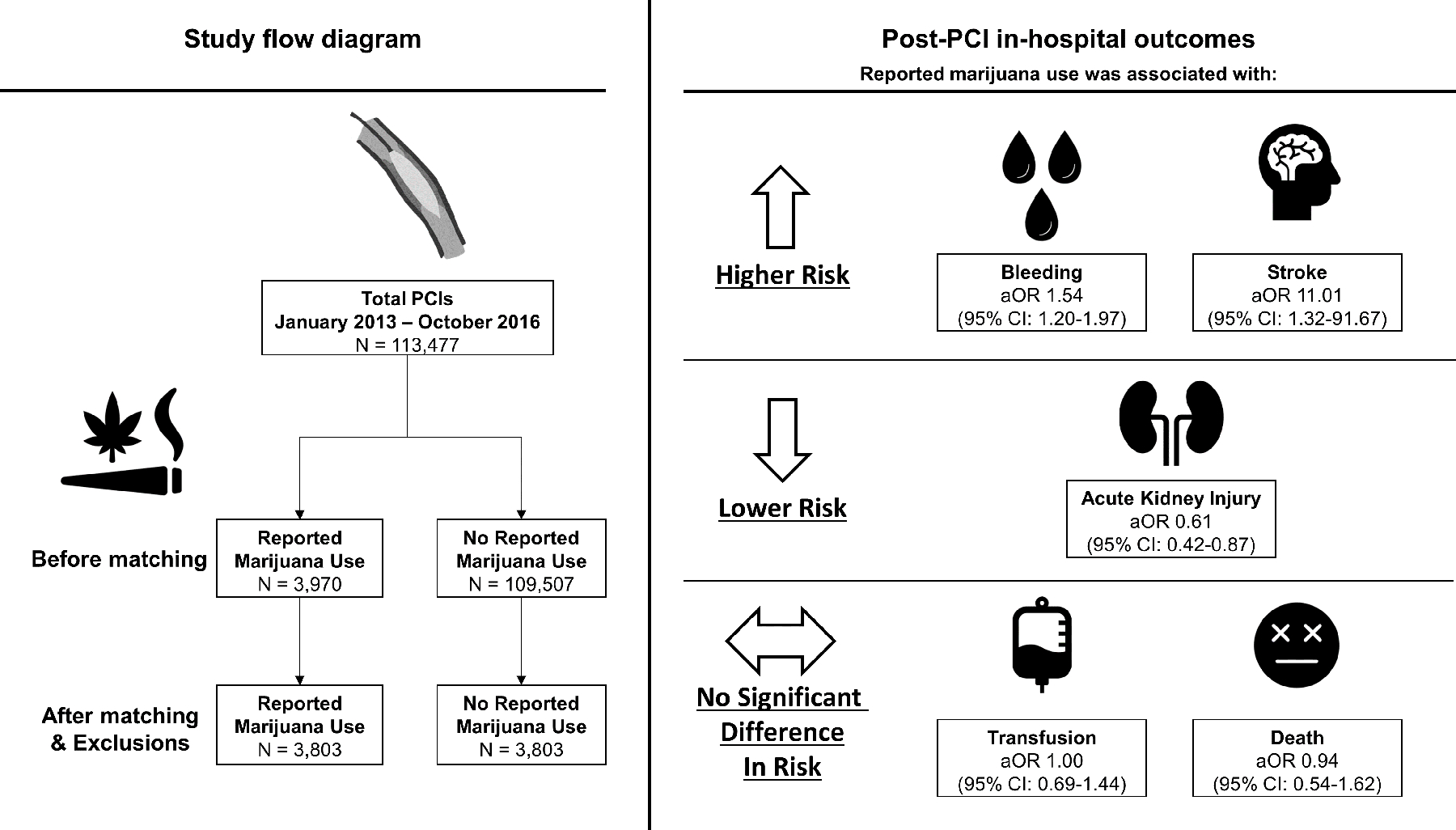
Study flow diagram and adjusted odds ratios of in-hospital outcomes after percutaneous coronary intervention in patients with or without reported marijuana use.
Prior to matching, patients with reported marijuana use were more likely to be younger (mean age 53.9 vs 65.8), men (79.2% vs 66.8%; p<0.001), and tobacco smokers (73.0% vs 26.8%; p<0.001). Patients with reported marijuana use were more likely to present with STEMI compared with patients without marijuana use (27.3% vs. 15.9%). Furthermore, patients who reported using marijuana were less likely to have cardiovascular comorbidities and traditional coronary artery disease risk factors, including diabetes mellitus, dyslipidemia, hypertension, or prior history of peripheral artery disease, heart failure, coronary artery bypass graft surgery, or CVA compared with those who did not use marijuana (Table 1). After propensity score matching, 3,803 patients were included in each study group. Absolute standardized differences between the two groups were less than 10% on all matched variables (Figure 1).
Table 1.
Patient characteristics before and after propensity score matching
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Reported marijuana use | Reported marijuana use | |||||
| No | Yes | P-value | No | Yes | P-value | |
| n= 109,507 (96.5%) | n= 3,970 (3.5%) | n= 3,803 (50.0%) | n= 3,803 (50.0%) | |||
|
| ||||||
| Men (%) | 73194 (66.8) | 3144 (79.2) | <0.001 | 3015 (79.3) | 3003 (79.0) | 0.756 |
| Age (mean (SD)) | 65.81 (11.81) | 53.85 (9.59) | <0.001 | 53.93 (10.03) | 53.91 (9.61) | 0.926 |
| Length of stay, days (Median (IQR)) | 2 (1, 3) | 2 (1, 3) | 0.527 | 2 (1, 3) | 2 (1, 3) | 0.766 |
| Race, n (%) | ||||||
| American Indian or Alaskan Native | 374 (0.3) | 23 (0.6) | 0.018 | 24 (0.6) | 20 (0.5) | 0.650 |
| Asian | 1271 (1.2) | 6 (0.2) | <0.001 | 5 (0.1) | 6 (0.2) | 1.000 |
| Black or African American | 11570 (10.6) | 1013 (25.5) | <0.001 | 963 (25.3) | 980 (25.8) | 0.674 |
| Native Hawaiian or Pacific Islander | 78 (0.1) | 3 (0.1) | 1 | 2 (0.1) | 2 (0.1) | 1.000 |
| White | 94732 (86.5) | 2860 (72.0) | <0.001 | 2743 (72.1) | 2732 (71.8) | 0.798 |
| Hispanic or Latino, n (%) | 1475 (1.4) | 60 (1.5) | 0.422 | 59 (1.6) | 58 (1.5) | 0.993 |
| Payer, n (%) | ||||||
| Indian Health Service | 55 (0.1) | 3 (0.1) | 0.641 | 3 (0.1) | 3 (0.1) | 1.000 |
| Medicaid | 13437 (12.6) | 1326 (37.3) | <0.001 | 1250 (36.5) | 1274 (37.4) | 0.446 |
| Medicare | 64112 (60.2) | 1257 (35.4) | <0.001 | 1154 (33.7) | 1213 (35.6) | 0.099 |
| Military Health Care | 1704 (1.6) | 64 (1.8) | 0.385 | 74 (2.2) | 57 (1.7) | 0.168 |
| Non-US Insurance | 255 (0.2) | 14 (0.4) | 0.097 | 16 (0.5) | 11 (0.3) | 0.450 |
| None | 3076 (2.8) | 418 (10.5) | <0.001 | 374 (9.8) | 394 (10.4) | 0.470 |
| Private Health Insurance | 78531 (73.8) | 1873 (52.7) | <0.001 | 1836 (53.5) | 1814 (53.2) | 0.802 |
| State-Specific Plan | 434 (0.4) | 36 (1.0) | <0.001 | 27 (0.8) | 32 (0.9) | 0.585 |
| Co-morbidities, n (%) | ||||||
| Cerebrovascular Disease | 17370 (15.9) | 422 (10.6) | <0.001 | 391 (10.3) | 409 (10.8) | 0.523 |
| Chronic Lung Disease | 21087 (19.3) | 908 (22.9) | <0.001 | 877 (23.1) | 881 (23.2) | 0.935 |
| Current/Recent Smoker* | 29375 (26.8) | 2896 (73.0) | <0.001 | 2804 (73.7) | 2780 (73.1) | 0.551 |
| Currently on Dialysis | 2794 (2.6) | 86 (2.2) | 0.143 | 79 (2.1) | 85 (2.2) | 0.693 |
| Diabetes Mellitus | 43532 (39.8) | 1113 (28.0) | <0.001 | 1063 (28.0) | 1073 (28.2) | 0.818 |
| Dyslipidemia | 88705 (81.1) | 2731 (68.9) | <0.001 | 2655 (69.9) | 2629 (69.2) | 0.522 |
| Hypertension | 93841 (85.7) | 3077 (77.5) | <0.001 | 2953 (77.6) | 2960 (77.8) | 0.869 |
| Peripheral Artery Disease | 17058 (15.6) | 516 (13.0) | <0.001 | 511 (13.4) | 502 (13.2) | 0.787 |
| Prior CABG | 20149 (18.4) | 389 (9.8) | <0.001 | 346 (9.1) | 380 (10.0) | 0.198 |
| Prior Heart Failure | 19503 (17.8) | 510 (12.8) | <0.001 | 493 (13.0) | 498 (13.1) | 0.892 |
| Prior Myocardial Infarction | 38122 (34.8) | 1431 (36.0) | 0.115 | 1386 (36.4) | 1380 (36.3) | 0.905 |
| Prior PCI | 50497 (46.1) | 1520 (38.3) | <0.001 | 1478 (38.9) | 1473 (38.7) | 0.925 |
| Prior Valve Surgery/Procedure | 2159 (2.0) | 37 (0.9) | <0.001 | 32 (0.8) | 37 (1.0) | 0.629 |
| Coronary Artery Disease Presentation, n (%) | <0.001 | 0.924 | ||||
| No symptom, no angina | 3963 (3.6) | 66 (1.7) | 62 (1.6) | 66 (1.7) | ||
| Non-STEMI | 25536 (23.3) | 1193 (30.1) | 1149 (30.2) | 1165 (30.6) | ||
| ST-Segment Elevation MI (STEMI) or equivalent | 17392 (15.9) | 1084 (27.3) | 976 (25.7) | 984 (25.9) | ||
| Stable angina | 10043 (9.2) | 216 (5.4) | 232 (6.1) | 211 (5.5) | ||
| Symptom unlikely to be ischemic | 2706 (2.5) | 59 (1.5) | 62 (1.6) | 58 (1.5) | ||
| Unstable angina | 49843 (45.5) | 1351 (34.0) | 1322 (34.8) | 1318 (34.7) | ||
CABG indicates coronary artery bypass grafting; PCI, percutaneous coronary intervention; and STEMI, ST-Elevation Myocardial infarction
Recent smoker is defined as smoking within 1 year.
Figure 1. Plot of absolute standardized differences before and after propensity score matching.
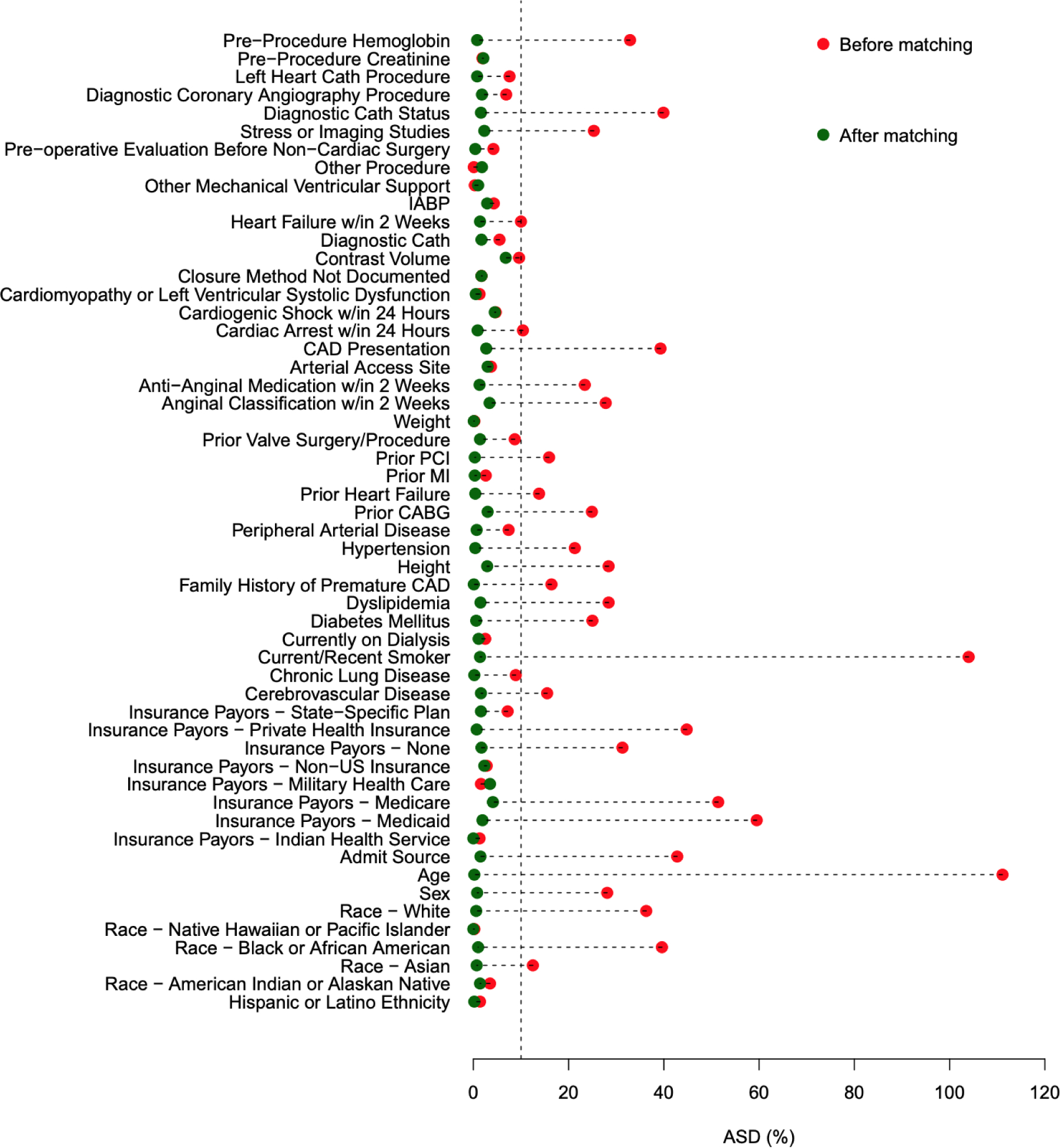
The absolute standardized differences before and after matching patients with and without a history of marijuana use. Abbreviations: CABG, coronary artery bypassing graft; CAD, coronary artery disease; IABP, intra-aortic balloon pump; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Procedural Medications
Prior to matching, patients with reported marijuana use were more likely to receive unfractionated heparin (85.6% vs 81.8%; p<0.001), glycoprotein IIb/IIIa inhibitors (28.5% vs 22.6%; p<0.001), prasugrel (18.2% vs 14.5%; p<0.001), ticagrelor (27.9% vs 22.8%; p<0.001) but less likely to receive clopidogrel (53.0% vs 59.6%; p<0.001) as procedural medications (Table 2). After matching, no significant differences in procedural medications were observed between patients with and without marijuana use.
Table 2.
Procedural medications in patients undergoing PCI with or without marijuana use, before and after propensity score matching.
| Before matching | ||||
| Reported marijuana use | ||||
| No | Yes | P-value | ASD (%) | |
| N (%) | n= 109,507 (96.5%) | n= 3,970 (3.5%) | ||
|
| ||||
| Aspirin (any) | 106711 (97.5) | 3868 (97.5) | 0.999 | 0.10% |
| Fondaparinux | 88 (0.1) | 4 (0.1) | 0.873 | 0.70% |
| Direct Thrombin Inhibitor (other) | 253 (0.2) | 6 (0.2) | 0.386 | 1.80% |
| Bivalirudin | 39464 (36.0) | 1359 (34.2) | 0.021 | 3.80% |
| Unfractionated Heparin (any) | 89546 (81.8) | 3398 (85.6) | <0.001 | 10.40% |
| Low Molecular Weight Heparins (any) | 2907 (2.7) | 114 (2.9) | 0.432 | 1.30% |
| Glycoprotein IIb/IIIa inhibitor (any) | 24713 (22.6) | 1130 (28.5) | <0.001 | 13.60% |
| Ticlopidine | 99 (0.1) | 1 (0.0) | 0.277 | 2.70% |
| Prasugrel | 15907 (14.5) | 721 (18.2) | <0.001 | 9.80% |
| Clopidogrel | 65241 (59.6) | 2104 (53.0) | <0.001 | 13.30% |
| Ticagrelor | 24965 (22.8) | 1106 (27.9) | <0.001 | 11.70% |
|
| ||||
| After Matching | ||||
| Reported marijuana use | ||||
| No | Yes | P-value | ASD (%) | |
| N (%) | n= 3,803 (50.0%) | n= 3,803 (50.0%) | ||
|
| ||||
| Aspirin (any) | 3705 (97.4) | 3711 (97.6) | 0.713 | 1.00% |
| Fondaparinux | 3 (0.1) | 4 (0.1) | 1.000 | 0.90% |
| Direct Thrombin Inhibitor (other) | 9 (0.2) | 5 (0.1) | 0.422 | 2.50% |
| Bivalirudin | 1291 (33.9) | 1299 (34.2) | 0.866 | 0.40% |
| Unfractionated Heparin (any) | 3276 (86.1) | 3256 (85.6) | 0.532 | 1.50% |
| Low Molecular Weight Heparins (any) | 111 (2.9) | 107 (2.8) | 0.837 | 0.60% |
| Glycoprotein IIb/IIIa inhibitor (any) | 1058 (27.8) | 1064 (28.0) | 0.898 | 0.40% |
| Ticlopidine | 2 (0.1) | 1 (0.0) | 1.000 | 1.30% |
| Prasugrel | 654 (17.2) | 685 (18.0) | 0.366 | 2.10% |
| Clopidogrel | 2066 (54.3) | 2035 (53.5) | 0.490 | 1.60% |
| Ticagrelor | 1081 (28.4) | 1058 (27.8) | 0.575 | 1.30% |
Procedural medications are defined as medications administered between 24 hours prior to and during the procedure. Abbreviations: ASD = absolute standardized difference.
In-hospital Outcomes
In the unmatched cohort, patients with reported marijuana use had a significantly higher rate of post-PCI bleeding (5.1 vs 3.5%; p<0.001) and a lower rate of post-PCI AKI (2.3% vs 3.4%; <0.001) compared with those who did not report using marijuana. However, there were no significant differences in post-PCI complications including stroke (0.3% vs 0.3% p=0.604), transfusion (2.3% vs 2.6%; p=0.316), and death (1.3% vs 1.6%; p=0.073) between the two groups (Table 3).
Table 3.
Post-PCI in-hospital outcomes before and after propensity matching
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Reported marijuana use | Reported marijuana use | |||||
| No | Yes | P-value | No | Yes | P-value | |
| n= 109,507 (96.5%) | n= 3,970 (3.5%) | n= 3,803 (50.0%) | n= 3,803 (50.0%) | |||
|
| ||||||
| In-Hospital Outcomes, n (%) | ||||||
| Bleeding | 3809 (3.5) | 204 (5.1) | <0.001 | 128 (3.4) | 196 (5.2) | <0.001 |
| CVA | 371 (0.3) | 11 (0.3) | 0.604 | 4 (0.1) | 11 (0.3) | 0.121 |
| Hemorrhagic CVA* | 62 (16.7) | 4 (36.4) | 1 (25.0) | 4 (36.4) | ||
| Blood Transfusion | 2805 (2.6) | 91 (2.3) | 0.316 | 85 (2.2) | 88 (2.3) | 0.876 |
| Acute Kidney Injury** | 3096/92453 (3.4) | 77/3426 (2.3) | <0.001 | 97/3366 (2.9) | 74/3367 (2.2) | 0.971 |
| Death | 1794 (1.6) | 50 (1.3) | 0.073 | 49 (1.3) | 49 (1.3) | 1.000 |
| Post-hoc Analysis | ||||||
| Hemodynamically Significant Arrhythmia | 1171 (1.1) | 68 (1.7) | <0.001 | 51 (1.3) | 64 (1.7) | 0.367 |
| Stent Thrombosis | 228 (0.2) | 12 (0.3) | 0.21 | 10 (0.3) | 12 (0.3) | 0.972 |
Percentage of hemorrhagic CVA reflects the total CVA events as denominators within each group.
Patients without post-procedural creatinine or pre-procedural creatinine and patients on dialysis were excluded in estimating event rate. The most common reason for exclusion was lack of post-procedural creatinine. For this reason, modified denominators are provided in the table. Missing rates for patients with and without marijuana use were 13.7% and 15.6% respectively. After matching, missing rates for post-procedural creatinine value for patients with and without marijuana use were 11.5% in both groups.
Abbreviations: CVA = cerebrovascular accident.
After matching, patients with reported marijuana use had significantly higher risks of bleeding (5.2% vs 3.4%; aOR 1.54 [95% CI, 1.20–1.97], p<0.001) and stroke (0.3% vs 0.1%; aOR 11.01 [95% CI, 1.32–91.67]; p=0.026), and a lower risk of AKI (2.2% vs 2.9%; aOR 0.61 [95% CI, 0.42–0.87]; p=0.007). Notably, among patients who experienced CVA, hemorrhagic strokes occurred in 36.4% (n/N=4/11) and 25% (n/N=1/4) of patients with and without a history of reported marijuana use, respectively (Table 3). There were no significant differences in the risks of transfusion (2.3 % vs 2.2%; aOR 1.00 [95% CI, 0.69–1.44]; p=0.876) and death (1.3% vs 1.3%; aOR 0.94 [95% CI, 0.54–1.62]; p=1.0) (Central Illustration, Figure 2). In a post-hoc analysis, there were no significant differences in the risks of hemodynamically significant arrhythmias (1.7% vs 1.3%; aOR 1.21 [95% CI 0.80–1.82]; p= 0.26) or stent thrombosis (0.3% vs 0.3%; aOR 1.02 [95% CI 0.40–2.58]; p=0.97) between the two groups (Table 3).
Figure 2. Adjusted odds ratios of in-hospital outcomes in the propensity score matched cohort.
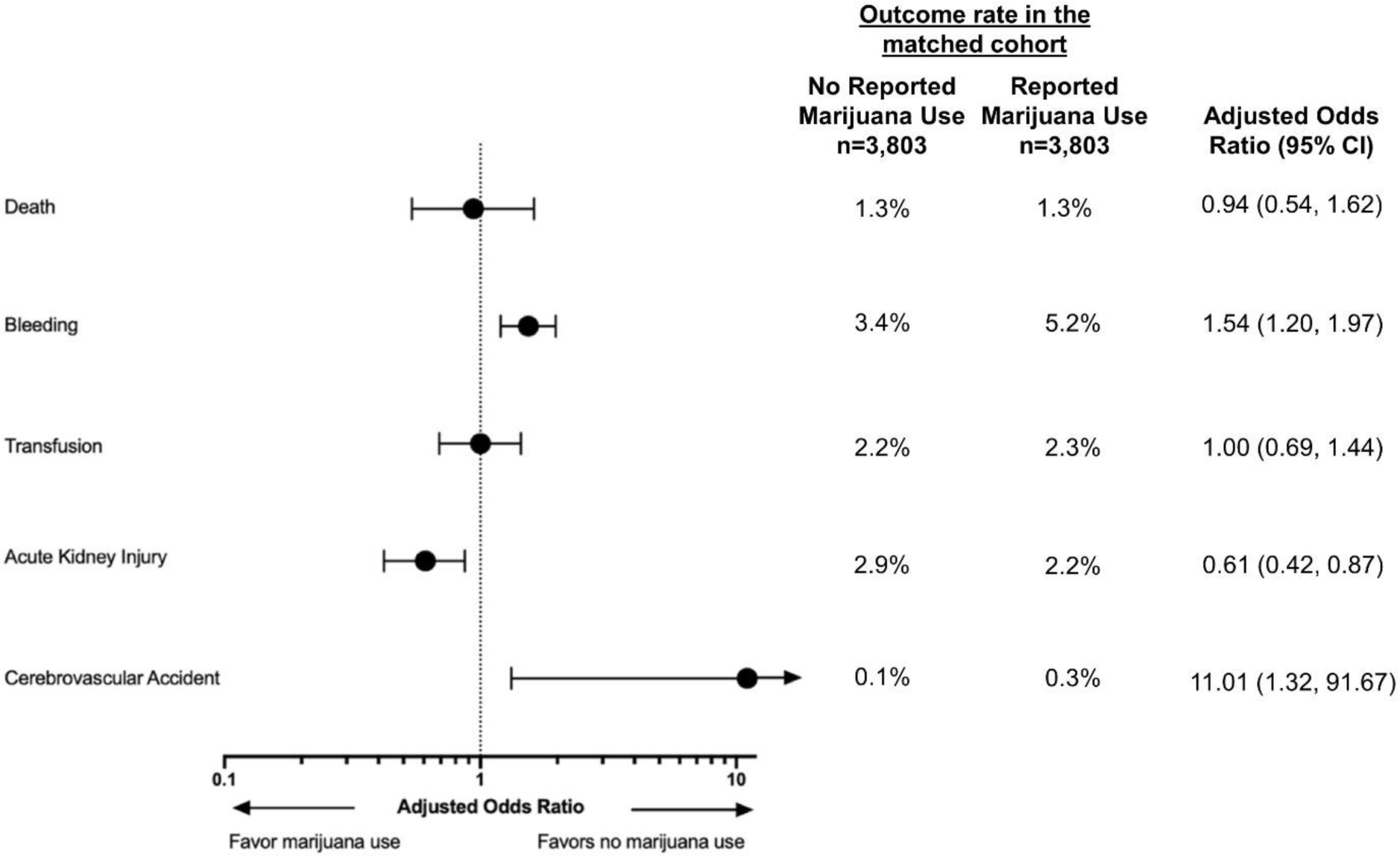
The forest plot depicts adjusted odds ratios and 95% confidence intervals (CIs) of in-hospital outcomes in the matched cohort.
Lastly, patients with reported marijuana use had significantly higher rates of both access site bleeding (2.6% vs 1.6%, p<0.0001) and non-access site bleeding (2.5% vs 1.9%, p=0.004) when compared with those without marijuana use (Supplementary Table 2). There were no significant differences in rates of femoral versus radial vascular access between the two groups after matching, with femoral access used in 65.6% and 66.0% of patients with and without reported marijuana use, respectively(Supplementary Table 3).
DISCUSSION
In this retrospective analysis of marijuana use and PCI outcomes in a large statewide multicenter registry of over 100,000 patients, we report three important findings. First, patients with reported marijuana use were more likely to present with STEMI, were on average 10 years younger, and had fewer cardiovascular comorbidities compared with patients without reported marijuana use. Second, patients with reported marijuana use had significantly higher risks of post-PCI bleeding and CVA, but a lower risk of AKI when compared with patients without marijuana use. Finally, there were no significant differences in the risks of in-hospital transfusion or death between patients with or without reported marijuana use.
The clinical characteristics of patients undergoing PCI with reported marijuana use markedly differed from those of patients without reported marijuana use. Consistent with prior studies, we found that patients with reported marijuana use were more frequently younger and had fewer cardiovascular comorbidities than patients without marijuana use (8–10). The lower prevalence of cardiovascular comorbidities and traditional cardiovascular risk factors, excluding tobacco use, among patients with marijuana use confounds the assessment of their cardiac event risk profile. Nevertheless, concomitant tobacco use likely increases the risks of acute coronary syndrome and may partially explain the younger age at the time of initial presentation. Moreover, some patients who use marijuana may also use other substances, such as cocaine, which could increase their risk for MI (18,19).
In addition to marijuana’s direct role in cardiovascular health, marijuana use may serve as a proxy for non-traditional cardiac risk factors. Patients with reported marijuana use were more likely to be uninsured, have Medicaid insurance, or identify as Black or African American. Indeed, barriers to substance use treatment and disparities in public health interventions may contribute to adverse cardiovascular health status (20–23). Of note, we were unable to account for differential environmental stressors such as structural racism, discrimination, and disparities in socioeconomic status and geography. Furthermore, there are likely unmeasured or unmeasurable cardiac risk factors associated with patients’ social experience that may impact outcomes.
Kwok and colleagues evaluated post-PCI in-hospital outcomes among patients with and without a diagnosis of cannabis dependence or cannabis use using the National Inpatient Sample administrative dataset (10). After adjusting for confounders using multivariable logistic regression, the authors reported no significant association between marijuana use and in-hospital mortality, stroke, or bleeding. However, they reported a decreased risk of vascular complications among patient with marijuana use compared with patients without marijuana use. In a propensity score-matched analysis, the authors reported similar findings except for a significantly higher risk of stroke among patients with marijuana use compared with those without marijuana use (10).
Building upon this prior body of work, we were able to overcome some of the limitations of analyses conducted with administrative data alone (10) by using granular peri-procedural demographic and clinical data. Similar to Kwok et al., we found no significant association between marijuana use and post-PCI in-hospital mortality. However, we reported a higher risk of stroke which was noted in the propensity-score matched analysis by Kwok et al but not in the multivariable logistic regression analysis. Finally, we reported a higher risk of bleeding associated with reported marijuana use. Although Kwok et al reported no significant association between marijuana use and bleeding, they found a lower risk of vascular complications which they may have been due to differences in radial versus femoral vascular access. Although it is possible that the increased risk of bleeding reported in our study may be due to unmeasured confounding, this association does not appear to be due to differences in vascular access sites between the two groups as the rates of radial and femoral access were similar after matching.
There are conflicting data surrounding the exact mechanism behind marijuana’s effect on clotting. Some studies suggest impaired platelet aggregation and others increased thrombogenesis with modulation of platelet function through the endocannabinoid system (2). Interestingly, despite increased population-level use of marijuana, our understanding of the in vivo effects of marijuana on platelet function and cardiovascular health remains limited. Previous research demonstrated that cannabinoid receptor agonists impaired collagen-induced platelet aggregation after consumption of Cannabis sativa (24). Moreover, higher doses of cannabinoids inhibited agonist-induced platelet aggregation more effectively, suggesting a dose-dependent association with bleeding risk (25). However, cannabinoids elevate inflammatory response by stimulating the sympathetic nervous system, which increases the expression of glycoprotein IIb-IIIa and P-selectin on platelet surface, activates factor VII, all leading to platelet aggregation (26,27). Even with an incomplete understanding between bleeding and marijuana use, our finding is clinically significant. In addition to effects on platelets, marijuana was reported to increase the incidence of atrial fibrillation and endothelial dysfunction, which may contribute to increased risks of CVA (28–31). A general population survey in Australia showed that marijuana use was associated with 3.3 times higher rate of CVA (29). In our analysis, patients with reported marijuana use had a higher risk of CVA; however, the confidence intervals were large due to the very small absolute number of events (11 vs. 4). As such, this finding may represent a spurious association and requires further evaluation in larger studies.
We also found that patients with reported marijuana use had lower risk of post-PCI AKI compared with patients without reported marijuana use. Previous analysis of the CARDIA study and NHANES did not reveal any association between cannabis use and eGFR (32,33). One possible explanation for our finding may be related to the effects of carbon monoxide generated from inhalation of smoke on the kidneys, as carbon monoxide reduces oxidative injury and decreases cell apoptosis (34). A previous study in mice demonstrated that activation of cannabinoid receptor type 2 reduces oxidative stress and inflammation during tubular injury. In addition, type 2 receptor activation antagonizes the effects of cannabinoid receptor type 1 activation which is associated with kidney injury (35,36). However, these represent hypothesized mechanisms, and as in any observational analysis, this finding may be due to the effect of unmeasured confounding. The relationship between marijuana use and kidney injury remains unclear and elucidating potential mechanisms by which marijuana use may be reno-protective will be an important area of future investigation, especially given the strong association of AKI with poor outcomes in the PCI population (37).
A recent scientific statement from the American Heart Association critically evaluated the safety and efficacy profile of marijuana where they noted limited cardiovascular benefits and potentially major harms (38). Currently, marijuana is considered a Schedule I drug at the federal level by the U.S. Drug Enforcement Administration making it illegal to conduct rigorous, controlled clinical trials of marijuana. With the increased marijuana use and its continued legalization across the United States, our findings underscore the importance of studying the effects of marijuana on cardiovascular disease. Due to significant barriers to marijuana-related research at this time, we encourage an open conversation between clinicians and patients regarding the limited scientific data available to help inform discussion about the potential risks and benefits of marijuana use.
Limitations
Our findings should be interpreted in the context of the following limitations. First, we used propensity matching to adjust for known confounders. However, given the nature of observational nature of analysis, propensity matching cannot account for unknown confounders and hence, our analysis cannot supplant a randomized study. Second, as marijuana use was obtained from patient medical records, there is a risk of reporting and ascertainment bias. Patients may not divulge their use of marijuana given that our study period was prior to the legalization of marijuana for recreational use. Also, if a patient was not asked about their use or their use was not recorded in the medical record, they would be considered a non-user. In our study, approximately 3–4% of patients reported using marijuana within 30 days prior to PCI and this finding may also be an underestimation of marijuana use. Nevertheless, our observations are based on the largest reported cohort of patients who use marijuana undergoing PCI and our findings remain important as the consequences of stroke and bleeding can be devastating. With Michigan’s legalization of recreational marijuana use in 2018, the prevalence of marijuana use among patients undergoing PCI is likely to be higher in current practice. Third, there may be a dose-dependent marijuana effect that we cannot account for as patients were not asked about frequency, dose, timing of the last dose relative to PCI, and concomitant use of other substances. Similarly, the cardiovascular effects of marijuana may differ by method of intake or formulation. Finally, the BMC2 PCI clinical registry is a state-wide collaborative quality improvement initiative and our findings may not be generalizable to other states that do not participate in such initiative (39).
CONCLUSIONS
This statewide retrospective observational study of patients who underwent PCI demonstrates that reported marijuana use was associated with higher risks of bleeding and CVA but lower risk of AKI. Despite the uncertainties surrounding use of marijuana, approximately 3–4% of patients presenting for PCI report recent marijuana use. As these estimates may be subject to under-reporting, the potential cardiovascular population at risk could be substantially higher. Especially as marijuana use continues to increase in the state of Michigan, and elsewhere, clinicians and patients should be aware of the increased risks of post-PCI complications in patients with marijuana use. The peri-procedural timeframe around PCI thus represents a window of opportunity for screening and counseling related to marijuana use. Further rigorous research evaluating the effects of marijuana on cardiovascular health is needed to provide evidence-based care for patients who use marijuana.
Supplementary Material
Clinical Perspectives.
WHAT IS KNOWN?
Marijuana use is increasing across the US. Its prevalence and safety profile among patients presenting for PCI are unclear.
WHAT IS NEW?
Approximately 3–4% of patients undergoing PCI reported using marijuana. Compared with those without marijuana use, patients with reported marijuana use had higher risks of post-PCI complications including CVA and bleeding, but a lower risk of AKI.
WHAT IS NEXT?
Future studies are necessary to understand mechanisms by which marijuana use is related to post-PCI outcomes and whether the effects of marijuana use are dose-dependent or related to the method of intake.
Funding source:
The BMC2 coordinating center is supported by a grant from Blue Cross Blue Shield of Michigan to the University of Michigan. The sponsor had no role in the study design or decision to publish this work.
Disclosures:
Dr. Yoo has received support from the Fogarty International Center and National Institute of Mental Health of the National Institutes of Health under Award Number D43 TW010543. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mr. Seth, Dr. Sukul, and Dr. Gurm receive salary support from Blue Cross Blue Shield of Michigan (BCBSM) for their roles in the Blue Cross Blue Shield of Cardiovascular Consortium. However, the opinions, beliefs and viewpoints expressed by the authors do not necessarily reflect those of BCBSM or any of its employees. Dr. Gurm receives research support from Blue Cross and Blue Shield of Michigan, the National Institutes of Health Center for Accelerated Innovations, and Michigan Translational Research and Commercialization for Life Sciences Innovation Hub. He is the co-founder of, owns equity in, and is a consultant to Calcium Solution. He also owns equity in Jiaxing Bossh Medical Technology Partnership and is a consultant for Osprey Medical. Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), receives research grant support from Amgen, serves on advisory boards for Amgen, AstraZeneca (AZ), Baxter Healthcare, Bayer, Boehringer Ingelheim, Cytokinetics, and Relypsa, and has participated on clinical endpoint committees for studies sponsored by Galmed, Novartis, and the NIH. All other authors do not have any relevant relationships to disclose.
Abbreviations:
- AKI
Acute Kidney Injury
- AMI
Acute Myocardial Infarction
- aOR
Adjusted Odds Ratio
- BMC2
Blue Cross Blue Shield Michigan Cardiovascular Consortium
- CARDIA
Coronary Artery Risk Development in Young Adults Study
- CVA
Cerebrovascular Accident
- NHANES
National Health and Nutrition Examination Survey
- NCDR
National Cardiovascular Data Registry
- PCI
Percutaneous Coronary Intervention
- STEMI
ST-Segment Elevation Myocardial Infarction
References
- 1.University of Michigan Center for Injury Prevention. Impact of Recreational Cannabis Legalization in Michigan: A Baseline Report. 2020. [Google Scholar]
- 2.Singla S, Sachdeva R, Mehta JL. Cannabinoids and atherosclerotic coronary heart disease. Clinical Cardiology 2012;35:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajesh M, Mukhopadhyay P, Haskó G, Liaudet L, MacKie K, Pacher P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. British Journal of Pharmacology 2010;160:688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han KH, Lim S, Ryu J et al. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovascular Research 2009;84:378–386. [DOI] [PubMed] [Google Scholar]
- 5.Frost L, Mostofsky E, Rosenbloom JI, Mukamal KJ, Mittleman MA. Marijuana use and long-term mortality among survivors of acute myocardial infarction. American Heart Journal 2013;165:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodondi N, Pletcher MJ, Liu K, Hulley SB, Sidney S. Marijuana Use, Diet, Body Mass Index, and Cardiovascular Risk Factors (from the CARDIA Study). American Journal of Cardiology 2006;98:478–484. [DOI] [PubMed] [Google Scholar]
- 7.DeFilippis EM, Bajaj NS, Singh A et al. Marijuana Use in Patients With Cardiovascular Disease: JACC Review Topic of the Week. J Am Coll Cardiol 2020;75:320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai R, Patel U, Sharma S, Amin P, Bhuva R, Patel MS. Recreational marijuana use and acute myocardial infarction: insights from nationwide inpatient sample in the United States. Cureus 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson-Sasso CP, Tompkins C, Kao DP, Walker LA. Marijuana use and short-term outcomes in patients hospitalized for acute myocardial infarction. PLoS One 2018;13:e0199705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwok CS, Alraies MC, Mohamed M, et al. Rates, predictors and the impact of cannabis misuse on in-hospital outcomes among patients undergoing percutaneous coronary intervention (from the National Inpatient Sample). Int J Clin Pract 2020;74(5):e13477. Doi: 10.1111/ijcp.13477. [DOI] [PubMed] [Google Scholar]
- 11.Kline-Rogers E, Share D, Bondie D et al. Development of a multicenter interventional cardiology database: The Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) experience. Journal of Interventional Cardiology 2002;15:387–392. [DOI] [PubMed] [Google Scholar]
- 12.Song C, Sukul D, Seth M et al. Outcomes After Percutaneous Coronary Intervention in Patients With a History of Cerebrovascular Disease: Insights From the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. Circ Cardiovasc Interv 2018;11:e006400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moscucci M, Rogers EK, Montoye C et al. Association of a continuous quality improvement initiative with practice and outcome variations of contemporary percutaneous coronary interventions. Circulation 2006;113:814–822. [DOI] [PubMed] [Google Scholar]
- 14.NCDR CathPCI Registry v4.4 Coder’s Data Dictionary [cited 2020 July 27]; Availabe from: https://www.ncdr.com/WebNCDR/docs/default-source/public-data-collection-documents/cathpci_v4_codersdictionary_4-4.pdf?sfvrsn=b84d368e_2
- 15.Slocum N, Grossman PM, Moscucci M et al. The changing definition of contrast-induced nephropathy and its clinical implications: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Am Heart J 2012;163(5):829–34 [DOI] [PubMed] [Google Scholar]
- 16.Harjal KJ, Raizada A, Shenoy C et al. A comparison of contemporary definitions of contrast nephropathy in patients undergoing percutaneous coronary intervention and a proposal for a novel nephropathy grading system. Am J Cardiol 2008;101(6):812–9 [DOI] [PubMed] [Google Scholar]
- 17.Sukul D, Bhatt DL, Seth M et al. Appropriateness and Outcomes of Percutaneous Coronary Intervention at Top-Ranked and Nonranked Hospitals in the United States. JACC Cardiovasc Interv 2018;11:342–350. [DOI] [PubMed] [Google Scholar]
- 18.Havranek EP, Mujahid MS, Barr DA et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2015;132:873–98. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay JA, Stotts AL, Green CE, Herin DV, Schmitz JM. Cocaine dependence and concurrent marijuana use: a comparison of clinical characteristics. Am J Drug Alcohol Abuse 2009;35:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis B, Hoffman L, Garcia CC, Nixon SJ. Race and socioeconomic status in substance use progression and treatment entry. J Ethn Subst Abuse 2018;17:150–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schauer GL, Peters EN, Rosenberry ZR, Kim H. Trends in and Characteristics of Marijuana and Menthol Cigarette Use Among Current Cigarette Smokers, 2005–2014. Nicotine Tob Res 2018;20:362–369. [DOI] [PubMed] [Google Scholar]
- 22.Yancy CW. Cardiovascular Disease Disparities: The Gap Remains. JAMA Cardiol 2018;3:1183. [DOI] [PubMed] [Google Scholar]
- 23.Scherer M, Voas RB, Furr-Holden D. Marijuana as a predictor of concurrent substance use among motor vehicle operators. J Psychoactive Drugs 2013;45:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Angelis V, Koekman AC, Weeterings C et al. Endocannabinoids control platelet activation and limit aggregate formation under flow. PLoS One 2014;9:e108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Formukong EA, Evans AT, Evans FJ. The Inhibitory Effects of Cannabinoids, The Active Constituents of Cannabis sativa L. on Human and Rabbit Platelet Aggregation. Journal of Pharmacy and Pharmacology 1989;41:705–709. [DOI] [PubMed] [Google Scholar]
- 26.Deusch E, Kress HG, Kraft B, Kozek-Langenecker SA. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesthesia and Analgesia 2004;99:1127–1130. [DOI] [PubMed] [Google Scholar]
- 27.Heiden D, Rodvien R, Jones R, Mielke CH Jr. Effect of oral delta-9-tetrahydrocannabinol on coagulation. Thrombosis Research 1980;17:885–889. [DOI] [PubMed] [Google Scholar]
- 28.Volpon LC, De Melo Sousa CLM, Moreira SKK, Teixeira SR, De Carvalho Panzeri Carlotti AP. Multiple Cerebral Infarcts in a Young Patient Associated with Marijuana Use. Journal of Addiction Medicine 2017;11:405–407. [DOI] [PubMed] [Google Scholar]
- 29.Hemachandra D, McKetin R, Cherbuin N, Anstey KJ. Heavy cannabis users at elevated risk of stroke: Evidence from a general population survey. Australian and New Zealand Journal of Public Health 2016;40:226–230. [DOI] [PubMed] [Google Scholar]
- 30.Singh NN, Pan Y, Muengtaweeponsa S, Geller TJ, Cruz-Flores S. Cannabis-related stroke: Case series and review of literature. Journal of Stroke and Cerebrovascular Diseases 2012;21:555–560. [DOI] [PubMed] [Google Scholar]
- 31.Zachariah SB. Stroke after heavy marijuana smoking. Stroke 1991;22:406–408. [DOI] [PubMed] [Google Scholar]
- 32.Lu C, Papatheodorou SI, Danziger J, Mittleman MA. Marijuana Use and Renal Function Among US Adults. Am J Med 2018;131:408–414. [DOI] [PubMed] [Google Scholar]
- 33.Ishida JH, Auer R, Vittinghoff E et al. Marijuana Use and Estimated Glomerular Filtration Rate in Young Adults. Clin J Am Soc Nephrol 2017;12:1578–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csongradi E, Juncos LA, Drummond HA, Vera T, Stec DE. Role of carbon monoxide in kidney function: is a little carbon monoxide good for the kidney? Curr Pharm Biotechnol 2012;13:819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park F, Potukuchi PK, Moradi H, Kovesdy CP. Cannabinoids and the kidney: effects in health and disease. Am J Physiol-Renal 2017;313(5):F1124–32. Doi: 10.1152/ajprenal.00290.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukhopadhyay P, Rajesh M, Pan H, et al. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radical Bio Med 2010;48(3):457–67. Doi: 10.1016/j.freeradbiomed.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kooiman J, Seth M, Nallamothu BK, Heung M, Humes D, Gurm HS. Association between acute kidney injury and in-hospital mortality in patients undergoing percutaneous coronary interventions. Circ Cardiovasc Interv 2015;8:e002212. [DOI] [PubMed] [Google Scholar]
- 38.Page RL II, Allen LA, Kloner RA et al. Medical Marijuana, Recreational Cannabis, and Cardiovascular Health. Circulation. 2020; 142:00–00 [DOI] [PubMed] [Google Scholar]
- 39.Share DA, Campbell DA, Birkmeyer N et al. How A Regional Collaborative of Hospitals and Physicians In Michigan Cut Costs And Improved The Quality of Care. Health Affairs 2011;Vol 30, No [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


